Abstract
Scope
Zinc deficiency results in immune dysfunction and promotes systemic inflammation. The objective of this study was to examine the effects of zinc deficiency on cellular immune activation and epigenetic mechanisms that promote inflammation. This work is potentially relevant to the aging population given that age-related immune defects, including chronic inflammation, coincide with declining zinc status.
Methods and results
An in vitro cell culture system and the aged mouse model were used to characterize immune activation and DNA methylation profiles that may contribute to the enhanced proinflammatory response mediated by zinc deficiency. Zinc deficiency up-regulated cell activation markers ICAM1, MHC class II, and CD86 in THP1 cells, that coincided with increased IL1β and IL6 responses following LPS stimulation. A decreased zinc status in aged mice was similarly associated with increased ICAM1 and IL6 gene expression. Reduced IL6 promoter methylation was observed in zinc deficient THP1 cells, as well as in aged mice and human lymphoblastoid cell lines derived from aged individuals.
Conclusion
Zinc deficiency induced inflammatory response in part by eliciting aberrant immune cell activation and altered promoter methylation. Our results suggested potential interactions between zinc status, epigenetics, and immune function, and how their dysregulation could contribute to chronic inflammation.
Keywords: zinc, inflammation, aging, epigenetics, DNA methylation
1. Introduction
Zinc is an essential micronutrient and a key component for the function of numerous proteins, including zinc-containing metalloenzymes and zinc-associated transcription factors [1]. It is required for many biological processes, including growth and development, neurological function, reproduction, and immunity. There is little storage for zinc in the human body, and bioavailable zinc from food or supplements must replenish the zinc pool at a regular basis. As a consequence, alterations in zinc uptake, retention, or secretion can quickly lead to zinc deficiency. In particular, since zinc is critical for the development and function of the immune system, zinc deficiency results in a wide range of immune defects including lymphopenia, dysregulation and impairment of both adaptive and innate immune, and increased susceptibility to infectious diseases [2–4]. At the same time, zinc has anti-inflammatory properties, and zinc deficiency promotes systemic inflammation. We and others have shown that zinc deficiency was associated with increased inflammation in cell culture models, as well as in murine models of microbial sepsis, allergic airway inflammation, and aging [5–9]. However to date the precise mechanisms by which zinc deficiency contributes to enhanced inflammation is not well understood.
The elderly population is particularly at risk for zinc deficiency. A decline in zinc status is observed with age, likely due to a combination of inadequate dietary zinc intake, and age-related alteration in the utilization, distribution, and absorption of zinc [10–13]. Importantly, immune defects associated with aging share many similar features as zinc deficiency. One of the many hallmarks of aging is chronic inflammation. Increases in inflammatory mediators in the blood are significant predictors of morbidity and mortality in aged individuals, and chronic inflammation can contribute to the promotion of many age-related diseases, including cardiovascular disease, type-2 diabetes, cancer and autoimmune diseases [14, 15]. Reduced zinc status with age may be one of the factors contributing to age-related chronic inflammation.
Zinc homeostasis is involved in the signaling events in a variety of immune cells, where changes in intracellular zinc can trigger immune cell signaling and activation [16–19]. Altered intracellular zinc levels due to zinc deficiency could potentially result in aberrant signaling, inappropriate cell activation, and dysregulated proinflammatory response [20, 21]. During aging, cellular signaling regulation and immune activation have been shown to be altered [22, 23]. Age-related zinc deficiency may play a role in the dysregulation, as a reduction in intracellular zinc in immune cells was associated with aberrant immune activation and increased inflammatory response in aged mice [8]. In addition, age-related epigenetic alterations, including changes in DNA methylation profiles, can result in aberrant gene expression, and potentially play a role in chronic inflammation [24–27]. We had previously shown that age-related changes in DNA methylation status resulted in dysregulated zinc transporter expression [8]. Similarly, others have shown that zinc deficiency may enhance proinflammatory response via epigenetic mechanisms [28]. However, the role of age-related zinc deficiency on DNA methylation changes, and their contribution to chronic inflammation, remains to be clarified.
Increasing evidence indicates zinc deficiency likely enhance inflammatory response via multiple mechanisms. One of the focuses of our laboratory is to understand the underlying cellular and epigenetic mechanisms by which zinc deficiency mediates the chronic inflammatory immune response. The goal of this current study is to characterize changes in immune activation and DNA methylation profiles during the course of zinc deficiency that may contribute to the induction of proinflammatory response. We hypothesize that zinc deficiency, induced using an in vitro cell culture system as well as in the aged mouse model, can promote chronic inflammation in part by triggering immune cell activation, as well as by altering DNA methylation status of genes involved in the induction of a proinflammatory response.
2. Materials and Methods
2.1 Cell culture, in vitro zinc depletion, and LPS treatments
Human monocytic cell line THP-1 was obtained from American Type Tissue Collection (Manassas, VA). Cells were grown in RPMI 1640 culture medium with 10% fetal bovine serum (FBS), and maintained in humidified incubators with 5% CO2 at 37°C. To create zinc deficient (ZD) culture media, zinc was removed from FBS as previously described [8]. Briefly, FBS was incubated with Chelex 100 (10% w/v) (Sigma, St. Louis, MO) overnight at 4°C with constant stirring. Zinc adequate (ZA) control media was made by replenishing zinc (4 µM ZnSO4) in ZD media to levels similar to culture media made with non-Chelex treated FBS. Calcium and magnesium were added back to all Chelex-treated media to levels similar to non-Chelex-treated media. Mineral concentrations of calcium, magnesium, iron, copper, and zinc in ZA and ZD media were verified by inductively coupled plasma optical emission spectrometry (ICP-OES).
THP-1 cells were cultured in ZA media (4 µM ZnSO4) or ZD media (0 µM ZnSO4) for up to 3 weeks prior to the evaluation of proinflammatory response in the respective groups. Proinflammatory response was induced using lipopolysaccharide (LPS) (Sigma). ZA or ZD THP-1 cells were seeded in 24-well tissue culture plates at 5×105 cells/well, and treated with 0 or 100 ng/ml LPS for 24h for flow cytometry and proinflammatory cytokines analyses. In groups treated with DNA demethylating agent, 5-aza-2'-deoxycytidine (AZA) (Sigma), 5 µM AZA was used and cells were incubated with AZA for 48h prior to LPS treatment. Cells and culture supernatants were collected 24h post-LPS treatment for subsequent analyses.
2.2. Animal study
Young (2mo) and aged (26mo) female C57Bl/6 mice were purchased from the aged rodent colonies at the National Institute on Aging (NIA) (Bethesda, MD, USA). Mice were housed in a temperature- and humidity-controlled environment and were fed a zinc adequate diet containing 30 mg/kg zinc [29]. Food and water were provided ad libitum. Following one week acclimation period, mice were euthanized and spleens were collected and stored in RNALater (Life Technologies, Grand Island, NY) for subsequent DNA and RNA isolation. The animal protocol (ACUP#4204) was approved by the Oregon State University Institutional Laboratory Animal Care and Use Committee.
2.3 Human aging repository DNA panel
NIA aging cell repository DNA panel and matching young controls were purchased from Coriell Cell Repositories (Camden, NJ). The aging panel contained genomic DNA isolated from human lymphoblastic cell lines derived from ten individuals between 92–101 years old (5 females, 5 males). Genomic DNA isolated from lymphoblastic cell lines derived from ten young individuals (between 20–28 years old, 5 females and 5 males) were used as young control.
2.4 Total and intracellular zinc measurements
Total and intracellular zinc concentrations in ZA and ZD THP1 cells were determined as previously described [8]. Total zinc concentrations were determined ICP-OES, where samples (THP1 cell pellets containing 5×106 cells each) were digested in 0.5 ml 70% ultrapure nitric acid and incubated overnight. Incubated samples were diluted with Chelex-treated nanopure water to a final concentration of 7% nitric acid, centrifuged and analyzed using the Prodigy High Dispersion ICP-OES instrument (Teledyne Leeman Labs, Hudson, NH, USA) against known standards. Intracellular zinc levels were determined using FluoZin-3 acetoxymethyl ester (FluoZin-3), a cell permeable, zinc-specific fluorescent indicator that measures intracellular free zinc (Molecular Probes, Eugene, OR, USA). Cells were labeled with 1 µM FluoZin-3 for 30 min at 37°C and washed once in phosphate-buffered saline. Intracellular zinc levels, as determined by FluoZin-3 mean fluorescence intensity, were analyzed by flow cytometry.
2.5 Flow cytometry analyses of cell activation markers
THP-1 cells (untreated and LPS-treated) were resuspended in flow cytometry buffer (PBS, 2% FBS, 1mM EDTA), and incubated with antibodies specific against CD54 (ICAM1), CD14, CD86, and HLA-DR (MHC class II) for 30 min on ice in the dark (eBioscience, San Diego, CA). Unstained cells were used as negative control. Labeled cells were washed twice and resuspended in buffer for flow cytometry analysis. Data were acquired using FACSCalibur (BD Biosciences, San Jose, CA). Data were analyzed using Summit software (DakoCytomation, Fort Collins, CO).
2.6 Proinflammatory cytokine measurements
THP-1 culture supernatants were collected 24h post-LPS treatment for cytokine analysis by ELISA. Quantification of human cytokines IL-1β and IL-6 were detected using cytokine-specific Ready-SET-Go!® ELISA kits from eBioscience.
2.7 Gene expression analyses
Total RNA was isolated from the spleens of young and old mice using TRIzol (Life Technologies), and reverse transcribed into cDNA using SuperScript III First-Strand Synthesis SuperMix for quantitative real-time PCR (Life Technologies). The following PCR primers were used for real-time PCR: mouse IL6 (forward: GAGGATACCACTCCCAACAGACC, reverse: AAGTGCATCATCGTTGTTCATACA), mouse ICAM1 (forward: TTCACACTGAATGCCAGCTC, reverse: GTCTGCTGAGACCCCTCTTG), and mouse 18S ribosomal RNA (18S) (forward: CCGCAGCTAGGAATAATGGAAT, reverse: CGAACCTCCGACTTTCGTTCT). Real-time PCR reactions were done using Fast SYBR Green Mastermix (Life Technologies) on 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Gene copies were determined using the standard curve method. A standard curve was generated from serial dilutions of purified plasmid DNA that encoded for each gene of interest. Data represent the copy number of the gene of interest normalized to the copy number of 18S housekeeping gene, and normalized fold-change relative to control was shown.
2.8 DNA methylation analyses
Genomic DNA was isolated from THP1 cells and mouse spleens using DNeasy Blood and Tissue kit (Qiagen, Valencia, CA). For pyrosequencing, genomic DNA was treated with sodium bisulfite using EpiTech Bisulfite Kit (Qiagen). Pyrosequencing PCR and sequencing primers were designed using PyroMark Assay Design version 2.0.1 software (Qiagen). Human IL6 pyrosequencing primers were designed to encompass the IL6 promoter region where the methylation status was associated with IL6 gene regulation [25]: Forward primer (AGGGTTTTTGAATTAGTTTGATTTAATAAG), biotinylated reverse primer (CTCCCTCTCCCTATAAATCTTAAT), and sequencing primer (ATTTAATAAGAAATTTTTGGGTG). Mouse IL6 pyrosequencing primers were designed to encompass analogous promoter region as in human IL6: Biotinylated forward primer (TGTTTAGGTTGGGTGTTG), reverse primer (ACCCTAAAAAACATAAACACTCTTC), and sequencing primer (AAACTAAAACTAATATTATTTAAAC). PCR amplification of bisulfite-converted genomic DNA was done using PyroMark PCR kit (Qiagen) under conditions as specified by the manufacturer. PCR products were submitted to Stanford University Protein and Nucleic Acid Facility (Palo Alto, CA) for pyrosequencing. Quantitative methylation analyses were done using PyroMark Q23 version 2.0.6 software (Qiagen). For global DNA methylation analyses, total DNA methylation in genomic DNA was measured using SuperSense Methylated DNA Quantification Kit (Epigentek, Brooklyn, NY), and was reported as relative fluorescence units (RFU) per 200 ng genomic DNA.
2.9 Statistical analyses
Statistical analyses were done using GraphPad Prism Version 5.02 (GraphPad, La Jolla, CA). All data were reported as mean ± SEM. P values were determined using unpaired t test, one-way ANOVA, or two-way ANOVA where appropriate. Bonferroni post-hoc tests were performed to determine differences among the means when there was a significant main effect in one-way or two-way ANOVA. Statistical significance was defined as P ≤ 0.05.
3. Results
3.1 Zinc deficiency induced increased cell activation and increased proinflammatory response in THP1 cells
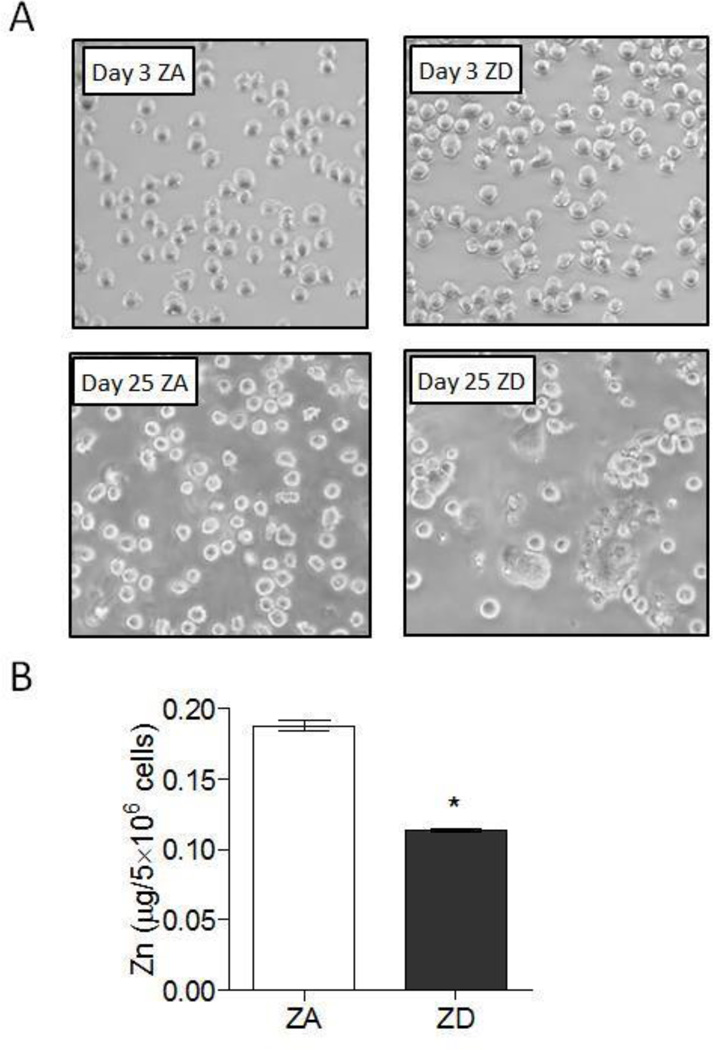
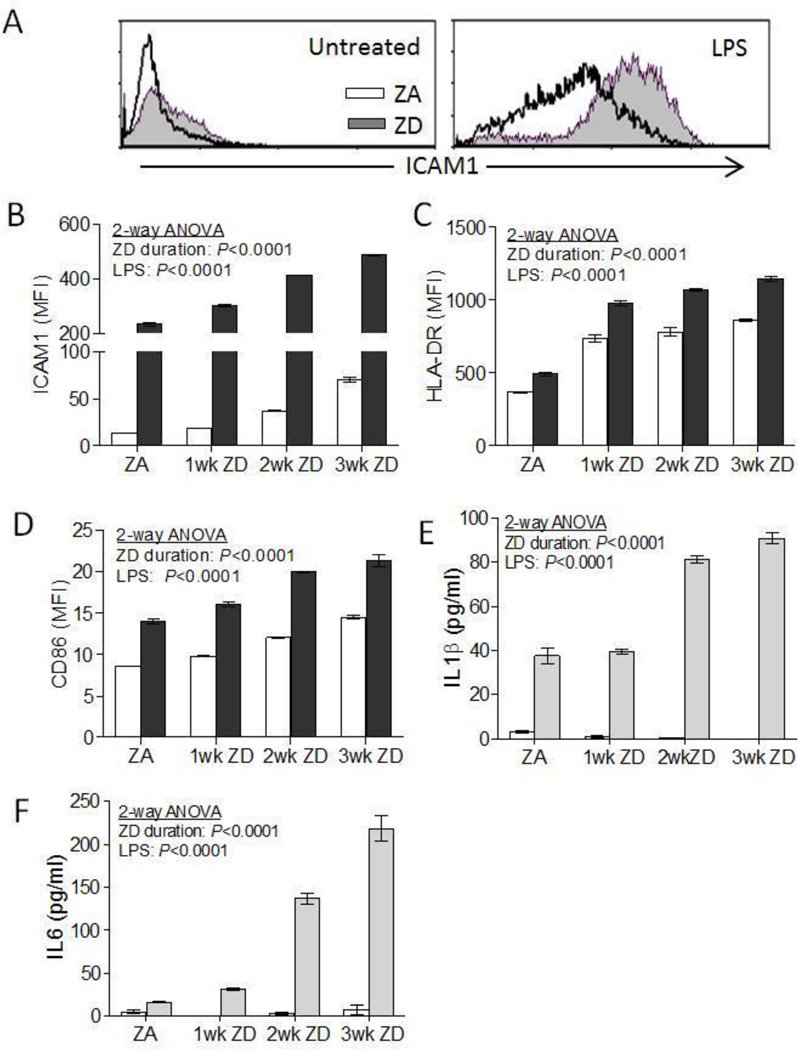
Macrophages and their monocyte precursors play an important role in the initiation, maintenance, and regulation of the inflammatory process [30, 31]. THP1 is a human acute monocytic leukemia cell line that can develop a macrophage-like phenotype and function upon stimulation. When activated with phorbol 12-myristate 13-acetate (PMA), THP1 monocytes acquire a macrophage-like phenotype and function that is characterized by the differentiation into adherent, well spread macrophages. This differentiation and activation is accompanied by the up-regulation of cell surface expression of intercellular adhesion molecule-1 (ICAM1), MHC class II (HLA-DR), and costimulator molecule CD86, among others. These hallmarks serve as surrogate markers for cell activation that primes the macrophages for the rapid and robust induction of a proinflammatory response upon exposure to an inflammatory stimulus such as LPS [32–34]. In the absence of any differentiation signal or proinflammatory stimulation, THP1 cells cultured in zinc adequate (ZA) media maintained characteristics of an undifferentiated monocyte phenotype, where cells remained rounded and in suspension. In contrast, we observed that THP1 cells cultured in zinc deficient (ZD) media had increasing cell adherence and altered cell morphology over time. By day 25, ZD THP1 cells were highly adherent, with distinctly flattened morphology that resembled activated macrophages despite the absence of PMA treatment or any other stimuli (Figure 1a). Increased cell adherence correlated with reduced total and intracellular zinc content (Figure 1b and supplemental Figure 1, respectively). Flow cytometry analyses showed that increased cell adherence in ZD THP1 cells was associated with a significant and progressive increase in the expression of cell activation markers ICAM1, HLA-DR, and CD86 when compared to ZA THP1 cells. Upon LPS stimulation, all three cell activation markers were further upregulated, and were significantly higher in ZD THP1 cells compared to ZA THP1 cells (Figure 2a–d). Our data suggested that zinc deficiency resulted in the activation of THP1 monocytes to acquire a macrophage-like phenotype.
Figure 1. THP1 cells cultured in zinc deficient media had increased cell adherence and decreased total zinc.
THP1 cells were cultured in zinc adequate (ZA) or zinc deficient (ZD) media for up to 25d. (A) Representative images showing ZA and ZD THP1 cell morphology at 3d and 25d. (B) Total zinc in ZA and ZD THP1 cells were determined by ICP-OES after two weeks culture in ZA and ZD media, respectively (n=3 per group). Data represent mean ± SEM. *P < 0.01 vs. ZA. Results are representative of three independent experiments.
Figure 2. Zinc deficiency induced macrophage-like cell activation and increased proinflammatory response in THP1 cells.
THP1 cells were cultured in ZA or ZD media for up to 3 weeks, followed by LPS stimulation (100 ng/ml) for 24h (n=3–4 per group). Cell surface expression of activation markers ICAM1, HLA-DR, and CD86 were analyzed by flow cytometry. (A) Representative flow cytometry histograms showing ICAM1 expression in untreated and LPS-treated ZA and ZD THP1 cells. (B) ICAM1, (C) HLA-DR, and (D) CD86 cell surface expression in THP1 cells cultured in ZA media, or ZD media for 1, 2, and 3 weeks followed by 24h LPS treatment (100 ng/ml) (black bars). Control cells were left untreated (white bars). (E) IL1β and (F) IL6 proinflammatory cytokines production in culture supernatant in LPS-treated (gray bars) and untreated (white bars) ZA and ZD THP1 cells. Data represent mean ± SEM. Results are representative of three independent experiments. MFI, mean fluorescence intensity.
We next tested whether increased cell activation mediated by zinc deficiency was accompanied by an increase in proinflammatory response in THP1 cells. IL1β and IL6 production were determined in both untreated and LPS-stimulated ZA and ZD THP1 cells. There was no significant production of IL1β and IL6 in unstimulated ZA and ZD THP1 cells (Figure 2e, f). Upon LPS stimulation, production of IL1β and IL6 was observed in ZA THP1 cells. LPS-treated ZD THP1 cells had significantly increased IL1β and IL6 production compared to ZA THP1 cells after 2 weeks culture in ZD media. IL6 production in ZD THP1 cells was further increased after 3 weeks culturing period (Figure 2e, f). Similar temporal increase in TNFα and MCP1 was observed in ZD THP1 cells (data not shown).
3.2 Zinc deficiency resulted in decreased IL6 promoter methylation
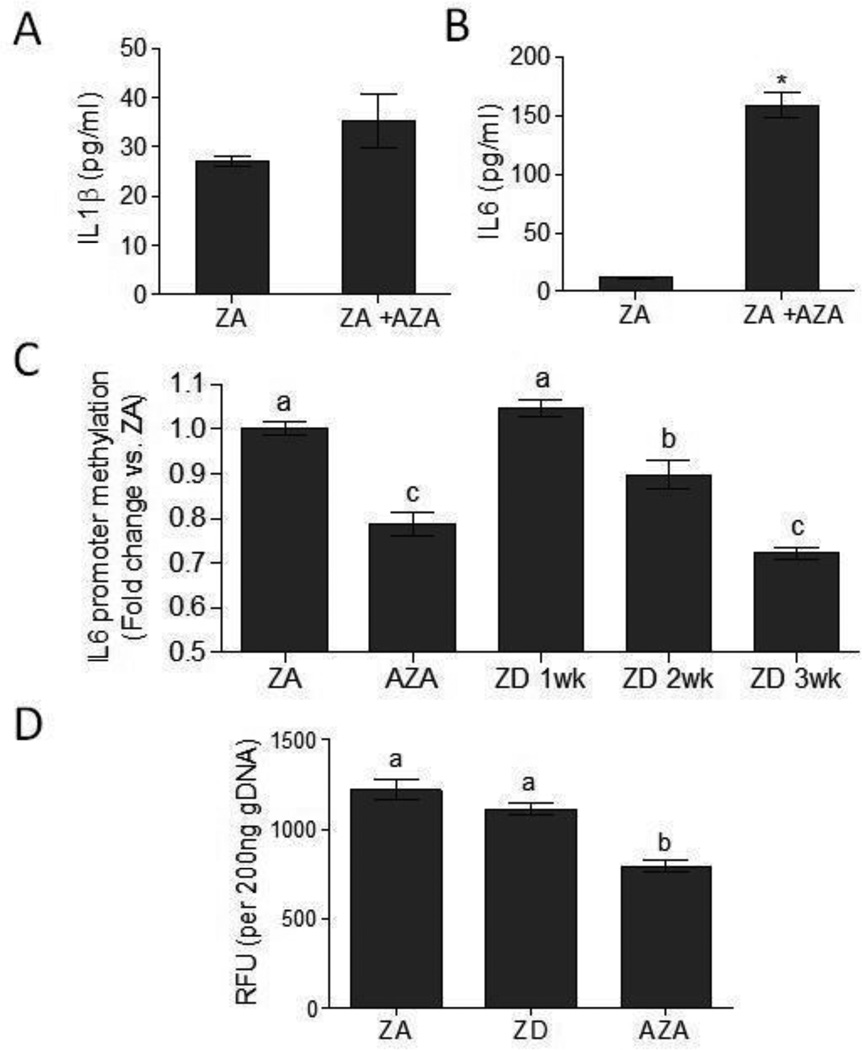
Epigenetics, including DNA methylation, are important mechanisms in the regulation of inflammatory genes [27]. In particular, the methylation status of proinflammatory cytokine genes may play a role in the pathogenesis of inflammatory diseases [25, 35, 36], and DNA demethylation via the use of 5-aza-2'-deoxycytidine (AZA) have been shown to induce the production of proinflammatory cytokines [36–38] Zinc deficiency has been proposed to have effects on the epigenome. In particular, zinc deficiency may alter DNA methylation profiles, as zinc likely has an important function in maintaining methylation status in the cell [9, 39]. To determine how methylation status affects the proinflammatory response, THP1 cells were treated with the DNA demethylation agent, AZA, for 48h prior to LPS stimulation. DNA demethylation significantly increased the production of LPS-induced proinflammatory cytokine IL6 but not IL1β (Figure 3a, b). We therefore focused our subsequent DNA methylation analyses on IL6. We targeted a specific IL6 promoter region that methylation status has shown correlation with IL6 gene regulation, and was associated with the pathogenesis of rheumatoid arthritis, a proinflammatory autoimmune disease. [25]. As expected, AZA treatment resulted in a significant decrease in IL6 promoter methylation compared to untreated ZA control (Figure 3c). We further showed that zinc deficiency resulted in progressive IL6 promoter demethylation in ZD THP1 cells. A significant decrease in IL6 promoter methylation was observed in ZD THP1 cells after two weeks culture in ZD media compared to ZA control (Figure 3c). A significant further decrease in IL6 promoter methylation was observed in THP1 cells cultured for three weeks in ZD media. Decreased IL6 promoter methylation coincided with both increased IL6 production and reduced cellular zinc level. Our data suggested that zinc deficiency resulted in a decrease in IL6 promoter methylation that could contribute to enhanced IL6 response. There were no significant differences in total DNA methylation levels between ZA and ZD groups (Figure 3d). Thus ZD-mediated decrease in DNA methylation was site-specific, and not attributed to a global decrease in DNA methylation.
Figure 3. Zinc deficiency resulted in decreased IL6 promoter methylation.
(A) IL1β and (B) IL6 proinflammatory cytokines production in ZA THP1 cells left untreated, or treated with 5 µM AZA for 48h prior to LPS stimulation (100 ng/ml) for 24h (n=3 per group). Data represent mean ± SEM. *P<0.01 vs. ZA. Results are representative of two independent experiments. (C) IL6 promoter methylation in ZA, ZD, and AZA-treated THP1 cells (n=5–9 per group). Data represent mean promoter methylation fold-change ± SEM vs. ZA. Means without a common letter differ, P < 0.05. (D) Global DNA methylation in ZA, ZD (3 wk), and AZA-treated THP1 cells (n=4 per group). Data represents mean relative fluorescence units (RFU) ± SEM per 200 ng DNA. Means without a common letter differ, P < 0.05. Results are representative of two independent experiments.
Aging was associated with increased ICAM1 and IL6 gene expression and decreased IL6 promoter methylation
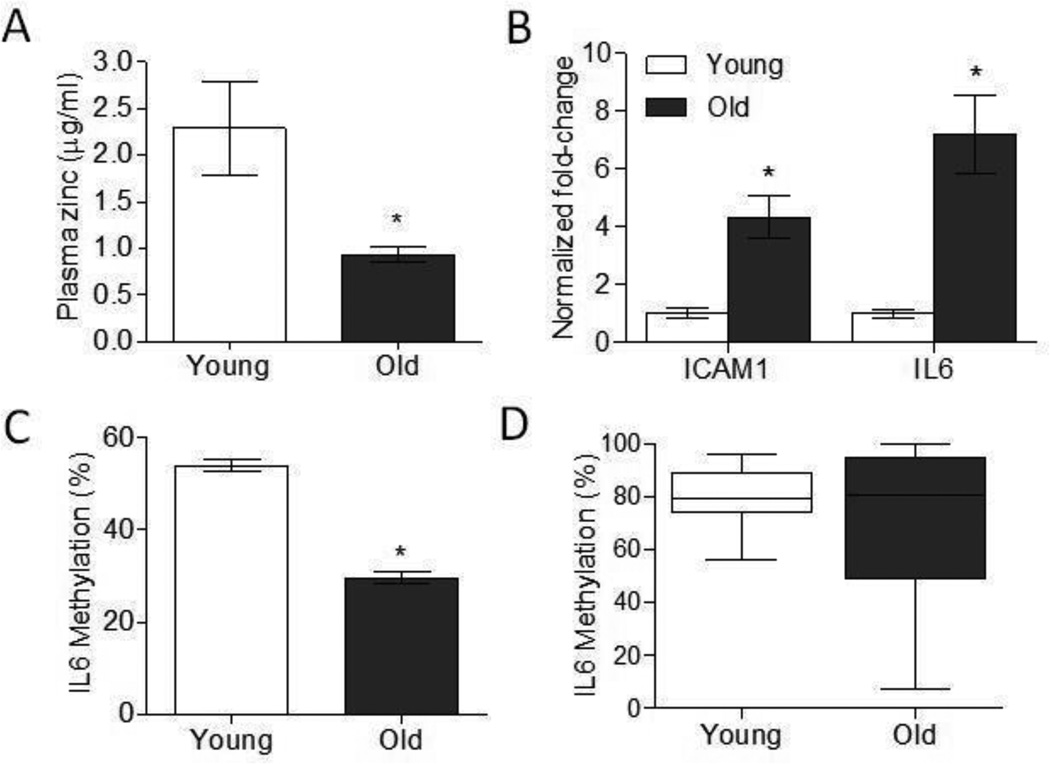
We had previously shown that age-related decline in zinc status in mice, particularly in immune subsets, may be one potential mechanism that contributes to increased susceptibility to inflammation with age [8]. To determine if the effects of zinc deficiency we observed in THP1 cells extend to immune cells in aged mice, we examined ICAM1 and IL6 gene expression, as well as IL6 promoter methylation in the spleens of young (2mo) and aged (26mo) mice. Aged mice had significantly reduced serum zinc levels compared to young mice despite being fed a zinc adequate diet ([8, 29] and Figure 4a). Similar to what was observed in zinc deficient THP1 cells, spleens from aged mice had a significant increase in ICAM1 and IL6 gene expression (Figure 4b). Moreover, aged mice had significantly decreased IL6 promoter methylation compared to young mice (Figure 4c). We next determined if reduced IL6 promoter methylation was similarly observed in the elderly human population. IL6 promoter methylation status was examined using DNA isolated from human lymphoblastic cell lines derived from young (20–28 yr) and aged (92–101 yr) individuals (Figure 4d). IL6 promoters in the majority of young individuals (8 out of 10) were highly methylated (≥ 75% methylation). In contrast, IL6 promoter methylation was highly heterogeneous among older individuals, and in general exhibit lower IL6 methylation compared to young individuals (5 out of 10 individuals with ≥ 75% methylation). Our data suggested that age-related immune and epigenetic alterations shared similarities to hallmarks of zinc deficiency, and the decline in zinc status with age may contribute to immune dysfunction and chronic inflammation.
Figure 4. Aging was associated with increased ICAM1 and IL6 gene expression and decreased IL6 promoter methylation.
(A) Plasma zinc concentrations in young (2 mo) and aged (26 mo) mice (n=6 per group). Data represent mean ± SEM. *P < 0.05 vs. young. (B) ICAM1 and IL6 gene expression in the spleens of young (2mo) and aged (26mo) mice (n=6 per group). Data represent mean normalized fold-change ± SEM vs. young. *P < 0.01 vs. young. (C) IL6 promoter methylation in the spleens of young (2mo) and aged (26mo) mice (n=6 per group). Data represent mean methylation percentage ± SEM. *P < 0.01 vs. young. (D) IL6 promoter methylation in human lymphoblastic cell lines derived from young (20–28 yr) and aged (92–101 yr) individuals represented as box and whiskers plot (n=10 per group). Whiskers represent maximum and minimum values.
Discussion
Zinc deficiency results in significant adverse health outcomes due to the necessity of zinc in numerous cellular functions, its role in protein structures and functions, and its involvement in gene regulation as well as intracellular signaling. In the context of the immune system, zinc deficiency results in a diverse array of immune defects, attributed to the dysfunction of numerous immune subsets involved in both adaptive and innate immunity. In particular, zinc deficiency has been associated with increases in inflammatory cytokines and other markers of inflammation. However to date, the precise mechanisms by which zinc deficiency contributes to increased inflammation remain to be clarified. In this study, we examined previously unexplored cellular and epigenetic mechanisms by which zinc deficiency promotes inflammation. Our data suggested that zinc deficiency contributed to increased immune cell activation that primed THP1 monocytes for the induction of a proinflammatory response. At the same time, DNA methylation status was altered by zinc deficiency. We showed that zinc deficient THP1 cells had decreased IL6 promoter methylation that likely contributed to increased IL6 production. Both mechanisms were observed with aging, suggesting a potential link by which zinc deficiency contributes to increased inflammation with age.
Macrophages and their monocyte precursors play an important role in the inflammatory process [30, 31]. In particular, dysregulation in the activation, function, and trafficking of monocytes and macrophages can contribute to chronic inflammatory response such as those observed with age [22, 40, 41]. Previous reports indicated that zinc deficiency can modulate monocytes differentiation, as well as proinflammatory cytokine signaling and expression [20, 28, 42]. In these studies, zinc deficiency was achieved via the use of a membrane permeable zinc chelator that resulted in rapid chelation and removal of intracellular zinc, and monocyte differentiation to macrophage was accomplished in the presence of differentiation agents such as PMA or 1α, 25-dihydroxyvitamin D3. In this current study, we for the first time were able to show that a physiologically relevant and gradual zinc depletion could lead to aberrant immune cell activation and differentiation in the absence differentiation signal. Moreover, this loss in intracellular zinc may contribute to increased monocyte responsiveness to the induction of a proinflammatory response. It has been reported recently that in primary peripheral blood mononuclear cells, Chelex-treated medium could augment cytokine production independent from its capability in zinc removal [21]. We did not observe this in our THP1 cells system where our untreated ZA and ZD THP1 cells, both similarly exposed to Chelex-treated media, did not produce spontaneous proinflammatory cytokines in the absence of LPS stimulation. Differences in results may be attributed to the use of very different cell culture system that differed in their sensitivity to Chelex. In the context of aging and immunosenescence, it has been observed that zinc deficiency results in a wide spectrum of immune defects that shares remarkable similarities to those associated with aging. A decline in zinc status during aging is potentially one of the factors resulting in the progressive decline and dysregulation of immune function with age [43]. In particular, zinc deficiency may play a role in promoting age-related chronic inflammation, one of the underlying etiologies of many age-related diseases [9]. In our current study, a similar increase in cellular activation was observed in aged mice, suggesting a potential mechanism where age-related zinc deficiency could prime immune cells for the induction of a proinflammatory response.
Epigenetics, including DNA methylation and histone modifications, plays a critical role in the regulation of gene expression, including the control of various immune cell populations [44–47]. Dysregulation in the epigenetic processes can result in altered gene expression via transcriptional activation or repression. Epigenetic dysregulation is associated with aging, characterized by alterations in DNA methylation and histone modifications [26, 48, 49]. Among other factors, nutritional deficiencies may play an important role in age-related epigenetic modifications that result in increased susceptibility to age-related diseases including chronic inflammation, autoimmunity, and cancer [50]. In particular, increasing evidence suggests that zinc deficiency can affect the epigenome. Zinc deficiency has been associated with increased chromatin accessibility of proinflammatory cytokine promoters [28], as well decreased DNA and histone methylation [39]. Oxidative stress that can be induced by zinc deficiency has also been shown to alter DNA methylation [51]. In this study we explored the effect of zinc deficiency on DNA methylation in the context of inflammation. We showed, for the first time, that zinc deficiency induced a progressive demethylation of the IL6 promoter in THP1 cells that correlated with increased IL6 expression. Our results further suggested that IL6 promoter methylation may also be reduced with age in human population. One limitation in the use of DNA derived from human lymphoblastoid cell lines was that it may not accurately reflect what is occurring in primary blood cells. An important future direction would include repeating our studies with the use of primary human PBMCs or monocytes to determine the promoter methylation status in young and old subjects. In this study, we focused on IL6 as it was most responsive to AZA treatment. It would be of significant interest to examine the effects of zinc deficiency on DNA methylation status of other proinflammatory cytokines including IL1β, IL8, and TNFα that could also be regulated epigenetically in future studies [28, 36]. Our results suggested a potential epigenetic link between zinc deficiency and age-related chronic inflammation, where zinc deficiency associated with age may result in promoter hypomethylation and increased expression of proinflammatory cytokines. A chronic inflammatory state, in turn, can promote additional epigenetic alterations including aberrant DNA methylation [52], resulting in further dysregulation of zinc homeostasis by altering promoter methylation and expression of zinc transporters [8, 53, 54]. Currently it is unknown whether zinc deficiency results in depletion of all intracellular zinc, or if particular zinc pools are more affected. Further, it is not well understood the mechanisms by which zinc deficiency affects epigenetic targets that result in DNA methylation alterations. Some postulated epigenetic mechanisms include the effects of zinc deficiency on chromatin access [28], alteration of methyl donor pools [39], as well as alterations in zinc-containing enzymes such as DNA methyltransferases and histone deacetylases [55, 56]. However, the key link between zinc and DNA methylation remains to be elucidated. It is likely that zinc deficiency impacts DNA methylation status via multiple mechanisms. An important future direction of this work is to understand the mechanism by which zinc deficiency alter DNA methylation, and examine how zinc supplementation may impact these mechanisms.
Results from this study contribute to our understanding of the role of zinc deficiency in promoting inflammation. An important area of continuing research will be to clarify interactions between zinc status, epigenetics, and immune function, and how their dysregulation contribute to chronic inflammation. Given the aging population is highly susceptible to zinc deficiency and chronic inflammation, identifying the mechanisms by which zinc status influence the inflammatory process could aid in developing strategies in reducing the disease burden associated with chronic inflammation in the elderly population.
Supplementary Material
Acknowledgements
This work was supported by funding from Oregon Agricultural Experiment Station (OR00735), Environmental Health Science Center at Oregon State University (NIEHS P30 ES00210), and Bayer Consumer Care AG, Switzerland.
Abbreviations
- 18S
18S ribosomal RNA
- AZA
5-aza-2'-deoxycytidine
- FBS
fetal bovine serum
- FluoZin-3
FluoZin-3 acetoxymethyl ester
- ICAM1
intercellular adhesion molecule-1
- ICP-OES
inductively coupled plasma optical emission spectrometry
- MFI
mean fluorescence intensity
- PMA
phorbol 12-myristate 13-acetate
- ZA
zinc-adequate
- ZD
zinc-deficient
Footnotes
Conflict of Interest
The authors have declared no conflict of interest.
References
- 1.King JC. Zinc: an essential but elusive nutrient. Am J Clin Nutr. 2011;94:679S–684S. doi: 10.3945/ajcn.110.005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr. 2004;24:277–298. doi: 10.1146/annurev.nutr.24.012003.132454. [DOI] [PubMed] [Google Scholar]
- 3.Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68:447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 4.Fischer Walker C, Black RE. Zinc and the risk for infectious disease. Annu Rev Nutr. 2004;24:255–275. doi: 10.1146/annurev.nutr.23.011702.073054. [DOI] [PubMed] [Google Scholar]
- 5.Golovine K, Uzzo RG, Makhov P, Crispen PL, et al. Depletion of intracellular zinc increases expression of tumorigenic cytokines VEGF, IL-6 and IL-8 in prostate cancer cells via NF-kappaB-dependent pathway. Prostate. 2008;68:1443–1449. doi: 10.1002/pros.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knoell DL, Julian MW, Bao S, Besecker B, et al. Zinc deficiency increases organ damage and mortality in a murine model of polymicrobial sepsis. Crit Care Med. 2009;37:1380–1388. doi: 10.1097/CCM.0b013e31819cefe4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang C, Murgia C, Leong M, Tan LW, et al. Anti-inflammatory effects of zinc and alterations in zinc transporter mRNA in mouse models of allergic inflammation. Am J Physiol Lung Cell Mol Physiol. 2007;292:L577–L584. doi: 10.1152/ajplung.00280.2006. [DOI] [PubMed] [Google Scholar]
- 8.Wong CP, Magnusson KR, Ho E. Increased inflammatory response in aged mice is associated with age-related zinc deficiency and zinc transporter dysregulation. J Nutr Biochem. 2013;24:353–359. doi: 10.1016/j.jnutbio.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong CP, Ho E. Zinc and its role in age-related inflammation and immune dysfunction. Mol Nutr Food Res. 2012;56:77–87. doi: 10.1002/mnfr.201100511. [DOI] [PubMed] [Google Scholar]
- 10.Hotz C, Peerson JM, Brown KH. Suggested lower cutoffs of serum zinc concentrations for assessing zinc status: reanalysis of the second National Health and Nutrition Examination Survey data (1976–1980) Am J Clin Nutr. 2003;78:756–764. doi: 10.1093/ajcn/78.4.756. [DOI] [PubMed] [Google Scholar]
- 11.Ravaglia G, Forti P, Maioli F, Nesi B, et al. Blood micronutrient and thyroid hormone concentrations in the oldest-old. J Clin Endocrinol Metab. 2000;85:2260–2265. doi: 10.1210/jcem.85.6.6627. [DOI] [PubMed] [Google Scholar]
- 12.Ervin RB, Kennedy-Stephenson J. Mineral intakes of elderly adult supplement and non-supplement users in the third national health and nutrition examination survey. J Nutr. 2002;132:3422–3427. doi: 10.1093/jn/132.11.3422. [DOI] [PubMed] [Google Scholar]
- 13.Mares-Perlman JA, Subar AF, Block G, Greger JL, Luby MH. Zinc intake and sources in the US adult population: 1976–1980. J Am Coll Nutr. 1995;14:349–357. doi: 10.1080/07315724.1995.10718520. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad A, Banerjee S, Wang Z, Kong D, et al. Aging and inflammation: etiological culprits of cancer. Curr Aging Sci. 2009;2:174–186. doi: 10.2174/1874609810902030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat Res. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Haase H, Ober-Blobaum JL, Engelhardt G, Hebel S, et al. Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. J Immunol. 2008;181:6491–6502. doi: 10.4049/jimmunol.181.9.6491. [DOI] [PubMed] [Google Scholar]
- 17.Kitamura H, Morikawa H, Kamon H, Iguchi M, et al. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol. 2006;7:971–977. doi: 10.1038/ni1373. [DOI] [PubMed] [Google Scholar]
- 18.Yu M, Lee WW, Tomar D, Pryshchep S, et al. Regulation of T cell receptor signaling by activation-induced zinc influx. J Exp Med. 2011;208:775–785. doi: 10.1084/jem.20100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaigne-Delalande B, Lenardo MJ. Divalent cation signaling in immune cells. Trends Immunol. 2014 doi: 10.1016/j.it.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruber K, Maywald M, Rosenkranz E, Haase H, et al. Zinc deficiency adversely influences interleukin-4 and interleukin-6 signaling. J Biol Regul Homeost Agents. 2013;27:661–671. [PubMed] [Google Scholar]
- 21.Mayer LS, Uciechowski P, Meyer S, Schwerdtle T, et al. Differential impact of zinc deficiency on phagocytosis, oxidative burst, and production of pro-inflammatory cytokines by human monocytes. Metallomics. 2014;6:1288–1295. doi: 10.1039/c4mt00051j. [DOI] [PubMed] [Google Scholar]
- 22.Hearps AC, Martin GE, Angelovich TA, Cheng WJ, et al. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell. 2012;11:867–875. doi: 10.1111/j.1474-9726.2012.00851.x. [DOI] [PubMed] [Google Scholar]
- 23.Fulop T, Le Page A, Fortin C, Witkowski JM, et al. Cellular signaling in the aging immune system. Curr Opin Immunol. 2014;29C:105–111. doi: 10.1016/j.coi.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Agrawal A, Tay J, Yang GE, Agrawal S, Gupta S. Age-associated epigenetic modifications in human DNA increase its immunogenicity. Aging (Albany NY) 2010;2:93–100. doi: 10.18632/aging.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nile CJ, Read RC, Akil M, Duff GW, Wilson AG. Methylation status of a single CpG site in the IL6 promoter is related to IL6 messenger RNA levels and rheumatoid arthritis. Arthritis Rheum. 2008;58:2686–2693. doi: 10.1002/art.23758. [DOI] [PubMed] [Google Scholar]
- 26.Valinluck V, Sowers LC. Inflammation-mediated cytosine damage: a mechanistic link between inflammation and the epigenetic alterations in human cancers. Cancer Res. 2007;67:5583–5586. doi: 10.1158/0008-5472.CAN-07-0846. [DOI] [PubMed] [Google Scholar]
- 27.Bayarsaihan D. Epigenetic mechanisms in inflammation. J Dent Res. 2011;90:9–17. doi: 10.1177/0022034510378683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wessels I, Haase H, Engelhardt G, Rink L, Uciechowski P. Zinc deficiency induces production of the proinflammatory cytokines IL-1beta and TNFalpha in promyeloid cells via epigenetic and redox-dependent mechanisms. J Nutr Biochem. 2013;24:289–297. doi: 10.1016/j.jnutbio.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Wong CP, Song Y, Elias VD, Magnusson KR, Ho E. Zinc supplementation increases zinc status and thymopoiesis in aged mice. J Nutr. 2009;139:1393–1397. doi: 10.3945/jn.109.106021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:281–286. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- 31.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernatchez SF, Atkinson MR, Parks PJ. Expression of intercellular adhesion molecule-1 on macrophages in vitro as a marker of activation. Biomaterials. 1997;18:1371–1378. doi: 10.1016/s0142-9612(97)00072-0. [DOI] [PubMed] [Google Scholar]
- 33.Santegoets SJ, van den Eertwegh AJ, van de Loosdrecht AA, Scheper RJ, de Gruijl TD. Human dendritic cell line models for DC differentiation and clinical DC vaccination studies. J Leukoc Biol. 2008;84:1364–1373. doi: 10.1189/jlb.0208092. [DOI] [PubMed] [Google Scholar]
- 34.Theus SA, Cave MD, Eisenach KD. Activated THP-1 cells: an attractive model for the assessment of intracellular growth rates of Mycobacterium tuberculosis isolates. Infect Immun. 2004;72:1169–1173. doi: 10.1128/IAI.72.2.1169-1173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishida K, Kobayashi T, Ito S, Komatsu Y, et al. Interleukin-6 gene promoter methylation in rheumatoid arthritis and chronic periodontitis. J Periodontol. 2012;83:917–925. doi: 10.1902/jop.2011.110356. [DOI] [PubMed] [Google Scholar]
- 36.Tekpli X, Landvik NE, Anmarkud KH, Skaug V, et al. DNA methylation at promoter regions of interleukin 1B, interleukin 6, and interleukin 8 in non-small cell lung cancer. Cancer immunology, immunotherapy : CII. 2013;62:337–345. doi: 10.1007/s00262-012-1340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullivan KE, Reddy AB, Dietzmann K, Suriano AR, et al. Epigenetic regulation of tumor necrosis factor alpha. Molecular and cellular biology. 2007;27:5147–5160. doi: 10.1128/MCB.02429-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wessels I, Fleischer D, Rink L, Uciechowski P. Changes in chromatin structure and methylation of the human interleukin-1beta gene during monopoiesis. Immunology. 2010;130:410–417. doi: 10.1111/j.1365-2567.2009.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallwork JC, Duerre JA. Effect of zinc deficiency on methionine metabolism, methylation reactions and protein synthesis in isolated perfused rat liver. J Nutr. 1985;115:252–262. doi: 10.1093/jn/115.2.252. [DOI] [PubMed] [Google Scholar]
- 40.Ingersoll MA, Platt AM, Potteaux S, Randolph GJ. Monocyte trafficking in acute and chronic inflammation. Trends Immunol. 2011;32:470–477. doi: 10.1016/j.it.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lumeng CN, Liu J, Geletka L, Delaney C, et al. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J Immunol. 2011;187:6208–6216. doi: 10.4049/jimmunol.1102188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubben S, Honscheid A, Winkler K, Rink L, Haase H. Cellular zinc homeostasis is a regulator in monocyte differentiation of HL-60 cells by 1 alpha,25-dihydroxyvitamin D3. J Leukoc Biol. 2010;87:833–844. doi: 10.1189/jlb.0409241. [DOI] [PubMed] [Google Scholar]
- 43.Haase H, Rink L. The immune system and the impact of zinc during aging. Immun Ageing. 2009;6:9. doi: 10.1186/1742-4933-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bluml S, Zupkovitz G, Kirchberger S, Seyerl M, et al. Epigenetic regulation of dendritic cell differentiation and function by oxidized phospholipids. Blood. 2009;114:5481–5489. doi: 10.1182/blood-2008-11-191429. [DOI] [PubMed] [Google Scholar]
- 45.Lal G, Bromberg JS. Epigenetic mechanisms of regulation of Foxp3 expression. Blood. 2009;114:3727–3735. doi: 10.1182/blood-2009-05-219584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Northrop JK, Thomas RM, Wells AD, Shen H. Epigenetic remodeling of the IL-2 and IFN-gamma loci in memory CD8 T cells is influenced by CD4 T cells. J Immunol. 2006;177:1062–1069. doi: 10.4049/jimmunol.177.2.1062. [DOI] [PubMed] [Google Scholar]
- 47.Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol. 2009;9:91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- 48.Issa JP. CpG-island methylation in aging and cancer. Curr Top Microbiol Immunol. 2000;249:101–118. doi: 10.1007/978-3-642-59696-4_7. [DOI] [PubMed] [Google Scholar]
- 49.Kawakami K, Nakamura A, Ishigami A, Goto S, Takahashi R. Age-related difference of site-specific histone modifications in rat liver. Biogerontology. 2009;10:415–421. doi: 10.1007/s10522-008-9176-0. [DOI] [PubMed] [Google Scholar]
- 50.Park LK, Friso S, Choi SW. Nutritional influences on epigenetics and age-related disease. Proc Nutr Soc. 2012;71:75–83. doi: 10.1017/S0029665111003302. [DOI] [PubMed] [Google Scholar]
- 51.Hitchler MJ, Domann FE. An epigenetic perspective on the free radical theory of development. Free Radic Biol Med. 2007;43:1023–1036. doi: 10.1016/j.freeradbiomed.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gasche JA, Hoffmann J, Boland CR, Goel A. Interleukin-6 promotes tumorigenesis by altering DNA methylation in oral cancer cells. Int J Cancer. 2011;129:1053–1063. doi: 10.1002/ijc.25764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coneyworth LJ, Mathers JC, Ford D. Does promoter methylation of the SLC30A5 (ZnT5) zinc transporter gene contribute to the ageing-related decline in zinc status? Proc Nutr Soc. 2009;68:142–147. doi: 10.1017/S0029665109001104. [DOI] [PubMed] [Google Scholar]
- 54.Fujishiro H, Okugaki S, Yasumitsu S, Enomoto S, Himeno S. Involvement of DNA hypermethylation in down-regulation of the zinc transporter ZIP8 in cadmium-resistant metallothionein-null cells. Toxicol Appl Pharmacol. 2009;241:195–201. doi: 10.1016/j.taap.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 55.Fatemi M, Hermann A, Pradhan S, Jeltsch A. The activity of the murine DNA methyltransferase Dnmt1 is controlled by interaction of the catalytic domain with the N-terminal part of the enzyme leading to an allosteric activation of the enzyme after binding to methylated DNA. Journal of molecular biology. 2001;309:1189–1199. doi: 10.1006/jmbi.2001.4709. [DOI] [PubMed] [Google Scholar]
- 56.Somoza JR, Skene RJ, Katz BA, Mol C, et al. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure. 2004;12:1325–1334. doi: 10.1016/j.str.2004.04.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.