Abstract
Several studies have reported that the overexpression of Sirtuin 1 (SIRT1) was associated with poor prognosis in various human cancers. However, little is known regarding the prognostic value of SIRT1 in lung adenocarcinoma. Therefore, the aim of this study is to evaluate the role of SIRT1 in the prognosis of lung adenocarcinoma patients. Using a tissue microarray, we detected SIRT1 expression by immunohistochemistry in lung adenocarcinoma tissue, as well as in corresponding noncancerous tissues (NCTs). A high expression level of SIRT1 was observed in 74.7% (56/75) of patients with lung adenocarcinoma and 6.7% (5/75) of NCTs (P<0.001). SIRT1 expression was significantly associated with high pathological stage. Importantly, we found that SIRT1 expression was associated with worse overall survival in these lung adenocarcinoma patients (67.0 months vs 104.5 months; P=0.005). In addition, anaplastic lymphoma kinase, epidermal growth factor receptor, vascular endothelial growth factor (VEGF), and Survivin expression were evaluated by fluorescent in situ hybridization or immunohistochemistry, respectively. We found that VEGF and Survivin were both highly expressed in the lung adenocarcinoma tissues, as compared to NCTs. Moreover, the SIRT1 and VEGF expression statuses were significantly positively correlated (r=0.238, P=0.039), while SIRT1 and Survivin expression status were not significantly correlated (r=0.220, P=0.058). Correlation analysis showed a positive correlation between VEGF and Survivin expression (r=0.436, P<0.001). However, we found that VEGF and Survivin expression were not associated with the prognosis of lung adenocarcinoma patients (P=0.334; P=0.433, respectively). Taken together, our findings suggest that SIRT1 plays a role in the progression of lung adenocarcinoma and may be a significant prognostic indicator for lung adenocarcinoma patients.
Keywords: SIRT1, VEGF, Survivin, prognosis, lung adenocarcinoma
Introduction
Silent mating-type information regulation 2 homolog 1 (SIRT1) is one of the mammalian homologs of silent information regulator 2 (Sir2), which functions as a nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylase.1,2 SIRT1 deacetylates not only histones, but also many nonhistone proteins, such as p53, p73, androgen receptor (AR), Ku70, NF-κB, E2F1, and FOXO, which are involved in cell growth, apoptosis, cell senescence, and tumorigenesis.3–7 Upregulation of SIRT1 has been reported in various human malignancies including prostate cancer, breast cancer, lung cancer, lymphoma, colon cancer, and gastric cancer.8–11 However, the role of SIRT1 in malignant tumors is controversial. On the one hand, SIRT1 might act as a tumor promoter by inhibiting tumor suppressor genes such as p53. It was reported that SIRT1 could bind and deacetylate p53, resulting in repressing p53-mediated transactivation.12 On the other hand, SIRT1 might also act as a tumor suppressor by repressing several oncogenes or oncoproteins such as Survivin.13 Wang et al13 reported that SIRT1 negatively regulated Survivin expression, leading to reduced tumor cell proliferation and increased apoptosis. Thus, SIRT1 can serve as a tumor promoter or suppressor, depending on the oncogenic pathways specific to particular tumors.
Several studies reported that overexpression of SIRT1 was associated with poor prognosis in various human cancers, including hepatocellular carcinoma,14 diffuse large B-cell lymphoma,15 gastric carcinoma,16,17 breast carcinoma,18,19 soft-tissue sarcomas,20 and colorectal carcinoma.21 However, it was also reported that overexpression of SIRT1 was associated with good prognosis in colorectal carcinoma, as well as in head and neck squamous cell carcinoma.22,23 However, despite the ongoing evaluation of SIRT1 as a therapeutic target, little is known regarding the prognostic value of SIRT1 in lung adenocarcinoma. Therefore, the aim of this study is to evaluate the role of SIRT1 in the prognosis of lung adenocarcinoma patients. Here, we examined the expression of SIRT1 and related proteins such as Survivin and vascular endothelial growth factor (VEGF), and their relationship to clinicopathological features and prognosis in lung adenocarcinoma patients.
Materials and methods
Clinical samples
Lung adenocarcinoma tissues and corresponding noncancerous tissue (NCT) sections containing HLug-Ade150Sur-01 (75 cancer cases and 75 NCTs) were provided by Outdo Bio-tech Co., Ltd. (Shanghai, People’s Republic of China). None of the patients received chemotherapy or radiotherapy prior to surgery. The experiments were approved by the Ethics Committee of Jinling Hospital (Nanjing, People’s Republic of China) and were conducted in compliance with the Declaration of Helsinki. Disease histology was determined in accordance with the criteria of the World Health Organization. Pathologic staging was performed in accordance with the current International Union against Cancer tumor–lymph node metastasis classification.
Immunohistochemistry
Lung adenocarcinoma tissue samples were deparaffinized in xylene. Heat-mediated antigen retrieval was applied using citrate buffer (BioGenex Laboratories, Inc., San Ramon, CA, USA). The immunostaining began with SIRT1 (rabbit monoclonal, ab32441; Abcam plc, Cambridge, UK), Survivin (rabbit monoclonal, ab134170; Abcam plc), and VEGF (mouse monoclonal, ab1316; Abcam plc) as primary antibodies for 60-minute staining, and it continued with the MaxVision™ HRP-Polymer anti-Rabbit IHC Kit (KIT-5005; Maxim) for 15 minutes, according to the manufacturers’ protocol, and it was followed by diaminobenzidine (D-5637; Sigma-Aldrich Co., St Louis, MO, USA) for visualization. All sections were counterstained with hematoxylin.
Evaluation of immunoreactivity
Every section was evaluated and scored independently by two pathologists. The H-score method was used in this trial. We multiplied the percentage score by the staining intensity score. The percentage of positively stained cells was scored as “−” (0%), “+” (1%–25%), “++” (26%–50%), or “+++” (51%–100%). Intensity was scored as “−” (negative), “+” (weak), “++” (moderate) and “+++” (strong). Immunohistochemical scoring was performed without prior knowledge of the clinical response. Immunostained sections were scanned using a microscope (Axiovert 200; Carl Zeiss Meditec AG, Jena, Germany).
Fluorescent in situ hybridization
Fluorescent in situ hybridization (FISH) was performed on 4 μm paraffin-embedded tissue sections following deparaffinization and digestion. The slides were washed in saline–sodium citrate buffer, fixed in 10% buffered formalin for 5 minutes, dehydrated in graded alcohol, and allowed to air dry. Hybridization was performed using a dual-color break-apart rearrangement probe (ZSGB-BIO, Beijing, People’s Republic of China) for the EGFR and ALK gene on chromosome 7 and 2, respectively. The probes were denatured by incubation at 78°C for 5 minutes in a humidified box, after which they were hybridized overnight at 42°C. The following day, the cover glass was removed and the slides were washed in posthybridization washing-buffer (Vysis) at 72°C for 2 minutes. After drying, 4′-6-diamidino-2-phenylindole (DAPI) (Vysis) was applied and the slides were protected from light at −20°C until reading. Automated scanning, capture, and scoring of interphase FISH was analyzed by the CytoVision® System (Applied Imaging Corp., San Jose, CA, USA).
Statistical analyses
SPSS Statistics 19.0 (IBM Corporation, Armonk, NY, USA) was used for the statistical analysis. Data were analyzed using one-way analysis of variance or a Student’s t-test. Data are presented as the mean ± standard deviation of three independent experiments. The χ2 tests were used to compare the distribution of demographic variables between the high and low SIRT1 expression groups. The log-rank test was used to assess the statistical significance of Kaplan–Meier plots. Univariate and multivariate analyses were performed using the Cox proportional hazards regression model to identify the most significant variables for predicting overall survival (OS). For immunohistochemistry (IHC) data, the two-related samples test between lung adenocarcinoma tissues and NCTs was used, and the statistical significance of the correlation between SIRT1, Survivin, and VEGF expression level in lung adenocarcinoma tissues or in NCTs was estimated by Spearman’s rank correlation analysis; statistical significance was again defined as P<0.05 or P<0.001.
Results
Patient characteristics
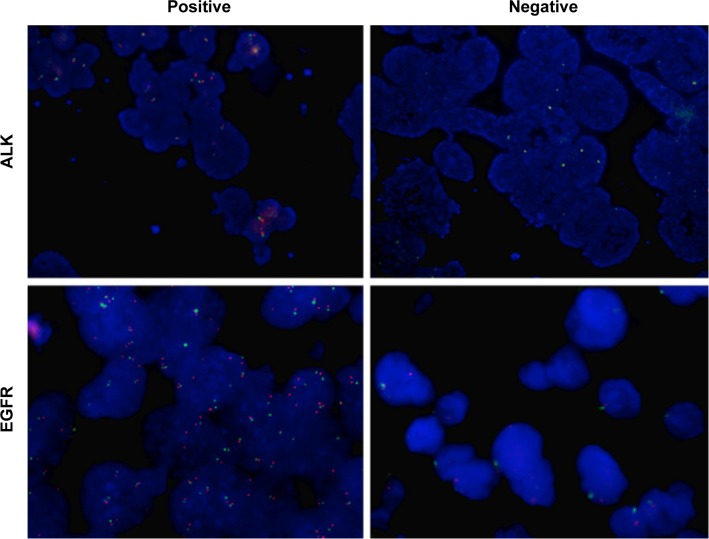
The clinicopathological features of 75 lung adenocarcinoma patients are summarized in Table 1. In all, 75 patients with a median age of 59 years (range, 20–84 years) were included in the final analyses. Among these cases, 39 (52.0%) patients were male and 36 (48.0%) patients were female. According to the Union for International Cancer Control cancer staging systems 7th edition stage grouping criteria, 46 (61.3%) cases were stage I–II and 19 (25.3%) were stage III–IV. In addition, we detected ALK and EGFR expression of the 75 lung adenocarcinoma patients using FISH. Representative figures of ALK- and EGFR-positive or -negative expression were shown in Figure 1. Briefly, eight (10.7%) cases positively expressed ALK, while 67 (89.3%) cases negatively expressed ALK. There are 29 (38.7%) patients with EGFR-positive and 46 (61.3%) patients with EGFR-negative expression (Table 1).
Table 1.
Relationship between the expression of SIRT1 and the clinicopathological features of lung cancer patients
| Variables | Total (n=75)
|
SIRT1 high (n=56)
|
SIRT1 low (n=19)
|
P-value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Sex | 0.318 | ||||||
| Male | 39 | 52.0 | 31 | 55.4 | 8 | 42.1 | |
| Female | 36 | 48.0 | 25 | 44.6 | 11 | 57.9 | |
| Age | 0.232 | ||||||
| ≤59 years | 38 | 50.7 | 26 | 46.4 | 12 | 21.5 | |
| >59 years | 36 | 48.0 | 29 | 51.8 | 7 | 36.8 | |
| NA* | 1 | 1.3 | 1 | 1.8 | 0 | 0 | |
| Histological grade | 0.028* | ||||||
| I | 13 | 17.3 | 6 | 10.7 | 7 | 36.8 | |
| II | 47 | 62.7 | 37 | 66.1 | 10 | 52.6 | |
| III | 15 | 20.0 | 13 | 23.2 | 2 | 10.5 | |
| TNM stage | 0.223 | ||||||
| I–II | 46 | 61.3 | 33 | 58.9 | 13 | 68.4 | |
| III–IV | 19 | 25.3 | 17 | 30.4 | 2 | 10.5 | |
| NA | 10 | 13.3 | 6 | 10.7 | 4 | 21.1 | |
| ALK | 1.000 | ||||||
| Positive | 8 | 10.7 | 6 | 10.7 | 2 | 10.5 | |
| Negative | 67 | 89.3 | 50 | 89.3 | 17 | 89.5 | |
| EGFR | 0.201 | ||||||
| Positive | 29 | 38.7 | 24 | 42.9 | 5 | 26.3 | |
| Negative | 46 | 61.3 | 32 | 57.1 | 14 | 73.7 | |
| Survivin | 0.057 | ||||||
| High | 49 | 65.3 | 40 | 71.4 | 9 | 47.4 | |
| Low | 26 | 34.7 | 16 | 28.6 | 10 | 52.6 | |
| VEGF | 0.039* | ||||||
| High | 50 | 66.7 | 41 | 73.2 | 9 | 47.4 | |
| Low | 25 | 33.3 | 15 | 26.8 | 10 | 52.6 | |
Notes:
denotes statistical significance.
Abbreviations: SIRT1, silent mating-type information regulation 2 homolog 1; n, sample number; N, total number; NA, not available; TNM, tumor–node–metastasis; ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; VEGF, vascular endothelial growth factor; NA, not available.
Figure 1.
ALK and EGFR expression in lung adenocarcinoma tissues by fluorescent in situ hybridization.
Notes: Representative figures of ALK-positive (patient number 27), ALK-negative (patient number 9), EGFR-positive (patient number 32), and EGFR-negative (patient number 45) expression, respectively (magnification: 200×).
Abbreviations: ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor.
The association between SIRT1 expression and clinical characteristics of lung adenocarcinoma patients
The expression status of SIRT1 was determined in 75 lung adenocarcinoma tissues and corresponding NCTs by IHC. As shown in Figure 2, the expression of SIRT1 in the tumor tissues and NCTs of patient number 9 were high, while the SIRT1 expression of patient number 34 was low. In total, a high expression level of SIRT1 was observed in 74.7% (56/75) of patients with lung adenocarcinoma and 6.7% (5/75) of NCTs (P<0.001). As shown in Table 2, SIRT1 expression was significantly increased in lung adenocarcinoma tissues when compared with NCTs (P<0.001). Moreover, we also assessed the association between SIRT1 expression status and the clinical characteristics of lung adenocarcinoma patients (Table 1). We found that SIRT1 expression was significantly associated with pathological stage (P=0.028), but it was not related to sex, age, tumor–node–metastasis (TNM) stage, and ALK and EGFR expression status (Table 1).
Figure 2.
SIRT1, Survivin, and VEGF expression in lung adenocarcinoma tissues by immunohistochemical staining.
Notes: SIRT1, Survivin, and VEGF expression in the tumor tissues or corresponding NCTs of (A) patient number 9 and (B) patient number 34, respectively (magnification: 200×).
Abbreviations: NCTs, noncancerous tissues; SIRT1, silent mating-type information regulation 2 homolog 1; VEGF, vascular endothelial growth factor.
Table 2.
SIRT1, VEGF, and Survivin expression in a lung cancer and NCT microarray
| Lung cancers (n=75)
|
NCTs (n=75)
|
P-value | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| SIRT1 | <0.001* | ||||
| 0 | 7 | 9.3 | 50 | 66.7 | |
| + | 12 | 16.0 | 20 | 26.7 | |
| ++ | 40 | 53.3 | 5 | 6.7 | |
| +++ | 16 | 21.3 | 0 | 0 | |
| VEGF | <0.001* | ||||
| 0 | 10 | 13.3 | 34 | 45.3 | |
| + | 15 | 20.0 | 27 | 36.0 | |
| ++ | 18 | 24.0 | 10 | 13.3 | |
| +++ | 32 | 42.7 | 4 | 5.3 | |
| Survivin | <0.001* | ||||
| 0 | 9 | 12.0 | 32 | 42.7 | |
| + | 17 | 22.7 | 32 | 42.7 | |
| ++ | 11 | 14.7 | 9 | 12.0 | |
| +++ | 38 | 50.7 | 2 | 2.7 | |
Note: The plus signs indicate the level of expression, and the asterisk indicates statistical significance.
Abbreviations: SIRT1, silent mating-type information regulation 2 homolog 1; VEGF, vascular endothelial growth factor; NCT, noncancerous tissue; n, sample number; N, total number.
SIRT1 expression predicted the prognosis of lung adenocarcinoma
The association between SIRT1 expression status and OS in lung adenocarcinoma patients was studied by log-rank test. Patients with a high expression of SIRT1 had markedly shorter OS than those with low SIRT1 expression (67.0 months vs 104.5 months, respectively; P=0.005). The Kaplan–Meier survival curve is shown in Figure 3. Moreover, univariate analysis for the predefined variables showed that age, TNM stage, and SIRT1 expression were significantly associated with OS in lung adenocarcinoma patients. During the multivariate analysis, age and TNM stage were independent adverse prognostic factors for OS (Table 3). However, multivariate analysis showed that SIRT1 expression was not an independent predictor of poor prognosis (P=0.087; hazard ratio =0.347; 95% confidence interval =0.103–1.167). Taken together, our data suggested that the expression of SIRT1 correlated with OS, but it was not an independent predictor of poor prognosis in lung adenocarcinoma.
Figure 3.
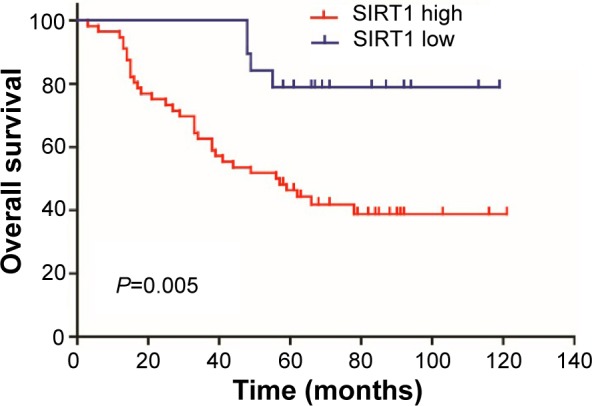
The association of SIRT1 with overall survival in lung adenocarcinoma.
Note: High expression of SIRT1 was associated with worse overall survival in lung adenocarcinoma (P=0.005).
Abbreviation: SIRT1, silent mating-type information regulation 2 homolog 1.
Table 3.
Univariate and multivariate Cox regression analysis of overall survival in lung cancer patients
| Variables | Univariate analysis
|
Multivariate analysis
|
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Sex (male vs female) | 1.115 (0.584–2.131) | 0.741 | ||
| Age | 1.032 (1.002–1.063) | 0.038* | 1.049 (1.014–1.085) | 0.006* |
| Histological grade (I+II vs III) | 0.607 (0.286–1.288) | 0.194 | ||
| TNM stage (I+II vs III+IV) | 0.235 (0.115–0.479) | 0.000* | 0.190 (0.086–0.418) | 0.000* |
| ALK (negative vs positive) | 1.913 (0.586–6.243) | 0.282 | ||
| EGFR (negative vs positive) | 0.682 (0.356–1.308) | 0.250 | ||
| Survivin (negative vs positive) | 0.756 (0.374–1.531) | 0.437 | ||
| VEGF (negative vs positive) | 0.702 (0.340–1.450) | 0.339 | ||
| SIRT1 (negative vs positive) | 0.251 (0.089–0.709) | 0.009* | 0.347 (0.103–1.167) | 0.087 |
Note:
indicates statistical significance.
Abbreviations: HR, hazard ratio; CI, confidence interval; TNM, tumor–node–metastasis; ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; VEGF, vascular endothelial growth factor; SIRT1, silent mating-type information regulation 2 homolog 1.
The association between SIRT1 and Survivin or VEGF expression in lung adenocarcinoma
This study had reported that the overexpression of Survivin and VEGF may be important in the tumorigenesis of small cell lung cancer (SCLC), and that overexpression can be used to indicate the poorer prognosis of SCLC.24 Thus, we detected the expression of Survivin and VEGF in the 75 lung adenocarcinoma patients. Figure 2 showed the expression level of Survivin and VEGF in the tumor tissues and NCTs in two representative patients. As shown in Table 1, the χ2 tests showed that SIRT1 expression was significantly associated with VEGF expression status (P=0.039), but not Survivin expression status (P=0.057). In addition, VEGF and Survivin expression were both significantly increased in lung adenocarcinoma tissues when compared with NCTs (P<0.001; P<0.001, respectively). We then analyzed the correlation between SIRT1 and VEGF or Survivin expression at the tumor microarray (Table 4). A significant and positive correlation was found between SIRT1 and VEGF expression status using the lung adenocarcinoma tissue array (r=0.238, P=0.039), while SIRT1 expression status was not significantly associated with Survivin expression status (r=0.220, P=0.058). Moreover, correlation analysis showed a positive correlation between VEGF and Survivin expression (r=0.436, P<0.001; Table 5).
Table 4.
Correlation analysis of SIRT1 with VEGF or Survivin expression at tumor microarray
| Variables | Tumor microarray (n=75)
|
|
|---|---|---|
| SIRT1 (high) | SIRT1 (low) | |
| VEGF (high) | 41 | 9 |
| VEGF (low) | 15 | 10 |
| r | 0.238 | |
| P | 0.039* | |
| Survivin (high) | 40 | 9 |
| Survivin (low) | 16 | 10 |
| r | 0.220 | |
| P | 0.058 | |
Note: The asterisk indicates statistical significance.
Abbreviations: SIRT1, silent mating-type information regulation 2 homolog 1; VEGF, vascular endothelial growth factor; n, sample number.
Table 5.
Correlation analysis of VEGF with Survivin expression at tumor microarray
| Variables | Tumor microarray (n=75) | |
|---|---|---|
| Survivin (high) | Survivin (low) | |
| VEGF (high) | 41 | 9 |
| VEGF (low) | 15 | 10 |
| r | 0.436 | |
| p | <0.001* | |
Note: The asterisk indicates statistical significance.
Abbreviations: VEGF, vascular endothelial growth factor; n, sample number.
VEGF and Survivin expression were not associated with overall survival of lung adenocarcinoma
We then calculated the association between VEGF or Survivin expression status and OS in lung adenocarcinoma patients. As shown in Figure 4, patients with a high expression of VEGF and Survivin had shorter OS than those with low VEGF or Survivin expression (VEGF: 73.2 months vs 83.4 months; Survivin: 73.8 months vs 82.2 months, respectively). However, the association between VEGF or Survivin expression status and OS were not statistically significant (P=0.334; P=0.433, respectively).
Figure 4.

The association of VEGF and Survivin with overall survival in lung adenocarcinoma.
Notes: The expression of (A) VEGF and (B) Survivin was not associated with overall survival in lung adenocarcinoma (P=0.334; P=0.433, respectively).
Abbreviation: VEGF, vascular endothelial growth factor.
Discussion
In the present study, it was revealed that the expression of SIRT1 correlated with OS and was a predictor of poor prognosis in lung adenocarcinoma. We found that SIRT1 expression was significantly increased in lung adenocarcinoma tissues compared with NCTs and it was significantly associated with pathological stage. Patients with high SIRT1 expression had a significantly shorter OS than those with low SIRT1 expression. Recently, several studies have shown that the overexpression of SIRT1 was associated with poor prognosis in various human cancers, such as gastric carcinoma16 and breast carcinoma.18 In contrast, SIRT1 expression was also reported to be associated with good prognosis for several human cancers, such as head and neck squamous cell carcinoma.23 Interestingly, the role of SIRT1 expression in colorectal cancer has been controversial. Jung et al22 reported that SIRT1 overexpression is a good prognostic factor for colorectal cancer, while Chen et al21 showed that high levels of SIRT1 expression enhance tumorigenesis and are associated with a poor prognosis in colorectal carcinoma. The association between SIRT1 expression and the prognosis of lung cancer was also investigated. In non-SCLC (NSCLC), high expression of SIRT1 was significantly associated with shorter progression-free survival and OS.25,26 Moreover, patients with high SIRT1 expressions had a significantly higher chance of being resistant to chemotherapy than those with low SIRT1 expression.25 However, little is known regarding the prognostic value of SIRT1 in lung adenocarcinoma. Here, we found that patients with high SIRT1 expression had a significantly shorter OS than those with low SIRT1 expression. These findings are consistent with previous reports indicating that SIRT1 is a tumor promoter in lung adenocarcinoma.27
Survivin functions as an inhibitor of apoptosis and plays a crucial role in regulating cell proliferation, apoptosis, and angiogenesis.28 Overexpression of Survivin has been implicated in tumor progression in various human cancers, including lung cancer.29 Previous studies have shown that the expression of VEGF is correlated with the expression of Survivin in many cancers,30 and overexpression of Survivin and VEGF were also reported to predict poor prognosis of both SCLC and NSCLC.24,31 In the present study, we found that VEGF and Survivin were both highly expressed in the lung adenocarcinoma tissue when compared with NCTs. Correlation analysis showed a positive correlation between VEGF and Survivin expression. However, we also found that VEGF and Survivin expression were not associated with the prognosis of lung adenocarcinoma patients. There are reports that Survivin is, independently, a poor prognostic marker in lung adenocarcinoma, and that high Survivin expression has a significantly shorter disease-free survival and shorter OS.32 However, other studies show that Survivin is a prognostic marker of advanced-stage NSCLC, but not of early-stage NSCLC.33 In the present study, most of the patients are early-stage lung adenocarcinoma, which could explain why Survivin is not associated with OS. Moreover, correlation analysis showed that SIRT1 and VEGF expression status were significantly and positively correlated, while SIRT1 and Survivin expression status were not significantly correlated. This was consistent with the view that SRT1720, a well-known agonist of SIRT1, significantly increased the amount of VEGF secreted from cancer cells, and it promoted cancer cell migration and lung metastasis in breast cancer.34 However, the direct effects between SIRT1 and VEGF should be validated by large-scale association clinical studies and demonstrated by further studies in vitro and in vivo.
To our knowledge, little has been reported concerning the role of SIRT1 expression on the prognosis of lung adenocarcinoma. Our results suggest that SIRT1 expression was significantly increased in lung adenocarcinoma tissues compared with NCTs, and it was significantly associated with pathological stage. Patients with high SIRT1 expression had a significantly shorter OS than those with low SIRT1 expression. In addition, we found that VEGF and Survivin were both highly expressed; we also found that SIRT1 and VEGF, but not Survivin, expression status was significantly and positively correlated in lung adenocarcinoma tissues. Taken together, our findings suggest that SIRT1 plays a critical role in the progression of lung adenocarcinoma and it may be a significant prognostic indicator for lung adenocarcinoma patients.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (numbers 30972703, 81171653, 81201741, and 81301960), and the Innovation Talents Training Project of Changzhou Health Bureau.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Landry J, Sutton A, Tafrov ST, et al. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci U S A. 2000;97(11):5807–5811. doi: 10.1073/pnas.110148297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voelter-Mahlknecht S, Mahlknecht U. Cloning, chromosomal characterization and mapping of the NAD-dependent histone deacetylases gene sirtuin 1. Int J Mol Med. 2006;17(1):59–67. [PubMed] [Google Scholar]
- 3.Luo J, Nikolaev AY, Imai S, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107(2):137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 4.Pediconi N, Guerrieri F, Vossio S, et al. hSirT1-dependent regulation of the PCAF-E2F1-p73 apoptotic pathway in response to DNA damage. Mol Cell Biol. 2009;29(8):1989–1998. doi: 10.1128/MCB.00552-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu M, Liu M, Sauve AA, et al. Hormonal control of androgen receptor function through SIRT1. Mol Cell Biol. 2006;26(21):8122–8135. doi: 10.1128/MCB.00289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23(12):2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunet A, Sweeney LB, Sturgill JF, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303(5666):2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 8.Derr RS, van Hoesel AQ, Benard A, et al. High nuclear expression levels of histone-modifying enzymes LSD1, HDAC2 and SIRT1 in tumor cells correlate with decreased survival and increased relapse in breast cancer patients. BMC Cancer. 2014;14:604. doi: 10.1186/1471-2407-14-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han L, Liang XH, Chen LX, Bao SM, Yan ZQ. SIRT1 is highly expressed in brain metastasis tissues of non-small cell lung cancer (NSCLC) and in positive regulation of NSCLC cell migration. Int J Clin Exp Pathol. 2013;6(11):2357–2365. [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Bhatia R. Roles of SIRT1 in leukemogenesis. Curr Opin Hematol. 2013;20(4):308–313. doi: 10.1097/MOH.0b013e328360ab64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huffman DM, Grizzle WE, Bamman MM, et al. SIRT1 is significantly elevated in mouse and human prostate cancer. Cancer Res. 2007;67(14):6612–6618. doi: 10.1158/0008-5472.CAN-07-0085. [DOI] [PubMed] [Google Scholar]
- 12.Langley E, Pearson M, Faretta M, et al. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J. 2002;21(10):2383–2396. doi: 10.1093/emboj/21.10.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang RH, Zheng Y, Kim HS, et al. Interplay among BRCA1, SIRT1, and Survivin during BRCA1-associated tumorigenesis. Mol Cell. 2008;32(1):11–20. doi: 10.1016/j.molcel.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen HC, Jeng YM, Yuan RH, Hsu HC, Chen YL. SIRT1 promotes tumorigenesis and resistance to chemotherapy in hepatocellular carcinoma and its expression predicts poor prognosis. Ann Surg Oncol. 2012;19(6):2011–2019. doi: 10.1245/s10434-011-2159-4. [DOI] [PubMed] [Google Scholar]
- 15.Jang KY, Hwang SH, Kwon KS, et al. SIRT1 expression is associated with poor prognosis of diffuse large B-cell lymphoma. Am J Surg Pathol. 2008;32(10):1523–1531. doi: 10.1097/PAS.0b013e31816b6478. [DOI] [PubMed] [Google Scholar]
- 16.Cha EJ, Noh SJ, Kwon KS, et al. Expression of DBC1 and SIRT1 is associated with poor prognosis of gastric carcinoma. Clin Cancer Res. 2009;15(13):4453–4459. doi: 10.1158/1078-0432.CCR-08-3329. [DOI] [PubMed] [Google Scholar]
- 17.Noguchi A, Kikuchi K, Zheng H, et al. SIRT1 expression is associated with a poor prognosis, whereas DBC1 is associated with favorable outcomes in gastric cancer. Cancer Med. 2014;3(6):1553–1561. doi: 10.1002/cam4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee H, Kim KR, Noh SJ, et al. Expression of DBC1 and SIRT1 is associated with poor prognosis for breast carcinoma. Hum Pathol. 2011;42(2):204–213. doi: 10.1016/j.humpath.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Wu M, Wei W, Xiao X, et al. Expression of SIRT1 is associated with lymph node metastasis and poor prognosis in both operable triple-negative and non-triple-negative breast cancer. Med Oncol. 2012;29(5):3240–3249. doi: 10.1007/s12032-012-0260-6. [DOI] [PubMed] [Google Scholar]
- 20.Kim JR, Moon YJ, Kwon KS, et al. Expression of SIRT1 and DBC1 is associated with poor prognosis of soft tissue sarcomas. PLoS One. 2013;8(9):e74738. doi: 10.1371/journal.pone.0074738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Sun K, Jiao S, et al. High levels of SIRT1 expression enhance tumorigenesis and associate with a poor prognosis of colorectal carcinoma patients. Sci Rep. 2014;4:7481. doi: 10.1038/srep07481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung W, Hong KD, Jung WY, et al. SIRT1 expression is associated with good prognosis in colorectal cancer. Korean J Pathol. 2013;47(4):332–339. doi: 10.4132/KoreanJPathol.2013.47.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noguchi A, Li X, Kubota A, et al. SIRT1 expression is associated with good prognosis for head and neck squamous cell carcinoma patients. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(3):385–392. doi: 10.1016/j.oooo.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Chen P, Zhu J, Liu DY, Li HY, Xu N, Hou M. Over-expression of survivin and VEGF in small-cell lung cancer may predict the poorer prognosis. Med Oncol. 2014;31(1):775. doi: 10.1007/s12032-013-0775-5. [DOI] [PubMed] [Google Scholar]
- 25.Zhang T, Rong N, Chen J, et al. SIRT1 expression is associated with the chemotherapy response and prognosis of patients with advanced NSCLC. PLoS One. 2013;8(11):e79162. doi: 10.1371/journal.pone.0079162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noh SJ, Baek HA, Park HS, et al. Expression of SIRT1 and cortactin is associated with progression of non-small cell lung cancer. Pathol Res Pract. 2013;209(6):365–370. doi: 10.1016/j.prp.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Hokka D, Maniwa Y, Ohbayashi C, Itoh T, Hayashi Y. Sirt1 is a tumor promoter in lung adenocarcinoma. Oncol Lett. 2014;8(1):387–393. doi: 10.3892/ol.2014.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8(1):61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 29.Wang M, Liu BG, Yang ZY, Hong X, Chen GY. Significance of survivin expression: Prognostic value and survival in stage III non-small cell lung cancer. Exp Ther Med. 2012;3(6):983–988. doi: 10.3892/etm.2012.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanwar JR, Kamalapuram SK, Kanwar RK. Targeting survivin in cancer: the cell-signalling perspective. Drug Discov Today. 2011;16(11–12):485–494. doi: 10.1016/j.drudis.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Tang XP, Li J, Yu LC, et al. Clinical significance of survivin and VEGF mRNA detection in the cell fraction of the peripheral blood in non-small cell lung cancer patients before and after surgery. Lung Cancer. 2013;81(2):273–279. doi: 10.1016/j.lungcan.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Sun PL, Jin Y, Kim H, et al. Survivin expression is an independent poor prognostic marker in lung adenocarcinoma but not in squamous cell carcinoma. Virchows Arch. 2013;463(3):427–436. doi: 10.1007/s00428-013-1462-9. [DOI] [PubMed] [Google Scholar]
- 33.Zhang LQ, Wang J, Jiang F, Xu L, Liu FY, Yin R. Prognostic value of survivin in patients with non-small cell lung carcinoma: a systematic review with meta-analysis. PLoS One. 2012;7(3):e34100. doi: 10.1371/journal.pone.0034100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki K, Hayashi R, Ichikawa T, et al. SRT1720, a SIRT1 activator, promotes tumor cell migration, and lung metastasis of breast cancer in mice. Oncol Rep. 2012;27(6):1726–1732. doi: 10.3892/or.2012.1750. [DOI] [PubMed] [Google Scholar]