Abstract
Introduction
Significant evidence has demonstrated that Type 2 diabetes mellitus and related pre-cursors are associated with diminished neurocognitive function and risk of dementia among older adults. However, very little research has examined relations of glucose regulation to neurocognitive function among older adults free of these conditions. The primary aim of this investigation was to examine associations among fasting glucose, glucose tolerance, and neurocognitive function among non-diabetic older adults. The secondary aim was to examine age, gender, and education as potential effect modifiers.
Methods
The study employed a cross-sectional, correlational study design. Participants were 172 older adults with a mean age of 64.43 years (SD = 13.09). The sample was 58% male and 87% White. Participants completed an oral glucose tolerance test as part of a larger study. Trained psychometricians administered neuropsychological tests that assessed performance in the domains of response inhibition, nonverbal memory, verbal memory, attention and working memory, visuoconstructional abilities, visuospatial abilities, psychomotor speed and executive function, and motor speed and manual dexterity. Linear multiple regressions were run to test study aims.
Results
No significant main effects of fasting glucose and 2-hour glucose emerged for performance on any neurocognitive test; however, significant interactions were present. Higher fasting glucose was associated with poorer short-term verbal memory performance among men, but unexpectedly better response inhibition and long-term verbal memory performance for participants over age 70. Higher 2-hour glucose values were associated with reduced divided attention performance among participants with less than a high school education.
Conclusions
Mixed findings suggest that glucose levels may be both beneficial and deleterious to neurocognition among non-diabetic older adults. Additional studies with healthy older adults are needed to confirm this unexpected pattern of associations; however, findings have implications for the importance of maintaining healthy glucose levels in older adulthood.
Keywords: Type 2 diabetes, neurocognitive function, impaired fasting glucose, impaired glucose tolerance, older adults
Optimal neurocognitive function is a critical component of quality of life in older adulthood (Levine & Croog, 1984; Hollenger, 1991). A preponderance of evidence suggests older adults with Type 2 diabetes mellitus (DM) perform more poorly than individuals without Type 2 DM on tests of neurocognitive function (Awad, Gagnon, & Messier, 2004; Elias, Elias, D’Agostino, et al., 1997; Hiltunen, Keinanen-Kiukaanniemi, & Laara, 2001; Ryan, 2005). Most notably, diminished performance has been demonstrated for learning and memory tests, but decrements have also been noted for attention, executive function, psychomotor speed, neurocognitive speed, and problem solving performance (Fischer, de Frias, Yeung, & Dixon, 2009; Ryan, 2005; Whitehead, Dixon, Hultsch, & MacDonald, 2011)). Furthermore, individuals with Type 2 DM are at higher risk for neurocognitive decline and dementia than their non-diabetic counterparts (Biessels, van der Heide, Kamal, Bleys, & Gispen, 2002; Cukierman, Gerstein, & Williamson, 2005; Elias et al., 1997; Ryan, 2005).
Prior to the onset of Type 2 DM, pre-clinical disease states, such as impaired fasting glucose (IFG) and impaired glucose tolerance (IGT), are also linked to reduced cognitive performance. Each of these conditions represents an intermediate stage between normal glucose metabolism and diabetes. IFG is defined by elevated fasting glucose values ≥ 100 and < 126 mg/dL (IFG). IGT is defined by sustained, elevated glucose values ≥ 140 and < 200 mg/dL (IGT) two hours post-ingestion during an oral glucose tolerance test. IFG has been associated with poorer delayed recall and lower mental status performance among older adults (Convit, Wolf, Tarshish, & de Leon, 2005; DiBonito et al., 2007). Similarly, older adults with IGT have demonstrated lower mental status and poorer verbal learning, and memory performance than older adults with normal glucose levels (Convit et al., 2003; Kaplan, Greenwood, Winocur, & Wolever, 2000; Ryan, 2001; Vanhanen et al., 1998). Decrements in working memory, verbal memory, and executive function performance have also been associated with IFG and IGT (Lamport, Lawton, Mansfield, & Dye, 2009). Relatedly, studies have demonstrated poorer cognitive performance among non-diabetic individuals with insulin resistance (IR) as compared to their non-diabetic counterparts without (IR) (Bruehl et al., 2010).
A preponderance of evidence points to Type 2 DM and pre-diabetic states as important correlates of neurocognitive performance; however, very few studies have examined linear relations of glucose to neurocognitive function among older adults along the continuum of glucose regulation from normal to pre-diabetic. Among those, poorer glucose regulation was associated with poorer attention/control and working memory performance (Gagnon et al., 2010; Messier et al., 2010). As increasing numbers of older adults progress from healthy glucose regulation to glucose dysregulation, it will be critical to understand how even slightly elevated levels of fasting glucose and suboptimal glucose regulation influence neurocognitive function. The majority of previous studies that have examined glucose and neurocognitive function have excluded non-diabetic older adults. Thus, the influence of normal and slightly impaired fasting glucose and glucose tolerance values on neurocognitive function in this population is not well documented.
Within the available literature, few studies have examined how relations between fasting glucose, glucose tolerance, and neurocognitive function may be modified based on membership in specific age, sex, and education subgroups. Prior research has demonstrated that relations of other cardiovascular risk factors (e.g., hypertension) to neurocognitive function may be moderated by demographic variables such as age, sex, and education, and these variables may represent specific vulnerability and resilience factors (Waldstein, Giggey, Thayer, & Zonderman, 2005; Waldstein & Katzel, 2001). It is critical to develop an understanding of the roles of potential effect modifiers to be able to 1) identify characteristics that place individuals at risk for glucose-related neurocognitive decrements, 2) identify protective factors that reduce the likelihood of developing glucose-related neurocognitive decrements, and 3) tailor interventions to individuals at greatest risk. Given that increasing age is a risk factor for Type 2 DM, (26.9% of adults age 65 and over affected vs. 11.3% of adults ages 20 to 64), it is plausible that age may interact with glucose to enhance vulnerability toward neurocognitive decrements. Moreover, there is evidence that the deleterious effects of IFG and IGT on neurocognitive performance are greater among older adults as compared to younger adults (Messier et al, 2003). Sex differences in the prevalence of Type 2 DM, on the other hand, are nominal, with 11.8% of men age 20 and older affected versus 10.8% of women (Cowie et al., 2006). Some evidence suggests that, among non-diabetic adults, fasting glucose levels are higher among men, but glucose tolerance is poorer among women (Huang, Shimel, Lee, Delancey, & Strother, 2007; Williams, Zimmet, Shaw, et al., 2003). Other evidence has suggested higher fasting glucose and lower glucose tolerance are associated with poor memory performance among women, but not men (Rolandsson, Backestrom, Eriksson, Hallmans, & Nilsson, 2008). Finally, we are unaware of any studies that have assessed differences according to educational attainment, which may be an important resilience factor. According to the brain-reserve hypothesis, greater educational attainment may afford individuals a degree of protection against brain insult that is greater than their counterparts with less education. Protective benefits include preservation of brain volume, intensity of brain metabolism, connectivity in neural networks, efficiency of brain functioning, and enhanced protection against cognitive symptoms (Satz, 1993). Consistent with this hypothesis, relations among fasting glucose, glucose tolerance, and neurocognitive function may vary as a function of educational attainment, such that individuals with more education have better performance.
To address the aforementioned gaps in the literature, the current study examined relations among fasting glucose, glucose tolerance, and various domains of neurocognitive function in a sample of non-diabetic older adults. In addition, the study examined whether age, sex, and education moderate the relations of fasting glucose and glucose tolerance to neurocognitive function.
Method
Participants
Participants were healthy, community-dwelling older adults that participated in an investigation of cardiovascular risk factors, neurocognitive function, and neuroimaging. Recruitment took place at the Baltimore Veterans Affairs Medical Center (B-VAMC), the Geriatric Research Education and Clinical Center at the B-VAMC, and through local advertisement in the greater Baltimore area for a study of cardiovascular risk factors, neuroimaging, and neurocognitive function (see Waldstein, Siegel, Lefkowitz, et al., 2004 and Waldstein, Brown, Maier, & Katzel, 2005 for detailed methods). Exclusion criteria for the study included DM (by self-report, or measured fasting glucose > 126 mg/dl and/or use of DM medications), self-reported history or clinical evidence of cardiovascular disease, systolic blood pressure (BP) ≥ 160 mmHg and/or diastolic blood pressure (BP) ≥ 100 mmHg (severe hypertension), other major medical disease (e.g., renal, hepatic, pulmonary), neurologic disease, stroke, known or suspected dementia (Mini-Mental State Examination score < 24) (Folstein, Folstein, & McHugh, 1975; Tombaugh, 1992), self-reported psychiatric disorder, heavy alcohol use (>14 drinks per week), severe head injury, or medications affecting central nervous system function. The total number of participants who completed the parent study protocol was 232. This analysis included a subset of participants with complete oral glucose tolerance test (OGTT), neuropsychological, and magnetic resonance imaging data for a final sample size of 172. All participants provided written informed consent in accordance with the guidelines of the Institutional Review Boards at the University of Maryland, Baltimore and University of Maryland, Baltimore County.
Measures
Thirteen neuropsychological tests were administered (see Lezak, Howieson, Loring, Hannay, & Fischer, 2004 for detailed descriptions of all tests). The Stroop Color-Word Test measured response inhibition, a dimension of executive function; the interference score was computed per Golden’s criteria (Golden & Freshwater, 2002). Nonverbal memory was assessed by the recall of line drawings using the Visual Reproductions I and II subtests of the Wechsler Memory Scale-Revised (WMS-R; Wechsler, 1987). Verbal memory was measured by the recall of story passages using the Logical Memory I and II subtests of the WMS-R (Wechsler, 1987). The Digits Forward and Digits Backward portions of Digit Span subtest from the Wechsler Adult Intelligence Scale – Revised (WAIS-R) were administered to measure attention and working memory (Wechsler, 1981). The Block Design subscale of the WAIS-R was administered to measure visuoconstructional abilities (Wechsler, 1981). The Judgment of Line Orientation Test was administered to measure visuospatial abilities (Benton, Hannay, & Varney, 1975). The Trail Making Test assessed psychomotor speed and executive function (e.g., set switching) (Reitan, 1978). Trailmaking A and B were administered. The Grooved Pegboard Test assessed motor speed and manual dexterity (Rourke, Yanni, MacDonald, & Young, 1973). Both dominant and non-dominant handed performance was assessed.
Procedure
Participants completed a multi-day, comprehensive medical evaluation that included a health history, a physical examination, a graded exercise treadmill test, and blood chemistries. Clinical assessment of glucose tolerance was completed with an oral glucose tolerance test (OGTT). An antecubital IV catheter was inserted for blood sampling at 10 and 5 minutes prior to oral ingestion of 75 grams of glucose. Blood samples were obtained at 0, 30, 60, 90, and 120 minutes after ingestion of glucose. Plasma was extracted and stored in a −70C freezer. Plasma glucose levels were measured using the glucose oxidase method on a Beckman Glucose Analyzer. Standardized neuropsychological tests were administered on a separate day by trained psychometricians.
Covariates
Covariates were selected based on their influence on neurocognitive function and typical inclusion in the relevant literature. Age and education were assessed in years. Body mass index (BMI) was calculated as the ratio of weight in kilograms to height in meters squared. Clinical assessment of blood pressure was performed on three occasions with participants in a seated position using an automated vital signs monitor (Dinamap Model #1846SX, Critikon, Tampa, FL) and appropriately sized occluding cuff. Blood pressure readings were averaged to yield an estimate of participants’ resting systolic and diastolic BP. Because systolic and diastolic BP are highly correlated, systolic hypertension is more common among older adults than diastolic hypertension, and our sample was more likely to have elevated systolic BP, not elevated diastolic BP, only systolic BP was included in the regression analyses to avoid multi-collinearity. Use of anti-hypertensive medications was collapsed into a single “yes/no” category (because coding methods precluded examination of specific medications). Depressive symptomatology was assessed with the Beck Depression Inventory (Beck, 1987).
Statistical analyses
All analyses were computed using SAS version 9.1 (Cary, NC). All variables were assessed for outliers, homoscedasticity, and assumptions of normality. Distributions for timed neuropsychological measures were normalized with a natural log transformation. Z-score transformations were applied to distributions for neuropsychological measures with “number correct” as scores. Both transformations were applied to satisfy the normality assumptions for the linear regression models. Fasting glucose and 2-hour glucose values were examined separately in the statistical analyses. To determine relations among fasting glucose, 2-hour glucose, and neurocognitive performance, two multiple regressions were run for each of the 13 neurocognitive outcome variables—one model each with fasting glucose as the main predictor and one with 2-hour glucose as the main predictor—for a total of 26 regressions. All outcomes were analyzed as continuous variables. Within these models, interaction terms were created to assess age, sex, and education as effect modifiers. To create interaction terms, age and education were dichotomized in the following manner: age ≤ 70/age > 70 and years of education ≤ 12/years of education > 12. Age was dichotomized at 70 years based on standard age cut-offs (i.e., young-old vs. old-old). This cut-off represents a meaningful transition period, with respect to cognitive decline, among older adults (Burnside, Ebersole, & Monea, 1979). Relatedly, the years of educational attainment cut-off was selected based on documented findings suggesting formal education is a buffer of cognitive decline (Satz, 1993). All models included the aforementioned covariates: age, education, sex, BMI, systolic blood pressure, anti-hypertensive medications, and depressive symptomatology.
Results
Sample characteristics
The mean age of participants was 64.43 (SD = 13.09). The sample was 58% male and 87% White. The mean educational attainment was 16.25 years (SD = 2.86). Twenty-one percent of the sample met criteria for IFG (≥ 100 and < 126 mg/dL) and 25.5% met criteria for IGT (≥ 140 and < 200 mg/dL). Just over 10% of participants met criteria for both IFG and IGT. Mean fasting glucose was in the normal range (M = 94.24 mg/DL, SD = 8.76). Mean systolic blood pressure approached the pre-hypertensive range (M = 128.77 mmHg, SD = 16.97) and mean BMI was in the overweight range (M = 27.68, SD = 4.68). Overall, lipid values were in the normal range, but 5.3% had previously undiagnosed or untreated hyperlipidemia based on an LDL-C > 160 mg/dl. Depressive symptomatology in the sample was low. All sample characteristics are listed in Table 1. Descriptive statistics for all neuropsychological variables are provided in Table 2.
Table 1.
Sample Characteristics (N = 172)
| Characteristic | M (SD) or % | Range |
|---|---|---|
| Age (years) | 64.43 (13.09) | 54–83 |
| Education (years) | 16.25 (2.86) | 8–24 |
| Sex (% male) | 58% | |
| Race (% White) | 87% | |
| Antihypertensive medications (% used) | 14% | |
| Impaired fasting glucose (% ≥100 and <126 mg/dL) | 21% | |
| Impaired glucose tolerance (% ≥140 and <200 mg/dL) | 25.5% | |
| Comorbid impaired fasting glucose and impaired glucose tolerance | 10.4% | |
| Fasting glucose (mg/dL) | 94.24 (8.76) | 75–124 |
| 2 hour glucose (mg/dL) | 133.01 (45.56) | 57–371 |
| Beck Depression Inventory total score | 3.71 (4.21) | 0–23 |
| Body mass index (kg/m2) | 27.68 (4.78) | 17.83–42.50 |
| Systolic blood pressure (mmHg) | 128.77 (16.97) | 95–178 |
| Diastolic blood pressure (mmHg) | 73.59 (8.93) | 50–102 |
Table 2.
Descriptive Statistics for Neuropsychological Tests (N = 172)
| Characteristic | M (SD) | Range |
|---|---|---|
| Stroop interference | −4.32 (6.84) | −24.65–14.22 |
| Visual Reproductions I | 33.09 (5.42) | 15–41 |
| Visual Reproductions II | 25.44 (8.45) | 0–40 |
| Logical Memory I | 24.91 (6.73) | 11–41 |
| Logical Memory II | 19.98 (7.85) | 1–38 |
| Digit Span Forward raw score | 8.17 (2.02) | 4–13 |
| Digit Span Backward raw score | 7.31 (2.48) | 2–14 |
| Block Design raw score | 26.33 (8.91) | 1–49 |
| Judgment of Line Orientation | 24.37 (4.32) | 6–30 |
| Trailmaking A (s) | 31.61 (11.11) | 11–86 |
| Trailmaking B (s) | 79.04 (38.27) | 32–356 |
| Grooved Pegboard Dominant Hand | 79.79 (14.01) | 56–155 |
| Grooved Pegboard Non-Dominant Hand | 86.89 (15.90) | 55–144 |
Linear regression findings
Significant and non-significant main effects, significant and non-significant interactions, and significant relations of covariates to neurocognitive outcomes are reported by glucose variable. Regression statistics are reported in Tables 3 and 4 where significant associations were present with respect to neurocognitive outcomes. Presentation of tables is organized by glucose variable (Table 3 = fasting glucose; Table 4 = 2-hour glucose).
Table 3.
Linear multiple regression: fasting glucose predicting Stroop interference scores, Logical Memory I scores, and Logical Memory II scores (N = 172)
| Stroop interference score | |||
|---|---|---|---|
| B | p | sr2 | |
| Fasting glucose | 0.36 | .48 | <.01 |
| Age | −35.93 | .001 | .05 |
| Education | 52.21 | .27 | .01 |
| Sex | 14.65 | .16 | .01 |
| Beck Depression Inventory | −0.31 | .01 | .03 |
| Systolic blood pressure | −0.02 | .02 | .02 |
| Body mass index | −0.16 | .14 | .01 |
| Anti-hypertensive medication | −0.76 | .52 | <.01 |
| Fasting glucose X age | 0.36 | .002 | .04 |
| Fasting glucose X sex | −0.50 | .17 | .01 |
| Fasting glucose X education | −0.15 | .31 | .01 |
| Total adjusted R2 | .20 | ||
|
| |||
| Logical Memory I | |||
| Fasting glucose | −0.01 | .93 | <.01 |
| Age | −2.10 | .11 | .01 |
| Education | −1.31 | .82 | <.01 |
| Sex | 2.36 | .06 | .02 |
| Beck Depression Inventory | −0.03 | .07 | .01 |
| Systolic blood pressure | −0.001 | .45 | <.01 |
| Body mass index | −0.01 | .67 | <.01 |
| Anti-hypertensive medication | −0.09 | .55 | <.01 |
| Fasting glucose X age | 0.02 | .15 | .01 |
| Fasting glucose X sex | −0.03 | .05 | .02 |
| Fasting glucose X education | 0.02 | .79 | <.01 |
| Total adjusted R2 | .09 | ||
|
| |||
| Logical Memory II | |||
| Fasting glucose | 0.01 | .87 | <.01 |
| Age | −2.91 | .03 | .03 |
| Education | 1.16 | .84 | <.01 |
| Sex | 1.85 | .14 | .01 |
| Beck Depression Inventory | −0.03 | .03 | .03 |
| Systolic blood pressure | −0.001 | .76 | <.01 |
| Body mass index | −0.01 | .57 | <.01 |
| Anti-hypertensive medication | 0.01 | .95 | <.01 |
| Fasting glucose X age | 0.03 | .04 | .02 |
| Fasting glucose X sex | −0.02 | .10 | .01 |
| Fasting glucose X education | −0.01 | .88 | <.01 |
| Total adjusted R2 | .12 | ||
Table 4.
Linear Multiple Regression: 2-hour Glucose Predicting Trail Making B scores and Visual Reproductions I scores (N = 172)
| Trailmaking B score | B | p | sr2 |
|---|---|---|---|
| 2 hour glucose | 0.01 | .06 | .02 |
| Age | 0.33 | .14 | .01 |
| Education | 0.87 | .11 | .01 |
| Sex | −0.22 | .25 | .01 |
| Beck Depression Inventory | 0.01 | .37 | <.01 |
| Systolic blood pressure | 0.002 | .001 | .07 |
| Body mass index | 0.02 | .01 | .03 |
| Anti-hypertensive medication | 0.10 | .15 | .01 |
| 2 hour glucose X age | −0.001 | .51 | <.01 |
| 2 hour glucose X sex | 0.001 | .45 | <.01 |
| 2 hour glucose X education | −0.01 | .04 | .02 |
| Total adjusted R2 | .20 | ||
|
| |||
| Visual Reproductions I | |||
| 2 hour glucose | −0.01 | .30 | .01 |
| Age | −1.17 | .01 | .03 |
| Education | −0.20 | .86 | <.01 |
| Sex | −0.09 | .82 | <.01 |
| Beck Depression Inventory | −0.03 | .10 | .01 |
| Systolic blood pressure | −0.001 | .41 | <.01 |
| Body mass index | 0.03 | .02 | .03 |
| Anti-hypertensive medication | −0.25 | .09 | .02 |
| 2 hour glucose X age | 0.01 | .03 | <.01 |
| 2 hour glucose X sex | 0.002 | .50 | <.01 |
| 2 hour glucose X education | 0.004 | .61 | <.01 |
| Total adjusted R2 | .12 | ||
Fasting glucose
After adjustment for all covariates that included age, sex, education, depressive symptomatology, systolic BP, BMI, and anti-hypertensive medication use, there were no significant main effects for fasting glucose on neurocognitive outcomes: Stroop interference (sr2 = .002, p = .48), Visual Reproductions I (sr2 = .001, p = .87), Visual Reproductions II (sr2 = .004, p = .35), Logical Memory I (sr2 = .001, p = .93), Logical Memory II (sr2 = .001, p = .87), Digits Forward (sr2 = .002, p = .50), Digits Backward (sr2 = .002, p = .57), Block Design (sr2 = .001, p = .85), Judgment of Line Orientation (sr2 = .01, p = .14), Trailmaking A (sr2 = .001, p = .79), Trailmaking B (sr2 = .003, p = .38), Grooved Pegboard dominant hand (sr2 = .004, p = .31), and Grooved Pegboard non-dominant hand (sr2 = .003, p = .39). Among the non-significant models, total adjusted R2 ranged from to .08 to .29.
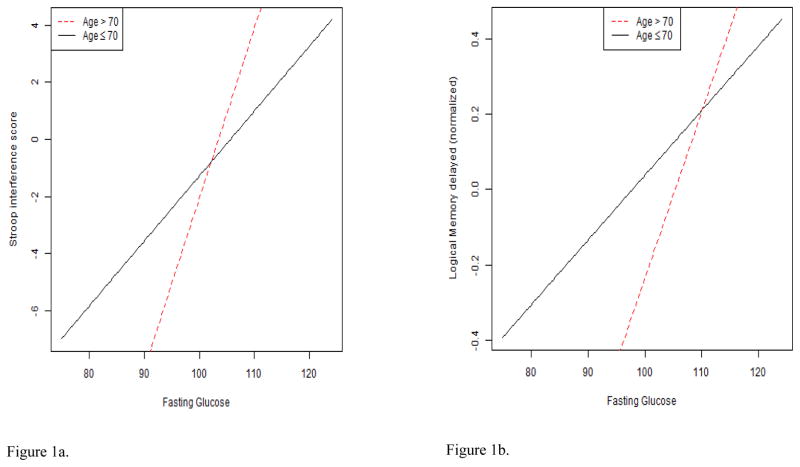
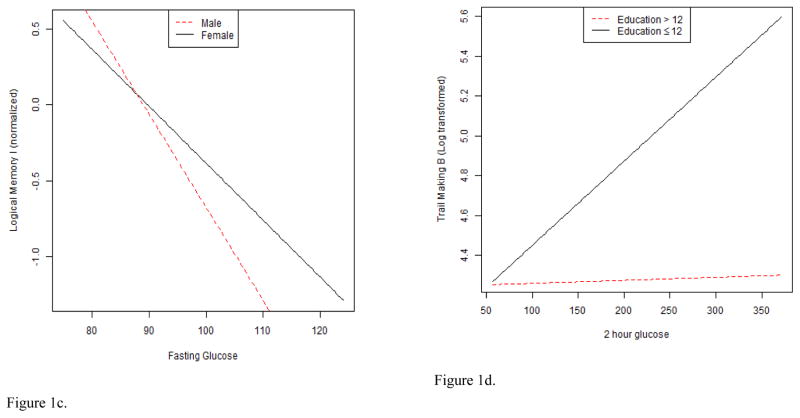
With respect to interactions, several findings were significant. The fasting glucose X age interaction was significantly associated with the Stroop interference score (B = 0.36; p = .002; sr2 = .04). A plot of the interaction illustrated that the slope of the positive linear association between fasting glucose and interference was steeper when participants were older (see Figure 1a). Similarly, there was a significant fasting glucose X age interaction for Logical Memory II (B = 0.03, p = .04; sr2 = .02). A plot of the interaction suggested the positive linear association between fasting glucose and long-term verbal memory performance was more pronounced when participants were older (see Figure 1b). Finally, there was a significant fasting glucose X sex interaction for Logical Memory I (B = −0.03, p = .05; sr2 = .02) (see Figure 1c). In this case, the negative linear association between fasting glucose and short-term verbal memory was more pronounced among men. Age, sex, and education did not emerge as significant effect modifiers of Visual Reproductions I and II, Digits Forward, Digits Backward, Block Design, Judgment of Line Orientation, Trailmaking A and B, and Grooved Pegboard dominant and non-dominant hand outcomes.
Figure 1.
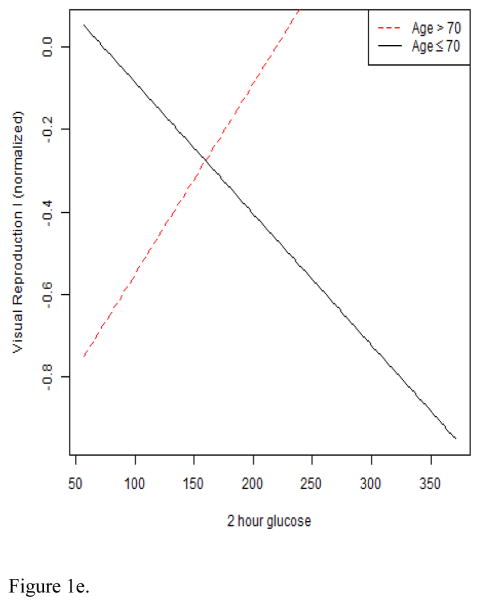
Figures 1a–1e. These figures illustrate interaction plots for significant glucose variables X age, sex, and education: a. relation of fasting glucose to Stroop interference performance modified by age; b. relation of fasting glucose to Logical memory II performance modified by age; c. relation of fasting glucose to Logical memory I performance modified by sex; d. relation of 2-hour glucose to Trailmaking B performance modified by education; e. relation of 2-hour glucose to Visual Reproductions I performance modified by age.
As expected, in the fasting glucose models, several covariates were associated with neurocognitive outcomes. Age was inversely associated with Stroop interference (B = −35.93, p = .001), Logical Memory II (B = −2.91, p = .03), and Visual Reproductions II performance (B = −2.67, p = .04). BDI was inversely associated with Stroop interference (B = −0.31, p = .01), Logical Memory II (B = −0.03, p = .03), and Visual Reproductions II (B = −0.03, p = .04). Systolic blood pressure was inversely associated with Stroop interference (B = −0.02, p = .02) and Block Design (B = −0.002, p = .03), and positively associated with Trailmaking B (B = 0.001, p = .001). Finally, BMI was positively associated with Grooved Pegboard non-dominant hand (B = 0.01, p = .02), Trailmaking B (B = 0.02, p = .01), Visual Reproductions I (B = 0.03, p = .02), and Digits Backward (B = −0.04, p = .004).
2-hour glucose
As with fasting glucose, after adjustment for all covariates, there were no significant main effects for 2-hour glucose on neurocognitive outcomes: Stroop interference (sr2 = .003, p = .40), Visual Reproductions I (sr2 = .01, p = .33), Visual Reproductions II (sr2 = .003, p = .42), Logical Memory I (sr2 = .002, p = .57), Logical Memory II (sr2 = .003, p = .45), Digits Forward (sr2 = .002, p = .52), Digits Backward (sr2 = .01, p = .28), Block Design (sr2 = .001, p = .86), Judgment of Line Orientation (sr2 = .01, p = .14), Trailmaking A (sr2 = .001, p = .85), Trailmaking B (sr2 = .02, p = .06), Grooved Pegboard dominant hand (sr2 = .01, p = .31), and Grooved Pegboard non-dominant hand (sr2 = .01, p = .10). Among the non-significant models, total adjusted R2 ranged from to .08 to .28.
With respect to interactions, a significant 2-hour glucose X education interaction emerged (B = −0.01, p = .04; sr2 = .02) for Trailmaking B. A plot of the interaction illustrated a positive linear association between 2-hour glucose and divided attention in the low education group, but a flat association in the high education group (see Figure 1d). A significant 2-hour glucose X age interaction for Visual Reproductions I scores was also noted (B = 0.01, p = .03; sr2 = .03). The interaction plot demonstrated a positive linear association between 2-hour glucose and Visual Reproductions I scores among older adults >70, but a negative association among older adults ≤ 70 (see Figure 1e). Age, sex, and education did not emerge as significant effect modifiers for Visual Reproductions II, Digits Forward, Digits Backward, Block Design, Judgment of Line Orientation, Trailmaking A, and Grooved Pegboard dominant and non-dominant hand outcomes.
In the 2-hour glucose models, several covariates were associated with neurocognitive outcomes. Age was positively associated with Grooved Pegboard dominant hand (B = 0.25, p = .003), Grooved Pegboard non-dominant hand (B = 0.28, p = .02), and inversely associated with Logical Memory II (B = −0.93, p = .04), and Visual Reproductions I performance (B = −1.17, p = .01). Education was inversely associated with Grooved Pegboard dominant hand performance (B = −0.43, p = .04). BDI was inversely associated with Stroop interference (B = −0.28, p = .02), Logical Memory II (B = −0.03, p = .03). Systolic blood pressure was inversely associated with Stroop interference (B = −0.02, p = .01) and Block Design (B = −0.002, p = .02), and positively associated with Trailmaking B (B = 0.002, p = .0001). BMI was positively associated with Grooved Pegboard non-dominant hand (B = 0.01, p = .01), Trailmaking B (B = 0.02, p = .01), and Digits Backward (B = 0.04, p = .01). Finally, use of anti-hypertensive medications was inversely associated with Block Design performance (B = −0.29, p = .03)
Discussion
Findings from the current study revealed significant interactive relations of fasting glucose, 2-hour glucose, and neurocognitive function modified by age, sex, and education. Higher levels of fasting glucose were associated with better response inhibition and long-term verbal memory performance for participants over age 70, but not among participants ≤ 70, and poorer short-term verbal memory performance among men, but not among women. In addition, we identified that higher 2-hour glucose values were associated with poorer set shifting performance among participants with less than a high school education, whereas this association was not observed among participants with a high school education or greater. Despite the absence of main effects of fasting glucose and 2-hour glucose, our interaction findings suggest a pattern of results wherein glucose may play a detectable role in neurocognitive outcomes among individuals with select vulnerability and resilience factors.
Numerous studies have demonstrated evidence of reduced cognitive performance among older adults with Type 2 DM (Awad et al., 2004; Cukierman et al., 2005; Ryan, 2005). Other findings suggest older adults with IFG and IGT show lower neurocognitive performance (Convit et al., 2003; Kaplan et al., 2000; Ryan, 2001; Vanhanen et al., 1998). Relatedly, non-diabetic individuals with evidence of insulin resistance have shown reductions in cognitive performance (Bruehl et al., 2010). Results pertaining to the relation of fasting glucose to neurocognitive function in the current study were not consistent with the majority of the literature; however, select findings for glucose tolerance were consistent.
The discrepancy between our findings and typical findings for the relations of fasting glucose to neurocognitive performance in the literature may be explained by our purposeful restriction of the range of glucose levels. Thus, fasting glucose levels in the sample may not have been sufficiently elevated to precipitate decrements in neurocognitive function. Another explanation for the beneficial association of higher glucose values with neurocognition in this relatively healthy sample may lie in the energy requirement of the brain, which is met almost exclusively by glucose (Siesjo, 1978). The brain has limited space for energy storage, but requires a high rate of glucose utilization. As such, the brain relies on a continuous glucose supply (Benton, Parker, & Donohoe, 1996). Consistent with our findings, a growing body of human and animal studies suggests that higher glucose levels may enhance neurocognitive performance. For example, one study found higher baseline levels of blood glucose among young adults were associated with benefits for neurocognitive tests that place higher demands on the brain, but not for those that were less challenging (Donohoe & Benton, 2000). Relatedly, glucose ingestion has been found to enhance subsequent memory performance in both younger and older adults (Hall, Gonderfrederick, Chewning, Silveira, & Gold, 1989; Riby, Meikle, & Glover, 2004); however, other neurocognitive domains have not been widely examined. The oldest adults (“old-old”) in our sample appeared to derive more of a neurocognitive benefit from higher fasting glucose levels than their “young-old” counterparts. Future research should not only explore the mechanism of glucose utilization during neurocognitive tasks, but also if this mechanism carries some compensatory benefit for neurocognitive function among the oldest.
Another alternative explanation for results that emerged in an unexpected direction could be the presence of nonlinear trends (e.g., quadratic and cubic) in glucose to cognitive function relations. Given aforementioned evidence that the brain relies on glucose for energy and requires a continuous supply (Benton et al., 1996, Siesjo, 1978), and elevated levels of glucose may be harmful cognitive function (Convit et al., 2005; DiBonito et al., 2007), it is plausible that both hypoglycemic and hyperglycemic states may be deleterious to cognitive function. For example, the association between fasting glucose and cognitive function might result in an inverted U-shaped association, whereby those individuals with the lowest and the highest fasting glucose values tend to have the poorest cognitive outcomes. Our sample size did not support the addition of more predictors and interactions (e.g., quadratic fasting glucose, quadratic fasting glucose X age); thus, it was not prudent to test quadratic and cubic trends. Given the selected effect modifiers that were analyzed and the documented difficulty with testing interactions once main effects have been partialed out (McClelland & Judd, 1993), we did not analyze these trends so as not to overfit the models. Future analysis of these trends is warranted with larger samples, however.
In contrast, the significant interaction between fasting glucose and sex suggested fasting glucose levels were deleterious to short-term verbal memory performance, particularly among men. Higher levels of cortisol are known to aggregate with higher fasting glucose levels and abdominal obesity (Bjorntorp & Rosmond, 2000), and have been associated with lower levels of memory performance and hippocampal atrophy (Sapolsky, 1999). Over a quarter (25.69%) of the men in our sample exceeded the criterion for abdominal obesity based on waist circumference (> 102 cm). It is plausible that abdominal fat among men helps to explain sex variation in the influence of fasting glucose on short-term and long-term verbal memory; however, we did not have sufficient power to examine this possibility among only 28 men.
Two-hour glucose values represent the glucose load remaining two hours after glucose ingestion. Two-hour glucose values showed a different pattern of results as compared to fasting glucose. In that regard, higher 2-hour glucose values were associated with poorer divided attention performance, but the association was education-dependent. Thus, when individuals had less than a high school education and a higher 2-hour glucose load, their divided attention performance was comparatively lower than those with at least a high school education. This result was consistent with prior evidence noting that poorer glucose tolerance is associated with poorer neurocognitive performance (Convit et al., 2005; DiBonito et al., 2007; Messier et al., 2010); however, it suggests that higher levels of education may afford some protection against the consequences of higher glucose load. Inversely, low education may represent a vulnerability factor in this association. In that regard, the distinction between having graduated from high school or not could potentially represent a meaningful cut-off with regard to 2-hour glucose and divided attention. This finding may, in part, relate to the brain-reserve hypothesis that posits greater educational attainment may buffer the progression of neuropathology and associated neurocognitive symptoms (Kaplan et al., 2000; Messier et al., 2003). Importantly, we did not directly test this hypothesis, and studies conducted on more highly educated older adults, such as this one, may mask the negative influence of poorer glucose tolerance on neurocognitive function. More socioeconomically heterogeneous samples of older adults may be ideal for examining the benefit of education in future studies.
Secondly, higher 2-hour glucose values were associated with better short-term nonverbal memory performance among older adults > 70 years, but not ≤ 70. Similar to our fasting glucose findings, older participants appeared to benefit from maintaining a higher glucose load over time. Again, the mechanisms through which higher glucose is more beneficial for neurocognitive function among the oldest warrant further exploration. This seemingly paradoxical association has also been noted in some studies that noted inverse relations between cholesterol and mortality (Brescianini, Maggi, Farchi, et al., 1997; Vischer, Safar, Safar, et al., 2009) and blood pressure and mortality (Busby, Campbell, & Robertson, 1994; Okumiya, Matsubayashi, Wada, et al., 1999; Vischer et al., 2009) in the old-old, and may be due to selective mortality or other factors such as frailty and malnutrition.
Potential biological mechanisms that explain relations between glucose abnormalities and neurocognitive deficits include neurological aberrations such as oxidative stress, accelerated ischemic brain damage, and impaired glucose utilization during cognitive tasks (Cukierman, Gerstein, & Williamson, 2005). Type 2 DM has been implicated in a number of brain alterations including reduced grey matter volume, increased ventricle volume, and increased white matter lesion volume (Jongen, van der Grond, Kappelle, et al., 2007). As a particularly potent risk factor for subclinical cerebrovascular disease and brain atrophy, Type 2 DM has been linked specifically to degree of white matter hyperintensities and cerebral atrophy in both cross-sectional and longitudinal studies (De Bresser, Tiehuis, van den Berg, et al., 2011; Tiehuis, van der Graaf, Visseren, et al., 2008; van Harten, de Leeuw, Weinstein, Scheltens, & Biessels, 2006). Each of these mechanisms is progressive, thus it is hypothesized that older adults even at slight risk for Type 2 DM demonstrate similar symptomatology, but less advanced sequelae than their diabetic counterparts. This hypothesis further underscores the need to examine subclinical neurological and neurocognitive changes in older adults at risk for IFG, IGT, and Type 2 DM.
Limitations
Given the potential for fasting glucose and glucose tolerance values to fluctuate, it is possible that data collection on a subsequent day may have yielded different results. The cross-sectional design did not enable us to establish temporal relations of our findings. Furthermore, due to the correlational nature of the study, it is plausible that a third variable may have accounted for observed associations that were less optimal among participants with less than a high school education. The sample size was limited to relatively healthy adults, which did not give us statistical power to examine membership in subgroups (i.e., participants with IFG, IGT, or both). In addition, the relatively small size and predominately White racial/ethnic background of the sample limits the generalizability of the findings. Examining these relationships in diverse samples is imperative as certain racial/ethnic groups are at greater risk for diabetes and other glucose abnormalities (Go, Mozaffarian, Roger, et al., 2013).
Given the number of univariate analyses run with our limited sample size, subsequent studies with larger sample sizes should consider data reduction techniques such as factor analysis to reduce the number of outcomes and help prevent Type 1 error. Factor analysis would enable analysis of potential underlying domains of cognitive function; however, it may also result in a loss of information specific to each cognitive outcome. We selected the univariate approach to maximize the information unique to each neuropsychological test and reduce the likelihood of Type II error. Although the pattern of results appears to be non-random and parallel to other documented findings, it is important to note that correction for Type I error, such as with a Bonferroni correction, would have yielded non-significant outcomes. Thus, the results presented here should be interpreted cautiously and viewed as preliminary with a need for replication in larger samples.
Conclusions
A future examination of fasting glucose, glucose tolerance, and neurocognitive function relations is warranted with IFG and IGT inclusion criteria to help further elucidate the neurocognitive trajectories associated with glucose in non-diabetic older adults. Our findings begin to suggest a need for targeted educational and practice-based interventions that encourage older adults to closely monitor glucose levels to reduce the risk of neurocognitive decrements. Indeed, this trend in the data has major implications for public health as millions of older Americans are overweight or obese, have IFG and IGT, and are projected to develop Type 2 DM. Moreover, longitudinal studies may be warranted to assess the value of obtaining higher levels of education or engaging in cognitively stimulating activities as tools to help buffer against glucose-related neurocognitive dysfunction. Our unexpected findings suggest that a moderate degree of glucose elevation may be indicated for optimal neurocognitive function among the oldest. Although a goal of increasing glucose levels is unlikely to be safe or practical, further understanding of the range of optimal levels is warranted.
Acknowledgments
“This work was supported by the [National Institute on Aging] under Grant [numbers R29 AG15112, RO1 AG5112, P30 AG02874, K24 AG00930, and T32 AG000029]; and [Department of Veterans Affairs] under [VA Merit Grant].”
Footnotes
Disclosure statement
There are no potential conflicts of interest in relation to the submitted manuscript.
Contributor Information
Regina Sims Wright, Email: rsims@udel.edu, School of Nursing, University of Delaware, 25 N. College Avenue, Newark, DE 19716, (302) 831-8364
Shellie-Anne T. Levy, Email: stlevy@howard.edu, Department of Psychology, Howard University, 525 Bryant St., NW, Washington, DC 20059, (617) 571-5928
Leslie I. Katzel, Email: lkatzel@grecc.umaryland.edu, Division of Gerontology & Geriatric Medicine, University of Maryland School of Medicine, Geriatric Research Education & Clinical Center, Baltimore Veterans Affairs Medical Center, Baltimore VA Medical Center, Room 4B-189, 655 West Baltimore St., Baltimore, MD 21201, (410) 605-7248
William F. Rosenberger, Email: wrosenbe@gmu.edu, Department of Statistics, George Mason University, 4400 University Dr., MS 4A7, Fairfax, VA 22030, (703) 993-3645
Zorayr Manukyan, Email: zorayr.manukyan@pfizer.com, Pfizer, 35 Cambridge Park Dr, Cambridge, MA 02140, (701) 538-4376
Keith E. Whitfield, Email: kwhit1@duke.edu, Department of Psychology & Neuroscience, Duke University, 9 Flowers Drive, Durham, NC, Box 90086, 417 Chapel Drive, Durham, NC 27708-0086, (919) 660-5769
Shari R. Waldstein, Email: waldstei@umbc.edu, Department of Psychology, University of Maryland Baltimore County, Geriatric Research Education & Clinical Center, Baltimore Veterans Affairs Medical Center, 1000 Hilltop Circle, Math/Psychology Building, Rm. 312, Baltimore, MD 21250, (410) 455-2848
References
- Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance type 2 diabetes and cognitive function. Journal of Clinical and Experimental Neuropsychology. 2004;26:1044–1080. doi: 10.1080/13803390490514875. [DOI] [PubMed] [Google Scholar]
- Beck AT. Beck Depression Inventory: Manual. New York: Psychological Corporation; 1987. [Google Scholar]
- Benton AL, Hannay HJ, Varney NR. Visual perception of line direction in patients with unilateral brain damage. Neurology. 1975;25:907–910. doi: 10.1212/wnl.25.10.907. [DOI] [PubMed] [Google Scholar]
- Benton D, Parker PY, Donohoe RT. The supply of glucose to the brain and cognitive functioning. Journal of Biosocial Science. 1996;28:463–479. doi: 10.1017/s0021932000022537. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, van der Heide LP, Kamal A, Bleys RL, Gispen WH. Ageing and diabetes: implications for brain function. European Journal of Pharmacology. 2002;441:1–14. doi: 10.1016/s0014-2999(02)01486-3. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Rosmond R. Neuroendocrine abnormalities in visceral obesity. International Journal of Obesity and Related Metabolic Disorders. 2000;24(Suppl):S80–S85. doi: 10.1038/sj.ijo.0801285. [DOI] [PubMed] [Google Scholar]
- Brescianini S, Maggi S, Farchi G, Mariotti S, Di Carlo A, Baldereschi M, Inzitari D. Low total cholesterol and increased risk of dying: are low levels clinical warning signs in the elderly? Results from the Italian Longitudinal Study on Aging. Journal of the American Geriatric Society. 1997;51:991–996. doi: 10.1046/j.1365-2389.2003.51313.x. [DOI] [PubMed] [Google Scholar]
- Bruehl H, Sweat V, Hassenstab J, Polyakov V, Convit A. Cognitive impairment in nondiabetic middle age and older adults is associated with insulin resistance. Journal of Clinical and Experimental Neuropsychology. 2010;32:487–493. doi: 10.1080/13803390903224928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnside IM, Ebersole P, Monea HE. Psychosocial caring throughout the life span. New York: McGraw Hill; 1979. [Google Scholar]
- Busby WJ, Campbell AJ, Robertson MC. Is low blood pressure in elderly people just a consequence of heart disease and frailty? Age and Ageing. 1994;23:69–74. doi: 10.1093/ageing/23.1.69. [DOI] [PubMed] [Google Scholar]
- Convit A, Wolf OT, Tarshish C, de Leon MJ. Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proceeds of the National Academy of the Sciences. 2003;100:2019–2022. doi: 10.1073/pnas.0336073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. Population: National Health and Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29:1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes-systematic overview of prospective observational studies. Diabetologia. 2005;48:2460–2469. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- De Bresser J, Tiehuis AM, van den Berg E, Reijmer YD, Jongen C, Kappelle LJ, et al. Progression of cerebral atrophy and white matter hyperintensities in patients with type 2 diabetes. Diabetes Care. 2011;33:1309–1314. doi: 10.2337/dc09-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bonito P, Di Fraia L, Di Gennaro L, Vitale A, Lapenta M, Scala A, et al. Impact of impaired fasting glucose and other metabolic factors on cognitive function in elderly people. Nutrition, Metabolic, and Cardiovascular Diseases. 2007;17:203–208. doi: 10.1016/j.numecd.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Donohoe RT, Benton D. Glucose tolerance predicts performance on tests of memory and cognition. Physiology & Behavior. 2000;71:395–401. doi: 10.1016/s0031-9384(00)00359-0. [DOI] [PubMed] [Google Scholar]
- Elias PK, Elias MF, D’Agostino RB, Cupples LA, Wilson PW, Silbershatz H, et al. NIDDM and blood pressure as risk factors for poor cognitive performance. The Framingham Study. Diabetes Care. 1997;20:1388–1395. doi: 10.2337/diacare.20.9.1388. [DOI] [PubMed] [Google Scholar]
- Fischer AL, de Frias CM, Yeung SE, Dixon RA. Short-term longitudinal trends in cognitive performance in older adults with type 2 diabetes. Journal of Clinical and Experimental Neuropsychology. 2009;31:809–822. doi: 10.1080/13803390802537636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state.’ A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Galanina N, Surampudi V, Ciltea D, Singh SP, Perlmuter LC. Blood glucose levels before and after cognitive testing in diabetes mellitus. Experimental Aging Research. 2008;34:152–161. doi: 10.1080/03610730701876979. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, et al. Executive summary: Heart disease and stroke statistics--2013 update: A report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- Golden CJ, Freshwater SM. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Chicago, Illinois: Stoelting Co; 2002. [Google Scholar]
- Hall JL, Gonderfrederick LA, Chewning WW, Silveira J, Gold PE. Glucose enhancement of performance on memory tests in young and aged humans. Neuropsychologia. 1989;27:1129–1138. doi: 10.1016/0028-3932(89)90096-1. [DOI] [PubMed] [Google Scholar]
- Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults: The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- Hiltunen LA, Keinanen-Kiukaanniemi SM, Laara EM. Glucose tolerance and cognitive impairment in an elderly population. Public Health. 2001;115:197–200. doi: 10.1038/sj/ph/1900758. [DOI] [PubMed] [Google Scholar]
- Hollenberg NK. Quality of life as a therapeutic endpoint. Drug Safety. 1991;6:83–93. doi: 10.2165/00002018-199106020-00001. [DOI] [PubMed] [Google Scholar]
- Huang TT, Shimel A, Lee RE, Delancey W, Strother ML. Metabolic risks among college students: prevalence and gender differences. Metabolic Syndrome and Related Disorders. 2007;5:365–372. doi: 10.1089/met.2007.0021. [DOI] [PubMed] [Google Scholar]
- Jongen C, van der Grond J, Kappelle LJ, Biessels GJ, Viergever MA, Pluim JPW. Automated measurement of brain and white matter lesion volume in type 2 diabetes mellitus. Diabetologia. 2007;50:1509–1516. doi: 10.1007/s00125-007-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaya AM, Barrett-Connor E, Gildengorin G, Yaffe K. Change in cognitive function by glucose tolerance status in older adults: a 4-year prospective study of the Rancho Bernardo study cohort. Archives of Internal Medicine. 2004;164:1327–1333. doi: 10.1001/archinte.164.12.1327. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Greenwood CE, Winocur G, Wolever TMS. Cognitive performance is associated with glucose regulation in healthy elderly persons and can be enhanced with glucose and dietary carbohydrates. American Journal of Clinical Nutrition. 2000;72:825–836. doi: 10.1093/ajcn/72.3.825. [DOI] [PubMed] [Google Scholar]
- Lamport DJ, Lawton CL, Mansfield MW, Dye L. Impairments in glucose tolerance can have a negative impact on cognitive function: a systematic research review. 2009 doi: 10.1016/j.neubiorev.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Levine S, Croog SH. What constitutes quality of life? A conceptualization of the dimensions of life quality in healthy populations and patients with cardiovascular disease. In: Wegner, editor. Assessment of Quality of Life in Clinical Trials of Cardiovascular Therapies. New York: Le Jacq Publishing Inc; 1984. pp. 396–403. [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment. Fourth ed. New York: Oxford University Press; 2004. [Google Scholar]
- McLelland GH, Judd CM. Statistical difficulties of detecting interactions and moderator effects. Psychological Bulletin. 1993;114:376–390. doi: 10.1037/0033-2909.114.2.376. [DOI] [PubMed] [Google Scholar]
- Messier C, Tsiakas M, Gagnon M, Desrochers A, Awad N. Effect of age and glucoregulation on cognitive performance. Journal of Clinical and Experimental Neuropsychology. 2010;32:809–821. doi: 10.1080/13803390903540323. [DOI] [PubMed] [Google Scholar]
- Okumiya K, Matsubayashi K, Wada T, Fujisawa M, Osaki Y, Doi Y, Yasuda N, Ozawa T. A U-shaped association between home systolic blood pressure and four-year mortality in community-dwelling older men. Journal of the American Geriatric Society. 1999;47:1415–1421. doi: 10.1111/j.1532-5415.1999.tb01559.x. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Trail making Test: Manual for Administration and Scoring. Tuscon, AZ: Reitan Neuropsychology Press; 1978. [Google Scholar]
- Riby LM, Meikle A, Glover C. The effects of age, glucose ingestion and gluco-regulatory control on episodic memory. Glucose and Ageing. 2004;33:483–487. doi: 10.1093/ageing/afh173. [DOI] [PubMed] [Google Scholar]
- Rolandsson O, Backestrom A, Eriksson S, Hallmans G, Nilsson LG. Increased glucose levels are associated with episodic memory in nondiabetic women. Diabetes. 2008;57:440–443. doi: 10.2337/db07-1215. [DOI] [PubMed] [Google Scholar]
- Rourke BP, Yanni DW, MacDonald GW, Young GC. Neuropsychological significance of lateralized deficits on the Grooved Pegboard Test for older children with learning disabilities. Journal of Consulting and Clinical Psychology. 1973;41:128–134. doi: 10.1037/h0035613. [DOI] [PubMed] [Google Scholar]
- Ryan CM. Diabetes, aging, and cognitive decline. Neurobiology of Aging. 2005;26(Suppl 1):21–25. doi: 10.1016/j.neurobiolaging.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Ryan C. Diabetes-associated cognitive dysfunction. In: Waldstein SR, Elias MF, editors. Neuropsychology of cardiovascular disease. Mahwah, NJ: Lawrence Erlbaum Associates; 2001. pp. 61–82. [Google Scholar]
- Sapolsky RM. Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Experimental Gerontology. 1999;34:721–732. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Satz P. Brain reserve capacity on symptom onset after brain injury: a formulation and review of evidence for threshold theory. Neuropsychology. 1993;3:273–295. [Google Scholar]
- Siesjo B. Brain Energy Metabolism. New York: Wiley; 1978. [Google Scholar]
- Tiehuis AM, van der Graaf Y, Visseren FL, Vincken KL, Biessels GJ, Appelman APA, Kappelle LJ, Mali WPTM. Diabetes increases atrophy and vascular lesions on brain MRI in patients with symptomatic arterial disease. Stroke. 2008;39:1600–1603. doi: 10.1161/STROKEAHA.107.506089. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, McIntyre NJ. The Mini-Mental State Examination: a comprehensive review. Journal of the American Geriatrics Society. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- Vanhanen M, Koivisto K, Kuusisto J, Mykkänen L, Helkala EL, Hänninen T, Riekkinen P, Sr, Soininen H, Laakso M. Cognitive function in an elderly population with persistent impaired glucose tolerance. Diabetes Care. 1998;21:398–402. doi: 10.2337/diacare.21.3.398. [DOI] [PubMed] [Google Scholar]
- Vischer UM, Safar ME, Safar H, Iaria P, Le Dudal K, Henry O, Herrmann FR, Ducimetière P, Blacher J. Cardiometabolic determinants of mortality in a geriatric population: is there a “reverse metabolic syndrome? Diabetes & Metabolism. 2009;35:108–114. 23. doi: 10.1016/j.diabet.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Giggey PP, Thayer JF, Zonderman AB. Nonlinear relations of blood pressure to cognitive function: the Baltimore Longitudinal Study of Aging. Hypertension. 2005;45:374–379. doi: 10.1161/01.HYP.0000156744.44218.74. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Katzel LI. Hypertension and cognitive function. In: Waldstein SR, Elias MF, editors. Neuropsychology of Cardiovascular Disease. Mahwah, NJ: Lawrence Erlbaum Associates; 2001. pp. 15–36.pp. 15–36. [Google Scholar]
- Waldstein SR, Siegel EL, Lefkowitz D, Maier KJ, Pelletier Brown JR, Obuchowski AM, et al. Stress-induced blood pressure reactivity and silent cerebrovascular disease. Stroke. 2004;35:1294–1298. doi: 10.1161/01.STR.0000127774.43890.5b. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Brown JRP, Maier KJ, Katzel LI. Diagnosis of hypertension and high blood pressure levels negatively affect cognitive function in older adults. Annals of Behavioral Medicine. 2005;29:174–180. doi: 10.1207/s15324796abm2903_3. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale - Revised Manual. New York: Psychological Corporation; 1987. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Revised Manual. New York: Psychological Corporation; 1981. [Google Scholar]
- Whitehead BP, Dixon RA, Hultsch DF, MacDonald SWS. Are neurocognitive speed and inconsistency similarly affected in type 2 diabetes? Journal of Clinical and Experimental Neuropsychology. 2011;33:647–657. doi: 10.1080/13803395.2010.547845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JW, Zimmet PZ, Shaw JE, de Courten MP, Cameron AJ, Chitson P, et al. Gender differences in the prevalence of impaired fasting glycaemia and impaired glucose tolerance in Mauritius. Does sex matter? Diabetic Medicine. 2003;20:915–920. doi: 10.1046/j.1464-5491.2003.01059.x. [DOI] [PubMed] [Google Scholar]