Abstract
BACKGROUND
Adenovirus (Ad)-mediated E2F-1 gene transfer induces apoptosis in cancer cells in vitro and in vivo, but clinical application of E2F-1 in cancer gene therapy remains controversial because of the oncogenic potential of E2F-1. This barrier can be circumvented by using the truncated form of the E2F-1 gene (E2Ftr) (amino acids 1 through 375), which lacks the E2F-1 transactivation domain and cell cycle-promoting effects.
METHODS
The authors constructed 3 adenoviral vectors that expressed E2Ftr under regulation of the tetracycline (Tet)-off system (AdTet-E2Ftr1, AdTet-E2Ftr2, and AdTet-E2Ftr3). These vectors were compared for E2Ftr expression and apoptosis induction in cancer cells and normal cells. E2Ftr antitumor activity in vivo also was assessed in a melanoma xenograft model.
RESULTS
One of the 3 vectors, AdTet-E2Ftr3, had the highest E2Ftr protein expression levels, which were correlated with the greatest induction of apoptosis and inhibition of cancer cell growth. E2Ftr induced apoptosis in a variety of cancer cell lines independent of p53 status with little cytotoxicity in normal cell lines. In a mouse melanoma xenograft model, AdTet-E2Ftr3 exhibited an approximately 80% decrease in tumor size compared with controls in vivo.
CONCLUSIONS
The current results indicated that AdTet-E2Ftr3 is a novel anticancer agent that has significant therapeutic activity in vitro and in vivo.
Keywords: adenovirus, E2F-1, truncated, cancer, tetracycline-off, apoptosis, in vivo
Adenovirus (Ad) vectors that express the transcription factor E2F-1 (Ad-E2F-1) efficiently induce apoptosis in cancer cells in vitro and in vivo.1–5 However, it is believed that E2F-1 is an oncogene, presumably by virtue of its ability to stimulate cell cycle progression from the G1 phase to the S-phase.6,7 Thus, the use of wild type (wt) E2F-1 in gene therapy has been controversial because of its oncogenic potential. To circumvent this barrier, we deleted the transactivation domain of wt E2F-1 and generated a truncated form of the E2F-1 gene (E2Ftr) (amino acids 1 through 375). Transactivation-deficient E2F-1 is unable to induce cell cycle progression compared with full-length E2F-1 but still is a potent inducer of apoptosis.8,9
To generate adenoviral vectors for efficiently transferring the therapeutic E2Ftr gene into tumors, we previously tried to use a cytomegalovirus (CMV) promoter to control E2Ftr. However, unlike an adenoviral vector that expresses the wt E2F-1 gene, the E2Ftr adenoviral vector could not be rescued from the plasmids with the adenoviral backbone in Ad host HEK-293 human embryonic kidney cells despite numerous attempts. It is likely that high levels of E2Ftr expression driven by the CMV promoter may be toxic to HEK-293 cells and may inhibit adenoviral replication. Next, we applied the tetracycline (Tet)-off regulated expression system10 to control the expression of E2Ftr in adenoviral vectors. We developed new vectors in which the Tet-controlled transactivator (tTA) and the E2Ftr gene were integrated into the same Ad construct. Under regulation of the Tet-responsive promoter (phcmv), E2Ftr is activated by the tTA only in the absence of Tet.11 This system overcame the toxic effects of transgenic gene on HEK-293 cells, allowing us to construct E2Ftr-expressing adenoviruses.
In this report, we demonstrate that the inducible expression system in our new vectors resulted in high-level E2Ftr expression in cancer cells. Compared with Ad-E2F-1–expressing wt E2F-1, vectors that expressed E2Ftr induced greater cancer cell cytotoxicity and lower cytotoxicity in normal cells. In addition, E2Ftr significantly suppressed tumor growth in a nude mouse melanoma tumor xenograft model. Our studies indicate that this novel Ad that expresses E2Ftr has potent antitumor effects with significant potential for clinical application.
MATERIALS AND METHODS
Cell Culture
The human epidermal melanocyte isolated from lightly pigmented adult skin (HEMa-LP) cell line was purchased from Cascade Biologics (Portland, Ore). The human foreskin fibroblast HS-27 line; the human melanoma SK-MEL-2 (mutant p53), SK-MEL-28 (wt p53), A2058, and DM6 cell lines; the human lung cancer A549 (wt p53) and H1299 (partial deletion p53) cell lines; and the HEK-293 cell line were purchased from the American Type Culture Collection (Rockville, Md). HEMa-LP cells were cultured in medium 254 and supplemented with human melanocyte growth supplement (HMGS)-2. SK-MEL-2 and SK-MEL-28 cells were cultured in α-minimal essential medium (α-MEM). HS-27 and A2058 cells were cultured in Dulbecco modified Eagle medium. DM6 cells were cultured in Iscove modified Dulbecco medium. A549, H1299, and HEK-293 cells were cultured in α-MEM. All media were supplemented with 10% heat-inactivated fetal bovine serum and penicillin/streptomycin (100 U/mL). All cell culture reagents were obtained from Gibco BRL (Bethesda, Md). Cells were cultured in a 5% CO2 incubator at 37°C.
Recombinant Adenoviral Vectors
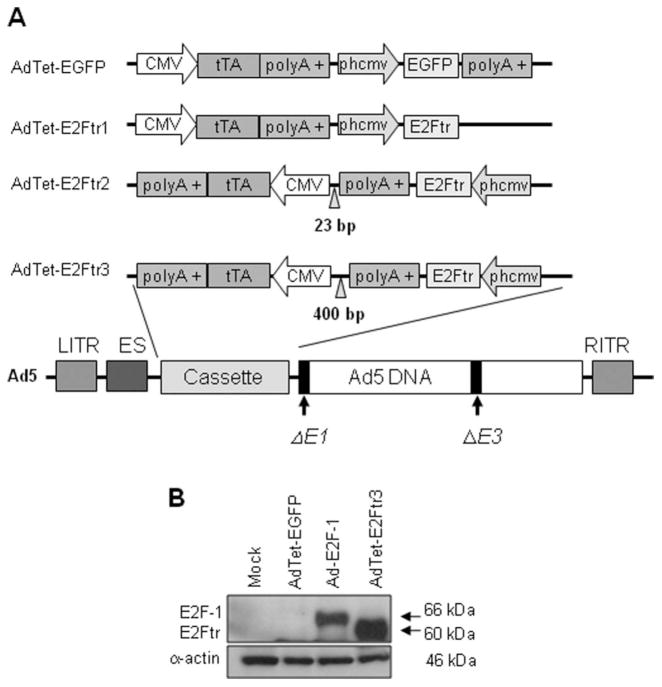
Six replication-defective, recombinant adenoviral vectors that were used in this study were deleted in the viral E1 gene. The Ad5CMV-E2F-1 vector (Ad-E2F-1) and the recombinant Ad serotype 5 vector encoding the Escherichia coli β-galactosidase (lacZ) gene controlled by the human CMV promoter (Ad5CMV-LacZ [Ad-LacZ]) vector contained the transgenes E2F-1 and nuclear-localized β-galactosidase, respectively, under control of the CMV promoter as described previously.2 We also constructed an AdTet that expressed the reporter-enhanced green fluorescent protein (AdTet-EGFP), AdTet-E2Ftr1, AdTet-E2Ftr2, and AdTet-E2Ftr3 (Fig. 1). The 3 vectors that expressed the same E2Ftr gene (amino acids 1 through 375) differ in structure or orientation of the expression cassette and are described in detail in our recent report.11
Figure 1.
This is a schematic diagram of 4 single, bicistronic adenovirus (Ad) vectors and confirms deletion of the E2F-1 transactivation domain. (A) The cytomegalovirus (CMV) promoter drives expression of the tetracycline (Tet)-controlled transactivator (tTA). The vector that expresses the reporter-enhanced green fluorescent protein (EGFP) or the truncated form of the E2F-1 gene (E2Ftr) is under the control of a synthetic minimal promoter composed of a Tet-responsive element (TRE) and a CMV mini-promoter (phcmv), which is silent unless activated by tTA. In the absence of Tet or doxycycline, tTA binds to phcmv and triggers the expression of EGFP or E2Ftr. When Tet is added to the medium, tTA is bound by Tet and is unable to bind to phcmv and activate the expression of EGFP or E2Ftr. The left and right inverted terminal repeat sequences (LITR and RITR, respectively), encapsidation signal (ES), and E1/E3-deleted genes are shown in the Ad structure. polyA indicates RNA with the addition of multiple adenosine monophosphates. (B) SK-MEL-2 cells were uninfected (Mock) or were infected with AdTet-EGFP, Ad-E2F-1, or AdTet-E2Ftr3 at a multiplicity of infection of 100; and, 36 hours postinfection, monoclonal antibody antihuman E2F-1 was used to detect both E2F-1 and E2Ftr expression. α-Actin was used to demonstrate equal loading for each lane.
Western Blot Analysis
Adenoviral infections and Western blot analyses were performed as described previously.2 The antibodies used in this reports were E2F-1 (KH95) sc-125, a mouse monoclonal antibody against human E2F-1 (Santa Cruz Biotechnology, Santa Cruz, Calif), mouse-antihuman-caspase-3/CPP32 monoclonal antibody (MoAb) (Transduction Laboratories, Lexington, Ky), mouse-antihuman MoAb poly(ADP-ribose) polymerase (PARP) and mouse-antihuman p53 (both from Calbiochem, San Diego, Calif), and rabbit-antihuman-α-actin polyclonal antibody (Sigma-Aldrich, St. Louis, Mo). Antimouse immunoglobulin (Ig) or antirabbit Ig peroxidase-linked, species-specific whole antibody (Amersham, Piscataway, NJ) was used to detect primary antibodies. Electrochemilumines-cent reagents were used to detect the signals according to the manufacturer’s instructions (Amersham).
Cell Viability and Cell Cycle Analysis
Cell viability was assessed 72 hours after infection or drug treatment by measuring the conversion of the tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) to formazan according to the manufacturer’s instructions (Boehringer Mannheim, Indianapolis, Ind). Cell cycle analysis was performed by flow cytometry on a fluorescence-activated cell sort scanner (FACScan; Becton Dickinson, San Jose, Calif). Cell cycle distribution was analyzed by using Cell Quest software (BD Biosciences, San Diego, Calif) as described elsewhere.2,12
Annexin V Staining
Annexin V staining was carried out according to the manufacturer’s instructions (Annexin V-Pe Apoptosis Kit; Pharmingen, San Diego, Calif) and as described previously.12 Cells were analyzed by FACScan flow cytometer (Becton Dickinson) with FlowJo software (Tree Star Inc., Ashland, Ore).
Melanoma Xenograft Study
Tumors were formed by subcutaneous injection of 5 × 106 A2058 melanoma cells into the bilateral flanks of athymic BALB/c nu/nu male mice (aged 6–8 weeks; Charles River Laboratories, Wilmington, Mass). Six days later, palpable tumors were randomized and injected directly with AdTet-E2Ftr3 (1 × 109 plaque forming units [pfu]) or with AdTet-EGFP (1 × 109 pfu; n = 6 for each group). Intratumoral injections were performed every 3 days for a total of 4 treatments. Each injection of purified virus was diluted in a total volume of 100 μL 0.9% NaCl solution. Tumors were measured every 3 days, and tumor volume was determined by externally measuring in 2 dimensions with a caliper. Volume (V) was determined according to the following equation: V = (L × W2)/2, where L is length and W is width of the tumor. Animal experiments were performed in accordance with institutional guidelines and were approved by the University of Louisville Institutional Animal Care and Use Committee.
Immunohistochemistry
Tumors were excised 24 hours after the fourth injection in vivo, fixed in 10% formalin, embedded in paraffin blocks, and processed for histologic analysis and detection of E2Ftr expression and cleaved caspase-3. A mouse-antihuman-E2F-1 MoAb (KH95) sc-125 (Santa Cruz Biotechnology) was used to detect E2Ftr. A rabbit-antihuman cleaved caspase-3 (Asp175)(5A1E) MoAb (Cell Signaling, Danvers, Mass) was used to detect cleaved caspase-3. Photographs were taken with at ×20 magnification and were analyzed with NIS-Elements BR 3.0 software (Nikon Instruments, Melville, NY)
Statistical Analysis
The results of the in vitro assays and tumor growth in mice from 2 treatment groups were analyzed with the Student t test for unpaired data using a 1-way ordinary parametric analysis of variance. P < .05 was considered statistically significant.
RESULTS
Increased E2Ftr Expression Enhances Cancer Cell Death
Figure 1A illustrates the 4 adenoviruses that we constructed, ie, 1 that expressed the reporter-enhanced green fluorescent protein (EGFP) and 3 that encoded the therapeutic E2Ftr gene. The Tet-off system was used to regulate EGFP and E2Ftr expression in these vectors. A Western blot analysis confirmed deletion of the E2F-1 transactivation domain, which resulted in an approximately 60-kDa product compared with wt E2F-1 which is approximately 66-kDa (Fig. 1B).
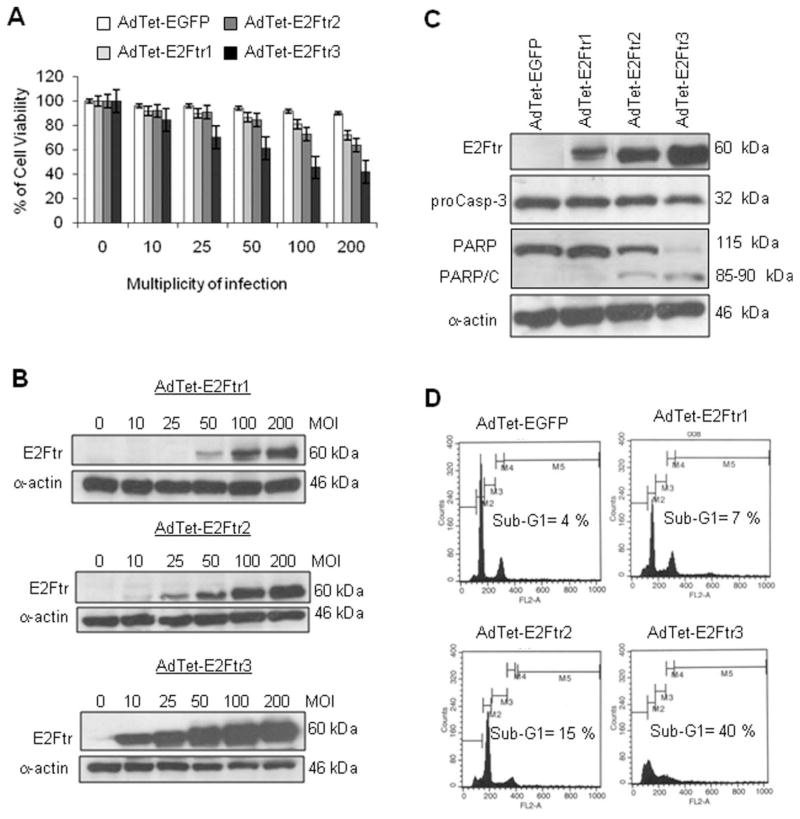
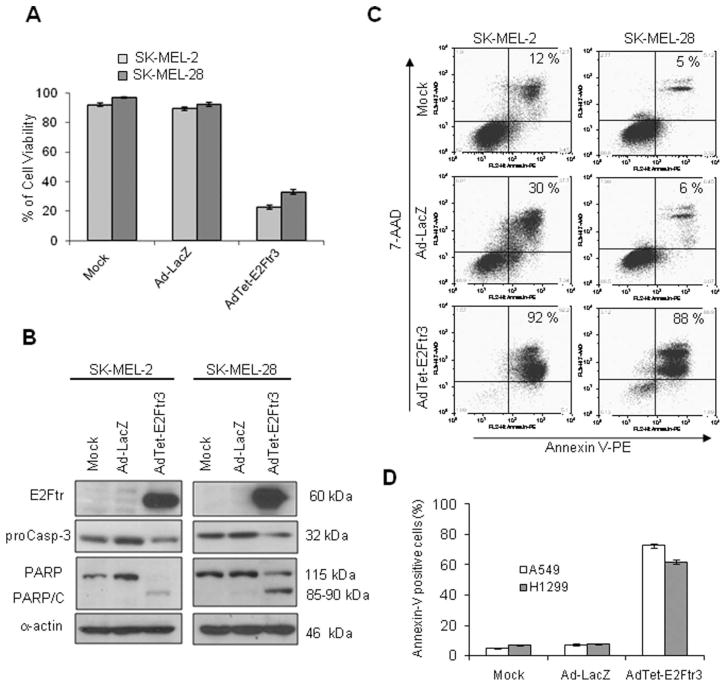
E2Ftr constructs were assessed for their ability to induce cancer cell killing in SK-MEL-2 cells. We observed that cancer cell cytotoxicity increased in a virus dose-dependent manner in cancer cells that were infected with AdTet-E2Ftr1, AdTet-E2Ftr2, and AdTet-E2Ftr3 (Fig. 2A). In contrast, cells that were infected with the control AdTet-EGFP vector exhibited little cytotoxicity. Cancer cell cytotoxicity was correlated with E2Ftr protein levels (Fig. 2A,B). Among 3 the vectors, AdTet-E2Ftr3 produced the greatest cancer cell cytotoxicity and the greatest E2Ftr transgene expression in Western blot analysis (Fig. 2B).
Figure 2.
Three adenovirus (Ad) vectors that expressed the truncated form of the E2F-1 gene (E2Ftr) were evaluated for their ability to induce melanoma cell killing and apoptosis. SK-MEL-2 cells were infected with Ad vectors that expressed E2Ftr under regulation of the tetracycline (Tet)-off system (AdTet-E2Ftr1, AdTet-E2Ftr2, and AdTet-E2Ftr3 or AdTet that expressed the reporter-enhanced green fluorescent protein [AdTet-EGFP]) at multiplicities of infection (MOIs) of 0, 10, 25, 50, 100, or 200. Three days after infection, cells were used for 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) and Western blot assays. (A) The MTT assay was used to determine cell survival. Each point represents the mean of 3 independent experiments (±standard deviation [bars]). (B) Monoclonal antibody (MoAb) antihuman E2F-1 was used to detect E2Ftr. SK-MEL-2 cells were infected with the vectors mentioned above at an MOI of 100. Three days after infection, the cells were used for a Western blot assay and cell cycle analysis. (C) MoAb antihuman poly (ADP-ribose) polymerase (PARP) was used to detect PARP, and MoAb antihuman caspase-3/CPP3 was used to detect caspase-3 (proCasp-3). α-Actin was used to demonstrate equal loading for each lane. PARP/C indicates PARP cleavage. (D) For cell cycle analysis, cells were stained with propidium iodide and analyzed as describe in the text (see Materials and Methods).
To investigate whether apoptosis is involved in E2Ftr-induced cancer cell death, melanoma cells were infected with the Ad vectors at a multiplicity of infection (MOI) of 100. We performed Western blot analysis to detect activation of the caspases pathway. Induction of apoptosis was confirmed by detection of PARP cleavage; PARP is a substrate of caspase-3, a well characterized apoptosis marker.5,13 No PARP cleavage was detected in control AdTet-EGFP–infected or AdTet-E2Ftr1–infected cells. Low levels of PARP cleavage were observed in cells that were infected with AdTet-E2Ftr2. Significant PARP cleavage was detected in AdTet-E2Ftr3–infected cells (Fig. 2C). A decrease in proenzyme caspase-3/cysteine protease (CP) P32 (CPP32) levels also was observed in cells that were infected with E2Ftr-expressing vectors compared with AdTet-EGFP. Among the vectors, AdTet-E2Ftr3 produced the greatest decrease in caspase-3/ CPP32 and the most significant PARP cleavage.
By using flow cytometry, we also observed that an increase in the sub-G1 population (consistent with apoptosis) was correlated with E2Ftr expression levels in Ad-infected cells. AdTet-E2Ftr3 induced the greatest sub-G1 population (40%), whereas AdTet-E2Ftr1 and AdTet-E2Ftr2 induced 7% and 15% sub-G1 cells, respectively (Fig. 2D). The baseline level of sub-G1 cells was 4% in cells that were treated with the control AdTet-EGFP vector. These data demonstrated that AdTet-E2Ftr3 infection was associated with the most potent reduction in cell proliferation and induction of apoptosis, which were related to the greatest E2Ftr transgene expression levels.
Comparison of Adenoviruses Expressing E2Ftr and E2F-1
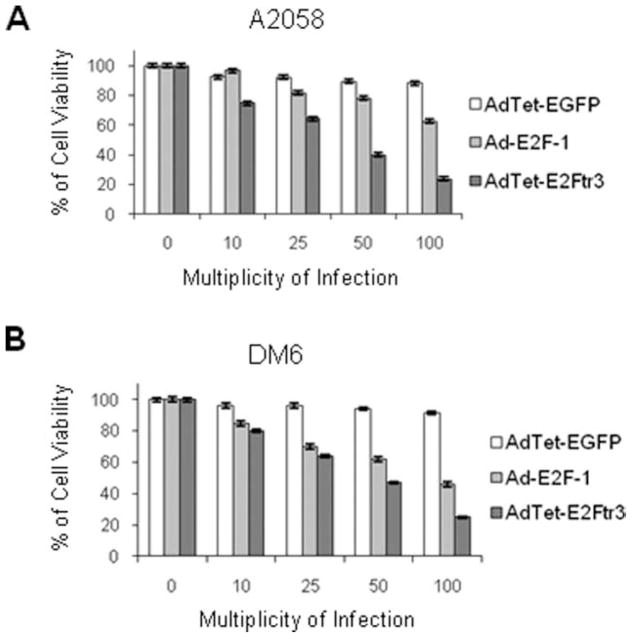
Next, we compared AdTet-E2Ftr3 with Ad-E2F-1, which expresses the full-length wt E2F-1 and has been used extensively in cancer gene therapy by us and others.1–3,5,14 We tested the vectors with A2058 cells, which induce tumors in BALB/c nude mice, and with DM6 cells, which are used in a rat melanoma model for isolated limb perfusion (ILP).15,16 Both cell lines were infected with AdTet-E2Ftr3, Ad-E2F-1, or AdTet-EGFP at increasing MOI. Three days after infection, a cell viability assay revealed dose-dependent cell killing with the Ad-E2F-1 and AdTet-E2Ftr3 viruses, but little cytotoxicity was observed in AdTet-EGFP–infected cells (Fig. 3A,B). AdTet-E2Ftr3 infection produced a greater reduction in cell viability compared with Ad-E2F-1 infection in both cell lines. These results demonstrate that AdTet-E2Ftr3 is a stronger inducer of cancer cell death than Ad-E2F-1.
Figure 3.
An E2F-1 adenovirus (Ad-E2F-1) vector was compared with an Ad vector that expressed the truncated form of the E2F-1 gene (E2Ftr) under regulation of the tetracycline (Tet)-off system (AdTet-E2Ftr3) in an assay that inhibited cell proliferation. (A) A2058 cells and (B) DM6 cells were infected with AdTet-expressing enhanced green fluorescent protein (AdTet-EGFP), Ad-E2F-1, or AdTet-E2Ftr3 at multiplicities of infection of 0, 10, 25, 50, or 100. Seventy-two hours after infection, the cells were used for a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay to determine cell survival. Each point represents the mean of 3 independent experiments (±standard deviation [bars]).
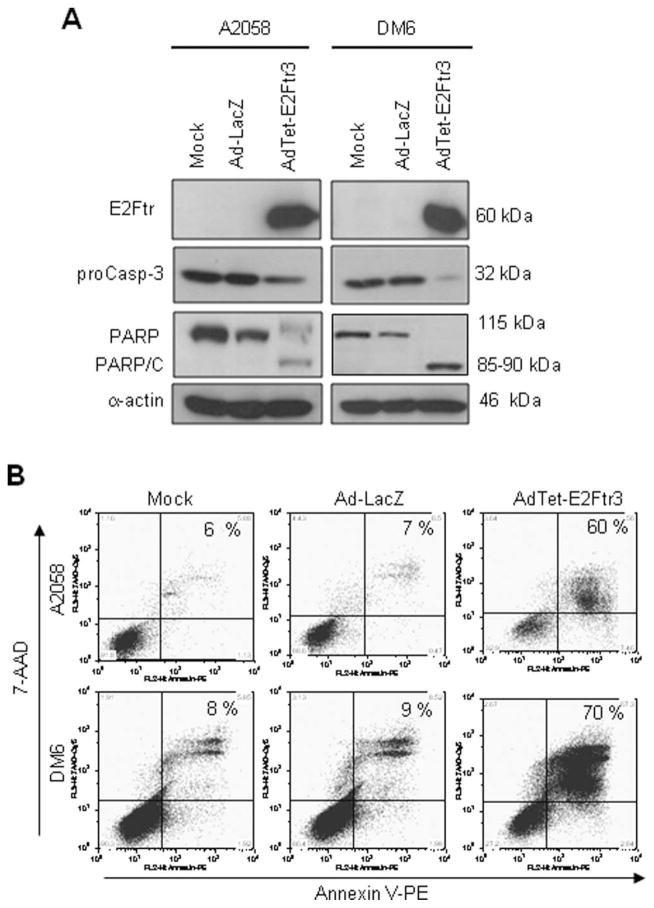
To study the cytotoxicity induced by AdTet-E2Ftr3 further, both cell lines (A2058 and DM6) were infected with Ad-LacZ, AdTet-E2Ftr3, or uninfected (mock) vector. After 72 hours, caspase-3 activation was analyzed. AdTet-E2Ftr3 induced PARP cleavage (85–90 kDa), as expected, and decreased the proenzyme caspase-3/CPP32 in both A2058 cells and DM6 cells. In contrast, neither a decrease in proenzyme caspase-3 nor PARP cleavage was evident in mock-infected or Ad-LacZ–infected cells (Fig. 4A).
Figure 4.
The induction of apoptosis is illustrated by the truncated form of the E2F-1 gene (E2Ftr) in melanoma cells that were used in tumor xenograft models. A2058 cells and DM6 cells were uninfected (Mock) or were infected with a recombinant Ad serotype 5 vector encoding the Escherichia coli β-galactosidase (lacZ) gene controlled by the human cytomegalovirus (CMV) promoter (Ad5CMV-LacZ [Ad-LacZ]) or with an Ad vector that expressed E2Ftr under regulation of the tetracycline (Tet)-off system (AdTet-E2Ftr3) at a multiplicity of infection of 100. Seventy-two hours after infection, the cells were used for Western blot assays or Annexin V staining. (A) Monoclonal antibody (MoAb) antihuman E2F-1 was used to detect E2Ftr, antihuman poly (ADP-ribose) polymerase (PARP) was used to detect PARP, and MoAb antihuman caspase-3/CPP3 was used to detect caspase-3 (proCasp-3). α-Actin was used to demonstrate equal loading for each lane. PARP/C indicates PARP cleavage. (B) To determine the percentage of apoptosis, cells were stained with Annexin V-phycoerythrin (PE) and were analyzed as describe in the text (see Materials and Methods). 7-AAD indicates 7-aminoactinomycin D.
We used an Annexin V-phycoerythrin (PE) kit to determine the percentage of cells that underwent apopto-sis. AdTet-E2Ftr3 induced 60% apoptosis in A2058 cells and 70% apoptosis in DM6 cells at 72 hours postinfection, whereas little apoptosis was observed in mock-infected or Ad-LacZ–infected cells (Fig. 4B). We used Ad-LacZ as a control instead AdTet-EGFP, because EGFP expression caused a strong background in flow cytometry analysis. These data suggested that cytotoxicity induced by AdTet-E2Ftr3 in A2058 cells and DM6 cells was caused at least in part by caspase-3 activation and apoptosis.
E2Ftr-Induced Apoptosis Does Not Require Functional p53
Mutations in the p53 gene are the most frequent genetic events in a wide variety of tumors.17 We investigated whether the p53 gene may play a role in E2Ftr-mediated apoptosis. To this end, SK-MEL-2 (mutant p53) cells and SK-MEL-28 (wt p53) cells were transduced with AdTet-E2Ftr3. A cell viability assay revealed that AdTet-E2Ftr3 infection caused cancer cell death in both cell lines; in contrast, no significant cytotoxicity was observed in mock-infected or Ad-LacZ–infected cells (Fig. 5A). Western blot analysis confirmed activation of the caspase pathway. We observed that both cell lines infected with AdTet-E2Ftr3 had an apoptosis-specific PARP cleavage fragment (85–90 kDa) as well as a decrease in the proenzyme caspase-3/CPP32, whereas neither a decrease of proenzyme caspase-3 nor PARP cleavage was present in mock-infected or Ad-LacZ–infected cells (Fig. 5B). Although there was significant PARP cleavage in SK-MEL-28 cells that were treated with E2Ftr, uncleaved PARP levels remained high. It is possible that PARP may be produced continually in E2Ftr-treated SK-MEL-28 cells. A similar result was observed when cyclin-dependent kinase 2 was inactivated in v-myc myelocytomastosis viral-related oncogene, neuroblastoma derived (MYCN)-overexpressing cancer cells.18
Figure 5.
The role of p53 status on apoptosis induced by the truncated form of the E2F-1 gene (E2Ftr) is illustrated. SK-MEL-2 (mutant p53) cells and SK-MEL-28 (wild type [wt] p53) cells were uninfected (Mock) or were infected with either a recombinant adenovirus (Ad) serotype 5 vector encoding the Escherichia coli β-galactosidase (lacZ) gene controlled by the human cytomegalovirus (CMV) promoter (Ad5CMV-LacZ [Ad-LacZ]) or with an Ad vector that expressed E2Ftr under regulation of the tetracycline (Tet)-off system (AdTet-E2Ftr3) at a multiplicity of infection of 100. Seventy-two hours after infection, the cells were used for a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) and Western blot analyses or for Annexin V staining. (A) An MTT assay was used to determine cell survival. Each point represents the mean of 3 independent experiments (±standard deviation [bars]). (B) Monoclonal antibody (MoAb) antihuman E2F-1 was used to detect E2Ftr, MoAb antihuman poly (ADP-ribose) polymerase (PARP) was used to detect PARP, and MoAb antihuman caspase-3/CPP32 was used to detect caspase-3 (proCasp-3). α-Actin was used to demonstrate equal loading for each lane. PARP/C indicates PARP cleavage. (C) For Annexin V-phycoerythrin (PE) staining, cells were stained and analyzed as described in the text (see Materials and Methods). 7-AAD indicates 7-aminoactinomycin D. (D) A549 (wt p53) and H1299 (partial deletion of p53) lung cancer cells were treated as described above and in an apoptosis assay.
Apoptosis was validated further by Annexin V staining. SK-MEL-2 cells exhibited 92% apoptosis, whereas SK-MEL-28 cells exhibited 88% apoptosis 72 hours after infection with AdTet-E2Ftr3 (Fig. 5C). Few apoptotic cells were detected in mock-infected or Ad-LacZ–infected cells at 72 hours (Fig. 5C). Similar induction of caspases activation and apoptosis was observed previously with Ad-E2F-1 infection.11
We also investigated the role of p53 in E2Ftr-induced apoptosis in lung cancer cells. A549 (wt p53) cells and H1299 (partial deletion of p53) cells were infected with Ad-LacZ, AdTet-E2Ftr3, or mock vector. Three days after infection, the percentage of apoptotic cells was determined as described above. Similar to what we observed in melanoma cells, the infection of A549 and H1299 cells with AdTet-E2Ftr3 resulted in 75% and 62% apoptosis, respectively (Fig. 5D). These results demonstrated that apoptosis induced by E2Ftr does not require the presence of functional p53.
Normal Cells Are Resistant to E2Ftr-Induced Apoptosis
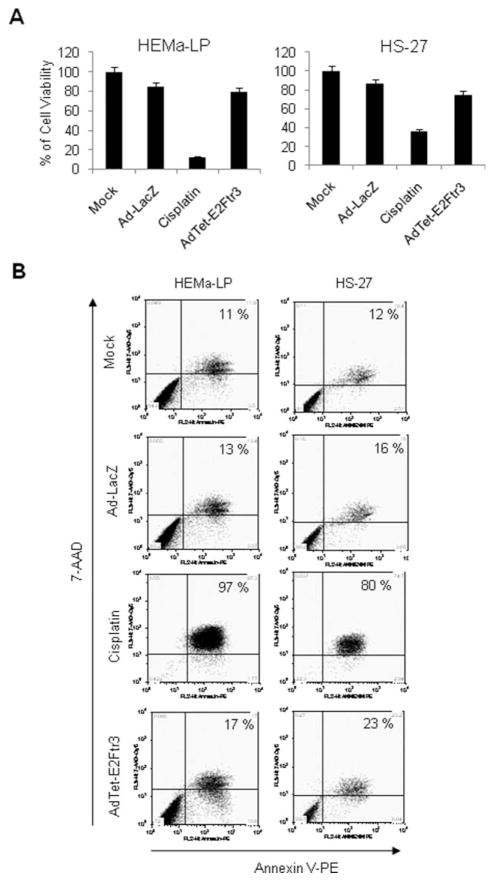
The application of adenoviruses that express proapoptotic genes may result in adverse effects on normal cells in patients.19,20 Therefore, we assessed the cytotoxic effect of AdTet-E2Ftr3 in the normal human cell lines HEMa-LPs and HS-27. Cisplatin, which is used as a chemotherapeutic agent in the treatment of melanoma, was used as a positive control for apoptosis at a final concentration of 25 μM. We demonstrated previously that cisplatin at this concentration induced ~50% cytotoxicity in melanoma cells.2 Infection of HEMa-LP or HS-27 cells with Ad-LacZ did not induce significant reduction in cell viability (Fig. 6A). Cisplatin produced a substantial reduction in the percentage of viable HEMa-LP and HS-27 cells, as expected, resulting in 15% and 36% remaining viable cells, respectively. In contrast, with AdTet-E2Ftr3 infection, 75% and 80% of cells remained viable, respectively, indicating only modest cytotoxicity to normal cells (Fig. 6A). In accordance with these results, Annexin V staining indicated that cisplatin induced 97% and 80% apoptosis in HEMa-LP cells and HS-27 cells, respectively; whereas AdTet-E2Ftr3 induced 17% and 23% apoptosis, respectively (Fig. 6B).
Figure 6.
The cytotoxic effect of transduction in normal cell lines of an adenovirus (Ad) vector that expressed the truncated form of the E2F-1 gene (E2Ftr) under regulation of the tetracycline (Tet)-off system (AdTet-E2Ftr3) is illustrated. Normal HEMa-LP cells and HS-27 cells were uninfected, or infected with a recombinant Ad serotype 5 vector encoding the Escherichia coli β-galactosidase (lacZ) gene controlled by the human cytomegalovirus (CMV) promoter (Ad5CMV-LacZ [Ad-LacZ]) or with AdTet-E2Ftr3 at a multiplicity of infection of 100, or were treated with cisplatin 25 μM. Seventy-two hours after treatments, cell viability or apoptosis was analyzed by an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay or Annexin V staining, respectively. (A) For MTT, each point represents the mean of 3 independent experiments (±standard deviation [bars]). (B) For Annexin V staining, cells were stained and analyzed as describe in the text (see Materials and Methods). 7-AAD indicates 7-aminoactinomycin D; PE, phycoerythrin.
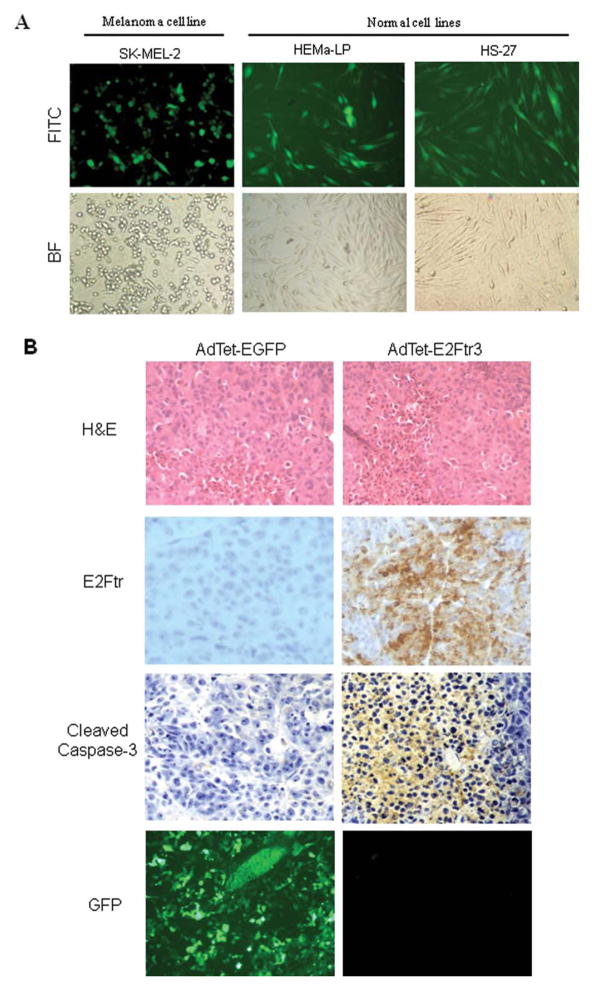
To exclude the possibility that the low toxicity induced in normal cells by AdTet-E2Ftr3 was not caused by poor infection efficiency in HEMa-LP and HS-27 cells, we applied AdTet-EGFP to determine adenoviral infection, because EGFP expression analysis is the most convenient method for evaluating transduction efficiency. HEMa-LP, HS-27, and SK-MEL-2 cells were infected with AdTeT-EGFP. Twenty-four hours after infection, the expression levels of EGFP were evaluated in all 3 cell lines as normalized to the cell count (% EGFP positive), and we observed similar infection efficiency, which was approximately 47% in SK-MEL-2 cells, 45% in HEMa-LP cells, and 41% in HS-27 cells (Fig. 7A). Therefore, it is unlikely that the low toxicity induced by AdTet-E2Ftr3 in normal cells was caused by poor transduction efficiency. These data suggest that AdTet-E2Ftr3 has a low toxicity to normal cells.
Figure 7.
Normal cell transduction efficiency and tumor immunohistochemistry were evaluated. (A) SK-MEL-2, HEMa-LP, and HS-27 cells were transduced with an adenovirus (Ad) vector under regulation of the tetracycline (Tet)-off system (AdTet) that expressed enhanced green fluorescent protein (AdTet-EGFP) at a multiplicity of infection of 100. After 24 hours, the cells were examined for EGFP expression under fluorescence microscopy. Photomicrographs were taken with Kodak MDS 290 software at ×20 magnification. FITC indicates fluorescein isothiocyanate; BF, bright field. (B) Immunohistochemistry of melanoma tumors is shown 24 hours after the last treatment with AdTet-EGFP or with the AdTet vector that expressed the truncated form of the E2F-1 gene (E2Ftr) (AdTet-E2Ftr3) (hematoxylin and eosin [H&E] staining; original magnification, ×20). EGFP expression was observed with fluorescence microscopy. Monoclonal antibody antihuman E2F-1 was used to detect E2Ftr expression, and polyclonal antihuman cleaved caspase-3 (Asp175; 5A1E) was used to confirm apoptosis. GFP indicates green fluorescent protein.
E2Ftr Overexpression Induces Caspase-3 Activation in a Tumor Xenograft Model
AdTet-E2Ftr3 was assessed further in vivo in a melanoma mouse subcutaneous xenograft model. A2058 cells were injected into the flanks of nude mice. Six days later, palpable tumors were randomized and were injected directly with AdTet-E2Ftr3 or AdTet-EGFP. Intratumoral injections were performed every 3 days for a total of 4 treatments. Tumors from the mice that were injected with AdTet-E2Ftr3 or AdTet-EGFP were harvested 24 hours after the last treatment and were subjected to histopathologic analysis. Immunohistochemical analysis revealed strong E2Ftr expression in tumors from mice that were injected with AdTet-E2Ftr3, which was associated with a significant number of cleaved caspase-3-positive cells in tumors (Fig. 7B). In contrast, E2Ftr and cleaved caspase-3 were not detected in the tumors from mice that were injected with AdTet-EGFP. However, AdTet-EGFP treatment resulted in high levels of EGFP expression in tumor cells. These results indicate that efficient AdTet-E2Ftr3 transduction also strongly activates caspase-3 in solid tumors.
AdTet-E2Ftr3 Has Potent Antitumor Activity In Vivo
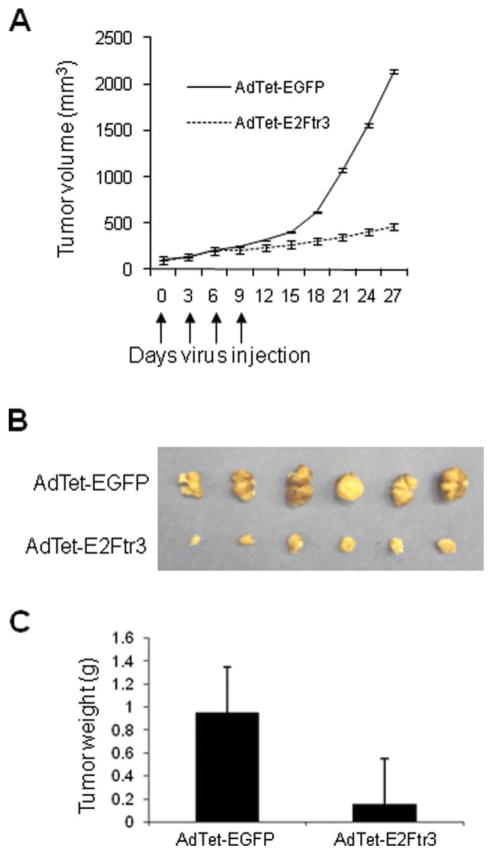
We also evaluated the antitumor activity of AdTet-E2Ftr3. The protocol described above (see Materials and Methods) produced strong tumor suppression in all 6 mice (100%) that were injected with AdTet-E2Ftr3 by Day 27 (Fig. 8A). The reduction in tumor size for those mice was ~80% compared with the reduction observed in control AdTet-EGFP–treated mice, and the difference was statistically significant (P < .05). Twenty-seven days after the first Ad injection, the tumors were excised. The photographs reveal that tumors from the mice that were injected with AdTet-EGFP were significantly larger than tumors from the mice that were injected with AdTet-E2Ftr3 (Fig. 8B). Tumors from the AdTet-EGFP treatment group measured 1.2 ± 0.4 g, whereas tumors from the AdTet-E2Ftr3 treatment group measured 0.2 ± 0.04 g (Fig. 8C). E2Ftr treatment resulted in a strong decrease in tumor size, and the difference between the 2 treatments was statistically significant (P < .05). We previously assessed the antitumor activity of Ad-E2F-1 in the same tumor model that was used in the current study. In that assessment, treatment with Ad-E2F-1 reduced tumor size in ~37% in infected group compared with controls.2 The results reported here clearly demonstrated that Ad that encodes E2Ftr strongly inhibits tumor growth and has greater potential antitumor activity compared with Ad that encodes wt E2F-1.
Figure 8.
In vivo antitumor effects of treatment with an adenovirus (Ad) vector that expressed the truncated form of the E2F-1 gene (E2Ftr) are illustrated. (A) After subcutaneous administration of 5 × 106 A2058 cells, palpable tumors were randomized to receive a local injection of either an Ad that expressed E2Ftr under regulation of the tetracycline (Tet)-off system (AdTet) with enhanced green fluorescent protein (AdTet-EGFP) or the vector AdTet-E2Ftr3 on Days 0, 3, 6, and 9 (vertical arrows). The total viral dose was 4 × 2×1 × 109 plaque forming units. Tumor volume (V) was plotted against time and was determined by the equation V = (L × W2)/2, where L is the length, and W is the width of the tumor. (B) On Day 27, tumors were excised and photographed: The relative mass of tumors is shown. (C) Tumors also were weighed on Day 27. The results are expressed as the mean from each treatment group (±standard error [bars]). The differences in tumor size or weight in animals that were treated with AdTet-E2Ftr3 were statistically significant by the end of the experiments compared with controls (Day 27; P < .05).
DISCUSSION
Despite numerous studies indicating that E2F-1 has potent antitumor activity in vitro and in vivo,1,2,4 its use in cancer clinical trials remains controversial because of its oncogenic potential.6,7 Nevertheless, there is strong evidence indicating that truncated E2F-1, which lacks the transactivation domain, has no oncogenic properties. Studies have demonstrated that E2F-1 mutants that lack the transactivation domain were unable to immortalize normal human foreskin keratinocytes and NIH-3T3 cells, and they inhibited colony formation in soft agar.9,21 E2F-1 mutants that contained only the DNA-binding domain decreased protein levels of cyclins A and D8 and induced apoptosis at a higher percentage than E2F-1 in SaOs-2 cells.9,22 Therefore, the controversy surrounding the oncogenic potential of E2F-1 could be obviated by using a truncated form of E2F-1 like E2Ftr, which lacks the oncogenic-related transactivation domain. We report here that Ad-mediated E2Ftr gene transfer significantly induced apoptosis in cancer cells and resulted in strong tumor suppression in mice.
Our previous attempt to construct an Ad-E2Ftr vector with the CMV promoter was unsuccessful, probably because high-level expression of E2Ftr is toxic to HEK-293 cells and inhibits Ad replication.11 Then, we modified the Tet-off inducible expression system to regulate E2Ftr and generated 3 Ad vectors, ie, AdTet-E2Ftr1, AdTet-E2Ftr2, and AdTet-E2Ftr3. All 3 E2Ftr vectors expressed the same truncated E2F, but AdTet-E2Ftr3 expressed the highest E2Ftr protein and exhibited the strongest cancer cell-killing activity (Fig. 2). AdTet-E2Ftr3 also had stronger cancer cytotoxicity compared with wt E2F-1 (Fig. 3A,B). In addition, we observed that E2Ftr protein was more stable and accumulated to higher levels in cells compared with wt E2F-1 (Fig. 1B, unpublished data). Higher E2Ftr protein accumulated in cancer cells may lead to stronger cytotoxic effects, which are under further investigation. It is noteworthy that AdTet-E2Ftr3 infection resulted in little toxicity to normal cells (Fig. 6B), indicating that this vector may be suitable for cancer gene therapy.
Caspase-3 is known as an executioner of the apoptotic process.13,23 Previously, we reported that the overexpression of E2F-1 lead to caspase-3 activation.24 Because E2Ftr lacks the transactivation domain, it may act in a manner different from that of its counterpart wt E2F-1. In the current study, we observed that AdTet-E2Ftr3-mediated cancer cell death also was associated with the activation of caspase-3 (Figs. 2C, 4A, 5B, 7B). Although the detailed mechanism of E2Ftr-induced apoptosis remains largely unknown, it is likely that caspase-3 activation is a common mechanism in both E2Ftr-induced and E2F-1-induced apoptosis.
We cannot exclude other cell death mechanisms that are involved in E2Ftr-induced cell death, such as mitotic catastrophe and terminal growth arrest. In fact, we observed that E2Ftr induced autophagy in cancer cells (unpublished data). Because E2Ftr does not have the transactivation domain but still binds to DNA, it is reasonable to hypothesize that E2Ftr may bind to and repress the promoters of genes that normally are activated by wt E2F-1 for cell proliferation.
A previous study demonstrated that the infection of prostate cancer cells with Ad-E2F-1 resulted in increases in p53 compared with controls.25 We questioned whether E2Ftr expression affects the p53 levels. Western blot analysis revealed that p53 levels were not affected by AdTet-E2Ftr3 treatment in SK-MEL-28 or SK-MEL-2 melanoma cells (data not shown), presumably because the transcription domain was deleted in E2Ftr. E2Ftr induced apoptosis in cells with both functional and nonfunctional p53. Because cancer cells with mutated p53 generally are more resistant to conventional treatments, such as chemotherapy or radiation therapy, the ability of E2Ftr to kill cancer cells regardless of p53 status is a relative advantage.
Previously, we observed that Ad-E2F-1-treated mice had a reduced tumor size by approximately 37%.2 Here, we report an approximately 80% reduction in tumor size in AdTet-E2Ftr3-treated mice (Fig. 8A). In those mice, AdTet-E2Ftr3 injections resulted in high levels of E2Ftr expression and strongly induced caspase-3 activation in tumors (Fig. 7B). These results indicate that AdTet-E2Ftr3 can activate the apoptotic pathway efficiently and can inhibit the growth of solid tumors. Thus, AdTet-E2Ftr3 may be safer and more efficient than Ad-E2F-1 in cancer gene therapy.
The systemic administration of Ad has many potential drawbacks, including effective delivery of the virus to the tumor site(s), hepatic and other potential toxicity, and immune response against the virus.26 We acknowledge the potential for immune response or pre-existing immunity against adenoviruses in patients that can render repeated doses of adenoviral gene therapy vectors less effective. Innate immunity also can result in an acute inflammatory response, which may can result in severe, potentially fatal, hepatic toxicity and other complications when very high viral particles (>3 × 1013 viral particles) are delivered systemically.27–29 However, melanoma may be an ideal human cancer in which to test Ad-mediated cancer gene therapy. In-transit melanoma (regional cutaneous and subcutaneous metastases) is a relatively common clinical entity for which local or regional gene therapy may be effective. Regional delivery of chemotherapy using ILP often is used clinically for the treatment of in-transit melanoma. ILP animal models using adenoviral vectors have been reported with promising results.30,31 ILP for in-transit melanoma has 2 features that somewhat mitigate the potential for either reduced transduction efficiency related to immunity or severe acute inflammatory reaction: 1) ILP for in-transit melanoma generally is used as a single treatment—it is rare that patients receive a second ILP treatment. Even in patients with pre-existing immunity, adenoviral transduction usually is efficient at the initial Ad vector administration.28 2) Adenoviral therapy will be confined to the extremity by vascular isolation and tourniquet placement around the proximal extremity. Thus, significant systemic leaks are rare, and the potential for severe acute inflammatory response and potential hepatic toxicity is lessened. Therefore, the problems with immunity against the vector for this type of treatment are less of a concern than for systemic administration of Ad. The study of AdTet-E2Ftr3 in a preclinical ILP animal model may set the stage for human clinical trials.
In summary, we demonstrated for the first time that E2Ftr, controlled by a modified Tet-off promoter in an adenoviral vector, induces high-level transgene expression that results in potent antitumor effects in vitro and in vivo. E2Ftr expression induces the apoptosis pathway associated with PARP cleavage and activation of proenzyme caspase-3. The in vitro and in vivo antitumor efficacy of the AdTet-E2Ftr3 vector suggests that E2Ftr may be an effective agent for human cancer gene therapy.
Acknowledgments
CONFLICT OF INTEREST DISCLOSURES
Supported by National Institutes of Health grants R01CA129975 (to H.S.Z.) and R01CA90784 (to K.M.M.) both from the National Cancer Institute and by grants GMB081410 (to K.M.M.) and GRNT30983 (to H.S.Z.) both from the Kentucky Lung Cancer Research Program. A.G.G. is the recipient of a scholarship from the National Council of Science and Technology (CONACYT) of Mexico.
We thank Chuanlin Ding for Annexin V analysis and Andrew Marsh and Margaret Abby for editing.
References
- 1.Dong YB, Yang HL, Elliott MJ, et al. Adenovirus-mediated E2F-1 gene transfer efficiently induces apoptosis in melanoma cells. Cancer. 1999;86:2021–2033. doi: 10.1002/(sici)1097-0142(19991115)86:10<2021::aid-cncr20>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Dong YB, Yang HL, Elliott MJ, McMasters KM. Adenovirus-mediated E2F-1 gene transfer sensitizes melanoma cells to apoptosis induced by topoisomerase II inhibitors. Cancer Res. 2002;62:1776–1783. [PubMed] [Google Scholar]
- 3.Elliott MJ, Dong YB, Yang H, McMasters KM. E2F-1 up-regulates c-Myc and p14(ARF) and induces apoptosis in colon cancer cells. Clin Cancer Res. 2001;7:3590–3597. [PubMed] [Google Scholar]
- 4.Vorburger SA, Hetrakul N, Xia W, et al. Gene therapy with E2F-1 up-regulates the protein kinase PKR and inhibits growth of leiomyosarcoma in vivo. Mol Cancer Ther. 2005;4:1710–1716. doi: 10.1158/1535-7163.MCT-05-0036. [DOI] [PubMed] [Google Scholar]
- 5.Itoshima T, Fujiwara T, Waku T, et al. Induction of apoptosis in human esophageal cancer cells by sequential transfer of the wild-type p53 and E2F-1 genes: involvement of p53 accumulation via ARF-mediated MDM2 down-regulation. Clin Cancer Res. 2000;6:2851–2859. [PubMed] [Google Scholar]
- 6.Johnson DG, Schwarz JK, Cress WD, Nevins JR. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature. 1993;365:349–352. doi: 10.1038/365349a0. [DOI] [PubMed] [Google Scholar]
- 7.Muller H, Helin K. The E2F transcription factors: key regulators of cell proliferation. Biochim Biophys Acta. 2000;1470:M1–M12. doi: 10.1016/s0304-419x(99)00030-x. [DOI] [PubMed] [Google Scholar]
- 8.Fan J, Bertino JR. Functional roles of E2F in cell cycle regulation. Oncogene. 1997;14:1191–1200. doi: 10.1038/sj.onc.1200940. [DOI] [PubMed] [Google Scholar]
- 9.Bell LA, O’Prey J, Ryan KM. DNA-binding independent cell death from a minimal proapoptotic region of E2F-1. Oncogene. 2006;25:5656–5663. doi: 10.1038/sj.onc.1209580. [DOI] [PubMed] [Google Scholar]
- 10.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez-Gutierrez JG, Rao X-M, Garcia-Garcia A, Hao H, McMasters KM, Zhou HS. Developing adenoviral vectors encoding therapeutic genes toxic to host cells: comparing binary and single-inducible vectors expressing truncated E2F- 1. Virology. 2010;397:337–345. doi: 10.1016/j.virol.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez-Gutierrez JG, Souza V, Hao HY, et al. Adenovirus-mediated gene transfer of FKHRL1 triple mutant efficiently induces apoptosis in melanoma cells. Cancer Biol Ther. 2006;5:875–883. doi: 10.4161/cbt.5.7.2911. [DOI] [PubMed] [Google Scholar]
- 13.Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel human apoptotic protein with homology to Caeno-rhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem. 1994;269:30761–30764. [PubMed] [Google Scholar]
- 14.Kuhn H, Liebers U, Gessner C, et al. Adenovirus-mediated E2F-1 gene transfer in nonsmall-cell lung cancer induces cell growth arrest and apoptosis. Eur Respir J. 2002;20:703–709. doi: 10.1183/09031936.02.00294502. [DOI] [PubMed] [Google Scholar]
- 15.Bauer TW, Gutierrez M, Dudrick DJ, et al. A human melanoma xenograft in a nude rat responds to isolated limb perfusion with TNF plus melphalan. Surgery. 2003;133:420–428. doi: 10.1067/msy.2003.113. [DOI] [PubMed] [Google Scholar]
- 16.Canter RJ, Zhou R, Kesmodel SB, et al. Metaiodobenzyl-guanidine and hyperglycemia augment tumor response to isolated limb perfusion in a rodent model of human melanoma. Ann Surg Oncol. 2004;11:265–273. doi: 10.1245/aso.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 Mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 18.Molenaar JJ, Ebus ME, Geerts D, et al. Inactivation of CDK2 is synthetically lethal to MYCN over-expressing cancer cells. Proc Natl Acad Sci U S A. 2009;106:12968–12973. doi: 10.1073/pnas.0901418106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakaguchi M, Kataoka K, Abarzua F, et al. Overexpression of REIC/Dkk-3 in normal fibroblasts suppresses tumor growth via induction of interleukin-7. J Biol Chem. 2009;284:14236–14244. doi: 10.1074/jbc.M808002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang W, Wang W, Zhang Y, Liu S, Liu Y, Zheng D. Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced chemokine release in both TRAIL-resistant and TRAIL-sensitive cells via nuclear factor kappa B. FEBS J. 2009;276:581–593. doi: 10.1111/j.1742-4658.2008.06809.x. [DOI] [PubMed] [Google Scholar]
- 21.Melillo RM, Helin K, Lowy DR, Schiller JT. Positive and negative regulation of cell proliferation by E2F-1: influence of protein level and human papillomavirus oncoproteins. Mol Cell Biol. 1994;14:8241–8249. doi: 10.1128/mcb.14.12.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsieh JK, Fredersdorf S, Kouzarides T, Martin K, Lu X. E2F1-induced apoptosis requires DNA binding but not trans-activation and is inhibited by the retinoblastoma protein through direct interaction. Genes Dev. 1997;11:1840–1852. doi: 10.1101/gad.11.14.1840. [DOI] [PubMed] [Google Scholar]
- 23.Agniswamy J, Fang B, Weber IT. Conformational similarity in the activation of caspase-3 and -7 revealed by the unliganded and inhibited structures of caspase-7. Apoptosis. 2009;14:1135–1144. doi: 10.1007/s10495-009-0388-9. [DOI] [PubMed] [Google Scholar]
- 24.Yang HL, Dong YB, Elliott MJ, Liu TJ, McMasters KM. Caspase activation and changes in Bcl-2 family member protein expression associated with E2F-1-mediated apoptosis in human esophageal cancer cells. Clin Cancer Res. 2000;6:1579–1589. [PubMed] [Google Scholar]
- 25.Nguyen KH, Hachem P, Khor LY, et al. Adenoviral-E2F-1 radiosensitizes p53wild-type and p53null human prostate cancer cells. Int J Radiat Oncol Biol Phys. 2005;63:238–246. doi: 10.1016/j.ijrobp.2005.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bessis N, Garcia-Cozar FJ, Boissier MC. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 2004;11(suppl 1):S10–S17. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- 27.Somia N, Verma IM. Gene therapy: trials and tribulations. Nat Rev Genet. 2000;1:91–99. doi: 10.1038/35038533. [DOI] [PubMed] [Google Scholar]
- 28.Bangari DS, Mittal SK. Current strategies and future directions for eluding adenoviral vector immunity. Curr Gene Ther. 2006;6:215–226. doi: 10.2174/156652306776359478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartman ZC, Appledorn DM, Amalfitano A. Adenovirus vector induced innate immune responses: impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res. 2008;132:1–14. doi: 10.1016/j.virusres.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Wilt JH, Bout A, Eggermont AM, et al. Adenovirus-mediated interleukin 3 beta gene transfer by isolated limb perfusion inhibits growth of limb sarcoma in rats. Hum Gene Ther. 2001;12:489–502. doi: 10.1089/104303401300042384. [DOI] [PubMed] [Google Scholar]
- 31.Hannay J, Davis JJ, Yu D, et al. Isolated limb perfusion: a novel delivery system for wild-type p53 and fiber-modified oncolytic adenoviruses to extremity sarcoma. Gene Ther. 2007;14:671–681. doi: 10.1038/sj.gt.3302911. [DOI] [PubMed] [Google Scholar]