Abstract
Rationale
Drugs that interfere with cannabinoid CB1 transmission suppress food-motivated behaviors and may be useful as appetite suppressants, but there is uncertainty about the locus of action for the feeding-suppression effects of these drugs.
Objective
The present work was conducted to determine if two drugs that interfere with cannabinoid receptor transmission, AM251 and AM4113, have effects on food-reinforced behavior after administration into the lateral ventricle (intracerebroventricular (ICV)).
Results
Although systemic administration of both drugs can suppress food-reinforced behavior, neither AM251 (40, 80, and 160μg) nor AM4113 (60, 120, and 240μg) administered at various times prior to testing produced any suppression of food-reinforced operant responding on a fixed-ratio 5 schedule. Because the modulation of locomotion by drugs that act on CB1 receptors is hypothesized to be a forebrain effect, these drugs also were assessed for their ability to reverse the locomotor suppression produced by the CB1 agonist AM411. ICV administration of either AM251 or AM4113 reversed the locomotor suppression induced by the CB1 agonist AM411 in the same dose range that failed to produce any effects on feeding.
Conclusions
This indicates that both AM4113 and AM251, when administered ICV, can interact with forebrain CB1 receptors and are efficacious on forebrain-mediated functions unrelated to feeding. These results suggest that CB1 neutral antagonists or inverse agonists may not be affecting food-reinforced behavior via interactions with forebrain CB1 receptors located in nucleus accumbens or hypothalamus and that lower brainstem or peripheral receptors may be involved.
Keywords: Feeding, Motivation, Appetite, Rimonabant, Food intake, Brain
Cannabinoid CB1 receptor modulation has been widely demonstrated to influence feeding and food-motivated behaviors. CB1 agonists such as tetrahydrocannabinol, the active ingredient in marijuana, increase reports of hunger (Hollister 1971) as well as food intake in human (Foltin et al. 1986; Abel 1971; Mattes et al. 1994) and animal studies (Anderson-Baker et al. 1979; Brown et al. 1977; Williams and Kirkham 2002). Drugs that interfere with cannabinoid transmission, such as CB1 neutral antagonists and inverse agonists, have been shown to suppress food intake and food-reinforced behaviors (for review, see Salamone et al. 2007). CB1 inverse agonist-induced feeding suppression has been demonstrated in both satiated and food-deprived animals (Chen et al. 2004; Colombo et al. 1998; Shearman et al. 2003, McLaughlin et al. 2003, 2005, 2006) and also in clinical trials (Pi-Sunyer et al. 2006; Despres et al. 2005; Van Gaal et al. 2005). More recently, CB1 antagonists including AM4113, AM6527, and O-2050, which have little intrinsic biological activity in vitro in comparison with CB1 inverse agonists such as AM251 and SR141716 (rimonabant), have also attenuated feeding in deprived (Sink et al. 2008, 2009) and nondeprived animals (Chambers et al. 2007; Gardner and Mallet 2006).
While it now seems clear that these CB1 antagonists and inverse agonists decrease food intake and food-reinforced behavior, there is still controversy as to the primary locus of action for these effects. CB1 agonists injected directly into hypothalamic nuclei related to feeding or into nucleus accumbens, a region involved with various aspects of motivated behavior, have been shown to induce hyperphagia (Jamshidi and Taylor 2001; Williams and Kirkham 1999; Verty and Mallet 2005; Soria-Gomez et al. 2007), effects that were blocked by CB1 inverse agonists. However, none of these studies was able to demonstrate an effect of a CB1 inverse agonist alone on feeding. Although a few papers have shown forebrain injections of CB1 inverse agonists to have anorectic properties (Werner and Koch 2003; Verty et al. 2004a, b), it has also been argued that feeding-related actions of CB1 inverse agonists may not depend substantially on forebrain sites. Gomez et al. (2002) observed decreases in food intake after systemic, but not intraventricular, administration of SR141716. Furthermore, capsaicin-induced deafferentation of peripheral vagal nerves innervating the gut abolished the changes in feeding elicited by systemic administration of a CB1 agonist and the inverse agonist SR141716 (Gomez et al. 2002), suggesting that CB1 receptor ligands may modulate feeding via sites located on these peripheral sensory nerve terminals.
The present set of studies was designed to determine if the suppression of food-reinforced behavior could be produced by administration of CB1 antagonist/inverse agonist drugs into the lateral ventricles. To this end, we assessed the effect of intracerebroventricular (ICV) administration of the CB1 inverse agonist AM251 and the CB1 antagonist AM4113 on fixed-ratio 5 (FR5) operant responding, a food-reinforced task previously used to characterize the actions of systemically administered CB1 inverse agonists such as AM251, SR141716, and AM1387 (McLaughlin et al. 2003, 2006) and the CB1 antagonists AM4113 and AM6527 (Sink et al. 2008, 2009). A control experiment also investigated the effects of ICVadministration of AM251 on FR5 operant responding and 18-h chow consumption in nondeprived animals. In order to validate the forebrain efficacy of the ICV drug injections, parallel experiments studied the ability of AM251 and AM4113 to reverse the locomotor suppression induced by the CB1 agonist AM411. CB1 agonist-induced locomotor suppression has been shown to be mediated via CB1 receptors in the basal ganglia and related motor regions of the brain (Sanudo-Pena et al. 1999; Sanudo-Pena and Walker 1998). It was hypothesized that ICV administration of AM251 and AM4113 would attenuate the locomotor suppression induced by the CB1 agonist AM411, which would demonstrate that AM251 and AM4113 could exert behavioral actions after ICV administration in the dose range used. Furthermore, it was hypothesized that AM251 and AM4113 should suppress food-reinforced FR5 responding in the same dose range that had locomotor effects if they are both active on forebrain mechanisms related to food motivation, while a lack of effect would indicate a lack of forebrain efficacy for the suppression of food-reinforced behavior.
Materials and methods
Animals
Adult male Sprague–Dawley rats (Harlan Sprague– Dawley, Indianapolis, IN, USA; starting weights 300– 325 g) were housed in a colony maintained at 23°C, with a 12-h light/dark cycle (lights on 07:00). With the exception of post-surgical animals, which were singly housed, all rats were caged in pairs. Rats subject to operant testing were food deprived to 85% of their free-feeding body weight for initial operant training and then allowed modest weight growth (i.e., an additional 5– 10%) during the experiment. Water was available ad libitum in the home cages. Animal protocols were approved by the University of Connecticut Institutional Animal Care and Use Committee, and the studies were conducted according to National Institutes of Health (NIH) guidelines.
Drugs
For IP administration, AM411 (synthesized in the Makriyannis laboratory at the Center for Drug Discovery, Northeastern University) was dissolved in a vehicle of dimethylsulfoxide (DMSO; Fisher, Waltham, MA, USA), Tween-80 (Fisher), and 0.9% saline in a 1:1:8 ratio. This mixture also served as the vehicle control for the IP drug treatments. For ICV administration, AM251 and AM4113 (synthesized at the Center for Drug Discovery, Northeastern University) were dissolved in 100% DMSO, which also served as control for ICV treatments. Doses and pretreatment times for AM411, AM251, and AM4113 were chosen based upon pilot studies and previously published research (McLaughlin et al. 2003, 2005; Sink et al. 2008).
Surgery
Rats were anesthetized at 1.0 mL/kg, i.p. with a solution containing 93.0 mg/mL ketamine and 1.4 mg/mL xylazine (both from Phoenix Scientific, Inc., St. Joseph, MO, USA). For ICV implantations, rats received unilateral implantations of guide cannulae made with 25-gauge extra-thin-wall stainless steel tubing (Small parts, Inc., Miami Lakes, FL, USA). Guide cannulae were inserted into the lateral ventricle (AP, −0.5 mm from bregma; ML, ±1.0 mm from midline; DV, −3.0 mm from the skull surface; incisor bar set 0.0 mm above the interaural line). The guide cannulae were secured to the skull with stainless steel screws and cranioplastic cement. Stainless steel stylets were inserted into the guide cannulae to maintain patency of the cannulae until injection. All animals were housed in separate cages following surgery and allowed at least 7 days of recovery.
Operant testing
Lever pressing sessions were conducted in Med Associates operant boxes (28×23×23 cm; Med Associates, St. Albans, VT, USA). Rats were first trained in 30-min sessions on a continuous reinforcement schedule in which every lever press was reinforced with a 45-mg food pellet (Bioserv, Frenchtown, NJ, USA) for 5 days. Following this initial training period, rats were then switched to an FR5 schedule in which every fifth lever press was reinforced. Rats were trained until stable baseline levels had been achieved (i.e., consistent responding over a 4-day period >1,200 responses per 30 min) prior to surgery. Following a post-surgical recovery period of 1 week, rats resumed training until stable baseline was re-established (approximately 2 weeks) before beginning testing.
Locomotor activity
For assessment of locomotion, rats were placed in small activity chambers (28×28×28 cm) inside soundproof shells. The floor of each chamber consists of two wire mesh panels (27×13 cm) connected through the center by a metal rod, which serves as a fulcrum for the floor panels. Locomotion by rats produces a slight deflection of one or more floor panels, which closes one or more of four microswitches mounted on the exterior of the chamber. Microswitch closure sends a signal to an external computer running a custom program by means of an interface card (Med Associates, St. Albans, VT, USA). Each microswitch closure was processed as a single activity count.
Histology
Following behavioral testing, each animal was anesthetized with CO2 and perfused intracardially with physiological saline followed by 3.7% formalin solution. The brains were stored in formalin and then cryoprotected in a 30% sucrose solution for 3 days before being cut with a cryostat in 50-μm slices and mounted on glass microscope slides. Mounted tissue was stained with cresyl violet and examined by an observer blind to the experimental condition. Any animal with improper cannula placement or significant damage around the injection site was excluded from the behavioral analyses.
Experimental procedures
Experiments 1–4: effects of lateral ventricle administration of CB1 inverse agonist/antagonists on food-reinforced behavior (FR5 operant responding) in food-deprived rats
Rats were thoroughly trained on the FR5 operant schedule (see above) before drug testing began, and different groups of rats were used for each experiment. All experiments used a within-subjects design, with all rats receiving all drug treatments in their particular experiment in a randomly varied order (one treatment per week; no treatment sequences were repeated across different animals in the same experiment). Baseline training (i.e., nondrug) sessions were conducted four additional days per week. The following treatments and testing times were used for each experiment:
| Experiment 1 | AM251: vehicle, 40, 80, and 160 μg AM251 ICV (30 min before testing; n = 16) |
| Experiment 2 | AM4113: vehicle, 60, 120, and 240μg AM4113 ICV (30 min before testing; n = 11) |
| Experiment 3 | AM251: vehicle and 160 μg AM251 ICV (10, 20, and 30 min before testing; n =15) |
| Experiment 4 | AM4113: vehicle and 240 μg AM4113 ICV (10, 20, and 30 min before testing; n = 16) |
Experiment 5: effects of lateral ventricle administration of AM251on food-reinforced behavior (FR5 operant responding) and 18-h chow intake in nondeprived rats
Prior to surgery, rats (n=7) were trained under food deprivation until consistently high levels of lever pressing (1,500 or more per 30-min session) were achieved on a FR5 operant schedule. Following 7 days of post-surgical recovery, the rats resumed FR5 operant sessions, which were always conducted between 3 and 5p.m. They were also given access to a preweighed amount of chow (about 30 g) in their home cages. Each morning, between 9 and 10a.m., the cage was carefully inspected for any remaining chow, which was then removed, weighed, and replaced with another 30 g of preweighed chow. Animals were trained on FR5 and given free access to weighed chow 6 days each week. In order to maintain operant responding levels, rats were food-deprived on the day of the week that they received no operant training. Testing began 1 week after post-surgical operant training resumed. Each test day, chow in the home cages was weighed between 9 and 10a.m., replaced with 30 g of fresh chow, and weighed again during the operant test session. Rats were injected ICV with vehicle, 40, 80, or 160μg AM251 either 10 or 30 min prior to the start of the operant test session. Every rat received each dose in a randomly varied order (one treatment per week; no treatment sequences were repeated across different animals in the same experiment). Home cage chow was weighed the morning after injection, and the difference between the chow weight recorded during the operant session and that recorded the following morning was considered as the animal's 18-h chow consumption.
Experiments 6–9: reversal of the effect of systemic administration of the CB1 agonist AM411 on locomotion by lateral ventricle administration of AM4113 or AM251
Animals were placed into the locomotion assessment chambers (see description above) for 30-min sessions. The chambers were novel to the subjects at the time of testing to ensure a high baseline of locomotor counts. Rats (n=6– 8 per group) received either 1.0 mL/kg vehicle or 5.0 mg/kg AM411 IP 30 min before testing plus AM251(40, 80, or 160μg), AM4113 (60, 120, or 240μg), or vehicle administered ICV either 10 or 30 min prior to testing.
Statistical analysis
Number of lever presses from experiments 1 and 2 were analyzed using analysis of variance (ANOVA) with repeated measures on the dose variable (four levels). Experiments 3 and 4 were analyzed in a 2 (dose)×3 (pretreatment time) ANOVA with repeated measures on both pretreatment time and dose. Experiment 5 was analyzed using repeated measures multivariate analysis of variance with operant responses and18-h chow intake as dependent variables. Locomotor counts from experiments 7 and 8 were subjected to a two-factor ANOVA with four levels on the dose factor and two levels on the pretreatment time factor. Whenever the overall ANOVA term was significant, non-orthogonal planned comparisons using the overall error term were used to compare each treatment with control conditions. The alpha level for each comparison was kept at 0.05 because the number of comparisons was restricted to the number of treatments minus one (Keppel 1982).
Results
Experiments 1–4: effects of lateral ventricle administration of CB1 inverse agonist/antagonists on food-reinforced behavior (FR5 operant responding) in food-deprived rats
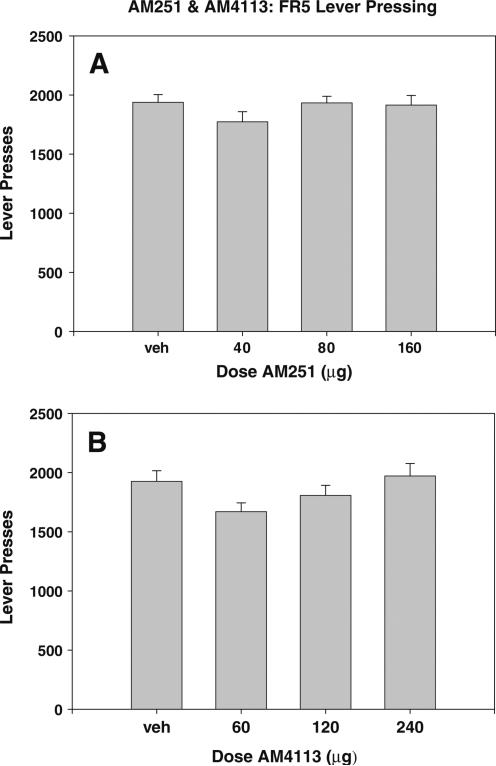
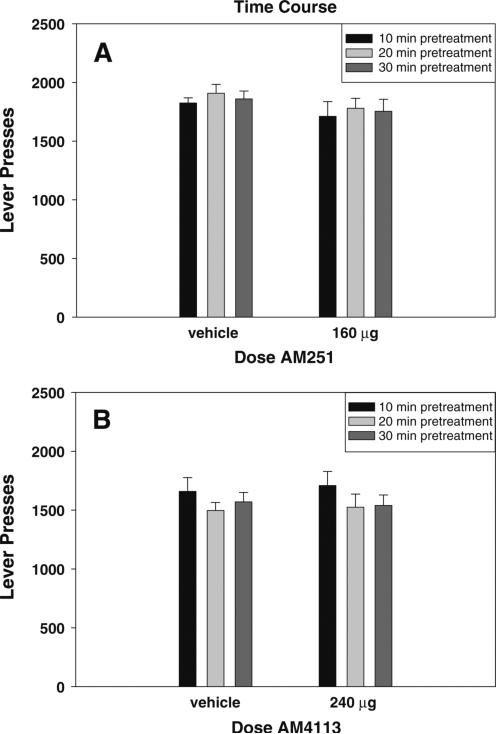
The results of experiments 1 and 2 are shown in Fig. 1. Neither AM251 nor AM4113 administered ICV produced any significant effect on FR5 operant responding for food in experiments manipulating ICV dosage [Fig. 1; AM251: F(3,45)=0.783, n.s.; AM4113: F(3,30)=2.693, n.s.]. Similarly, the results from experiments 3 and 4 (shown in Fig. 2) demonstrate that varying the amount of elapsed time between injection and testing did not significantly alter the lack of effect of AM251 and AM4113 on lever pressing for food pellets [Fig. 2; AM251: F(2,47)=0.374, n.s.; AM4113: F(2,59)=1.371, n.s.].
Fig. 1.
Effects of cannabinoid CB1 receptor antagonist/inverse agonist AM251 on FR5 responding for food pellets. Mean (±SEM) number of lever presses after ICV administration of vehicle or various doses of a AM251 and b AM4113 30 min prior to testing. Neither drug produced any significant changes in lever pressing for food at any of the doses tested
Fig. 2.
Effects of cannabinoid CB1 receptor inverse agonist AM251 and antagonist AM4113 on FR5 responding for food pellets. Mean (±SEM) number of lever presses after ICV administration of vehicle or the highest dose tested of a AM251 (160μg) and b AM4113 (240μg) either 10, 20, or 30 min prior to testing. Neither drug produced significant changes in lever pressing for food at any of pre-treatment intervals tested
Experiment 5: effects of lateral ventricle administration of AM251 on food-reinforced behavior (FR5 operant responding) and 18-h chow intake in nondeprived rats
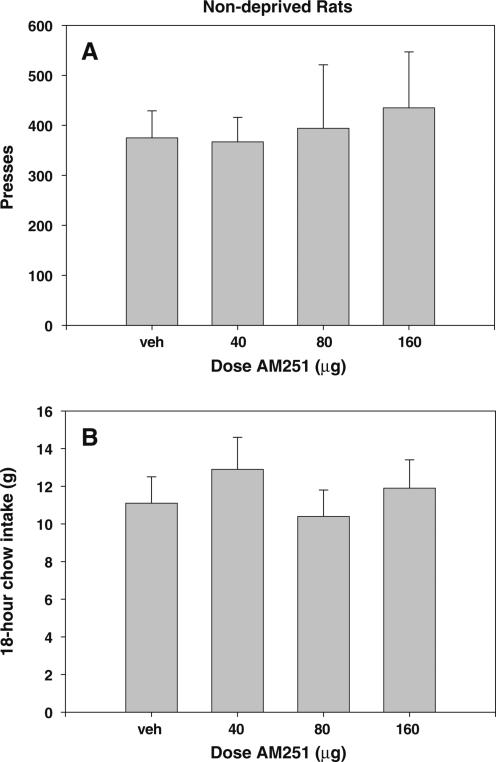
In experiment 5, AM251 did not produce any significant effect on lever pressing or 18-h chow intake of nondeprived animals when injected ICV either 10 or 30 min prior to testing. Therefore, data from both groups were pooled for analysis. The pooled data are shown in Fig. 3 ((a) lever presses: F(3,18)=0.858, n.s.; (b) chow consumption: F (3,18)=0.565, n.s.).
Fig. 3.
Effects of cannabinoid CB1 receptor inverse agonist AM251 on FR5 responding for food pellets in nondeprived animals and chow intake during the 18 h following the operant session. a Mean (±SEM) number of lever presses. b Mean (±SEM) 18-h chow intake. There were no significant differences in lever pressing for food or 18-h chow intake at any of the doses tested
Experiments 6–9: reversal of the effect of systemic administration of the CB1 agonist AM411 on locomotion by lateral ventricle administration of CB1 antagonist AM4113 or inverse agonist AM251
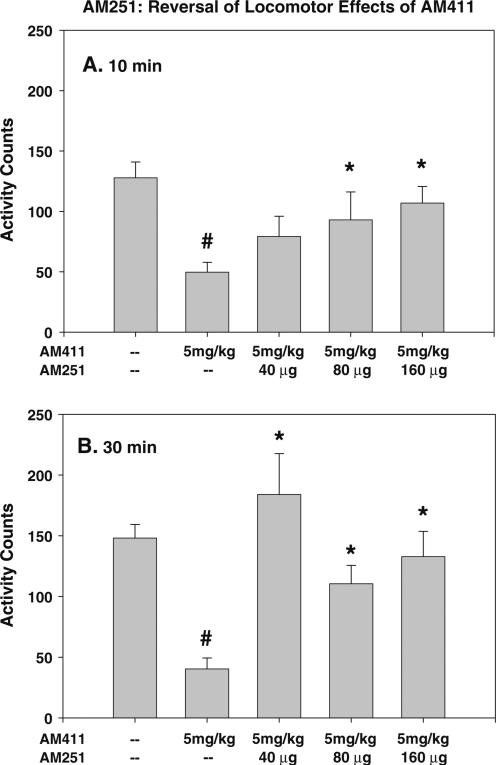
Results from experiments 7 and 8 are summarized in Figs. 4 and 5. In both of these experiments, the overall ANOVAs were significant, and planned comparisons revealed that AM411 produced a significant suppression of locomotor activity. Both AM4113 and AM251 given ICV either 10 or 30 min prior to testing produced a significant increase in locomotor activity in animals co-administered AM411 [AM251 10-min pretreatment: F (4,32)=4.406, p=0.006; AM251 30-min pretreatment: F(4, 30)=8.316, p<0.001; AM4113 10-min pretreatment: F(4,34)=9.184, p<0.001; AM4113 30-min pretreatment: F(4,30)=22.305, p<0.001]. For both drugs, planned comparisons showed that all doses in the 30-min pretreatment groups and all doses except the lowest dose for each drug treatment in the 10-min pretreatment groups attenuated the AM411-induced locomotor suppression.
Fig. 4.
ICV administration of cannabinoid CB1 receptor inverse agonist AM251 reverses locomotor suppression induced by CB1 agonist AM411. Mean (±SEM) locomotor counts following a 10 min or b 30 min pretreatment. Overall ANOVA was significant for both pretreatment times. AM411 produced a significant suppression of locomotion compared with vehicle, which was reversed by AM251
Fig. 5.
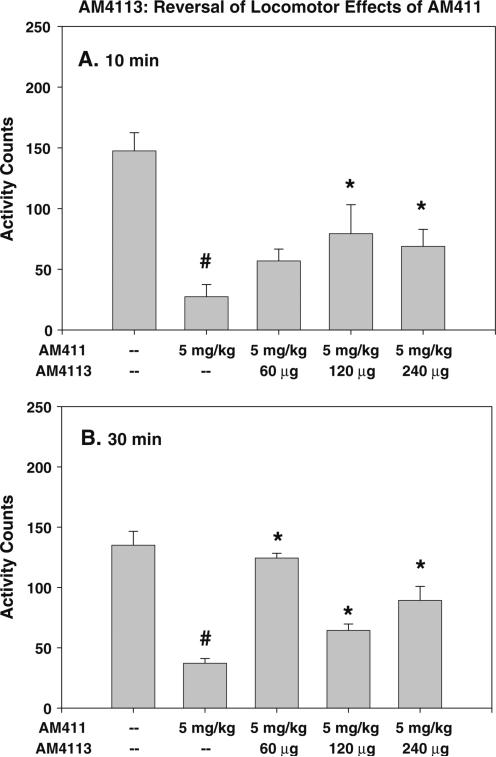
ICV administration of cannabinoid CB1 receptor antagonist AM4113 reverses locomotor suppression induced by CB1 agonist AM411. Mean (±SEM) locomotor counts following a 10 min or b 30 min pretreatment. Overall ANOVA was significant for both pretreatment times. AM411 produced a significant suppression of locomotion compared with vehicle, which was reversed by AM4113
Discussion
The studies described above were conducted to determine if the effects of AM251 and AM4113 on food-reinforced behavior are due to actions on the forebrain. Thus, the effects of administration of AM251and AM4113 into the lateral ventricles were examined by employing a FR5 schedule with food reinforcement. This task has previously been used to characterize the effects of systemic administration of drugs that interfere with CB1 receptor transmission, including rimonabant, AM251, AM1387, and, more recently, AM4113 (Chambers et al. 2007; McLaughlin et al. 2003, 2006; Sink et al. 2008, 2009; Salamone et al. 2007). In those previous studies, AM251, AM1387, rimonabant, and AM4113 given intraperitoneally all potently reduced food-reinforced lever pressing (Chambers et al. 2007; McLaughlin et al. 2003, 2006; Sink et al. 2008). These previous results are in agreement with an extensive literature illustrating CB1 inverse agonist or antagonist-induced suppression of food intake or appetitive behaviors related to food motivation (for review, see Salamone et al. 2007). In the present studies, however, neither AM251 nor AM4113 produced any change in operant responding for food pellets when injected into the lateral ventricles, even at doses up to nearly one tenth of the systemic doses that decrease feeding and food-reinforced behaviors. A control experiment in nondeprived animals similarly yielded no indication of an effect on lever pressing for food pellets or subsequent 18-h chow intake. However, lateral ventricle administration of AM4113 or AM251 significantly attenuated CB1 agonist-induced locomotor suppression, which is thought to involve CB1 receptors located within motor circuitry in the forebrain and midbrain, including basal ganglia structures such as nucleus accumbens and neostriatum, as well as pallidal and nigral areas (Sanudo-Pena et al. 1999; Sanudo-Pena and Walker 1998). Thus, the present results from studies involving locomotor activity indicate that AM4113 and AM251 are capable of exerting some central actions (i.e., effects on locomotion) after administration into the lateral ventricle. Taken together, this pattern of results suggests that CB1 antagonists and inverse agonists may reduce food-motivated behavior primarily by actions on areas outside the forebrain. In view of reports showing potency differences between the effects of drugs injected into the lateral or third ventricles vs. the fourth ventricle (Fan et al. 2004; Blevins et al. 2004), the present studies cannot conclusively rule out a possible locus of action for CB1 antagonists in a lower brainstem area such as the parabrachial nucleus (DiPatrizio and Simansky 2008). Moreover, the present results are consistent with the suggestion of Gomez et al. (2002) that peripheral sites, particularly those located on vagus nerve terminals, also may be important. Gomez et al. (2002) reported that systemic administration of SR 141716A (rimonabant) suppressed feeding, but ICV administration of rimonabant at doses up to 10.0μg had no effect. Furthermore, capsaicin-induced deafferentation of the vagal nerve terminals innervating the gastrointestinal tract abolished the feeding-related effects of the systemically administered CB1 ligands.
The present experiments extend the scope of the Gomez et al. (2002) paper by demonstrating that AM251 (up to 160.0μg) and AM4113 (up to 240.0μg) produced little effect on food-motivated behaviors when administered directly into the brain. They also strengthen the validity of the previous studies by providing a control experiment demonstrating the efficacy of the ICV drug injection methods on behaviors thought to be mediated via centrally located CB1 receptors (i.e., locomotor activity). Significant CB1 receptor expression is observed at several peripheral sites, including the cell bodies of vagal nerve afferents in the nodose ganglion, which has projections to the gastrointestinal tract (Partosoedarso et al. 2003). This peripheral location of CB1 receptors suggests another potential mechanism by which systemically administered CB1 antagonists or inverse agonists might influence ingestive behaviors. In further support of this hypothesis, levels of expression and distribution of CB1 receptors in these populations are regulated by satiety state (Burdyga et al. 2004). In satiated rats, there is very little CB1 mRNA or protein expression within the nodose ganglion. Fasted rats, however, have substantially upregulated mRNA and protein expression in the nodose ganglion and increased distribution of the CB1 receptor to the caudal pole, which contains neurons projecting to the gut. The terminals of these vagal afferent neurons expressing CB1 receptors also express CCK1, orexin, leptin, ghrelin, and MCH receptors (Burdyga et al. 2006). These receptors bind to neuropeptide hormones involved in the regulation of hunger and satiety.
It should be mentioned that the present results, as well as those of Gomez (Gomez et al. 2002), do not agree with the findings obtained by Verty et al. (2004a, b) or Werner and Koch (2003). These papers reported that CB1 inverse agonists SR141716 (Verty et al. 2004a, b) and AM281 (Werner and Koch 2003) significantly reduced food intake when administered ICV. In seeking to reconcile our results with these previous findings, it was noted that our study and that of Gomez et al. (2002) were performed in food-deprived animals, while both of the studies finding significance upon ICV administration of CB1 inverse agonists were performed in nondeprived animals. This observation led to a control experiment in which we tested the effects of ICV AM251 in nondeprived animals, measuring their performance on operant responding for preferred sucrose pellets and subsequent 18-h chow intake. However, we were unable to find any significant effects of AM251 on either measure at doses from 40.0 to 160.0μg. Data from Werner and Koch (2003) demonstrated suppression of food intake following ICV administration of AM281 for up to 6 h. However, from 6 to 24 h, intake in these groups exceeded that of the controls, possibly due to rebound feeding (Werner and Koch 2003). This evidence, plus corroborating pharmacokinetic data showing brain uptake of intravenously administered radiolabeled AM251 to be nearly maximal at 30 min post-injection and only one half of the maximal value 8 h following administration (Gatley et al. 1996), leads to the possibility that our experimental parameters were not ideal for observing suppression of chow intake since chow was only measured at a single time point 18 h after drug administration. However, we would have still expected to see some decrease in operant responding since this was measured within the first hour of drug treatment. Furthermore, food intake suppression following a single systemic treatment with CB1 inverse agonist AM251 has been documented to begin within 15 min of injection and to last up to 6 days in nondeprived rats (Chambers et al. 2004). Thus, any short-term suppression of intake after central administration of CB1 inverse agonists or antagonists might only partially account for the hypophagic effects observed upon systemic administration. SR141716A and AM251 have similar binding affinity for CB1 receptors (i.e., in the low nanomolar range), although these two drugs differ in terms of their pharmacological profile at non-CB1/non-CB2 cannabinoid-binding receptors such as GPR55 (Lauckner et al. 2008; Ryberg et al. 2007), and also may differ in terms of actions on novel uncharacterized non-CB1 receptors (Haller et al. 2002; Hajos et al. 2001; Haller et al. 2004).
An important future study that could further clarify this issue would be measurement of food intake or food-motivated behaviors following systemic administration of a CB1 inverse agonist or antagonist with poor blood–brain barrier penetrability. If food-intake suppression could be observed following systemic injection with such a compound, this would lend strong evidence supporting the role of peripheral CB1 receptors in the modulation of feeding. It has been reported that LH-21 is such a compound and that systemic injection of this purported CB1 antagonist with poor blood–brain barrier penetrability did indeed decrease feeding (Pavon et al. 2006, 2008). However, continued pharmacological evaluation of this compound will be necessary for determining which of its effects are central and which are peripheral (Chen et al. 2008; Pavón et al. 2008), and additional compounds need to be evaluated. Recently, the novel CB1 antagonist AM6545 was developed to explore the behavioral actions of peripherally active CB1 antagonists. Although this drug has poor penetrability into the brain compared to AM251, it has been shown to suppress food-reinforced FR5 responding at doses of 8.0 and 16.0 mg/kg IP (Salamone et al. 2009).
In summary, interference with forebrain CB1 receptor transmission by administration of AM251 or AM4113 into the lateral ventricle failed to induce any changes in food-reinforced behavior. ICV administration of these drugs did appear to exert some central actions, as both drugs significantly reversed a behavior thought to be mediated via central CB1 receptors (i.e., reversal of CB1 agonist-induced locomotor suppression) in the same dose range that was ineffective in terms of actions on food-reinforced behavior. The implications of a possible peripheral mechanism for the hypophagic actions of CB1 antagonists and inverse agonists are intriguing with respect to the use of these drugs as treatments for obesity. A CB1 antagonist or inverse agonist compound with poor brain penetrability could be a clinically useful appetite suppressant and may also engage peripherally mediated metabolic effects that contribute to weight loss (Herling et al. 2008; Bensaid et al. 2003; Cota et al. 2003; Jbilo et al. 2005; Osei-Hyiaman et al. 2005; Ravinet Trillou et al. 2003) while avoiding centrally mediated psychiatric side effects which have limited the clinical usefulness of the CB1 inverse agonist rimonabant (Christensen et al. 2007; Curioni and Andre 2006). Pharmaceutical companies are recognizing these limitations, and even now, a novel CB1 antagonist that specifically acts in the periphery (TM38837) is currently under investigation by 7TM Pharma as an obesity treatment (7TM Pharma 2008).
Acknowledgments
This research was supported by grants to JDS and AM from the US NIH/NIDA.
Contributor Information
K. S. Sink, Department of Psychology, University of Connecticut, Storrs, CT 06269-1020, USA Yerkes National Primate Center and the Center for Behavioral Neuroscience, Emory University, 954 Gatewood Drive, Atlanta, GA 30329, USA.
K. N. Segovia, Department of Psychology, University of Connecticut, Storrs, CT 06269-1020, USA
E. J. Nunes, Department of Psychology, University of Connecticut, Storrs, CT 06269-1020, USA
L. E. Collins, Department of Psychology, University of Connecticut, Storrs, CT 06269-1020, USA
V. K. Vemuri, Center for Drug Discovery, Northeastern University, 360 Huntington Avenue, Boston, MA 02115, USA
G. Thakur, Center for Drug Discovery, Northeastern University, 360 Huntington Avenue, Boston, MA 02115, USA
A. Makriyannis, Center for Drug Discovery, Northeastern University, 360 Huntington Avenue, Boston, MA 02115, USA
J. D. Salamone, Department of Psychology, University of Connecticut, Storrs, CT 06269-1020, USA
References
- 7TM Pharma [September 20, 2008];7TM Pharma announces the selection of a new pre-clinical development candidate, TM38837, for treatment of obesity and Type 2 diabetes. http://www.7tm.com/News.aspx?M=News&PID=42&NewsID=33.
- Abel EL. Effects of marihuana on the solution of anagrams, memory and appetite. Nature. 1971;231:260–261. doi: 10.1038/231260b0. [DOI] [PubMed] [Google Scholar]
- Anderson-Baker WC, McLaughlin CL, Baile CA. Oral and hypothalamic injections of barbiturates, benzodiazepines and cannabinoids and food intake in rats. Pharmacol Biochem Behav. 1979;11:487–491. doi: 10.1016/0091-3057(79)90030-3. [DOI] [PubMed] [Google Scholar]
- Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G, Oury-Donat F, Soubrie P. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol. 2003;63:908–914. doi: 10.1124/mol.63.4.908. [DOI] [PubMed] [Google Scholar]
- Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol. 2004;287:R87–96. doi: 10.1152/ajpregu.00604.2003. [DOI] [PubMed] [Google Scholar]
- Brown JE, Kassouny M, Cross JK. Kinetic studies of food intake and sucrose solution preference by rats treated with low doses of delta9-tetrahydrocannabinol. Behav Biol. 1977;20:104–110. doi: 10.1016/s0091-6773(77)90606-x. [DOI] [PubMed] [Google Scholar]
- Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci. 2004;24:2708–2715. doi: 10.1523/JNEUROSCI.5404-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1289–97. doi: 10.1152/ajpgi.00543.2005. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Sharkey KA, Koopmans HS. Cannabinoid (CB) 1 receptor antagonist, AM 251, causes a sustained reduction of daily food intake in the rat. Physiol Behav. 2004;82:863–869. doi: 10.1016/j.physbeh.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Vemuri VK, Peng Y, Wood JT, Olzewska T, Pittman QJ, Makriyannis A, Sharkey K. A neutral CB1 receptor antagonist reduces weight gain in rat. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2185–2193. doi: 10.1152/ajpregu.00663.2007. [DOI] [PubMed] [Google Scholar]
- Chen RZ, Huang RR, Shen CP, MacNeil DJ, Fong TM. Synergistic effects of cannabinoid inverse agonist AM251 and opioid antagonist nalmefene on food intake in mice. Brain Res. 2004;999:227–230. doi: 10.1016/j.brainres.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Chen RZ, Frassetto A, Lao JZ, Huang RR, Xiao JC, Clements MJ, Walsh TF, Hale JJ, Wang J, Tong X, Fong TM. Pharmacological evaluation of LH-21, a newly discovered molecule that binds to cannabinoid CB1 receptor. Eur J Pharmacol. 2008;584:338–342. doi: 10.1016/j.ejphar.2008.02.029. [DOI] [PubMed] [Google Scholar]
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998;63:PL113–PL117. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thone-Reineke C, Ortmann S, Tomassoni F, Cervino C, Nisoli E, Linthorst AC, Pasquali R, Lutz B, Stalla GK, Pagotto U. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curioni C, Andre C. Rimonabant for overweight or obesity. Cochrane Database Syst Rev. 2006;4:CD006162. doi: 10.1002/14651858.CD006162.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres JP, Golay A, Sjostrom L, Rimonabant in Obesity-Lipids Study Group Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- DiPatrizio NV, Simansky KJ. Activating parabrachial cannabinoid CB1 receptors selectively stimulates feeding of palatable foods in rats. J Neurosci. 2008;28:9702–9709. doi: 10.1523/JNEUROSCI.1171-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P, Cone RD. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci. 2004;7:335–336. doi: 10.1038/nn1214. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Brady JV, Fischman MW. Behavioral analysis of marijuana effects on food intake in humans. Pharmacol Biochem Behav. 1986;25:577–582. doi: 10.1016/0091-3057(86)90144-9. [DOI] [PubMed] [Google Scholar]
- Gardner A, Mallet PE. Suppression of feeding, drinking, and locomotion by a putative cannabinoid receptor 'silent antagonist'. Eur J Pharmacol. 2006;530:103–106. doi: 10.1016/j.ejphar.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Gatley SJ, Gifford AN, Volkow ND, Lan R, Makriyannis A. 123I-labeled AM251: a radioiodinated ligand which binds in vivo to mouse brain cannabinoid CB1 receptors. Eur J Pharmacol. 1996;307:331–338. doi: 10.1016/0014-2999(96)00279-8. [DOI] [PubMed] [Google Scholar]
- Gomez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, Cippitelli A, Nava F, Piomelli D, Rodriguez de Fonseca F. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Haller J, Bakos N, Szirmay M, Ledent C, Freund TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur J Neurosci. 2002;16:1395–1398. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Varga B, Ledent C, Freund TF. CB1 cannabinoid receptors mediate anxiolytic effects: convergent genetic and pharmacological evidence with CB1-specific agents. Behav Pharmacol. 2004;15:299–304. doi: 10.1097/01.fbp.0000135704.56422.40. [DOI] [PubMed] [Google Scholar]
- Herling AW, Kilp S, Elvert R, Haschke G, Kramer W. Increased energy expenditure contributes more to the body weight-reducing effect of rimonabant than reduced food intake in candy-fed Wistar rats. Endocrinology. 2008;149:2557–2566. doi: 10.1210/en.2007-1515. [DOI] [PubMed] [Google Scholar]
- Hollister LE. Hunger and appetite after single doses of marihuana, alcohol, and dextroamphetamine. Clin Pharmacol Ther. 1971;12:44–49. doi: 10.1002/cpt197112144. [DOI] [PubMed] [Google Scholar]
- Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol. 2001;134:1151–1154. doi: 10.1038/sj.bjp.0704379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jbilo O, Ravinet-Trillou C, Arnone M, Buisson I, Bribes E, Peleraux A, Penarier G, Soubrie P, Le Fur G, Galiegue S, Casellas P. The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J. 2005;19:1567–1569. doi: 10.1096/fj.04-3177fje. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and analysis: a researcher's handbook. Englewood Cliffs; Prentice-Hall: 1982. [Google Scholar]
- Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci U S A. 2008;105:2699–2704. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes RD, Engelman K, Shaw LM, Elsohly MA. Cannabinoids and appetite stimulation. Pharmacol Biochem Behav. 1994;49:187–195. doi: 10.1016/0091-3057(94)90475-8. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston K, Swezey L, Wisniecki A, Aberman J, Tardif DJ, Betz AJ, Ishiwari K, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonists SR 141716A and AM 251 suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav Pharmacol. 2003;14:583–588. doi: 10.1097/00008877-200312000-00002. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston KM, Limebeer CL, Parker LA, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonist AM 251 produces food avoidance and behaviors associated with nausea but does not impair feeding efficiency in rats. Psycho-pharmacology (Berl) 2005;180:286–293. doi: 10.1007/s00213-005-2171-0. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Qian L, Wood JT, Wisniecki A, Winston KM, Swezey LA, Ishiwari K, Betz AJ, Pandarinathan L, Xu W, Makriyannis A, Salamone JD. Suppression of food intake and food-reinforced behavior produced by the novel CB1 receptor antagonist/inverse agonist AM 1387. Pharmacol Biochem Behav. 2006;83:396–402. doi: 10.1016/j.pbb.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L, Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partosoedarso ER, Abrahams TP, Scullion RT, Moerschbaecher JM, Hornby PJ. Cannabinoid1 receptor in the dorsal vagal complex modulates lower oesophageal sphincter relaxation in ferrets. J Physiol. 2003;550:149–158. doi: 10.1113/jphysiol.2003.042242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavon FJ, Bilbao A, Hernandez-Folgado L, Cippitelli A, Jagerovic N, Abellan G, Rodriguez-Franco MA, Serrano A, Macias M, Gomez R, Navarro M, Goya P, Rodriguez de Fonseca F. Antiobesity effects of the novel in vivo neutral cannabinoid receptor antagonist 5-(4-chlorophenyl)-1-(2, 4-dichlorophenyl)-3-hexyl-1H-1, 2, 4-triazole-LH 21. Neuropharmacology. 2006;51:358–366. doi: 10.1016/j.neuropharm.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Pavón FJ, Serrano A, Pérez-Valero V, Jagerovic N, Hernández-Folgado L, Bermúdez-Silva FJ, Macías M, Goya P, de Fonseca FR. Central versus peripheral antagonism of cannabinoid CB1 receptor in obesity: effects of LH-21, a peripherally acting neutral cannabinoid receptor antagonist, in Zucker rats. J Neuroendocrinol. 2008;20(suppl 1):116–123. doi: 10.1111/j.1365-2826.2008.01693.x. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J, RIO-North America Study Group Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- Ravinet Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP, Soubrie P. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol. 2003;284:R345–53. doi: 10.1152/ajpregu.00545.2002. [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, McLaughlin PJ, Sink K, Makriyannis A, Parker LA. Cannabinoid CB1 receptor inverse agonists and neutral antagonists: effects on food intake, food-reinforced behavior and food aversions. Physiol Behav. 2007;91:383–388. doi: 10.1016/j.physbeh.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Randall P, Hosmer S, Sink KS, Segovia KN, Stopper CM, Port RG, Vemuri VK, Wood J, Thakur G, Makriyannis A. Central vs. peripheral effects of cannabinoid CB1 antagonists/inverse agonists on food-reinforced operant responding: Effects of the peripherally active CB1 antagonist AM6545. 2009 Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2009. 2009. (in press) [Google Scholar]
- Sanudo-Pena MC, Walker JM. A novel neurotransmitter system involved in the control of motor behavior by the basal ganglia. Ann N Y Acad Sci. 1998;860:475–479. doi: 10.1111/j.1749-6632.1998.tb09081.x. [DOI] [PubMed] [Google Scholar]
- Sanudo-Pena MC, Tsou K, Walker JM. Motor actions of cannabinoids in the basal ganglia output nuclei. Life Sci. 1999;65:703–713. doi: 10.1016/s0024-3205(99)00293-3. [DOI] [PubMed] [Google Scholar]
- Shearman LP, Rosko KM, Fleischer R, Wang J, Xu S, Tong XS, Rocha BA. Antidepressant-like and anorectic effects of the cannabinoid CB1 receptor inverse agonist AM251 in mice. Behav Pharmacol. 2003;14:573–582. doi: 10.1097/00008877-200312000-00001. [DOI] [PubMed] [Google Scholar]
- Sink KS, McLaughlin PJ, Wood JA, Brown C, Fan P, Vemuri VK, Pang Y, Olzewska T, Thakur GA, Makriyannis A, Parker LA, Salamone JD. The novel cannabinoid CB(1) receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology. 2008;33:946–966. doi: 10.1038/sj.npp.1301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Vemuri VK, Wood J, Makriyannis A, Salamone JD. Oral bioavailability of the novel cannabinoid CB1 antagonist AM6527: effects on food-reinforced behavior and comparisons with AM4113. Pharmacol Biochem Behav. 2009;91:303–306. doi: 10.1016/j.pbb.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria-Gomez E, Matias I, Rueda-Orozco PE, Cisneros M, Petrosino S, Navarro L, Di Marzo V, Prospero-Garcia O. Pharmacological enhancement of the endocannabinoid system in the nucleus accumbens shell stimulates food intake and increases c-fos expression in the hypothalamus. Br J Pharmacol. 2007;151:1109–1116. doi: 10.1038/sj.bjp.0707313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S, RIO-Europe Study Group Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- Verty AN, McFarlane JR, McGregor IS, Mallet PE. Evidence for an interaction between CB1 cannabinoid and oxytocin receptors in food and water intake. Neuropharmacology. 2004a;47:593–603. doi: 10.1016/j.neuropharm.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Verty AN, McFarlane JR, McGregor IS, Mallet PE. Evidence for an interaction between CB1 cannabinoid and melanocortin MCR-4 receptors in regulating food intake. Endocrinology. 2004b;145:3224–3231. doi: 10.1210/en.2004-0059. [DOI] [PubMed] [Google Scholar]
- Verty AN, Mallet PE. Paraventricular hypothalamic CB1 cannabinoid receptors are involved in the feeding stimulatory effects of delta9-tetrahydrocannabinol. Neuropharmacology. 2005;49:1101–1109. doi: 10.1016/j.neuropharm.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Werner NA, Koch JE. Effects of the cannabinoid antagonists AM281 and AM630 on deprivation-induced intake in lewis rats. Brain Res. 2003;967:290–292. doi: 10.1016/s0006-8993(02)04274-9. [DOI] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1999;143:315–317. doi: 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Reversal of delta 9-THC hyperphagia by SR141716 and naloxone but not dexfenfluramine. Pharmacol Biochem Behav. 2002;71:333–340. doi: 10.1016/s0091-3057(01)00694-3. [DOI] [PubMed] [Google Scholar]