The aim of this study was to describe clinicopathologic features of patients with breast cancer brain metastasis (BCBM); to evaluate survival after diagnosis of BCBM; and to compare estrogen receptor, progesterone receptor, and HER2 expression in the paired primary and brain tumors. The results show that younger age, solitary brain lesion, and continuation of HER2-targeted therapy in HER2-positive BCBM patients are predictors of better survival.
Keywords: Breast cancer, Brain metastases, Survival, Hormone receptor, HER2
Abstract
Background.
The aim of this study was to describe clinicopathologic features of patients with breast cancer brain metastasis (BCBM); to evaluate survival after diagnosis of BCBM; and to compare estrogen receptor (ER), progesterone receptor (PR), and HER2 expression in the paired primary and brain tumors.
Materials and Methods.
We identified 140 consecutive patients who underwent craniotomy for BCBM (either for diagnostic purpose or with therapeutic intent) at the University of Texas MD Anderson Cancer Center between 2002 and 2009.
Results.
Most patients had invasive ductal histology (91%), grade 3 tumors (67%), and positive axillary lymph node (64%). Of the tumors, 56% were ER-negative, 62% were PR-negative, 44% were HER2-positive, and 28% were triple negative (TN). Brain metastasis (BM) was solitary in 51% of patients. Median interval from breast cancer diagnosis to BM was 46 months; median survival after BM was 14.1 months. In the univariate analysis, younger age, solitary brain metastasis, and ER or PR positivity in the breast tumors were associated with longer survival. There was a statistical trend toward increased survival in HER2-positive patients compared with HER2-negative patients (18 vs. 11 months). In the multivariate analysis, predictors for longer survival included younger age, solitary brain lesion, and HER2 positivity in the breast cancer. Biomarkers were evaluated in paired primary and brain tumors in 35 patients for ER status, 34 for PR status, and 36 for HER2 status. Discordant rates were 28% for ER, 20% for PR, and 3% for HER2.
Conclusion.
Compared with unselected breast cancer patients at the same institution, patients with breast cancer who had brain metastases had a higher proportion of hormone receptor-negative, HER2-positive, and TN tumors. Younger age, solitary brain lesion, and HER2 expression were independent predictors of better survival in patients with BCBM. HER2 status was highly concordant between the paired primary and brain tumors, whereas changes of ER and PR status occurred in a substantial proportion of the patients. These findings are important for making effective treatment decisions for patients with BCBM.
Implications for Practice:
This study was performed to assess clinical outcomes in a cohort of patients with histologically diagnosed breast cancer brain metastasis (BCBM) and to compare biomarker expressions in the paired primary and brain tumors. The results show that younger age, solitary brain lesion, and continuation of HER2-targeted therapy in HER2-positive BCBM patients are predictors of better survival. HER2 expression was highly concordant between the primary and brain tumors, whereas changes of ER or PR status occurred in a substantial proportion of the patients. Larger series are warranted to confirm these data and to determine the value of performing a brain biopsy and reassessing HER2, ER, and PR expression in the setting of BCBM.
Introduction
Breast cancer is one of the most common causes of brain metastasis (BM). Approximately 10%–16% of patients with breast cancer are affected by BM during the course of their disease [1]. Recently, a trend toward an increased rate of BM (range of 25%–34%) has been observed [2]. This may be attributed to the increased awareness of BM by patients and clinicians, the increased use of more sensitive neuroimaging techniques, and the introduction of more effective systemic treatment, particularly anti-HER2 therapy, that led to prolonged survival [3].
The current standard of care for patients with breast cancer brain metastasis (BCBM) is whole-brain radiotherapy, with or without surgical resection, or stereotactic radiosurgery. In addition, systemic treatments including chemotherapy and targeted therapy are included [4]. Once BM occurs, the prognosis is generally poor, with 1- and 2-year survival rates of only 20% and 2%, respectively [5]. The clinical outcome relies on many factors related to the patient, the disease, and the treatment regimens.
Hormone receptor ([HR]; estrogen receptor [ER], progesterone receptor [PR]) and HER2 expressions are used as predictors of response to hormone- and HER2-targeted treatments, respectively [6, 7]. In clinical practice, the assessment of these predictive and prognostic factors is performed predominantly in the breast tumor, and these results are also applied to select treatment for the metastatic disease; however, distant metastases, particularly BM, usually develop years after the initial diagnosis of breast cancer, often after many intervening therapies. Recent studies have shown varying degrees of discordance in ER, PR, and HER2 expression between the primary and metastatic diseases [8], but little information exists about the degree and the clinical implication of discordance in the brain metastases.
The aim of this retrospective study was to describe the clinicopathologic features of a cohort of patients with histologically diagnosed BCBM; to define the outcomes of patients with BM; and to compare ER, PR, and HER2 expression in the paired primary and brain tumors.
Materials and Methods
Data Collection
We identified 140 consecutive patients who underwent craniotomy for BCBM (either for diagnostic purpose or with therapeutic intent) at the University of Texas MD Anderson Cancer Center (MDACC) Department of Neurosurgery between 2002 and 2009 (during the same period of time, a total of 1,140 patients with BCBM were evaluated at MDACC). Clinicopathologic information was collected from the electronic medical record, including the patient demographics, tumor characteristics, biomarker status, dates of diagnosis of breast cancer and subsequent brain metastases, number of brain lesions based on magnetic resonance imaging study, and data on BCBM treatment and clinical outcomes. ER and PR status was determined by immunohistochemical testing according to the College of American Pathologists guideline. Tumors with HER2 immunohistochemical staining intensity of 3+ were considered positive, whereas those with 2+ staining intensity were further evaluated by fluorescent in situ hybridization (FISH). Follow-up took place through September 30, 2012. This study was approved by the institutional review board of MDACC.
Statistical Analysis
Time to brain metastasis was defined as the time from the date of breast cancer diagnosis to the date of BCBM diagnosis. Overall survival after BCBM was defined as the time from the date of BCBM diagnosis to the date of death from any cause or last follow-up. Survival after BCBM was estimated using the Kaplan-Meier method and compared between groups using the log-rank test. Univariate and multivariate Cox proportional hazards analyses were performed to evaluate the effects of age; interval from breast cancer diagnosis to BM; status of ER, PR, and HER2; and number of brain lesions on length of survival following diagnosis of BCBM. The concordance analysis of the biomarker results for the paired primary tumors and brain metastases was performed using Cohen’s κ statistic. A p value ≤.05 was considered statistically significant.
Results
Patient and Tumor Characteristics
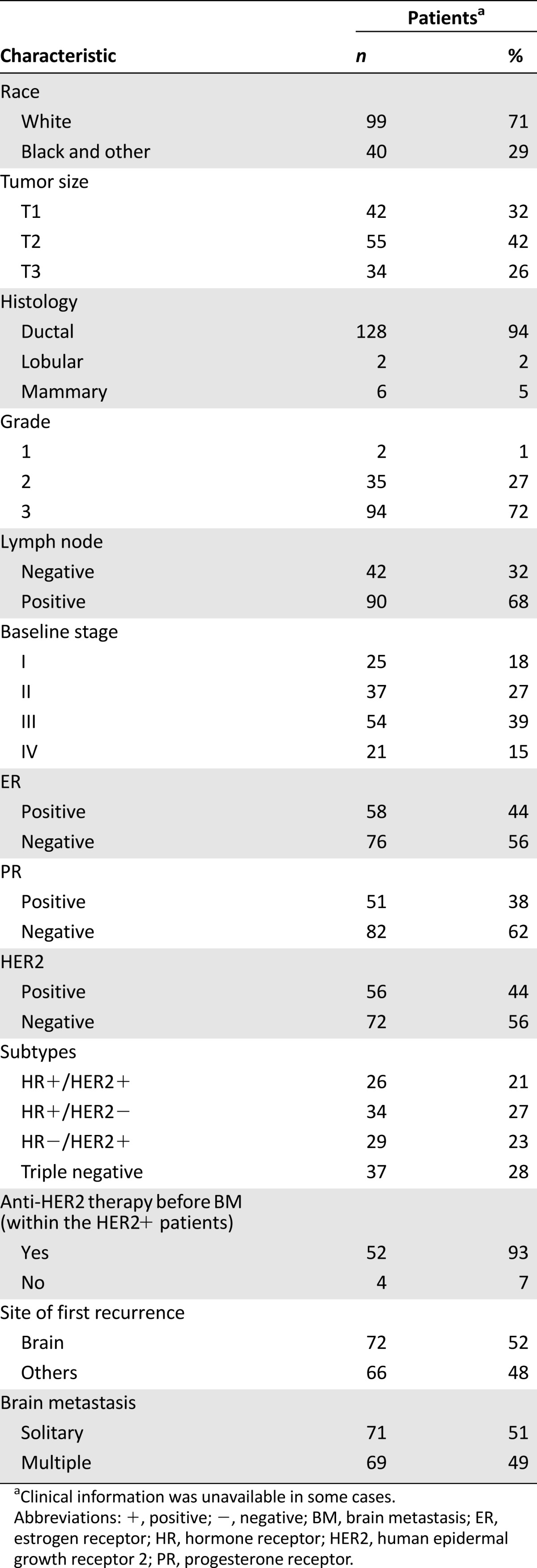
The study group included 140 consecutive patients who received craniotomy for BCBM at MDACC from 2002 to 2009. Patient and tumor characteristics are summarized in Table 1. The median age at diagnosis of breast cancer was 46 years (range: 24–73 years). Most patients had invasive ductal histology (n = 128, 94%) and grade 3 tumors (n = 94, 72%). Moreover, 68% of patients had T2 (n = 55) or T3 (n = 34) disease, and 68% of patients (n = 90) had positive axillary lymph nodes. In addition, 39% (n = 54) of patients had locally advanced disease, and 15% (n = 21) had metastases at the time of diagnosis. HR and HER2 status were known in 96% and 91% of patients, respectively. Among patients with known HR and HER2 status (n = 126), a total of 56% of the tumors were ER negative (ER−; n = 76), 62% were PR negative (n = 82), and 44% were HER2-positive (HER2+; n = 56). With regard to the four subtypes formed by the joint values of HR and HER2 status, 21% (n = 26) of the tumors were HR+HER2+, 27% (n = 34) were HR+HER2−, 23% (n = 29) were HR−/HER2+, and 28% (n = 37) were triple negative (TN). Of the 56 patients with HER2+ breast tumors, 52 (93%) received anti-HER2 therapy before BM.
Table 1.
Patient and tumor characteristics
The median age at diagnosis of BCBM was 53 years (range: 25–81 years). The median interval from breast cancer diagnosis to BM was 46 months (range: 0–266 months). Patients with TN tumors and stage IV disease had significantly shorter intervals to BM compared with those with non-TN tumors (median interval: 27 vs. 49 months; p = .004) and stage I, II, or III disease (median interval: 19 vs. 52 months; p < .001). No marked difference in the BM-free interval was noted between patients who were HER2-positive and those with HER2-negative disease (46 vs. 41 months; p = .92).
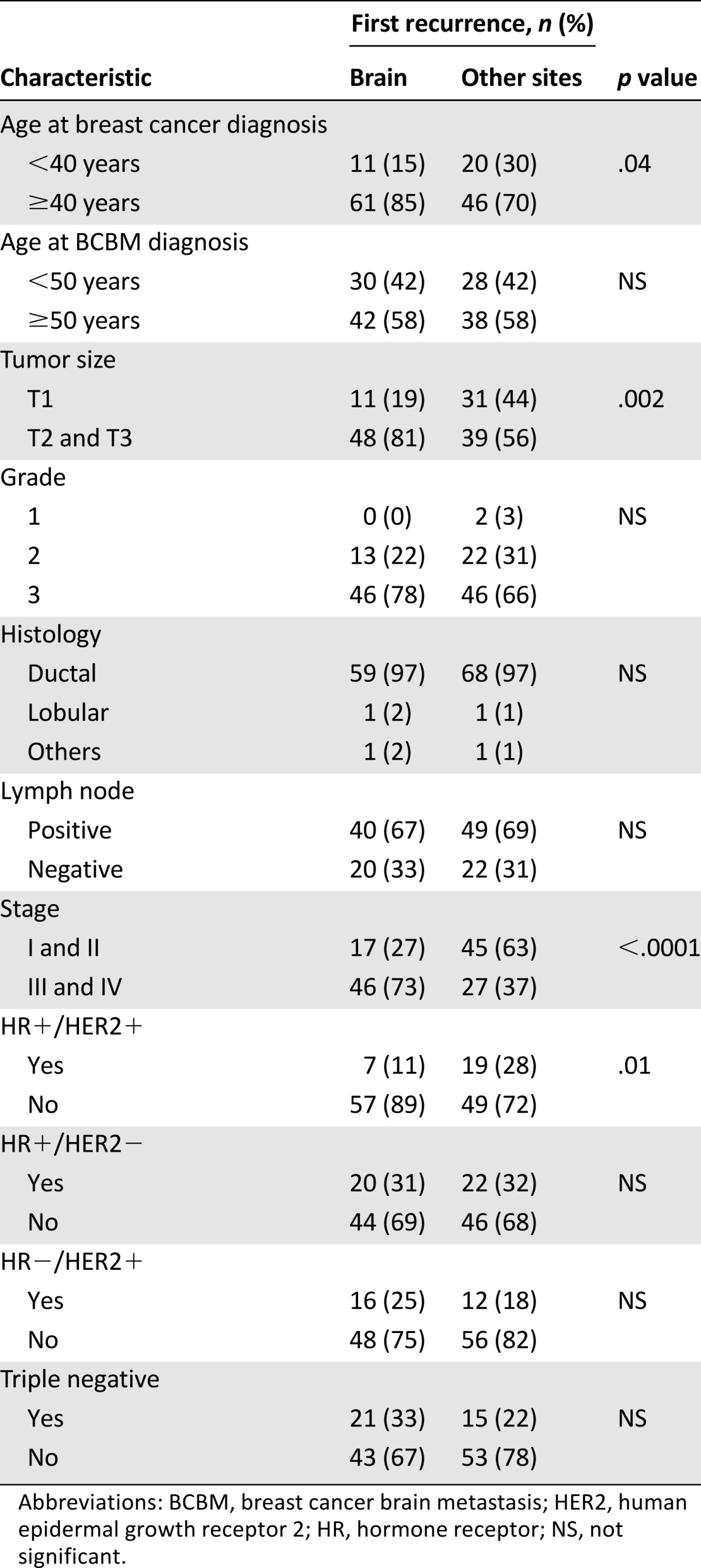
Overall, 51% (n = 71) of patients had a solitary brain metastasis. Brain was the first site of recurrence in 52% (n = 72) of patients. Older age (≥40 years) at diagnosis of breast cancer (p = .04), larger tumor size (T2 and T3; p = .002), advanced baseline stage (III and IV; p < .0001), and HR+HER2+ subtype (p = .01) were more frequently found in patients who developed BM as a first recurrence compared with those who had a first recurrence at other sites in the body (Table 2).
Table 2.
Comparison of clinicopathologic parameters in patients with breast cancer who developed brain metastases as the first site of recurrence with those who had a first recurrence at other sites of the body
Brain Metastasis Treatment
Most patients received multimodality treatment. Significantly, all patients (n = 140) underwent craniotomy either as initial therapy or at the time of brain tumor recurrence. Furthermore, 36% (n = 50) of patients also underwent stereotactic radiosurgery, and 78% of patients had brain radiation therapy, with 108 patients receiving whole-brain radiotherapy and 2 patients receiving focal brain radiation. Regarding systemic treatment, 71% (n = 100) of patients received more than one cycle of chemotherapy after the diagnosis of BCBM. Among patients with HR-positive tumors, 52% (n = 34) received adjuvant hormonal therapy. Among patients with HER2-positive tumors, 74% (n = 43) received HER2-targeted therapy after the diagnosis of BCBM.
Survival Following BCBM
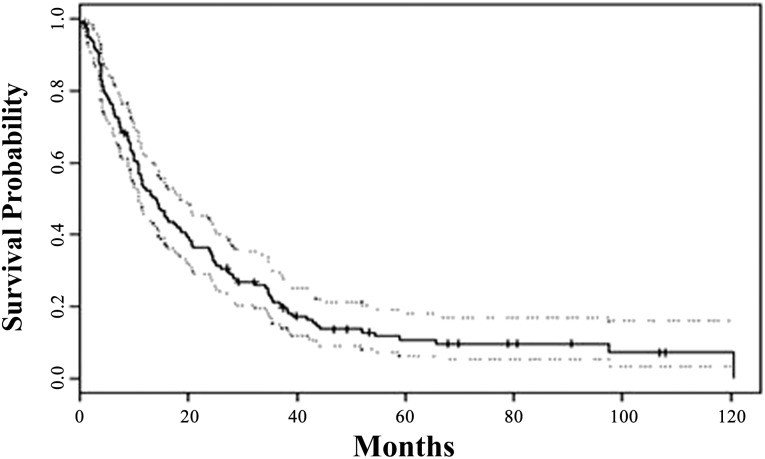
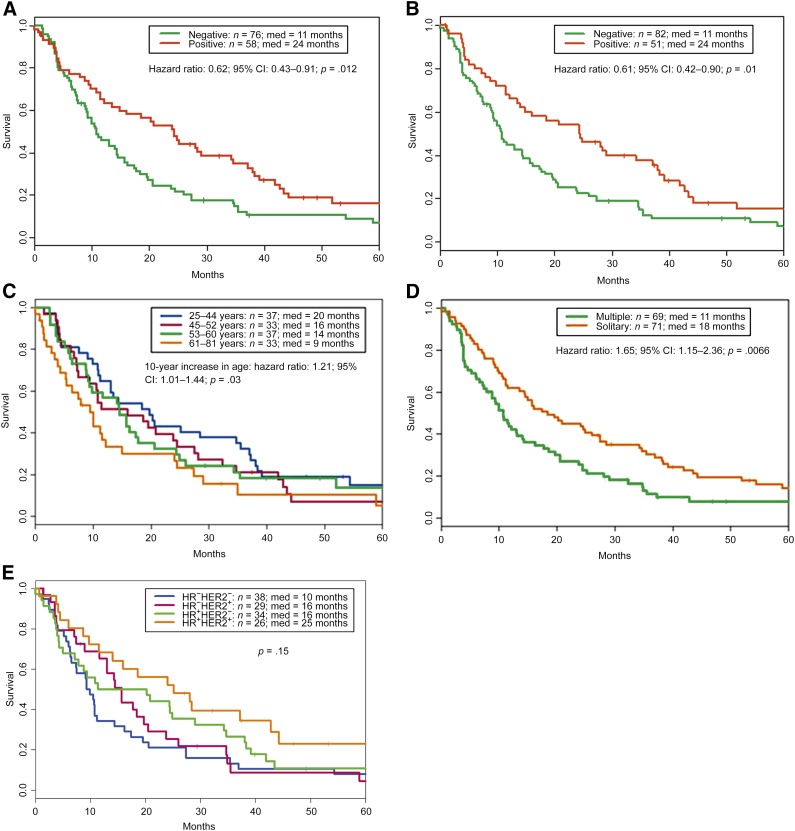
By the end of follow-up, 123 patients (88%) had died. The median overall survival after BM was 14 months (range: 0–121 months) (Fig. 1). In the univariate analysis, ER-positive and PR-positive patients had longer survival after the diagnosis of BM than patients who were ER negative (24 vs. 11 months; p = .01) (Fig. 2A) or PR-negative (24 vs. 11 months; p = .01) (Fig. 2B). There was a trend toward increased survival in HER2-positive and non-TN patients compared with HER2-negative and TN patients, respectively, but this did not reach statistical significance (18 months for HER2-positive vs. 11 months for HER2-negative, p = .26; 16 months for non-TN vs. 10 months for TN, p = .12). Additional predictors of prolonged survival in univariate analysis included younger age at diagnosis of BM (p = .03 for age as a continuous variable) (Fig. 2C) and solitary brain metastasis (18 vs. 11 months; p = .01) (Fig. 2D). The overall survival following BM was not significantly different when patients were grouped based on the joint values of HR and HER2 status (p = .15) (Fig. 2E). In addition, no difference in survival after BM was observed between patients with stage IV disease and patients with stage I, II, or III disease (median survival was 11.0 months for patients with stage IV disease vs. 15.5 months for patients with stage I, II, or III disease).
Figure 1.
Kaplan-Meier survival estimate from the time of diagnosis of brain metastasis. The median overall survival was 14 months (solid line), and the 95% confidence interval was 11–19 months (dotted lines).
Figure 2.
Kaplan-Meier survival estimates according to the status of ER (A), PR (B), age at diagnosis of brain metastasis (C), number of brain metastases (D), and subtypes of breast cancer based on the joint values of HR and HER2 status (E). The p values were determined using the log-rank test.
Abbreviations: +, positive; −, negative; CI, confidence interval; ER, estrogen receptor; HER2, human epidermal growth receptor 2; HR, hormone receptor; med, median survival; PR, progesterone receptor.
In the multivariate Cox analysis, predictors of longer survival were HER2 positivity in the breast tumors, younger age at diagnosis of BM, and solitary brain lesion (Table 3).
Table 3.
Multivariate Cox analysis of survival after diagnosis of brain metastases
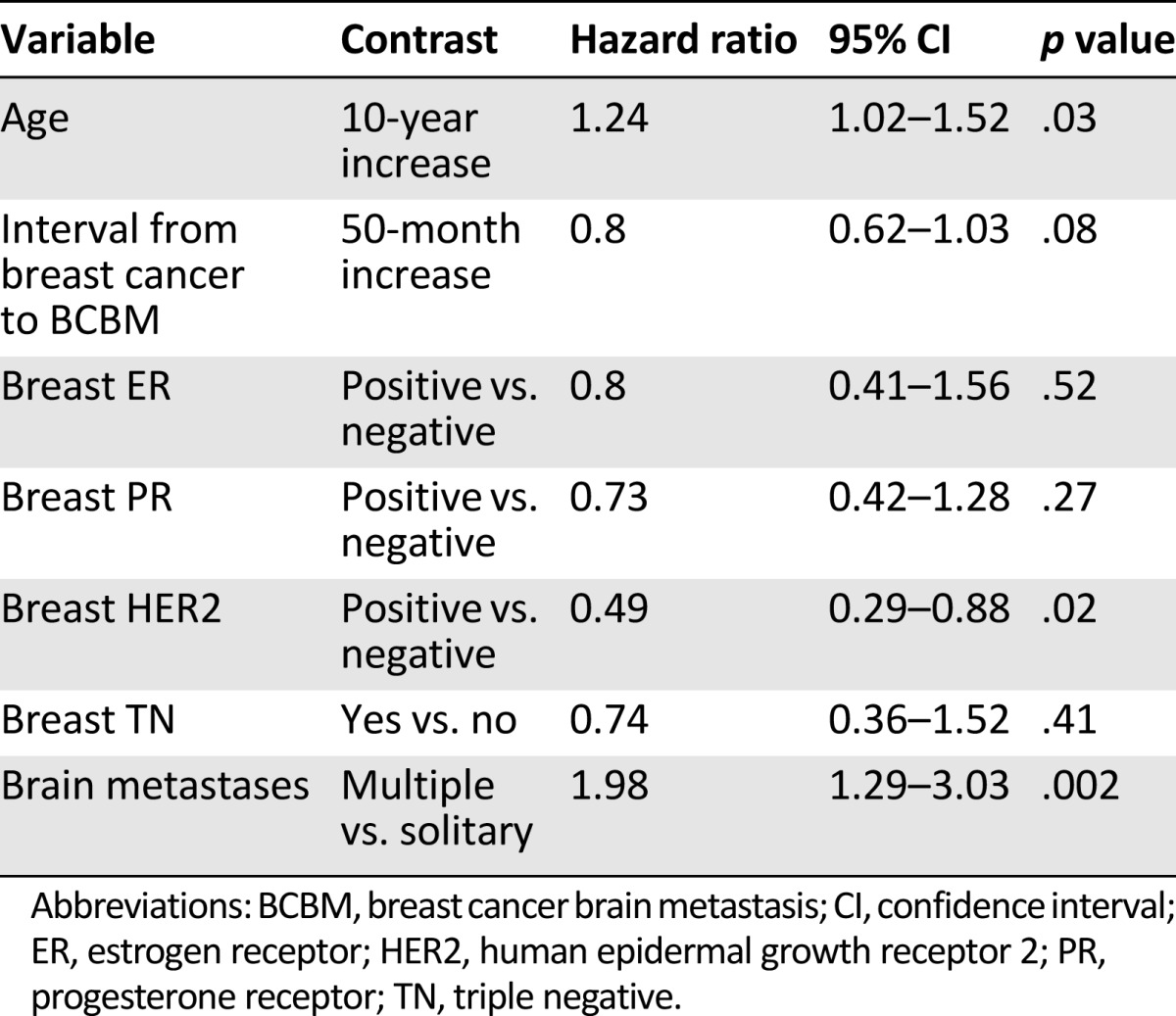
Patients With Survival Longer Than 36 Months Following BM
Of the 140 patients, 19% (n = 27) were alive at least 36 months following diagnosis of BM, and 7% (n = 10) were alive at least 60 months after this diagnosis. Of these 27 patients, 77% (n = 20) had ER-positive disease, 70% (n = 18) had PR-positive disease, 44% (n = 11) had HER2-positive disease, and 74% (n = 20) had a solitary brain lesion.
Paired Analysis of HR and HER2 Expression
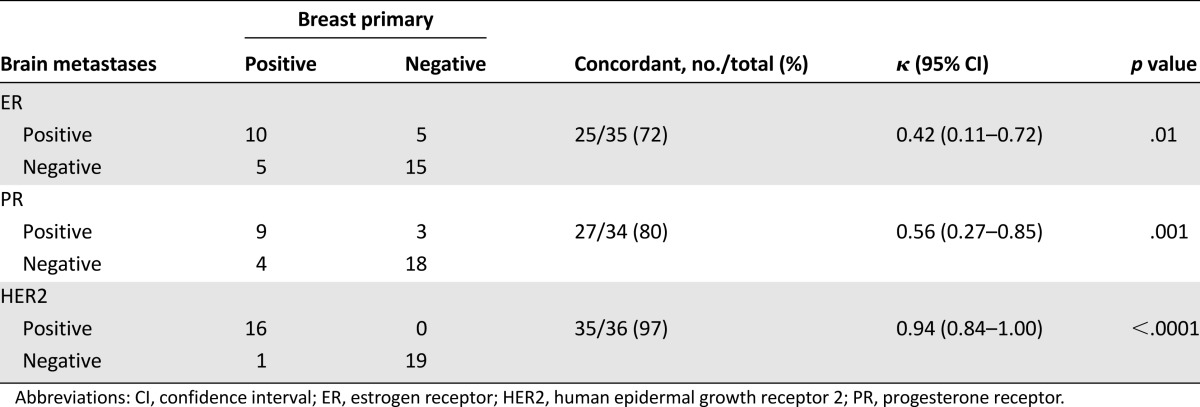
In 35 patients, ER status was known for both the primary tumor and BM. Discordance in ER status was seen in 28% (n = 10) of patients. Among these, 14% (n = 5) had an ER-positive primary tumor but ER-negative metastases; 14% (n = 5) had an ER-negative primary tumor but ER-positive metastases (Table 4). In 34 patients, PR status was known for both the primary tumor and BM. Discordance in PR status was seen in 20% (n = 7) of patients. Among these, 11% (n = 4) of patients had a PR-positive primary tumor but PR-negative metastases; 9% (n = 3) had a PR-negative primary tumor but PR-positive metastases (Table 4).
Table 4.
Paired analysis of hormone receptors and HER2 expression in the breast tumors and corresponding brain metastases
In clinical practice, HR status is used to determine patient eligibility for hormone therapy [9]; therefore, we also analyzed how frequently the HR status changed between the primary tumor and BM. Among 18 patients with an HR-positive primary tumor, 4 patients (22%) changed to negative status (positive/negative) and 14 patients remained HR positive (positive/positive) in BM. Among 17 patients with an HR-negative primary tumor, 7 patients (41%) changed to positive status (negative/positive) and 10 patients remained HR negative (negative/negative) in BM. There was no significant difference in the median survival among these four subgroups (16 months for positive/negative, 29 months for positive/positive, 11 months for negative/positive, and 20 months for negative/negative).
In 36 patients, HER2 status was known for both the primary tumor and BM. Discordance in HER2 status was observed in only 1 (3%) of these 36 patients. This patient had HER2-positive breast tumor but HER2-negative BM. None of the patients with a HER2-negative primary tumor developed HER2-positive BM (Table 4).
Discussion
In this cohort study of 140 breast cancer patients with histologically confirmed BM, we found that, consistent with prior studies, most patients had high-grade tumors (grade 3, 67%) with positive nodal status (64%) at the time of breast cancer diagnosis. Among these patients, ∼60% were HR negative, 44% were HER2-positive, and 28% were TN. Hess and Esteva retrospectively studied 11,011 women diagnosed with breast cancer between 1997 and 2012 at MDACC who had data on HR and HER2 status [10]. The authors reported that among this unselected group of breast cancer patients, 26% were HR negative, 17% were HER2-positive, and 20% were TN. Consequently, the proportions of HR-negative, HER2-positive, and TN tumors noted in patients with breast cancer who had brain metastases were much higher than those in the unselected group of breast cancer patients at the same institution. Our results are thus in agreement with the observations of other studies and support the notion that negative HR, positive HER2, and TN status are risk factors for BCBM [5].
Three decades ago, DiStefano et al. [11] reported median overall survival of 4 months in a study of 101 patients with BCBM who were followed at MDACC. Later, Altundag et al. [12] reported median survival of 6.8 months in a cohort of 420 patients diagnosed with BCBM between 1994 and 2004 at MDACC. It is interesting to note that in the present study, median survival following BM had increased to 14 months. This was much higher than the previously published MDACC data and also higher than what has been reported in recent studies performed at other institutions [2, 13–16]. The reasons behind this better survival may include the availability of more sensitive imaging techniques and the use of more efficacious neurosurgical, radiosurgical, and systemic treatment modalities. It is also noteworthy that in comparison to other studies, our cohort included a much higher proportion of patients with only a single brain lesion, and all 140 patients in our cohort underwent craniotomy of their BM. It has been well established that achieving local control in the brain confers improved survival [17]. Two randomized trials demonstrated that in patients with BCBM, surgical resection plus radiotherapy led to longer survival compared with radiotherapy alone [18, 19]. In line with our data, superior median survival of 19 months was reported by Brogi et al. in a selected group with craniotomy performed in all 209 patients included following a diagnosis of BM [20].
In the current study, we found that, in addition to patient age and number of brain metastases, HER2 status was a strong predictor of survival after BM. Patients with HER2-positive disease survived significantly longer than those who had HER2-negative disease (18 vs. 11 months; p = .02). This observation is consistent with findings from recent studies that examined the effects of HER2 expression on the outcome of patients after the diagnosis of BM. In a cohort of 60 patients treated with stereotactic radiosurgery, O’Meara et al. [21] demonstrated an extended 1-year survival rate in HER2-positive patients treated with trastuzumab compared with similar patients with HER2-negative disease (78% vs. 55%; p = .02). Similarly, Kirsch et al. [22] retrospectively studied a cohort of 108 patients with BCBM and reported significantly better median survival in patients with HER2-positive tumors compared with those with HER2-negative tumors (22 vs. 9 months; p = .0002). To further address whether this survival advantage was attributed to HER2-targeted therapy, we divided the HER2-positive patients into subgroups of those who received anti-HER2 treatment after BCBM and those who did not and then compared their survival rates. We found that HER2-positive patients who received HER2-targeted therapy had a median survival of 21 months, which was markedly longer than survival of both HER2-negative patients (11 months; p = .09) and those who had HER2-positive disease but did not receive HER2-targeted therapy (7 months; p = .003) following BM (data not shown). This observation, together with data from other studies [14, 22–24], strongly indicates that the survival benefit is not due to HER2-associated biological properties of the tumors but rather largely reflects better disease control related to anti-HER2 therapy.
How anti-HER2 therapy extends the survival of patients with HER2-positive disease is not completely clear. Previously, it was believed that the survival advantage resulted exclusively from sustained antitumor activity at extracranial sites [25] because trastuzumab, the most commonly used anti-HER2 antibody, cannot effectively penetrate the brain-blood barrier (BBB). Recently, however, multiple lines of evidence suggest that trastuzumab may also contribute to intracranial tumor control in patients with BCBM. In animal models, brain metastases measuring >5 mm are associated with longer junctions between endothelial cells, impaired interactions between pericytes and glia, and disruption of the BBB in the peritumoral vessels [26]. Whole-brain radiotherapy, the current standard of care for patients with BM, has been shown to breach the BBB and facilitate delivery of therapeutic agents into brain [27]. In a pilot study, Stemmler et al. reported a five- to sixfold increase in the trastuzumab level in cerebrospinal fluid after completion of whole-brain radiotherapy in patients with BCBM [28]. In keeping with these findings, studies by Park et al. showed significant prolongation of time to progression of intracranial tumors in patients who received trastuzumab after BM was diagnosed [29]. Lapatinib is a novel tyrosine kinase inhibitor directed against epidermal growth factor receptor (EGFR) and HER2. It has a much lower molecular weight than trastuzumab (943 vs. 148,000 Da) and has been shown to cross the BBB. A recent phase II study has shown that 66% of patients with previously untreated brain metastases from HER2-positive metastatic breast cancer had a central nervous system response to a combination therapy of lapatinib plus capecitabine [30]. Given the intracranial antitumor effect of anti-HER2 therapy, it is important to establish HER2 status not only in the primary tumor but also in the BM to assist in treatment selection.
In the paired biomarkers analysis, we found high concordance between HER2 status of the primary and metastatic brain tumors (97% concordance by immunohistochemistry [IHC] and FISH; 35 of 36 paired breast and brain tumors). The only discordant samples were from a patient with HER2-positive breast cancer who, after being treated with neoadjuvant chemotherapy, mastectomy, and chest wall radiation, subsequently developed HER2-negative BM. Similar to our findings, highly concordant HER2 status between the breast and corresponding brain disease was also found in studies by Fuchs et al. (97% concordance by IHC and FISH; 28 of 29 paired breast and brain tumors) [31] and Lear-Kaul et al. (100% concordance by FISH; 10 of 10 paired breast and brain tumors) [32]. Taken together, these data suggest that HER2 testing of primary breast tumors is predictive of the HER2 status of BM, and a biopsy of brain lesion only for the purpose of determining HER2 status might not be always needed, although larger series are warranted to confirm these data.
In this study, discordant ER and PR status, with both loss and acquisition of ER and PR, was identified in 28% and 20% of paired primary and brain tumors, respectively. Other studies found levels of HR discordance (18%–54%) similar to our results [8]. The reason for the discordant HR status is not clearly understood. One explanation is intratumoral genomic heterogeneity. Breast cancer is a molecularly heterogeneous disease, with cytogenetically unrelated clones found unevenly distributed in half of all breast tumors [33]. A clone that has the potential to metastasize may be present as a small proportion in the primary tumor that cannot be sampled or detected. During disease progression, this particular clone evolves, expands, and forms metastatic deposits that are genetically different from the majority of cells in the primary tumor [34]. Prior treatment such as chemotherapy may also elicit changes in HR status [35]. It is certainly possible that in a small percentage of cases, the discordance is solely the result of suboptimal reproducibility of the measurement methods. The prognostic impact of discordance between expression of receptors in the primary and metastatic tumors is not clear. A retrospective study by Lindström et al. showed that discordant cases have worse survival [36], whereas a prospective trial by Amir et al. failed to demonstrate adverse effects on clinical outcome [37]. In our study, 4 of 18 patients with an HR-positive primary tumor changed to HR-negative status in the BM, whereas 7 of 17 patients with an HR-negative primary tumor changed to HR-positive status in the BM; loss or gain of HR expression in the brain metastases appears to have no significant effect on survival after diagnosis of BCBM. Further studies with larger numbers of patients are warranted to confirm this finding.
Conclusion
The results of the current study demonstrate that younger age, solitary brain metastasis, and HER2 positivity of the breast tumors are independent predictors of better survival in patients with BCBM. Expression of HER2 is highly concordant between the paired primary and brain tumors, whereas changes of ER and PR status were observed in a substantial proportion of patients. These findings are important for making effective treatment decisions for patients with BCBM.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgment
This work was supported by the Sheila Wynne Research Fund.
Footnotes
For Further Reading: Polly Niravath, Yee Lu Tham, Tao Wang et al. A Phase II Trial of Capecitabine Concomitantly With Whole-Brain Radiotherapy Followed by Capecitabine and Sunitinib for Brain Metastases From Breast Cancer. The Oncologist 2015;20:13.
Abstract
Background. Brain metastasis from breast cancer presents a significant threat to women’s health and quality of life. Capecitabine and sunitinib have shown some activity in this setting; therefore, we conducted a single-arm phase II trial with these agents.
Methods. Patients with breast cancer and central nervous system (CNS) metastases received whole-brain radiotherapy concurrently with capecitabine (1,000 mg/m2 per day for 14 consecutive days), followed by concomitant capecitabine (2,000 mg/m2 per day for 2 weeks followed by a 1-week break) and sunitinib (37.5 mg daily, continuously). The primary endpoint was progression-free survival (PFS).
Results. Of 25 planned patients that would be required to detect a 4-month improvement (from 5 to 9 months) in median PFS with 80% power, 12 were enrolled, and the study was then closed for slow accrual. Median PFS was 4.7 months, and median overall survival was 10 months. In the CNS, 25% had progressive disease, and 83% experienced extra-CNS progression. The most common side effects were fatigue and nausea.
Conclusion. In 12 evaluable patients studied, concurrent capecitabine and whole-brain radiation followed by capecitabine and sunitinib did not extend PFS over historical rates and was associated with significant toxicity. Our study was small and closed due to slow accrual.
Related Commentary: Susan E. Bates. Central Nervous System Metastasis From Breast Cancer. The Oncologist 2015;20:3–4.
Author Contributions
Conception/Design: Qi Shen, Aysegul A. Sahin, Dima Suki, Kenneth D. Aldape, Raymond Sawaya, Nuhad K. Ibrahim
Provision of study material or patients: Qi Shen, Aysegul A. Sahin, Dima Suki, Raymond Sawaya, Nuhad K. Ibrahim
Collection and/or assembly of data: Qi Shen, Aysegul A. Sahin, Dima Suki, Raymond Sawaya, Nuhad K. Ibrahim
Data analysis and interpretation: Qi Shen, Aysegul A. Sahin, Kenneth R. Hess, Nuhad K. Ibrahim
Manuscript writing: Qi Shen, Aysegul A. Sahin, Kenneth R. Hess, Nuhad K. Ibrahim
Final approval of manuscript: Qi Shen, Aysegul A. Sahin, Kenneth R. Hess, Dima Suki, Kenneth D. Aldape, Raymond Sawaya, Nuhad K. Ibrahim
Disclosures
The authors indicated no financial relationships.
References
- 1.Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 2.Lee SS, Ahn JH, Kim MK, et al. Brain metastases in breast cancer: Prognostic factors and management. Breast Cancer Res Treat. 2008;111:523–530. doi: 10.1007/s10549-007-9806-2. [DOI] [PubMed] [Google Scholar]
- 3.Lin NU, Winer EP. Brain metastases: The HER2 paradigm. Clin Cancer Res. 2007;13:1648–1655. doi: 10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- 4.Leyland-Jones B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J Clin Oncol. 2009;27:5278–5286. doi: 10.1200/JCO.2008.19.8481. [DOI] [PubMed] [Google Scholar]
- 5.Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22:3608–3617. doi: 10.1200/JCO.2004.01.175. [DOI] [PubMed] [Google Scholar]
- 6.Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 7.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 8.Sari E, Guler G, Hayran M, et al. Comparative study of the immunohistochemical detection of hormone receptor status and HER-2 expression in primary and paired recurrent/metastatic lesions of patients with breast cancer. Med Oncol. 2011;28:57–63. doi: 10.1007/s12032-010-9418-2. [DOI] [PubMed] [Google Scholar]
- 9.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 10.Hess KR, Esteva FJ. Effect of HER2 status on distant recurrence in early stage breast cancer. Breast Cancer Res Treat. 2013;137:449–455. doi: 10.1007/s10549-012-2366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiStefano A, Yong Yap Y, Hortobagyi GN, et al. The natural history of breast cancer patients with brain metastases. Cancer. 1979;44:1913–1918. doi: 10.1002/1097-0142(197911)44:5<1913::aid-cncr2820440554>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 12.Altundag K, Bondy ML, Mirza NQ, et al. Clinicopathologic characteristics and prognostic factors in 420 metastatic breast cancer patients with central nervous system metastasis. Cancer. 2007;110:2640–2647. doi: 10.1002/cncr.23088. [DOI] [PubMed] [Google Scholar]
- 13.Niwińska A, Murawska M, Pogoda K. Breast cancer brain metastases: Differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole-brain radiotherapy (WBRT) Ann Oncol. 2010;21:942–948. doi: 10.1093/annonc/mdp407. [DOI] [PubMed] [Google Scholar]
- 14.Eichler AF, Kuter I, Ryan P, et al. Survival in patients with brain metastases from breast cancer: The importance of HER-2 status. Cancer. 2008;112:2359–2367. doi: 10.1002/cncr.23468. [DOI] [PubMed] [Google Scholar]
- 15.Arslan UY, Oksuzoglu B, Aksoy S, et al. Breast cancer subtypes and outcomes of central nervous system metastases. Breast. 2011;20:562–567. doi: 10.1016/j.breast.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa K, Yoshii Y, Nishimaki T, et al. Treatment and prognosis of brain metastases from breast cancer. J Neuro-Oncol. 2008;86:231–238. doi: 10.1007/s11060-007-9469-1. [DOI] [PubMed] [Google Scholar]
- 17.Wroński M, Arbit E, McCormick B. Surgical treatment of 70 patients with brain metastases from breast carcinoma. Cancer. 1997;80:1746–1754. doi: 10.1002/(sici)1097-0142(19971101)80:9<1746::aid-cncr8>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 18.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 19.Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al. Treatment of single brain metastasis: Radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33:583–590. doi: 10.1002/ana.410330605. [DOI] [PubMed] [Google Scholar]
- 20.Brogi E, Murphy CG, Johnson ML, et al. Breast carcinoma with brain metastases: Clinical analysis and immunoprofile on tissue microarrays. Ann Oncol. 2011;22:2597–2603. doi: 10.1093/annonc/mdr022. [DOI] [PubMed] [Google Scholar]
- 21.O’Meara WP, Seidman AD, Yamada Y, et al. Impact of HER2 status in breast cancer patients receiving stereotactic radiosurgery for brain metastases. J Clin Oncol. 2005;23:637. [Google Scholar]
- 22.Kirsch DG, Ledezma CJ, Mathews CS, et al. Survival after brain metastases from breast cancer in the trastuzumab era. J Clin Oncol. 2005;23:2114–2116; author reply 2116–2117. doi: 10.1200/JCO.2005.05.249. [DOI] [PubMed] [Google Scholar]
- 23.Church DN, Modgil R, Guglani S, et al. Extended survival in women with brain metastases from HER2 overexpressing breast cancer. Am J Clin Oncol. 2008;31:250–254. doi: 10.1097/COC.0b013e31815a43c4. [DOI] [PubMed] [Google Scholar]
- 24.Dawood S, Broglio K, Esteva FJ, et al. Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol. 2008;19:1242–1248. doi: 10.1093/annonc/mdn036. [DOI] [PubMed] [Google Scholar]
- 25.Gori S, Rimondini S, De Angelis V, et al. Central nervous system metastases in HER-2 positive metastatic breast cancer patients treated with trastuzumab: Incidence, survival, and risk factors. The Oncologist. 2007;12:766–773. doi: 10.1634/theoncologist.12-7-766. [DOI] [PubMed] [Google Scholar]
- 26.Gerstner ER, Fine RL. Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: Establishing a treatment paradigm. J Clin Oncol. 2007;25:2306–2312. doi: 10.1200/JCO.2006.10.0677. [DOI] [PubMed] [Google Scholar]
- 27.van Vulpen M, Kal HB, Taphoorn MJ, et al. Changes in blood-brain barrier permeability induced by radiotherapy: Implications for timing of chemotherapy? (Review) Oncol Rep. 2002;9:683–688. [PubMed] [Google Scholar]
- 28.Stemmler HJ, Schmitt M, Willems A, et al. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs. 2007;18:23–28. doi: 10.1097/01.cad.0000236313.50833.ee. [DOI] [PubMed] [Google Scholar]
- 29.Park IH, Ro J, Lee KS, et al. Trastuzumab treatment beyond brain progression in HER2-positive metastatic breast cancer. Ann Oncol. 2009;20:56–62. doi: 10.1093/annonc/mdn539. [DOI] [PubMed] [Google Scholar]
- 30.Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): A single-group phase 2 study. Lancet Oncol. 2013;14:64–71. doi: 10.1016/S1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs IB, Loebbecke M, Buhler H, et al. HER2 in brain metastases: Issues of concordance, survival, and treatment. J Clin Oncol. 2002;20:4130–4133. doi: 10.1200/JCO.2002.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Lear-Kaul KC, Yoon HR, Kleinschmidt-DeMasters BK, et al. Her-2/neu status in breast cancer metastases to the central nervous system. Arch Pathol Lab Med. 2003;127:1451–1457. doi: 10.5858/2003-127-1451-NSIBCM. [DOI] [PubMed] [Google Scholar]
- 33.Heim S, Teixeira MR, Dietrich CU, et al. Cytogenetic polyclonality in tumors of the breast. Cancer Genet Cytogenet. 1997;95:16–19. doi: 10.1016/s0165-4608(96)00322-6. [DOI] [PubMed] [Google Scholar]
- 34.Kuukasjärvi T, Karhu R, Tanner M, et al. Genetic heterogeneity and clonal evolution underlying development of asynchronous metastasis in human breast cancer. Cancer Res. 1997;57:1597–1604. [PubMed] [Google Scholar]
- 35.Rasbridge SA, Gillett CE, Seymour AM, et al. The effects of chemotherapy on morphology, cellular proliferation, apoptosis and oncoprotein expression in primary breast carcinoma. Br J Cancer. 1994;70:335–341. doi: 10.1038/bjc.1994.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindström LS, Karlsson E, Wilking UM, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol. 2012;30:2601–2608. doi: 10.1200/JCO.2011.37.2482. [DOI] [PubMed] [Google Scholar]
- 37.Amir E, Miller N, Geddie W, et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J Clin Oncol. 2012;30:587–592. doi: 10.1200/JCO.2010.33.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]