Abstract
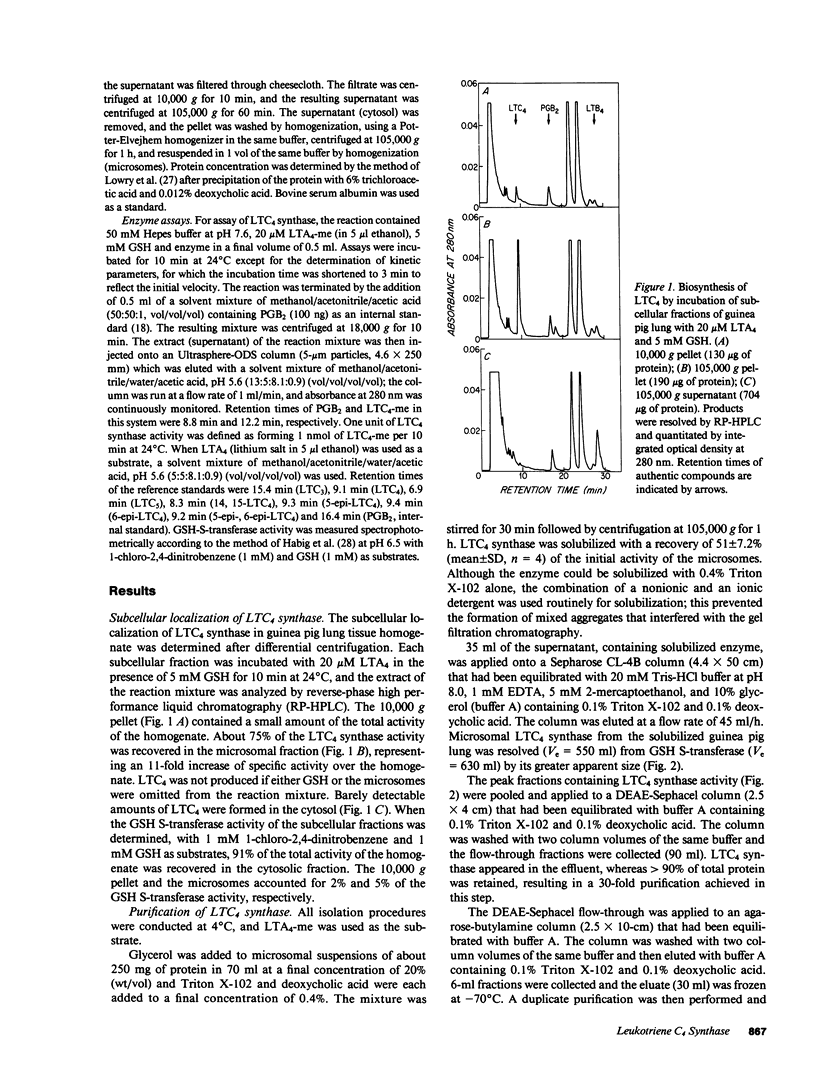
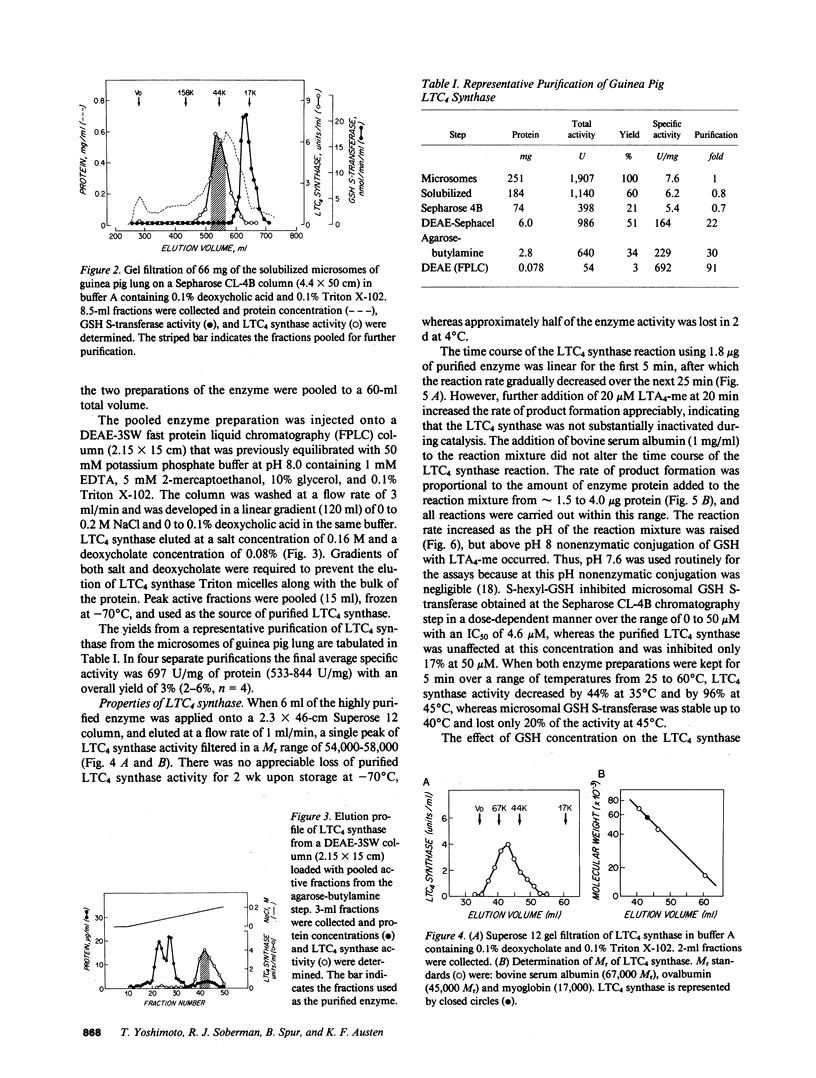
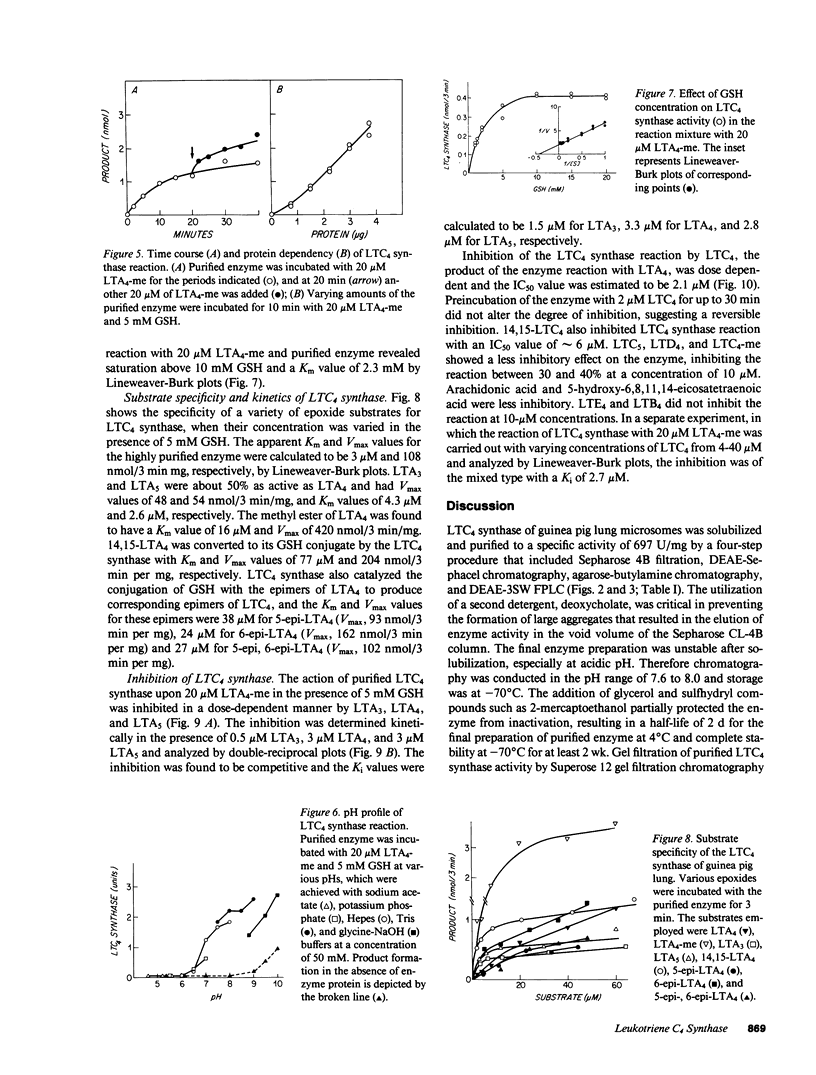
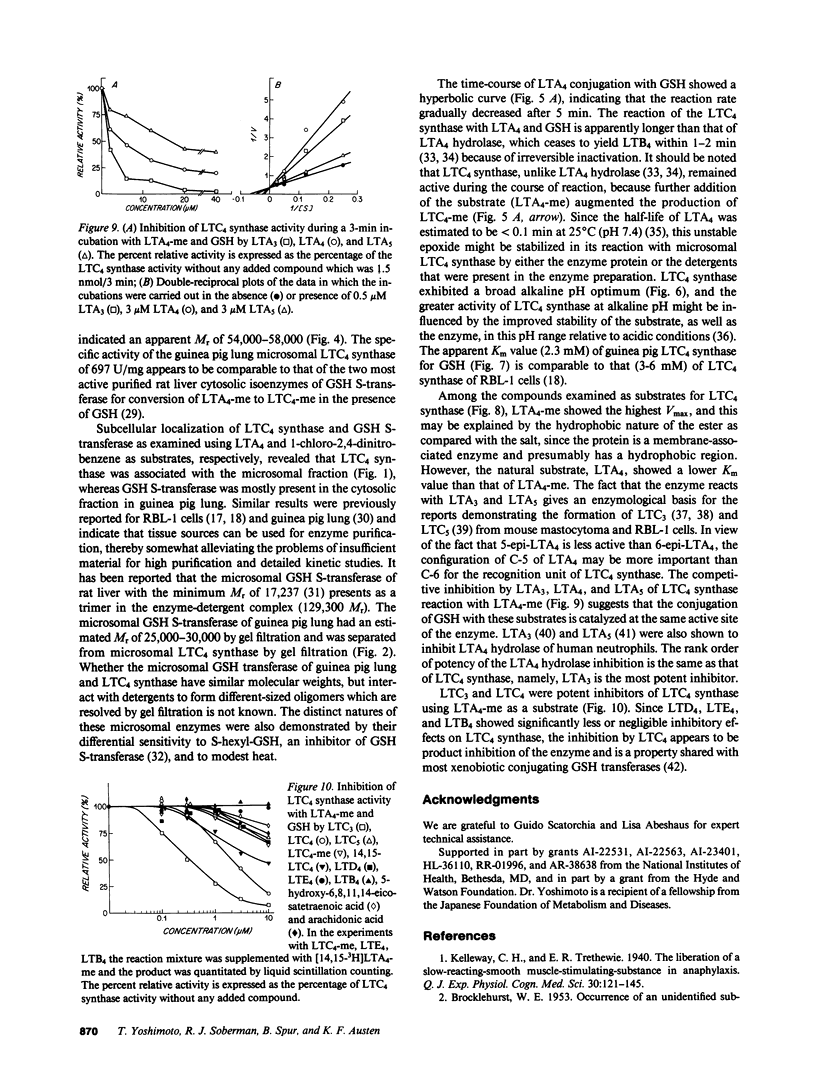
Leukotriene C4 (LTC4) synthase, which conjugates LTA4 and LTA4-methyl ester (LTA4-me) with glutathione (GSH) to form LTC4 and LTC4-me, respectively, has been solubilized from the microsomes of guinea pig lung and purified 91-fold in four steps to a specific activity of 692 nmol/10 min per mg protein using LTA4-me as substrate. LTC4 synthase of guinea pig lung was separated from microsomal GSH S-transferase by Sepharose CL-4B chromatography and further purified by DEAE-Sephacel chromatography, agarose-butylamine chromatography, and DEAE-3SW fast-protein liquid chromatography. It was also differentiated from the microsomal GSH S-transferase, which utilized 1-chloro-2,4-dinitrobenzene as a substrate, by its heat lability and relative resistance to inhibition by S-hexyl-GSH. The Km value of guinea pig lung LTC4 synthase for LTA4 was 3 microM and the Vmax was 108 nmol/3 min per microgram; the Km values for LTA3 and LTA5 were similar, and the Vmax values were about one-half those obtained with LTA4. The conversion of LTA4-me to LTC4-me was competitively inhibited by LTA3, LTA4, and LTA5, with respective Ki values of 1.5, 3.3, and 2.8 microM, suggesting that these substrates were recognized by a common active site. IC50 values for the inhibition of the conjugation of 20 microM LTA4-me with 5 mM GSH were 2.1 microM and 0.3 microM for LTC4 and LTC3, respectively. In contrast, LTD4 was substantially less inhibitory (IC50 greater than 40 microM), and LTE4 and LTB4 had no effect on the enzyme, indicating that the mixed type product inhibition observed was specific for sulfidopeptide leukotrienes bearing the GSH moiety.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askelöf P., Guthenberg C., Jakobson I., Mannervik B. Purification and characterization of two glutathione S-aryltransferase activities from rat liver. Biochem J. 1975 Jun;147(3):513–522. doi: 10.1042/bj1470513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M. K., Brashler J. R., Morton D. R., Jr Solubilization and characterization of the leukotriene C4 synthetase of rat basophil leukemia cells: a novel, particulate glutathione S-transferase. Arch Biochem Biophys. 1984 May 1;230(2):455–465. doi: 10.1016/0003-9861(84)90426-0. [DOI] [PubMed] [Google Scholar]
- Badr K. F., Baylis C., Pfeffer J. M., Pfeffer M. A., Soberman R. J., Lewis R. A., Austen K. F., Corey E. J., Brenner B. M. Renal and systemic hemodynamic responses to intravenous infusion of leukotriene C4 in the rat. Circ Res. 1984 May;54(5):492–499. doi: 10.1161/01.res.54.5.492. [DOI] [PubMed] [Google Scholar]
- Drazen J. M., Austen K. F., Lewis R. A., Clark D. A., Goto G., Marfat A., Corey E. J. Comparative airway and vascular activities of leukotrienes C-1 and D in vivo and in vitro. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4354–4358. doi: 10.1073/pnas.77.7.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eling T. E., Danilowicz R. M., Henke D. C., Sivarajah K., Yankaskas J. R., Boucher R. C. Arachidonic acid metabolism by canine tracheal epithelial cells. Product formation and relationship to chloride secretion. J Biol Chem. 1986 Sep 25;261(27):12841–12849. [PubMed] [Google Scholar]
- Evans J. F., Nathaniel D. J., Zamboni R. J., Ford-Hutchinson A. W. Leukotriene A3. A poor substrate but a potent inhibitor of rat and human neutrophil leukotriene A4 hydrolase. J Biol Chem. 1985 Sep 15;260(20):10966–10970. [PubMed] [Google Scholar]
- Fitzpatrick F. A., Morton D. R., Wynalda M. A. Albumin stabilizes leukotriene A4. J Biol Chem. 1982 May 10;257(9):4680–4683. [PubMed] [Google Scholar]
- Goetze A. M., Fayer L., Bouska J., Bornemeier D., Carter G. W. Purification of a mammalian 5-lipoxygenase from rat basophilic leukemia cells. Prostaglandins. 1985 May;29(5):689–701. doi: 10.1016/0090-6980(85)90130-3. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hammarström S. Conversion of 5,8,11-eicosatrienoic acid to leukotrienes C3 and D3. J Biol Chem. 1981 Mar 10;256(5):2275–2279. [PubMed] [Google Scholar]
- Hammarström S. Leukotriene C5: a slow reacting substance derived from eicosapentaenoic acid. J Biol Chem. 1980 Aug 10;255(15):7093–7094. [PubMed] [Google Scholar]
- Izumi T., Shimizu T., Seyama Y., Ohishi N., Takaku F. Tissue distribution of leukotriene A4 hydrolase activity in guinea pig. Biochem Biophys Res Commun. 1986 Feb 26;135(1):139–145. doi: 10.1016/0006-291x(86)90953-8. [DOI] [PubMed] [Google Scholar]
- Jakoby W. B. The glutathione S-transferases: a group of multifunctional detoxification proteins. Adv Enzymol Relat Areas Mol Biol. 1978;46:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- Jakschik B. A., Morrison A. R., Sprecher H. Products derived from 5,8,11-eicosatrienoic acid by the 5-lipoxygenase-leukotriene pathway. J Biol Chem. 1983 Nov 10;258(21):12797–12800. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee C. W., Lewis R. A., Corey E. J., Austen K. F. Conversion of leukotriene D4 to leukotriene E4 by a dipeptidase released from the specific granule of human polymorphonuclear leucocytes. Immunology. 1983 Jan;48(1):27–35. [PMC free article] [PubMed] [Google Scholar]
- Leitch A. G., Corey E. J., Austen K. F., Drazen J. M. Indomethacin potentiates the pulmonary response to aerosol leukotriene C4 in the guinea pig. Am Rev Respir Dis. 1983 Oct;128(4):639–643. doi: 10.1164/arrd.1983.128.4.639. [DOI] [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F., Drazen J. M., Clark D. A., Marfat A., Corey E. J. Slow reacting substances of anaphylaxis: identification of leukotrienes C-1 and D from human and rat sources. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3710–3714. doi: 10.1073/pnas.77.6.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Jensson H., Alin P., Orning L., Hammarström S. Transformation of leukotriene A4 methyl ester to leukotriene C4 monomethyl ester by cytosolic rat glutathione transferases. FEBS Lett. 1984 Oct 1;175(2):289–293. doi: 10.1016/0014-5793(84)80753-x. [DOI] [PubMed] [Google Scholar]
- Morgenstern R., DePierre J. W., Jörnvall H. Microsomal glutathione transferase. Primary structure. J Biol Chem. 1985 Nov 15;260(26):13976–13983. [PubMed] [Google Scholar]
- Morris H. R., Taylor G. W., Piper P. J., Tippins J. R. Structure of slow-reacting substance of anaphylaxis from guinea-pig lung. Nature. 1980 May 8;285(5760):104–106. doi: 10.1038/285104a0. [DOI] [PubMed] [Google Scholar]
- Murphy R. C., Hammarström S., Samuelsson B. Leukotriene C: a slow-reacting substance from murine mastocytoma cells. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4275–4279. doi: 10.1073/pnas.76.9.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathaniel D. J., Evans J. F., Leblanc Y., Léveillé C., Fitzsimmons B. J., Ford-Hutchinson A. W. Leukotriene A5 is a substrate and an inhibitor of rat and human neutrophil LTA4 hydrolase. Biochem Biophys Res Commun. 1985 Sep 16;131(2):827–835. doi: 10.1016/0006-291x(85)91314-2. [DOI] [PubMed] [Google Scholar]
- Orning L., Hammarström S., Samuelsson B. Leukotriene D: a slow reacting substance from rat basophilic leukemia cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2014–2017. doi: 10.1073/pnas.77.4.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S. P., MacGlashan D. W., Jr, Schulman E. S., Schleimer R. P., Hayes E. C., Rokach J., Adkinson N. F., Jr, Lichtenstein L. M. Arachidonic acid metabolism in purified human lung mast cells. J Immunol. 1984 Apr;132(4):1972–1979. [PubMed] [Google Scholar]
- Rouzer C. A., Samuelsson B. On the nature of the 5-lipoxygenase reaction in human leukocytes: enzyme purification and requirement for multiple stimulatory factors. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6040–6044. doi: 10.1073/pnas.82.18.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rådmark O., Shimizu T., Jörnvall H., Samuelsson B. Leukotriene A4 hydrolase in human leukocytes. Purification and properties. J Biol Chem. 1984 Oct 25;259(20):12339–12345. [PubMed] [Google Scholar]
- Shimizu T., Rådmark O., Samuelsson B. Enzyme with dual lipoxygenase activities catalyzes leukotriene A4 synthesis from arachidonic acid. Proc Natl Acad Sci U S A. 1984 Feb;81(3):689–693. doi: 10.1073/pnas.81.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda N., Kaneko S., Yoshimoto T., Yamamoto S. Purification of arachidonate 5-lipoxygenase from porcine leukocytes and its reactivity with hydroperoxyeicosatetraenoic acids. J Biol Chem. 1986 Jun 15;261(17):7982–7988. [PubMed] [Google Scholar]
- Wu C. Conversion of leukotrienes A4 to C4 in cell-free systems. Biochem Biophys Res Commun. 1986 Jan 14;134(1):85–92. doi: 10.1016/0006-291x(86)90530-9. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Soberman R. J., Lewis R. A., Austen K. F. Isolation and characterization of leukotriene C4 synthetase of rat basophilic leukemia cells. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8399–8403. doi: 10.1073/pnas.82.24.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]


