This study presents the largest reported series of patients treated with pemetrexed. The results show that although well-tolerated, pemetrexed had limited efficacy. They also suggest a role for neutrophil-lymphocyte in the prognostication of advanced urothelial cancer, which may be relevant when constructing prognostic models in future studies. There is an urgent need to develop novel therapies for this lethal disease.
Keywords: Pemetrexed, Bladder cancer, Urothelial carcinoma
Abstract
Background.
Pemetrexed is a commonly used treatment for platinum-resistant advanced urothelial carcinoma (UC) based on objective response rates of 8% and 28% in two small phase II studies. To address the discrepancy in reported response rates and to assess efficacy and toxicity outside of a clinical trial setting, we performed a large retrospective analysis of pemetrexed use at Memorial Sloan Kettering Cancer Center. We also investigated candidate prognostic factors for overall survival in this setting to explore whether the neutrophil-lymphocyte ratio (NLR) had independent prognostic significance.
Patients and Methods.
Patients receiving pemetrexed for platinum-resistant advanced UC between 2008 and 2013 were identified. The Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) were used to determine response rate. Kaplan-Meier and Cox regression analyses were used to examine the association of various factors with efficacy and survival outcomes. Hematologic toxicity and laboratory abnormalities were recorded.
Results.
One hundred and twenty-nine patients were treated with pemetrexed. The objective response rate was 5% (95% confidence interval: 1%–9%), and the median duration of response was 8 months. Median progression-free survival (PFS) was 2.4 months, and the 6-month PFS rate was 14%. There was no significant difference in response rate by age, Eastern Cooperative Oncology Group (ECOG) performance status, or number of prior therapies. On multivariable analysis, ECOG performance status (p < .01), liver metastases (p = .02), and NLR (p < .01) had independent prognostic significance for overall survival.
Conclusion.
This 129-patient series is the largest reported data set describing pemetrexed use in advanced UC. Activity was modest, although discovery of molecular biomarkers predictive of response would be valuable to identify the small subset of patients who do gain significant benefit. Overall, the data highlight the urgent need to develop novel therapies for these patients.
Implications for Practice:
Pemetrexed is a commonly used treatment option for platinum-resistant advanced urothelial cancer, but there are limited data on its efficacy and toxicity. This study presents the largest reported series of 129 patients treated with this agent. The results show that although well-tolerated, pemetrexed had limited efficacy, with an objective response rate of 5% and median progression-free survival of 2.4 months. The data also suggest a role for neutrophil-lymphocyte ratio in the prognostication of advanced urothelial cancer, which may be relevant when constructing prognostic models in future studies.
Introduction
Urothelial carcinoma (UC) is diagnosed in approximately 78,000 individuals and accounts for 17,000 deaths in the U.S. each year [1]. The disease normally arises in the bladder, but a minority (<10%) originate in the renal pelvis or upper urinary tracts [1]. The most commonly used first-line treatment for locally advanced or metastatic UC is cisplatin/gemcitabine combination chemotherapy, for which there is a 50% response rate, median progression-free survival of 8 months, and median overall survival of 14 months [1]. Long-term survival is rare, with the majority of patients eventually succumbing to their disease, and only 13% surviving beyond 5 years.
There are no Food and Drug Administration (FDA)-approved second-line treatment options after progression on platinum-based chemotherapy for advanced UC. Vinflunine is approved for use in Europe based on a phase III clinical trial in which it was compared with best supportive care and showed improvements in response rate (9% vs. 0%, p = .01), median progression-free survival (3.0 vs. 1.5 months, p = .001), and a nonstatistically significant improvement in median overall survival (6.9 vs. 4.6 months, p = .28) [2]. This agent is not available in the U.S.; approval was not granted because the registration trial did not meet the predetermined primary endpoint of improved overall survival.
Commonly used chemotherapy options in platinum-resistant advanced UC include paclitaxel and docetaxel, for which single-arm phase II trials reported response rates of 10%–20% and median overall survival (OS) of 6–9 months [3, 4]. Pemetrexed (an intravenously administered antifolate antimetabolite) is commonly used based on modest activity and good tolerability reported in two single-arm phase II trials [5, 6]. The first study enrolled 45 patients and reported a 28% response rate, median progression-free survival (PFS) of 2.9 months, and median OS of 9.6 months [6]. The second trial enrolled 13 patients and reported an 8% response rate, with 2 patients (15%) achieving disease control for ≥6 months [5]. This trial was intended as a two-stage design but did not meet minimal threshold of activity at interim analysis to continue to the second stage of accrual.
A number of recent publications have attempted to define the most robust prognostic factors in advanced UC [7–11], and candidates include age (65 years or younger vs. older than 65 years), Eastern Cooperative Oncology Group (ECOG) performance status, line of chemotherapy (second or third line vs. fourth or line later), presence of liver metastases, presence of any visceral metastases, time from prior chemotherapy (3 months or less vs. more than 3 months), hemoglobin (10 g/dL or less vs. more than 10 g/dL), albumin (lower limit of normal or less vs. above the lower limit of normal), and white blood cell count (lower limit of normal or less vs. above the lower limit of normal). We investigated the prognostic significance of these factors, as well as neutrophil-lymphocyte ratio (NLR), in this setting. High NLR is considered a marker of systemic inflammation, which may drive cancer proliferation by increasing levels of circulating angiogenic and growth factors while decreasing effective cell mediated immunity [12–14]. Previous work in multiple malignancies has shown NLR to have prognostic significance independent of other well-known risk factors [15, 16]. In localized bladder and upper tract urothelial cancer, it was shown to have independent prognostic significance at cut-off values of 2.5 and 3.0 in different studies [17–20].
In an attempt to clarify the discrepancy in pemetrexed efficacy reported in these two trials, as well as to gain an improved understanding of the effectiveness of this commonly used treatment option outside of the clinical trial setting, we performed a retrospective review of outcomes for pemetrexed use in platinum-resistant advanced UC at Memorial Sloan-Kettering Cancer Center. We also examined prognostic factors for overall survival in these patients.
Materials and Methods
Study Population
Patients were included who received single-agent pemetrexed for platinum-resistant metastatic UC (defined here as progressive disease after prior platinum-based chemotherapy in the perioperative or metastatic setting with no specific time interval stipulated). Using our institutional database, all patients who received pemetrexed chemotherapy between 2008 and 2013 and had a documented diagnosis of urothelial carcinoma were identified (n = 195). Of these, 58 were excluded because they received pemetrexed for stage IV non-small cell lung cancer and had a synchronous diagnosis of localized UC. Six patients who had not received prior platinum chemotherapy were excluded: two had received pemetrexed as first-line systemic therapy for metastatic UC, and the other four received pemetrexed as second-line therapy or later after prior first-line treatment with a non-platinum-containing regimen. One patient was excluded because he received only one cycle of pemetrexed and was then switched to a clinical trial at another institution. Another patient was excluded because he was treated with a cisplatin/pemetrexed doublet in the second-line setting. Overall, 129 patients were included in the final analysis.
After receiving institutional review board and ethics board approval, medical records were reviewed to record demographic information and treatment details including gender, age, ECOG performance status, extent of metastatic disease, prior therapies, and baseline laboratory values. Pemetrexed treatment details were recorded including number of cycles, treatment response, date of progression, and date of death. Toxicity data were recorded when possible given the retrospective nature of the study. Specifically, hematologic toxicity rates, febrile neutropenia, frequency and reason for dose reduction, and reasons for treatment discontinuation were recorded.
Treatment and Outcomes
Response to therapy was investigator-assessed, and objective responses were confirmed as per Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) by review of all cross-sectional imaging obtained for response assessment from time of starting pemetrexed therapy to discontinuation of drug by the same research radiologist (J.L.C.) [21]. Prognostic variables described in the prior published literature (as discussed in the Introduction) were recorded and analyzed including NLR. Because the prior literature does not agree on a cut-point for NLR, we investigated it using two previously identified cut-points of 2.5 and 3, as well as on the continuous scale.
Statistical Analysis
Response rates were compared between different prognostic subgroups using Fisher’s exact test. PFS was defined as time from start of pemetrexed to date of disease progression or death. OS was defined as time from start of pemetrexed to date of death. Patients who were still alive or lost to follow-up were censored at date of last follow up. PFS and OS were estimated using the Kaplan-Meier method and by-group comparisons were made using the log-rank test. Variables with potential prognostic significance for overall survival were defined a priori and assessed on univariable analysis. Significant variables on univariable analysis were included in a multivariable Cox regression model. Statistical significance was defined by a p value of <.05. All analyses were conducted using SAS software version 9.2 (SAS Institute, Cary, NC, http://www.sas.com) or R version 3.1.0 (R Core Development, Vienna, Austria, http://www.R-project.org/).
Results
Baseline Patient Characteristics
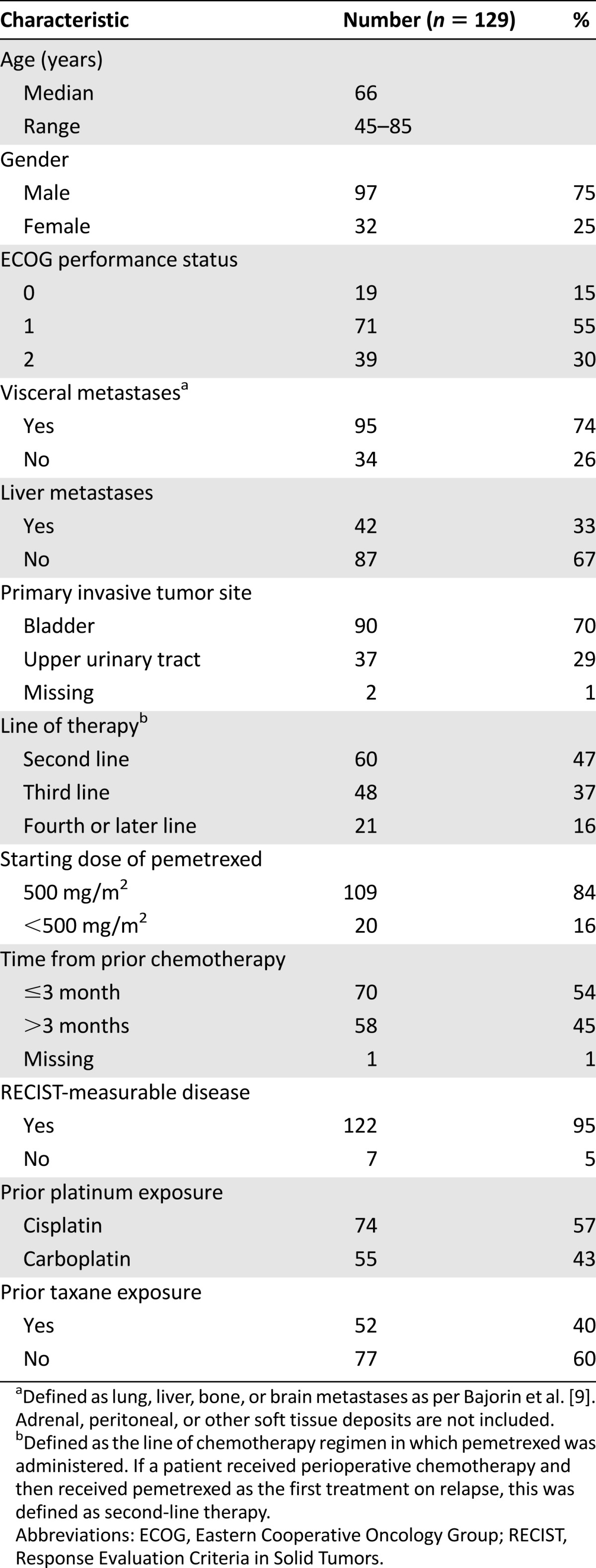
In the final analysis, 129 patients who received single-agent pemetrexed (with vitamin B12 and folic acid support) for platinum-resistant advanced UC between 2008 and 2013 were included. The baseline characteristics are presented in Table 1. At the time of commencing pemetrexed, 33% (n = 42) had liver metastases, and 74% (n = 95) had any visceral metastases (lung, liver, bone, or brain). Of all the patients, 33% (n = 43) had received prior neo-adjuvant platinum-based chemotherapy (data not shown) and 95% (n = 122) had RECIST-measurable disease at baseline (the other 7 patients were excluded from the objective response portion of the analysis). A total of 109 (84%) patients were treated at a dose of 500 mg/m2, which is the FDA-approved dose for use in lung cancer and which was also the dose used in the aforementioned phase II clinical trials for UC [5, 6]. Using a recently published nomogram described by Pond et al. [7], we calculated the expected 6-month PFS for each patient in our cohort. The expected mean 6-month PFS rate was 18%, median 6-month PFS was 17%, and the interquartile range was 10%–20%.
Table 1.
Baseline patient characteristics
Efficacy
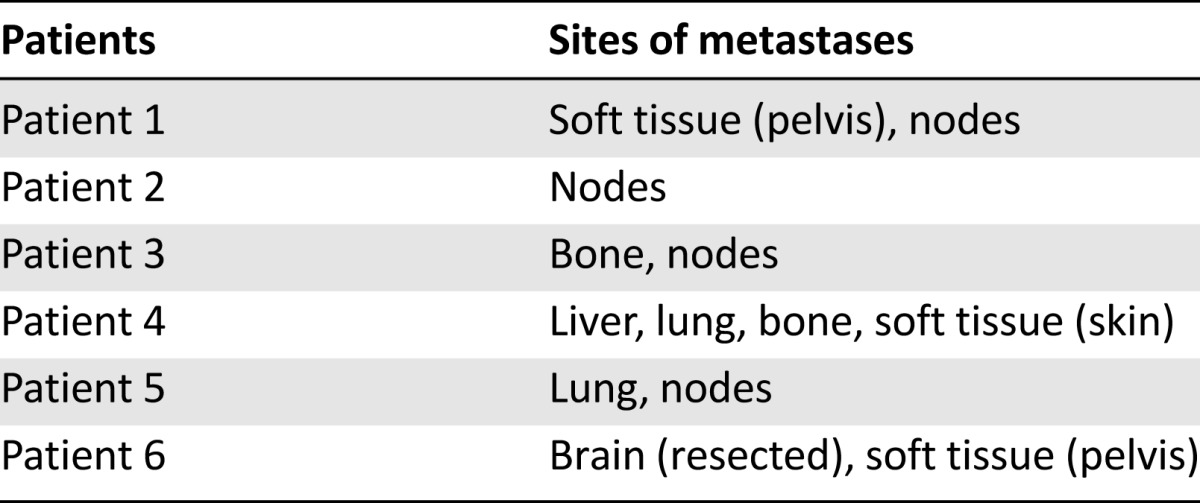
Of 122 patients with RECIST-measurable disease, 6 had a partial response to pemetrexed to give an overall response rate of 5% (95% confidence interval [CI]: 1%–9%; Table 2). The sites of disease for the six patients who responded are outlined in Table 3. Median response duration (defined as time from start of therapy to time of disease progression in those patients who responded) was 8 months (range, 6–18 months). There were no complete responses. At the time of data analysis, 126 patients had progressed, and 109 had died. Median PFS for the whole cohort was 2.4 months (95% CI: 2.0–2.8), 6-month PFS rate was 14% (95% CI: 9%–21%) (Table 2), and median overall survival was 6.7 months (95% CI: 5.6–8.2 months). There was no significant difference in the objective response rate when patients were stratified by ECOG performance status, line of therapy, age, burden of metastatic disease (visceral vs. nonvisceral), starting pemetrexed dose (500 mg/m2 vs. <500 mg/m2), or time from prior chemotherapy (Tables 2 and 3).
Table 2.
Efficacy outcomes for 129 patients treated with pemetrexed
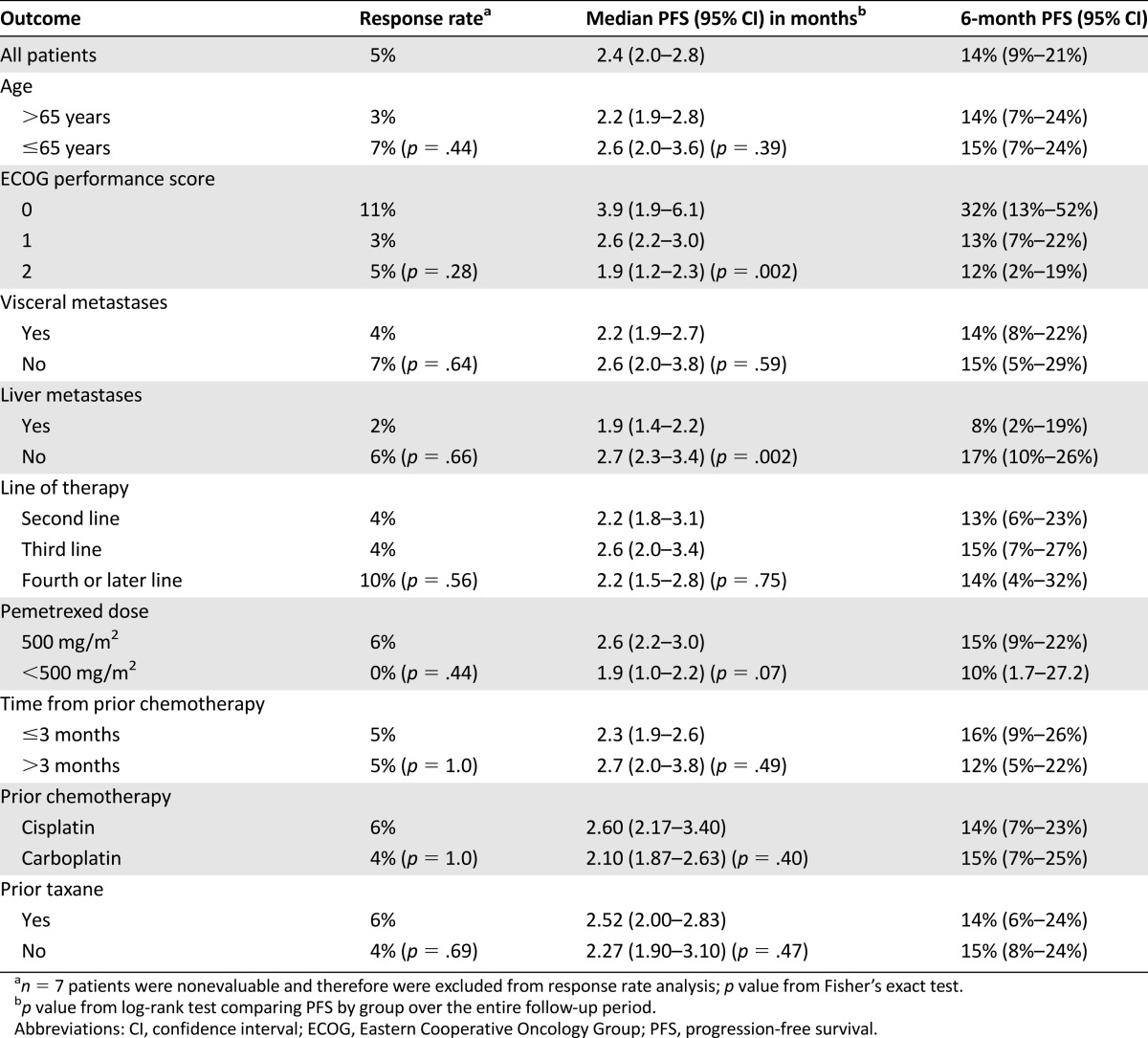
Table 3.
Sites of disease for patients achieving partial response on pemetrexed
PFS was improved in patients with ECOG performance status of 0 (6-month PFS 32%) as compared with those with performance status of 1 (6-month PFS 13%) and 2 (6-month PFS 12%) (log-rank p = .03) and in those without versus with liver metastases (6-month PFS 17% vs. 8%, log-rank p = .002) (Table 2). Of note, among the six patients who were excluded from the overall analysis because they had not received prior platinum, two partial responses were seen. Response durations were 8 and 17 months among these patients (data not shown). Both of these patients had received prior single-agent gemcitabine for metastatic disease, and one had also received prior paclitaxel.
Treatment Administration and Toxicity
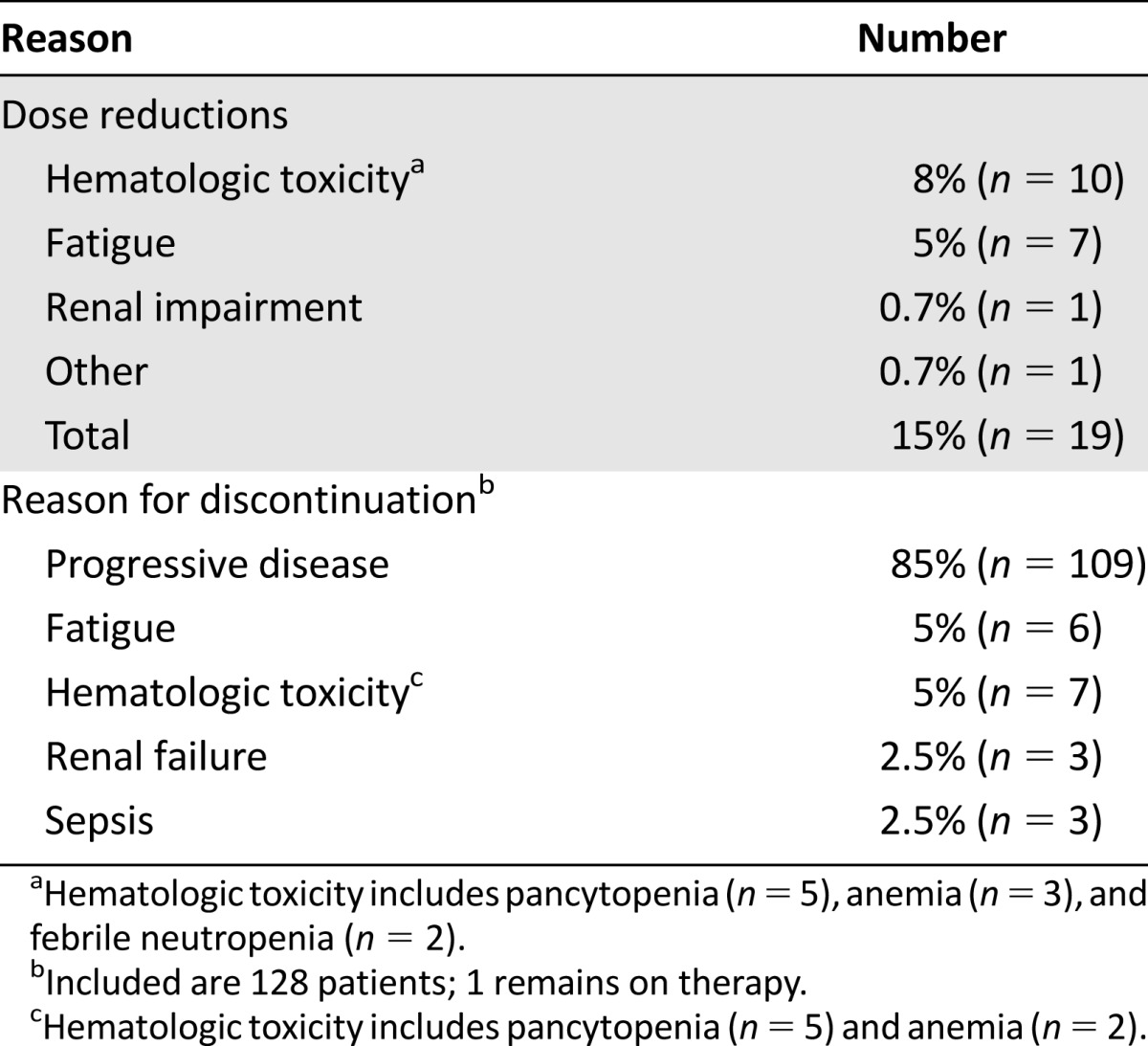
The median number of pemetrexed doses administered was 3 (range, 1–21), and 39% (n = 50) received ≥4 doses. Follow-up laboratory data were available in 126 of 129 patients, and the rates of laboratory toxicities (hematologic and nonhematologic) are reported in Table 4. Grade 3–4 anemia, neutropenia, and thrombocytopenia occurred in 26%, 13%, and 8% of patients, respectively. Of all the patients, 5% developed febrile neutropenia, and 15% (n = 19) required dose reductions during their treatment course (Table 5). The majority of these were for hematologic toxicity (8%, n = 10) and fatigue (5%, n = 7). In total, 85% (n = 109) of patients eventually discontinued chemotherapy because of progressive disease. The others discontinued for hematologic toxicity, fatigue, renal failure, or sepsis (Table 5). In the rare cases in which pemetrexed was discontinued because of renal failure (n = 3) and sepsis (n = 3), it was not clear whether the problems were caused by pemetrexed.
Table 4.
Laboratory toxicity rates for patients treated with pemetrexed
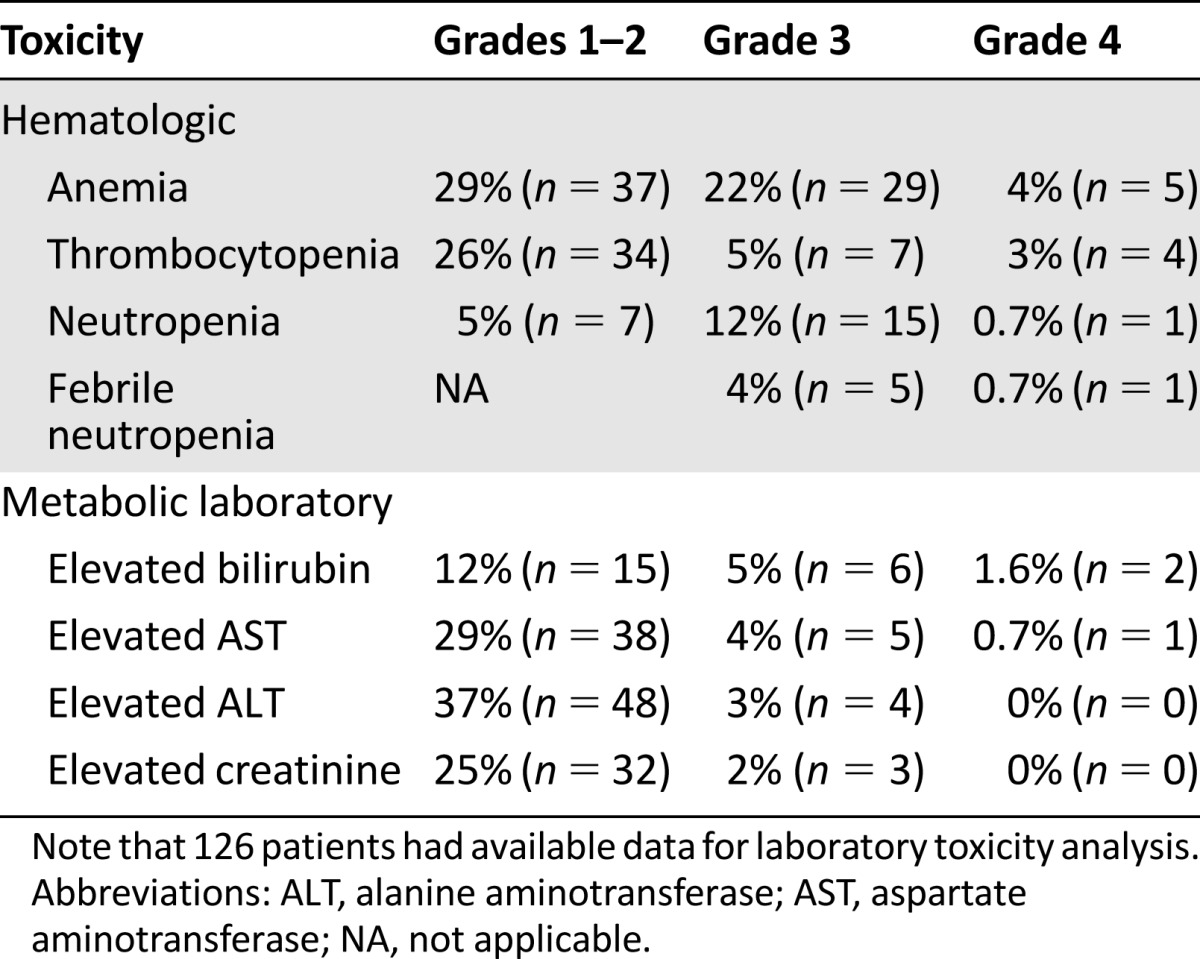
Table 5.
Reasons for dose reductions and treatment discontinuations
Factors Prognostic for Overall Survival
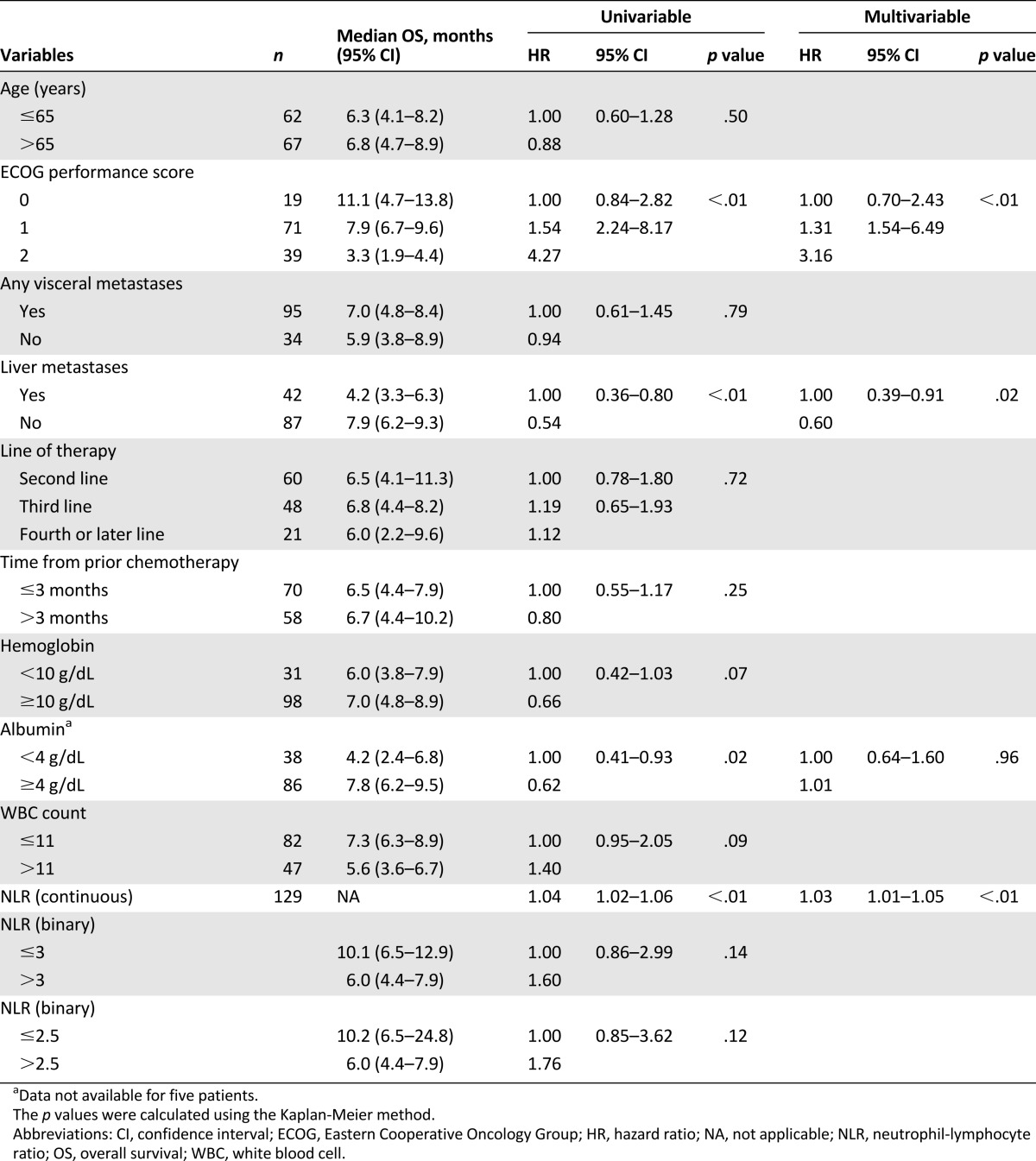
Univariable and multivariable Cox regression analysis for factors associated with overall survival are presented in Table 6. On univariable analysis, ECOG performance status, absence of liver metastases, albumin ≥ 4 g/dL, and lower NLR all conferred improved prognosis. Neither tested cut-point for NLR was statistically significant in this study on univariable analysis (NLR ≤ 3: p = .12; NLR ≤ 2.5: p = .14). On multivariable analysis, ECOG performance status, liver metastases, and NLR (as a continuous variable) remained independently significant (Table 6).
Table 6.
Analysis of prognostic factors for overall survival
Discussion
We report efficacy and toxicity data in the largest published series of patients treated with pemetrexed agent for advanced UC. The reported response rate of 5% is disappointing and highlights the urgent need for novel therapeutics in this disease. The main limitations of the study are its retrospective nature and the fact that it was a single-center experience.
The 5% response rate reported here (95% CI: 1%–9%) is in keeping with the results of the phase II study reported by Galsky et al. (response rate 8%, 90% upper limit of confidence: 29%) but significantly lower than that reported by Sweeney et al. (response rate 28%, 95% confidence interval: 16%–43%). Our cohort and that reported by Galsky et al. [5] had higher rates of baseline visceral metastases (74% and 62%, respectively) compared with 42% in the trial reported by Sweeney et al. [6], which is a possible explanation for this discrepancy. That said, even for the 34 patients in our cohort without visceral metastases, the response rate was only 7%. Similarly, 60% of patients in the Sweeney et al. [6] cohort had ECOG performance status of 0 compared with 15% here. Again, however, when our data were analyzed by subgroup, the response rate was only 11% among the 19 patients with ECOG performance status 0. Indeed, as presented in Table 2, response rates were <15% regardless of the line of therapy, age, ECOG performance status, site of metastases, and time from prior chemotherapy. These lower response rates may also be due to the use of pemetrexed outside of a clinical trial setting with all the inherent (and often unmeasurable) differences in patient population.
Interestingly, of the 6 patients excluded from our cohort because they had not received prior platinum chemotherapy, there were 2 responses (33%), raising the possibility of increased efficacy for pemetrexed in platinum-naïve patients. All of the patients in the Galsky et al. [5] trial received prior platinum therapy, whereas 8 of 47 (17%) in the Sweeney et al. [6] paper did not. These studies did not report response rates stratified by prior platinum therapy, and so it is not possible to ascertain whether the higher response rate seen by Sweeney et al. was due to responses in platinum-naïve patients. Similarly, in another 64-patient phase II study evaluating the combination of pemetrexed and gemcitabine for a mostly platinum-naïve population, the response rate was 28% [22].
Recent data suggest that the 6-month PFS rate is a more robust predictor of overall survival and treatment efficacy than response rate in advanced UC and so should be reported in analyses of treatment efficacy [7, 23]. In a large meta-analysis of phase II clinical trials in this setting, the aggregate 6-month PFS rate was 22% (95% CI: 19%–26%), and as mentioned in the Baseline Patient Characteristics section, a nomogram was created to predict the likelihood of individual patients achieving 6-month PFS [7]. Using the aforementioned nomogram, the mean predicted 6-month PFS rate for our cohort was 18%, and the interquartile range was 10%–20%, suggesting that our patients’ measurable prognostic factors were broadly similar to those entering second-line clinical trials for urothelial cancer [7]. Our observed 6-month PFS rate of 14% (95% CI: 9%–21%) is also in keeping with predicted outcomes based on the nomogram, although trending toward a lower value, which may be because our patients were treated outside of a clinical trial setting and also because many of our patients were treated in the third-line setting or later, whereas the patients used to create the nomogram were treated in the second-line setting. Absence of liver metastases and better ECOG performance status predicted higher likelihood of remaining progression-free in our patients, but these factors are likely to be indicative of more indolent biology rather than predictive biomarkers of treatment benefit.
Pemetrexed was generally well tolerated, with 85% continuing treatment until time of disease progression. Dose reductions were required in 15% of patients mostly for hematologic toxicity or fatigue. Febrile neutropenia was seen in 5% and grade 3–4 thrombocytopenia in 8% compared with rates of 3%–15% and 9%–23% in the previously mentioned phase II studies of pemetrexed in this setting [5, 6]. Of note, among the 34 patients (26%) who developed grade 3–4 anemia (defined as hemoglobin levels of <8.0 g/dL) while on pemetrexed, 31 had grade 1–2 anemia at baseline, which clearly contributed to this high rate.
Given the recent publications of multiple prognostic factors in UC, we sought to validate known factors and identify novel factors in this homogenously treated group of patients. Our data confirm that liver metastases rather than any visceral metastases have independent prognostic significance. Time from prior chemotherapy was recently shown to have independent significance in a large analysis of prognostic factors in this setting, although it did not have significance in our cohort. NLR was shown to have independent prognostic significance in our data set, which is keeping with data from multiple other cancer types and settings. We could not confirm the statistical significance of previously identified NLR cut-points of 2.5 and 3 in our data set [17–19]. However, the fact that NLR remained statistically significant on multivariable analysis when analyzed as a continuous variable indicates that it may have biologic significance in this setting and should be considered for future studies attempting to construct prognostic scores in this disease. C-reactive protein, another marker of systemic inflammation, has also been shown to have prognostic significance in localized bladder cancer and may be worth investigating in the advanced setting [24, 25].
Overall, these data point to pemetrexed, despite being well tolerated, as having at best modest efficacy in an unselected population of platinum-resistant advanced UC. Correlative tissue studies to identify molecular predictors of response to this and other agents may help improve their therapeutic index going forward [26]. We were unable to perform molecular tumor profiling of the responders in our cohort because archival tissue and/or patient consent were unavailable. Indeed a molecular predictor of response to pemetrexed would be highly valuable to identify the small proportion of patients who do respond because they achieve significant benefit from treatment with response durations ranging from 6 to 18 months in this data set. Another promising approach to help direct patients to the most appropriate therapy in the setting of multiple therapeutic options, all with modest likelihood of response, is the use of parallel mouse xenograft studies to allow real-time in vivo testing of tumor sensitivity to a variety of agents [27–29].
A number of promising agents are under investigation for treatment of platinum-resistant advanced UC. Nab-paclitaxel is being further studied (NCT02033993) after a 48-patient single-arm phase II study, and encouraging activity was seen with a response rate of 28% and a 6-month PFS rate of 49% [30]. The PD-L1 monoclonal antibody MPDL3280A is also under investigation, and preliminary experience from a phase I trial reported objective responses in 16 of 30 (52%) UC patients with PD-L1-positive tumors [31]. The search for active agents will continue in coming years.
Conclusion
The data presented here give clinicians a detailed assessment of the efficacy and toxicity of this commonly used treatment option outside of a clinical trial setting. Given the lack of convincing benefit from currently available therapies, enrollment to clinical trials of novel therapies should be encouraged. The data also suggest a role for NLR in the prognostication of advanced platinum-resistant UC, which may be relevant when constructing prognostic models in future studies.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Footnotes
For Further Reading: David D. Chism, Michael E. Woods, Matthew I. Milowsky. Neoadjuvant Paradigm for Accelerated Drug Development: An Ideal Model in Bladder Cancer. The Oncologist 2013;18:933-940.
Recent recommendations to use the neoadjuvant setting in breast cancer as an accelerated drug development pathway make a similar approach in bladder cancer very appealing. The current article will review the rationale for consideration of bladder cancer as the ideal neoadjuvant model for accelerated drug development. Several factors including the ease of bladder tumor tissue collection performed as standard of care, the use of pathologic response as an intermediate marker for overall outcome, and a richer understanding of the important molecular pathways involved in bladder cancer development and progression make the neoadjuvant paradigm particularly relevant. The ability to conduct clinical trials that require fewer patients and efficiently explore disease biology will undoubtedly lead to the development of novel therapies and have a profound effect on every day medical practice.
Author Contributions
Conception/Design: Richard M. Bambury, David J. Benjamin, Joshua L. Chaim, Emily C. Zabor, John Sullivan, Ilana R. Garcia-Grossman, Ashley M. Regazzi, Irina Ostrovnaya, Aryln Apollo, Han Xiao, Martin H. Voss, Gopa Iyer, Dean F. Bajorin, Jonathan E. Rosenberg
Provision of study material or patients: Aryln Apollo, Han Xiao, Martin H. Voss, Gopa Iyer, Dean F. Bajorin, Jonathan E. Rosenberg
Collection and/or assembly of data: Richard M. Bambury, David J. Benjamin, Joshua L. Chaim, Emily C. Zabor, John Sullivan, Ilana R. Garcia-Grossman, Ashley M. Regazzi, Aryln Apollo, Han Xiao, Martin H. Voss, Gopa Iyer, Dean F. Bajorin, Jonathan E. Rosenberg
Data analysis and interpretation: Richard M. Bambury, David J. Benjamin, Joshua L. Chaim, Emily C. Zabor, John Sullivan, Ilana R. Garcia-Grossman, Ashley M. Regazzi, Irina Ostrovnaya, Aryln Apollo, Han Xiao, Martin H. Voss, Gopa Iyer, Dean F. Bajorin, Jonathan E. Rosenberg
Manuscript writing: Richard M. Bambury, David J. Benjamin, Emily C. Zabor, Martin H. Voss, Gopa Iyer, Jonathan E. Rosenberg
Final approval of manuscript: Richard M. Bambury, David J. Benjamin, Joshua L. Chaim, Emily C. Zabor, John Sullivan, Ilana R. Garcia-Grossman, Ashley M. Regazzi, Irina Ostrovnaya, Aryln Apollo, Han Xiao, Martin H. Voss, Gopa Iyer, Dean F. Bajorin, Jonathan E. Rosenberg
Disclosures
Jonathan E. Rosenberg: Lilly (C/A, uncompensated), Oncogenex (C/A), Bristol-Myers Squibb (C/A), Merck (C/A, OI), Boehringer Ingelheim (C/A, compensated); Dean F. Bajorin: Bristol-Myers Squibb (C/A), Novartis (C/A), Merck (C/A), Genentech (C/A), BioCancell (C/A), Roche (C/A), Astellas (C/A), Novartis (RF), Dendreon (RF), Genentech, Amgen (RF), Merck (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 2.Bellmunt J, Théodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27:4454–4461. doi: 10.1200/JCO.2008.20.5534. [DOI] [PubMed] [Google Scholar]
- 3.Vaughn DJ, Broome CM, Hussain M, et al. Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J Clin Oncol. 2002;20:937–940. doi: 10.1200/JCO.2002.20.4.937. [DOI] [PubMed] [Google Scholar]
- 4.McCaffrey JA, Hilton S, Mazumdar M, et al. Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J Clin Oncol. 1997;15:1853–1857. doi: 10.1200/JCO.1997.15.5.1853. [DOI] [PubMed] [Google Scholar]
- 5.Galsky MD, Mironov S, Iasonos A, et al. Phase II trial of pemetrexed as second-line therapy in patients with metastatic urothelial carcinoma. Invest New Drugs. 2007;25:265–270. doi: 10.1007/s10637-006-9020-9. [DOI] [PubMed] [Google Scholar]
- 6.Sweeney CJ, Roth BJ, Kabbinavar FF, et al. Phase II study of pemetrexed for second-line treatment of transitional cell cancer of the urothelium. J Clin Oncol. 2006;24:3451–3457. doi: 10.1200/JCO.2005.03.6699. [DOI] [PubMed] [Google Scholar]
- 7.Pond GR, Agarwal N, Bellmunt J, et al. A nomogram including baseline prognostic factors to estimate the activity of second-line therapy for advanced urothelial carcinoma. BJU Int. 2014;113:E137–E143. doi: 10.1111/bju.12564. [DOI] [PubMed] [Google Scholar]
- 8.Sonpavde G, Pond GR, Fougeray R, et al. Time from prior chemotherapy enhances prognostic risk grouping in the second-line setting of advanced urothelial carcinoma: A retrospective analysis of pooled, prospective phase 2 trials. Eur Urol. 2013;63:717–723. doi: 10.1016/j.eururo.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17:3173–3181. doi: 10.1200/JCO.1999.17.10.3173. [DOI] [PubMed] [Google Scholar]
- 10.Apolo AB, Ostrovnaya I, Halabi S, et al. Prognostic model for predicting survival of patients with metastatic urothelial cancer treated with cisplatin-based chemotherapy. J Natl Cancer Inst. 2013;105:499–503. doi: 10.1093/jnci/djt015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galsky MD, Moshier E, Krege S, et al. Posttreatment prognostic nomogram for patients with metastatic urothelial cancer completing first-line cisplatin-based chemotherapy. Urol Oncol. 2014;32:48. doi: 10.1016/j.urolonc.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 12.de Jager CP, Wever PC, Gemen EF, et al. The neutrophil-lymphocyte count ratio in patients with community-acquired pneumonia. PLoS One. 2012;7:e46561. doi: 10.1371/journal.pone.0046561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Zahorec R. Ratio of neutrophil to lymphocyte counts: Rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy (Tlacene Vyd) 2001;102:5–14. [PubMed] [Google Scholar]
- 15.Walsh SR, Cook EJ, Goulder F, et al. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 16.Keizman D, Gottfried M, Ish-Shalom M, et al. Pretreatment neutrophil-to-lymphocyte ratio in metastatic castration-resistant prostate cancer patients treated with ketoconazole: Association with outcome and predictive nomogram. The Oncologist. 2012;17:1508–1514. doi: 10.1634/theoncologist.2012-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azuma T, Matayoshi Y, Odani K, et al. Preoperative neutrophil-lymphocyte ratio as an independent prognostic marker for patients with upper urinary tract urothelial carcinoma. Clin Genitourin Cancer. 2013;11:337–341. doi: 10.1016/j.clgc.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Krane LS, Richards KA, Kader AK, et al. Preoperative neutrophil/lymphocyte ratio predicts overall survival and extravesical disease in patients undergoing radical cystectomy. J Endourol. 2013;27:1046–1050. doi: 10.1089/end.2012.0606. [DOI] [PubMed] [Google Scholar]
- 19.Gondo T, Nakashima J, Ohno Y, et al. Prognostic value of neutrophil-to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology. 2012;79:1085–1091. doi: 10.1016/j.urology.2011.11.070. [DOI] [PubMed] [Google Scholar]
- 20.Hermanns T, Bhindi B, Wei Y, et al. Pre-treatment neutrophil-to-lymphocyte ratio as predictor of adverse outcomes in patients undergoing radical cystectomy for urothelial carcinoma of the bladder. Br J Cancer. 2014;111:444–451. doi: 10.1038/bjc.2014.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 22.von der Maase H, Lehmann J, Gravis G, et al. A phase II trial of pemetrexed plus gemcitabine in locally advanced and/or metastatic transitional cell carcinoma of the urothelium. Ann Oncol. 2006;17:1533–1538. doi: 10.1093/annonc/mdl154. [DOI] [PubMed] [Google Scholar]
- 23.Galsky MD, Krege S, Lin CC, et al. Relationship between 6- and 9-month progression-free survival and overall survival in patients with metastatic urothelial cancer treated with first-line cisplatin-based chemotherapy. Cancer. 2013;119:3020–3026. doi: 10.1002/cncr.28145. [DOI] [PubMed] [Google Scholar]
- 24.Gakis G, Stenzl A. C-reactive protein in bladder cancer: Where do we stand? Nat Rev Urol. 2013;10:182. doi: 10.1038/nrurol.2011.145-c1. [DOI] [PubMed] [Google Scholar]
- 25.Gakis G, Todenhöfer T, Renninger M, et al. Development of a new outcome prediction model in carcinoma invading the bladder based on preoperative serum C-reactive protein and standard pathological risk factors: The TNR-C score. BJU Int. 2011;108:1800–1805. doi: 10.1111/j.1464-410X.2011.10234.x. [DOI] [PubMed] [Google Scholar]
- 26.Van Allen EM, Mouw KW, Kim P et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov 2014;4:1140–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott CL, Becker MA, Haluska P, et al. Patient-derived xenograft models to improve targeted therapy in epithelial ovarian cancer treatment. Front Oncol. 2013;3:295. doi: 10.3389/fonc.2013.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott CL, Mackay HJ, Haluska P., Jr Patient-derived xenograft models in gynecologic malignancies. Am Soc Clin Oncol Educ Book. 2014;34:e258–e266. doi: 10.14694/EdBook_AM.2014.34.e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stebbing J, Paz K, Schwartz GK, et al. Patient-derived xenografts for individualized care in advanced sarcoma. Cancer. 2014;120:2006–2015. doi: 10.1002/cncr.28696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ko YJ, Canil CM, Mukherjee SD, et al. Nanoparticle albumin-bound paclitaxel for second-line treatment of metastatic urothelial carcinoma: A single group, multicentre, phase 2 study. Lancet Oncol. 2013;14:769–776. doi: 10.1016/S1470-2045(13)70162-1. [DOI] [PubMed] [Google Scholar]
- 31.Thomas Powles NJV, Fine GD, Eder JP et al. Inhibition of PD-L1 by MPDL3280A and clinical activity in pts with metastatic urothelial bladder cancer. Paper presented at: ASCO Annual Meeting; 2014; Chicago, IL. [Google Scholar]


