Using data from the Surveillance, Epidemiology, and End Results database, overall and cause-specific survival were estimated according to insurance status within 3 years after diagnosis of patients diagnosed with non-Hodgkin lymphoma (NHL) in the U.S. in the period 2007–2011. Lack of insurance and Medicaid only were associated with significantly lower survival for patients with NHL. Further evaluation of the reasons for this disparity and implementation of comprehensive coverage for medical care are urgently needed.
Keywords: Non-Hodgkin lymphoma, Survival analysis, Health care disparities, Diffuse large B-cell lymphoma
Abstract
Background.
New treatment options and supportive care measures have greatly improved survival of patients with non-Hodgkin lymphoma (NHL) but may not be affordable for those with no insurance or inadequate insurance.
Methods.
Using data from the Surveillance, Epidemiology, and End Results database, we estimated overall and cause-specific survival according to insurance status within 3 years after diagnosis of patients diagnosed with NHL in the U.S. in the period 2007–2011. Because NHL is a heterogeneous condition, we also examined survival in diffuse large B-cell lymphoma (DLBCL).
Results.
Survival was higher for patients with non-Medicaid insurance compared with either uninsured patients or patients with Medicaid. For patients with any NHL, the 3-year survival estimates were 68.0% for uninsured patients, 60.7% for patients with Medicaid, and 84.9% for patients with non-Medicaid insurance. Hazard ratios (HRs) for uninsured and Medicaid-only patients compared with insured patients were 1.92 (95% confidence interval [CI]: 1.76–2.10) and 2.51 (95% CI: 2.36–2.68), respectively. Results were similar for patients with DLBCL, with survival estimates of 68.5% for uninsured patients (HR: 1.78; 95% CI: 1.57–2.02), 58%, for patients with Medicaid (HR: 2.42; 95% CI: 2.22–2.64), and 83.3% for patients with non-Medicaid insurance. Cause-specific analysis showed survival estimates of 80.3% for uninsured patients (HR: 1.83; 95% CI: 1.62–2.05), 77.7% for patients with Medicaid (HR: 2.23; 95% CI: 2.05–2.42), and 90.5% for patients with non-Medicaid insurance.
Conclusion.
Lack of insurance and Medicaid only were associated with significantly lower survival for patients with NHL. Further evaluation of the reasons for this disparity and implementation of comprehensive coverage for medical care are urgently needed.
Implications for Practice:
Patients with non-Hodgkin lymphoma who have no insurance or who have only Medicaid are at increased risk of death in the first 5 years after diagnosis. Practitioners should be aware of this risk and provide support to these patients to reduce the risk of mortality due to noncompliance for social reasons (i.e., lack of ability to pay for treatment). A multidisciplinary approach including social work support may be helpful in providing optimal care to patients who are uninsured.
Introduction
Survival for patients with non-Hodgkin lymphoma (NHL) [1–4] has improved greatly in the past several decades to the point that long-term survival is the expected outcome in many cases, especially for children and young adults [5, 6]. New treatment options [7–10] and supportive care measures [11–14] have been shown to improve survival in clinical trials, and epidemiologic evidence suggests that the increased survival seen in clinical trials has translated into increased survival on the population level [1–6]. In particular, the use of rituximab in B-cell NHL, especially diffuse large B-cell lymphoma (DLBCL), has improved survival significantly since its introduction [7–10], and use of highly active retroviral therapy has improved survival in HIV-positive patients [12, 13], including those with lymphoma. Evidence shows, however, that the observed increases in survival have not disseminated equally throughout the population [2, 15, 16]. In the U.S. in particular, an issue leading to poorer outcomes for some patients may be lack of insurance or inadequate insurance, which may lead to patients delaying medical care or receiving less than standard of care treatment. In the past, it has been difficult to measure this effect directly; however, the latest version of the Surveillance, Epidemiology, and End Results (SEER) database included information on insurance status of patients diagnosed in 2007 and later, allowing for direct comparison of survival on the population level for the first time. In this paper, we examine survival for patients with NHL by insurance status.
Methods
Data were extracted from the SEER18 database. The SEER18 database includes data from 18 regional cancer registries throughout the U.S. Registries are chosen for their high quality and epidemiologically significant populations. Together, the SEER registries draw on a base population of ∼86 million people (28% of the total U.S. population) [17]. The population within the SEER registry is similar to the general U.S. population in most respects, although there is deliberate oversampling of some minority ethnic groups and a higher proportion of foreign-born persons than in the general U.S. population. In addition, it has been suggested that outcomes may be slightly better in the SEER registries than in the general population [18].
Survival up to 3 years from diagnosis was estimated for patients aged 15–64 years who were diagnosed in 2007–2011 and followed with respect to vital status until the end of 2011. Age-specific (15–44 and 45–64 years at diagnosis) and age-standardized survival was estimated for point estimates of survival. Age standardization was performed according to the International Cancer Survival Standard [19] using three age groups (15–44, 45–54, and 55–64 years). Because there may be differences in the overall health of patients on Medicaid or without insurance compared with those with private insurance, we evaluated overall and cause-specific survival. In cause-specific survival, death from NHL (including DLBCL) was counted as an event. Patients dying from other causes were censored at the date of death. Patients with no cause of death listed were excluded from the cause-specific analysis (145 patients with NHL, 59 with DLBCL).
Kaplan-Meier curves were calculated for absolute and cause-specific survival for up to 36 months after diagnosis, both overall and for each age group examined.
Because stage (Ann Arbor stage I, II, III, and IV), age, race (white, black, other), and sex can affect prognosis in patients with NHL, a Cox proportional hazards analysis was used to estimate the effect of insurance on overall and cause-specific survival in NHL overall and DLBCL correcting for these variables.
Infection with HIV is a poor prognostic indicator in patients with NHL. Although a code for HIV status is available in the SEER database for NHL, it is not completed in ∼99% of cases. Consequently, we attempted to indirectly estimate the frequency of HIV infection by analyzing cause-specific survival for HIV and infection-related conditions. In this analysis, death from HIV or other infection was counted as an event, and patients dying from other causes were censored at the date of death.
Patients were categorized according to their insurance type including no insurance, Medicaid, other insurance including Medicare and private insurance, and “information missing.” According to the coding in the SEER database, insurance type was recorded at the time of initial diagnosis or treatment of the condition. Patients without insurance or who were “self-pay” were coded as “no insurance.” Patients with Medicaid, Medicaid HMO (health maintenance organization), or Indian Health Services insurance were coded as “Medicaid.” Patients with private insurance, Medicare, any combination of Medicare plus supplemental insurance, or Veterans Affairs or military insurance were coded as “other insurance.” Patients who were coded as “insured-no specifics” were included in the “other insurance” category. Because the majority of patients aged ≥65 would be eligible for Medicare and thus the rate of uninsured patients aged ≥65 years would be extremely small, survival was evaluated for patients aged 15–64 years only.
All calculations were carried out using SAS software (version 9.2; SAS Institute, Cary, NC, http://www.sas.com). Macros developed for population-based survival analysis [20] were used to estimate survival at 1–3 years after diagnosis. Cox proportional hazards models were estimated using standard SAS procedures. Statistical significance was tested two-sided with α = .05 and no multiple-comparison corrections.
Results
A total of 37,253 patients diagnosed with NHL in 2007–2011 were identified, including 16,245 with DLBCL. Of this population, 2,159 (5.8%) and 4,468 (12%) of patients with NHL had no insurance or had Medicaid only, respectively (Table 1). Younger patients (aged 15–44 years) were more likely to be uninsured or to have Medicaid only, at 8% and 16.7%, respectively, compared with 5.1% and 10.5%, respectively, for older patients (aged 45–64 years). Patients with DLBCL were slightly more likely to be uninsured or to have Medicaid only, at 6.3% and 13.6%, respectively, compared with all NHL patients.
Table 1.
Numbers of patients diagnosed in 2007–2011 by age and insurance status

Median age at presentation was 50 years for patients without insurance, 51 years for patients with Medicaid, and 54 years for patients with non-Medicaid insurance, both for NHL overall and for DLBCL.
Patients without insurance or with Medicaid were more likely to be diagnosed with stage IV disease for NHL or stage III or IV disease for DLBCL compared with patients with other insurance. Patients with NHL with Medicaid only were slightly more likely to be diagnosed with later stage disease compared with patients with no insurance, although the difference was not statistically significant for older patients (Table 2). No statistically significant difference in stage of presentation was observed between patients with no insurance and patients with Medicaid only for DLBCL.
Table 2.
Number of patients diagnosed in 2007–2011 at each stage by insurance type
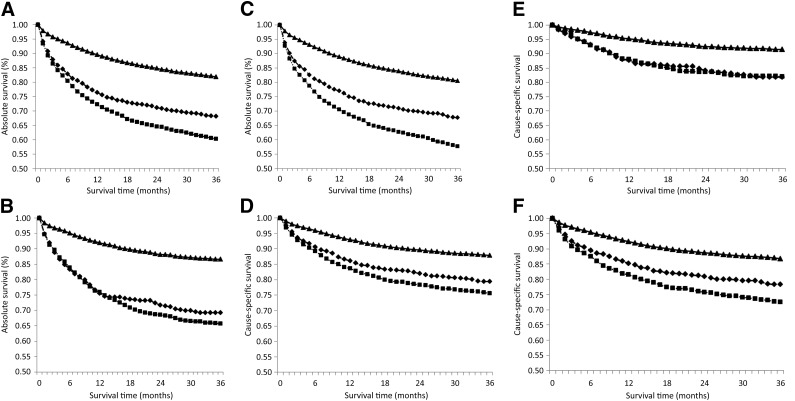
For patients with NHL, 3-year age-adjusted absolute survival was significantly lower for patients without insurance or with Medicaid only, at 68.0% and 60.7%, respectively, compared with 84.9% for patients with non-Medicaid insurance (supplemental online Table 1). Absolute survival was lower for patients without insurance or with Medicaid only compared with patients with other insurance within 1 month after diagnosis (Fig. 1A). By 4 months after diagnosis, a disparity could be observed between patients with Medicaid and patients with no insurance, and patients with Medicaid continued to have lower survival estimates for the rest of the period studied (Fig. 1A). There was less difference between uninsured patients and patients with Medicaid among those aged 15–44 years (Fig. 1B), but a significant difference in survival estimates was observed from the first month after diagnosis for patients with Medicaid or uninsured patients compared with patients with non-Medicaid insurance (Fig. 1A–1C).
Figure 1.
Kaplan-Meier curves for survival within 36 months after diagnosis with non-Hodgin lymphoma by insurance type for absolute survival of all patients (A), patients aged 15–44 years (B), and patients aged 45–64 years (C) and by cause-specific survival of all patients (D), patients aged 15–44 years (E), and patients aged 45–64 years (F). The p value for uninsured versus non-Medicaid insurance is <.0001 for all ages. Non-Medicaid insurance is designated by a solid line with triangular markers, uninsured is designated by a dashed line with diamond-shaped markers, and Medicaid is designated by a dotted line with square markers.
Cause-specific survival followed a similar pattern, with overall 3-year cause-specific survival for all patients with NHL being 88.4%, 80.3%, 77.7%, and 90.5%, respectively, for all patients, uninsured, Medicaid, and other insurance (supplemental online Table 1). The differences in cause-specific survival between uninsured patients and those insured with non-Medicaid insurance were less than the differences in overall survival (Fig. 1D) but remained highly statistically significant (p < .0001). Interestingly, survival was almost equal for patients with Medicaid and patients without insurance for those aged 15–44 years, although lower survival was observed for those with Medicaid in the 45–64 age group (Fig. 1E, 1F).
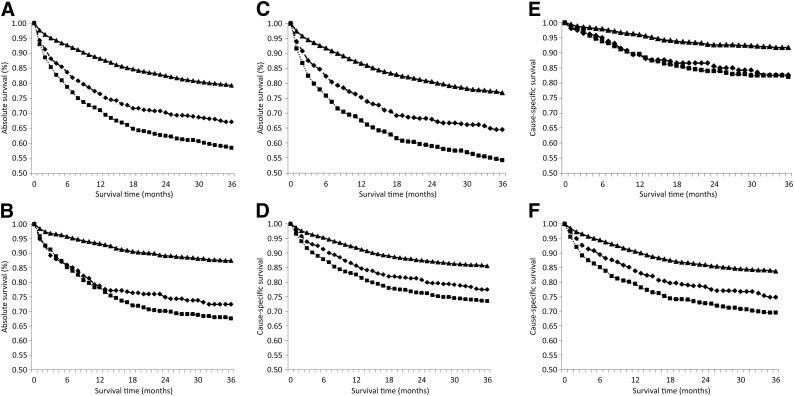
When the analysis was restricted to patients with DLBCL, overall 3-year absolute survival was lower at 78.3%. Again, survival was lower for patients with no insurance or with Medicaid only at 68.5% and 58%, respectively, than for patients with non-Medicaid insurance, for whom 3-year absolute survival was 83.3% (supplemental online Table 2). A difference in survival between patients with non-Medicaid insurance and patients with Medicaid or without insurance was evident within the first month after diagnosis, and the disparity increased over time (Fig. 2A). For younger patients, survival was similar for patients with Medicaid and uninsured patients for the first year after diagnosis, but lower survival was observed for patients with Medicaid after this time (Fig. 2B), whereas the curves separated sooner for older patients (Fig. 2C). Cause-specific 3-year survival was 86.3% for all patients, 79.4% for uninsured patients, 75.3% for patients with Medicaid only, and 88.8% for patients with other insurance (supplemental online Table 2). Again, the differences were slightly less for cause-specific versus absolute survival (Fig. 2D), but the p value for uninsured versus non-Medicaid insurance was highly significant for all comparisons (p < .0001). Interestingly, no difference in survival was observed for patients with Medicaid versus those without insurance for younger patients (Fig. 2E), but older patients with Medicaid had lower cause-specific survival than those with no insurance (Fig. 2F).
Figure 2.
Kaplan-Meier curves for survival within 36 months after diagnosis with diffuse large B-cell lymphoma by insurance type for absolute survival of all patients (A), patients aged 15–44 years (B), and patients aged 45–64 years (C) and cause-specific survival of all patients (D), patients aged 15–44 years (E), patients aged 45–64 years (F). The p value for uninsured versus non-Medicaid insurance is <.0001 for all ages. Non-Medicaid insurance is designated by a solid line with triangular markers, uninsured is designated by a dashed line with diamond-shaped markers, and Medicaid is designated by a dotted line with square markers.
A Cox proportional hazards analysis was performed to estimate the effect of insurance on absolute and cause-specific survival, adjusting for age, sex, race, and stage. The p values for the comparison of survival and cause-specific survival between patients with non-Medicaid insurance and those without insurance or with Medicaid only were <.0001 for all age groups, both NHL overall and DLBCL, and for both cause-specific and overall survival. Compared with non-Medicaid insurance, after adjustment for all variables, the hazard ratios (HRs) for uninsured patients and those with Medicaid only were 1.92 (95% confidence interval [CI]: 1.76–2.10) and 2.51 (95% CI: 2.36–2.68), respectively, for patients with NHL (Table 3). Results were similar for cause-specific survival, with the hazard ratios for patients with NHL being 1.83 (95% CI: 1.62–2.05) and 2.23 (95% CI: 2.05–2.42) for no insurance and Medicaid, respectively, versus non-Medicaid insurance (Table 3).
Table 3.
Cox proportional hazards model (hazard ratio and 95% confidence interval extracted) to estimate the effect of insurance on overall and cause-specific survival for both age groups and NHL and DLBCL adjusted for sex, race, and stage using non-Medicaid insurance as a reference group
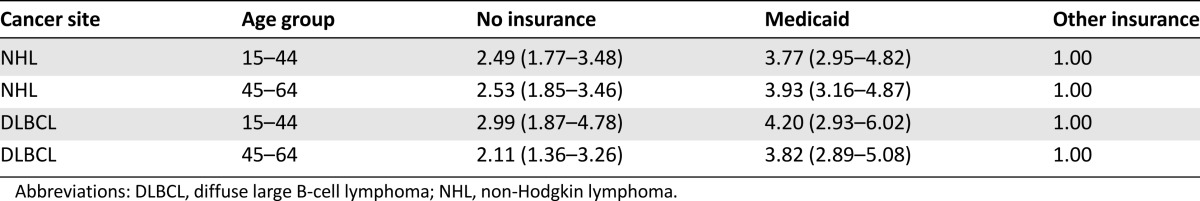
Because HIV infection is associated with more aggressive and less chemoresponsive NHL, we analyzed cause-specific survival for HIV infection and infectious illness. Compared with patients with non-Medicaid insurance, patients with no insurance and Medicaid had increased HIV- and infection-related cause-specific mortality, with hazard ratios of 2.53 (95% CI: 1.85–3.46) and 3.93 (95% CI: 3.16–4.87), respectively, for patients aged 45–64 years (Table 4).
Table 4.
Cox proportional hazards model (hazard ratio and 95% confidence interval extracted) to estimate the effect of insurance on HIV and infection-related survival for both age groups and NHL and DLBCL adjusted for sex, race, and stage using patients with non-Medicaid insurance as the reference group
Discussion
To our knowledge, this paper is the first to describe differences in survival for patients with NHL based on insurance status using a large, population-based database. Although 3-year absolute survival was as high as 80.4% for NHL and 78.3% for DLBCL, very large variation was seen by insurance type, with differences in survival as high as 25.3% between uninsured patients or those with Medicaid only and those with non-Medicaid insurance.
The data demonstrate a strong association between lack of insurance and poor outcome. Indeed, the survival estimates for patients without insurance are not dissimilar to survival of patients diagnosed in the late 1970s or early 1980s. There may be multiple reasons for this discrepancy. First, patients without insurance may be less willing to seek medical assistance for problems they perceive as minor, such as a nonpainful swelling of lymph nodes, and thus may present with higher stage disease than patients with private insurance or Medicare who may be more inclined to both present earlier for medical issues and to seek preventative care, which could identify problems earlier (although there are no screening tests for NHL). Consequently, patients with private insurance may have their disease discovered sooner and may have a lower stage of disease at diagnosis and thus have a better prognosis, especially for DLBCL. Our results show a small difference in stage at presentation for patients without insurance or with Medicaid only, suggesting that this issue contributes to the difference in survival, although the data suggest that the contribution is relatively small.
Patients without insurance would be much more likely to have difficulty in obtaining treatment for their condition. Modern treatment of NHL often involves the use of expensive biological agents such as rituximab in the outpatient setting and may be too expensive for patients without insurance to afford. Myeloid growth factors may improve outcomes for patients with NHL by decreasing the risk of chemotherapy-associated neutropenic fever and allowing increased chemotherapy dose intensity [21], but, again, they are expensive, and patients without insurance may be unable to obtain these medications, potentially delaying therapy or increasing their risk of neutropenic fevers. Indeed, a survey of physicians on the issue of barriers to the use of rituximab in lymphoma and chronic lymphocytic leukemia demonstrated that a substantial percentage of physicians in the U.S. reported that inability of patients to pay for the medication was a major barrier to therapy with rituximab, with lack of insurance or lack of coverage for the medication reported as the reason for not using, for delaying, or for reducing the dose of rituximab in 33% of cases for which such modifications were necessary [22]. Even if therapy can be provided without charge, adjuvant therapy that makes the treatment tolerable, such as antinausea medications, may be near to impossible for a patient without insurance to obtain. In addition, hematopoietic stem cell transplant is often considered for second-line treatment after failure of initial therapy and may be difficult or impossible for a patient without insurance to obtain because of the cost.
Finally, patients without health insurance are unlikely to receive regular care for chronic medical issues such as hypertension or diabetes. Consequently, they may present with multiple comorbid conditions that complicate their lymphoma treatment and worsen their overall health. Our results demonstrate that the nonlymphoma mortality among patients without insurance and with NHL is quite high because the differences between cause-specific mortality for patients with no insurance versus non-Medicaid insurance were substantially lower than the difference in all-cause mortality between these two groups. Low-grade lymphomas may have a prolonged natural history, during which time the patient's overall health may have a greater effect on his or her survival than the lymhoma itself. Uninsured patients are more likely to have untreated or inadequately treated comorbid illness and thus die with, but not of, NHL compared with privately insured patients. In addition, active comorbid problems may make treatment of NHL more difficult and, therefore, death from NHL more likely. A patient with cardiac issues, for example, may not be able to tolerate doxorubicin, a component of the standard first-line treatment of many lymphomas, and thus may be more likely to die of NHL. Similarly, patients with hepatitis B cannot be treated with rituximab unless antivirals can be administered, adding complications and risk to the treatment and increasing the risk of both NHL and nonmalignant death.
The finding that patients with Medicaid do no better than or worse than patients without insurance is unexpected. Several factors may be involved in the etiology of this finding. First, some patients may be ineligible for Medicaid, despite having no other insurance and few resources, if they are immigrants, especially undocumented immigrants. Although information concerning vital status of patients in the SEER database is considered extremely reliable due to its verification with the national death index, people born outside the U.S. who are diagnosed with a serious illness may return to their home countries, and thus their deaths are undercounted because they are not entered into the national death index. Consequently, deaths in the uninsured population may actually be undercounted. It is also notable that patients with Medicaid were at slightly higher stage at presentation than either uninsured patients or patients with non-Medicaid insurance and were slightly older than uninsured patients; however, regression analysis suggests that this explains only a minority of the differences observed. Medicaid eligibility varies state to state, but Medicaid is generally available to people who live in extreme poverty (i.e., have incomes less than poverty level and have no financial resources such as ownership of property) or are considered disabled. Patients with HIV and a CD4 count of <200 are qualified for Medicaid, although patients with higher CD4 counts are not generally qualified unless they meet income requirements. Our results demonstrated that death due to HIV and infectious illness is higher in patients with Medicaid compared with those with either no insurance or with non-Medicaid insurance, especially in the older patient group. Higher rates of HIV infection and especially HIV infection with low CD4 counts would be expected to lead to both lower absolute survival, because HIV increases the risk of infectious mortality, and cause-specific mortality, because HIV-related lymphomas tend to be less treatable, especially in the presence of a low CD4 count. Moreover, patients with Medicaid may be even more likely than uninsured patients to have severe comorbidities that either interfere with treatment of NHL or lead to higher nonlymphoma mortality in this population.
Extreme poverty may make compliance with medical care difficult for reasons including difficulty paying for transportation to appointments and lack of understanding of the medical system. Medicaid may compensate providers less than other forms of insurance, and a number of providers do not accept it, limiting options for patients with Medicaid. In addition, undocumented immigrants may be eligible only for a form of Medicaid that covers “emergency” but not nonemergent treatment, for example, it would cover chemotherapy for an acute malignancy but not stem cell transplant and thus would severely limit options for treatment in the relapse or refractory setting. Finally, some forms of Medicaid cover hospital care only, such that patients have no insurance for outpatient treatment but may still be listed as having “Medicaid” if they were diagnosed in the hospital. Consequently, patients with Medicaid are relatively likely to have incomplete insurance. In summary, a number of factors may influence the low survival estimates observed for patients with Medicaid; however, the finding is extremely concerning because it may indicate inadequacy in this insurance system.
Previous studies have noted decreased survival in patients with no insurance. A study using data from the Virginia cancer registry comparing survival for acute myeloblastic leukemia in patients with and without insurance showed higher mortality and lower rates of treatment among those without insurance, with mortality no longer being significant after adjustment for nontreatment; this finding suggests that lack of treatment, rather than disease characteristics, explained the survival difference in this condition [23]. Another study using the National Cancer Data Base showed lower rates of chemotherapy and biological therapy among patients without insurance or with Medicaid alone [24] among patients diagnosed with DLBCL in the early 21st century. Several studies demonstrated later stage at presentation among patients who were uninsured or who had Medicaid only for several tumor types [25–27]. In addition, studies of survival in nonhematologic cancers have generally found a correlation between being uninsured or having nonprivate insurance and lower survival [28, 29]. Finally, a recent study examining survival among patients with DLBCL by insurance type using a hospital-based database showed inferior survival for patients with no insurance or Medicaid only [30]. It should be noted that prior studies examining the effect of insurance on survival in hematologic malignancies have not used the SEER database and thus have been limited by smaller patient numbers. This increases the risk of a false negative result and potentially biases the sample, for example, including only patients who were hospitalized. Our study is the first to demonstrate this finding on a national level and in an unselected population.
Studies of survival for patients with Medicaid compared with private insurance or all non-Medicaid patients have generally shown disparity. One study showed lower survival in patients with Medicaid, particularly for those who were enrolled in Medicaid at the time of diagnosis or after diagnosis (as opposed to before diagnosis) [31]. Another showed decreased survival for patients with Hodgkin lymphoma, but not acute myeloblastic leukemia, among Medicaid beneficiaries as opposed to all other patients [32]. As noted, at least one study has shown lower survival estimates for patients with Medicaid and DLBCL [30]. Finally, a study of colorectal cancer patients showed lower survival for patients with nonprivate insurance, including Medicaid. This study, using the hospital based National Cancer Data Base, was able to demonstrate a higher rate of comorbid illness among patients who were uninsured or insured only with Medicaid [29], supporting the hypothesis that some of the difference in survival between Medicaid and non-Medicaid insured patients may be related to differences in comorbid illness.
As noted, patients with Medicaid tend to be highly impoverished. Some [15, 33], but not all [34], studies from countries with universal public health systems have noted lower survival in various cancers for patients with low socioeconomic status (although generally less dramatic differences than seen in our data). These findings suggest that universal insurance, although necessary for optimal population-level survival, is not sufficient for optimal population-level survival and that further systematic changes in social patterns may be necessary to prevent unnecessary deaths from highly treatable and curable malignancies such as NHL.
In interpreting our results, some limitations should be considered. First, although a field code for HIV infection is included in the SEER data, it is almost never completed. Although indirect evidence (i.e., number of deaths due to HIV infection) suggests that HIV infection is more common among patients with Medicaid, the exact level of difference is unclear because of the lack of direct data. In addition, a significant number of patients did not have insurance status recorded, and the effect of missing data are difficult to determine. Finally, insurance status was coded at diagnosis or start of treatment. It is possible that some patients changed categories over time, for example, lost insurance or gained some level of insurance, most likely Medicaid, during their treatment course. Consequently, differences between uninsured and insured patients may be underestimated.
Conclusion
Our results demonstrate substantially decreased survival for NHL patients with no insurance or Medicaid on a population level. The survival differences are large and persistent across different ages and persist when only DLBCL is considered, demonstrating that differences in lymphoma subtype do not explain the differences in survival. The reasons for the disparity are likely multifactorial but very likely include decreased access to treatment, especially for uninsured patients. There is an urgent need to improve care for patients without insurance and with Medicaid who have this highly treatable cancer.
See http://www.TheOncologist.com for supplemental material available online.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Supplementary Material
Acknowledgment
Some of the data contained in this paper were presented previously at the 2013 American Society for Hematology annual meeting.
Author Contributions
Conception/Design: Dianne Pulte, Lina Jansen, Hermann Brenner
Collection and/or assembly of data: Lina Jansen, Hermann Brenner
Data analysis and interpretation: Dianne Pulte, Lina Jansen, Hermann Brenner
Manuscript writing: Dianne Pulte, Lina Jansen, Hermann Brenner
Final approval of manuscript: Dianne Pulte, Lina Jansen, Hermann Brenner
Disclosures
Dianne Pulte: Selexys, Apopharma (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Pulte D, Jansen L, Gondos A, et al. Survival of patients with non-Hodgkin lymphoma in Germany in the early 21st century. Leuk Lymphoma. 2013;54:979–985. doi: 10.3109/10428194.2012.734616. [DOI] [PubMed] [Google Scholar]
- 2.Pulte D, Gondos A, Brenner H. Ongoing improvement in outcomes for patients diagnosed as having Non-Hodgkin lymphoma from the 1990s to the early 21st century. Arch Intern Med. 2008;168:469–476. doi: 10.1001/archinternmed.2007.125. [DOI] [PubMed] [Google Scholar]
- 3.Pulte D, Gondos A, Brenner H. Expected long-term survival of older patients diagnosed with non-Hodgkin lymphoma in 2008-2012. Cancer Epidemiol. 2012;36:e19–e25. doi: 10.1016/j.canep.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Pulte D, Gondos A, Brenner H. Long-term survival of patients diagnosed with non-Hodgkin lymphoma after a previous malignancy. Leuk Lymphoma. 2009;50:179–186. doi: 10.1080/10428190802645061. [DOI] [PubMed] [Google Scholar]
- 5.Pulte D, Gondos A, Brenner H. Trends in 5- and 10-year survival after diagnosis with childhood hematologic malignancies in the United States, 1990-2004. J Natl Cancer Inst. 2008;100:1301–1309. doi: 10.1093/jnci/djn276. [DOI] [PubMed] [Google Scholar]
- 6.Pulte D, Gondos A, Brenner H. Trends in survival after diagnosis with hematologic malignancy in adolescence or young adulthood in the United States, 1981-2005. Cancer. 2009;115:4973–4979. doi: 10.1002/cncr.24548. [DOI] [PubMed] [Google Scholar]
- 7.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 8.Coiffier B, Haioun C, Ketterer N, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: A multicenter phase II study. Blood. 1998;92:1927–1932. [PubMed] [Google Scholar]
- 9.Hainsworth JD, Litchy S, Burris HA, 3rd, et al. Rituximab as first-line and maintenance therapy for patients with indolent non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20:4261–4267. doi: 10.1200/JCO.2002.08.674. [DOI] [PubMed] [Google Scholar]
- 10.Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: An open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–1210. doi: 10.1016/S0140-6736(12)61763-2. [DOI] [PubMed] [Google Scholar]
- 11.Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367:520–531. doi: 10.1056/NEJMoa1200920. [DOI] [PubMed] [Google Scholar]
- 12.Castillo JJ, Beltran BE, Bibas M, et al. Prognostic factors in patients with HIV-associated peripheral T-cell lymphoma: A multicenter study. Am J Hematol. 2011;86:256–261. doi: 10.1002/ajh.21947. [DOI] [PubMed] [Google Scholar]
- 13.Lim ST, Karim R, Tulpule A, et al. Prognostic factors in HIV-related diffuse large-cell lymphoma: Before versus after highly active antiretroviral therapy. J Clin Oncol. 2005;23:8477–8482. doi: 10.1200/JCO.2005.02.9355. [DOI] [PubMed] [Google Scholar]
- 14.Lee SM, Radford JA, Dobson L, et al. Recombinant human granulocyte colony-stimulating factor (filgrastim) following high-dose chemotherapy and peripheral blood progenitor cell rescue in high-grade non-Hodgkin’s lymphoma: Clinical benefits at no extra cost. Br J Cancer. 1998;77:1294–1299. doi: 10.1038/bjc.1998.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frederiksen BL, Dalton SO, Osler M, et al. Socioeconomic position, treatment, and survival of non-Hodgkin lymphoma in Denmark—a nationwide study. Br J Cancer. 2012;106:988–995. doi: 10.1038/bjc.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pulte D, Redaniel MT, Brenner H, et al. Changes in survival by ethnicity of patients with cancer between 1992-1996 and 2002-2006: Is the discrepancy decreasing? Ann Oncol. 2012;23:2428–2434. doi: 10.1093/annonc/mds023. [DOI] [PubMed] [Google Scholar]
- 17.SEER data, 1973-2011. Available at http://seer.cancer.gov/data/. Accessed July 1, 2014.
- 18.Mariotto A, Capocaccia R, Verdecchia A, et al. Projecting SEER cancer survival rates to the US: An ecological regression approach. Cancer Causes Control. 2002;13:101–111. doi: 10.1023/a:1014380323037. [DOI] [PubMed] [Google Scholar]
- 19.Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer. 2004;40:2307–2316. doi: 10.1016/j.ejca.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Brenner H, Gefeller O, Hakulinen T. Period analysis for ‘up-to-date’ cancer survival data: Theory, empirical evaluation, computational realisation and applications. Eur J Cancer. 2004;40:326–335. doi: 10.1016/j.ejca.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Lyman GH. Guidelines of the National Comprehensive Cancer Network on the use of myeloid growth factors with cancer chemotherapy: A review of the evidence. J Natl Compr Canc Netw. 2005;3:557–571. doi: 10.6004/jnccn.2005.0031. [DOI] [PubMed] [Google Scholar]
- 22.Baer Ii WH, Maini A, Jacobs I. Barriers to the access and use of rituximab in patients with non-Hodgkin’s lymphoma and chronic lymphocytic leukemia: A physician survey. Pharmaceuticals (Basel) 2014;7:530–544. doi: 10.3390/ph7050530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradley CJ, Dahman B, Jin Y, et al. Acute myeloid leukemia: How the uninsured fare. Cancer. 2011;117:4772–4778. doi: 10.1002/cncr.26095. [DOI] [PubMed] [Google Scholar]
- 24.Flowers CR, Fedewa SA, Chen AY, et al. Disparities in the early adoption of chemoimmunotherapy for diffuse large B-cell lymphoma in the United States. Cancer Epidemiol Biomarkers Prev. 2012;21:1520–1530. doi: 10.1158/1055-9965.EPI-12-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halpern MT, Ward EM, Pavluck AL, et al. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: A retrospective analysis. Lancet Oncol. 2008;9:222–231. doi: 10.1016/S1470-2045(08)70032-9. [DOI] [PubMed] [Google Scholar]
- 26.Ward EM, Fedewa SA, Cokkinides V, et al. The association of insurance and stage at diagnosis among patients aged 55 to 74 years in the national cancer database. Cancer J. 2010;16:614–621. doi: 10.1097/PPO.0b013e3181ff2aec. [DOI] [PubMed] [Google Scholar]
- 27.Smith EC, Ziogas A, Anton-Culver H. Association between insurance and socioeconomic status and risk of advanced stage Hodgkin lymphoma in adolescents and young adults. Cancer. 2012;118:6179–6187. doi: 10.1002/cncr.27684. [DOI] [PubMed] [Google Scholar]
- 28.Bristow RE, Powell MA, Al-Hammadi N, et al. Disparities in ovarian cancer care quality and survival according to race and socioeconomic status. J Natl Cancer Inst. 2013;105:823–832. doi: 10.1093/jnci/djt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robbins AS, Pavluck AL, Fedewa SA, et al. Insurance status, comorbidity level, and survival among colorectal cancer patients age 18 to 64 years in the National Cancer Data Base from 2003 to 2005. J Clin Oncol. 2009;27:3627–3633. doi: 10.1200/JCO.2008.20.8025. [DOI] [PubMed] [Google Scholar]
- 30.Han X, Jemal A, Flowers CR, et al. Insurance status is related to diffuse large B-cell lymphoma survival. Cancer. 2014;120:1220–1227. doi: 10.1002/cncr.28549. [DOI] [PubMed] [Google Scholar]
- 31.Koroukian SM, Bakaki PM, Raghavan D. Survival disparities by Medicaid status: An analysis of 8 cancers. Cancer. 2012;118:4271–4279. doi: 10.1002/cncr.27380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yung RL, Chen K, Abel GA, et al. Cancer disparities in the context of Medicaid insurance: A comparison of survival for acute myeloid leukemia and Hodgkin’s lymphoma by Medicaid enrollment. The Oncologist. 2011;16:1082–1091. doi: 10.1634/theoncologist.2011-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jembere N, Campitelli MA, Sherman M, et al. Influence of socioeconomic status on survival of hepatocellular carcinoma in the Ontario population: A population-based study 1990–2009. PLoS One. 2012;7:e40917. doi: 10.1371/journal.pone.0040917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darmawikarta D, Pole JD, Gupta S, et al. The association between socioeconomic status and survival among children with Hodgkin and non-Hodgkin lymphomas in a universal health care system. Pediatr Blood Cancer. 2013;60:1171–1177. doi: 10.1002/pbc.24386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.