Abstract
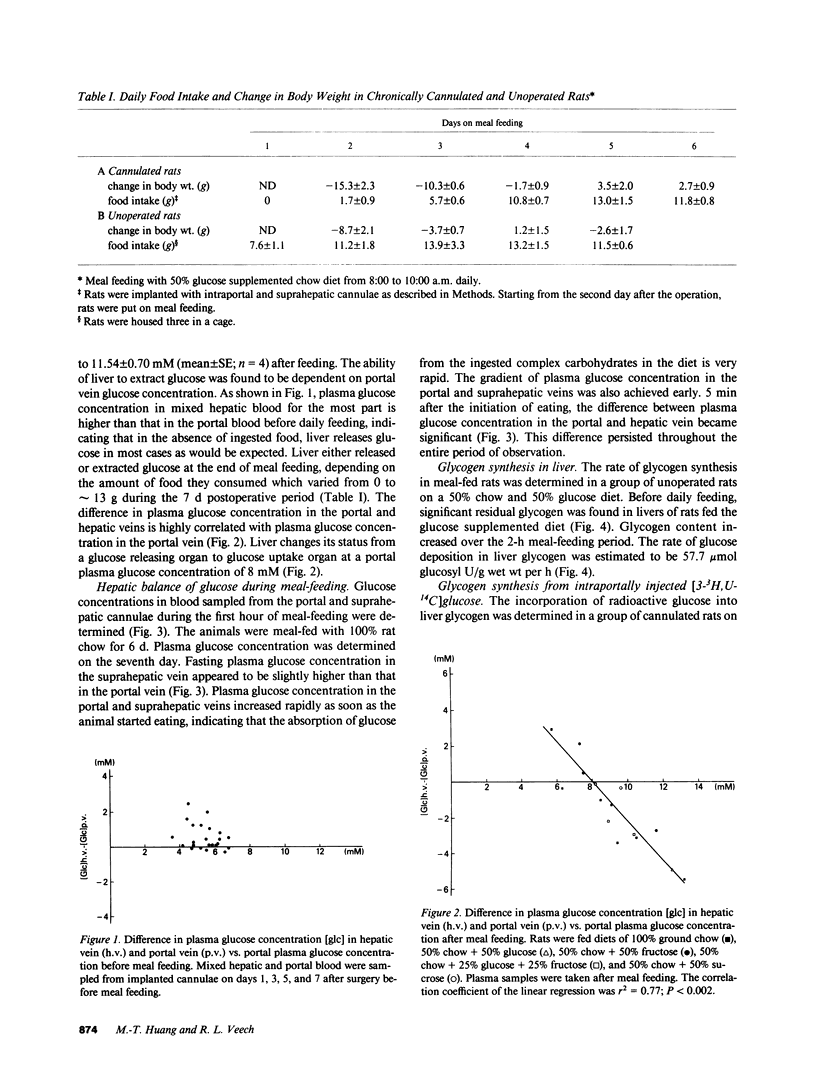
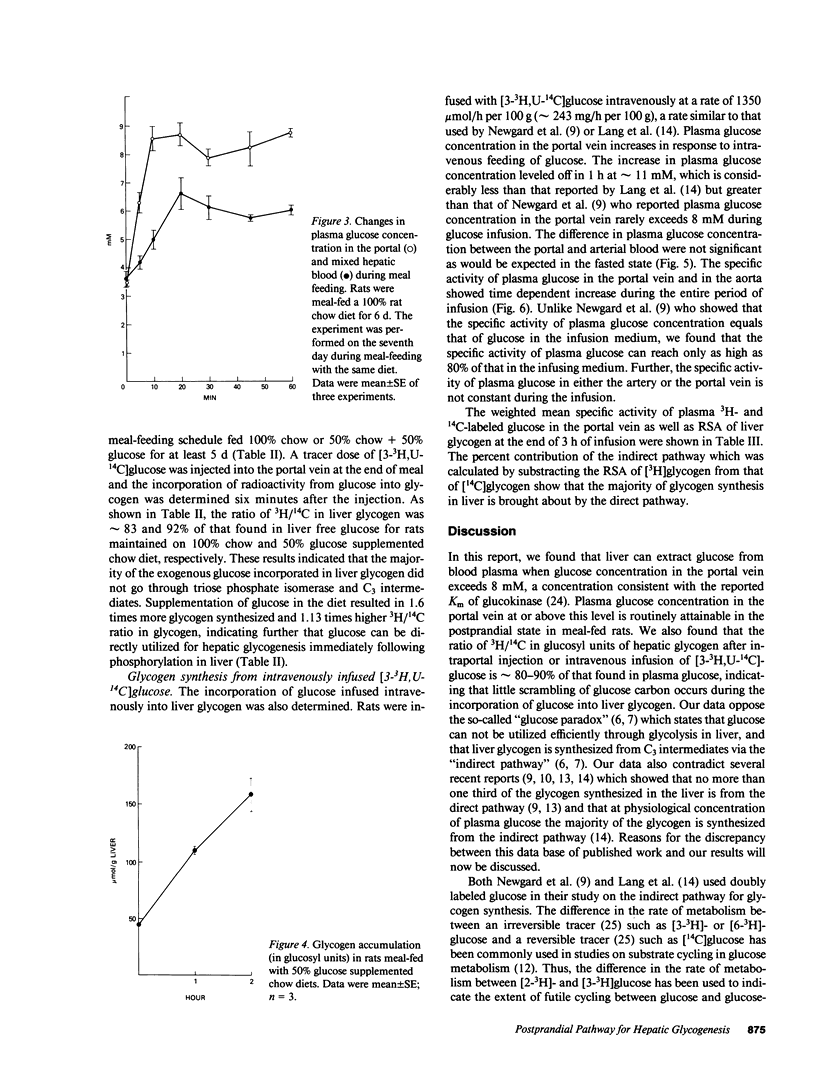
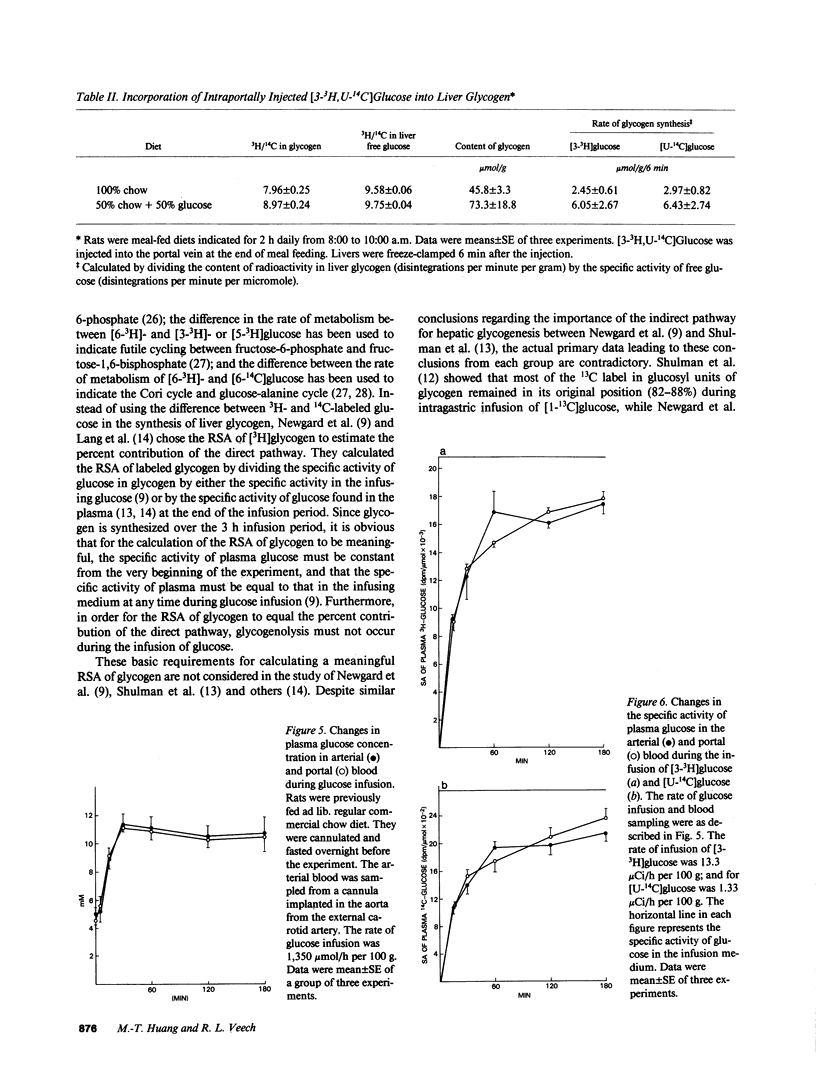
The pathway for hepatic glycogen synthesis in the postprandial state was studied in meal-fed rats chronically cannulated in the portal vein. Plasma glucose concentration in the portal vein was found to be 4.50 +/- 1.01 mM (mean +/- SE; n = 3) before a meal and 11.54 +/- 0.70 mM (mean +/- SE; n = 4) after a meal in rats meal-fed a diet consisting of 100% commercial rat chow for 7 d. The hepatic-portal difference of plasma glucose concentration showed that liver released glucose in the fasted state and either extracted or released glucose after feeding depending on plasma glucose concentration in the portal vein. The concentration of portal vein glucose at which liver changes from glucose releasing to glucose uptake was 8 mM, the Km of glucokinase [E.C. 2.7.1.12]. The rate of glycogen synthesis in liver during meal-feeding was found to be approximately 1 mumol glucosyl U/g wet wt/min in rats meal-fed a 50% glucose supplemented chow diet. The relative importance of the direct vs. indirect pathway for the replenishment of hepatic glycogen was determined by the incorporation of [3-3H,U-14C]glucose into liver glycogen. Labeled glucose was injected into the portal vein at the end of meal-feeding. The ratio of 3H/14C in the glucosyl units of glycogen was found to be 83-92% of the ratio in liver free glucose six minutes after the injection, indicating that the majority of exogenous glucose incorporated into glycogen did not go through glycolysis. The percent contribution of the direct versus indirect pathway was quantitated from the difference in the relative specific activity (RSA) of [3H] and [14C]-glycogen in rats infused with [3-3H,U-14C]glucose. No significant difference was found between the RSA of [3H]glycogen and [14C]glycogen, indicating further that the pathway for glycogen synthesis in liver from exogenous glucose is from the direct pathway. Our results do not support the thesis that the majority of liver glycogen is synthesized from glucose-6-phosphate derived from gluconeogenesis. Reasons for the discrepancy between current findings and other reports supporting the indirect pathway for glycogen synthesis in the liver are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP J. S., STEELE R., ALTSZULER N., DUNN A., BJERKNES C., DEBODO R. C. EFFECTS OF INSULIN ON LIVER GLYCOGEN SYNTHESIS AND BREAKDOWN IN THE DOG. Am J Physiol. 1965 Feb;208:307–316. doi: 10.1152/ajplegacy.1965.208.2.307. [DOI] [PubMed] [Google Scholar]
- COOK M., LORBER V. Conversion of 1-C14-Mannose and 1-C14-glucose to liver and muscle glycogen in the intact rat. J Biol Chem. 1952 Nov;199(1):1–8. [PubMed] [Google Scholar]
- Clark D. G., Storer G. B., Topping D. L. Inhibition of the substrate cycle glucose:glucose 6-phosphate by physiological concentrations of fructose in perfused rat liver. Biochem Biophys Res Commun. 1980 Mar 13;93(1):155–161. doi: 10.1016/s0006-291x(80)80259-2. [DOI] [PubMed] [Google Scholar]
- Dunn A., Chenoweth M., Schaeffer L. D. Estimation of glucose turnover and the Cori cycle using glucose-6-t-14C. Biochemistry. 1967 Jan;6(1):6–11. doi: 10.1021/bi00853a002. [DOI] [PubMed] [Google Scholar]
- HERS H. G. The conversion of fructose-1-C14 and sorbitol-1-C14 to liver and muscle glycogen in the rat. J Biol Chem. 1955 May;214(1):373–381. [PubMed] [Google Scholar]
- Hellerstein M. K., Greenblatt D. J., Munro H. N. Glycoconjugates as noninvasive probes of intrahepatic metabolism: pathways of glucose entry into compartmentalized hepatic UDP-glucose pools during glycogen accumulation. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7044–7048. doi: 10.1073/pnas.83.18.7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman A., Castaing D., Morin J., Pfister-Lemaire N., Assan R. A new technique for hepatic portal vein catheterization in freely moving rats. Am J Physiol. 1984 Jun;246(6 Pt 1):E544–E547. doi: 10.1152/ajpendo.1984.246.6.E544. [DOI] [PubMed] [Google Scholar]
- Hostetler K. Y., Landau B. R. Estimation of the pentose cycle contribution to glucose metabolism in tissue in vivo. Biochemistry. 1967 Oct;6(10):2961–2964. doi: 10.1021/bi00862a001. [DOI] [PubMed] [Google Scholar]
- Huang M. T., Lardy H. A. Effects of thyroid states on the Cori cycle, glucose--alanine cycle, and futile cycling of glucose metabolism in rats. Arch Biochem Biophys. 1981 Jun;209(1):41–51. doi: 10.1016/0003-9861(81)90254-x. [DOI] [PubMed] [Google Scholar]
- Jungermann K., Katz N. Functional hepatocellular heterogeneity. Hepatology. 1982 May-Jun;2(3):385–395. doi: 10.1002/hep.1840020316. [DOI] [PubMed] [Google Scholar]
- Katz J., McGarry J. D. The glucose paradox. Is glucose a substrate for liver metabolism? J Clin Invest. 1984 Dec;74(6):1901–1909. doi: 10.1172/JCI111610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Rognstad R. Futile cycles in the metabolism of glucose. Curr Top Cell Regul. 1976;10:237–289. doi: 10.1016/b978-0-12-152810-2.50013-9. [DOI] [PubMed] [Google Scholar]
- Katz J., Rostami H., Dunn A. Evaluation of glucose turnover, body mass and recycling with reversible and irreversible tracers. Biochem J. 1974 Jul;142(1):161–170. doi: 10.1042/bj1420161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Wals P. A., Golden S., Rognstad R. Recycling of glucose by rat hepatocytes. Eur J Biochem. 1975 Dec 1;60(1):91–101. doi: 10.1111/j.1432-1033.1975.tb20979.x. [DOI] [PubMed] [Google Scholar]
- Kuwajima M., Newgard C. B., Foster D. W., McGarry J. D. The glucose-phosphorylating capacity of liver as measured by three independent assays. Implications for the mechanism of hepatic glycogen synthesis. J Biol Chem. 1986 Jul 5;261(19):8849–8853. [PubMed] [Google Scholar]
- Lang C. H., Bagby G. J., Blakesley H. L., Johnson J. L., Spitzer J. J. Plasma glucose concentration determines direct versus indirect liver glycogen synthesis. Am J Physiol. 1986 Nov;251(5 Pt 1):E584–E590. doi: 10.1152/ajpendo.1986.251.5.E584. [DOI] [PubMed] [Google Scholar]
- Newgard C. B., Hirsch L. J., Foster D. W., McGarry J. D. Studies on the mechanism by which exogenous glucose is converted into liver glycogen in the rat. A direct or an indirect pathway? J Biol Chem. 1983 Jul 10;258(13):8046–8052. [PubMed] [Google Scholar]
- Newgard C. B., Moore S. V., Foster D. W., McGarry J. D. Efficient hepatic glycogen synthesis in refeeding rats requires continued carbon flow through the gluconeogenic pathway. J Biol Chem. 1984 Jun 10;259(11):6958–6963. [PubMed] [Google Scholar]
- Rémésey C., Demigné C., Aufrère J. Inter-organ relationships between glucose, lactate and amino acids in rats fed on high-carbohydrate or high-protein diets. Biochem J. 1978 Feb 15;170(2):321–329. doi: 10.1042/bj1700321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sable-Amplis R., Abadie D. Permanent cannulation of the hepatic portal vein in rats. J Appl Physiol. 1975 Feb;38(2):358–359. doi: 10.1152/jappl.1975.38.2.358. [DOI] [PubMed] [Google Scholar]
- Scofield R. F., Kosugi K., Schumann W. C., Kumaran K., Landau B. R. Quantitative estimation of the pathways followed in the conversion to glycogen of glucose administered to the fasted rat. J Biol Chem. 1985 Jul 25;260(15):8777–8782. [PubMed] [Google Scholar]
- Shulman G. I., Rothman D. L., Smith D., Johnson C. M., Blair J. B., Shulman R. G., DeFronzo R. A. Mechanism of liver glycogen repletion in vivo by nuclear magnetic resonance spectroscopy. J Clin Invest. 1985 Sep;76(3):1229–1236. doi: 10.1172/JCI112078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloop C. H., Krause B. R. Portal and aortic blood sampling technique in unrestrained rats. Physiol Behav. 1981 Mar;26(3):529–533. doi: 10.1016/0031-9384(81)90183-9. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Passonneau J. V. Factors affecting the turnover of cerebral glycogen and limit dextrin in vivo. J Neurochem. 1973 Jun;20(6):1543–1554. doi: 10.1111/j.1471-4159.1973.tb00272.x. [DOI] [PubMed] [Google Scholar]



