Abstract
A fundamental challenge for any complex nervous system is to regulate behavior in response to environmental challenges. Three measures of behavioral regulation were tested in a panel of 8 inbred rat strains. These measures were; 1) sensation seeking as assessed by locomotor response to novelty and the sensory reinforcing effects of light onset, 2) attention and impulsivity, as measured by a choice reaction time task, and 3) impulsivity as measured by a delay discounting task. Deficient behavioral regulation has been linked to a number of psychopathologies, including ADHD, Schizophrenia, Autism, drug abuse and eating disorders. Eight inbred rat strains (August Copenhagen Irish, Brown Norway, Buffalo, Fischer 344, Wistar Kyoto, Spontaneous Hypertensive Rat, Lewis, Dahl Salt Sensitive) were tested. With n=9 for each strain, we observed robust strain differences for all tasks; heritability was estimated between 0.43 and 0.66. Performance of the 8 inbred rat strains on the choice reaction time task was compared to the performance of out bred Sprague Dawley (n=28) and Heterogeneous strain rats (n=48). The results indicate a strong genetic influence on complex tasks related to behavioral regulation and indicate that some of measures tap common genetically-driven processes. Furthermore, our results establish the potential for future studies aimed at identifying specific alleles that influence variability for these traits. Identification of such alleles could contribute to our understanding of the molecular genetic basis of behavioral regulation, which is of fundamental importance and likely contributes to multiple psychiatric disorders.
Keywords: Inbred rat strains, heritability, novelty, habituation, choice, reaction time, impulsivity, delay discounting, ADHD, Drug Abuse, heterogeneous stock rats
A fundamental challenge for any complex nervous system is to regulate behavior in response to environmental challenges. Examples of poor behavioral regulation has been linked to a number of different psychopathologies, including ADHD, Schizophrenia, Autism and drug abuse (Barch & Braver, 2009, Semrud-Clikeman et al., 2010). The environmental contingencies present during human evolution may have selected for different temperaments and personalities, some of which may not align well with the requirements of the modern world. For example, modern problems of behavioral regulation such as drug abuse and obesity may result from genetic predispositions that were well adapted in prior environmental conditions. In this paper we examine behavior regulation in rats using procedures that measure sensation seeking, attention, and choice between delayed and immediate rewards.
Two behavioral paradigms that have been hypothesized to model aspects of sensation seeking are locomotor response to a novel environment (LRN) (Dellu et al., 1993, Dellu et al., 1996) and responding to produce a sensory reinforcer (SR) (Gancarz et al., 2012c, Olsen & Winder, 2009). In the LRN procedure a novel environment evokes locomotor activity, thus exposure to novel stimulation is involuntary (Bardo et al., 1996, Meyer et al., 2010). In the SR procedure exposure to novel stimulation is voluntary. Both of these procedures measure the magnitude of the response to novel stimulation and the rapidity of habitation to novel stimulation, though the procedures differ in that the LRN procedure measures both approach and avoidance of novel stimuli and the SR procedure measures only approach responses to novel stimuli.
Foraging for important resources is an important aspect of behavioral regulation. Two laboratory behavioral paradigms that measure important components of foraging behavior are choice reaction time (CRT) and delay discounting (DD). The CRT task measures stimulus control. Rats were required to regulate their behavior in response to a visual stimulus in order to obtain water reinforcers. Slow reaction times and high variability indicate poor stimulus control, perhaps due to impaired attention. Premature and incorrect responses indicate poor stimulus control due to poor inhibition and impulsivity. Slow, variable reactions times and premature responses are core symptoms of Attention Deficit Hyperactivity Disorder (ADHD), and ADHD is associated with drug addiction (Charach et al., 2011). Efficient regulation of behavior by stimuli increases the likelihood of obtaining important resources.
In DD, rats chose between small but immediate or large but delayed amounts of water. The DD procedure used in this paper presents a sequential choice situation that was designed to be similar to those that animals would encounter while foraging for important resources in patchy environments (Kacelnik et al., 2011, Stevens & Stephens, 2010). Rats chose between an immediate but small reward by staying in a rapidly depleting patch and a delayed but larger reward by investing the time needed to change to a full patch. Longer delays between patches make staying in the old depleting patch a better choice, while shorter delays between patches make staying in the old depleting patch longer a worse choice. Greater DD is indicated by longer stays in the depleting patch with smaller rewards. Optimal foraging theory predicts that evolutionary processes will select animals that make choices on this task that maximize the long term rate of intake (Stevens & Stephens, 2010). Performance on DD tasks has been used as a measure of impulsivity and has been shown to be associated with drug abuse in humans (Mackillop et al., 2011) and drug self-administration in animals (Perry & Carroll, 2008, Perry et al., 2005).
The first goal of this study was to determine the impact of genetics on multiple domains of behavioral-regulation in the rat and to determine if there were correlations among them, which could reflect the presence of pleiotropic alleles. The second goal of this study was to collect preliminary data to determine the feasibility of a genome wide association study (GWAS) aimed at identifying genetic polymorphisms associated with these behavioral phenotypes. The proposed GWAS would test measures of behavioral regulation in out bred Heterogeneous stock (HS) rats. HS rats are the most highly recombinant rat intercross available, making them an ideal rat model for GWAS studies (see, Solberg Woods et al., 2010, Solberg Woods et al., 2012). The HS colony was initiated in 1984 using eight inbred rat strains: (ACI/N, BN/SsN, BUF/N, F344/N, M520/N, MR/N, WKY/N, and WN/N) (Hansen & Spuhler, 1984). The panel of inbred rat strains used in the current study includes proxies for 5 (ACI, BN, BUF, F344, WKY) of the 8 original founder strains. Although the M520 strain is currently available, the other 2 original founders of the HS stock no longer are. Three additional strains that are not closely related to the founders of the rats were used in the present study (SHR, LEW, & SS). The SHR strain was chosen because it has been widely used as animal model of ADHD (Sagvolden & Johansen, 2012), the LEW strain was chosen because it has been reported that there are differences in discounting between LEW and F344 rats (Anderson & Woolverton, 2005), and the SS strain was chosen because it is often used as the background strain for the development of genetic knock-outs (see: http://rgd.mcw.edu/wg/physgenknockouts). This last strain is important because we plan to create knock-out rats based on the results of the proposed GWAS study to experimentally test the results of the GWAS. Finally, in order to further support the use of HS rats in the GWAS, we have also tested out bred HS and Sprague Dawley rats on the CRT task for comparison to the inbred rat strains.
Material and Methods
Animals
Seventy-two male rats, nine each from eight inbred strains: August Copenhagen Irish (ACI/SegHsd), Brown Norway, (BN/SsNOlaHsd), Buffalo, (BUF/Cr/Crl), Fischer (F344/NCrHsd), Lewis (LEW/Crl), Spontaneous Hypertensive Rat (SHR/NCrl), Wistar Kyoto, (WKY/NCrl), and Dahl salt sensitive (SS/JrHsd) were tested. In addition, 28 out bred male Sprague Dawley (Hsd:SD), and 24 male & 24 female out bred heterogeneous stock (NMcwi:HS) rats were tested on the CRT task for comparison to the inbred rat strains.
All inbred rats were between 7-8 weeks of age when they arrived at the colony. BUF, LEW, WKY, and SHR rats were obtained from Charles River Laboratories (Wilmington, MA). ACI, BN, DSS, and F344 rats were obtained from Harlan Laboratories (Indianapolis, IN). Inbred rats from both sources arrived at the colony at the same time and all 72 rats were tested simultaneously. The out bred SD rats were purchased from Harlan Laboratories and arrived in the colony at 7-8 weeks of age. The HS rats were obtained from Dr. Solberg-Woods, who maintains a colony of HS rats at the Medical College of Wisconsin. The HS rats were shipped to the University of Buffalo at 3-4 weeks of age and testing began when they reached 8 weeks of age.
Rats were housed in pairs in plastic cages (42.5 cm × 22.5 cm × 19.25 cm). Lights were on in the colony room from 6:00 pm to 8:00 am. Behavioral testing occurred 6 days/week during the dark phase of the light/dark cycle between the hours of 9:00 am and 12:00 am. Food (Harlan Teklad Laboratory Diet #8604, Harlan Inc., Indianapolis, IN) was continuously available. Access to water was restricted to 20 min following testing. Animals were adapted to the colony and the water restriction for 1 week before the start of testing. Animals were treated in compliance with the Guide for the Care and Use of Laboratory Animals and the experiments were conducted in accordance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at the University at Buffalo, The State University of New York.
Apparatus
Locomotor response to a novel environment (LRN)
Behavior was recorded using eight infrared motion-sensor systems (Hamilton-Kinder) fitted outside a standard plastic cage (42.5 × 22.5 × 19.25 cm); this procedure has been previously described (Gancarz et al., 2012c). The activity-monitoring system monitored each of the beams at a frequency of 0.01 s to determine whether the beams were interrupted.
Sensory reinforcement (SR)
Behavior was tested in 24 locally constructed experimental chambers; this procedure has been previously described (Lloyd et al., 2012b). The back and two side walls of the test chambers were aluminum. The top and front of the chambers were made of Plexiglas. Flooring was made of parallel stainless steel rods. Each test chamber had three snout poke holes located in the left, right, and rear aluminum walls. Infrared photo detectors were used to record snout pokes. Three stimulus lights were located above each snout poke hole and a fourth stimulus light was located in the ceiling of the test chamber.
Choice reaction time (CRT) and delay discounting (DD)
Behavior was measured in a separate set of 24 locally constructed experimental chambers; this procedure has been previously described by Richards et al. (1997). The test panel had two water dispensers located on either side of a centrally located snout-poke hole. Stimulus lights were mounted above the two water dispensers and the center snout poke hole. Sonalert tone generators were mounted above the left and right stimulus lights. The left Sonalert emitted a continuous pure tone at 2.9 kHz and the right Sonalert emitted a pulsed 1.9 kHz tone.
The water dispenser and stimulus lights were arranged so that they were level with the rat's eyes when the rat's snout interrupted an infrared beam in the center snout-poke hole. Snout pokes and head entries into the water dispensers were monitored with infrared detectors. Precise amounts of water were delivered to the left and right water feeders by syringe pumps (PHM- 100; MED Associates, East Fairfield, VT). All test chambers were housed in light and sound attenuating chambers. An 800 MHz Pentium II computer connected to a Med Associates interface controlled the 16 chambers. The MED-PC IV software package was used to program and control experimental contingencies as well as collect data. The complete system operated at a temporal resolution of 0.01 s.
Procedures
The inbred rats were tested in the following order: LRN procedure (one session), SR procedure (20 sessions), CRT procedure (37 sessions) and finally DD procedure (12 sessions). One test session was performed per day. Testing on the next procedure began immediately after completion of the previous procedure. The out bred rats were tested only on the CRT procedure.
LRN
Each rat was placed into an activity monitor for 30 min and locomotor activity was recorded. Because we have only 8 locomotor test chambers, locomotor activity testing was done in groups of 8 spread across 3 consecutive days.
The primary dependent measures were locomotor counts, operationally defined as the total number of horizontal and vertical beam breaks and within session habituation of locomotor activity. Data from the 30 min test sessions were divided into five 6-min epochs (i.e., 0–6 min, 7–12 min, etc.). This analysis revealed clear within-session declines in activity, indicating habituation to the novel environment. For each individual rat, the number of responses that occurred in each epoch was divided by the total responses that occurred in the test session to determine the proportion of responding that occurred in the epoch. Since the proportions must sum to 1.0, this analysis describes how the animals distributed their responding across the test session and is independent of the absolute rates of responding. The difference between the proportion of activity in the first and last epochs provided a quantitative measure of the rate of habituation, with larger values indicating greater habituation. Without this proportion conversion differences in habituation are confounded with differences in baseline activity levels (Leussis & Bolivar, 2006).
SR
The animals were first pre-exposed to dark experimental chambers in 30-min test sessions. One test session was performed per day. Snout pokes had no programmed consequences during pre-exposure. Following the ten session pre-exposure/familiarization phase, the animals were tested for light contingent responding for ten 30 min sessions. One test session was performed per day. The test chambers were dark during testing except when a response contingent visual stimulus was presented. One of the snout poke holes was designated as active and snout pokes into this hole resulted in illumination of the ceiling stimulus light for 5 s according to a variable interval (VI) 1 min schedule of reinforcement. On a VI 1 min schedule snout pokes produced the visual stimulus (VS) on the average of every 60 s. The ceiling light produced a luminance of 53 lux as measured from either the left or right snout poke hole. Snout pokes to the inactive alternative had no programmed consequences. The animals were tested six days a week. See Lloyd et al. (2012a) for a detailed description of the SR procedure.
The primary dependent measures were active and inactive responding and within session habituation of active responding. Active responding was the number of responses to the alternative that produced the SR. Inactive responding was the number of responses to the alternative that had no programmed effect. Habituation was measured as described above for locomotor activity. The averaged data for test sessions 2-10 of the light contingent testing phase were used for analysis. The data were averaged across tests sessions 2-10 because for some strains (i.e., BN & WKY) the number of responses that occurred in individual test sessions was very low. Session 1 was not included in this analysis because the animals had not had the opportunity to learn that responding to the active alternative produced light onset.
CRT
We have previously used similar versions of this procedure to test the effects of methamphetamine (Sabol et al., 2003) and prenatal ethanol exposure (Hausknecht et al., 2005) on attention. Rats initiated test trials by holding their snout in the center snout hole until either the left or right stimulus light was turned on. The amount of time required for the rat to hold its snout in the center snout-poke hole before the onset of the imperative stimulus (left or right stimulus lights) was called the hold time. Once the hold time criterion was reached and the imperative stimulus was presented, the rat had 3 s to respond (by removing its snout and inserting in into one of the two feeder holes), or the trial ended (the imperative stimulus was turned off) and the response was counted as an omission. If the rat made a correct response, the rat received a water reinforcer (30 uL) and the trial ended. If the rat made an incorrect response, the trial ended without reinforcement. The stimulus lights were the only sources of illumination in the test box. Training occurred 6 days a week. Sessions lasted for 30 min or 100 trials, whichever occurred first.
The hold time was progressively increased during initial training to have a final average value of 2.6 s. The hold time on any particular trial was variable ranging from a minimum of 0.06 s to a maximum of 10.5 s as determined by an exponential distribution with a mean of 2.6 s (Fleshler & Hoffman, 1962). The hold time was cumulative; for example, if the hold time was 4 s, the rat could meet this requirement by holding its snout in the hole for 2 s on two different occasions. A 2900 CPS tone was turned on for the duration of each snout poke response into the center hole. The feedback tone occurred independently of any other contingency and was in effect for the duration of the test session.
Preliminary training consisted of 20 sessions with a minimal hold time, so that only a brief snout poke into the center hole was required to initiate the trial. During this initial training period the animals also learned to respond correctly to the left and right stimulus lights. During sessions 20 to 31 the average hold time was gradually increased to 2.6 s and made variable. Sessions 32-36 were used for analysis.
The primary dependent variables were the mean reaction time (RT), reaction time standard deviation (RT-SD), deviation from the mode (DEVM), premature responses, and proportion correct. RT was the mean time elapsed from onset of the imperative stimulus to withdrawal of the snout from the center snout poke hole for each rat. RT-SD was the standard deviation of the RT for each rat. DEVM was the difference between the RT mean and the MODE of each rat distribution. The Half-Range Mode method was used to calculate the mode. Originally described by Hedges and Shah (2003), use of the Half-Range Mode method to determine the deviation from the mode of RT distributions is discussed in Spencer et al., (2009, p. 810). The DEVM metric was designed to measure lapses of attention (See Hausknecht et al., 2005, Sabol et al., 2003, Spencer et al., 2009) for further explanation). Premature responses (false alarms) were defined as a withdrawal from the center hole followed by a snout poke response into the left or right water feeder holes prior to the presentation of the stimulus. The number of premature responses was divided by the total number of trials completed to provide an estimate of premature responses that was not biased by the number of trials completed. Proportion correct was the number of correct responses in a session divided by the total number of completed trials.
DD
The patch depletion procedure is based on the more complex adjusting amount procedure that we previously developed (Richards et al., 1997). The patch depletion procedure was designed to rapidly determine the effect of delay on reward value. In the patch depletion procedure rats consume water at both the left and right water feeders (patches). Rats receive successively smaller amounts of water every 4 s by remaining at the same feeder. The amount of water is initially 0.15 mL and is then decreased by 20% after each delivery from the same feeder. For example, if the animals remain in the same patch (indicated by detection of the head in the feeder) they would receive 0.150 mL at 0 s, 0.120 mL at 4 s, 0.096 mL at 8 s, 0.077mL at 12 s, 0.061 mL at 16 s, etc. The rats can reset the amount of water to the initial maximum of 0.15 mL by switching to the alternative water feeder. However, changing to the opposite patch results in a delay to activation of the alternative feeder. During the delay, water is not available at either feeder.
A change in patch is indicated by a snout poke into the alternative non-active feeder. If a 0 s delay is being tested, the stimulus light associated with the old feeder is turned off and the stimulus light associated with the new feeder is illuminated simultaneously with the delivery of 0.15 mL of water. The obtained patch change time is the time from the last head detection in the previously active patch to the head poke into the currently active patch. If an 8 s delay is being tested, the stimulus light associated with the previous patch is turned off and the pulsed 1.9 kHz tone is presented. The delay to activation of the new feeder lasts for 8 s (starting from the time of the last head detection in the previously active patch) before the stimulus light above the new feeder is illuminated. The first snout poke into the new feeder after onset of the stimulus light results in the delivery of 0.15 mL of water.
Delays of 0, 8, and 16 s were tested. A different delay was tested during each session. The delays were tested in the following sequence (0, 8, 16, 0, 16, 8 s). This sequence was repeated for a total of 12 test sessions. Sessions lasted for 10 minutes.
The primary dependent variables were area under curve (AUC) and k. The AUC measure provides a simple measure of discounting which is not tied to a particular discount function (Myerson et al., 2001). However, much importance has been given to the hypothesis that discount functions are hyperbolic in shape (Ainslie, 1975). Hyperbolic discount functions indicate a varying rate of discounting while exponential discount functions indicate a constant rate of discounting. Discount functions for single parameter hyperbolic and exponential discount functions were obtained by fitting the median change points for each of the 8 strains at the 0, 8 and 16 s delays. For the 0 s delay the median obtained delay for the strain was used as the delay value. The hyperbolic equation was, V = 150/1+kD and the exponential equation was, V = 150e-kD. For both equations V is the value of the water available at the alternative feeder, 150 is the amount of water available at the alternative feeder, D is the length of the experimenter imposed delay to obtaining water at the alternative feeder and k is a free parameter that describes the steepness of the discount function. Larger values of k indicate greater discounting.
A non-linear curve-fitting program (Microcal Origin, version 6.0) was used to fit the hyperbolic and exponential discount functions and obtain the best fitting values of k. This program used an iterative Levenberg-Marqardt algorithm to determine the values of k that minimized chi square values describing the difference between predicted and obtained points. Smaller chi square values indicated better fits.
Statistical analysis
LRN
Total locomotor activity and locomotor habituation were analyzed using a one factor between subject analysis of variance with strain as the between subject factor. Significant F-values were followed by Tukey post-hoc tests to determine homogeneous subsets. Genetic effect size (h2) was estimated by dividing the sum of squares between strains by the total sum of squares (Meyer et al., 2010, Wilhelm & Mitchell, 2009).
SR
Active responding and habituation of active responding were analyzed to determine homogeneous subsets and h2 as described above for locomotor activity.
CRT
Five dependent measures derived from the CRT task (RT, RT-SD, DEVM, premature responses, and proportion correct) were analyzed to determine homogeneous subsets and h2 as described above for locomotor activity.
DD
The AUC values for each rat were analyzed to determine homogeneous subsets and h2 as described above for locomotor activity. The goodness of fit for k values obtained with hyperbolic and exponential equations fitted to the medians of the discount points obtained for each of the 8 strains were evaluated by comparing the obtained chi square values.
Results
LRN
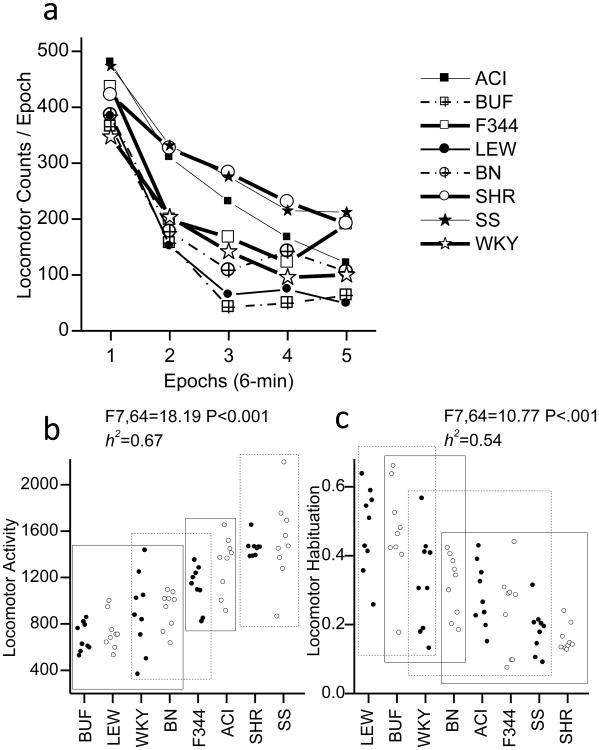
Average locomotor activity for each strain across the 30 min test session is shown in terms of 6-min epochs in Fig 1a. Examination of the locomotor activity across the test session indicates obvious within session declines in activity. There was a significant effect of strain and high heritability for both total locomotor activity (F7,64 = 18.19, P <.001; h2=0.67; see Fig 1b) and within session habituation (F7,64 = 10.77, P <.001; h2=0.54; see Fig 1c) of locomotor activity.
Fig 1. Locomotor activity in a novel environment.
A) within session pattern of locomotor activity in a novel environment plotted as five 6-min epochs. B) total locomotor activity for each rat. C) degree to which locomotor activity decreased between the first epoch of the session and the last epoch of the test session for each rat. Circles indicate the data from individual animals. The X-axis indicates strain. The rectangles in plots B and C indicate homogenous subsets as indicated by Tukey post hoc tests. The significance of the F-test for the single factor of strain and heritability (h2) are indicted.
SR
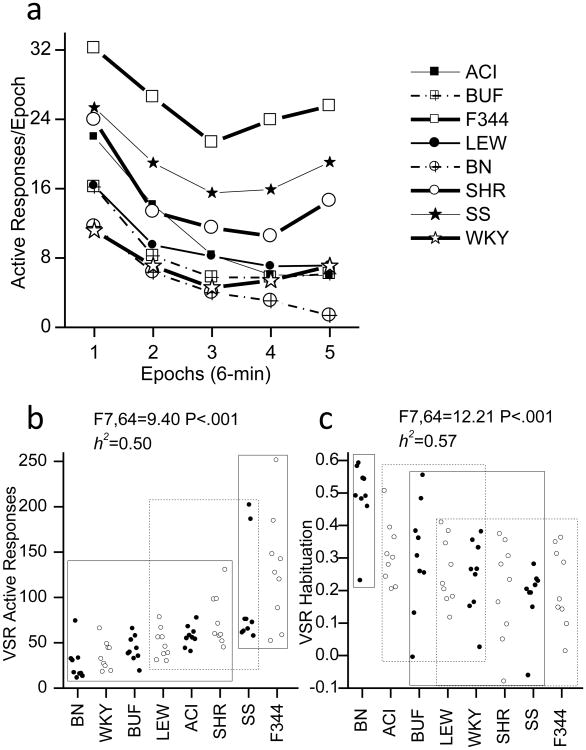
Introduction of response contingent light onset increased the rate of active responding (Fig 2) but did not significantly affect the rate of inactive responding (Data not shown). The selective effects of response contingent light onset on active responding replicate previous results obtained in out bred Sprague Dawley rats (Gancarz et al., 2012a, Gancarz et al., 2012b, Gancarz et al., 2012c, Lloyd et al., 2012a, Lloyd et al., 2012b) This indicates that the visual stimulus was a reinforcer. Similar to locomotor activity in the novel environment, responding for the VSR generally decreased from the beginning of the test session to the end of the test session (Fig 2a). There was a significant effect of strain and high heritability for both active responding (F7,64 = 9.40, P <.001; h2=0.50; see Fig 2b) and habituation (F7,64 = 12.21, P <.001; h2=0.57; see Fig 2c).
Fig 2. Sensory Reinforcement.
A) within session pattern of active responding to produce a visual stimulus reinforcer (VSR) plotted as five 6-min epochs. B) total active responses for each rat. C) degree to which active responding decreased between the first epoch of the session and the last epoch of the test session for each rat. Circles indicate the data from individual animals. The X-axis indicates strain. The rectangles in plots B and C indicate homogenous subsets as indicated by Tukey post hoc tests. The significance of the F-test for the single factor of strain and heritability (h2) are indicted.
CRT
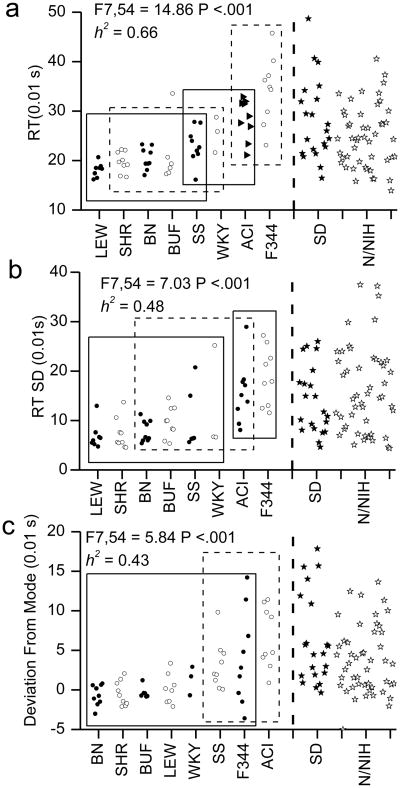
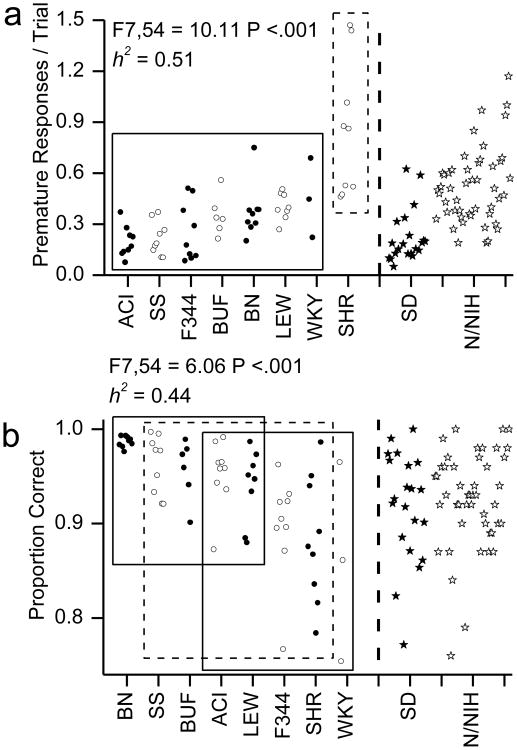
Six WKY rats, three BUF rats and one LEW rat failed to complete an average of 20 trials per test session and were excluded from the analysis. The data from the CRT task were divided into two subsets hypothesized to be related to attention deficit symptoms and hyperactivity/impulsivity deficit symptoms of ADHD. Significant effects of strain and high heritability were found for all three measures related to attention and for both hyperactivity/impulsivity related measures. The attention related measures were RT (F7,54 = 14.86, P <.001; h2=0.66; see Fig 3a), RT-SD (F7,54 = 7.03, P <.001; h2=0.48; see Fig 3b) and DEVM (F7,54 = 5.84, P <.001; h2=0.43; see Fig 3c). The hyperactivity/impulsivity related measures were premature responses (F7,54 = 10.11, P <.001; h2=0.51; see Fig 4a) and proportion correct (F7,54 = 6.06, P <.001; h2=0.44; see Fig 4b).
Fig 3. Choice reaction time measures related to attention.
Attention related measures from the choice reaction time task (CRT). A) Reaction time (RT) for each rat. B) Reaction time standard deviation (RT-SD) for each rat. C) The deviation from the mode (DEVM) for each rat. Circles indicate the data of individual animals from the 8 inbred strains. The X-axis indicates strain. Values for 28 Sprague Dawley (dark stars) and 48 N/NIH (open stars) out bred rats are shown to the right of the dashed line for comparison with the 8 inbred strains. The rectangles in plots indicate homogenous subsets as indicated by Tukey post hoc tests. The significance of the F-test for the single factor of strain and heritability (h2) are indicted.
Fig 4. Choice Reaction time measures related to hyperactivity and impulsivity.
Hyperactivity/impulsivity related measures from the choice reaction time task are shown. A) Premature responses for each rat. B) The proportion of correct responses. Circles indicate the data from individual animals. The X-axis indicates strain. Values for 28 Sprague Dawley (dark stars) and 48 HS (open stars) out bred rats are shown to the right of the dashed line for comparison with the 8 inbred strains. The rectangles in plots indicate homogenous subsets as indicated by Tukey post hoc tests. The significance of the F-test for the single factor of strain and heritability (h2) are indicted.
Performance of all of the inbred rats regardless of strain was compared to the performance of out bred SD and HS rats (See Figs 3 and 4). The resulting means and standard errors for each of the 5 measures are shown in Table 1. For the attention related measures, a significant effect of rat type (Inbred, SD or HS) was found for IRT (F2,131=3.33, P <.05), RTSD (F2,131=7.27, P <.01), and DevM (F2,131=8.77, P <.001). Tukey tests indicated that; inbred rats had faster IRT times than the SD rats but were not significantly different from the HS rats, inbred rats had less variability (smaller RTSDs) than the HS rats and were not significantly different from the SD rats, and inbred rats had smaller DevM than SD rats but were not significantly different from HS rats. For the impulsivity/hyperactivity measures of premature responses and proportion correct, only premature responses produced a significant effect of rat type (F2,131=11.93, P <.001). Post hoc Tukey tests indicated that inbred rats had had significantly fewer premature responses than the HS rats but significantly more than the SD rats. As expected, a high degree of variation was found within both out bred strains. Importantly, the variation within the HS rats generally encompassed the variation found between the inbred strains, indicating this resource will likely be useful for fine-mapping genetic loci involved in these traits (see, Solberg Woods et al., 2010, Solberg Woods et al., 2012).
Table 1. Choice Reaction time performance of inbred rats averaged across strains compared to out bred Sprague Dawley and HS rats.
| Attention Related | Hyper/Impulsivity | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Group | IRT | RTSD | DevM | Prem | Correct |
| Inbred | 23.72 | 10.902 | 1.770 | .375 | .934 |
| Strains | .860 | .933 | .557 | .031 | .008 |
| SD | 28.13a | 14.092 | 6.238a | .222ab | .918 |
| Out bred | 1.478 | 1.603 | .957 | .053 | .014 |
| HS | 24.83 | 16.244a | 3.793 | .520ab | .914 |
| Out bred | .978 | 1.060 | .633 | .035 | .010 |
Note: Bolded values are means and italic values below the means are standard error or of the mean.
: indicates significantly different from inbred strain.
: indicates significant difference between SD and HS.
DevM = deviation from the mode, Correct = proportion correct, Prem = premature responses on the CRT procedure.
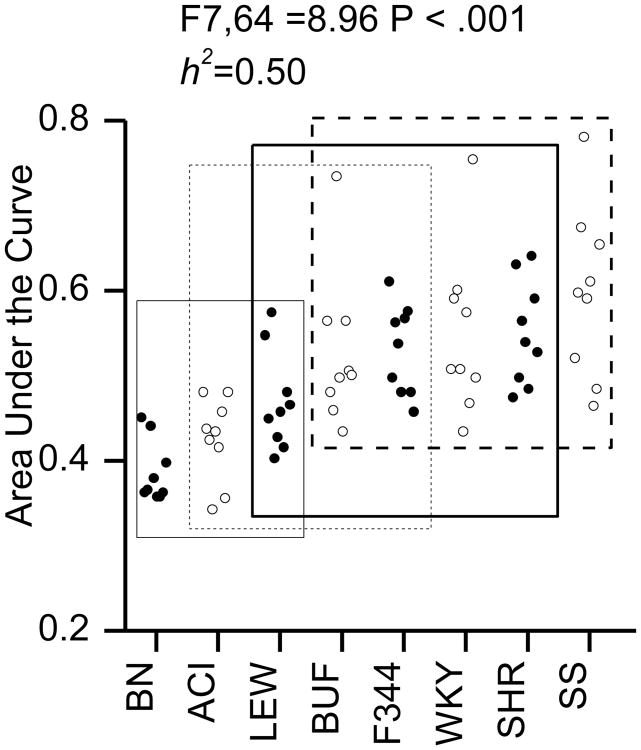
DD
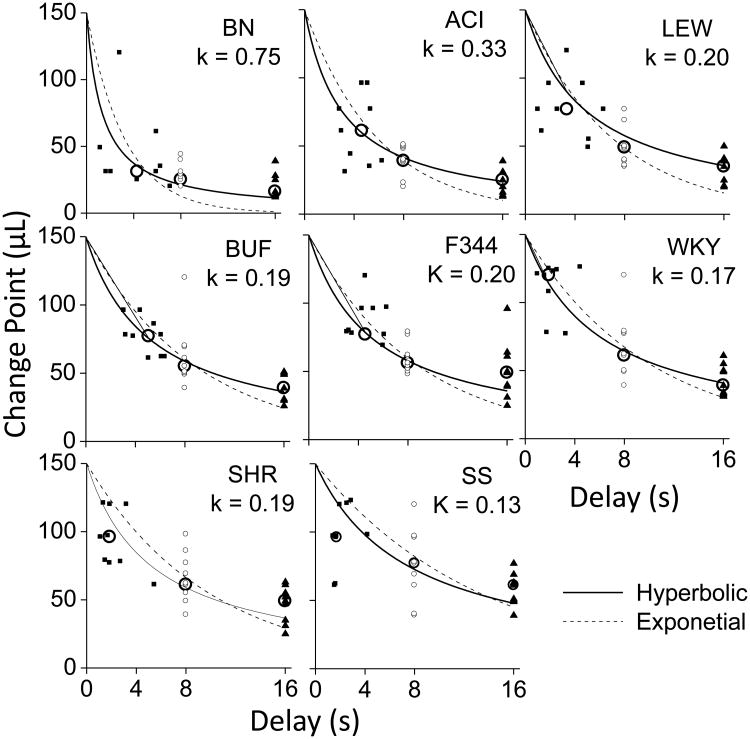
All of the rats quickly learned to switch between the two feeders. There was a significant effect of strain and high heritability for the AUC measure (F7,64 = 8.96, P <.001; h2=0.50; see Fig 5). Fig 6 shows that the rats proved to be sensitive to the imposed delays of 8 and 16 s. The hyperbolic discount function fit the data for each strain better than the exponential discount function. For each strain, the chi-square values for the hyperbolic fits were smaller than the chi-square values for the exponential fits. The chi-square values for the hyperbolic fit ranged between 4.4 and 434 with a median value of 72.2 and the chi-square values for the exponential fit ranged between 65.5 and 781 with a median value of 271.9. A Wilcoxon signed rank test confirmed that the difference between the chi-square values for the hyperbolic and exponential fits were significantly different (P = 0.012).
Fig 5. Delay discounting Area Under the Curve Measure.
Area under the curve (AUC) is plotted as function of strain. The X-axis indicates strain. Circles indicate the data from individual animals. Rectangles in plots indicate homogenous subsets as indicated by Tukey post hoc tests. The significance of the F-test for the single factor of strain and heritability (h2) are indicted.
Fig 6. Best fitting hyperbolic and exponential discount functions.
Best fitting hyperbolic and exponential discount functions. Individual animal change values for each strain are shown when the programmed delays were 0, 8 and 16 s. The X-axis indicates how long it took the animal to switch between the two water sources. Solid squares indicate data for individual animals when the programmed delay was 0 s, open circles indicate data for individual data when the programmed delay was 8 s and solid triangles indicate data when the programmed delay was 16 s. Large open circles indicate median values of the for each each strain. For the 0 s delay the values vary along the X-axis indicating how long it took the animals to switch when no delay was imposed (0 s). All animals switched in less than 8 s so that the time to switch was determined by the Experimenter imposed delays of 8 and 16 s. Hyperbolic (solid lines) and exponential (dashed lines) curves were fit to the medians of the individual change values for each strain. Individual k values resulting from the hyperbolic fit are shown for each strain.
Between task correlations
Table 2 shows the between task correlation computed using the average values for each strain (n=8). The significance values in the table are meant only to indicate association that may be of importance. Interpretation of the significance values in Table 2 needs to take into account that 54 correlations are presented and the probability of one of them reaching a high level of significance by chance alone is high.
Table 2.
Spearman nonparametric correlation coefficients for 8 inbred rat strains across the four behavioral challenges.
| LRN | SR | CRT | DD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||
| LHab | Rs | SRHab | RT | SD | DevM | Correct | Prem | AUC | k | |
| LRN | -.929 | .643 | -.429 | .119 | .095 | .310 | -.036 | -.286 | .476 | -.286 |
| .001 | .086 | .289 | .779 | .823 | .456 | .933 | .493 | .233 | .493 | |
| LHab | - | .524 | .143 | -.119 | -.167 | .132 | .143 | -.584 | .333 | |
| .071 | .183 | .736 | .779 | .693 | .756 | .736 | .160 | .420 | ||
| Rs | -.786 | .143 | .190 | .571 | -.240 | -.381 | .548 | -.333 | ||
| .021 | .736 | .651 | .139 | .568 | .352 | .160 | .420 | |||
| SRHab | -.286 | -.048 | -.357 | .527 | -.048 | -.833 | .690 | |||
| .493 | .911 | .385 | .180 | .911 | .010 | .058 | ||||
| RT | .881 | .667 | -.299 | -.381 | .214 | -.286 | ||||
| .004 | .071 | .471 | .352 | .610 | .493 | |||||
| SD | .730 | -.108 | -.619 | -.071 | .024 | |||||
| .037 | .799 | .102 | .867 | .955 | ||||||
| DevM | -.228 | -.690 | .143 | -.119 | ||||||
| .588 | .058 | .736 | .779 | |||||||
| Correct | -.527 | -.455 | .395 | |||||||
| .180 | .257 | .333 | ||||||||
| Prem | .190 | -.190 | ||||||||
| .651 | .651 | |||||||||
| AUC | -.952 | |||||||||
| .001 | ||||||||||
Note: Bolded values indicate a Spearman correlation coefficient with r > 0.6 using n = 8. P-values for these correlations are indicated in smaller print; nominally significant P-values are underlined. Abbreviations: LHab = habituation of locomotor activity on LRN procedure. Rs = active responding, SRHab = habituation of active responding on SR procedure. RT = reaction time, SD = reaction time standard deviation, DevM = deviation from the mode, Correct = proportion correct, Prem = premature responses on the CRT procedure. AUC = area under the obtained discount curve, k = describes steepness of best fitting hyperbolic discount curve.
Discussion
A primary goal of these studies was to determine the impact of genetics on the regulation of behavior in response to environmental challenges. The results clearly indicate that these behaviors are highly heritable among these inbred rat strains. We also show a high degree of variability between the founder strains of the HS colony and this variability is represented within the HS colony, indicating that this resource should prove useful for a future GWAS.
Novelty
Both the LRN and SR procedures measure how the rats regulate their behavior in response to novel stimulation and are related to the boldness-shyness continuum used to characterize the personality differences in a variety of species including humans (Aron et al., 2012, Kagan et al., 1988, Sloan Wilson et al., 1994). Novelty tests such as LRN (Dellu et al., 1993, Dellu et al., 1996), SR (Gancarz et al., 2012b, Olsen & Winder, 2009) and novel object exploration (Bardo et al., 1996, Meyer et al., 2010) have been suggested to model aspects of human sensation seeking, which has also been linked to a tendency to use drugs of abuse (Zuckerman, 2008). There are numerous studies indicating that LRN predicts drug self-administration in animals (Piazza et al., 1989, Piazza et al., 2000), although there are exceptions (Gancarz et al., 2012b, Meyer et al., 2010).
In the LRN procedure novel stimulation is imposed upon the animal, while in the SR procedure the animal learns to make a response that produces novel stimulation. It has previously been shown that the pattern of activity observed during voluntary exposure to novel environments is very different from the pattern of activity observed during forced exposure (Welker, 1957). Bardo and colleagues (Bardo et al., 1996, Meyer et al., 2010) characterize locomotor response in a novel environment as “inescapable novelty,” emphasizing that locomotor activity measured in a novel enclosure may reflect attempts to escape. These authors make a distinction between tests of inescapable novelty such as LRN and tests of “free choice novelty” such as novel object exploration. According to this distinction, the SR procedure would fall into the “free choice” category since the animal voluntarily emits an investigatory response that produces the sensory stimulus. The present study indicated that activity and responding in the LRN and SN procedures may be associated (r=0.643; P = 0.09, n=8, Table 2). We have previously reported a modest correlation between LRN and SR (r = 0.42, P < 0.01, n= 93) using out bred Sprague Dawley rats (Gancarz et al., 2012c). These results provide some support for the hypothesis that responses to inescapable novel stimuli and free choice novel stimuli may have a common genetic basis.
We found that both the LRN and SN procedures were heritable, with h2 ranging from 0.5 to 0.66 (Figs 1 & 2). Prior studies have also demonstrated the heritability of both LRN and SR. Meyer et al. (2010) tested 12 rat strains on a LRN procedure and found a strong effect of strain. The Meyer et al. study included the 8 strains tested in the current paper. The rank order of effects reported in the Meyer et al. study do not match the results reported in this paper. A Spearmen rank order correlation between the Meyer et al. results and present paper produced a nonsignificant correlation coefficient of r = 0.28. Perhaps the most salient difference is the ranking of the LEW rats, with the Meyer study finding them to have the highest levels of activity, and the present study finding them to have the 2nd to lowest ranking. Given this salient difference it is notable that Wilhelm and Mitchell (2009) tested 3 of the strains tested in the present paper (LEW/Crl, BN/Crl & F344/NCrl), and also ranked the LEW rats as least active in accord with the present results. These differences may be due to differences in dependent measures and testing procedures used and/or that the LEW/NHsd rats used in the Meyer et al. study were obtained from Harlan and the while the Wilhelm and Mitchell study and the present study used LEW/Crl rats purchased from Charles Rivers.
While the LRN procedure measures general activation (both approach and avoidance) induced by a novel environment, the SR procedure can be said to measure the reinforcing effects (approach) of novel sensory stimuli. A previous study found a strong effect of strain on SR among twelve inbred strains (Harrington, 1981). Only two of the strains used in the Harrington study are similar to the strains used in the current study: ACI/Har and F344/DuHar. However, while the F344/NCrHsd rats in the current study had the highest levels of SR responding, Harrington reported that F344/DuHar showed intermediate levels of responding. Furthermore, in the Harrington study ACI/Har had rates of SR responding that were equivalent to the F344/DuHar, while in the current study the F344/NCrHsd rats clearly have higher rates of responding than ACI/SegHsd. The between study differences in LRN and SR could be explained by genetic and phenotypic differences between strains due to genetic drift over time or environmental differences. For example genetic phenotypic differences between WKY/NHsd rats (from Harlan) and WKY/NCrl rats (from Charles River) have been well documented (Sagvolden et al., 2009).
An important aspect of novel sensory stimuli is that their effects rapidly habituate with continued exposure (Lloyd et al., 2012a). Often described as the oldest form of learning, habituation has been interpreted as learning to ignore irrelevant stimuli. Figs 1c and 2c clearly indicate that there are heritable differences in the rate of habituation to novel stimuli. Strain differences in habituation have previously been observed in rodents (Bolivar, 2009, Bolivar et al., 2000, Leussis & Bolivar, 2006). These authors hypothesize that habituation is related to memory. For example, in tests of object recognition memory, animals are exposed to two novel objects. After a delay the animals are tested with one of the old (now familiar) objects and a novel object. Degree of preference for the novel object over the familiar object is considered a measure of how well the animal remembers the familiar object (Antunes & Biala, 2012). It is arguable that habituation in the LRN and SR procedures is also a measure of memory in that the animal responds less as the sensory stimulus or environmental context becomes familiar.
Stimulus Control
The CRT procedure measures how precisely the rats are able to regulate their behavior in response to important discriminative stimuli. This procedure measures behavioral phenotypes related to attention and hyperactivity/impulsivity. The attention related impairments of individuals with ADHD are characterized by slower, more variable reaction times resulting from momentary lapses of attention (Uebel et al., 2010). The hyperactivity/impulsivity symptoms of ADHD are reflected in the more frequent occurrence of false alarms and careless errors (Toplak et al., 2012).
The attention related phenotypes are RT, RT-SD and DEVM. Slow and variable RTs indicate poor attention, possibly due to more frequent lapses of attention. Fig 3 shows that there was substantial heritability for all of the attention related measures (h2=0.43-0.66). As may be expected, the three measures of attention were strongly correlated (Table 2). According to this analysis, the ACI and F344 strains which had slow (RT) and variable RT-SD & Deviations from the mode showed an ADHD phenotype relative to the other strains on the attention related phenotypes. Interestingly, the SHR strain, which has been hypothesized to be an animal model of ADHD (Sagvolden & Johansen, 2012), did not show an ADHD-like profile on the attention related measures. Inspection of Fig 3 shows that the out bred SD and HS rats generally had larger variability on the attention related measures than the inbred strains. The high degree of variability in the HS rats indicates that these traits can be fine-mapped using these rats (see, Solberg Woods et al., 2010, Solberg Woods et al., 2012).
The hyperactivity/impulsivity measures were also highly heritable (h2=0.44-0.51; Fig 4). Unlike the attention related measures (Fig 3), the SHR rats had a clear ADHD-like profile for the premature responses and proportion correct measures. In general, the attention-related and hyperactivity measures were negatively correlated (Table 2). According to this analysis the SHR rats have hyperactivity/impulsivity symptoms but not the attention-related symptoms of ADHD. Post hoc Tukey tests (Fig 3) indicated that most effects of strain were due to the large number of premature responses in the SHR rats. Inspection of Fig 4 shows that with the exception of the SHR rats variability in the inbred strains was within the range of variability found in the out bred SD and HS rats. These results indicate that SHR rats are outliers with respect to premature responding.
Delay Discounting
The DD procedure measures how rats regulate their behavior when confronted with choices between small but immediate versus large but delayed reinforcers. It is often, although not always, the case that choosing the delayed large reinforcer will result in a greater overall rate of return. The tendency to choose the immediate small reinforcer over the delayed and, in the long run, more profitable, large reinforcer reflects discounting of reinforcing value by delay. DD is considered to be a measure of “choice impulsivity” and has been reported to predict drug self-administration in rats (Perry & Carroll, 2008, Perry et al., 2005). Similarly, human drug abusers have been found to discount delayed rewards more than controls (Mackillop et al., 2011).
The procedure used to measure DD in this paper can be viewed as an operant simulation of foraging in patchy environments. Behavioral ecologists (Kacelnik et al., 2011, Stevens & Stephens, 2010) have noted similarities and dissimilarities between laboratory measures of delay discounting and models of animals foraging in patchy environments. They point out that a salient difference between the two approaches is that commonly used delay discounting tasks use discreet one-shot choices between two alternatives while patch foraging models use sequential choices between staying in a patch and leaving for a new patch. It is arguable that evolutionary selection of choice predispositions involving delayed outcomes is more likely to have involved sequential choices than the one shot-choice situations commonly studied in the laboratory. Thus, we have modified the one-shot choice adjusting amount procedure that we previously developed (Richards et al., 1997) to use sequential choices.
The sequential choice patch DD procedure used in this paper has important advantages over the original adjusting amount procedure in that delay discounting can be rapidly tested. For example, in the current paper discounting was characterized in 12 test sessions of 10 min each; in contrast, the adjusting amount procedure requires at least 45 test sessions of 1 hr each. The efficiency of the patch DD procedure is attractive for genetic studies, in which large sample sizes are often required. A disadvantage of the sequential choice patch DD procedure is that choosing the smaller but more immediate alternative is sometimes a better choice, while in the adjusting amount DD procedure choosing the smaller but more immediate alternative is always a worse choice. Perhaps the ecological validity of the patch DD procedure is what makes it relatively easy for the rats to learn while the arbitrary nature of the adjusting amount procedure is what makes it hard for the rats to learn.
We observed strong heritability for AUC (h2 = 0.50). In addition, we provide evidence that the discount functions obtained using the patch DD procedure was better described by a hyperbolic function than an exponential function. Hyperbolic discounting indicates that discount rate decreases as delay to reinforcement increases rather than at a constant (exponential) rate. This is important because hyperbolic discount functions can be used to explain preference reversals that are observed during the delay to an expected outcome while exponential discount functions cannot (Ainslie, 1975).
A recent study by Wilhelm and Mitchell (2009) used an adjusting amount procedure to test 6 inbred rat strains and reported strong effects of strain. Three of the strains used by Wilhelm and Mitchell were also used in the present study (LEW, BN & F344). The results of the Wilhelm and Mitchell study do not completely correspond to the results of the present study. Wilhelm and Mitchell found that the F344/NCrl rats (from Charles River) had the highest rate of discounting while the present results indicate that the F344/NHsd rats (from Harlan) had the lowest rate of discounting. One point of agreement between the Wilhelm and Mitchell study and the present study is that both found that discounting in LEW/NCrl (from Charles River) was not significantly different from discounting in F344 rats. Other studies have consistently reported that LEW rats discount more than F344 rats (Anderson & Diller, 2010, Anderson & Woolverton, 2005, Huskinson & Anderson, 2012, Huskinson et al., 2012, Madden et al., 2008, Stein et al., 2013). In contrast to the Wilhelm and Mitchell study, which used F344/NCrl and LEW/NCrl rats (from Charles River), these studies all used F344/NHsd and LEW/NHsd rats (from Harlan) while the present study compared F344/NHsd rats (from Harlan) and Lew/NCrl rats (from Charles River). Because of the documented possibility of genetic and/or phenotypic differences between inbred rats obtained from Harlan and Charles River vendors (i.e., WKY; Sagvolden et al., 2009) it not possible to precisely evaluate the differences in DD observed in these studies. The results from the DD and LRN studies described above indicate the behavioral phenotype of LEW/NCrl rats (from Charles River) and LEW/NHsd rats (from Harlan) may be different. As is reviewed by Mitchell (2011), in addition to vendor differences there were considerable differences in testing procedures that may also have affected the results. However, it notable that despite differences in rat sources and procedures both the Wilhelm and Mitchell and the present study report substantial heritability of DD.
Finally, as was mentioned above, the procedure used to measure DD in this paper can be viewed as an operant simulation of foraging in patchy environments. The marginal value theorem was developed to predict the behavior of animals foraging in patchy environments (Charnov, 1976). The theorem predicts that animals will choose to leave a patch at the time that produces the greatest overall rate of intake. The optimal patch residence time is determined by the rate of depletion in the patch and the time required for travel to a new patch. The present results with domestic rats indicate that within species genetic differences strongly influence patch residence time. Thus, it appears that strain differences in domestic rats can produce consistent biases in decision making which may direct individual animals away from (or toward) optimality. Although it is unclear if similar genetic effects would be observed in wild rats (that are presumably more strongly selected for optimal foraging), these results suggest that genetically determined biases in decision making may underlie personality differences described as “impulsivity” in humans.
Between task associations
Most of the significant correlations found in Table 2 involve performance within the same task. There was an unexpected correlation between habituation on the SR task and DD (Table 2). The direction of the association indicates that faster habituation to the reinforcing effects of light onset was associated with greater discounting of the reinforcing effects of water by delay. Although this result was unexpected and may be due to chance, an interpretation of this result is that it reflects strain dependent differences in memory processes. As was indicated in the previous discussion of the SR task, it is possible that the rate of habituation to novel stimuli may be related to recognition memory, with better recognition memory leading to faster habituation. According to this interpretation, the correlation between habituation on the SR task and DD suggests that rats with poor recognition memory have lower rates of discounting. So, fast habituating rats such as BN are more likely to remember the delay and to have more discounting while slow habituating rats such as SS are less likely to remember the delay and to have less discounting.
The absence of a between task genetic correlation between premature responding on the CRT task and DD is of interest because both premature responding and delay discounting are used as operational definitions of impulsivity. Robinson et al. (2009) reported that Lister out bred rats selected for having a greater number of premature responses also had greater DD. However, others have reported no relationship between premature responses and DD (Lovic et al., 2011). In addition, brain lesions have been found to produce opposite effects on premature responding and DD (Chudasama et al., 2003, Uslaner & Robinson, 2006). The failure to find a genetic correlation between these two measures is consistent with theoretical interpretations (De Wit & Richards, 2004) and empirical studies (Reynolds et al., 2008, Sonuga-Barke, 2002), indicating that these two procedures measure separate impulsivity related processes.
Conclusion
Our survey of a panel of eight inbred rat strains indicates strong genetic influences on complex tasks thought to measure behavioral regulation. We also showed that two out bred strains (SD and HS) show high levels of variability for some of these traits. The HS rat has been shown to be useful for fine-mapping multiple complex traits to only a few Megabases (Johannesson et al., 2009, Solberg Woods et al., 2010, Solberg Woods et al., 2012). In the current study, we show large behavioral differences between the inbred strains that make up the HS colony, as well as a high degree of behavioral variability within the HS colony. These finding indicate that HS rats will likely be a useful resource for fine-mapping genetic loci that underlie these behavioral traits.
The present study has some limitations. Although there was a large effect of strain on all of the behavioral measures, the scatter plots showing individual subject performance indicate that there was also considerable inter-subject variability within strains. The source of this variability is presumed to be due to non-genetic differences or measurement error; however, it is also possible that there are polymorphisms that are segregating within one or more of these strains. In addition, since not all strains were obtained from the same vendor, it is possible that some of the effects we are attributing to genetics are in fact due to environmental differences between the vendors. There is also strong possibility that the genotypes of inbred strains may be substantially different between vendors. Finally, we do not know if small procedural differences would alter the rank order of the strains in this study. Indeed, when complimentary data were available from other studies with substantial behavioral expertise, the rank order of the strains appeared to be different. These differences may reflect environmental, procedural or genetic differences between studies, as it is known that some behaviors are very sensitive to such effects (Crabbe et al., 1999).
In summary, we have shown that a number of behavioral regulation measures that are related to sensation seeking, attention and decision making are highly heritable and can be measured efficiently in large cohorts of rats. The effects of genotype in these studies are much larger than those that would commonly be expected with drugs or other kinds of environmental interventions. These results increase confidence that studies aimed at identifying genetic polymorphisms associated with these behavioral phenotypes would be successful.
Acknowledgments
This research was supported by DA10688 to JBR, and The Chicago Biomedical Consortium with support from the Searle Funds at The Chicago Community Trust, The Conte Center for Computational Neuropsychiatric Genomics (NIH P50MH94267), DA021336, GM097737, MH079103, to AAP.
Footnotes
Supplemental information: N/A
References
- Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Anderson KG, Diller JW. Effects of acute and repeated nicotine administration on delay discounting in Lewis and Fischer 344 rats. Behavioural Pharmacology. 2010;21:754–764. doi: 10.1097/FBP.0b013e328340a050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KG, Woolverton WL. Effects of clomipramine on self-control choice in Lewis and Fischer 344 rats. Pharmacol Biochem Behav. 2005;80:387–393. doi: 10.1016/j.pbb.2004.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes M, Biala G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cognitive Processing. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron EN, Aron A, Jagiellowicz J. Sensory processing sensitivity: a review in the light of the evolution of biological responsivity. Pers Soc Psychol Rev. 2012;16:262–282. doi: 10.1177/1088868311434213. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS. Context, goals, and behavioral regulation in schizophrenia: Psychological and neural mechanisms. In: Salzinger K, Serper MR, editors. Behavioral mechanisms and psychopathology: Advancing the explanation of its nature, cause, and treatment. American Psychological Association; Washington, DC US: 2009. pp. 41–76. [Google Scholar]
- Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res. 1996;77:23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ. Intrasession and intersession habituation in mice: From inbred strain variability to linkage analysis. Neurobiology of Learning and Memory. 2009;92:206–214. doi: 10.1016/j.nlm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar VJ, Caldarone BJ, Reilly AA, Flaherty L. Habituation of activity in an open field: A survey of inbred strains and F1 hybrids. Behavior Genetics. 2000;30:285–293. doi: 10.1023/a:1026545316455. [DOI] [PubMed] [Google Scholar]
- Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: comparative meta-analyses. J Am Acad Child Adolesc Psychiatry. 2011;50:9–21. doi: 10.1016/j.jaac.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Charnov EL. Optimal foraging, the marginal value theorem. Theor Popul Biol. 1976;9:129–136. doi: 10.1016/0040-5809(76)90040-x. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- de Wit H, Richards JB. Dual determinants of drug use in humans: reward and impulsivity. Nebr Symp Motiv. 2004;50:19–55. [PubMed] [Google Scholar]
- Dellu F, Mayo W, Piazza PV, Le Moal M, Simon H. Individual differences in behavioral responses to novelty in rats. Possible relationship with the sensation-seeking trait in man. Personality Individual Differences. 1993;4:411–418. [Google Scholar]
- Dellu F, Mayo W, Vallee M, Maccari S, Piazza PV, Le Moal M, Simon H. Behavioral reactivity to novelty during youth as a predictive factor of stress-induced corticosterone secretion in the elderly--a life-span study in rats. Psychoneuroendocrinology. 1996;21:441–453. doi: 10.1016/0306-4530(96)00017-0. [DOI] [PubMed] [Google Scholar]
- Fleshler M, Hoffman HS. A progression for generating variable-interval schedules. J Exp Anal Behav. 1962;5:529–530. doi: 10.1901/jeab.1962.5-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancarz AM, Ashrafioun L, San George MA, Hausknecht KA, Hawk LW, Jr, Richards JB. Exploratory studies in sensory reinforcement in male rats: effects of methamphetamine. Exp Clin Psychopharmacol. 2012a;20:16–27. doi: 10.1037/a0025701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancarz AM, Robble MA, Kausch MA, Lloyd DR, Richards JB. Sensory reinforcement as a predictor of cocaine and water self-administration in rats. Psychopharmacology (Berl) 2012b doi: 10.1007/s00213-012-2907-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancarz AM, Robble MA, Kausch MA, Richards JB. Association between locomotor response to novelty and light reinforcement: Sensory reinforcement as an animal model of sensation seeking. Behavioural Brain Research. 2012c;230:380–388. doi: 10.1016/j.bbr.2012.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C, Spuhler K. Development of the National Institutes of Health genetically heterogeneous rat stock. Alcohol Clin Exp Res. 1984;8:477–479. doi: 10.1111/j.1530-0277.1984.tb05706.x. [DOI] [PubMed] [Google Scholar]
- Harrington GM. The Har strains of rats: origins and characteristics. Behav Genet. 1981;11:445–468. doi: 10.1007/BF01070003. [DOI] [PubMed] [Google Scholar]
- Hausknecht KA, Acheson A, Farrar AM, Kieres AK, Shen RY, Richards JB, Sabol KE. Prenatal alcohol exposure causes attention deficits in male rats. Behav Neurosci. 2005;119:302–310. doi: 10.1037/0735-7044.119.1.302. [DOI] [PubMed] [Google Scholar]
- Hedges SB, Shah P. Comparison of mode estimation methods and application in molecular clock analysis. BMC Bioinformatics. 2003;4:31. doi: 10.1186/1471-2105-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskinson SL, Anderson KG. Effects of acute and chronic administration of diazepam on delay discounting in Lewis and Fischer 344 rats. Behavioural Pharmacology. 2012;23:315–330. doi: 10.1097/FBP.0b013e3283564da4. [DOI] [PubMed] [Google Scholar]
- Huskinson SL, Krebs CA, Anderson KG. Strain differences in delay discounting between Lewis and Fischer 344 rats at baseline and following acute and chronic administration of d-amphetamine. Pharmacology, Biochemistry and Behavior. 2012;101:403–416. doi: 10.1016/j.pbb.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesson M, Lopez-Aumatell R, Stridh P, Diez M, Tuncel J, Blazquez G, Martinez-Membrives E, Canete T, Vicens-Costa E, Graham D, Copley RR, Hernandez-Pliego P, Beyeen AD, Ockinger J, Fernandez-Santamaria C, Gulko PS, Brenner M, Tobena A, Guitart-Masip M, Gimenez-Llort L, Dominiczak A, Holmdahl R, Gauguier D, Olsson T, Mott R, Valdar W, Redei EE, Fernandez-Teruel A, Flint J. A resource for the simultaneous high-resolution mapping of multiple quantitative trait loci in rats: the NIH heterogeneous stock. Genome Res. 2009;19:150–158. doi: 10.1101/gr.081497.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacelnik A, Vasconcelos M, Monteiro T, Aw J. Darwin's “tug-of-war” vs. Starlings' “horse-racing”: How adaptations for sequential encounters drive simultaneous choice. Behavioral Ecology and Sociobiology. 2011;65:547–558. [Google Scholar]
- Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Bolivar VJ. Habituation in rodents: a review of behavior, neurobiology, and genetics. Neurosci Biobehav Rev. 2006;30:1045–1064. doi: 10.1016/j.neubiorev.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Lloyd DR, Gancarz AM, Ashrafioun L, Kausch MA, Richards JB. Habituation and the Reinforcing Effectiveness of Visual Stimuli. Behav Processes. 2012a;91:184–191. doi: 10.1016/j.beproc.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DR, Kausch MA, Gancarz AM, Beyley LJ, Richards JB. Effects of novelty and methamphetamine on conditioned and sensory reinforcement. Behav Brain Res. 2012b;234:312–322. doi: 10.1016/j.bbr.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovic V, Saunders BT, Yager LM, Robinson TE. Rats prone to attribute incentive salience to reward cues are also prone to impulsive action. Behav Brain Res. 2011;223:255–261. doi: 10.1016/j.bbr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafo MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl) 2011;216:305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Smith NG, Brewer AT, Pinkston JW, Johnson PS. Steady-state assessment of impulsive choice in Lewis and Fischer 344 rats: Between-condition delay manipulations. Journal of the Experimental Analysis of Behavior. 2008;90:333–344. doi: 10.1901/jeab.2008.90-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AC, Rahman S, Charnigo RJ, Dwoskin LP, Crabbe JC, Bardo MT. Genetics of novelty seeking, amphetamine self-administration and reinstatement using inbred rats. Genes Brain Behav. 2010;9:790–798. doi: 10.1111/j.1601-183X.2010.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SH. The genetic basis of delay discounting and its genetic relationship to alcohol dependence. Behavioural Processes. 2011;87:10–17. doi: 10.1016/j.beproc.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. Journal of the Experimental Analysis of Behavior. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM, Winder DG. Operant sensation seeking engages similar neural substrates to operant drug seeking in C57 mice. Neuropsychopharmacology. 2009;34:1685–1694. doi: 10.1038/npp.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Penfold RB, Patak M. Dimensions of impulsive behavior in adolescents: laboratory behavioral assessments. Exp Clin Psychopharmacol. 2008;16:124–131. doi: 10.1037/1064-1297.16.2.124. [DOI] [PubMed] [Google Scholar]
- Richards JB, Mitchell SH, de Wit H, Seiden LS. Determination of discount functions in rats with an adjusting-amount procedure. J Exp Anal Behav. 1997;67:353–366. doi: 10.1901/jeab.1997.67-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Economidou D, Theobald DE, Mar AC, Murphy ER, Robbins TW, Dalley JW. Behavioural characterisation of high impulsivity on the 5-choice serial reaction time task: specific deficits in ‘waiting’ versus ‘stopping’. Behav Brain Res. 2009;196:310–316. doi: 10.1016/j.bbr.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Sabol KE, Richards JB, Broom SL, Roach JT, Hausknecht K. Effects of stimulus salience and methamphetamine on choice reaction time in the rat: central tendency versus distribution skew. Behav Pharmacol. 2003;14:489–500. doi: 10.1097/00008877-200311000-00001. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB. Rat models of ADHD. Curr Top Behav Neurosci. 2012;9:301–315. doi: 10.1007/7854_2011_126. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Wøien G, Walaas SI, Storm-Mathisen J, Bergersen LH, Hvalby Ø, Jensen V, Aase H, Russell VA, Killeen PR, DasBanerjee T, Middleton FA, Faraone SV. The spontaneously hypertensive rat model of ADHD—The importance of selecting the appropriate reference strain. Neuropharmacology. 2009;57:619–626. doi: 10.1016/j.neuropharm.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Walkowiak J, Wilkinson A, Butcher B. Executive functioning in children with Asperger syndrome, ADHD-combined type, ADHD-predominately inattentive type, and controls. Journal of Autism and Developmental Disorders. 2010;40:1017–1027. doi: 10.1007/s10803-010-0951-9. [DOI] [PubMed] [Google Scholar]
- Sloan Wilson D, Clark AB, Coleman K, Dearstyne T. Shyness and boldness in humans and other animals. Trends Ecol Evol. 1994;9:442–446. doi: 10.1016/0169-5347(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Solberg Woods LC, Holl K, Tschannen M, Valdar W. Fine-mapping a locus for glucose tolerance using heterogeneous stock rats. Physiol Genomics. 2010;41:102–108. doi: 10.1152/physiolgenomics.00178.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg Woods LC, Holl KL, Oreper D, Xie Y, Tsaih SW, Valdar W. Fine-mapping diabetes-related traits, including insulin resistance, in heterogeneous stock rats. Physiol Genomics. 2012;44:1013–1026. doi: 10.1152/physiolgenomics.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Psychological heterogeneity in AD/HD--a dual pathway model of behaviour and cognition. Behav Brain Res. 2002;130:29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- Spencer SV, Hawk LW, Jr, Richards JB, Shiels K, Pelham WE, Jr, Waxmonsky JG. Stimulant treatment reduces lapses in attention among children with ADHD: The effects of methylphenidate on intra-individual response time distributions. Journal of Abnormal Child Psychology. 2009;37:805–816. doi: 10.1007/s10802-009-9316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JS, Johnson PS, Renda CR, Smits RR, Liston KJ, Shahan TA, Madden GJ. Early and prolonged exposure to reward delay: Effects on impulsive choice and alcohol self-administration in male rats. Experimental and Clinical Psychopharmacology. 2013;21:172–180. doi: 10.1037/a0031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JR, Stephens DW. The adaptive nature of impulsivity. In: Madden GJ, Bickel WK, editors. Impulsivity: The behavioral and neurological science of discounting. American Psychological Association; Washington, DC US: 2010. pp. 361–387. [Google Scholar]
- Toplak ME, Sorge GB, Flora DB, Chen W, Banaschewski T, Buitelaar J, Ebstein R, Eisenberg J, Franke B, Gill M, Miranda A, Oades RD, Roeyers H, Rothenberger A, Sergeant J, Sonuga-Barke E, Steinhausen HC, Thompson M, Tannock R, Asherson P, Faraone SV. The hierarchical factor model of ADHD: invariant across age and national groupings? J Child Psychol Psychiatry. 2012;53:292–303. doi: 10.1111/j.1469-7610.2011.02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebel H, Albrecht B, Asherson P, Borger NA, Butler L, Chen W, Christiansen H, Heise A, Kuntsi J, Schafer U, Andreou P, Manor I, Marco R, Miranda A, Mulligan A, Oades RD, van der Meere J, Faraone SV, Rothenberger A, Banaschewski T. Performance variability, impulsivity errors and the impact of incentives as gender-independent endophenotypes for ADHD. J Child Psychol Psychiatry. 2010;51:210–218. doi: 10.1111/j.1469-7610.2009.02139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner JM, Robinson TE. Subthalamic nucleus lesions increase impulsive action and decrease impulsive choice - mediation by enhanced incentive motivation? Eur J Neurosci. 2006;24:2345–2354. doi: 10.1111/j.1460-9568.2006.05117.x. [DOI] [PubMed] [Google Scholar]
- Welker WI. ‘Free’ versus ‘forced’ exploration of a novel situation by rats. Psychological Reports. 1957;3:95–108. [Google Scholar]
- Wilhelm CJ, Mitchell SH. Strain differences in delay discounting using inbred rats. Genes Brain Behav. 2009;8:426–434. doi: 10.1111/j.1601-183X.2009.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Sensation Seeking and Risky Behavior. American Psychological Association; Washington, DC: 2008. [Google Scholar]