Abstract
Theories of ADHD increasingly highlight the role of neuropsychological impairment in ADHD; however, a consistent and identifiable pattern of performance on tests is not well established. The NIH EXAMINER battery provides measures of common variance across multiple executive function tests within specific domains and was used to characterize which executive functions are most affected in children with ADHD. Thirty-two children (24 male), ages 8–15 years (M=12.02, SD=2.29), diagnosed with ADHD and no comorbid disorder completed the NIH EXAMINER battery. Sixty age and gender matched healthy controls were chosen from a database of participants enrolled in the NIH EXAMINER multi-site study. Children with ADHD performed worse on the working memory score compared with the controls. No differences were found on the cognitive control or fluency scores. For children with ADHD, poorer working memory performance predicted parent report of child learning problems. Cognitive control and fluency scores did not predict learning problems. In summary, working memory emerges as a primary impairment in children with ADHD who have no comorbid disorders. Furthermore, working memory weaknesses may underlie the academic problems often seen in children with ADHD.
Keywords: executive function, ADHD, working memory, NIH EXAMINER, learning problems, children
Attention Deficit/Hyperactivity Disorder (ADHD) is one of the most common childhood mental health disorders, with current estimates indicating that 9% of children in the United States aged 5–17 years have been diagnosed at some point (Akinbami, Liu, Pastor, & Reuben, 2011). Diagnosis is made on the basis of symptoms of inattention, impulsivity, and motor restlessness, which must be observed before 7 years and cause impairment in at least two settings (American Psychiatric Association, 2000). Currently, three subtypes are recognized: “inattentive,” “hyperactive-impulsive,” and “combined.”
Children with ADHD demonstrate more academic problems compared with typically developing peers, including lower grades and lower scores on achievement tests (Frazier, Youngstrom, Glutting, & Watkins, 2007). Higher rates of school-based services, grade retention, and school dropout are also found among children with ADHD (Langberg et al., 2011; Loe & Feldman, 2007; Massetti et al., 2008; Molina et al., 2009). Learning disorder is a particularly common comorbid diagnosis, with 20–25% of children with ADHD also meeting criteria for learning disorder (Pliszka, 2000). The effects of ADHD symptoms on school performance, however, remain after controlling for learning disorders (Currie & Stabile, 2006) and behavior problems (Giannopulu, Escolano, Cusin, Citeau, & Dellatolas, 2008). Research suggests that academic difficulties are more related to inattention symptoms than hyperactivity symptoms (Breslau et al., 2009; Chhabildas, Pennington, & Willcutt, 2001; Rabiner & Coie, 2000). Attention problems predict poor academic progress (Fergusson & Horwood, 1995; Fergusson, Lynskey, & Horwood, 1997; Merrell & Tymms, 2001; Galera, Melchior, Chastang, Bouvard, & Fombonne, 2009) in children with ADHD as well as in children below the diagnostic threshold (Breslau, et al., 2009; Currie & Stabile, 2006).
Theories increasingly highlight the role of executive function impairments in individuals with ADHD (Barkley, 1997; Berger & Posner, 2000; Castellanos & Tannock, 2002; Nigg, Willcutt, Doyle, & Sonuga-Barke, 2005; Pennington & Ozonoff, 1996). Executive function refers to a range of higher-level skills critical for successful functioning in everyday life and for learning and adaptive development in children. Impairments in executive functions can lead to poor attention and planning, difficulties generating and implementing strategies, inability to utilize feedback, and inflexibility of thinking (Anderson, Anderson, Northam, Jacobs, & Catroppa, 2001). Poor performance on executive function tasks is associated with abnormalities in the prefrontal cortex and associated subcortical and posterior structures (Moore, Schettler, Killiany, Rosene, & Moss, 2012; Petrides, 2000; Ravizza & Ciranni, 2002; Stern et al., 2000). Moreover, neuroimaging reveals deficits in neural activity within the fronto-striatal and fronto-parietal circuits of individuals with ADHD (Arnsten, 2009; Arnsten & Li, 2005; Dickstein, Bannon, Castellanos, & Milham, 2006; Seidman, Valera, & Makris, 2005). Recent meta-analyses provide evidence of a consistent pattern of hypoactivation in frontal brain regions of individuals with ADHD compared with controls (Cortese et al., 2012; Dickstein, et al., 2006).
Research on ADHD has particularly emphasized impairments in inhibitory control (Barkley, 1997; Nigg, 2000) and working memory (Castellanos & Tannock, 2002; Martinussen, Hayden, Hogg-Johnson, & Tannock, 2005). Barkley’s (1997) model of ADHD emphasizes inhibitory control (i.e. inhibition of prepotent response, stopping an ongoing response, and interference control) as the primary neuropsychological impairment, which underlies secondary impairments in working memory and related functions. However, working memory—a limited capacity system that temporarily stores and manipulates information while performing complex tasks (Baddeley, 2003, 2010)—has also been proposed as a “core” deficit in ADHD (Rapport, Chung, Shore, & Isaacs, 2001). According to the functional working memory model of ADHD, inhibition is a downstream product of working memory because stimuli must gain access to the working memory system before a response can be inhibited (Kofler, Rapport, Bolden, & Altro, 2008; Rapport, et al., 2001). Furthermore, research indicates that working memory is important for learning and acquiring academic skills (Alloway & Alloway, 2010; Gathercole, Pickering, Ambridge, & Wearing, 2004). This is likely due to the impact working memory can have on many activities important for classroom learning, such as remembering instructions or keeping track of progress on complex tasks (Alloway, Gathercole, & Elliott, 2010). Indeed, working memory deficits have been linked to learning problems and poor school performance in children with and without ADHD (Alloway & Alloway, 2010; Alloway, Elliott, & Place, 2010).
A meta-analysis indicated that ADHD is consistently associated with moderate weaknesses in response inhibition and working memory as well as impulsivity, vigilance, organization, and some measures of planning (Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005). Despite a large literature examining executive function in children with ADHD, the existence of a “core” executive function deficit or an identifiable pattern of performance on tests remains elusive (Doyle, 2006; Wodka et al., 2008). Problems in concept definition and test development may also have hampered hypothesis testing and replication of specific executive function deficits (Stefanatos & Baron, 2007). Certainly, clinical investigators are faced with several challenges, including the overwhelming number of tasks reputed to measure executive function (Chiappe, Hasher, & Siegel, 2000). To make test selection more complicated, different investigators might use the same task, but with subtle variations, such as specific stimulus set, number of trials, or mode of administration.
The psychometric properties of executive function tasks pose another challenge to clinical investigators. Construct validity refers to how well an instrument measures what it purports to measure. Neuropsychological instruments have been criticized for being multifactorial, drawing on numerous non-executive component skills (Willcutt, et al., 2005). A survey of neuropsychologists by Rabin and colleagues (Rabin, Barr, & Burton, 2005) listed clock drawing, Rey-Osterrieth Complex Figure, and block design among the top 20 “executive tasks.” Although executive skills are important to performing these tests, it is impossible to disentangle systematically the component skills. Most tasks designed to assess some aspect of executive functioning also involve varying degrees of information processing speed, motor speed, language processing, spatial processing, and fundamental perceptual and motor skills. Thus, it is difficult to determine if poor performance on a test of working memory or response inhibition is due to a weakness in that construct or to a weakness in another skill required to perform the task. Furthermore, the low to moderate reliability estimates of many available executive function measures suggests that future studies should obtain a measure of common variance among multiple measures of a specific executive function domain (Willcutt, et al., 2005). Thus, use of a reliable and valid battery of domain specific executive functions that provides measures of common variance (i.e., factor scores) among multiple measures within a specific domain is necessary to meaningfully characterize which executive function skills are most affected in children with ADHD.
The present study used the NIH EXAMINER battery, developed to assess executive functions reliably and validly across a range of ages and disorders (Kramer et al., 2013, this series), to examine executive function in children with ADHD and healthy controls. The battery includes measures of spatial and verbal working memory, set-shifting, inhibition, phonemic and semantic fluency, planning, and insight. Working memory, cognitive control, and fluency scores were created by combining individual test scores using item response theory, which allows for meaningful comparisons at all ability levels on the same scale. The cognitive control measures overlap with measures in the NIH Toolbox Cognition Battery, which includes similar set-shifting and inhibition measures (Weintraub et al., 2013). The NIH EXAMINER provides a more comprehensive and in-depth measurement of executive functions than the Toolbox, however, including working memory measures that tap both verbal and spatial modalities and emphasize updating processes.
The present study examined differences in the working memory, cognitive control, and fluency scores between children with ADHD and healthy controls. Based on the literature, we predicted that children with ADHD would have significantly worse working memory scores compared with the controls. Because the cognitive control score incorporates measures of inhibitory control, we also predicted that children with ADHD would have significantly worse cognitive control scores compared with controls. Furthermore, given that children with ADHD demonstrate more academic problems compared with typically developing peers, we also examined the associations between the working memory, cognitive control, and fluency scores and parent report of learning problems in children with ADHD. We predicted that poorer working memory would be associated with more learning problems.
Method
Participants
This investigation was part of a larger multi-site study to develop the NIH EXAMINER battery of executive function (Kramer, 2011). Children diagnosed with ADHD were recruited from the Behavioral Neurology Program at Boston Children’s Hospital (BCH). Parents completed a health and development history-parent form and an ADHD Rating Scale-IV. To be eligible, a child needed to be diagnosed with ADHD by a pediatric neurologist according to DSM-IV criteria, which included a clinical interview with the child and caregiver to document symptoms, and teacher questionnaire ratings to confirm impairment across multiple settings. Subtype of ADHD was determined by the number and duration of symptoms. An ADHD, Combined Type diagnosis required at least six inattention and six hyperactivity-impulsivity symptoms, which persisted for at least six months. An ADHD, Predominantly Inattentive Type diagnosis required at least six inattention symptoms but fewer than six hyperactivity-impulsivity symptoms, which persisted for six months. In addition, ratings on the ADHD Rating Scale-IV had to exceed the 90th percentile on the inattention or hyperactive-impulsivity subscales using appropriate age and gender norms.
Participants also needed to speak English and have an IQ score equal to or greater than 85 on the Wechsler Abbreviated Scale of Intelligence (WASI). Children were excluded if they had any neurological condition (i.e., head injury, epilepsy, brain tumor, genetic disorder), visual or hearing impairment, a developmental disorder, a psychiatric disorder (other than ADHD), or were on any psychoactive medication (other than short-acting stimulants). Children were also excluded if they had a learning disorder based on a previous assessment, were receiving special education, or scored below a standard score of 90 on the Wide Range Achievement Test – Fourth Edition (WRAT-4) Word Reading subtest.
The study team reviewed the medical charts of patients seen in the Behavioral Neurology Program for a diagnosis of ADHD. Families of 86 eligible patients were mailed letters describing the study and were followed up by phone. Thirty-three families reported that they were not interested or not able to participate due to other commitments. Eleven children were not eligible because they aged out of the study age range. Four patients were scheduled for testing but failed to attend the session. Two patients did not score over the 90th percentile on the ADHD rating scale-IV. Two patients were invited in for testing but scored below a standard score of 85 on the WASI.
Thirty-two children diagnosed with ADHD ages 8 to 15 years (Mean=12.02, SD=2.29) were enrolled and completed computer-based and paper and pencil tests of executive function at BCH. The sample included twenty-four boys and eight girls with an ethnic breakdown of: 90.6% Caucasian and 9.4% African American or Black. With regard to ADHD subtype, 71.9% had combined type and 28.1% inattentive type. With regard to medication, 81.3% were prescribed short-acting stimulant medication to treat ADHD symptoms; however, parents were instructed to withhold their child’s medication on the day of testing. The study was approved by the BCH’s Institutional Review Board. All children received gift cards for their participation.
Sixty healthy control children ages 8–16 years (Mean=11.75, SD=2.53) were chosen from the database of participants recruited by other institutions participating in the larger multi-site study. All participating sites administered tests using standardized instructions and procedures to minimize differences across setting. The control sample consisted of forty-four males and sixteen females with an ethnic breakdown of: 80% Caucasian and 18.3% African American or Black. One participant’s race was unknown. Controls were selected to roughly match the ADHD participants with respect to age and gender. This ensured minimal group demographic differences; however, variable matching was not exact for all cases. Of the controls included, three were enrolled and tested at the University of California–San Francisco; forty-seven were enrolled and tested at the Developmental Cognitive Neuroscience Laboratory at the University of Nebraska–Lincoln; and ten were enrolled and tested at the University of South Carolina Neuropsychology and Human Development Lab. Controls were excluded if they had current drug use, major psychiatric disorder, B12 deficiency or other metabolic syndrome, hypothyroidism, known HIV, renal failure, respiratory failure, or significant systemic medical illnesses.
Procedure
Eligible participants were administered paper and pencil and computer-based neuropsychological tests measuring different aspects of executive function. Parents and guardians were asked to participate in objective interviews and questionnaires. Children enrolled at BCH were also administered a screening of intelligence (IQ). The full assessment took approximately 2.5 hours to complete.
Measures
Families enrolled through BCH were administered multiple screening measures. A health and developmental history form included questions regarding the participant’s birth, family, and medical history. The ADHD Rating Scale-IV was used to assess current ADHD symptoms. This behavior rating scale includes items related to the 18 symptoms of ADHD based on the DSM-IV-TR (American Psychiatric Association, 2000). Items are scored on a 0 (never or rarely) to 3 (very often) basis. Normative data based on age and gender are available, and the psychometric properties are well established (Collett, Ohan, & Myers, 2003; DuPaul, Power, Anastopoulos, & Reid, 1998). General cognitive ability was assessed using the two-subtest form of the WASI, which consists of Vocabulary and Matrix Reasoning (Wechsler, 1999). The two subtest form provides a measure of Full Scale IQ that is highly correlated with that derived from the Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV).
Learning problems were assessed using the Learning Problems subscale from the Conners 3-Parent Rating Scale (Conners 3-P; Conners, 2008). The Conners 3-P assesses behaviors in children ages 6–18, and has links to the DSM-IV-TR. A revision of the Conners Rating Scale –Revised, it provides a thorough assessment of ADHD and co-morbidities. The parent form includes 110 items and provides an up-to-date normative sample and validity scales. The Learning Problems scale of the Conners 3-P consists of 9 items that inquire about learning difficulties within the areas of reading, spelling, and math, as well as about problems that span across academic areas (e.g., problems with the ability to remember concepts). The Conners 3-T Learning Problems subscale (which contains 6 of the 9 items from the Conners 3-P) was moderately to highly correlated (r=.66-.92, p<.01) with the Learning Problems scale from the Behavior Assessment System for Children, Second Edition Teacher Report (BASC-2 TRS-C and BASC-2 TRS-A; Reynolds & Kamhaus 2004), indicating decent construct validity (Conners, 2008).
Academic achievement in reading was also assessed in children with ADHD and controls. The majority of participants were administered the Word Reading subtest from the WRAT-4 (Wilkinson & Robertson, 2006). The controls who were tested at the University of South Carolina completed the Letter-Word Identification subtest from Woodcock-Johnson III Tests of Achievement (Woodcock, McGraw, & Mather, 2001). Both of these tests of single word reading ability have good reliability and validity, are normed for both young and older children, and can be administered efficiently.
The NIH EXAMINER was used to evaluate executive function, and specifically we used the working memory, cognitive control, and fluency Scores. The executive measures that contributed to these scores are listed below. Additional measures were administered as part of the larger multi-site study to develop the NIH EXAMINER battery but are not listed because they were not included in the current analyses. Please see Kramer et al (2013, this series) for more detailed information about each test.
Working Memory
Contributing to the working memory score were the 1-Back and 2-Back tests of spatial working memory, which are based on the classic n-back paradigm and require updating processes, and the Dot Counting Test of verbal working memory, modeled after the counting span task by Case et al (Case, Kurland, & Goldberg, 1982). On the 1-Back and 2-Back tests, participants were shown a series of white squares that appeared in different locations on a black screen and indicated whether each square was presented in the same or different location as the one previous (for 1-Back) or from two screens ago (2-Back). On the Dot Counting Test, participants viewed a series of screens (2–7) with blue dots, green dots, and blue triangles and counted the blue dots. At the end of each trial, the participant recalled the number of blue dots from each screen in order.
Cognitive Control
The cognitive control score included measures from the Flanker Test (Krueger et al., 2009) and the Antisaccade Test (Hellmuth, Mirsky, et al., 2012) as measures of inhibition; a Set-Shifting Test that required switching between matching simple stimuli based on shape or color; and an error measure that combined dysexecutive errors across the battery (see Kramer et al, 2013, for details). On the Flanker Test, participants looked at a fixation point in the center of the screen and were presented a row of five arrows. The target (center) arrow was either congruent or incongruent to the non-target arrows and participants were instructed to indicate whether the target arrow is facing left or right. On the Antisaccade Test, participants were instructed to move their eyes upon presentation of a laterally presented stimulus in the same direction (pro-saccade) or in the opposite direction (anti-saccade). Performance was measured by accuracy on the anti-saccade trials. The Set-Shifting Test required participants to match a stimulus by cue to shape or color to two constant stimuli (red triangle and blue rectangle) in the lower corners of the computer screen.
Fluency
The fluency score combined accurate responses across measures of phonemic and category fluency. On phonemic fluency, participants were given 60 seconds to name as many words as they could that were not the names of people or places or numbers, first with the letter “F” and then the letter “L.” On category fluency, participants were given 60 seconds to name as many items belonging to the category animals, then to the category vegetables.
Statistical Analysis
Demographic comparisons between children with ADHD and controls were made using two-tailed t tests (equal variance). Item response theory was used to create composite scores from the multiple tests included in the NIH EXAMINER battery (Kramer et al., this series).
Analysis of covariance (ANCOVA) techniques compared the working memory, cognitive control, and fluency scores between children with ADHD and controls. Given that the study included a broad age range, and the executive function scores produced by the EXAMINER scoring program are not age-referenced, age was a covariate in all analyses. A hierarchical regression analysis examined the additive effects of the cognitive control, fluency, and working memory scores on parent report of learning problems. Age was a covariate in the model. All analyses were conducted using PASW Statistics 18 software.
Results
Demographic comparisons across groups
Table 1 depicts demographic information of all the participants by group. Independent sample t-tests compared age and word reading performance between children with ADHD and controls. There was not a significant difference in age, t(90)=−.52, p = .61, or in word reading ability, t(87)=−.83, p = .41).
Table 1.
Demographic Information on Participants.
| ADHD | Healthy Controls | |
|---|---|---|
| Number | 32 | 60 |
| Age | M=12.02 (2.29) | M=11.75 (2.53) |
| Sex | 24 males | 44 males |
| 8 females | 16 females | |
| Race | 90.6% Caucasian | 80% Caucasian |
| 9.4% AA or Black | 18.3% AA or Black | |
| Single Word Reading Test (SS) | M=109.21 (12.41) | M=106.60 (15.17) |
M=mean (Standard Deviation); SS=standard score
Associations among Executive Function Scores and Covariates
Intercorrelations among the variables are shown in Table 2. All executive function scores were moderately correlated with each other. Age was positively correlated with all three executive function scores. Sex was not significantly correlated with any variables. Word reading was positively correlated with the fluency score and negatively correlated with learning problems. Learning problems was also moderately correlated with both worse fluency and working memory scores. All participants with ADHD had a WASI two-subtest IQ score that was average or better (M=108.53, SD=11.14, Range 88–129). None of the executive function scores were significantly associated with IQ.
Table 2.
Correlations among Variables
| Cognitive Control |
Fluency | Working memory |
Age | Sex | Reading Test (SS) |
|
|---|---|---|---|---|---|---|
| Fluency | .49** | -- | ||||
| Working Memory | .50** | .29** | -- | |||
| Age | .73** | .56** | .42** | -- | ||
| Sex | .13 | .17 | .08 | .12 | -- | |
| Reading Test (SS) | .17 | .23* | .10 | .06 | −.00 | -- |
| Learning Problems | −.04 | −.45** | −.51** | −.23 | .12 | −.51** |
Note.
p < 0.10,
p< 0.05,
p< 0.01,
Sex coded as 1=boys, 2=girls; SS=Standard Score
Group Differences in Executive Function
Differences in executive function scores (cognitive control, fluency, and working memory) between children with ADHD and controls was examined using a series of analyses of covariance (ANCOVA) controlling for age. Analyses are presented in Table 3. Results for the cognitive control and fluency scores indicated nonsignificant group effects. Results for the working memory score indicated a significant effect of group. Children with ADHD exhibited significantly worse performance on working memory tasks compared with controls.
Table 3.
Summary of ANCOVA Results for Group Differences in Executive Function Factor Scores
| Measure | Factor | ANCOVA statistics |
|---|---|---|
| Cognitive Control | ||
| Age | F=101.20, df 1,89, p<.01, η2=.53 | |
| Group | F=0.00, df 1,89, p=.99, η2=.00 | |
| Fluency | ||
| Age | F=39.65, df 1,89, p>.01, η2=.38 | |
| Group | F=2.48, df 1,89, p=.12, η2=.03 | |
| Working Memory | ||
| Age | F=22.70, df 1,89, p=.00, η2=.20 | |
| Group | F=10.26, df 1,88, p<.01, η2=.11 | |
Associations among Executive Function Factors and Learning Problems
Table 4 shows the four-step hierarchical regression analysis examining the additive effects of the executive function scores on parent report of learning problems in children with ADHD. Age was entered at step one, cognitive control at step two, fluency at step three, and working memory at step four.
Table 4.
Summary of Hierarchical Regression Analysis for Predicting Learning Problems.
| Fixed Effects | |||||||
|---|---|---|---|---|---|---|---|
| Predictors | B | SE | β | t | R | R2 | ΔR2 |
| Step 1 | .23 | .06 | |||||
| Age | −.56 | .42 | −.23 | −1.32 | |||
| Step 2 | .27 | .07 | .02 | ||||
| Age | −.79 | .54 | −.33 | −1.47 | |||
| Cognitive Control | 1.33 | 1.88 | .16 | .71 | |||
| Step 3 | .46 | .21* | .14* | ||||
| Age | −.20 | .57 | −.08 | −.35 | |||
| Cognitive Control | .35 | 1.83 | .04 | .19 | |||
| Fluency | −4.12 | 1.88 | −.42 | −2.20* | |||
| Step 4 | .67 | .45** | .24** | ||||
| Age | −.30 | .49 | −.13 | −.61 | |||
| Cognitive Control | 3.14 | 1.76 | .38 | 1.79† | |||
| Fluency | −2.79 | 1.65 | −.29 | −1.70 | |||
| Working Memory | −5.81 | 1.71 | −.60 | −3.40** | |||
Note.
p < 0.10,
p < 0.05,
p < 0.01
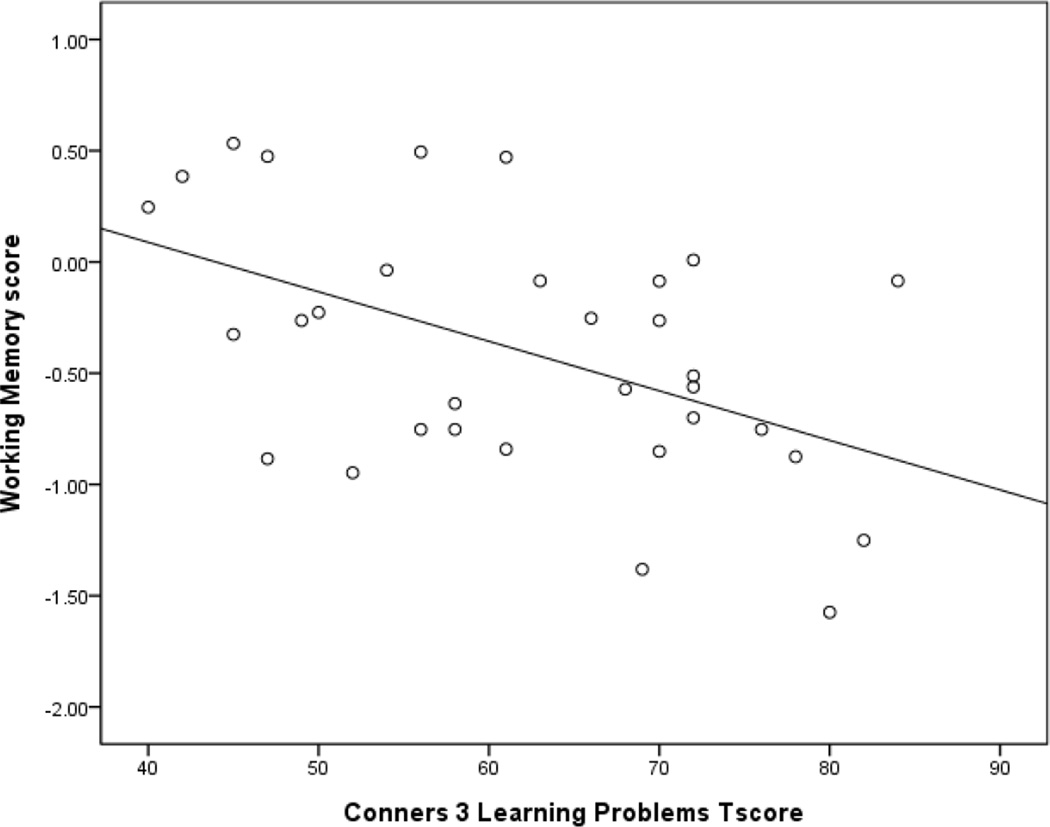
In the first step, the model was not statistically significant, F(1, 30)=1.738, p=.197 and Age explained only 5.5% of the variation in parent ratings of learning problems. Introducing cognitive control in step two only explained an additional 1.6% of the variation in learning problems and the model was not significant, F(2,29)=1.103, p=.345. In step three, Fluency explained an additional 13.7% of the variation in learning problems, and the model was not significant, F(3,28)=2.440, p=.085. Finally, the addition of working memory to the regression model explained an additional 23.8% of the variation in learning problems, and the model was statistically significant F(4,27)=5.410, p=.002. When all four variables were included in the step four of the regression model, working memory was the only significant predictor of learning problems. Children with poorer working memory had significantly more parent report of learning problems (See Figure 1). Together, the four independent variables accounted for 44.5% of the variation in parent report of learning problems.
Figure 1.
Working Memory and Parent Ratings of Learning Problems in Children with ADHD
Discussion
The present study compared executive functions in children with ADHD and healthy controls using the NIH EXAMINER battery to overcome many of the psychometric and test development issues which have hampered replication of specific executive function deficits in prior studies. The NIH EXAMINER battery, designed to be a reliable and valid battery of domain specific executive function, used item response theory to create comparable measures of working memory, cognitive control, and fluency by combining across multiple measures. Our findings indicate that working memory ability was significantly worse in children with ADHD compared with age and gender matched healthy control children. This is consistent with literature implicating working memory impairment as a core deficit in children with ADHD (Kasper, Alderson, & Hudec, 2012; Martinussen, et al., 2005; Rapport, 2001). According to the working memory model of ADHD, if present, inhibition is a downstream product of working memory impairment (Alderson, Rapport, & Kofler, 2007; Kofler, et al., 2008).
Contrary to our prediction, cognitive control was not significantly worse in children with ADHD compared with controls. This is somewhat unexpected given the literature proposing the related concept of inhibitory control as a primary deficit in children with ADHD (Barkley, 1997). However, the cognitive control factor may not be capturing the same concept of inhibition as described in prior studies. In fact, multiple types of inhibitory processes have been proposed, suggesting that inhibitory control is not a unitary construct that cuts across both cognitive and behavioral domains (Engelhardt, Nigg, Carr, & Ferreira, 2008; Logan, Cowan, & Davis, 1984; Nigg, 2000, 2001). In fact, Nigg (2000) distinguished between eight different kinds of inhibition that fall within the categories of executive inhibitory processes, motivational inhibitory processes, and automatic attentional inhibition processes. Inhibitory deficits in children with ADHD more often occur on tasks requiring suppression of a prepotent motor response (e.g., basic go/no-go task) and are less common on tasks requiring suppression of a conflicting response (e.g., flanker test; Nigg, 2001). Another study of adolescents and adults with ADHD also failed to demonstrate cognitive inhibition problems compared with controls (Engelhardt, et al., 2008).
Another reason for the lack of significant difference in cognitive control between children with ADHD and controls may be due to this sample of children with ADHD. Children were selected to have relatively “pure” ADHD, with no co-occurring learning or behavioral disorders. Still, the literature has demonstrated that substantial comorbidity exists between ADHD and other learning and behavioral disorders (Angold, Costello, & Erkanli, 1999). Furthermore, research suggests that impairment of inhibitory control processes may be worse in children with ADHD that is comorbid with other disorders, particularly learning disorders (Purvis & Tannock, 2000; Willcutt et al., 2001). Our results suggest that cognitive control may not be impaired in children with a relatively “pure” form of ADHD that is not comorbid with other disorders.
Working memory was also the only factor significantly associated with parent report of learning problems, which is consistent with prior research establishing working memory as a predictor of academic success (Alloway & Alloway, 2010; Alloway, et al., 2010; Gathercole, et al., 2004). Behaviors associated with working memory, such as holding information in mind and managing and manipulating multiple pieces of information simultaneously, are important for learning and acquiring knowledge. Consequently, a primary deficit in working memory may underlie the academic problems found in children with ADHD compared with healthy peers.
When considering academic outcomes, the literature distinguishes between academic knowledge and school grades, which may combine effort, behavior, homework completion, and knowledge (Bowers 2011; Randall and Englehard 2009). Research suggests that working memory is associated with learning and acquiring academic knowledge as measured by standardized achievement tests (Miller, Nevado-Montenegro, & Hinshaw, 2012), whereas organization/planning and inhibition are associated with school grades (Langberg, Dvorsky & Evans, 2013; Langberg et al. 2011). Assessment of academic knowledge in the present study was limited because children were excluded if diagnosed with a learning disorder or if word reading ability was below average; however, results still indicated working memory to be associated with learning. Thus, findings emphasize that working memory can have a measureable impact on learning even in the absence of a learning disorder. It is possible that children with ADHD and no comorbid learning or behavioral disorder may be better at compensating for working memory weaknesses when it comes to school performance. Unfortunately, we did not obtain information about school grades from children in the study sample, so we were unable to examine the effects of executive function scores on grades. Future studies should include multiple measures of academic outcomes, including both school grades and academic achievement scores, to further explore the impact of executive function deficits on school functioning.
With respect to intervention, stimulant medication is effective at diminishing parent and teacher rated inattention and hyperactivity symptoms (Swanson, Baler, & Volkow, 2011; Swanson et al., 1993); however, the impact of stimulants on working memory has been less clear. A review indicated that methylphenidate improved performance in working memory in only about 50% of studies (Pietrzak, Mollica, Maruff, & Snyder, 2006). Furthermore, many children treated with stimulants demonstrate improvements on attention and behavior ratings but make only marginal improvements on academic outcomes (Marcus & Durkin, 2011; Rapport, Denney, DuPaul, & Gardner, 1994). Thus, additional intervention options for working memory should continue to be explored.
More recently, computerized training of working memory in children with ADHD has yielded improvements on tests of working memory, response inhibition, and complex reasoning, as well as a significant reduction in the number of parent-rated inattention and hyperactivity/impulsivity symptoms (Klingberg et al., 2005). Only a few studies examined how computerized training of working memory my impact academic performance. Two studies indicated promising results for improvements in math (Holmes, Gathercole, & Dunning, 2009) and reading comprehension (Dahlin, 2011). However, another study examining children with ADHD and comorbid learning disorder did not find improvement in academic scores following working memory training (Gray et al., 2012). A recent meta-analysis of studies of working memory training demonstrates short term gains in working memory, but generalization to other contexts remains in question (Mervy-Lervag & Hulme, 2013). The current findings provide justification for additional studies to examine the potential benefit of working memory training on academic outcomes in children with ADHD.
Within the context of these findings, this study has several potential limitations which provide direction for future research. First, the sample size was very small which limited our ability to examine certain variables that may be of interest. For example, research suggests that males and females with ADHD may have different neuropsychological profiles and should be examined together and separately (O’Brien, Dowell, Mostofsky, Denckla, & Mahone, 2010). Our sample was too small to examine males and females separately. We were also unable to examine differences in executive function by subtypes of ADHD. The literature suggests that the executive function deficit profile may differ by subtype (Nigg, et al., 2005), and this would be worth exploring with the use of the NIH EXAMINER battery.
Another limitation was the broad age range of the sample (ages 8–16 years), which spanned important developmental periods (i.e., middle childhood through adolescence). Behavior regulation and executive function gradually improve over the course of development, and it is likely that children with ADHD also show variation in executive function deficits at different stages of development (Brocki, Fan, & Fossella, 2008). Our sample size did not allow for examination of executive function deficits at different ages. Future studies should include enough participants to examine executive function differences across development.
Finally, the current study did not include a measure of intelligence in the control group. Children with ADHD who had IQ scores below 85 were excluded, but we were unable to see if group differences remained after controlling for IQ. Then again, IQ shares significant variance with working memory (Ackerman, Beier, & Boyle, 2005), and it is possible that covarying for IQ would eliminate the group difference due to shared variance. Nonetheless, future studies should include a measure of IQ to compare across groups.
In conclusion, our findings indicate that, on a valid and reliable battery of executive function tests, children with ADHD and no comorbid disorders perform significantly worse on tests of working memory ability compared with healthy control children. Furthermore, poorer working memory is associated with parent report of learning problems in children with ADHD, even in the absence of a learning disorder diagnosis. Thus, working memory impairment appears to be one of the mechanisms for poor academic outcomes in children with ADHD.
Acknowledgments
This work was supported by the National Institutes of Health (NIH-NINDS-05-02) and the National Institute on Aging (Possin: K23AG037566).
Footnotes
The authors report no conflicts of interest.
References
- Ackerman PL, Beier ME, Boyle MO. Working memory and intelligence: the same or different constructs? Psychological Bulletin. 2005;131(1):30–60. doi: 10.1037/0033-2909.131.1.30. [DOI] [PubMed] [Google Scholar]
- Akinbami LJ, Liu X, Pastor PN, Reuben CA. Attention deficit hyperactivity disorder among children aged 5–17 years in the United States, 1998–2009. NCHS Data Brief. 2011;70:1–8. Retrieved from http://www.cdc.gov/nchs/products/databriefs.htm. [PubMed] [Google Scholar]
- Alderson RM, Rapport MD, Kofler MJ. Attention-deficit/hyperactivity disorder and behavioral inhibition: a meta-analytic review of the stop-signal paradigm. Journal of Abnormal Child Psychology. 2007;35(5):745–758. doi: 10.1007/s10802-007-9131-6. [DOI] [PubMed] [Google Scholar]
- Alloway TP, Alloway RG. Investigating the predictive roles of working memory and IQ in academic attainment. Journal of Experimental Child Psychology. 2010;106(1):20–29. doi: 10.1016/j.jecp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Alloway TP, Elliott J, Place M. Investigating the relationship between attention and working memory in clinical and community samples. Child Neuropsychology. 2010;16(3):242–254. doi: 10.1080/09297040903559655. [DOI] [PubMed] [Google Scholar]
- Alloway TP, Gathercole SE, Elliott J. Examining the link between working memory behavior and academic attainment in children with ADHD. Developmental Medicine & Child Neurology. 2010;52(7):632–636. doi: 10.1111/j.1469-8749.2009.03603.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Text rev. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Anderson VA, Anderson P, Northam E, Jacobs R, Catroppa C. Development of executive functions through late childhood and adolescence in an Australian sample. Developmental Neuropsychology. 2001;20(1):385–406. doi: 10.1207/S15326942DN2001_5. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A. Comorbidity. Journal of Child Psychology and Psychiatry. 1999;40(1):57–87. Retrieved from http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1469-7610/issues. [PubMed] [Google Scholar]
- Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs. 2009;23(Suppl 1):33–41. doi: 10.2165/00023210-200923000-00005. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biological Psychiatry. 2005;57(11):1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nature Reviews Neuroscience. 2003;4(10):829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Current Biology. 2010;20(4):R136–R140. doi: 10.1016/j.cub.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Berger A, Posner MI. Pathologies of brain attentional networks. Neuroscience & Biobehavioral Reviews. 2000;24(1):3–5. doi: 10.1016/s0149-7634(99)00046-9. Retrieved from http://www.sciencedirect.com/science/article/pii/S0149763499000469. [DOI] [PubMed] [Google Scholar]
- Bowers AJ. What’s in a grade? The multidimensional nature of what teacher-assigned grades assess in high school. Educational Research and Evaluation. 2011;17(3):141–159. [Google Scholar]
- Breslau J, Miller E, Breslau N, Bohnert K, Lucia V, Schweitzer J. The impact of early behavior disturbances on academic achievement in high school. Pediatrics. 2009;123(6):1472–1476. doi: 10.1542/peds.2008-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocki K, Fan J, Fossella J. Placing neuroanatomical models of executive function in a developmental context: imaging and imaging--genetic strategies. Annals of the New York Academy of Science. 2008;1129:246–255. doi: 10.1196/annals.1417.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R, Kurland DM, Goldberg J. Operational efficiency and the growth of short-term memory span. Journal of Experiemental Child Psychology. 1982;33(3):386–404. [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nature Reviews Neuroscience. 2002;3(8):617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Chhabildas N, Pennington BF, Willcutt EG. A comparison of the neuropsychological profiles of the DSM-IV subtypes of ADHD. Journal of Abnormal Child Psychology. 2001;29(6):529–540. doi: 10.1023/a:1012281226028. [DOI] [PubMed] [Google Scholar]
- Chiappe P, Hasher L, Siegel LS. Working memory, inhibitory control, and reading disability. Memory & Cognition. 2000;28(1):8–17. doi: 10.3758/bf03211570. [DOI] [PubMed] [Google Scholar]
- Collett B, Ohan J, Myers K. Ten-year review of rating scales V: Scales assessing attention-deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42(9):1015–1037. doi: 10.1097/01.CHI.0000070245.24125.B6. Retrieved from http://www.mdconsult.com/das/article/body/403646378-2/jorg=journal&source=&sp=14043839&sid=0/N/375590/1.html?issn=0890-8567. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners 3rd Edition. North Tonawanda, NY: Multi-Health Systems; 2008. [Google Scholar]
- Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, Castellanos FX. Toward Systems Neuroscience of ADHD: A Meta-Analysis of 55 fMRI Studies. American Journal of Psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie J, Stabile M. Child mental health and human capital accumulation: the case of ADHD. Journal Health Economics. 2006;25(6):1094–1118. doi: 10.1016/j.jhealeco.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Dahlin KE. Effects of working memory training on reading in children with special needs. Reading and Writing. 2011;24(4):479–491. [Google Scholar]
- Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. Journal of Child Psychologyand Psychiatry. 2006;47(10):1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Doyle AE. Executive functions in attention-deficit/hyperactivity disorder. Journal of Clinical Psychiatry. 2006;(67 Suppl 8):21–26. Retrieved from http://www.psychiatrist.com/default2.asp#. [PubMed]
- DuPaul G, Power T, Anastopoulos A, Reid R. ADHD Rating Scale-IV: Checklists, norms, and clinical interpretation. New York: Guilford Press; 1998. [Google Scholar]
- Engelhardt PE, Nigg JT, Carr LA, Ferreira F. Cognitive inhibition and working memory in attention-deficit/hyperactivity disorder. Journal ofAbnorm Psychology. 2008;117(3):591–605. doi: 10.1037/a0012593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ. Early disruptive behavior, IQ, and later school achievement and delinquent behavior. Journal of Abnormal Child Psychology. 1995;23(2):183–199. doi: 10.1007/BF01447088. Retrieved from http://rd.springer.com/journal/volumesAndIssues/10802. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Lynskey MT, Horwood LJ. Attentional difficulties in middle childhood and psychosocial outcomes in young adulthood. Journal of Child Psychology and Psychiatry. 1997;38(6):633–644. doi: 10.1111/j.1469-7610.1997.tb01690.x. Retrieved from http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1469-7610. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Youngstrom EA, Glutting JJ, Watkins MW. ADHD and achievement: meta-analysis of the child, adolescent, and adult literatures and a concomitant study with college students. Journal of Learning Disabilities. 2007;40(1):49–65. doi: 10.1177/00222194070400010401. Retrieved from http://ldx.sagepub.com/ [DOI] [PubMed] [Google Scholar]
- Galera C, Melchior M, Chastang JF, Bouvard MP, Fombonne E. Childhood and adolescent hyperactivity-inattention symptoms and academic achievement 8 years later: the GAZEL Youth study. Psychological Medicine. 2009;39(11):1895–1906. doi: 10.1017/S0033291709005510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole SE, Pickering SJ, Ambridge B, Wearing H. The structure of working memory from 4 to 15 years of age. Developmental Psychology. 2004;40(2):177–190. doi: 10.1037/0012-1649.40.2.177. [DOI] [PubMed] [Google Scholar]
- Giannopulu I, Escolano S, Cusin F, Citeau H, Dellatolas G. Teachers’ reporting of behavioural problems and cognitive-academic performances in children aged 5–7 years. British Journal of Educational Psychology. 2008;78(Pt 1):127–147. doi: 10.1348/000709907X204372. [DOI] [PubMed] [Google Scholar]
- Gray SA, Chaban P, Martinussen R, Goldberg R, Gotlieb H, Kronitz R, Tannock R. Effects of a computerized working memory training program on working memory, attention, and academics in adolescents with severe LD and comorbid ADHD: a randomized controlled trial. Journal of Child Psychology and Psychiatry. 2012;53(12):1277–1284. doi: 10.1111/j.1469-7610.2012.02592.x. [DOI] [PubMed] [Google Scholar]
- Hellmuth J, Mirsky J, Heuer HW, Matlin A, Jafari A, Garbutt S, Boxer AL. Multicenter validation of a bedside antisaccade task as a measure of executive function. Neurology. 2012;78(23):1824–1831. doi: 10.1212/WNL.0b013e318258f785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes J, Gathercole SE, Dunning DL. Adaptive training leads to sustained enhancement of poor working memory in children. Developmental Science. 2009;12(4):F9–F15. doi: 10.1111/j.1467-7687.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Kasper LJ, Alderson RM, Hudec KL. Moderators of working memory deficits in children with attention-deficit/hyperactivity disorder (ADHD): a meta-analytic review. Clinical Psychology Review. 2012;32(7):605–617. doi: 10.1016/j.cpr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlstrom K, Westerberg H. Computerized training of working memory in children with ADHD--a randomized, controlled trial. Journal American Academy of Child and Adolescent Psychiatry. 2005;44(2):177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Bolden J, Altro TA. Working memory as a core deficit in ADHD: Preliminary findings and implications. The ADHD Report. 2008;16:8–14. [Google Scholar]
- Kramer J. Executive Abilities: Measures and Instruments for Neurobehavioral Evaluation and Research (EXAMINER) 2011 Retrieved December 13, 2012, from http://examiner.ucsf.edu/EXAMINER%20User%20Manual.pdf.
- Krueger CE, Bird AC, Growdon ME, Jang JY, Miller BL, Kramer JH. Conflict monitoring in early frontotemporal dementia. Neurology. 2009;73(5):349–355. doi: 10.1212/WNL.0b013e3181b04b24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg JM, Dvorsky MR, Evans SW. What specific facets of executive function are associated with academic functioning in youth with attention-deficit/hyerpactivity disorder? Journal of Abnormal Child Psychology. 2013 doi: 10.1007/s10802-013-9750-z. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg JM, Molina BS, Arnold LE, Epstein JN, Altaye M, Hinshaw SP, Hechtman L. Patterns and predictors of adolescent academic achievement and performance in a sample of children with attention-deficit/hyperactivity disorder. Journal of Clincal Child and Adolescent Psychology. 2011;40(4):519–531. doi: 10.1080/15374416.2011.581620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loe IM, Feldman HM. Academic and educational outcomes of children with ADHD. Journal of Pediatric Psychology. 2007;32(6):643–654. doi: 10.1093/jpepsy/jsl054. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. Journal Experimental Psychology: Human Perception and Performance. 1984;10(2):276–291. doi: 10.1037//0096-1523.10.2.276. Retrieved from http://psycnet.apa.org/index.cfm?fa=browsePA.volumes&jcode=xhp. [DOI] [PubMed] [Google Scholar]
- Marcus SC, Durkin M. Stimulant adherence and academic performance in urban youth with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(5):480–489. doi: 10.1016/j.jaac.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(4):377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- Massetti GM, Lahey BB, Pelham WE, Loney J, Ehrhardt A, Lee SS, Kipp H. Academic achievement over 8 years among children who met modified criteria for attention-deficit/hyperactivity disorder at 4–6 years of age. Journal of Abnormal Child Psychology. 2008;36(3):399–410. doi: 10.1007/s10802-007-9186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melby-Lervåg M, Hulme C. Is working memory training effective? A meta-analytic review. Developmental psychology. 2013;49(2):270–291. doi: 10.1037/a0028228. [DOI] [PubMed] [Google Scholar]
- Merrell C, Tymms PB. Inattention, hyperactivity and impulsiveness: their impact on academic achievement and progress. British Journal of Educational Psychology. 2001;71(Pt 1):43–56. doi: 10.1348/000709901158389. Retrieved from http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)2044-8279. [DOI] [PubMed] [Google Scholar]
- Miller M, Nevado-Montenegro AJ, Hinshaw SP. Childhood executive function continues to predict outcomes in young adult females with and without childhood-diagnosed ADHD. Journal of Abnormal Child Psychology. 2012;40:657–668. doi: 10.1007/s10802-011-9599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BS, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, Houck PR. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(5):484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TL, Schettler SP, Killiany RJ, Rosene DL, Moss MB. Impairment in Delayed Nonmatching to Sample Following Lesions of Dorsal Prefrontal Cortex. Behavioral Neuroscience. 2012;126(6):772–780. doi: 10.1037/a0030493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT. On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychological Bulletin. 2000;126(2):220–246. doi: 10.1037/0033-2909.126.2.220. Retrieved from http://psycnet.apa.org/index.cfm?fa=browsePA.volumes&jcode=bul. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Is ADHD a disinhibitory disorder? Psychology Bulletin. 2001;127(5):571–598. doi: 10.1037/0033-2909.127.5.571. Retrieved from http://psycnet.apa.org/index.cfm?fa=browsePA.volumes&jcode=bul. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biological Psychiatry. 2005;57(11):1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- O’Brien JW, Dowell LR, Mostofsky SH, Denckla MB, Mahone EM. Neuropsychological profile of executive function in girls with attention-deficit/hyperactivity disorder. Archives of Clinical Neuropsychology. 2010;25(7):656–670. doi: 10.1093/arclin/acq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. 1996;37(1):51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. Retrieved from http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1469-7610) [DOI] [PubMed] [Google Scholar]
- Petrides M. The role of the mid-dorsolateral prefrontal cortex in working memory. Experimental Brain Research. 2000;133(1):44–54. doi: 10.1007/s002210000399. Retrieved from http://www.springer.com/biomed/neuroscience/journal/221. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Mollica CM, Maruff P, Snyder PJ. Cognitive effects of immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. [Review] Neuroscience and Biobehavioral Review. 2006;30(8):1225–1245. doi: 10.1016/j.neubiorev.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Pliszka SR. Patterns of psychiatric comorbidity with attention-deficit/hyperactivity disorder. Child & Adolescent Psychiatric Clinics of North America. 2000;9(3):525–540. vii. Retrieved from http://www.childpsych.theclinics.com/issues. [PubMed] [Google Scholar]
- Purvis KL, Tannock R. Phonological processing, not inhibitory control, differentiates ADHD and reading disability. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(4):485–494. doi: 10.1097/00004583-200004000-00018. [DOI] [PubMed] [Google Scholar]
- Rabin LA, Barr WB, Burton LA. Assessment practices of clinical neuropsychologists in the United States and Canada: a survey of INS, NAN, and APA Division 40 members. Archives ofClinical Neuropsychology. 2005;20(1):33–65. doi: 10.1016/j.acn.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Rabiner D, Coie JD. Early attention problems and children’s reading achievement: a longitudinal investigation. The Conduct Problems Prevention Research Group. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(7):859–867. doi: 10.1097/00004583-200007000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall J, Engelhard G. Examining teacher grades using rasch measurement theory. Journal of Educational Measurement. 2009;46(1):1–18. [Google Scholar]
- Rapport MD. Bridging theory and practice: conceptual understanding of treatments for children with attention deficit hyperactivity disorder (ADHD), obsessive-compulsive disorder (OCD), autism, and depression. Journal of Clinical Child Psychology. 2001;30(1):3–7. doi: 10.1207/S15374424JCCP3001_2. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Chung KM, Shore G, Isaacs P. A conceptual model of child psychopathology: implications for understanding attention deficit hyperactivity disorder and treatment efficacy. Journal of Clinical Child Psychology. 2001;30(1):48–58. doi: 10.1207/S15374424JCCP3001_6. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Denney C, DuPaul GJ, Gardner MJ. Attention deficit disorder and methylphenidate: normalization rates, clinical effectiveness, and response prediction in 76 children. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33(6):882–893. doi: 10.1097/00004583-199407000-00015. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Ciranni MA. Contributions of the prefrontal cortex and basal ganglia to set shifting. Journal of Cognitive Neuroscience. 2002;14(3):472–483. doi: 10.1162/089892902317361985. [DOI] [PubMed] [Google Scholar]
- Reynolds C, Kamphaus R. Behavior Assessment for Children, (BASC-2) Circle Pines, MN: American Guidance Service; 2004. [Google Scholar]
- Rogers M, Hwang H, Toplak M, Weiss M, Tannock R. Inattention, working memory, and academic achievement in adolescents referred for attention deficit/hyperactivity disorder (ADHD) Child Neuropsychology. 2011;17(5):444–458. doi: 10.1080/09297049.2010.544648. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57(11):1263–1272. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Stefanatos GA, Baron IS. Attention-deficit/hyperactivity disorder: a neuropsychological perspective towards DSM-V. Neuropsychology Review. 2007;17(1):5–38. doi: 10.1007/s11065-007-9020-3. [DOI] [PubMed] [Google Scholar]
- Stern CE, Owen AM, Tracey I, Look RB, Rosen BR, Petrides M. Activity in ventrolateral and mid-dorsolateral prefrontal cortex during nonspatial visual working memory processing: evidence from functional magnetic resonance imaging. Neuroimage. 2000;11(5 pt 1):392–399. doi: 10.1006/nimg.2000.0569. [DOI] [PubMed] [Google Scholar]
- Swanson J, Baler RD, Volkow ND. Understanding the effects of stimulant medications on cognition in individuals with attention-deficit hyperactivity disorder: a decade of progress. Neuropsychopharmacology. 2011;36:207–226. doi: 10.1038/npp.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM, McBurnett K, Wigal T, Pfiffner LJ, Williams L, Christian DL. Effects of stimulant medication on children with attention deficit disorder: a ‘review of reviews’. Exceptional Children. 1993;60(2):154–162. Retrieved from http://www.questia.com/library/p367/exceptional-children. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, Texas: Harcourt Assessment; 1999. [Google Scholar]
- Weintraub S, Bauer PJ, Zelazo PD, Wallner-Allen K, Dikmen SS, Heaton RK, Gershon RC. Nih toolbox cognition battery (cb): introduction and pediatric data. Monographs of the Society for Research on Child Development. 2013;78(4):1–15. doi: 10.1111/mono.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test - 4 (WRAT-4) Lutz, FL: Psychological Assessment Resources; 2006. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Boada R, Ogline JS, Tunick RA, Chhabildas NA, Olson RK. A comparison of the cognitive deficits in reading disability and attention-deficit/hyperactivity disorder. Journal of Abnormal Psychology. 2001;110:157–172. doi: 10.1037//0021-843x.110.1.157. Retrieved from http://www.apa.org/pubs/journals/abn/index.aspx. [DOI] [PubMed] [Google Scholar]
- Wodka EL, Mostofsky SH, Prahme C, Gidley Larson JC, Loftis C, Denckla MB, Mahone EM. Process examination of executive function in ADHD: sex and subtype effects. Clinical Neuropsychology. 2008;22(5):826–841. doi: 10.1080/13854040701563583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock R, McGraw K, Mather N. Woodcock-Johnson Third Edition, Tests of Achievement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]