Abstract
In vivo, bone marrow-derived multipotent mesenchymal stromal cells (MSC) have been identified at sites of tumors, suggesting that specific signals mobilize and activate MSC to migrate to areas surrounding tumors. The signals and migratory mechanisms that guide MSC are not well understood. Here, we investigated the migration of human MSC induced by conditioned medium of Huh-7 hepatoma cells (Huh-7 CM). Using a transwell migration system, we showed that human MSC migration was increased in the presence of Huh-7 CM. Using a human cytokine antibody array, we detected increased levels of MIP-1δ and MIP-3α in Huh-7 CM. Recombinant chemokines MIP-1δ and MIP-3α induced MSC migration. Anti-MIP-1δ and anti-MIP-3α antibodies added to Huh-7 CM decreased MSC migration, further suggesting that MIP-1δ and MIP-3α were implicated in the Huh-7 CM-induced MSC migration. By real-time polymerase chain reaction, we observed an absence of chemokine receptors CCR2 and CXCR2 and low expression of CCR1, CCR5, and CCR6 in MSC. Expression of these chemokine receptors was not regulated by Huh-7 CM. Furthermore, matrix metalloproteinase 1 (MMP-1) expression was strongly increased in MSC after incubation with Huh-7 CM, suggesting that MSC migration depends on MMP-1 activity. The signaling pathway MAPK/ERK was activated by Huh-7 CM but its inhibition by PD98059 did not impair Huh-7 CM-induced MSC migration. Further, long-term incubation of MSC with MIP-1δ increased α-smooth muscle actin expression, suggesting its implication in the Huh-7 CM-induced evolvement of MSC into myofibroblasts. In conclusion, we report that two inflammatory cytokines, MIP-1δ and MIP-3α, are able to increase MSC migration in vitro. These cytokines might be responsible for migration and evolvement of MSC into myofibroblasts around tumors.
Introduction
Multipotent mesenchymal stromal cells (MSC) are often considered as mesenchymal stem cells with highly proliferative capacity and multipotent differentiation potential. MSC can be differentiated in vitro into cell lineages derived from mesoderm such as osteoblasts, adipocytes, and chondrocytes [1]. In addition, MSC display immunomodulatory properties [2–6] and therefore have been investigated for their potential application in autoimmune diseases [7], cell-based immunotherapy in bone marrow and solid-organ transplantation [8–10], as antifibrotic agent in chronic liver diseases [11], and as cell therapies in regenerative medicine [12].
We recently demonstrated that MSC secrete anti-inflammatory molecules such as IL-1Ra, allowing an anti-fibrotic effect that attenuates liver fibrosis in mice [13]. Human MSC do not develop into tumors [14], but some reports indicate that MSC participate in the pathogenesis of cancer by transforming into cancer-associated fibroblasts (CAFs) [15–19]. Some studies indicated that, when injected systemically, MSC migrate to sites of inflammation and diseased tissues [20–23]. The in vivo tropism toward gliomas has been extensively studied. It appears that targeting gliomas by MSC is possible [24], but the ability of MSC to migrate over long distances to gliomas is still under debate [25,26]. In vitro, MSC migrate to conditioned medium from gliomas [27], breast cancer [28], and colorectal cancer [29] cells. Related to their tumor-homing properties, MSC have gained attention as potential therapeutic vehicles to deliver anti-tumor agents for cancer therapies [30–32]. However, one major problem encountered with MSC is their poor migratory property once transplanted. Further insights of the mechanisms regulating MSC migration should help manipulate MSC for their use in specific applications.
So far, soluble factors, such as chemokines, seem to play an important role in inducing MSC migration and the attraction of MSC to tumors. Several chemokines and factors have been identified by systemic screening of conditioned medium of tumor cells, such as SDF-1 [29,33,34], IL-8 [35], MCP-1 [36], and platelet-derived growth factor BB (PDGF-BB) [26,37]. Further, extracellular proteolysis through matrix metalloproteinase (MMP) seems to be involved in the migratory activity of MSC [27,38,39]. Results from studies analyzing chemokines receptor expression in MSC populations are contradictory [35,40–43]. However, several of these receptors were shown to be implicated in MSC migration in vitro such as CXCR1 and CXCR2 [35], as well as CCR2 and CXCR4 [36].
With the aim of further identifying chemokines involved in MSC migration, we analyzed the human hepatoma cell line conditioned medium (Huh-7 CM), which increased MSC migration similarly to PDGF-BB. In this study, we showed that several chemokines were present in the Huh-7 CM. Two of them, MIP-1δ and MIP-3α, increased MSC migration. This Huh-7 CM-induced migration was not regulated through differential expression of the chemokine receptors. We further observed that αSMA expression was induced in MSC after long-term treatment by Huh-7 CM and MIP-1δ. We concluded that in vitro migration of MSC can be stimulated by chemokines MIP-1δ and MIP-3α and that prolonged treatment of MSC with recombinant MIP-1δ favors the differentiation toward myofibroblasts. Hence, in vivo MIP-1δ and MIP-3α might be important chemoattractants that attract MSC to sites of injury and further favor differentiation into myofibroblasts.
Materials and Methods
Human bone marrow-derived multipotent MSC isolation and culture
Human adult bone marrow cells were collected from femoral heads of patients undergoing total hip arthroplasty after informed consent. This research project was approved by local ethics committees of the University Hospitals of Geneva, Switzerland. Cells were isolated from bone fragments, cultured, and characterized as previously described [44]. Medium used for culture was Iscove's modified Dulbecco's medium (Cambrex), supplemented with 10% fetal calf serum (FCS) (Gibco-Invitrogen), 100 IU/mL penicillin, 100 μg/mL streptomycin (P-S) (Gibco-Invitrogen), dithiothreitol (DTT; Sigma), and 10 ng/mL PDGF-BB (PeproTech EC Ltd.). Cells were expanded as previously described [45,46]. MSC were used between passages three and five.
Preparation of Huh-7, HepG2, and primary human hepatocyte conditioned media
Human hepatoma cells, Huh-7 and HepG2, as well as primary human hepatocytes, were cultured at subconfluence in a 150 cm2 bottle in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% FCS and P-S for 48 h. Conditioned media were harvested, filtered, and stored at −20°C until use.
Measurement of cytokines in Huh-7 CM
Human cytokine antibody array (C series 1000; Raybiotech, Inc.) was used for the qualitative assessment of 60 cytokines in Huh-7 CM and control medium, as indicated by the manufacturer.
Cell migration assays
The migration capacity of MSC was analyzed using Costar transwell migration chambers (6.5 mm diameter inserts) with 8.0 μm pore size (Corning Incorporated). These transwell chambers (referred to as upper compartment) were inserted into a 24-well tissue culture plate (referred to as lower compartment). Cells were cultured for at least 4 h in DMEM 5% FCS before trypsinization. After trypsinization, cells were washed and 6×103 cells per 40 μL DMEM 5% FCS were plated in the upper compartment. One milliliter control medium (DMEM 5% FCS) containing recombinant human growth factors or cytokines MIP-1δ, MIP-3α, IGFBP-2, or PDGF-BB (20 ng/mL, all from Peprotech), angiogenin (Novoprotein) (100 ng/mL), inhibitor PD98059 (50 μM), or conditioned medium were added in the lower compartment. Neutralizing antibodies against human MIP-1δ (R&D Systems) (10 μg/mL) [47] and MIP-3α (Peprotech) (10 μg/mL) [48] as well as control IgG were used. After 18 h of migration, cells were fixed with methanol at −20°C for 2 min. Nonmigrating cells in the upper compartment were wiped off from the membrane with a cotton swab. Migrating cells were found on the lower side of the membrane and were colored with Hoechst. The total number of migrating cells was quantified by counting blue nuclei using Metamorph software. A migrating index was calculated by expressing the number of migrating cells as a fold increase of migrated cells in control condition (DMEM 5% FCS), which was set at 1.
Cell proliferation assay
MSC were seeded at 5,000 cells per well in a 24-well plate. After overnight incubation, cells were rinsed with phosphate-buffered saline (PBS) and incubated for 4 h in DMEM 5% FCS. Then, cells were exposed to control medium or medium containing MIP-1δ and MIP-3α (50 ng/mL) for 48 h. Cells were then rinsed, fixed with paraformaldehyde (PFA) 4%, and stained with Ki67 antibody. Ki67-positive cells were counted and expressed as a ratio of the total number of cells.
Cell differentiation assay
Differentiation assay was performed as previously described [44]. Briefly, MSC were trypsinized and seeded at high density (25,000 cells per cm2). MSC were exposed to either control medium or MIP-1δ and MIP-3α. Adipogenic differentiation was induced with adipogenic differentiation medium for 3 weeks. Cells were fixed with cold 10% formalin for 1 h, washed twice with water, and stained with Oil-red-O solution (Sigma) for 2 h at room temperature, to reveal triglyceride droplets in the cytoplasm. Cells were washed twice, coverslipped, and observed under an optical microscope (Zeiss Axiophot1; Carl Zeiss AG).
Mitogen-activated protein kinase/extracellular signal-related kinase activation assay
MSC were seeded at 3×104 cells per well on a 24-well plate. After overnight incubation, cells were rinsed twice with PBS and incubated for 4 h in medium without FCS. Then, cells were exposed to conditioned medium or medium containing growth factors for 30 min. Cells were rinsed with ice-cold PBS containing 1 mM orthovanadate (Na3Vo4). MSC were scrapped in DTT loading buffer 2×(0.4 M Tris pH 6.8, 20% glycerol, 4% SDS, 10% DTT), snap frozen, boiled at 95°C for 5 min, and submitted to electrophoresis.
SDS-polyacrylamide gel electrophoresis and western blot analysis
Whole cell extract proteins were separated on 12% SDS-polyacrylamide gel and electro-blotted on polyvinylidene fluoride (PVDF) membranes (Milipore). Membranes were blocked for 1 h with either 5% nonfat dry milk or 5% bovine serum albumin (BSA) in TBST (tris-buffered saline 150 mM NaCl, 20 mM Tris–HCl, pH 7.5) containing 0.1% Tween 20. Membranes were incubated overnight with the primary antibodies diluted in TBST with nonfat dry milk or BSA. Thereafter, membranes were washed in TBST and further incubated with secondary peroxidase-conjugated (HRP-goat anti-mouse or goat anti-rabbit; Bio-Rad) IgG antibody. Protein bands were visualized by the enhanced chemiluminescence system as recommended by the manufacturer (UptiLight HRP Blot Substrate-Reagent A). Antibodies against phosphorylated and total ERK were purchased from Cell Signaling, and anti-GAPDH was purchased from Santa Cruz Biotechnology. Antibody against MMP-1 was purchased from Abcam Antibody against αSMA and was a kind gift of Prof. C. Chaponnier (Geneva). The intensity of signals was evaluated by analyzing the optical density of the spots using Quantity One software.
Real-time polymerase chain reaction
Total RNA was extracted from expanded MSC and from MSC treated by control condition, PDGF-BB, or Huh-7 CM for 48 h using the RNeasy™ Mini Kit. cDNA was synthesized from 100 ng total RNA with SuperScript™. For real-time polymerase chain reaction (PCR), the following SYBR Green human QuantiTect Primers (from Qiagen) were used: MMP-1 (QT00014581), MMP-2 (QT00088396), TIMP1 (QT00084168), CCR2 (QT00000224), CXCR2 (QT00000518), and CCR6 (QT01666140). Other specific primers (Invitrogen) were used: CCR1 (forward TTTGGTGTCATCACCAGCAT and reverse GCCTGAAACAGCTTCCACTC) and CCR5 (forward CTGAGACATCCGTTCCCCTA and reverse CTGCGATTTGCTTCACATTG). Normalization was performed using three genes: glyceraldehyde 3-phosphate dehydrogenase (GAPDH), tata binding protein (TBP), and transferrin receptor protein 1 (TfR1).
Statistical analysis
All values are presented as mean±SD. For statistical analyses, the Student's t-test was used. A P value <0.05 was considered statistically significant.
Results
Huh-7 cell conditioned medium and PDGF-BB induce MSC migration
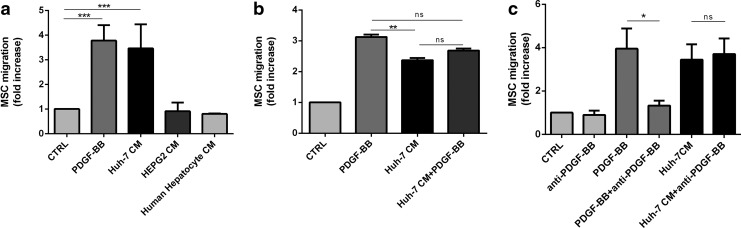
To investigate MSC migration, we exposed MSC to conditioned medium of primary hepatocytes and of two different human hepatoma cell lines, Huh-7 and HepG2. We used PDGF-BB, a known inducer of migration, as a positive control. The migration, assessed using a transwell system, was increased by PDGF-BB (3.9±0.9-fold) and Huh-7 CM (3.5±1.3) compared with control (Fig. 1a). CM from hepatoma cell line HepG2 and from primary human hepatocytes did not activate MSC migration. Addition of PDGF-BB to Huh-7 CM did not potentiate MSC migration (2.7±0.2) compared with Huh-7 CM alone (Fig. 1b). Addition of an anti-PDGF-BB antibody in Huh-7 CM did not affect cell migration, suggesting that PDGF-BB was not the responsible factor in the Huh-7 CM (Fig. 1c). Other recombinant factors, reported to induce MSC migration, such as SDF-1 [49], IL-8 [35], and TGF-β1 [50], did not affect migration in our assay (data not shown).
FIG. 1.
Huh-7 CM and PDGF-BB induces MSC migration. (a) 6×103 human MSC per condition were seeded in transwell with 8 μm pores. After 24 h of incubation, cells were rinsed, fixed by methanol, and stained by Hoechst. Nucleus were counted after whipping of nonmigrating cells on the top of the transwell. Incubation with PDGF-BB (20 ng/mL) and Huh-7 CM reached, respectively, 3.9±0.9 and 3.5±1.3-fold increased migration compared with control condition. HepG2 CM as well as conditioned medium from primary human hepatocytes did not induce MSC migration. The data are presented as mean values±SD from four independent experiments. (b) Combining Huh-7 CM and PDGF-BB did not potentiate the number of migrating cells. (c) Huh-7 CM were preincubated for 30 min with antibodies (0.5 μg/mL) against human PDGF-BB and then added to the migration assay for 24 h. For (b) and (c), mean values±SD of three independent experiments were performed as duplicated experiments. Migration of MSC under Huh-7 CM and Huh-7 CM with the anti-PDGF-BB antibody is not significantly different (*P=0.148). (**P≤0.01); (***P≤0.001); n.s., no significance. MSC, mesenchymal stromal cells; PDGF-BB, platelet derived growth factor BB.
Huh-7 cells produce angiogenin, IGFBP-2, MIP-1δ, and MIP-3α
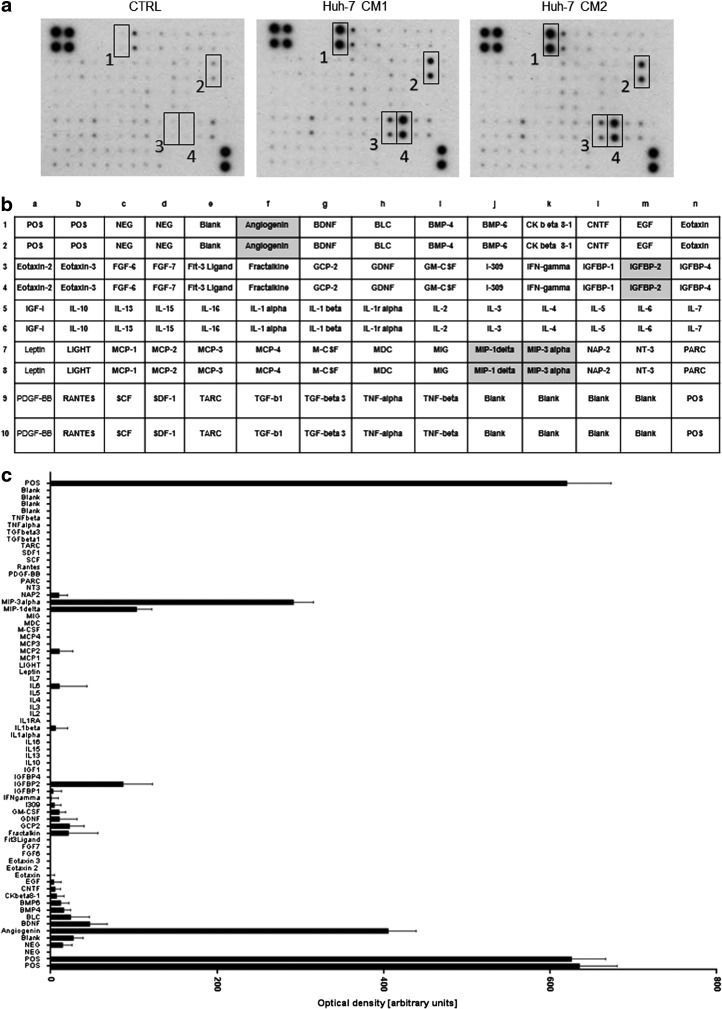
To identify cytokines potentially involved in MSC migration induced by Huh-7 cells, we analyzed the cytokine profile of Huh-7 CM. We found that angiogenin, IGFBP-2, MIP-1δ (CCL15), and MIP-3α (CCL20) were secreted by Huh-7 cells (Fig. 2a, b). Intensity of the signals was evaluated by analyzing the optical density of the spots (Fig. 2c). As shown in the graph, chemokines known to be important for MSC migration, such as PDGF-BB, TGF-β1, SDF-1, IGF-1, IL-6, and IL-1β, were not present in the Huh-7 CM, suggesting that they are not responsible for Huh-7-induced MSC migration.
FIG. 2.
Huh-7 cells produce increased levels of angiogenin, IGFBP-2, MIP-1δ, and MIP-3α. (a) Membranes of the human inflammatory cytokine antibody array were incubated for 2 h with control medium (DMEM 5% FCS) and two different preparations of Huh-7 CM. Several chemokines with increased levels such as MIP-1δ, MIP-3α, and angiogenin were detected in Huh-7. (b) Cytokines and their position on the membranes are shown in the table. (c) The graph shows the intensity of signals that was evaluated by analyzing the optical density of the spots. The experiment was performed twice with two different preparations of Huh-7 CM. DMEM, Dulbecco's modified Eagle medium.
MIP-1δ and MIP-3α are involved in the increased migration of MSC
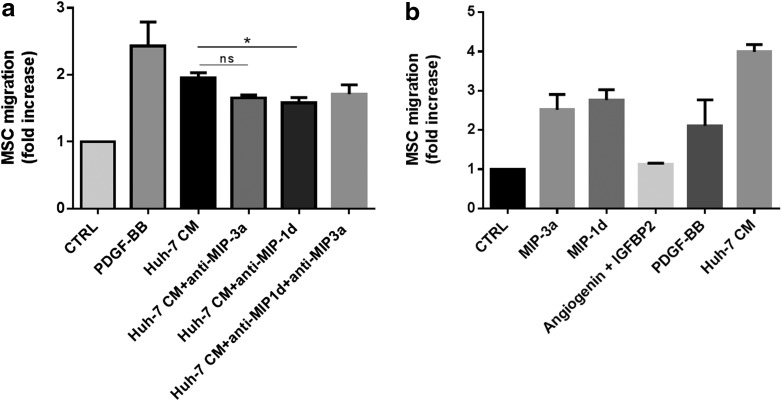
To investigate whether MIP-1δ and MIP-3α were involved in the Huh-7 CM-induced migration of MSC, we performed a migration assay in the presence of blocking antibodies directed against MIP-1δ and MIP-3α. As shown in Figure 3a, in experiments using MSC from four different donors, antibodies against MIP-1δ and MIP-3α slightly decreased MSC migration. However, inhibition of MSC migration was statistically significant only for MIP-1δ (Fig. 3a). No additive effect was observed when both antibodies are added simultaneously. Further, using recombinant proteins MIP-1δ and MIP-3α, MSC migration was induced in three out of five experiments when using a physiological concentration of recombinant proteins (50 ng/mL), whereas the use of angiogenin (100 ng/mL) and IGFBP-2 (20 ng/mL) did not modulate MSC migration (Fig. 3b). These results suggest that both chemokines MIP-1δ and MIP-3α are able to induce migration of MSC and also that Huh-7 CM most likely contains other factors increasing migration. We further investigated whether MIP-1δ and MIP-3α modulated adipogenic differentiation and proliferation of MSC, but these activities were not affected (data not shown).
FIG. 3.
Recombinant chemokines increased transwell migration of MSC in three out of five experiments. (a) Huh-7 CM were pre-incubated for 30 min with antibodies (10 μg/mL) against human MIP-3α and MIP-1δ. MSC were then treated with control medium PDGF-BB, Huh-7 CM, or Huh-7 CM containing the antibody against MIP-3α or MIP-1δ or both antibodies and migration was performed. Data are presented as mean±SD of three independent experiments each performed as duplicate (*P<0.05). (b) Transwell migration of MSC in control medium containing either recombinant MIP-3α, MIP-1δ, angiogenin, and IGFBP-2 or PDGF-BB compared with Huh-7 CM. Data are presented as mean±SD of three independent experiments each performed as duplicate (*P<0.05). n.s., no significance.
Expression of chemokine receptors and proteins involved in ECM degradation in MSC
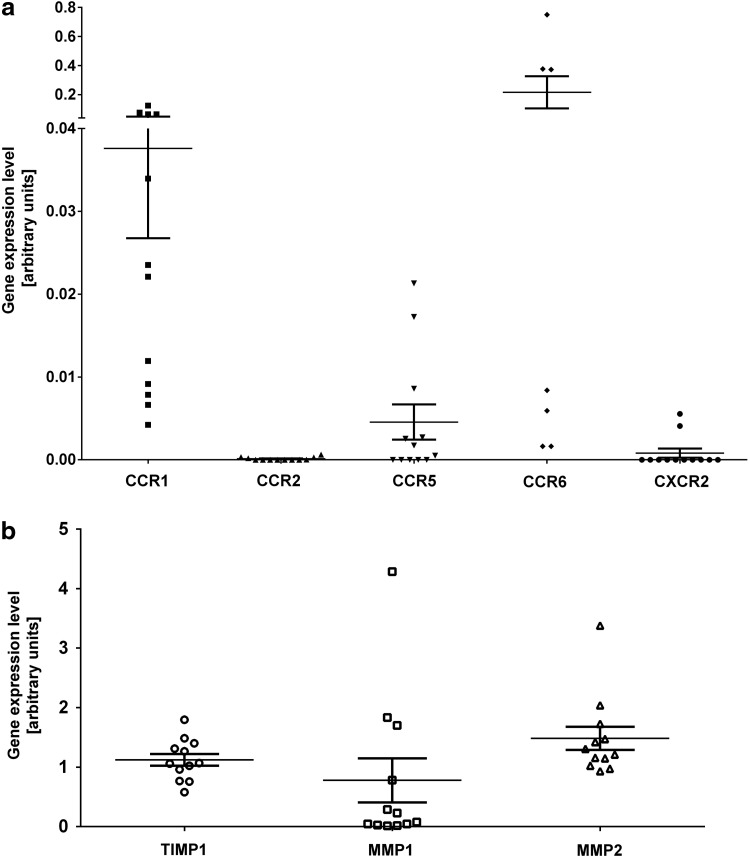
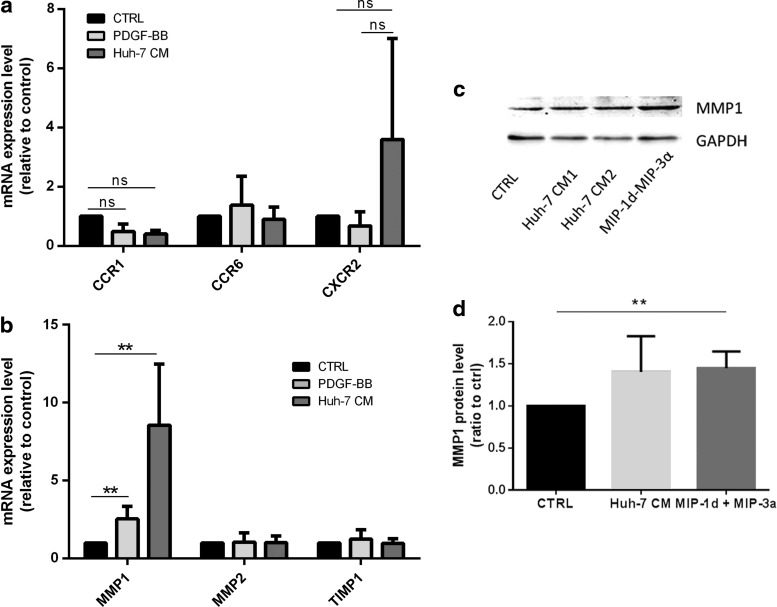
The chemokine response can be regulated not only at the level of production of a ligand but also at the level of chemokine receptor expression in the target cell [51]. We thus investigated the expression level by real-time PCR not only of several cognate receptors for the identified chemokines but also of receptors CXCR2, CCR2 and the MMP-1, MMP-2 and their inhibitor TIMP-1, which was shown to be relevant in MSC migration and invasion [27,52]. Chemokine receptors CCR1, CCR6, and CCR5 were expressed at low levels in MSC at various passages from eight different donors. CCR6 was expressed at relatively high levels in four out of seven donors (Fig. 4a). Receptors, CCR2 and CXCR2 expressed at the lowest levels were considered absent. MMP-1, MMP-2, and TIMP-1 were strongly expressed in all MSC populations tested (Fig. 4b). To evaluate whether Huh-7 CM regulates the expression of chemokine receptors for MIP-3α, MIP-1δ, and IL-8 in MSC, we performed reverse transcription quantitative real time polymerase chain reaction (RT-qPCR) of MSC exposed to Huh-7 CM, PDGF-BB, and control medium for 48 h. We observed that expression of CCR1, the receptor for MIP-1δ, did not significantly change in both PDGF-BB and Huh-7 CM-treated cells. Further, the expression level of CCR6, CCR1, and CXCR2 was not significantly affected as well (Fig. 5a). Further, MMP-1, but not MMP-2 or TIMP-1, was significantly increased after exposure to Huh-7 CM and PDGF-BB (Fig. 5b). We further analyzed protein levels for MMP-1. MSC from three different donors were incubated with control, Huh-7 CM, MIP-1δ, and MIP-3α for 72 h and protein expression of MMP-1 was analyzed by western blotting. We showed an increase in MMP-1 protein level (Fig. 5c), which was quantified (Fig. 5d).
FIG. 4.
MSC express low levels of chemokine receptors and high levels of proteins involved in EM degradation. MSC isolated from at least six different donors and expanded for three to five passages have been used to perform real-time PCR on total RNA extractions. (a) Expression level of all five chemokine receptors CCR1, CCR2, CCR5, CCR6, and CXCR2. Expression level of CCR6 was expressed at higher levels in three of seven donors. (b) Expression level of the matrix metallo proteinase 1 (MMP-1) and 2 (MMP-2) as well as TIMP-1. PCR, polymerase chain reaction.
FIG. 5.
Expression of chemokine receptors and of proteins involved in EM degradation after incubation with Huh-7 CM. MSC isolated from four different donors have been treated with control condition (CTRL), PDGF-BB, and Huh-7 CM for 24 h. (a) Total RNA were extracted, and real-time PCR was performed on CCR1, CCR6, and CXCR2. (b) Real-time PCR was performed for MMP-1, MMP-2, and TIMP-1. All experimental data are mean±SD from at least three independent experiments (**P<0.02). (c) MSC were seeded at a density of 30,000 cells per well in a 24-well plate. Cells were incubated with control medium (CTRL), control medium containing MIP-1δ and MIP-3α, as well as Huh-7 CM. Western blotting were performed using an anti-MMP1 antibody. GAPDH was used as loading control. This figure shows one representative experiment out of three. (d) The graph presents intensity of signals that was evaluated by analyzing the optical density of the spots. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. n.s., no significance.
MIP-1δ and MIP-3α mediates activation of ERK pathway in MSC
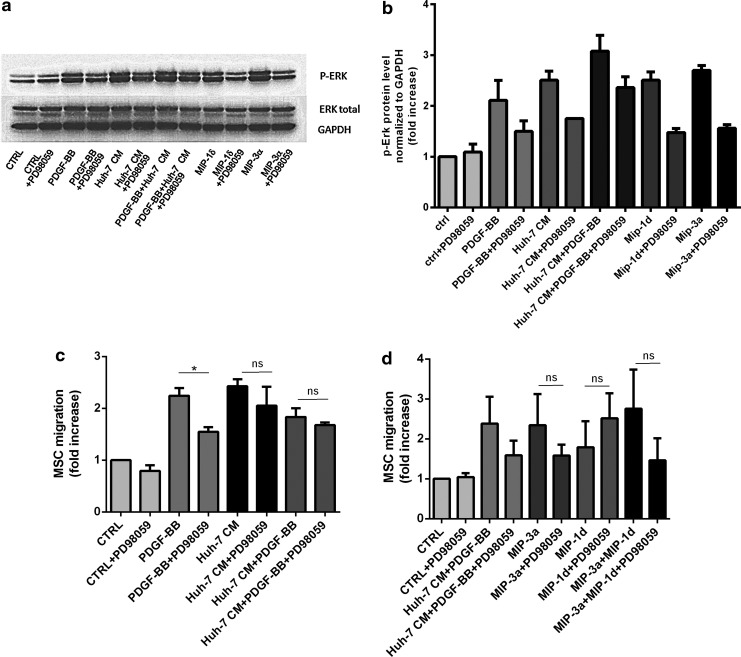
Considering that ligand binding to CCR1 and CCR6 receptor activates ERK signaling, and that ERK signaling is, in turn, implicated in MSC migration [53,54], we investigated the activation of the ERK pathway. We observed an increased phosphorylation of ERK after 30 min of stimulation with PDGF-BB, Huh-7 CM, as well as MIP-1δ and MIP-3α, when compared with control medium. Adding the inhibitor of MAPK/ERK kinase (MEK) PD98059 decreased phosphorylation of ERK to a basal level of phosphorylation in all stimulated conditions (Fig. 6a, b). Further, the role of ERK phosphorylation in migration was tested by exposing MSC to PD98059 during the migration assay. We observed that PD98059 was most efficiently interfering with migration in PDGF-BB-stimulated MSC (Fig. 6c); however, although not significantly, MSC migration was also decreased in Huh-7 CM, MIP-1δ and MIP-3α-treated cells (Fig. 6d), suggesting that treatment with Huh-7 CM, MIP-1δ, and MIP-3α activates other signaling pathways involved in MSC migration.
FIG. 6.
Huh-7 CM, MIP-1δ, and MIP-3α mediate activation of ERK pathway in MSC. (a) 30×103 MSC per well were seeded into 24-well plates. Two days later, MSC are incubated in control medium (CTRL) containing PDGF-BB (20 ng/mL), Huh-7 CM, MIP-1δ, or MIP-3α with or without MAP kinase inhibitor PD98059 (50 μM). Cells were rinsed with PBS containing orthovanadate, and proteins were loaded on 12% SDS/polyacrylamide gel. Phospho-ERK and ERK total were detected using polyclonal antibodies. GAPDH is shown as a control for loading. (b) The p-ERK signal was quantified by densitometry and normalized to GAPDH. Error bars represent the SD from duplicates of one representative experiment out of three independent experiments. (c, d) Migration assay was performed by seeding 6×103 MSC per well at the top of membrane inserts with 8 μm pores. PD98059 was added or not during 24 h of migration. Cells that have migrated on the lower side of the membrane were counted and expressed as fold increase to the control condition. (b, c) Represent mean values±SD from three independent experiments. (*, P≤0.05); n.s., no significance. PBS, phosphate-buffered saline.
MIP-1δ induces αSMA expression in MSC
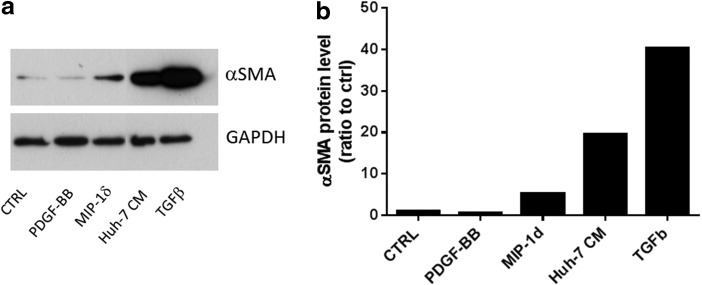
Bone marrow fibrocyte migration into CCl4-damaged liver is regulated by CCR1 and CCR2 [55]. As MIP-1δ is secreted by Huh-7 cells and induces MSC migration, we investigated its implication in the differentiation of MSC into myofibroblasts. Treatment of MSC with TGF-β1 and Huh-7 CM strongly induced αSMA expression in MSC after 5 days of culture (Fig. 7a, b). In addition, MIP-1δ treatment for 5 days (but not MIP-3α, data not shown) increased αSMA expression in MSC (Fig. 7a, b), suggesting that prolonged exposure to MIP-1δ is implicated in the evolvement of MSC into myofibroblasts.
FIG. 7.
MIP-1δ induces α-SMA expression in MSC after 5 days of exposure. MSC were seeded at a density of 30,000 cells per well in a 24-well plate. Cells were incubated with control medium (CTRL), control medium containing PDGF-BB (25 ng/mL), MIP-1δ (25 ng/mL), or TGF-β (50 ng/mL) as well as with Huh-7 CM. (a) Western blotting was performed using an anti-αSMA antibody. GAPDH was used as loading control. This figure shows one representative experiment out of three. (b) The graph shows the intensity of signals of the western blot spots that was evaluated by analyzing the optical density of the spots.
Discussion
MSC migration is controlled by common mechanisms identified in leukocytes, and it has been proposed that exploiting such cellular mechanisms may help improve cellular therapies [56]. However, the mechanisms involved in MSC and leukocytes migration might be different, since the expression of adhesion molecules such as P-selectins, necessary for tethering in leukocytes, was not unequivocally demonstrated in MSC [40,57]. Other studies showed the importance of CD44 in MSC migration [58]. Conversion of the native CD44 glycoform on MSC into hematopoietic cell E-selectin/L-selectin ligand increases the homing capacity of MSC into the bone marrow [59]. The involvement of chemokines, cytokines, and growth factors and their receptors in the attraction of MSC into wounds or tumors still need to be clarified both in vitro and in vivo.
Here, we investigated the implication of chemokines and factors in molecular trafficking mechanisms of MSC using a hepatoma cell conditioned medium that we found to induce MSC migration. In line with others [60], we observed that conditioned medium from the human cell line Huh-7 was able to induce efficient migration in MSC similar to PDGF-BB, whereas conditioned medium from freshly isolated human hepatocytes and another hepatoma cell line, HepG2, did not. Therefore, the effect observed with the Huh-7 CM is specific to this type of cell line and is probably related to their unique secretory profile that we have analyzed. We attempted to identify chemokines or growth factors involved in the activation of migration of MSC specific to Huh-7 CM. Using a chemokine antibody array, we detected increased levels of angiogenin, MIP-1δ (CCL15), MIP-3α (CCL20), and IGFBP-2 in the Huh-7 CM. Factors previously reported to induce MSC migration such as PDGF-BB [26,61], TGF-β [62], IL-1β [63], TNF-α [64], MCP-1 [28], and SDF-1 [33,65] were not increased in Huh-7 CM compared with control medium, excluding the possibility of an association between these factors and the induced migration in this setting. Neither angiogenin nor IGFBP-2 has an effect on MSC migration, which is not surprising since angiogenin is relevant for angiogenesis and more likely to be involved in inducing tumor vascularization [66] and IGFBP-2 was shown to act mainly on mitogenic and survival capacity of cells [67,68]. We focused on MIP-1δ and MIP-3α, both of which are cytokines regulating immune cell migration [69]. MIP-1δ stimulated chemotactic migration in primary endothelial cells. It was further proposed to play a role in angiogenesis [47] and recently considered a serum biomarker for hepatocellular carcinoma [70]. Using recombinant MIP-1δ and MIP-3α in the transwell migration assay, we showed that these soluble factors increased MSC migration and that specific blocking antibodies against MIP-1δ and MIP-3α slightly decreased MSC migration, with a significant result only with anti-MIP-1δ antibody.
Cell migration in response to chemokines has been shown to be modulated through the regulation of receptor expression. In neuronal stem cells, IL-10 or IL-4 up-regulated CXCR4 and CCR5 chemokine receptor expression, thereby increasing the migratory response to IL-4 and regulated on activation normal T cell expressed (RANTES) [71]. In human MSC, modulation of chemokine receptor expression was observed after exposure to several factors [56]. Treatments with cytokines TNF-α and IFN-β increased expression of chemokines receptors CXCR4 and CCR3, leading to increased migration in response to SDF-1 in vitro [72]. However, the chemokine receptor's expression profile in human MSC is broad and the expression of a chemokine receptor is not necessarily related to a function in migration [35]. Discrepancies exist between studies from different groups about the expression of CCR2 and its function [35,42]. CCR1 expression and its involvement in migration had also been reported [73]; however, others did not detect CCR1 [40].
We analyzed several receptors, such as CXCR2, CCR2, and CCR5, which were not related to the factors that we found to be increased in the Huh-7 CM. CXCR2 and CCR2 are receptors for IL-8 and MCP-1, respectively. Both were shown to be involved in MSC migration [28,33]. In our experiment, both receptors were considered absent. Others have demonstrated the expression of CCR2 and CXCR2 by FACS analysis in bone marrow-derived MSC [64,74]. These discrepancies might be related to different time periods of expansion of MSC in various media, which may select subpopulations of MSC with different receptor expressions. Such subpopulations might also further reflect the broad range of functions that MSC are expected to fulfill. Further, we detected low but consistent expression of CCR5 and CCR1 and highly variable expression of CCR6. Interestingly, the observation that the expression of receptors remained unchanged on treatment with Huh-7 CM and PDGF-BB suggests that migration of MSC was independent of mechanisms regulating their expression. As mentioned, the expression of CCR6 was highly variable in MSC populations from different donors, suggesting a regulation of this receptor independently of migration. Further, in our experiments, expression of the receptor for IL-8, CXCR2, which was previously shown to be involved in MSC migration [33], was highly variable, although not significantly increased in three independent experiments. This result leads us to hypothesize that Huh-7-induced migration of MSC might not be mainly regulated by receptor expression. However, we only analyzed receptor expression at 24 h after treatment, which might be too short to observe long-term regulatory mechanisms.
Cell migration is a complex phenomenon, also implicating dynamic modifications of the extracellular matrix through the release of metalloproteinases [75]. In MSC, previous studies revealed that MMP-1 was implicated in MSC migration to gliomas and induced by glioma conditioned medium [27], and that the migration/invasion capacity of hMSCs via MMP-1 was regulated by the Wnt signaling pathway [39]. Exposure of MSC to factors such as IL-1β or TGF-β increased the production of MMPs [52]. In this study, we also observed an up-regulation of MMP-1 by PDGF-BB and, to a higher extent, by the Huh-7 CM. This up-regulation was specific for MMP-1 since the expression of MMP-2 remained unchanged. Whether the identified inflammatory chemokines are involved in the up-regulation of MMP-1 remains to be investigated.
Several studies have shown that MSC migration is regulated through the activation of the MAPK/ERK signaling pathway [49,76]. We found that Huh-7 CM activated the ERK pathway similarly to PDGF-BB. However, PDGF-BB-induced migration was significantly impaired after inhibition of ERK phosphorylation by MEK inhibitor PD98059, whereas Huh-7 CM, MIP-1δ, and MIP-3α-induced migration was not, suggesting that other pathways are implicated under these conditions. In MSC, activation of several other pathways had been demonstrated. In human umbilical cord blood-derived MSC, Akt, ERK, and p38 signaling pathways were shown to be activated on SDF-1-induced migration [76]. Sphingosine-1-phosphate-induced MSC mobilization and migration requires cooperation of MMPs with the RhoA/Rho kinase and MAP/ERK signaling pathways [38]. Interestingly, MSC migration induced by TNF-α increased expression of p-ERK and p38; however, only the inhibitor SB203580 and not PD98059 suppressed migration of MSC [77]. Here, the induction of MSC migration by MIP-1δ and MIP-3α may activate several signaling pathways, also including the cooperation of MMP-1.
We further observed that αSMA expression was increased after prolonged culture of MSC with Huh-7 CM and MIP-1δ. At first sight, this observation appears as counterintuitive, since increased αSMA expression is accompanied with increased adhesion strength and contractibility of the cell [78], which lead to reduced cell migration [79]. However, we observed αSMA expression after 5 days, suggesting that migration and αSMA expression are sequential events where MSC migrate first and then start expressing αSMA. In tumors, tumor-derived factors not only activate local fibroblasts but may also attract circulating precursor cells, contributing to a tumor permissive microenvironment [80,81]. It has been proposed that MSC are a source of CAFs, which contain αSMA-positive contractile stromal cells with increased secretion of extracellular matrix and increased expression of SDF-1 [82–84]. Interestingly, MSC exposed to conditioned medium of human breast cancer cells adopted a CAF-like phenotype [83]. MSC showed increased migration when exposed to the MDAMB231-conditioned medium without previous exposure of MSC, whereas MSC that had been exposed for several days to tumor conditioned medium migrated less, suggesting that adopting a CAF-like phenotype decreases migration. It will be of interest to further analyze whether MSC under long-term incubation with Huh-7 CM or MIP-1δ adopt a CAF phenotype.
In conclusion, we identified two chemokines, MIP-1δ and MIP-3α in the Huh-7 CM that are implicated in the Huh-7 CM-induced MSC migration; one of them, MIP-1δ, is involved in the induction of αSMA expression. Both MIP-1δ and MIP-3α activated the ERK pathway, but inhibition of this pathway in MSC did not significantly impair migration, suggesting the activation of other pathways. Activation of MSC migration was independent of regulation of receptors expression. Further, prolonged exposure to MIP-1δ induced a myofibroblast phenotype in MSC, characterized by increased levels of αSMA. This might be relevant for clinical application of MSC, since MSC are recruited to sites of injury or tumors producing chemokines and factors. MSC might be susceptible to undergo phenotypic changes once exposed to micro-environmental conditions. Characterizing chemokine receptors in MSC as well as analyzing inflammatory chemokines expressed by tumors might be important for patient-tailored therapies.
Acknowledgments
This study was funded by a research grant no. 05-1-II from the Research and Development Budget of the University Hospitals of Geneva, Switzerland to C.G.G. The authors gratefully acknowledge Corinne Sinigaglia, Nadine Pernin, and David Matthey-Doret for their technical help and Sergei Startchik and Olivier Brun (Bioimaging Core Facility, Faculty of Medicine, Geneva, Switzerland) for their help in using microscope and Metamorph settings for the quantification of cell migration.
Author Disclosure Statement
The authors disclose that no competing financial interests exist.
References
- 1.Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luria EA. and Ruadkow IA. (1974). Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol 2:83–92 [PubMed] [Google Scholar]
- 2.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S. and Gianni AM. (2002). Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99:3838–3843 [DOI] [PubMed] [Google Scholar]
- 3.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E. and Dazzi F. (2003). Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 101:3722–3729 [DOI] [PubMed] [Google Scholar]
- 4.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V. and Uccelli A. (2006). Human mesenchymal stem cells modulate B-cell functions. Blood 107:367–372 [DOI] [PubMed] [Google Scholar]
- 5.Glennie S, Soeiro I, Dyson PJ, Lam EW. and Dazzi F. (2005). Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 105:2821–2827 [DOI] [PubMed] [Google Scholar]
- 6.Benvenuto F, Ferrari S, Gerdoni E, Gualandi F, Frassoni F, Pistoia V, Mancardi G. and Uccelli A. (2007). Human mesenchymal stem cells promote survival of T cells in a quiescent state. Stem Cells 25:1753–1760 [DOI] [PubMed] [Google Scholar]
- 7.Tyndall A. and Uccelli A. (2009). Multipotent mesenchymal stromal cells for autoimmune diseases: teaching new dogs old tricks. Bone Marrow Transplant 43:821–828 [DOI] [PubMed] [Google Scholar]
- 8.Casiraghi F, Azzollini N, Todeschini M, Cavinato RA, Cassis P, Solini S, Rota C, Morigi M, Introna M, et al. (2012). Localization of mesenchymal stromal cells dictates their immune or proinflammatory effects in kidney transplantation. Am J Transplant 12:2373–2383 [DOI] [PubMed] [Google Scholar]
- 9.Dahlke MH, Hoogduijn M, Eggenhofer E, Popp FC, Renner P, Slowik P, Rosenauer A, Piso P, Geissler EK, et al. (2009). Toward MSC in solid organ transplantation: 2008 position paper of the MISOT study group. Transplantation 88:614–619 [DOI] [PubMed] [Google Scholar]
- 10.Le Blanc K. (2006). Mesenchymal stromal cells: tissue repair and immune modulation. Cytotherapy 8:559–561 [DOI] [PubMed] [Google Scholar]
- 11.Meier RP, Muller YD, Morel P, Gonelle-Gispert C. and Buhler LH. (2013). Transplantation of mesenchymal stem cells for the treatment of liver diseases, is there enough evidence?. Stem Cell Res 11:1348–1364 [DOI] [PubMed] [Google Scholar]
- 12.Fouraschen SM, Pan Q, de Ruiter PE, Farid WR, Kazemier G, Kwekkeboom J, Ijzermans JN, Metselaar HJ, Tilanus HW, de Jonge J. and van der Laan LJ. (2012). Secreted factors of human liver-derived mesenchymal stem cells promote liver regeneration early after partial hepatectomy. Stem Cells Dev 21:2410–2419 [DOI] [PubMed] [Google Scholar]
- 13.Meier RP, Mahou R, Morel P, Meyer J, Montanari E, Muller YD, Christofilopoulos P, Wandrey C, Gonelle-Gispert C. and Buhler LH. (2014). Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice. J Hepatol pii: [DOI] [PubMed] [Google Scholar]
- 14.Casiraghi F, Remuzzi G, Abbate M. and Perico N. (2013). Multipotent mesenchymal stromal cell therapy and risk of malignancies. Stem Cell Rev 9:65–79 [DOI] [PubMed] [Google Scholar]
- 15.Bergfeld SA. and DeClerck YA. (2010). Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Cancer Metastasis Rev 29:249–261 [DOI] [PubMed] [Google Scholar]
- 16.Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, Alison MR. and Wright NA. (2004). Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res 64:8492–8495 [DOI] [PubMed] [Google Scholar]
- 17.Ishii G, Sangai T, Oda T, Aoyagi Y, Hasebe T, Kanomata N, Endoh Y, Okumura C, Okuhara Y, et al. (2003). Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction. Biochem Biophys Res Commun 309:232–240 [DOI] [PubMed] [Google Scholar]
- 18.Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, et al. (2011). Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 19:257–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, Andreeff M. and Marini F. (2009). Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One 4:e4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chamberlain G, Smith H, Rainger GE. and Middleton J. (2011). Mesenchymal stem cells exhibit firm adhesion, crawling, spreading and transmigration across aortic endothelial cells: effects of chemokines and shear. PLoS One 6:e25663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knoop K, Kolokythas M, Klutz K, Willhauck MJ, Wunderlich N, Draganovici D, Zach C, Gildehaus FJ, Boning G, et al. (2011). Image-guided, tumor stroma-targeted 131I therapy of hepatocellular cancer after systemic mesenchymal stem cell-mediated NIS gene delivery. Mol Ther 19:1704–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Cao F, De A, Cao Y, Contag C, Gambhir SS, Wu JC. and Chen X. (2009). Trafficking mesenchymal stem cell engraftment and differentiation in tumor-bearing mice by bioluminescence imaging. Stem Cells 27:1548–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin J, Kim JK, Moon JH, Beck S, Piao D, Jin X, Kim SH, Lim YC, Nam DH, et al. (2011). hMSC-mediated concurrent delivery of endostatin and carboxylesterase to mouse xenografts suppresses glioma initiation and recurrence. Mol Ther 19:1161–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryu CH, Park SH, Park SA, Kim SM, Lim JY, Jeong CH, Yoon WS, Oh WI, Sung YC. and Jeun SS. (2011). Gene therapy of intracranial glioma using interleukin 12-secreting human umbilical cord blood-derived mesenchymal stem cells. Hum Gene Ther 22:733–743 [DOI] [PubMed] [Google Scholar]
- 25.Bexell D, Gunnarsson S, Svensson A, Tormin A, Henriques-Oliveira C, Siesjo P, Paul G, Salford LG, Scheding S. and Bengzon J. (2012). Rat multipotent mesenchymal stromal cells lack long-distance tropism to 3 different rat glioma models. Neurosurgery 70:731–739 [DOI] [PubMed] [Google Scholar]
- 26.Hata N, Shinojima N, Gumin J, Yong R, Marini F, Andreeff M. and Lang FF. (2010). Platelet-derived growth factor BB mediates the tropism of human mesenchymal stem cells for malignant gliomas. Neurosurgery 66:144–156; discussion 156–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho IA, Chan KY, Ng WH, Guo CM, Hui KM, Cheang P. and Lam PY. (2009). Matrix metalloproteinase 1 is necessary for the migration of human bone marrow-derived mesenchymal stem cells toward human glioma. Stem Cells 27:1366–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dwyer RM, Potter-Beirne SM, Harrington KA, Lowery AJ, Hennessy E, Murphy JM, Barry FP, O'Brien T. and Kerin MJ. (2007). Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res 13:5020–5027 [DOI] [PubMed] [Google Scholar]
- 29.Menon LG, Picinich S, Koneru R, Gao H, Lin SY, Koneru M, Mayer-Kuckuk P, Glod J. and Banerjee D. (2007). Differential gene expression associated with migration of mesenchymal stem cells to conditioned medium from tumor cells or bone marrow cells. Stem Cells 25:520–528 [DOI] [PubMed] [Google Scholar]
- 30.Bexell D, Scheding S. and Bengzon J. (2010). Toward brain tumor gene therapy using multipotent mesenchymal stromal cell vectors. Mol Ther 18:1067–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menon LG, Kelly K, Yang HW, Kim SK, Black PM. and Carroll RS. (2009). Human bone marrow-derived mesenchymal stromal cells expressing S-TRAIL as a cellular delivery vehicle for human glioma therapy. Stem Cells 27:2320–2330 [DOI] [PubMed] [Google Scholar]
- 32.Reagan MR. and Kaplan DL. (2011). Concise review: mesenchymal stem cell tumor-homing: detection methods in disease model systems. Stem Cells 29:920–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Picinich SC, Glod JW. and Banerjee D. (2010). Protein kinase C zeta regulates interleukin-8-mediated stromal-derived factor-1 expression and migration of human mesenchymal stromal cells. Exp Cell Res 316:593–602 [DOI] [PubMed] [Google Scholar]
- 34.Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ. and Janowska-Wieczorek A. (2006). Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells 24:1254–1264 [DOI] [PubMed] [Google Scholar]
- 35.Ringe J, Strassburg S, Neumann K, Endres M, Notter M, Burmester GR, Kaps C. and Sittinger M. (2007). Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem 101:135–146 [DOI] [PubMed] [Google Scholar]
- 36.Xu F, Shi J, Yu B, Ni W, Wu X. and Gu Z. (2010). Chemokines mediate mesenchymal stem cell migration toward gliomas in vitro. Oncol Rep 23:1561–1567 [DOI] [PubMed] [Google Scholar]
- 37.Fiedler J, Roderer G, Gunther KP. and Brenner RE. (2002). BMP-2, BMP-4, and PDGF-BB stimulate chemotactic migration of primary human mesenchymal progenitor cells. J Cell Biochem 87:305–312 [DOI] [PubMed] [Google Scholar]
- 38.Meriane M, Duhamel S, Lejeune L, Galipeau J. and Annabi B. (2006). Cooperation of matrix metalloproteinases with the RhoA/Rho kinase and mitogen-activated protein kinase kinase-1/extracellular signal-regulated kinase signaling pathways is required for the sphingosine-1-phosphate-induced mobilization of marrow-derived stromal cells. Stem Cells 24:2557–2565 [DOI] [PubMed] [Google Scholar]
- 39.Neth P, Ciccarella M, Egea V, Hoelters J, Jochum M. and Ries C. (2006). Wnt signaling regulates the invasion capacity of human mesenchymal stem cells. Stem Cells 24:1892–1903 [DOI] [PubMed] [Google Scholar]
- 40.Brooke G, Tong H, Levesque JP. and Atkinson K. (2008). Molecular trafficking mechanisms of multipotent mesenchymal stem cells derived from human bone marrow and placenta. Stem Cells Dev 17:929–940 [DOI] [PubMed] [Google Scholar]
- 41.Chamberlain G, Wright K, Rot A, Ashton B. and Middleton J. (2008). Murine mesenchymal stem cells exhibit a restricted repertoire of functional chemokine receptors: comparison with human. PLoS One 3:e2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM. and Silberstein LE. (2006). Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells 24:1030–1041 [DOI] [PubMed] [Google Scholar]
- 43.Von Luttichau I, Notohamiprodjo M, Wechselberger A, Peters C, Henger A, Seliger C, Djafarzadeh R, Huss R. and Nelson PJ. (2005). Human adult CD34-progenitor cells functionally express the chemokine receptors CCR1, CCR4, CCR7, CXCR5, and CCR10 but not CXCR4. Stem Cells Dev 14:329–336 [DOI] [PubMed] [Google Scholar]
- 44.Baertschiger RM, Serre-Beinier V, Morel P, Bosco D, Peyrou M, Clement S, Sgroi A, Kaelin A, Buhler LH. and Gonelle-Gispert C. (2009). Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PLoS One 4:e6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baertschiger RM, Bosco D, Morel P, Serre-Beinier V, Berney T, Buhler LH. and Gonelle-Gispert C. (2008). Mesenchymal stem cells derived from human exocrine pancreas express transcription factors implicated in beta-cell development. Pancreas 37:75–84 [DOI] [PubMed] [Google Scholar]
- 46.Suva D, Garavaglia G, Menetrey J, Chapuis B, Hoffmeyer P, Bernheim L. and Kindler V. (2004). Non-hematopoietic human bone marrow contains long-lasting, pluripotential mesenchymal stem cells. J Cell Physiol 198:110–118 [DOI] [PubMed] [Google Scholar]
- 47.Hwang J, Kim CW, Son KN, Han KY, Lee KH, Kleinman HK, Ko J, Na DS, Kwon BS, Gho YS. and Kim J. (2004). Angiogenic activity of human CC chemokine CCL15 in vitro and in vivo. FEBS Lett 570:47–51 [DOI] [PubMed] [Google Scholar]
- 48.Dohlman TH, Chauhan SK, Kodati S, Hua J, Chen Y, Omoto M, Sadrai Z. and Dana R. (2013). The CCR6/CCL20 axis mediates Th17 cell migration to the ocular surface in dry eye disease. Invest Ophthalmol Vis Sci 54:4081–4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao H, Priebe W, Glod J. and Banerjee D. (2009). Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells 27:857–865 [DOI] [PubMed] [Google Scholar]
- 50.Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, et al. (2009). TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med 15:757–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Locati M, Riboldi E, Otero K, Martinez FO, Riva F, Perrier P, Baviera S, Signorelli P, Bonecchi R, et al. (2001). Regulation of the chemokine system at the level of chemokine receptor expression and signaling activity. Immunobiology 204:536–542 [DOI] [PubMed] [Google Scholar]
- 52.Ries C, Egea V, Karow M, Kolb H, Jochum M. and Neth P. (2007). MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood 109:4055–4063 [DOI] [PubMed] [Google Scholar]
- 53.Kato T, Fujita Y, Nakane K, Mizutani K, Terazawa R, Ehara H, Kanimoto Y, Kojima T, Nozawa Y, Deguchi T. and Ito M. (2013). CCR1/CCL5 interaction promotes invasion of taxane-resistant PC3 prostate cancer cells by increasing secretion of MMPs 2/9 and by activating ERK and Rac signaling. Cytokine 64:251–257 [DOI] [PubMed] [Google Scholar]
- 54.Tang JM, Yuan J, Li Q, Wang JN, Kong X, Zheng F, Zhang L, Chen L, Guo LY, et al. (2012). Acetylcholine induces mesenchymal stem cell migration via Ca2+/PKC/ERK1/2 signal pathway. J Cell Biochem 113:2704–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scholten D, Reichart D, Paik YH, Lindert J, Bhattacharya J, Glass CK, Brenner DA. and Kisseleva T. (2011). Migration of fibrocytes in fibrogenic liver injury. Am J Pathol 179:189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maijenburg MW, van der Schoot CE. and Voermans C. (2012). Mesenchymal stromal cell migration: possibilities to improve cellular therapy. Stem Cells Dev 21:19–29 [DOI] [PubMed] [Google Scholar]
- 57.Ruster B, Gottig S, Ludwig RJ, Bistrian R, Muller S, Seifried E, Gille J. and Henschler R. (2006). Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood 108:3938–3944 [DOI] [PubMed] [Google Scholar]
- 58.Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, Demetriou A. and Wu GD. (2006). The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells 24:928–935 [DOI] [PubMed] [Google Scholar]
- 59.Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP. and Wohlgemuth R. (2008). Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med 14:181–187 [DOI] [PubMed] [Google Scholar]
- 60.Garcia MG, Bayo J, Bolontrade MF, Sganga L, Malvicini M, Alaniz L, Aquino JB, Fiore E, Rizzo MM, et al. (2011). Hepatocellular carcinoma cells and their fibrotic microenvironment modulate bone marrow-derived mesenchymal stromal cell migration in vitro and in vivo. Mol Pharm 8:1538–1548 [DOI] [PubMed] [Google Scholar]
- 61.Cheng P, Gao ZQ, Liu YH. and Xue YX. (2009). Platelet-derived growth factor BB promotes the migration of bone marrow-derived mesenchymal stem cells towards C6 glioma and up-regulates the expression of intracellular adhesion molecule-1. Neurosci Lett 451:52–56 [DOI] [PubMed] [Google Scholar]
- 62.Zhang F, Tsai S, Kato K, Yamanouchi D, Wang C, Rafii S, Liu B. and Kent KC. (2009). Transforming growth factor-beta promotes recruitment of bone marrow cells and bone marrow-derived mesenchymal stem cells through stimulation of MCP-1 production in vascular smooth muscle cells. J Biol Chem 284:17564–17574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carrero R, Cerrada I, Lledo E, Dopazo J, Garcia-Garcia F, Rubio MP, Trigueros C, Dorronsoro A, Ruiz-Sauri A, Montero JA. and Sepulveda P. (2012). IL1beta induces mesenchymal stem cells migration and leucocyte chemotaxis through NF-kappaB. Stem Cell Rev 8:905–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ponte AL, Marais E, Gallay N, Langonne A, Delorme B, Herault O, Charbord P. and Domenech J. (2007). The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells 25:1737–1745 [DOI] [PubMed] [Google Scholar]
- 65.Wynn RF, Hart CA, Corradi-Perini C, O'Neill L, Evans CA, Wraith JE, Fairbairn LJ. and Bellantuono I. (2004). A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 104:2643–2645 [DOI] [PubMed] [Google Scholar]
- 66.Tello-Montoliu A, Patel JV. and Lip GY. (2006). Angiogenin: a review of the pathophysiology and potential clinical applications. J Thromb Haemost 4:1864–1874 [DOI] [PubMed] [Google Scholar]
- 67.Giannini S, Cresci B, Manuelli C, Pala L. and Rotella CM. (2006). Diabetic microangiopathy: IGFBP control endothelial cell growth by a common mechanism in spite of their species specificity and tissue peculiarity. J Endocrinol Invest 29:754–763 [DOI] [PubMed] [Google Scholar]
- 68.Ranke MB. and Elmlinger M. (1997). Functional role of insulin-like growth factor binding proteins. Horm Res 48(Suppl. 4):9–15 [DOI] [PubMed] [Google Scholar]
- 69.Griffith JW, Sokol CL. and Luster AD. (2014). Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 32:659–702 [DOI] [PubMed] [Google Scholar]
- 70.Li Y, Wu J, Zhang W, Zhang N. and Guo H. (2013). Identification of serum CCL15 in hepatocellular carcinoma. Br J Cancer 108:99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guan Y, Jiang Z, Ciric B, Rostami AM. and Zhang GX. (2008). Upregulation of chemokine receptor expression by IL-10/IL-4 in adult neural stem cells. Exp Mol Pathol 85:232–236 [DOI] [PubMed] [Google Scholar]
- 72.Croitoru-Lamoury J, Lamoury FM, Zaunders JJ, Veas LA. and Brew BJ. (2007). Human mesenchymal stem cells constitutively express chemokines and chemokine receptors that can be upregulated by cytokines, IFN-beta, and Copaxone. J Interferon Cytokine Res 27:53–64 [DOI] [PubMed] [Google Scholar]
- 73.Huang J, Zhang Z, Guo J, Ni A, Deb A, Zhang L, Mirotsou M, Pratt RE. and Dzau VJ. (2010). Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ Res 106:1753–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith H, Whittall C, Weksler B. and Middleton J. (2012). Chemokines stimulate bidirectional migration of human mesenchymal stem cells across bone marrow endothelial cells. Stem Cells Dev 21:476–486 [DOI] [PubMed] [Google Scholar]
- 75.Ravanti L. and Kahari VM. (2000). Matrix metalloproteinases in wound repair (review). Int J Mol Med 6:391–407 [PubMed] [Google Scholar]
- 76.Ryu CH, Park SA, Kim SM, Lim JY, Jeong CH, Jun JA, Oh JH, Park SH, Oh WI. and Jeun SS. Migration of human umbilical cord blood mesenchymal stem cells mediated by stromal cell-derived factor-1/CXCR4 axis via Akt, ERK, and p38 signal transduction pathways. Biochem Biophys Res Commun 398:105–110 [DOI] [PubMed] [Google Scholar]
- 77.Fu X, Han B, Cai S, Lei Y, Sun T. and Sheng Z. (2009). Migration of bone marrow-derived mesenchymal stem cells induced by tumor necrosis factor-alpha and its possible role in wound healing. Wound Repair Regen 17:185–191 [DOI] [PubMed] [Google Scholar]
- 78.Hinz B, Dugina V, Ballestrem C, Wehrle-Haller B. and Chaponnier C. (2003). Alpha-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol Biol Cell 14:2508–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ronnov-Jessen L. and Petersen OW. (1996). A function for filamentous alpha-smooth muscle actin: retardation of motility in fibroblasts. J Cell Biol 134:67–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grisendi G, Bussolari R, Veronesi E, Piccinno S, Burns JS, De Santis G, Loschi P, Pignatti M, Di Benedetto F, et al. (2011). Understanding tumor-stroma interplays for targeted therapies by armed mesenchymal stromal progenitors: the Mesenkillers. Am J Cancer Res 1:787–805 [PMC free article] [PubMed] [Google Scholar]
- 81.Kalluri R. and Zeisberg M. (2006). Fibroblasts in cancer. Nature reviews. Cancer 6:392–401 [DOI] [PubMed] [Google Scholar]
- 82.Mishra PJ, Glod JW. and Banerjee D. (2009). Mesenchymal stem cells: flip side of the coin. Cancer Res 69:1255–1258 [DOI] [PubMed] [Google Scholar]
- 83.Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW. and Banerjee D. (2008). Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res 68:4331–4339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL. and Weinberg RA. (2005). Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121:335–348 [DOI] [PubMed] [Google Scholar]