Abstract
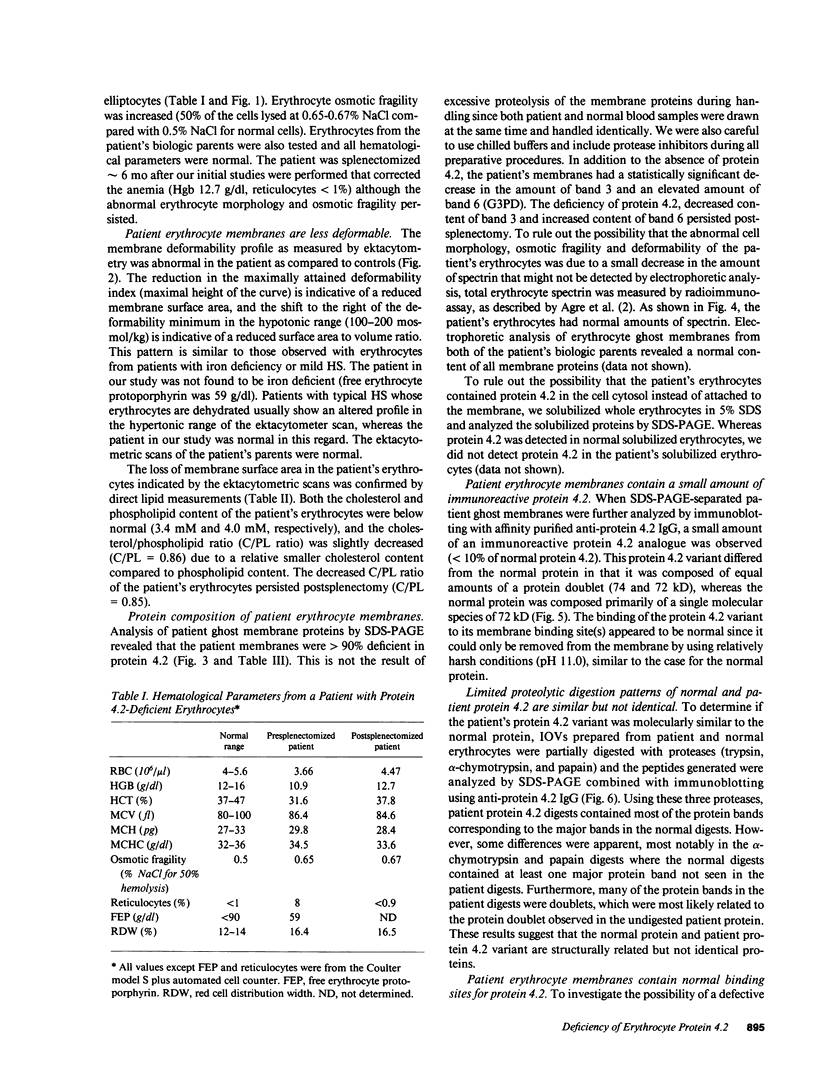
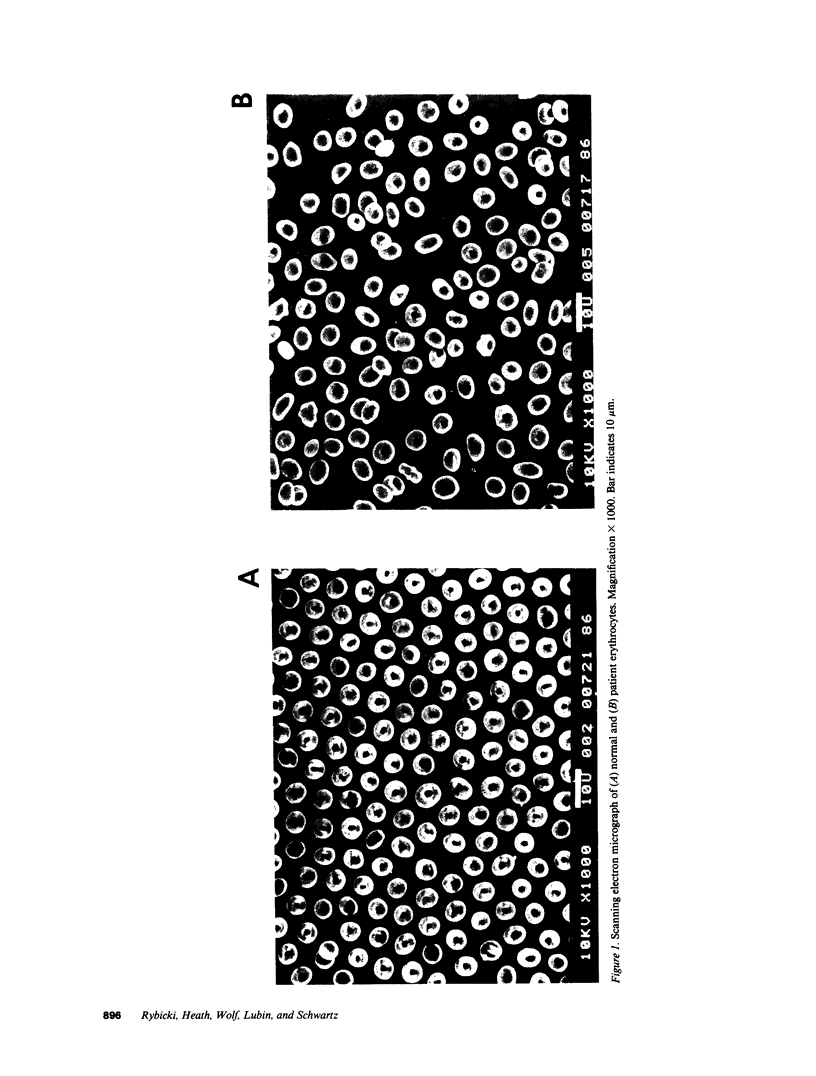
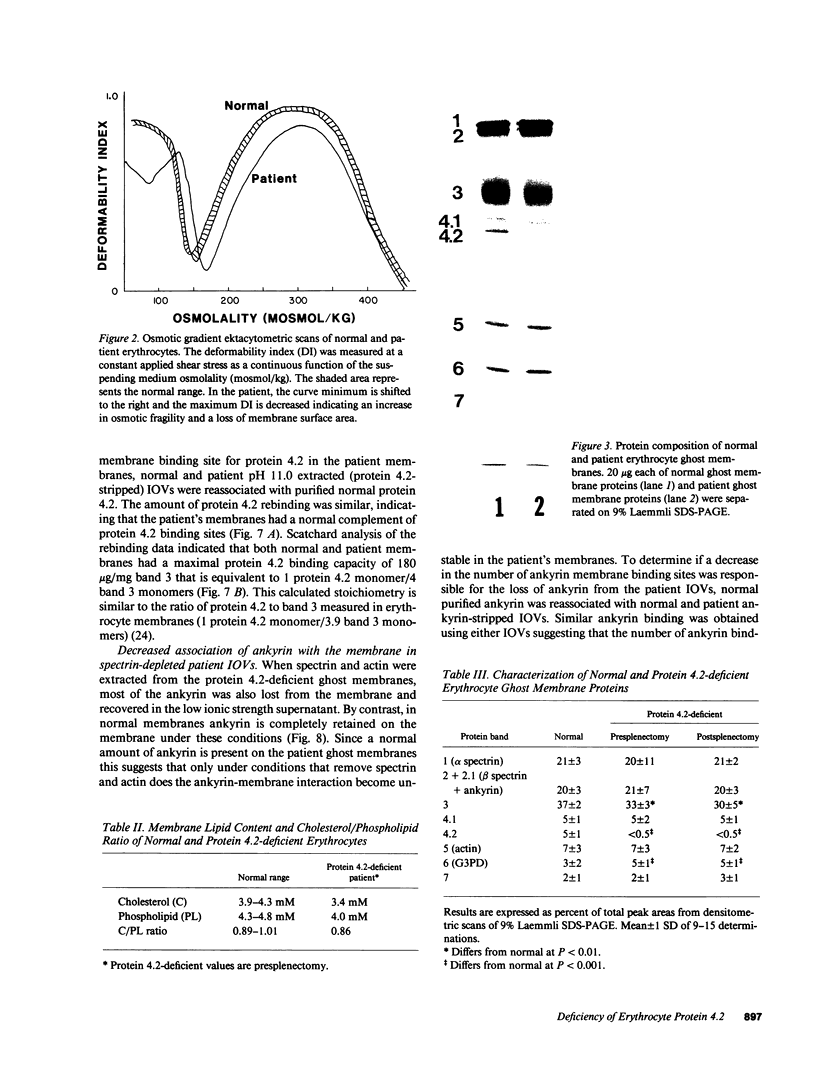
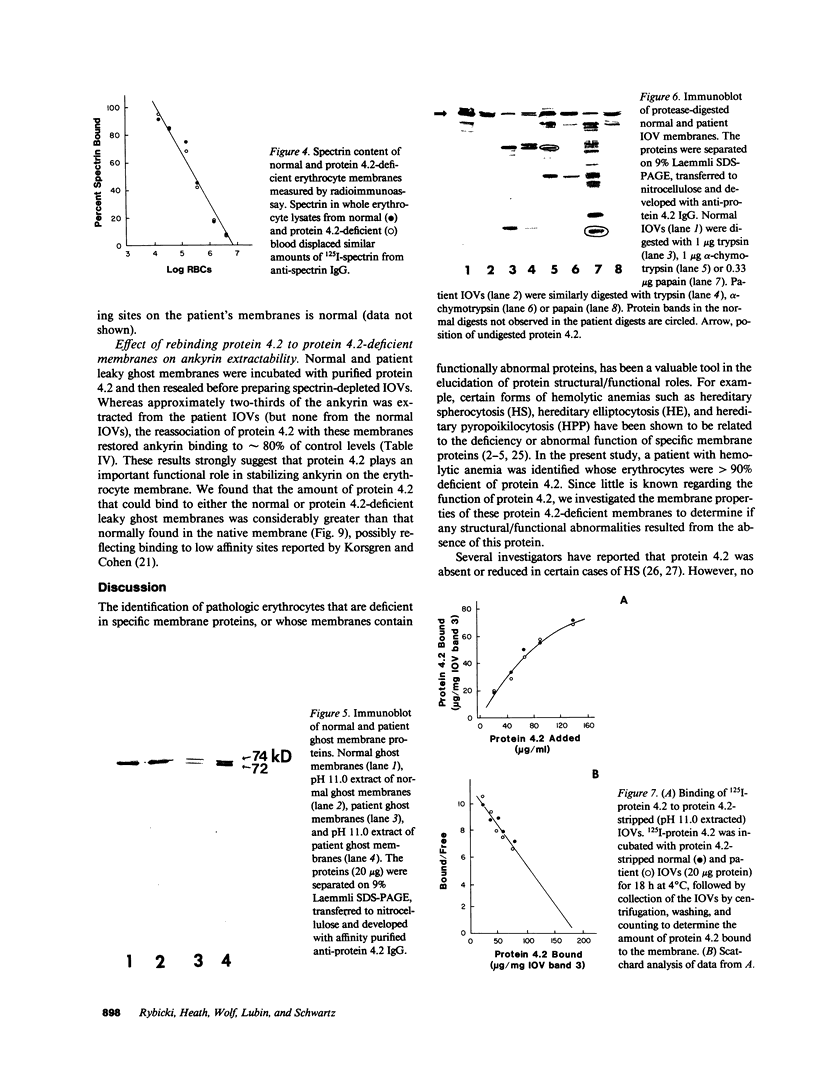
A patient with a mild hemolytic anemia and osmotically fragile, spherocytic erythrocytes was studied. Analysis of the erythrocyte membrane proteins by SDS-PAGE revealed a deficiency of protein 4.2 (less than 0.10% of normal). The protein 4.2-deficient erythrocytes contained normal amounts of all other membrane proteins, although the amount of band 3 was slightly reduced and the amount of band 6 (G3PD) was slightly elevated. The spectrin content of these cells was normal, as measured by both SDS-PAGE and radioimmunoassay. Erythrocytes from the patient's biologic parents were hematologically normal and contained normal amounts of protein 4.2. Immunological analysis using affinity purified antibodies revealed that the patient's protein 4.2 was composed of equal amounts of a 74-kD and 72-kD protein doublet, whereas the normal protein was composed primarily of a 72-kD monomer. Proteolytic digestion studies using trypsin, alpha-chymotrypsin and papain demonstrated that the patient's protein 4.2 was similar but not identical to the normal protein. Binding studies showed that the protein 4.2-deficient membranes bound purified protein 4.2 to the same extent as normal membranes, suggesting that the membrane binding site(s) for the protein were normal. Depleting the protein 4.2-deficient membranes of spectrin and actin resulted in a loss of nearly two-thirds of the membrane ankyrin, whereas similar depletion of normal membranes resulted in no loss of ankyrin. Repletion of the protein 4.2-deficient membranes with purified protein 4.2 before spectrin-actin extraction partially prevented the loss of ankyrin. These results suggest that protein 4.2 may function to stabilize ankyrin on the erythrocyte membrane.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Casella J. F., Zinkham W. H., McMillan C., Bennett V. Partial deficiency of erythrocyte spectrin in hereditary spherocytosis. 1985 Mar 28-Apr 3Nature. 314(6009):380–383. doi: 10.1038/314380a0. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bennett V., Branton D. Selective association of spectrin with the cytoplasmic surface of human erythrocyte plasma membranes. Quantitative determination with purified (32P)spectrin. J Biol Chem. 1977 Apr 25;252(8):2753–2763. [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. Association between ankyrin and the cytoplasmic domain of band 3 isolated from the human erythrocyte membrane. J Biol Chem. 1980 Jul 10;255(13):6424–6432. [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. The membrane attachment protein for spectrin is associated with band 3 in human erythrocyte membranes. Nature. 1979 Aug 9;280(5722):468–473. doi: 10.1038/280468a0. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Goodman S. R., Shiffer K. The spectrin membrane skeleton of normal and abnormal human erythrocytes: a review. Am J Physiol. 1983 Mar;244(3):C121–C141. doi: 10.1152/ajpcell.1983.244.3.C121. [DOI] [PubMed] [Google Scholar]
- Hargreaves W. R., Giedd K. N., Verkleij A., Branton D. Reassociation of ankyrin with band 3 in erythrocyte membranes and in lipid vesicles. J Biol Chem. 1980 Dec 25;255(24):11965–11972. [PubMed] [Google Scholar]
- Hayashi S., Koomoto R., Yano A., Ishigami S., Tsujino G. Abnormality in a specific protein of the erythrocyte membrane in hereditary spherocytosis. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1038–1044. doi: 10.1016/0006-291x(74)90801-8. [DOI] [PubMed] [Google Scholar]
- Iida H., Hasegawa I., Nozawa Y. Biochemical studies on abnormal erythrocyte membranes. Protein abnormality of erythrocyte membrane in biliary obstruction. Biochim Biophys Acta. 1976 Sep 7;443(3):394–401. doi: 10.1016/0005-2736(76)90459-4. [DOI] [PubMed] [Google Scholar]
- Korsgren C., Cohen C. M. Purification and properties of human erythrocyte band 4.2. Association with the cytoplasmic domain of band 3. J Biol Chem. 1986 Apr 25;261(12):5536–5543. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Palek J., Prchal J. T. Defective spectrin dimer-dimer association with hereditary elliptocytosis. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2072–2076. doi: 10.1073/pnas.79.6.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas N., Clark M. R., Jacobs M. S., Shohet S. B. Analysis of factors regulating erythrocyte deformability. J Clin Invest. 1980 Sep;66(3):563–573. doi: 10.1172/JCI109888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa Y., Noguchi T., Iida H., Fukushima H., Sekiya T. Erythrocyte membrane of hereditary spherocytosis: alteration in surface ultrastructure and membrane proteins, as inferred by scanning electron microscopy and SDS-disc gel electrophoresis. Clin Chim Acta. 1974 Aug 30;55(1):81–85. doi: 10.1016/0009-8981(74)90336-2. [DOI] [PubMed] [Google Scholar]
- Palek J., Liu S. C., Liu P. Y., Prchal J., Castleberry R. P. Altered assembly of spectrin in red cell membranes in hereditary pyropoikilocytosis. Blood. 1981 Jan;57(1):130–139. [PubMed] [Google Scholar]
- ROSE H. G., OKLANDER M. IMPROVED PROCEDURE FOR THE EXTRACTION OF LIPIDS FROM HUMAN ERYTHROCYTES. J Lipid Res. 1965 Jul;6:428–431. [PubMed] [Google Scholar]
- Steck T. L. Cross-linking the major proteins of the isolated erythrocyte membrane. J Mol Biol. 1972 May 14;66(2):295–305. doi: 10.1016/0022-2836(72)90481-0. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernia G., Mohandas N., Shohet S. B. Deficiency of skeletal membrane protein band 4.1 in homozygous hereditary elliptocytosis. Implications for erythrocyte membrane stability. J Clin Invest. 1981 Aug;68(2):454–460. doi: 10.1172/JCI110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. M., Reinhardt B. N., Branton D. Associations of erythrocyte membrane proteins. Binding of purified bands 2.1 and 4.1 to spectrin. J Biol Chem. 1980 Jul 25;255(14):7034–7039. [PubMed] [Google Scholar]
- Wolfe L. C., John K. M., Falcone J. C., Byrne A. M., Lux S. E. A genetic defect in the binding of protein 4.1 to spectrin in a kindred with hereditary spherocytosis. N Engl J Med. 1982 Nov 25;307(22):1367–1374. doi: 10.1056/NEJM198211253072203. [DOI] [PubMed] [Google Scholar]
- ZLATKIS A., ZAK B., BOYLE A. J. A new method for the direct determination of serum cholesterol. J Lab Clin Med. 1953 Mar;41(3):486–492. [PubMed] [Google Scholar]










