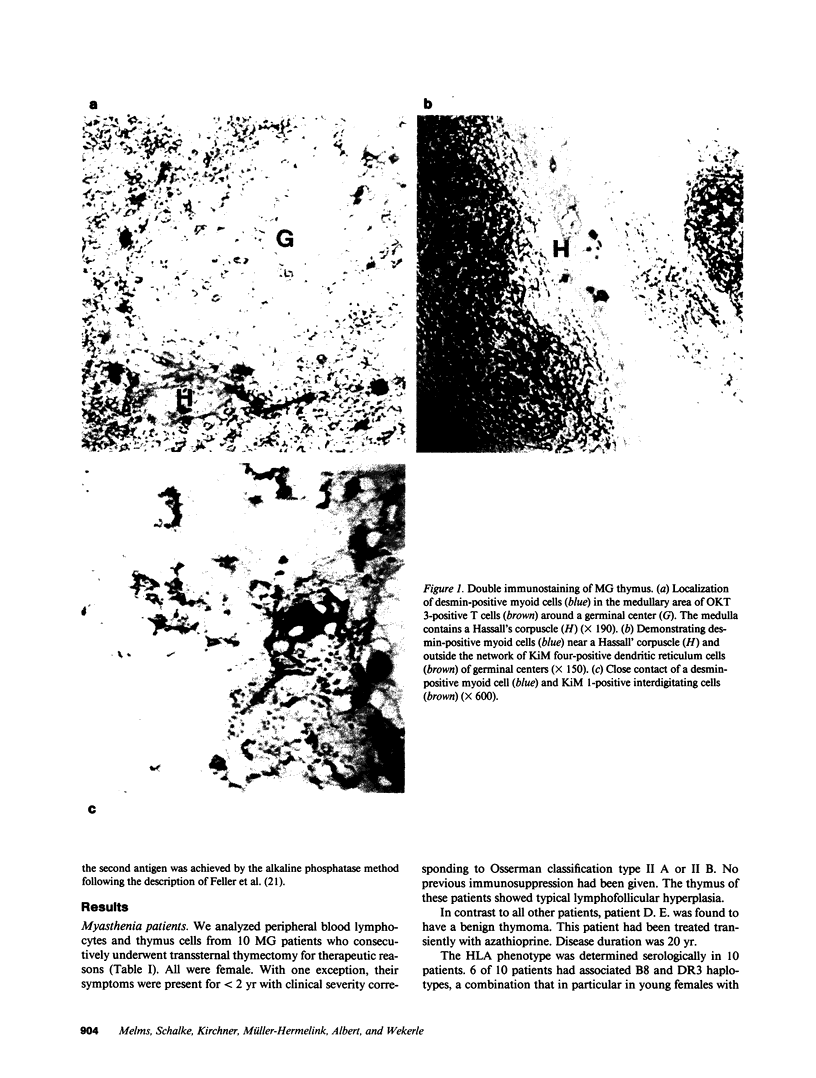
Abstract
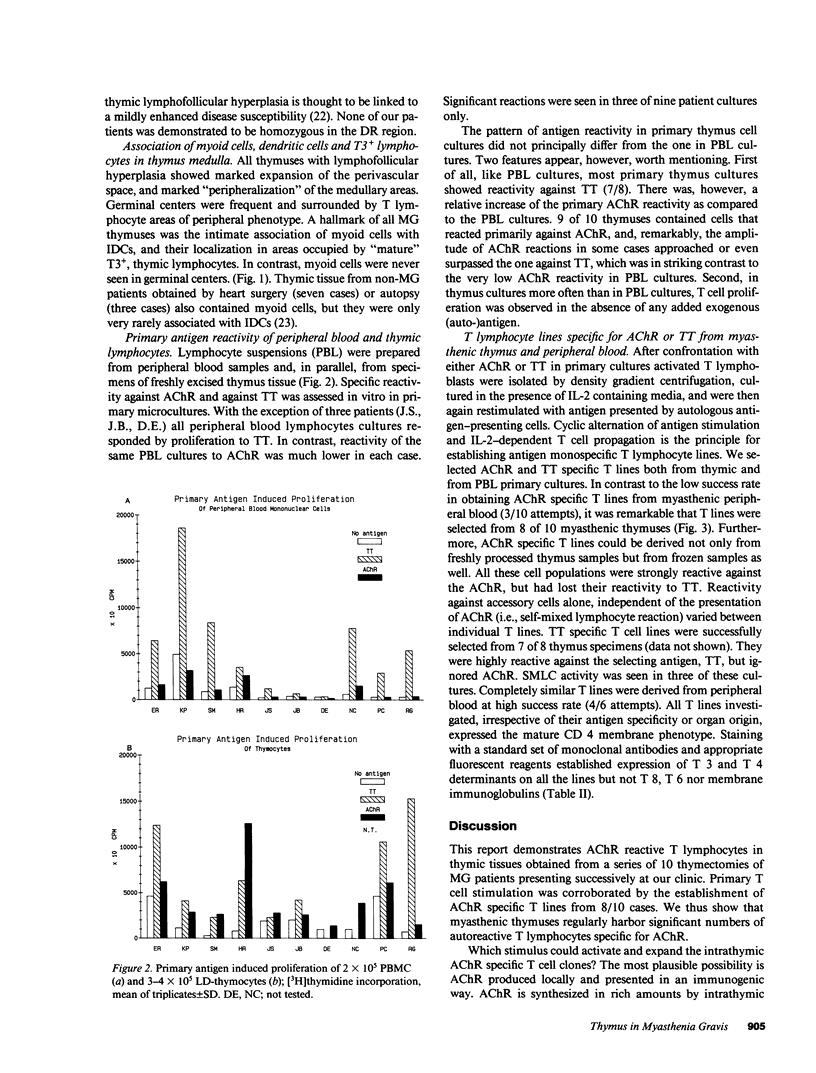
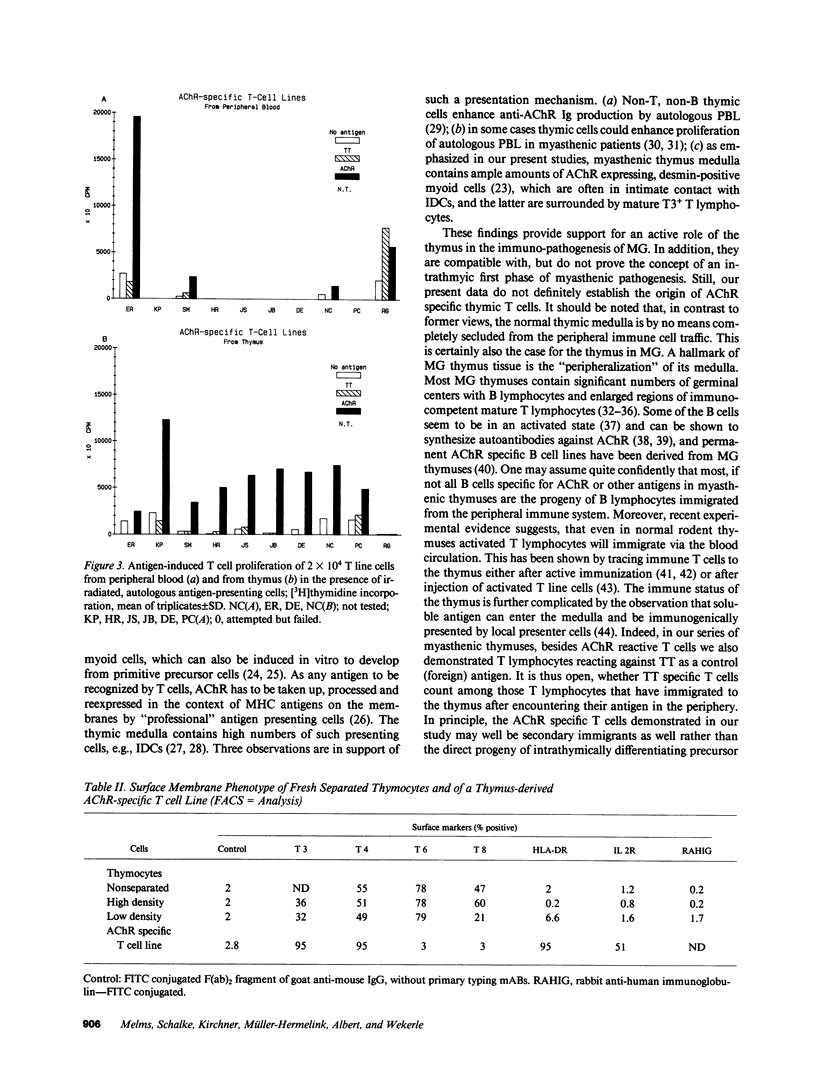
The thymus is believed to play a central role in the pathogenesis of Myasthenia gravis (MG). According to a previous hypothesis, MG is initiated within the thymus by immunogenic presentation of locally produced nicotinic acetylcholine receptor (AChR) to potentially autoimmune T cells. Data of 10 consecutive MG patients demonstrate two critical features of MG thymuses that support the concept of intrathymic activation of autoreactive, AChR-specific lymphocytes. Morphologically, the thymuses showed lympho-follicular hyperplasia in nine cases and benign thymoma in one case. The paramount feature revealed by immunohistological double marker analyses was the intimate association of myoid cells (antigen producing) with interdigitating reticulum cells (potentially antigen presenting cells), both of which were surrounded by T3+ lymphocytes in thymus medulla. All 10 thymuses contained T lymphocytes reactive with AChR. This was in contrast to the peripheral immune compartment (blood) where in only 3 of 10 patients, significant T cell responses to AChR were observed. AChR-specific T cell lines could be established from 8 of 10 thymuses, all members of the helper/inducer subset as indicated by the expression of markers T3 and T4.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Lisak R. P., Zweiman B., Abrahamsohn I., Penn A. S. The thymus in myasthenia gravis. Evidence for altered cell populations. N Engl J Med. 1974 Dec 12;291(24):1271–1275. doi: 10.1056/NEJM197412122912403. [DOI] [PubMed] [Google Scholar]
- Abramsky O., Aharonov A., Webb C., Fuchs S. Cellular immune response to acetylcholine receptor-rich fraction, in patients with myasthenia gravis. Clin Exp Immunol. 1975 Jan;19(1):11–16. [PMC free article] [PubMed] [Google Scholar]
- Aisenberg A. C., Wilkes B., Harris N. L., Frist W. H. The predominant lymphocyte in most thymomas and in nonneoplastic thymus from patients with myasthenia gravis is the cortical thymocyte. Clin Immunol Immunopathol. 1985 Apr;35(1):130–136. doi: 10.1016/0090-1229(85)90086-8. [DOI] [PubMed] [Google Scholar]
- Alpert L. I., Papatestas A., Kark A., Osserman R. S., Osserman K. A histologic reappraisal of the thymus in myasthenia gravis. A correlative study of thymic pathology and response to thymectomy. Arch Pathol. 1971 Jan;91(1):55–61. [PubMed] [Google Scholar]
- Ben-Nun A., Cohen I. R. Spontaneous remission and acquired resistance to autoimmune encephalomyelitis (EAE) are associated with suppression of T cell reactivity: suppressed EAE effector T cells recovered as T cell lines. J Immunol. 1982 Mar;128(3):1450–1457. [PubMed] [Google Scholar]
- Bofill M., Janossy G., Willcox N., Chilosi M., Trejdosiewicz L. K., Newsom-Davis J. Microenvironments in the normal thymus and the thymus in myasthenia gravis. Am J Pathol. 1985 Jun;119(3):462–473. [PMC free article] [PubMed] [Google Scholar]
- Castleman B. The pathology of the thymus gland in myasthenia gravis. Ann N Y Acad Sci. 1966 Jan 26;135(1):496–505. doi: 10.1111/j.1749-6632.1966.tb45497.x. [DOI] [PubMed] [Google Scholar]
- Compston D. A., Vincent A., Newsom-Davis J., Batchelor J. R. Clinical, pathological, HLA antigen and immunological evidence for disease heterogeneity in myasthenia gravis. Brain. 1980 Sep;103(3):579–601. doi: 10.1093/brain/103.3.579. [DOI] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Morgutti M., Sghirlanzoni A., Clementi F. Cellular immune response against acetylcholine receptor in myasthenia gravis: I. Relevance to clinical course and pathogenesis. Neurology. 1979 Apr;29(4):496–501. doi: 10.1212/wnl.29.4.496. [DOI] [PubMed] [Google Scholar]
- Drachman D. B., Adams R. N., Josifek L. F., Self S. G. Functional activities of autoantibodies to acetylcholine receptors and the clinical severity of myasthenia gravis. N Engl J Med. 1982 Sep 23;307(13):769–775. doi: 10.1056/NEJM198209233071301. [DOI] [PubMed] [Google Scholar]
- Feller A. C., Parwaresch M. R., Wacker H. H., Radzun H. J., Lennert K. Combined immunohistochemical staining for surface IgD and T-lymphocyte subsets with monoclonal antibodies in human tonsils. Histochem J. 1983 Jun;15(6):557–562. doi: 10.1007/BF01954146. [DOI] [PubMed] [Google Scholar]
- Fink P. J., Bevan M. J., Weissman I. L. Thymic cytotoxic T lymphocytes are primed in vivo to minor histocompatibility antigens. J Exp Med. 1984 Feb 1;159(2):436–451. doi: 10.1084/jem.159.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y., Monden Y., Hashimoto J., Nakahara K., Kawashima Y. Acetylcholine receptor antibody-producing cells in thymus and lymph nodes in myasthenia gravis. Clin Immunol Immunopathol. 1985 Jan;34(1):141–146. doi: 10.1016/0090-1229(85)90018-2. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Conti-Tronconi B., Kalies I., Bertrams J., Toyka K. V. Genetic restriction of autoreactive acetylcholine receptor-specific T lymphocytes in myasthenia gravis. J Immunol. 1985 Oct;135(4):2393–2399. [PubMed] [Google Scholar]
- Hohlfeld R., Kalies I., Kohleisen B., Heininger K., Conti-Tronconi B., Toyka K. V. Myasthenia gravis: stimulation of antireceptor autoantibodies by autoreactive T cell lines. Neurology. 1986 May;36(5):618–621. doi: 10.1212/wnl.36.5.618. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Toyka K. V., Heininger K., Grosse-Wilde H., Kalies I. Autoimmune human T lymphocytes specific for acetylcholine receptor. Nature. 1984 Jul 19;310(5974):244–246. doi: 10.1038/310244a0. [DOI] [PubMed] [Google Scholar]
- Kaiserling E., Stein H., Müller-Hermelink H. K. Interdigitating reticulum cells in the human thymus. Cell Tissue Res. 1974;155(1):47–55. doi: 10.1007/BF00220283. [DOI] [PubMed] [Google Scholar]
- Kamo I., Furukawa S., Tada A., Mano Y., Iwasaki Y., Furuse T., Ito N., Hayashi K., Satoyoshi E. Monoclonal antibody to acetylcholine receptor: cell line established from thymus of patient with Myasthenia gravis. Science. 1982 Feb 19;215(4535):995–997. doi: 10.1126/science.6297000. [DOI] [PubMed] [Google Scholar]
- Kao I., Drachman D. B. Thymic muscle cells bear acetylcholine receptors: possible relation to myasthenia gravis. Science. 1977 Jan 7;195(4273):74–75. doi: 10.1126/science.831257. [DOI] [PubMed] [Google Scholar]
- Kirchner T., Schalke B., Melms A., von Kügelgen T., Müller-Hermelink H. K. Immunohistological patterns of non-neoplastic changes in the thymus in Myasthenia gravis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;52(3):237–257. doi: 10.1007/BF02889966. [DOI] [PubMed] [Google Scholar]
- Kyewski B. A., Fathman C. G., Kaplan H. S. Intrathymic presentation of circulating non-major histocompatibility complex antigens. Nature. 1984 Mar 8;308(5955):196–199. doi: 10.1038/308196a0. [DOI] [PubMed] [Google Scholar]
- Levinson A. I., Zweiman B., Lisak R. P., Dziarski A., Moskovitz A. R. Thymic B-cell activation in myasthenia gravis. Neurology. 1984 Apr;34(4):462–468. doi: 10.1212/wnl.34.4.462. [DOI] [PubMed] [Google Scholar]
- Monden Y., Nakahara K., Kagotani K., Fujii Y., Masaoka A., Kawashima Y. Myasthenia gravis with thymoma: analysis of and postoperative prognosis for 65 patients with thymomatous myasthenia gravis. Ann Thorac Surg. 1984 Jul;38(1):46–52. doi: 10.1016/s0003-4975(10)62185-6. [DOI] [PubMed] [Google Scholar]
- Monden Y., Nakahara K., Kagotani K., Fujii Y., Nanjo S., Masaoka A., Kawashima Y. Effects of preoperative duration of symptoms on patients with myasthenia gravis. Ann Thorac Surg. 1984 Sep;38(3):287–291. doi: 10.1016/s0003-4975(10)62253-9. [DOI] [PubMed] [Google Scholar]
- Naparstek Y., Holoshitz J., Eisenstein S., Reshef T., Rappaport S., Chemke J., Ben-Nun A., Cohen I. R. Effector T lymphocyte line cells migrate to the thymus and persist there. Nature. 1982 Nov 18;300(5889):262–264. doi: 10.1038/300262a0. [DOI] [PubMed] [Google Scholar]
- Newsom-Davis J., Willcox N., Calder L. Thymus cells in myasthenia gravis selectively enhance production of anti-acetylcholine-receptor antibody by autologous blood lymphocytes. N Engl J Med. 1981 Nov 26;305(22):1313–1318. doi: 10.1056/NEJM198111263052203. [DOI] [PubMed] [Google Scholar]
- Olanow C. W., Wechsler A. S., Roses A. D. A prospective study of thymectomy and serum acetylcholine receptor antibodies in myasthenia gravis. Ann Surg. 1982 Aug;196(2):113–121. doi: 10.1097/00000658-198208000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opelz G., Keesey J., Glovsky M. M., Gale R. P. Autoreactivity between lymphocytes and thymus cells in myasthenia gravis. Arch Neurol. 1978 Jul;35(7):413–415. doi: 10.1001/archneur.1978.00500310015003. [DOI] [PubMed] [Google Scholar]
- Parwaresch M. R., Radzun H. J., Hansmann M. L., Peters K. P. Monoclonal antibody Ki-M4 specifically recognizes human dendritic reticulum cells (follicular dendritic cells) and their possible precursor in blood. Blood. 1983 Sep;62(3):585–590. [PubMed] [Google Scholar]
- Penn A. S., Jaretzki A., 3rd, Wolff M., Chang H. W., Tennyson V. Thymic abnormalities: antigen or antibody? Response to thymectomy in myasthenia gravis. Ann N Y Acad Sci. 1981;377:786–804. doi: 10.1111/j.1749-6632.1981.tb33776.x. [DOI] [PubMed] [Google Scholar]
- Radzun H. J., Parwaresch M. R., Feller A. C., Hansmann M. L. Monocyte/macrophage-specific monoclonal antibody Ki-M1 recognizes interdigitating reticulum cells. Am J Pathol. 1984 Dec;117(3):441–450. [PMC free article] [PubMed] [Google Scholar]
- Richman D. P., Antel J. P., Patrick J. W., Arnason B. G. Cellular immunity to acetylcholine receptor in myasthenia gravis: relationship to histocompatibility type and antigenic site. Neurology. 1979 Mar;29(3):291–296. doi: 10.1212/wnl.29.3.291. [DOI] [PubMed] [Google Scholar]
- Rüchel R., Watters D., Maelicke A. Molecular forms and hydrodynamic properties of acetylcholine receptor from electric tissue. Eur J Biochem. 1981 Oct;119(2):215–223. doi: 10.1111/j.1432-1033.1981.tb05597.x. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Raftery M. A. A simple assay for the study of solubilized acetylcholine receptors. Anal Biochem. 1973 Apr;52(2):349–354. doi: 10.1016/0003-2697(73)90036-5. [DOI] [PubMed] [Google Scholar]
- Stein H., Gerdes J., Schwab U., Lemke H., Mason D. Y., Ziegler A., Schienle W., Diehl V. Identification of Hodgkin and Sternberg-reed cells as a unique cell type derived from a newly-detected small-cell population. Int J Cancer. 1982 Oct 15;30(4):445–459. doi: 10.1002/ijc.2910300411. [DOI] [PubMed] [Google Scholar]
- Toyka K. V., Drachman D. B., Griffin D. E., Pestronk A., Winkelstein J. A., Fishbeck K. H., Kao I. Myasthenia gravis. Study of humoral immune mechanisms by passive transfer to mice. N Engl J Med. 1977 Jan 20;296(3):125–131. doi: 10.1056/NEJM197701202960301. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Beller D. I., Lu C. Y., Allen P. M. Antigen presentation: comments on its regulation and mechanism. J Immunol. 1984 Jan;132(1):1–5. [PubMed] [Google Scholar]
- Van Voorhis W. C., Valinsky J., Hoffman E., Luban J., Hair L. S., Steinman R. M. Relative efficacy of human monocytes and dendritic cells as accessory cells for T cell replication. J Exp Med. 1983 Jul 1;158(1):174–191. doi: 10.1084/jem.158.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A., Scadding G. K., Thomas H. C., Newsom-Davis J. In-vitro synthesis of anti-acetylcholine-receptor antibody by thymic lymphocytes in myasthenia gravis. Lancet. 1978 Feb 11;1(8059):305–307. doi: 10.1016/s0140-6736(78)90073-9. [DOI] [PubMed] [Google Scholar]
- Wekerle H., Hohlfeld R., Ketelsen U. P., Kalden J. R., Kalies I. Thymic myogenesis, T-lymphocytes and the pathogenesis of myasthenia gravis. Ann N Y Acad Sci. 1981;377:455–476. doi: 10.1111/j.1749-6632.1981.tb33753.x. [DOI] [PubMed] [Google Scholar]
- Wekerle H., Ketelsen U. P. Intrathymic pathogenesis and dual genetic control of myasthenia gravis. Lancet. 1977 Mar 26;1(8013):678–680. doi: 10.1016/s0140-6736(77)92118-3. [DOI] [PubMed] [Google Scholar]
- Wekerle H., Ketelsen U. P., Zurn A. D., Fulpius B. W. Intrathymic pathogenesis of myasthenia gravis: transient expression of acetylcholine receptors on thymus-derived myogenic cells. Eur J Immunol. 1978 Aug;8(8):579–582. doi: 10.1002/eji.1830080808. [DOI] [PubMed] [Google Scholar]
- Wekerle H., Müller-Hermelink H. K. The thymus in myasthenia gravis. Curr Top Pathol. 1986;75:179–206. doi: 10.1007/978-3-642-82480-7_6. [DOI] [PubMed] [Google Scholar]
- Wekerle T. H., paterson B., Ketelsen U., Feldman M. Striated muscle fibres differentiate in monolayer cultures of adult thymus reticulum. Nature. 1975 Aug 7;256(5517):493–494. doi: 10.1038/256493a0. [DOI] [PubMed] [Google Scholar]
- Wiersbowsky-Schmeel A., Helpap B., Totovic V., Grouls V. Thymus in myasthenia gravis: a light and electron microscopic study of a case with thymic follicular hyperplasia. Pathol Res Pract. 1984 Mar;178(4):323–331. doi: 10.1016/S0344-0338(84)80021-7. [DOI] [PubMed] [Google Scholar]