Abstract
Sugarcane is the most important crop for supplying sugar. Due to its high biomass, sugarcane needs to absorb a large amount of potassium (K) throughout its lifecycle. In South China, a deficiency of K available in soil restricts the production of sugarcane. Increasing the tolerance of sugarcane to low-K will be an effective approach for improving survival of the crop in this area. However, there is little information regarding the mechanism of tolerance to low-K stress in sugarcane. In this study, a customized microarray was used to analyze the changes in the level of transcripts of sugarcane genes 8 h, 24 h and 72 h after exposure to low-K conditions. We identified a total of 4153 genes that were differentially expressed in at least one of the three time points. The number of genes responding to low-K stress at 72 h was almost 2-fold more than the numbers at 8 h and 24 h. Gene ontology (GO) analysis revealed that many genes involved in metabolic, developmental and biological regulatory processes displayed changes in the level of transcripts in response to low-K stress. Additionally, we detected differential expression of transcription factors, transporters, kinases, oxidative stress-related genes and genes in Ca+ and ethylene signaling pathways; these proteins might play crucial roles in improving the tolerance of sugarcane to low-K stress. The results of this study will help to better understand the molecular mechanisms of sugarcane tolerance to low-K.
Introduction
Potassium (K) is one of the most important macronutrients for plants, playing vital functions in maintaining plasma membrane potential, ion homeostasis, enzyme activation, signal transduction, and many other physiological processes [1]. There are four forms of K in the soil: soluble K, exchangeable K, fixed K, and lattice K. However, plants can only take up soluble K from soil solutions. Generally, the concentration of K+ in soil solutions varies widely (from 0.1 to 6.0 mmol L-1) [2]. In many areas, the soil K+ concentration is extremely low, usually less than 0.3 mmol L-1 [3], and the growth of plants is inhibited by this deficiency. However, plants can initiate a series of physiological and molecular reactions to improve their tolerance to K deficiency. In recent years, a great deal of research has focused on the molecular mechanisms of K uptake, loading and transport in plants, and has demonstrated that genes in both high- and low-affinity K transport systems may be involved in K acquisition and homeostasis in plants under low-K conditions [2,4]. Many genes involved in high-affinity K transport, such as AtKUP3 [5], AtHAK5 [6,7], HvHAK1 [8] and OsHAK1 [9], are induced by low-K stress conditions, and K deficiency triggers the expression of K channel genes such as TaAKT1 in wheat [10]. In addition, genes involved in K signal transduction, such as calcium signaling genes (CBL-CIPK complexes) [11], ethylene signaling genes [12] and transcription factors [13], are known to play important roles in improving the tolerance of plants to low-K. Transcriptomic analysis of the response of Arabidopsis, rice, soybean and wild barley to K deficiency has revealed that genes related to metabolism, ion transport, signal transduction and protein phosphorylation are altered in the level of transcripts [14–17].
Sugarcane (Saccharum species hybrid L.) is an important crop for the production of sugar. Due to its high biomass, it demands a large amount of nutrients for optimum productivity. Generally, 1.00–2.50 kg of K2O will be absorbed in the production of a ton of cane, and approximately 790 kg ha-1 potassium per year will be removed from soil by sugarcane [18,19]. Previous work has indicated that the number, height and sucrose content of millable stalks at harvest will decline under low-K conditions [20]. Indeed, the available K in soil is often deficient in most areas of South China, which is the primary region where sugarcane is cultivated. Enhancement of K uptake under low-K conditions will therefore benefit cane production. Genetically improving the uptake capacity of K from soil is an effective method to enhance the K use efficiency of plants [21]. However, few details are known regarding the molecular mechanisms underlying K absorption, transport and regulatory pathways related to K uptake in sugarcane under low-K stress conditions. Recently, high-throughput technologies have been widely used to analyze the gene expression patterns of plants under low nutrient stress [22,23]. Transcriptome profiling of rice, Arabidopsis and soybean in response to low-K stress has previously been initiated using microarray and transcriptome sequencing, and many genes that respond low-K stress have been identified. Knowledge about these genes will provide valuable information on the molecular mechanism of K absorption and transport under low-K stress [14,16,17]. Here, a customized array was used to detect changes in the level of transcripts of sugarcane genes under low-K conditions. Many genes encoding transporters, kinases and transcription factors were differentially expressed in response to low-K conditions. These results will provide clues regarding the molecular mechanisms by which sugarcane is able to cope with K deficiency.
Materials and Methods
Plant growth conditions and low-K-induced stress
All setts of sugarcane cultivar ROC22 were cut into single-bud setts and then sterilized with 5% carbendazim for 10 min. All single-bud setts were planted in quartz for germination. Seedlings with four leaves were hydroponically cultured in a greenhouse under natural lighting. The greenhouse was controlled to maintain a temperature of 30°C. The nutrient solution used was modified Magnavaca’s solution [24] containing 1.5 mM NH4NO3, 1 mM KCl, 1 mM CaCl2, 0.5 mM Mg(NO3)2, 0.155 mM MgCl2, 0.045 mM KH2PO4, 1.643 mM MgSO4, 11.8 μM MnCl2, 0.61 μM (NH4)6Mo7O24, 33 μM H3BO3, 3.06 μM ZnSO4, 0.8 μM CuSO4, 0.077 mM FeSO4, and 0.077 mM Na2 EDTA (pH = 6.0). Six seedlings were planted in 10 L plastic basins, and a total of nine basins were planted: three for measuring the K concentration in the plants and six for microarray and qRT-PCR analysis. The nutrient solution was replaced every week. After two weeks, all plants (with 8–10 adventitious roots) were transferred to a low-K nutrient solution containing 0.1 mM KCl.
Determination of K content
The roots and shoots of three seedlings from three basins were separately collected at 0 h (Control), 8 h, 24 h, 72 h and 7 d after initiating low-K treatment. All plants were killed at 105°C and then dried in an oven at 75°C for 2 d. Dried shoots and roots were ground, and approximately 1-g samples were moist-ashed with H2SO4-H2O2. The concentration of K in each digest was measured using a flame photometer (Model 425, Sherwood Scientific Ltd, Cambridge, UK) [25]. The concentration of K in the roots and shoots was also calculated.
RNA extraction and microarray hybridization
Three adventitious roots (approximately 10 cm from the root tip) from each of six seedlings in six independent basins were collected at 0 h (CK), 8 h, 24 h and 72 h after initiating low-K treatment. A total of 18 adventitious roots were collected at each time point and were mixed to create one sample. Samples from four time points were immediately frozen in liquid nitrogen and stored at -80°C until RNA extraction. Total RNA was extracted using TRIZOL Reagent (Life Technologies, Carlsbad, CA, US) following the manufacturer’s instructions, and RNA integrity number (RIN), a measure of RNA quality, was determined as using an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, US). Total RNA was further purified with the RNeasy mini kit and RNase-Free DNaseSet (QIAGEN, GmBH, Germany). A customized sugarcane genome array ordered from Agilent Technologies was used in this study. The array contained 61,637 probes, which were designed by Shanghai Biotechnology Corporation according to sequences derived from transcriptome sequencing of sugarcane subjected to low-K, drought, salt and pathogenic stress (our previous unpublished research), as well as sugarcane genes deposited in GenBank (S1 Table, GPL19240 deposited in http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GPL19240). Total RNA was amplified and labeled with the One-Color Low Input Quick Amp Labeling Kit (Agilent Technologies, Santa Clara, CA, US) according to the manufacturer’s instructions. Labeled cRNA was purified with the RNeasy Mini Kit (QIAGEN, GmBH, Germany). Each slide was hybridized with 1.65 μg of Cy3-labeled cRNA using the Gene Expression Hybridization Kit (Agilent Technologies, Santa Clara, CA, US) in a Hybridization Oven (Agilent Technologies, Santa Clara, CA, US), according to the manufacturer’s instructions. After 17 h of hybridization, the slides were washed in staining dishes (Thermo Shandon, Waltham, MA, US) with the Gene Expression Wash Buffer Kit (Agilent Technologies, Santa Clara, CA, US), according to the manufacturer’s instructions. The slides were scanned with an Agilent Microarray Scanner (Agilent Technologies, Santa Clara, CA, US) with the following default settings: dye channel = green; scan resolution = 3μm, 20 bit. Data were extracted with Feature Extraction software 10.7 (Agilent Technologies, Santa Clara, CA, US). Raw data were normalized with the Quantile algorithm using Gene Spring Software 11.0 (Agilent Technologies, Santa Clara, CA, US).
Data processing and analysis
First data with low raw signals (less than 27) in all four arrays were filtered. Pair-wise comparisons were performed to assess changes in gene expression of 8 h vs. 0 h, 24 h vs. 0 h and 72 h vs. 0 h. A 2-fold change was used as the threshold for determining differentially expressed genes. A BLAST search was performed on sequences of differentially expressed genes against the nr database in NCBI. GO analysis was carried out according the sequences used for customizing the microarray and the web set found at http://www.geneontology.org (P<0.05). MeV 4.6.0 was used for hierarchical cluster analysis of differentially expressed genes.
Quantitative real-time (qRT)-PCR analysis
The remaining adventitious roots of six seedlings used for the microarray analysis at each time point were collected separately. Three of the six samples at each time points were used for qRT-PCR to validate the microarray results. RNA was extracted with the EasyPure Plant RNA Kit (TransGen Biotech, China). First-strand cDNA was then synthesized using the PrimeScript Reagent Kit with gDNA Eraser (TaKaRa, Japan) from 1 μg of total RNA in a 20-μl reaction volume. Fifteen differentially expressed genes with different functions were selected to validate the microarray results. Primer Premier 5.0 software (Lynnon Corporation) was used to design gene-specific primers (S2 Table). The qRT-PCR was performed using 0.5 μl of cDNA in a 25-μl reaction volume with SYBR Premix ExTaq (TOYOBO, Japan) on an ABIPRISM 7500 Sequence Detection System (Applied Biosystems). The qRT-PCR program was as follows: 95°C (10 s) followed by 45 cycles of 95°C (5 s), 60°C (30 s), and 72°C (30 s). The products were further analyzed by a dissociation curve program at 95°C (15 s) ramping down to 60°C (1 min) followed by 95°C (15 s). In each qRT-PCR experiment, each gene was analyzed in triplicate with different cDNAs synthesized from three biological replicates. The genes with unimodal dissociation curve were selected for subsequent analysis. Relative fold changes in gene expression were calculated using the comparative ΔΔCt method [26], and GAPDH was used as an endogenous reference gene. For microarray validation, the 2-ΔΔCt values were calculated for each gene in each sample and log2 transformed.
Statistical analysis
Significant differences in the K concentration in shoots or roots at 0 h, 8 h, 24 h, 72 h and 7 d after initiating low-K stress were examined using the IBM SPSS statistical software package (version 19), followed by Duncan’s Multiple Range Test (DMRT). Differences with P<0.05 were considered significant.
Results
Effect of the K concentration on sugarcane shoots and roots
To investigate the effects of low-K stress on sugarcane, the K concentration in shoots and roots was measured 0 h, 8 h, 24 h, 72 h and 7 d after exposure to low-K conditions (Fig 1). The K content of sugarcane shoots decreased slightly after the initiation of low-K stress; however there were no differences between the 0 h time point and any of the other four time points (Fig 1A). Interestingly, a significant difference in the K concentration at five time points was detected in sugarcane roots (P<0.05). The K concentration in roots decreased rapidly after only 8 h of exposure to low-K stress. After that time point it decreased further, reaching statistical significance after 24 h (Fig 1B). These results revealed that the K concentration might decrease earlier in roots compared with shoots during K deficiency.
Fig 1. K concentration in sugarcane shoots and roots under low-K stress.
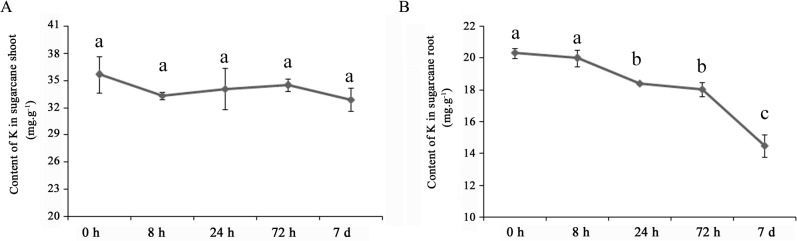
The K concentration of sugarcane was measured after 0 h, 8 h, 24 h, 72 h and 7 d of low-K stress. A) The K concentration in shoots at 0 h, 8 h, 24 h,72 h and 7d; B) The K concentration in roots at 0 h, 8 h, 24 h,72 h and 7d. The different lowercase letters above the error bar (standard error) indicate significant differences (P<0.05) in the K concentration between 0 h and the other four time points (8 h, 24 h, 72 h and 7d); n = 3.
Microarray analysis of genes that were differentially expressed under K deficiency
To determine the global transcriptome responses to K deficiency in sugarcane, a microarray containing 61,637 primers was customized according to sequences derived from transcriptome sequencing of sugarcane subjected to low-K, drought, salt and pathogenic stress, as well as sequences of sugarcane genes deposited in GenBank. The cDNAs synthesized from root RNA derived from samples obtained 0, 8, 24 and 72 h after the initiation of low-K treatment were hybridized to the customized microarrays. Of the 61,637 spots on the array, 41.75% of the probes produced signals of up to 27 in at least one of the four arrays and were used for further analysis. A scatter plot of normalized signal intensities from 8 h vs. 0 h, 24 h vs. 0 h and 72 h vs. 0 h revealed that the expression of the vast majority of transcripts remained unchanged (Fig 2A). A fold change cut-off value of 2.0 was used to identify genes that were responsive to low-K stress. A total of 1545 genes at 8 h, 1053 genes at 24 h and 3155 genes at 72 h were identified as being differentially expressed during low-K treatment (S3 Table and Fig 2). More genes responded to low-K stress at 72 h compared with 8 h and 24 h. Hierarchical cluster analysis showed that the expression pattern of genes at 24 h was similar to that at 72 h but different form that at 8 h (Fig 2B). This result suggested that genes that are differentially expressed at 8 h might be involved in short-term responses to K deficiency, while the long-term response to K deficiency was initiated after 24 h of exposure. All of the low-K-responsive genes are presented in S4–S9 Tables.
Fig 2. Gene expression detected by microarray after different periods of low-K stress.
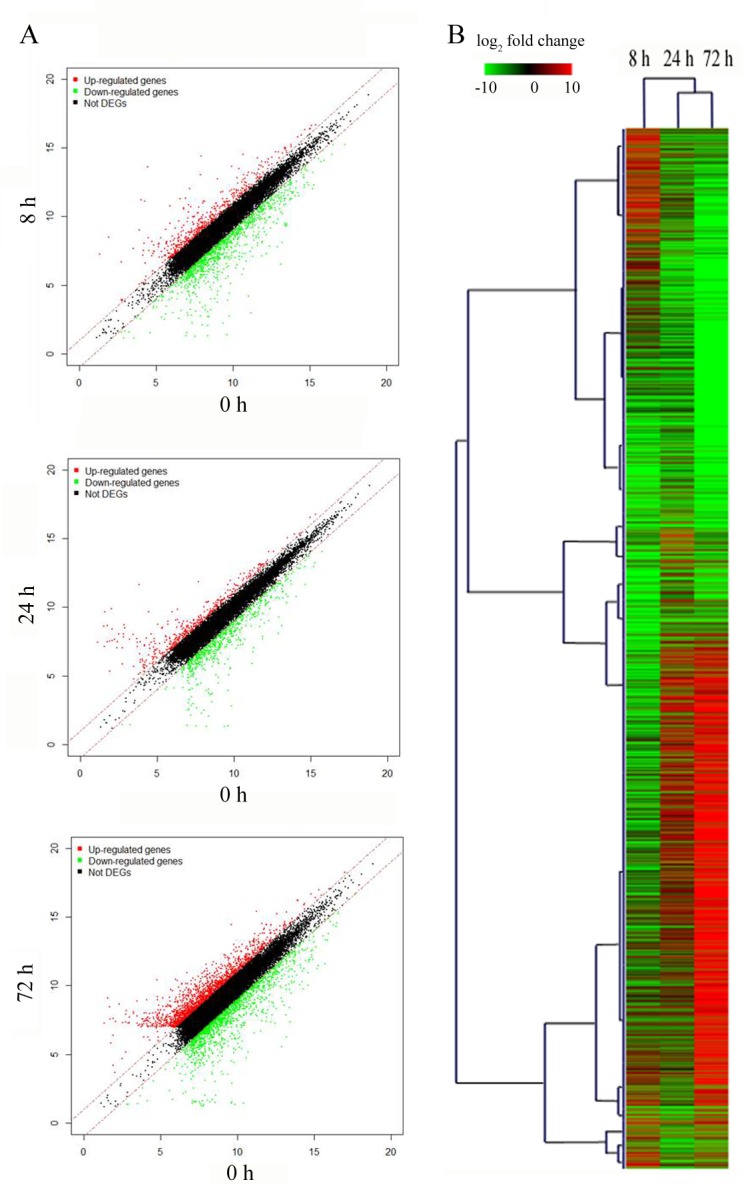
A) Scatter plots of the normalized signal intensities of approximately 21516 genes on the microarray. Log2 intensities for each spot on the microarray are plotted on the x and y axes with signals from root tips stressed for 0 h and 8 h, 0 h and 24, and 0 h and 72 h. The diagonal lines represent fold change cutoffs of 2. The red spots represent up-regulated genes and the green spots indicate down-regulated genes. B) Hierarchical cluster analysis of genes which were responsive to low-K stress. Genes are displayed using different colors, and relative expression levels are illustrated by a color gradient from low (green) to high (red).
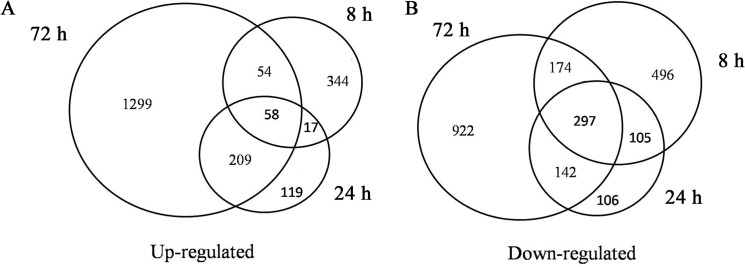
A Venn diagram was constructed to investigate the similarities and differences in gene expression changes at different time points. A total of 58 up-regulated genes and 297 down-regulated genes were found at all three time points (Fig 3). A greater percentage of genes were differentially expressed only at 72 h (80.19% up-regulated genes and 60.07% down-regulated genes) compared with 8 h (72.73% and 46.27%) and 24 h (29.53% and 16.38%), whereas 70.47% and 83.69% of the genes that were up- and down-regulated at 24 h also displayed transcriptional changes at 8 h or 72 h (Fig 3).
Fig 3. Venn diagrams of sugarcane genes showing changes in the level of transcripts in response to low-K stress.
Venn diagrams of up-regulated (A) and down-regulated (B) genes at different times points after the initiation of low-K treatment. Genes that were up-regulated at at least at one time point (8 h, 24 h and 72 h) were selected for the analysis in (A). Genes that were down-regulated at at least one time point (8 h, 24 h and 72 h) were selected for the analysis in (B).
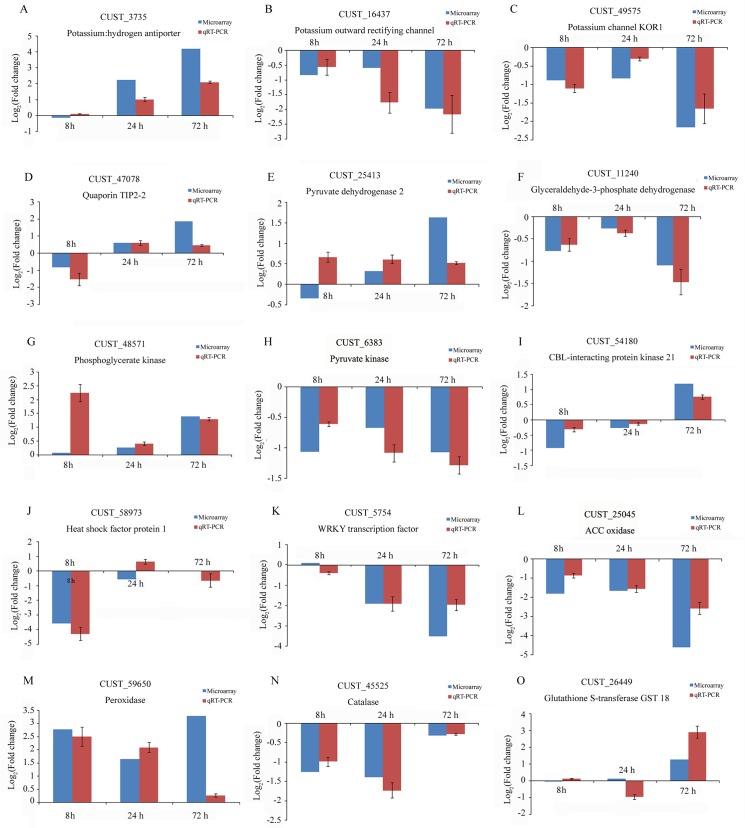
To confirm the validity of the microarray data, fifteen differentially expressed genes were selected, including four genes involved in transport, four genes involved in glycolysis and the citrate cycle, three genes involved in calcium signaling and transcriptional regulation, one gene involved in ethylene synthesis and three genes involved in oxidative stress. The expression patterns of these genes were monitored at four time points by qRT-PCR. For most of the fifteen genes, the expression patterns showed somewhat similar trends; however there were slight differences (Fig 4). For example, pyruvate dehydrogenase 2 (CUST_25413) was up-regulated at 72 h based on microarray analysis; however, qRT-PCR analysis revealed that the expression patterns of this gene were similar at the three time points and the level of transcript did not notably increase at 72 h. Conversely, the expression of phosphoglycerate kinase (CUST_48571) did not increase at 8 h based on microarray analysis; however, it was obviously up-regulated based on qRT-PCR. All the expression datasets have been deposited in the Gene Expression Omnibus (GSE61935) at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/geo/).
Fig 4. Quantitative real-time PCR confirmation of the transcriptomic profiles of selected genes.
The log2(LK/CK) values derived from the microarray data of 15 genes were compared with those from qRT-PCR.
Gene ontology analysis of genes that respond to low-K stress
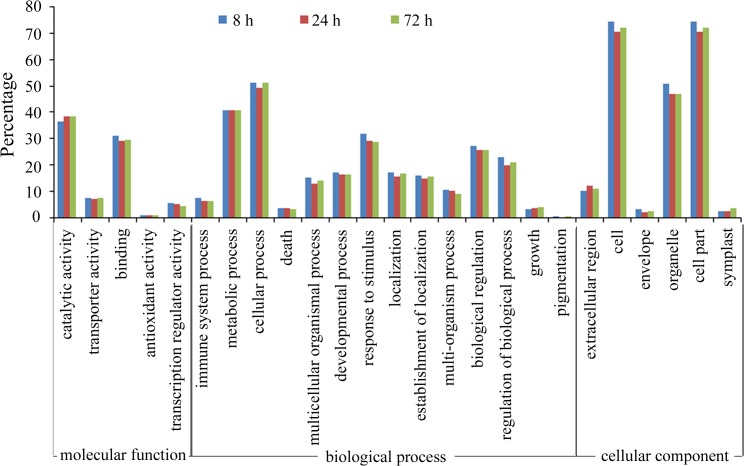
Gene Ontology (GO) analysis was used to evaluate the potential function of genes that were differentially expressed under low-K stress. Although the number of low-K responsive genes differed among the three time points, the percentages of genes in the different functional categories were similar (Fig 5). Based on enrichment of genes binned by molecular function, we found that most genes were associated with catalytic activity, binding, transport and transcriptional regulation, accounting for approximately 90% of molecular function (Fig 5). Enrichment analysis of GO terms based on biological processes revealed that the genes involved in metabolic processes, cellular processes, response to stimulus, biological regulation and developmental processes were significantly enriched (Fig 5). In addition, the products of genes that responded to low-K stress were involved in diverse cellular components. Genes in the cell parts category were the most enriched (approximately 70%), followed by organelle genes s (approximately 40%) (Fig 5). However, the percentage of genes in each category was slightly different among the three time points. At 8 h, the percentage of differentially expressed genes in 20 categories (four molecular function categories, 12 biological process categories and four cellular component categories) was higher than that at 24 h or 72 h. The same result was not observed for genes in the other five categories (catalytic activity, growth, pigmentation, symplast and extracellular region).
Fig 5. Distribution of the functional GO categories of sugarcane genes that were differentially expressed (P<0.05) after exposure to low-K stress for 8, 24 and 72 h.
Metabolic genes
It is known that K ions are a cofactor for many metabolic enzymes [27]. Transcriptional changes in genes involved in metabolic processes were detected in this study. GO analysis of differentially expressed genes revealed that approximately 40% of the genes found were involved in metabolic processes. Of these, approximately 12% were related to nitrogen metabolism, 10% to phosphorus metabolism, 18% to carbohydrate metabolism and 15% to secondary metabolism, together accounting for approximately 55% of genes in the metabolic category (Fig 6). We observed that many genes involved in carbohydrate metabolism participated in glycolysis and the citrate (TCA) cycle (Fig 7 and S4 Table). However, the expression patterns of these genes varied. The expression of pyruvate kinase (CUST_6383) rapidly decreased at 8 h, while genes encoding pyruvate decarboxylase (CUST_15254, CUST_44992), 6-phosphofructokinase (CUST_47734), glyceraldehyde-3-phosphate dehydrogenase (CUST_11240), and alcohol dehydrogenase (CUST_5247, CUST_2997) were down-regulated under low-K stress, especially at 72 h. However, the transcription of phosphoglycerate kinase (CUST_48571), pyruvate dehydrogenase (CUST_25413) and NAD-dependent isocitrate dehydrogenase (CUST_53982) increased at 72 h. Therefore, differences in the expression patterns of genes involved in glycolysis and the citrate cycle could disrupt energy production.
Fig 6. Functional classification of metabolic gene responses to low-K stress.
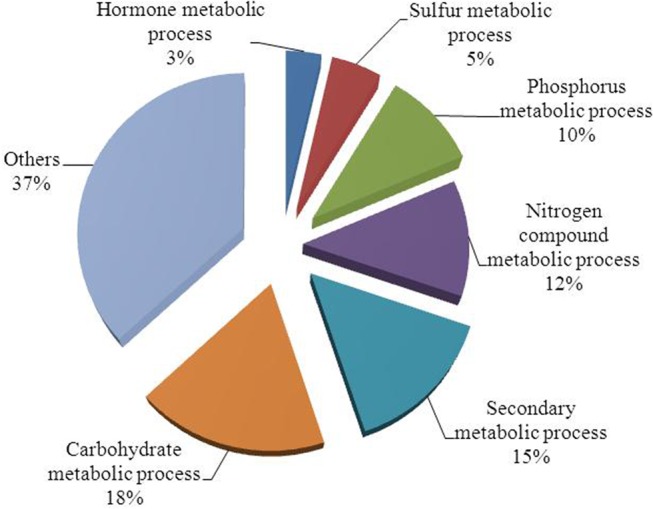
Fig 7. Genes involved in glycolysis and the TCA cycle that displayed altered expression in sugarcane under low-K stress.
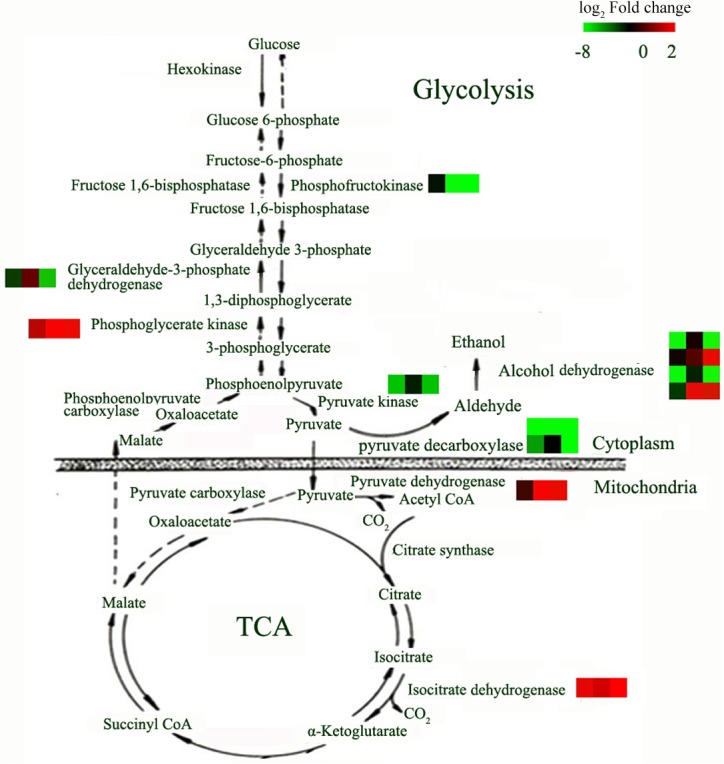
The relative expression levels at 8 h, 24 h and 72 h are shown adjacent to a color gradient from low (green) to high (red). The probes for the differentially expressed genes were CUST_47734 (phosphofructokinase), CUST_11240 (glyceraldehydes-3-phosphate dehydrogenase), CUST_48571 (phosphoglycerate kinase), CUST_6383 (pyruvate kinase), CUST_15254, CUST_44992 (pyruvate decarboxylase), CUST_5247, CUST_7632, CUST_2997, CUST_45329 (alcohol dehydrogenase), CUST_25413 (pyruvate dehydrogenase), and CUST_53982 (isocitrate dehydrogenase).
Meanwhile, some genes involved in the metabolism of cell wall components, such as cellulose synthase (CUST_40582, CUST_11102, CUST_9367, CUST_14077) and glycosyltransferase (CUST_14014, CUST_45535), were up-regulated at 72 h (S4 Table). In addition, genes involved in the metabolism of soluble acids, such as sucrose synthase (CUST_44128, CUST_42367, CUST_27228) and soluble acid invertase (CUST_59812), were up-regulated at both 24 h and 72 h (S4 Table).
In our datasets, approximately 3% of the differentially expressed genes in the metabolic category participated in hormone metabolic processes. Among these genes, a gene encoding 1-aminocyclopropane-1-carboxylate oxidase (ACC oxidase, CUST_25045) was rapidly down-regulated after 8 h of exposure to low-K stress (S4 Table). A similar result was detected in our qRT-PCR analysis which showed a continuous decrease in the level of transcript as the stress continued (Fig 4).
Transporters
Absorption and translocation of K in plants occur mainly via by high- and low-affinity K uptake systems, which are mediated by K transporters and channels, respectively [28]. Based on our microarray data, 17 genes related to K transport showed changes in the level of transcripts in response to low-K stress (Table 1). Four K transporters (CUST_1497, CUST_17819, CUST_36971 and CUST_7112) and two K channels (CUST_36270, CUST_41802) were markedly up-regulated after 72 h of exposure to low-K stress. However, four K transporters (CUST_20591, CUST_510, CUST_53063 and CUST_6380) and a K channel (CUST_49258) were rapidly down-regulated after 8 h of exposure to low-K conditions and their expression remained inhibited at 24 h and 72 h. According to cellular component GO analysis, seven out of nine K transporters (CUST_1497, CUST_20591, CUST_36971, CUST_510, CUST_53063, CUST_6380 and CUST_7112) and two out of eight K channels (CUST_27100 and CUST_36270) were likely located in the plasma membrane, while the other eight K transporters/channels might be located in different cellular organelles such as the nucleus, cytoplasm, vacuole, membrane or plasmodesma (S5 Table).
Table 1. Genes showing altered expression in sugarcane under low-K stress.
| ProbeName | log2(LK/CK) a | nr_accession | Description | Function | ||
|---|---|---|---|---|---|---|
| 8 h vs 0 h | 24 h vs 0 h | 72 h vs 0 h | ||||
| CUST_1497 | 1.06 | XP_003557711 | potassium transporter 22-like | potassium transport | ||
| CUST_17819 | 1.20 | XP_003576484 | potassium transporter 23-like | potassium transport | ||
| CUST_20591 | -1.25 | NP_001060637 | potassium transporter 9 | potassium transport | ||
| CUST_36971 | 1.42 | NP_001048012 | Potassium transporter 25 | potassium transport | ||
| CUST_42207 | -1.36 | NP_001053859 | probable potassium transporter 11 | potassium transport | ||
| CUST_510 | -1.69 | -2.06 | -1.36 | NP_001060637 | probable potassium transporter 9 | potassium transport |
| CUST_53063 | -1.56 | NP_001147472 | potassium transporter 10 | potassium transport | ||
| CUST_6380 | -2.12 | -1.79 | -2.25 | AEA08583 | high affinity potassium transporter | potassium transport |
| CUST_7112 | 1.12 | ADR51675 | potassium high-affinity transporter | potassium transport | ||
| CUST_25267 | -1.25 | AAX08090 | outward-rectifying potassium channel | potassium transport | ||
| CUST_16437 | -1.94 | AAW82753 | potassium outward rectifying channel | potassium transport | ||
| CUST_27100 | -1.06 | XP_003626309 | sodium/potassium/calcium exchanger | potassium transport | ||
| CUST_36270 | 1.08 | 1.10 | P0C550 | potassium channel AKT1 | potassium transport | |
| CUST_49258 | -1.74 | -1.03 | -1.56 | NP_001105120 | potassium channel protein ZMK2 | potassium transport |
| CUST_49575 | -2.18 | Q653P0 | Potassium channel KOR1 | potassium transport | ||
| CUST_41802 | 1.08 | XP_002868604 | potassium channel tetramerization domain-containing protein | potassium transport | ||
| CUST_55816 | -1.56 | -2.00 | -1.06 | NP_00104741 | potassium channel tetramerization domain-containing protein-like | potassium transport |
| CUST_42158 | 2.45 | ACQ83491 | CBL-interacting protein kinase 14 | kinases and phosphatase | ||
| CUST_51966 | 1.20 | ACQ83503 | CBL-interacting protein kinase 03 | kinases and phosphatase | ||
| CUST_20141 | -1.36 | ACQ83488 | CBL-interacting protein kinase 09 | kinases and phosphatase | ||
| CUST_13175 | -4.06 | -2.84 | -3.18 | ACQ83508 | CBL-interacting protein kinase 01 | kinases and phosphatase |
| CUST_7638 | -1.64 | -1.32 | ACQ83502 | CBL-interacting protein kinase 30 | kinases and phosphatase | |
| CUST_54180 | 1.18 | ACQ83498 | CBL-interacting protein kinase 21 | kinases and phosphatase | ||
| CUST_9371 | 1.32 | ACQ83514 | CBL-interacting protein kinase 24 | kinases and phosphatase | ||
| CUST_49630 | 1.36 | ACQ83509 | CBL-interacting protein kinase 15 | kinases and phosphatase | ||
| CUST_4771 | -1.60 | 1.16 | 3.36 | ACQ83496 | CBL-interacting protein kinase 20 | kinases and phosphatase |
| CUST_10946 | -1.51 | -2.47 | ACQ83486 | CBL-interacting protein kinase 19 | kinases and phosphatase | |
| CUST_28467 | 1.63 | NP_001056378 | probable protein phosphatase 2C 51 | kinases and phosphatase | ||
| CUST_40716 | -2.25 | -1.84 | -3.18 | ABB47942 | protein phosphatase 2C containing protein | kinases and phosphatase |
| CUST_36509 | -1.25 | ABF94415 | protein phosphatase 2C containing protein | kinases and phosphatase | ||
| CUST_36910 | -1.06 | NP_001151594 | catalytic/ protein phosphatase type 2C | kinases and phosphatase | ||
| CUST_60543 | -1.18 | NP_001148728 | protein phosphatase 2C | kinases and phosphatase | ||
| CUST_55757 | 1.41 | ACQ83549 | calcineurin B-Like protein 04 | calcineurin B-like protein | ||
| CUST_25359 | -1.29 | ACQ83548 | calcineurin B-Like protein 07 | calcineurin B-like protein | ||
| CUST_2176 | -2.40 | -1.29 | -2.84 | ACF95746 | MYB transcription factor MYBAS1 | transcription regulators |
| CUST_27453 | -2.56 | -1.84 | -2.84 | P20025 | Myb-related protein Zm38 | transcription regulators |
| CUST_28701 | -1.89 | ACG36390 | MYB59 | transcription regulators | ||
| CUST_61488 | -1.56 | -2.40 | EMT32647 | Myb-related protein MYBAS2 | transcription regulators | |
| CUST_58112 | -1.22 | -1.84 | -2.06 | AFO85372 | NAC1, partial | transcription regulators |
| CUST_470 | -1.51 | -1.56 | -1.47 | AAW62955 | NAC23 | transcription regulators |
| CUST_16274 | -3.06 | -1.89 | -2.47 | DAA37711 | putative NAC domain transcription factor | transcription regulators |
| CUST_45563 | -2.40 | -2.25 | -2.74 | DAA46404 | putative NAC domain transcription factor | transcription regulators |
| CUST_48354 | -1.18 | DAA55107 | putative NAC domain transcription factor | transcription regulators | ||
| CUST_54637 | 1.93 | DAA46243 | putative AP2/EREBP transcription factor | transcription regulators | ||
| CUST_12974 | -1.89 | -2.32 | AFW63369 | putative AP2/EREBP transcription factor | transcription regulators | |
| CUST_13792 | -1.06 | CAM35490 | ethylene responsive transcription factor | transcription regulators | ||
| CUST_20821 | -1.69 | -2.12 | NP_001147529 | ethylene-responsive element binding protein | transcription regulators | |
| CUST_46633 | -2.40 | -2.84 | NP_001146913 | ethylene-responsive factor-like protein | transcription regulators | |
| CUST_58973 | -3.64 | NP_001152657 | heat shock factor protein 1 | transcription regulators | ||
| CUST_41064 | -2.94 | -1.89 | -2.32 | ACM42161 | heat shock protein 70.58 | stress-related |
| CUST_22888 | 1.36 | AFV66576 | heat shock protein 70, | stress-related | ||
| CUST_60607 | 1.97 | AFK73383 | small heat-shock protein | stress-related | ||
| CUST_50618 | 1.19 | CAA78738 | heat shock protein hsp82 | stress-related | ||
| CUST_7987 | -1.23 | -1.21 | DAA41888 | class IV heat shock protein | stress-related | |
| CUST_57095 | 1.51 | NP_001104981 | glutathione S-transferase12 | oxidative stress-related | ||
| CUST_61600 | 1.42 | DAA43222 | TPA: glutathione S-transferase GST 11 | oxidative stress-related | ||
| CUST_26449 | 1.28 | NP_001104984 | glutathione S-transferase GST 18 | oxidative stress-related | ||
| CUST_27585 | -1.56 | AAL73498 | lipoxygenase | oxidative stress-related | ||
| CUST_27568 | 1.29 | ACG32380 | superoxide dismutase | oxidative stress-related | ||
| CUST_45525 | -1.25 | -1.40 | ABQ44283 | catalase | oxidative stress-related | |
| CUST_26339 | -1.06 | YP_006316948 | glutathione peroxidase | oxidative stress-related | ||
| CUST_43430 | 1.37 | AFW87396 | peroxiredoxin-5 | oxidative stress-related | ||
| CUST_56046 | 1.36 | 1.70 | NP_001106040 | peroxidase 70 precursor | oxidative stress-related | |
| CUST_45804 | 2.07 | DAA45509 | plant peroxidase family protein | oxidative stress-related | ||
Note: a The fold change of each gene was transformed into “log2”. The gene with log2(LK/CK) ≥ 1 were defined as up-regulated; The genes with log2(LK/CK) ≤ -1 were defined as down-regulated.
In addition to K transporter genes, we found that the level of transcripts of some genes involved in water transport changed in sugarcane roots during K deficiency. According to our microarray data, four aquaporin genes (CUST_56031, CUST_44135, CUST_52950 and CUST_47078) were rapidly down-regulated after 8 h of K starvation. However, the expression levels of these genes recovered after 24 h and subsequently increased as the stress continued (S5 Table).
Kinases and phosphatases
Protein kinases and phosphatases participate in a large number of distinct perception and signaling pathways that play important roles in cellular development. Based on our microarray data, a total of 405 genes encoding kinases and phosphatases were found to be transcriptionally altered by K starvation (S6 Table), accounting for approximately 10% of the low-K-responsive genes and suggesting that phosphorylation and dephosphorylation may be very important regulatory mechanisms for the sugarcane response to K deficiency. Of these genes, 11 CIPKs and 5 type 2C protein phosphatases were found to be differentially expressed in response to low-K stress (Table 1). Six CIPK genes (CUST_42158, CUST_51966, CUST_54180, CUST_9371, CUST_49630 and CUST_4771) were markedly up-regulated after 72 h of exposure to low-K conditions, and a protein phosphatase 2C (CUST_28467) was up-regulated after 8 h of low-K stress (Table 1). Increased expression of these kinase and phosphatase genes may regulate K uptake and K homeostasis in sugarcane during K deficiency. We also found that two members of the CBL family (CUST_55757, CUST_25359) were differentially expressed. CUST_55757 was up-regulated at 72 h, while CUST_25359 was down-regulated at 8 h (Table 1). There were also many other types of kinases that were differentially expressed in our microarray data, including an S-receptor-like serine/threonine-protein kinase (CUST_422), three MAPKKK family protein kinases (CUST_4402, CUST_29659 and CUST_49837), and three wall-associated receptor protein kinases (CUST_44285, CUST_85 and CUST_19991) (S6 Table). These kinases might participate in the regulation of the sugarcane response to low-K stress and form a complicated biological regulation network.
Transcriptional regulation
Our microarray results showed that 191 genes encoding transcriptional regulators were differentially expressed during low-K stress, including MYB (4), NAC (5), AP2-EREBP (2), WRKY (1), ERF (3), bHLH (2), TGA6 (1), E2F3 (1), RCBF3 (1) and HSF (1) family members (Table 1). The other genes were defined as putative transcription factors (S7 Table). Among the genes we identified, five transcription factors involved in ethylene signaling (CUST_54637, CUST_12974, CUST_13792, CUST_20821, and CUST_46633) were differentially expressed (Table 1). The expression of three ethylene-responsive transcription factors (CUST_12974, CUST_20821 andCUST_46633) was down-regulated in response to low-K stress within 8 h, and one (CUST_13792) showed marked down-regulation only at the 72 h time point. However, an AP2/ERF transcription factor (CUST_54637) was up-regulated in response to low-K stress at 8 h. In addition, four putative Myb family transcription factors (CUST_2176, CUST_27453, CUST_28701 and CUST_61488) and five NAC family transcription factors (CUST_16274, CUST_45563, CUST_48354, CUST_58112 and CUST_470) were found to be down-regulated at at least one of the three time points (8 h, 24 h, and 72 h) (Table 1). We also noticed that a heat shock factor protein (CUST_58973) was markedly down-regulated at 8 h. However, the heat shock proteins regulated by heat shock factor showed differential expression patterns in our datasets. Three heat shock proteins (CUST_22888 CUST_50618 and CUST_60607) were markedly up-regulated at 8 h or 72 h, whereas two members (CUST_41064 and CUST_7987) were down-regulated (Table 1).
Oxidative stress-related genes
Reactive oxygen species (ROS) play crucial roles in plant stress responses, development, response to pathogens, and many other physiological processes [29]. However, the excessive production of ROS will cause oxidative stress. In our study, a total of 44 oxidative stress-related genes were differentially expressed under low-K stress conditions (S8 Table and Table 1). At 8 h, 24 h and 72 h, the number of up-regulated genes was 3.75-, 1.75- and 3-fold greater than the number of down-regulated genes, respectively. Among these genes, a superoxide dismutase (CUST_26449) was rapidly up-regulated at 8 h of exposure to low-K conditions. Three glutathione S-transferases (CUST_57095, CUST_61600 and CUST_26449), a peroxiredoxin gene (CUST_43430) and two peroxidases (CUST_56046, CUST_45804) were up-regulated at 72 h. However, a catalase (CUST_45525) and a glutathione peroxidase (CUST_26339) were rapidly down-regulated at 8 h under low-K stress, while the expression of a lipoxygenase (CUST_27585) decreased at 24 h (Table 1).
Discussion
Potassium is an essential macronutrient that is crucial for plant growth and development. Previous work has demonstrated that plants respond rapidly to K deficiency. For example, hyperpolarization of the cell membrane potential occurs within a few minutes of a decrease in extracellular K, an event that represents the earliest detected event in the plant response to K deficiency and may act as one of the sensing-related signals for downstream responses [30]. After hyperpolarization, a series of biochemical and physiological reactions occur in plant cells that include both short- and long-term responses [31]. Transcriptional profiling of rice genes has revealed that many genes are differentially regulated 6 h after the start of low-K stress, and the number of differentially expressed genes increases with time [14]. Our work examining the relationship between the K content of sugarcane roots and changes in the level of transcripts also demonstrated a rapid response occurring after only 8 h of exposure to low-K stress. The K content of sugarcane roots continued to decrease after this time point, and the number of differentially expressed genes increased at 72 h (Fig 1 and Fig 3), which was consistent with the results of a transcriptional profiling study of rice genes under low-K stress [14]. However, many genes showed differential expression only at 72 h, and most of the genes that were differentially expressed at 24 h were also detected at 8 h or 72 h (Fig 2), suggesting that short-term responses to low-K stress might differ from long-term responses and that 24 h might be an important time point for initiating the long-term response in sugarcane. GO analysis of differentially expressed genes revealed that as the stress continued, higher percentages of genes in the catalytic activity and growth categories were detected especially at 72 h (Fig 5). These results indicated that more genes downstream of metabolic and regulatory networks might be activated under long-term stress, which would likely eventually affect sugarcane growth.
As cofactor for many enzymes, K ions participate in many metabolic processes [27]. K-activated enzymes are thought to act as K sensors in the cytoplasm [28]. Transcriptional profiling studies of genes under low-K stress in rice, soybean and Arabidopsis have revealed that genes involved in metabolic processes account for a higher percentage of genes relative to the total number of stress-response genes, and these studies have detected expression changes in many crucial enzymes that are crucial for metabolism, such as enzymes involved in N, S, and P assimilation, pyruvate synthesis and sugar metabolism [14,16,17]. These changes indicate that transcriptional regulation of metabolic enzymes may be very important for plants when adapting to K deficiency [32]. In this study, nearly half of the genes that were responsive to low-K stress were involved in metabolic processes, including genes related to C, N, P and secondary metabolism (Fig 5). Among the metabolic enzymes, pyruvate kinase acts as a central regulator of C/N metabolism [33]. The activity of pyruvate kinase can be directly inhibited after long-term K-deficiency, which induces a significant reduction in the cytoplasmic pyruvate content of root cells and leads to inhibition of glycolysis and many downstream metabolic processes (such as the TCA and GS/GOGAT/GDH cycles) [32]. Here, we detected the down-regulation of pyruvate kinase (CUST_6383) after short-term (8 h) low-K stress (S4 Table and Fig 7). This result suggested that the inhibition of pyruvate kinase occurs not only on the enzymatic level but also on the transcriptional level. The rapid inhibition of pyruvate kinase under low-K stress might be one of the earliest responses to K deficiency in sugarcane. Meanwhile, we also found several genes other than pyruvate kinase that are involved in glycolysis and the citrate (TCA) cycle differentially expressed in response to low-K stress. Changes in the levels of transcripts of genes in the glycolytic pathway such as pyruvate kinase, glyceraldehyde 3-phosphate dehydrogenase and short-chain alcohol dehydrogenase, were detected in Arabidopsis under low-P and low-N conditions [34,35], suggesting that transcriptional regulation of carbohydrate metabolism enzymes, especially those involved in glycolysis and the TCA cycle, might be essential for plant survival under low nutrient conditions.
In plants, K transporters and channels function in the absorption and translocation of K [28]. To date, many plant genes encoding K transporters and channels have been cloned, including AtAKT1, TaAKT1, AtHAK5, HvHAK1, and OsHAK1, and have been found to mediate the uptake of K under low-K conditions [7,9,10,36,37]. In our microarray experiment, we detected 17 genes related to K transport that were differentially expressed in response to low-K stress (Table 1 and S5 Table). According to cellular component GO analysis, the products of these genes were located in different cellular organelles (S5 Table), which suggested that low-K stress might not only affect the uptake of K but also the transport of K in cellular organelles. However, these genes showed differential expression changes, especially K transporters/channels located in the plasma membrane. The differential changes in the levels of transcripts involved in K transport might be a strategy employed by the plant to cope with K deficiency. The up-regulation of three K transporters (CUST_1497, CUST_36971 and CUST_7112) and a K channel AKT1 (CUST_36270) in the plasma membrane might be involved in K uptake in sugarcane under low-K conditions.
There are two primary signaling pathways that play important roles in regulating K uptake. The first well-known pathway is the Ca2+ signaling pathway, which is mediated by CBL-CIPK. Plant-specific serine/threonine protein kinases, or CIPK proteins, can exclusively interact with calcineurin B-Like protein and form a CBL-CIPK complex, which in turn constitutes a specific regulatory network of Ca2+ signaling in plant cells [38]. In plants, the CBL-CIPK complex (CBL1-CIPK23, CBL9-CIPK23, CBL4-CIPK6, CBL3-CIPK9) functions in response to low-K stress and is essential for regulating K uptake [11,39,40]. Additional work has revealed that phosphatase PP2CA also participates in the regulation of K uptake via K channels [41]. In sugarcane, two CBL genes, as well as many genes in the CIPK and PP2C families, were differentially expressed in response to low-K stress (Table 1), indicating that a CBL-CIPK-PP2CA pathway may also be involved in regulating K uptake under low-K stress.
The other signaling pathway responsible for low-K tolerance is mediated by ethylene. Ethylene content is significantly increased after only 6 h of K deficiency, and the transcription of genes involved in ethylene biosynthesis also increase in response to these conditions [42]. Under low-K stress, ethylene stimulates the production of ROS [12]. Then, ROS regulate the expression of AtHAK5 and stimulate root hair elongation, which enhances K uptake and stress tolerance [43]. Genes involved in the ethylene synthesis pathway, such as ACC synthase were identified in a study on comparative transcriptome profiling of wild barley genes responsive to low-K [15]. However, in our study, we found that an important gene in the ethylene synthesis pathway, ACC oxidase, was down-regulated at three time points (S4 Table and Fig 4). ACC oxidase functions to catalyze the conversion of 1-aminocyclopropane-1-carboxylic acid to ethylene, which is one of the most important steps in ethylene synthesis in plants. The down-regulation of ACC oxidase genes disrupts ethylene synthesis in sugarcane under conditions of low-K stress.
Commonly, transcription factors regulate the expression of stress response genes and help plants to overcome biotic and abiotic stress [44–46]. The differential expression of transcription factors, including many stress-related transcription factors, such as Myb, NAC, WRKY family transcription factors has been detected in rice, wild barley, soybean and Arabidopsis [14–17]. The AP2/ERF transcription factor RAP2.11 is an important component of the low-K signaling pathway. This transcription factor can be induced by ethylene and ROS and directly binds to the promoter of AtHAK5 to regulate its expression in response to low-K stress [13]. However, most of the ethylene-responsive transcription factors in our datasets, including AP2/ERF family members, were rapidly down-regulated in response to low-K stress (Table 1). The decrease in the expression of genes involved in both ethylene synthesis and the ethylene signaling pathway indicated that the function of ethylene in regulating low-K responses in sugarcane might be different from that in Arabidopsis.
ROS signals are commonly induced under nutrient deprivation conditions, including deficiencies in K, N, P and S [28,47]. ROS is an important signaling component; thus, its production and elimination are important for plant cells. In plants, peroxidase, cytochrome P450, and glutathione S-transferase participate in the production and elimination of ROS [48]. In our datasets, a number of genes involved in oxidative stress were differentially expressed, including lipoxygenase, glutathione S-transferase, superoxide dismutase and peroxidase. Meanwhile, a high percentage of genes involved in oxidative stress were up-regulated at the three time points, especially at 72 h (S8 Table). Up-regulation of genes in oxidative stress-related categories would help to eliminate the excessive ROS and maintain the balance of ROS in sugarcane, which might be a mechanism by which sugarcane can cope with low-K stress.
Conclusions
In the present study, we performed a microarray-based comparative investigation to assess expression changes in sugarcane genes in response to low-K stress. The microarray data showed that a total of 4153 genes were differentially expressed under low-K conditions and most of the changes appeared within 72 h. GO enrichment analysis revealed that genes involved in metabolic processes, cation binding, biological regulation, transport, and transcriptional regulation were enriched. Genes involved in the Ca+ signaling and ethylene pathways showed changes in the level of transcripts in response to low-K conditions, suggesting that they may play important roles in sugarcane responses to K deficiency. However, there were also many ambiguous tags and unannotated genes we were unable to explore in the current study. Future increases our knowledge regarding the sugarcane genome may resolve some of these ambiguities and lead to the discovery of large numbers of genes responsible for low-K tolerance. Although the experiments in which we profiled genes responsive to low-K stress were not replicated in the current study, these results will provide valuable clues for further studies. Further verification of these differentially expressed genes via transgenic technology could lead to the improvement of sugarcane resistance to K deficiency.
Supporting Information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We thank Zhiyu Sun and Hanqi Yin (Shanghai Biotechnology Corporation, China) for excellent technical assistance and valuable suggestions.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Earmarked Fund for China Agriculture Research System (CARS-20-1-4 and CARS-20-3-1), and the Science and Technology Planning Project of Guangdong Province, China (2012B020301008). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wang M, Zheng Q, Shen Q, Guo S. The critical role of potassium in plant stress response. Int J Mol Sci. 2013;14: 7370–7390. 10.3390/ijms14047370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ashley MK, Grant M, Grabov A. Plant responses to potassium deficiencies: a role for potassium transport proteins. J Exp Bot. 2006;57: 425–436. [DOI] [PubMed] [Google Scholar]
- 3. Schroeder JI, Ward JM, Gassmann W. Perspectives on the physiology and structure of inward-rectifying K+ channels in higher plants: biophysical implications for K+ uptake. Annu Rev Biophys Biomol Struct. 1994;23: 441–471. [DOI] [PubMed] [Google Scholar]
- 4. Grabov A. Plant KT/KUP/HAK potassium transporters: single family—multiple functions. Ann Bot. 2007;99: 1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim EJ, Kwak JM, Uozumi N, Schroeder JI. AtKUP1: an Arabidopsis gene encoding high-affinity potassium transport activity. Plant Cell. 1998;10: 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gierth M, Maser P, Schroeder JI. The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant Physiol. 2005;137: 1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qi Z, Hampton CR, Shin R, Barkla BJ, White PJ, Schachtman DP. The high affinity K+ transporter AtHAK5 plays a physiological role in planta at very low K+ concentrations and provides a caesium uptake pathway in Arabidopsis . J Exp Bot. 2008;59: 595–607. 10.1093/jxb/erm330 [DOI] [PubMed] [Google Scholar]
- 8. Fulgenzi FR, Peralta ML, Mangano S, Danna CH, Vallejo AJ, Puigdomenech P, et al. The ionic environment controls the contribution of the barley HvHAK1 transporter to potassium acquisition. Plant Physiol. 2008;147: 252–262. 10.1104/pp.107.114546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Banuelos MA, Garciadeblas B, Cubero B, Rodriguez-Navarro A. Inventory and functional characterization of the HAK potassium transporters of rice. Plant Physiol. 2002;130: 784–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buschmann PH, Vaidyanathan R, Gassmann W, Schroeder JI. Enhancement of Na+ uptake currents, time-dependent inward-rectifying K+ channel currents, and K+ channel transcripts by K+ starvation in wheat root cells. Plant Physiol. 2000;122: 1387–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, et al. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis . Cell. 2006;125: 1347–1360. [DOI] [PubMed] [Google Scholar]
- 12. Jung JY, Shin R, Schachtman DP. Ethylene mediates response and tolerance to potassium deprivation in Arabidopsis . Plant Cell. 2009;21: 607–621. 10.1105/tpc.108.063099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim MJ, Ruzicka D, Shin R, Schachtman DP. The Arabidopsis AP2/ERF transcription factor RAP2.11 modulates plant response to low-potassium conditions. Mol Plant. 2012;5: 1042–1057. 10.1093/mp/sss003 [DOI] [PubMed] [Google Scholar]
- 14. Ma TL, Wu WH, Wang Y. Transcriptome analysis of rice root responses to potassium deficiency. BMC Plant Biol. 2012;12: 161 10.1186/1471-2229-12-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeng J, He X, Wu D, Zhu B, Cai S, Nadira UA, et al. Comparative transcriptome profiling of two Tibetan wild barley genotypes in responses to low potassium. PLoS One. 2014;9: e100567 10.1371/journal.pone.0100567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang C, Chen H, Hao Q, Sha A, Shan Z, Chen L, et al. Transcript profile of the response of two soybean genotypes to potassium deficiency. PLoS One. 2012;7: e39856 10.1371/journal.pone.0039856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Armengaud P, Breitling R, Amtmann A. The potassium-dependent transcriptome of Arabidopsis reveals a prominent role of jasmonic acid in nutrient signaling. Plant Physiol. 2004;136: 2556–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wood R. The roles of nitrogen, phosphorus and potassium in the production of sugarcane in South Africa. Fertilizer Res. 1990;26: 89–98. [Google Scholar]
- 19. Gopalasundaram P, Bhaskaran A, Rakkiyappan P. Integrated nutrient management in sugarcane. Sugar Tech. 2012;14: 3–20. [Google Scholar]
- 20. Ashraf M, Afzal M, Ahmad R, Maqsood MA, Shahzad SM, Tahir MA, et al. Growth response of the salt-sensitive and the salt-tolerant sugarcane genotypes to potassium nutrition under salt stress. Arch Agron Soil Sci. 2012;58: 385–398. [Google Scholar]
- 21. Baligar VC, Fageria NK, He ZL. Nutrient use efficiency in plant. Commun Soil Sci Plant. 2007;32: 921–950. [Google Scholar]
- 22. Lian X, Wang S, Zhang J, Feng Q, Zhang L, Fan D, et al. Expression profiles of 10,422 genes at early stage of low nitrogen stress in rice assayed using a cDNA microarray. Plant Mol Biol. 2006;60: 617–631. [DOI] [PubMed] [Google Scholar]
- 23. Wasaki J, Shinano T, Onishi K, Yonetani R, Yazaki J, Fujii F, et al. Transcriptomic analysis indicates putative metabolic changes caused by manipulation of phosphorus availability in rice leaves. J Exp Bot. 2006;57: 2049–2059. [DOI] [PubMed] [Google Scholar]
- 24. Famoso AN, Clark RT, Shaff JE, Craft E, McCouch SR, Kochian LV. Development of a novel aluminum tolerance phenotyping platform used for comparisons of cereal aluminum tolerance and investigations into rice aluminum tolerance mechanisms. Plant Physiol. 2010;153: 1678–1691. 10.1104/pp.110.156794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bao S. Soil and agricultural chemistry analysis (in Chinese) Chinese Agriculture Publishing House; 1999. [Google Scholar]
- 26. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 27. Wyn Jones R, Pollard A. Proteins, enzymes and inorganic ions In: Lauchli A, Pirson A, editors. Encyclopedia of plant physiology. Berlin: Springer-Verlag; 1983. pp. 528–562. [Google Scholar]
- 28. Wang Y, Wu WH. Potassium transport and signaling in higher plants. Annu Rev Plant Biol. 2013;64: 451–476. 10.1146/annurev-arplant-050312-120153 [DOI] [PubMed] [Google Scholar]
- 29. Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55: 373–399. [DOI] [PubMed] [Google Scholar]
- 30. Nieves-Cordones M, Miller AJ, Aleman F, Martinez V, Rubio F. A putative role for the plasma membrane potential in the control of the expression of the gene encoding the tomato high-affinity potassium transporter HAK5. Plant Mol Biol. 2008;68: 521–532. 10.1007/s11103-008-9388-3 [DOI] [PubMed] [Google Scholar]
- 31. Schachtman DP, Shin R. Nutrient sensing and signaling: NPKS. Annu Rev Plant Biol. 2007;58: 47–69. [DOI] [PubMed] [Google Scholar]
- 32. Armengaud P, Sulpice R, Miller AJ, Stitt M, Amtmann A, Gibon Y. Multilevel analysis of primary metabolism provides new insights into the role of potassium nutrition for glycolysis and nitrogen assimilation in Arabidopsis roots.Plant Physiol. 2009;150: 772–785. 10.1104/pp.108.133629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith CR, Knowles VL, Plaxton WC. Purification and characterization of cytosolic pyruvate kinase from Brassica napus (rapeseed) suspension cell culture: implications for the integration of glycolysis with nitrogen assimilation. Eur J Biochem. 2000;267:4477–85. [DOI] [PubMed] [Google Scholar]
- 34. Wasaki J, Yonetani R, Kuroda S, Shinano T, Yazaki J, Fujii F, Shimbo K, et al. Transcriptomic analysis of metabolic changes by phosphorus stress in rice plant roots. Plant, Cell & Environment. 2003;26: 1515–1523. [Google Scholar]
- 35. Wang R, Okamoto M, Xing X, Crawford NM. Microarray analysis of the nitrate response in Arabidopsis roots and shoots reveals over 1,000 rapidly responding genes and new linkages to glucose, trehalose-6-phosphate, iron, and sulfate metabolism. Plant Physiol. 2003;132: 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Santa-Maria GE, Rubio F, Dubcovsky J, Rodriguez-Navarro A. The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell. 1997;9: 2281–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dennison KL, Robertson WR, Lewis BD, Hirsch RE, Sussman MR, Spalding EP. Functions of AKT1 and AKT2 potassium channels determined by studies of single and double mutants of Arabidopsis . Plant Physiol. 2001;127: 1012–1019. [PMC free article] [PubMed] [Google Scholar]
- 38. Yu Q, An L, Li W. The CBL-CIPK network mediates different signaling pathways in plants. Plant Cell Rep. 2014;33: 203–214. 10.1007/s00299-013-1507-1 [DOI] [PubMed] [Google Scholar]
- 39. Held K, Pascaud F, Eckert C, Gajdanowicz P, Hashimoto K, Corratgé-Faillie C, et al. Calcium-dependent modulation and plasma membrane targeting of the AKT2 potassium channel by the CBL4/CIPK6 calcium sensor/protein kinase complex. Cell Res. 2011;21: 1116–1130. 10.1038/cr.2011.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu LL, Ren HM, Chen LQ, Wang Y, Wu WH. A protein kinase, calcineurin B-like protein-interacting protein Kinase9, interacts with calcium sensor calcineurin B-like Protein3 and regulates potassium homeostasis under low-potassium stress in Arabidopsis . Plant Physiol. 2013;161: 266–277. 10.1104/pp.112.206896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lan WZ, Lee SC, Che YF, Jiang YQ, Luan S. Mechanistic analysis of AKT1 regulation by the CBL-CIPK-PP2CA interactions. Mol Plant. 2011;4: 527–536. 10.1093/mp/ssr031 [DOI] [PubMed] [Google Scholar]
- 42. Shin R, Schachtman DP. Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc Natl Acad Sci U S A. 2004;101: 8827–8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim MJ, Ciani S, Schachtman DP. A peroxidase contributes to ROS production during Arabidopsis root response to potassium deficiency. Mol Plant. 2010;3: 420–427. 10.1093/mp/ssp121 [DOI] [PubMed] [Google Scholar]
- 44. Golldack D, Lüking I, Yang O. Plant tolerance to drought and salinity: stress regulating transcription factors and their functional significance in the cellular transcriptional network. Plant Cell Rep. 2011;30: 1383–1391. 10.1007/s00299-011-1068-0 [DOI] [PubMed] [Google Scholar]
- 45. Chen L, Song Y, Li S, Zhang L, Zou C, Yu D. The role of WRKY transcription factors in plant abiotic stresses. Biochim Biophys Acta. 2012;1819: 120–128. 10.1016/j.bbagrm.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 46. Alves MS, Dadalto SP, Goncalves AB, De Souza GB, Barros VA, Fietto LG. Plant bZIP transcription factors responsive to pathogens: a review. Int J Mol Sci. 2013;14: 7815–7828. 10.3390/ijms14047815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shin R, Berg RH, Schachtman DP. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol. 2005;46: 1350–1357. [DOI] [PubMed] [Google Scholar]
- 48. Kim MJ, Ciani S, Schachtman DP. A peroxidase contributes to ROS production during Arabidopsis root response to potassium deficiency. Mol Plant. 2010;3: 420–427. 10.1093/mp/ssp121 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.