Abstract
Background
Multiplex analysis allows measurements of a large number of analytes simultaneously in each sample. Based on the Luminex multiplex technology (xMAP), kits for measuring multiple cytokines and chemokines (immunomodulators) are commercially available and are useful in investigations on inflammatory diseases. This study evaluated four multiplex kits (Bio-Plex, LINCOplex, Fluorokine, and Beadlyte) that contained 27, 29, 20 and 22 analytes each, respectively, for the analysis of immunomodulators in plasma of rheumatoid arthritis (RA) patients who underwent treatment with antibody against CD20 (rituximab), a B-cell reductive therapy.
Methods
Multiplex kits were tested on serial plasma samples obtained from six RA patients at baseline and multiple time points (3, 6, and 9 months) post-treatment with rituximab. The RA patients included in this study had previously failed therapy with disease modifying anti-arthritis drugs (DMARD) and treatment with anti-TNFα antibody (infliximab).
Results
Computer modeling and hierarchical cluster analysis of the multiplex data allowed a comparison of the performance of multiplex assay kits and revealed profiles of immunomodulators in the RA patients.
Conclusions
In plasma of RA patients who appeared to have benefited from rituximab treatment the profile of significantly elevated immunomodulators by at least two of the three kits (BioPlex, LINCOplex, Beadlyte), is as follows: IL-12p70, Eotaxin, IL-4, TNFα, Il-9, IL-1β, IFNγ, IL-10, IL-6, and IL-13. Immunomodulator profiling by multiplex analysis may provide useful plasma biomarkers for monitoring response to B-cell reductive therapy in RA patients.
Keywords: Cytokines, chemokines, rheumatoid arthritis, Luminex, multiplex suspension array
Introduction
Cytokines and chemokines are key modulators of the immune system. Levels of these proteins may be altered in a variety of diseases, particularly inflammatory diseases (1–3). Measurements of cytokines and chemokines in plasma/serum and other body fluids may provide insights into disease mechanisms and can have clinical application for disease diagnosis, prognosis and therapy. To date, several dozen distinct cytokines (3) and chemokines (1,2) have been described. Because these immunomodulatory molecules exhibit a certain amount of redundancy and promiscuity in their functions (1–3), measurements of a large number of cytokines and chemokines to generate molecular profiles are likely to provide more biologically relevant information than measuring one or a few of these. Amounts of these analytes can be measured by immunoassays e.g., ELISA (body fluids), flow cytometery (cells), and indirect immunofluorescence in cells and tissues (4). However, for studying profiles of large numbers of cytokines, these conventional procedures are labor intensive and have limited throughput. In addition, small sample volume may prohibit performing multiple tests on a single sample. Recent advances in multiplex technologies enable measurement of multiple analytes simultaneously. Multiplexing provides data on a large number of analytes, even when sample volumes are limited (5,6). Several multiplex detection systems are available (7,8). A popular system is based on the Luminex xMAP technology. This technology allows simultaneous measurements of up to a hundred analytes in a single test sample (6).
Blood cytokine levels are likely to be altered in a number of diseases including cancer (9,10), cardiovascular diseases (11,12), inflammatory diseases (13–15), and infectious diseases (13,14,16). Therefore, plasma levels of cytokines and chemokines may serve as useful biomarkers for patient stratification and for monitoring efficacy of therapy. Because, the underlying molecular mechanism of disease is often not clear, it is difficult to predict which individual cytokines might be more useful for measurement. Therefore, a practical choice is to use multiplex technology with the capability to measure a large number of immunomodulators simultaneously.
Rheumatoid arthritis (RA) is a systemic, potentially debilitating, chronic autoimmune disease characterized by inflammation and destruction of the joints. Rituximab, a chimeric anti-CD20 monoclonal antibody that is FDA approved for the treatment of non-Hodgkins lymphoma and RA, reduces antibody production by depleting B-cells. However, its effect on cytokines and chemokines is not understood. The purpose of this study was to evaluate changes that may occur over time in immunomodulator levels in RA patients undergoing rituximab therapy. The small sample volume required for multiplex testing also allowed for comparison of multiplex kits for the largest panels of analytes available from each manufacturer (Bio-Rad (BioPlex), LINCO (LINCOplex), R&D Systems (Fluorokine) and Upstate (Beadlyte) containing 27, 29, 21 and 22 cytokines and chemokines each, respectively. Such a comparison would be helpful to more accurately identify alterations in analyte concentrations in patient plasma.
Materials and Methods
Patients
Study subjects eligible for enrollment were adult (≥18 years), male or female, patients who had active, seropositive RA with at least 2 swollen and tender joints and functional class I, II, or III, as defined by the American College of Rheumatology (ACR) revised criteria, despite at least 3 months’ treatment with at least 1 DMARD, and who were able to give signed, informed consent. This study was approved by the Institutional Review Boards of the VA Northern California Health Care System and the University of California, Davis.
Patients were excluded if they had a history of hypersensitivity to murine proteins or a history of significant renal, hepatic, cardiac, or psychiatric impairment. Additional exclusion criteria were active infection (including seropositive hepatitis C) or a malignancy (except adequately treated basal cell or squamous cell carcinoma of the skin or in situ carcinoma of the cervix) within the previous 5 years. Female patients who were pregnant or were planning conception during the subsequent 2 years were also excluded. A total of seventeen patients were enrolled in the original study (17). Of the seventeen patients in the original study, plasma samples from 6 patients were available at multiple time points. For the rest of the patients, plasma samples had been exhausted during the original study. Therefore, in the present study we focused on samples from six patients for whom samples at different time points were available for the analysis of immunomodulator levels at baseline, and 3, 6, and 9 months.
Treatment
Patients continued with their baseline DMARDs and other concomitant medications at a stable dose throughout the study period. Rituximab was administered as an intravenous (i.v.) infusion weekly for 4 consecutive weeks according to a dose escalation schedule that was prospectively agreed to, prior to the availability of Phase IIa data (17) by federal regulatory authorities, as a condition of approval of the investigational new drug (IND) application for this study.
Prior to each infusion, patients received acetaminophen 650 mg orally, diphenhydramine 50 mg orally or intravenously, and dexamethasone 10 mg intravenously to attenuate possible infusion-related symptoms. During week 1, the dose of rituximab was 100 mg which was infused at a rate of 50 mg/h with incremental increases of 50 mg/h after 30 minutes as tolerated. During the second week, 375 mg/m2 of rituximab was infused at an initial rate of 100 mg/h with incremental increases of 100 mg/h every 30 minutes as tolerated up to a maximum infusion rate of 400 mg/h if the first dose was well tolerated. During weeks 3 and 4, a dose of 500 mg/m2 of rituximab was infused at the same incremental rates as tolerated during week 2.
During the infusions, the patients were closely observed for infusion-related symptoms such as transient fever, rigors, hypotension, and dyspnea, in which event the infusion could be slowed or temporarily stopped and restarted when symptoms resolved. In this study, however, there were no such symptoms necessitating divergence from the above schedule. No further rituximab infusions were administered during the subsequent follow-up period, although patients remained on their baseline therapy according to clinical need.
Clinical assessments
At baseline, a thorough history and physical examination was conducted and recorded together with baseline demographic data. At baseline and at weeks 5, 8, 16, and 28, the number of tender joints (tender joint count [TJC]) and swollen joints (swollen joint count [SJC]) were recorded for each patient. At these time points the global assessment score of disease activity was recorded for each patient by the physician and by the patient using a 100-mm horizontal visual analog score (VAS) where the left-hand extreme was labeled “no disease activity” and the right-hand extreme was labeled “maximum disease activity”. Similarly, at each assessment time point patients recorded their level of pain on a 100-mm VAS where the left-hand extreme was labeled “no pain” and the right-hand extreme was labeled “pain as bad as it could be”. In addition, patient-assessed disability was evaluated using the Multidimensional Health Assessment Questionnaire (17).
Blood samples were obtained at baseline and at follow-up visits for assessment of laboratory parameters including erythrocyte sedimentation rate (ESR), rheumatoid factor (RF), peripheral B and T cell counts, complement C3 and C4 levels, and immunoglobulin isotype (IgA, IgG, and IgM) and human anti-chimeric antibody (HACA) titers. Samples were processed and analyzed using standard techniques (17).
Healthy Controls
Control plasma samples were obtained randomly from fourteen healthy individuals regardless of sex but the donors were of advanced age (median age of 58 years). The median age of patients was 66 years.
Multiplex kits
Multiplex kits for measuring cytokines and chemokines on the Luminex platform were obtained from four different vendors. The kit manufacturers and the list of analytes included in each kit are shown in Table 1. Analytes that are common between the kits are indicated by asterisks. The kits were provided as generous gifts by the following manufacturers: Bio-Plex by Bio-Rad (Hercules, CA), Beadlyte by Upstate (Charlottesville, VA), LINCOplex by LINCO (St. Louis, MO), and Fluorokine by R&D Systems (Minneapolis, MN). The kits were used per the manufacturers’ instructions. Plasma samples were diluted using the appropriate sample diluents provided in each kit in accordance with the manufacturer’s instructions.
Table 1.
List of analytes from various panels
| No | Analytes | Bioplex | Beadlyte | LINCOplex | Fluorokine | Common |
|---|---|---|---|---|---|---|
| 1 | EGF | x | ||||
| 2 | ENA78 | x | ||||
| 3 | Eotaxin | x | x | x | ||
| 4 | FGF | x | ||||
| 5 | FGF basic | x | ||||
| 6 | Fractalkine | x | ||||
| 7 | G-CSF | x | x | x | x | * |
| 8 | GM-CSF | x | x | |||
| 9 | IFNg | x | x | x | x | * |
| 10 | IL-10 | x | x | x | x | * |
| 11 | IL-12p40 | x | x | |||
| 12 | IL-12p70 | x | x | x | ||
| 13 | IL-13 | x | x | x | ||
| 14 | IL-15 | x | x | x | ||
| 15 | IL-17 | x | x | x | ||
| 16 | IL-1α | x | x | x | ||
| 17 | IL-1β | x | x | x | x | * |
| 18 | IL-1ra | x | x | x | ||
| 19 | IL-2 | x | x | x | x | * |
| 20 | IL-3 | x | ||||
| 21 | IL-4 | x | x | x | x | * |
| 22 | IL-5 | x | x | x | x | * |
| 23 | IL-6 | x | x | x | x | * |
| 24 | IL-7 | x | x | x | ||
| 25 | IL-8 | x | x | x | x | * |
| 26 | IL-9 | x | ||||
| 27 | IP-10 | x | x | x | ||
| 28 | MCP-1 | x | x | x | x | * |
| 29 | MIP-1α | x | x | x | x | * |
| 30 | MIP-1β | x | x | x | ||
| 31 | PDGF-bb | x | ||||
| 32 | RANTES | x | x | x | ||
| 33 | sCD40L | x | ||||
| 34 | TGFα | x | ||||
| 35 | TNFα | x | x | x | x | |
| 36 | TPO | x | ||||
| 37 | VEGF | x | x | x | ||
| Total | 27 | 22 | 29 | 21 | 11 | |
Data Analysis
Concentrations (pg/ml) of different analytes in the plasma samples were determined by using the standard curves generated in the multiplex assays. MasterPlex QT software for quantitation was used for 5 point curve fitting to generate standard curves and analyze data according to manufacturer’s instructions (MiraiBio, Alameda, CA). This software uses "Levenberg-Marquardt" nonlinear least squares minimization algorithms for the curve fitting by 5PL equation and determines the high and low limits of detection (MiraiBio, user’s manual). Data points for analytes that were occasionally above or below the detection range were discarded. The analyte concentrations were transformed to log2 scale for further analysis to accommodate the dynamic range in the concentration values. Many of the analytes measured, using the Fluorokine panel, were not detectable. Therefore, results obtained with the Fluorokine kit were dropped from further analysis. Investigation of reproducible differences between treatments was performed with the Bioconductor and R software package. Robust linear regression was used on the observed versus theoretical quantiles to determine the linear transformation of these t-statistics would confer a normal distribution, and then scaled accordingly (18). Data were visualized with box-and-whisker plots and scatter plots. Intensities were adjusted to the same interquartile range. A linear model fit was determined for each analyte using the LIMMA package using R, and lists of analytes with the most evidence of differential expression were obtained. The per-panel data corresponding to analytes with a positive signal were combined in a two-step process to obtain an initial analytical data set. First, the initial data set consisted of data for all the analytes for which a signal was detected for at least one treatment for one patient. Second, the data from all the patients were combined into a single data set. The resulting data set consisted of analytes that exhibited modulation for at least one patient for at least one of the treatment tested. Differential measurements in patient samples across the four time points (baseline, and 3, 6 and 9 months) were detected by an F test. A separate F test, was performed on each analyte and each panel; p-values for different analytes were transformed to compensate for multiple comparisons using the False Discovery Rate (FDR) adjustment for multiple comparisons using the Benjamini-Hochberg procedure. After tranforming the p-values for these analytes, a table containing a select set of analytes for each panel was generated.
Fold changes (log2) and analyte concentrations (pg/ml ± standard error) are listed in Tables 3 and 4. Fold changes in Table 3 are derived from multivariate statistical analysis. This analysis allows a comparison of more than one statistical variable at a time and therefore increases the statistical dimensionality of the data to get a meaningful fold change and p.value for multiple comparisons. As an example, fold change for Eotaxin was calculated as follows: mean value of the normal is 94 pg/ml, while that of the Patient 1 at 3, 6 and 9 months are 959 pg/ml, 1505 pg/ml and 829 pg/ml, respectively (Table 4). Fold changes (log2) for patient 1 at 3, 6 and 9 months are 3.4 (log2 (959/94)), 4.0 (log2 (1505/94)) and 3.1 (log2 (829/94)), respectively (Table 3).
Table 3.
Fold changes of select analytes in patients classified by cluster analysis for BioPlex, LINCOplex and Beadlyte kits.
| Patient#/ Time Point |
1 3 Month |
1 6 Month |
1 9 Month |
3 0 Month |
3 3 Month |
3 6 Month |
3 9 Month |
6 0 Month |
6 3 Month |
6 6 Month |
|---|---|---|---|---|---|---|---|---|---|---|
| BioPlex | ||||||||||
| Eotaxin@ | 3.4 | 4.0 | 3.1 | 4.8 | 3.5 | 3.7 | 5.0 | 3.7 | 3.0 | 3.7 |
| G-CSF | 3.0 | 4.0 | 3.3 | 4.5 | 3.4 | 4.0 | 4.7 | 3.6 | 2.3 | 3.5 |
| IFNγ* | 5.3 | 5.9 | 5.0 | 5.9 | 4.5 | 5.0 | 5.8 | 4.4 | 3.2 | 5.0 |
| IL-10* | 3.1 | 5.6 | 3.0 | 6.6 | 5.2 | 5.8 | 6.5 | 5.2 | 3.0 | 3.6 |
| IL12p70@ | 6.0 | 7.6 | 6.6 | 6.6 | 5.2 | 5.7 | 5.8 | 6.3 | 4.3 | 3.8 |
| IL-13# | 5.4 | 5.8 | 5.0 | 6.1 | 5.2 | 5.3 | 4.7 | 4.4 | 2.9 | 3.2 |
| IL-1β* | 6.1 | 6.9 | 5.7 | 6.7 | 5.4 | 5.7 | 6.5 | 5.5 | 4.1 | 5.6 |
| IL-1ra | 5.7 | 5.7 | 4.5 | 6.2 | 4.8 | 5.1 | 6.1 | 4.5 | 3.6 | 4.9 |
| IL_2 | 5.6 | 6.6 | 4.2 | 7.2 | 6.2 | 6.7 | 7.1 | 6.3 | 5.3 | 5.7 |
| IL_4@ | 5.4 | 6.1 | 4.5 | 6.1 | 4.6 | 5.2 | 5.7 | 4.7 | 3.6 | 4.9 |
| IL-5 | 9.0 | 9.2 | 9.0 | 7.7 | 6.4 | 6.5 | 8.1 | 6.4 | 4.6 | 5.0 |
| IL-6* | 4.3 | 5.0 | 3.7 | 5.7 | 4.4 | 4.6 | 5.2 | 4.1 | 3.1 | 4.1 |
| IL-7 | 5.4 | 7.4 | 7.2 | 7.0 | 5.8 | 6.6 | 7.1 | 5.8 | 4.3 | 4.1 |
| IL-9* | 6.4 | 6.9 | 7.0 | 4.2 | 3.8 | 3.5 | 4.2 | 4.9 | 3.2 | 3.2 |
| Beadlyte | ||||||||||
| Eotaxin@ | 3.9 | 1.9 | 3.9 | 7.7 | 5.1 | 4.0 | 4.2 | 2.0 | 4.1 | 4.1 |
| GM-CSF | 4.2 | 2.5 | 4.2 | 12.9 | 6.9 | 5.4 | 6.2 | 3.1 | 5.5 | 5.3 |
| IL12p70@ | 4.4 | 0.8 | 4.4 | 9.4 | 6.1 | 5.8 | 5.7 | 1.7 | 4.5 | 3.7 |
| IL-13# | 4.7 | 2.0 | 4.7 | 7.9 | 5.8 | 6.0 | 6.4 | 3.0 | 4.6 | 4.7 |
| IL-3 | 8.4 | 2.7 | 8.4 | 9.2 | 8.8 | 9.2 | 9.2 | 3.8 | 8.3 | 8.9 |
| IL-4@ | 4.3 | 0.8 | 4.3 | 6.9 | 5.3 | 6.2 | 6.8 | 1.9 | 4.5 | 5.2 |
| TNFα% | 7.1 | 2.6 | 7.1 | 8.9 | 7.0 | 8.0 | 9.6 | 4.0 | 6.2 | 6.2 |
| LINCOplex | ||||||||||
| Eotaxin@ | 3.9 | 4.5 | 3.5 | 5.1 | 3.9 | 4.1 | 5.2 | 4.3 | 4.3 | 3.4 |
| IFNγ* | 4.5 | 5.1 | 4.2 | 5.0 | 3.6 | 4.2 | 5.0 | 4.4 | 3.7 | 2.7 |
| IL-10* | 2.4 | 4.8 | 2.1 | 5.9 | 4.3 | 4.8 | 5.5 | 2.7 | 4.2 | 2.6 |
| IL12p70@ | 4.9 | 6.6 | 5.6 | 5.7 | 3.7 | 4.5 | 4.3 | 2.6 | 5.1 | 3.1 |
| IL-15 | 2.8 | 3.3 | 2.5 | 4.8 | 3.8 | 3.8 | 4.9 | 3.5 | 3.5 | 2.6 |
| IL-1β* | 4.0 | 4.9 | 3.2 | 4.7 | 2.8 | 3.3 | 4.4 | 3.0 | 2.9 | 1.6 |
| IL-4@ | 5.2 | 5.8 | 4.3 | 5.9 | 4.3 | 4.9 | 5.6 | 4.5 | 4.5 | 3.1 |
| IL-6* | 4.0 | 4.9 | 3.3 | 5.6 | 4.0 | 4.4 | 5.0 | 3.7 | 3.9 | 2.5 |
| IL-9* | 5.7 | 6.7 | 6.6 | 3.8 | 3.5 | 3.1 | 3.9 | 4.2 | 3.1 | 2.9 |
| TNFα% | 4.2 | 5.6 | 3.3 | 5.5 | 3.5 | 4.5 | 5.6 | 3.4 | 2.9 | 1.7 |
Analytes shared by all three kits are denoted by @, those shared between Bioplex and LINCOplex are noted by *, # denotes analytes common between BioPlex and Beadlyte and % denotes those common between Beadlyte and LINCOplex. Fold changes listed in Table are derived by multi-variate statistics as described in the Materials and Methods.
Table 4.
Concentrations of the select analytes in healthy controls and patients BioPlex, LINCOplex and Beadlyte kits.
| Bio-Plex – Analyte Concentrations (pg/ml) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | Patient 3 | Patient 6 | |||||||||
| Proteins | Healthy | 3 Month | 6 Month | 9 Month | 0 Month | 3 Month | 6 Month | 9 Month | 0 Month | 3 Month | 6 Month |
| Eotaxin@ | 159 ± 32 | 965 ± 108 | 1515 ± 180 | 832 ± 66 | 2557 ± 125 | 1031 ± 2 | 1232 ± 194 | 2923 ± 170 | 1252 ± 55 | 733 ± 20 | 1229 ± 63 |
| G-CSF | 114 ± 19 | 657 ± 64 | 1353 ± 151 | 863 ± 121 | 1839 ± 12 | 894 ± 24 | 1341 ± 54 | 2245 ± 17 | 1036 ± 31 | 400 ± 6 | 929 ± 20 |
| IFNγ* | 338 ± 85 | 8227 ± 136 | 12637 ± 1042 | 6709 ± 392 | 12414 ± 89 | 4946 ± 310 | 6819 ± 382 | 11997 ± 1404 | 4634 ± 25 | 1964 ± 162 | 7007 ± 4 |
| IL-10* | 33 ± 7 | 181 ± 2 | 987 ± 149 | 170 ± 29 | 1953 ± 23 | 782 ± 3 | 1158 ± 34 | 1912 ± 401 | 736 ± 9 | 162 ± 17 | 249 ± 19 |
| IL12p70@ | 31 ± 6 | 1310 ± 121 | 3851 ± 771 | 1979 ± 466 | 1922 ± 46 | 728 ± 53 | 1038 ± 55 | 1171 ± 226 | 1571 ± 79 | 402 ± 26 | 289 ± 32 |
| IL-13# | 5 ± 1 | 127 ± 3 | 169 ± 18 | 91 ± 8 | 194 ± 13 | 107 ± 1 | 119 ± 4 | 87 ± 43 | 63 ± 2 | 22 ± 1 | 27 ± 2 |
| IL-1β* | 16 ± 6 | 478 ± 42 | 850 ± 162 | 346 ± 27 | 732 ± 27 | 278 ± 2 | 344 ± 18 | 642 ± 97 | 300 ± 19 | 121 ± 3 | 324 ± 2 |
| IL-1ra | 510 ± 178 | 11617 ± 1173 | 11826 ± 1614 | 4922 ± 534 | 16333 ± 502 | 6029 ± 31 | 7691 ± 712 | 15193 ± 1121 | 5229 ± 206 | 2629 ± 68 | 6465 ± 228 |
| IL_2 | 101 ± 47 | 1198 ± 83 | 2352 ± 423 | 434 ± 47 | 3621 ± 153 | 1806 ± 125 | 2578 ± 274 | 3354 ± 358 | 1863 ± 117 | 957 ± 58 | 1236 ± 59 |
| IL_4@ | 31 ± 10 | 644 ± 31 | 1070 ± 121 | 359 ± 37 | 1056 ± 3 | 382 ± 21 | 567 ± 44 | 860 ± 320 | 419 ± 14 | 197 ± 16 | 457 ± 37 |
| IL-5 | 1 ± 0 | 233 ± 3 | 282 ± 30 | 245 ± 42 | 98 ± 1 | 40 ± 5 | 42 ± 3 | 124 ± 10 | 40 ± 0 | 11 ± 1 | 15 ± 1 |
| IL-6* | 195 ± 64 | 1774 ± 132 | 2974 ± 278 | 1225 ± 157 | 4640 ± 195 | 1898 ± 160 | 2302 ± 231 | 3542 ± 645 | 1580 ± 13 | 791 ± 26 | 1605 ± 35 |
| IL-7 | 7 ± 2 | 149 ± 2 | 634 ± 87 | 542 ± 71 | 459 ± 24 | 195 ± 2 | 341 ± 28 | 503 ± 3 | 198 ± 1 | 69 ± 1 | 63 ± 0 |
| IL-9* | 255 ± 46 | 13722 ± 543 | 19265 ± 3152 | 20761 ± 3307 | 3045 ± 132 | 2353 ± 223 | 1816 ± 47 | 3048 ± 318 | 4859 ± 14 | 1538 ± 20 | 1506 ± 51 |
| Beadlyte - Concentrations (pg/ml) | |||||||||||
| Eotaxin@ | 68 ± 30 | 399 ± 234 | 399 ± 234 | 399 ± 234 | 5247 ± 3753 | 727 ± 55 | 5247 ± 3753 | 727 ± 55 | 917 ± 522 | 385 ± 11 | 907 ± 533 |
| GM-CSF | 4 ± 1 | 42 ± 22 | 42 ± 22 | 42 ± 22 | 14360 ± 13944 | 206 ± 50 | 14360 ± 13944 | 206 ± 50 | 192 ± 95 | 82 ± 15 | 177 ± 110 |
| IL12p70@ | 8 ± 0 | 81 ± 69 | 81 ± 69 | 81 ± 69 | 2647 ± 2162 | 389 ± 15 | 2647 ± 2162 | 389 ± 15 | 168 ± 62 | 71 ± 35 | 133 ± 97 |
| IL-13# | 6 ± 1 | 67 ± 50 | 67 ± 50 | 67 ± 50 | 653 ± 404 | 324 ± 39 | 653 ± 404 | 324 ± 39 | 104 ± 12 | 100 ± 8 | 112 ± 4 |
| IL-3 | 23 ± 14 | 1443 ± 1387 | 1443 ± 1387 | 1443 ± 1387 | 4451 ± 550 | 5000 ± 0 | 4451 ± 550 | 5000 ± 0 | 2880 ± 1112 | 2918 ± 1150 | 4030 ± 38 |
| IL-4@ | 11 ± 0 | 117 ± 98 | 117 ± 98 | 117 ± 98 | 907 ± 467 | 1044 ± 226 | 907 ± 467 | 1044 ± 226 | 285 ± 144 | 258 ± 117 | 402 ± 27 |
| TNFα% | 4 ± 1 | 204 ± 187 | 204 ± 187 | 204 ± 187 | 841 ± 487 | 1407 ± 721 | 841 ± 487 | 1407 ± 721 | 205 ± 36 | 243 ± 3 | 208 ± 39 |
| LINCOplex - Concentrations (pg/ml) | |||||||||||
| Eotaxin@ | 111 ± 28 | 978 ± 120 | 1570 ± 149 | 764 ± 47 | 2349 ± 43 | 975 ± 20 | 1167 ± 105 | 2451 ± 48 | 1281 ± 18 | 705 ± 18 | 1316 ± 30 |
| IFNγ* | 657 ± 127 | 10362 ± 270 | 15929 ± 1212 | 8651 ± 522 | 14847 ± 307 | 5620 ± 64 | 8802 ± 146 | 15247 ± 1483 | 6071 ± 82 | 3051 ± 148 | 9609 ± 3 |
| IL-10* | 40 ± 5 | 167 ± 2 | 910 ± 154 | 136 ± 18 | 1958 ± 110 | 639 ± 8 | 898 ± 31 | 1494 ± 263 | 608 ± 44 | 194 ± 25 | 204 ± 0 |
| IL12p70@ | 42 ± 2 | 1193 ± 47 | 3834 ± 1040 | 1887 ± 220 | 2087 ± 57 | 493 ± 5 | 889 ± 107 | 935 ± 503 | 1336 ± 21 | 346 ± 23 | 238 ± 9 |
| IL-15 | 22 ± 4 | 108 ± 16 | 148 ± 18 | 91 ± 15 | 438 ± 16 | 217 ± 6 | 209 ± 18 | 475 ± 29 | 170 ± 10 | 92 ± 12 | 180 ± 12 |
| IL-1β* | 29 ± 3 | 416 ± 5 | 752 ± 78 | 230 ± 36 | 634 ± 33 | 175 ± 10 | 258 ± 28 | 523 ± 92 | 188 ± 15 | 75 ± 2 | 207 ± 24 |
| IL-4@ | 33 ± 9 | 725 ± 21 | 1145 ± 184 | 389 ± 39 | 1204 ± 32 | 402 ± 23 | 599 ± 10 | 976 ± 206 | 454 ± 15 | 169 ± 4 | 440 ± 11 |
| IL-6* | 222 ± 66 | 1982 ± 157 | 3609 ± 440 | 1206 ± 22 | 5851 ± 378 | 1904 ± 122 | 2612 ± 67 | 3951 ± 573 | 1769 ± 35 | 706 ± 78 | 1587 ± 133 |
| IL-9* | 358 ± 59 | 11792 ± 2074 | 24116 ± 947 | 22345 ± 1968 | 3294 ± 112 | 2529 ± 150 | 2008 ± 7 | 3330 ± 20 | 4114 ± 80 | 1934 ± 46 | 1739 ± 85 |
| TNFα% | 107 ± 11 | 1631 ± 0 | 4475 ± 106 | 886 ± 17 | 4085 ± 483 | 984 ± 26 | 2050 ± 108 | 4635 ± 1704 | 696 ± 59 | 283 ± 2 | 926 ± 2 |
Analytes shared by all three kits are denoted by @, those shared between Bioplex and LINCOplex are noted by *, # denotes analytes common between BioPlex and Beadlyte and % denotes those common between Beadlyte and LINCOplex. Variation in the concentrations were obtained from the replicate measurements using the linear modeling (Materials & Methods).
Cluster analysis was based on Euclidean distance without standardization to identify the natural grouping of analyte expression profiles (19). All the data processing were performed using R statistical environment (20,21). Plots were generated with a combination of Matlab (22) and SigmaPlot (23).
Results
Levels of cytokines and chemokines in patient samples
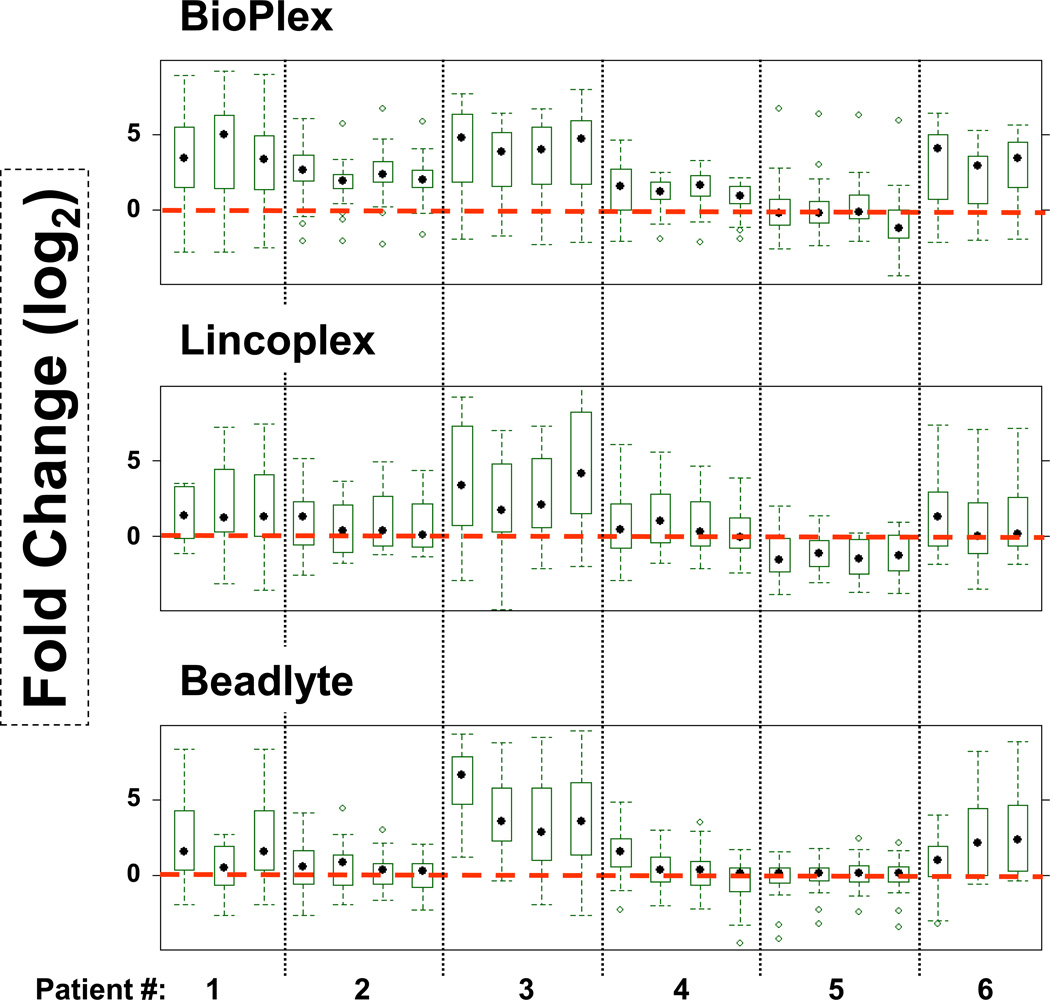
Kits were obtained from four different vendors as listed in Table 1. Eleven analytes overlap among the four kits. Values for analytes measured by the Fluorokine Kit were low in all samples, therefore, the results for this kit were not considered for further analysis. Removal of the Fluorokine kit from analysis also resulted in an increase in the total number of overlapping analytes between the other three kits (Bio-Plex, LINCOplex, and Beadlyte) to eighteen. Amounts of cytokines and chemokines in patients were compared to those measured in healthy individuals. Mean levels of fold changes (in log2 scale) of cytokines and chemokines as measured by a given kit are plotted in Fig. 1. The Bio-Plex kit displayed the clearest difference between healthy individuals (red dotted line) and patients (patients 1, 2, 3, 4 and 6). Assay kits from Beadlyte and LINCOplex highlighted patients 1, 3 and 6. Patient 5 was not different from the healthy samples by any of the assay kits. In our previous analysis, patients 1, 3 and 6 were three of the patients among seventeen who benefited the most from Rituximab treatment (17).
Figure 1.
Fold changes of analytes measured by multiplex kits: The results represent fold differences (Log2 Scale) of all the cytokines collectively between RA patients and healthy individuals (red dotted line; n=14) as measured using each kit. The first data point for each patient is pretreatment and the rest are post-treatment samples (3, 6 and 9 months). Sample (patient) number 1 is missing pretreatment and sample 6 is missing six month time point, respectively.
Detection of cytokines and chemokines in RA patients by different assay kits
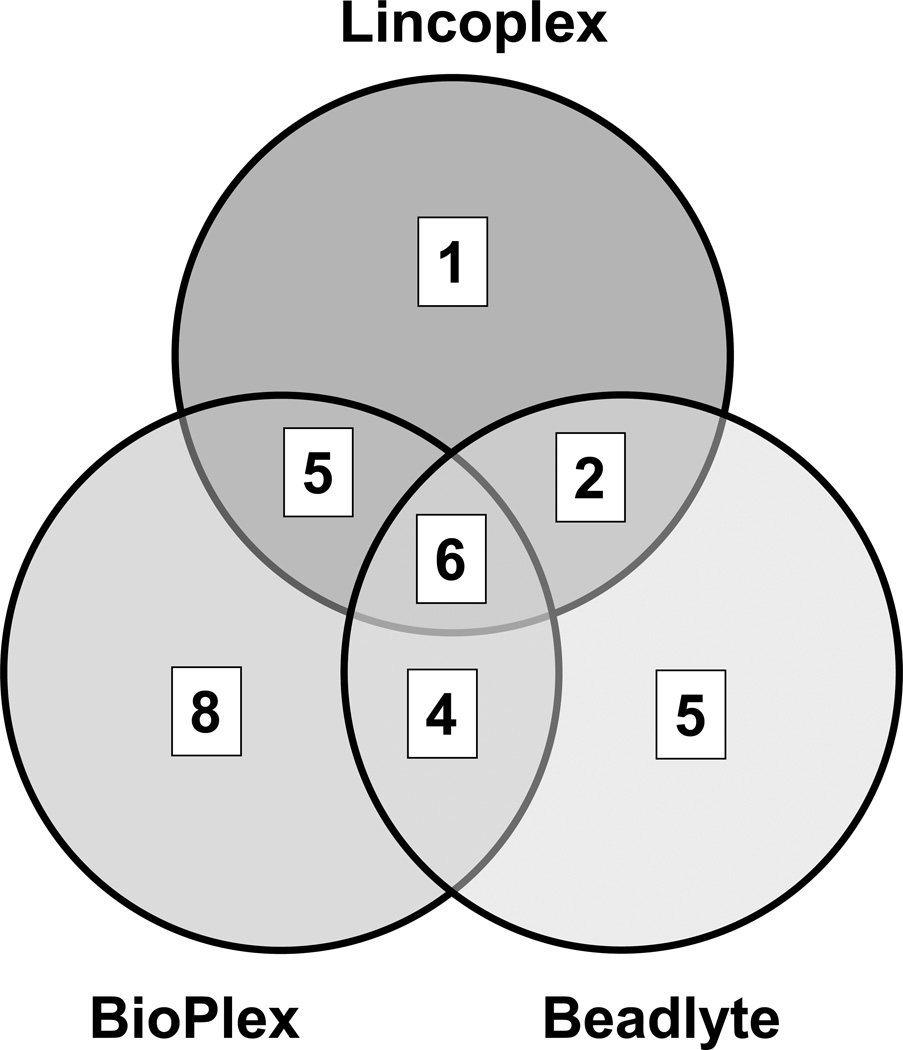
Comparison of cytokine and chemokine detection by individual assay kits is presented in Fig. 2; these data are based on the statistical analysis presented in Table 2. Note that these data do not represent individual patient cytokine/chemokine profiles but rather composite data of analytes in each patient sample (individual data are presented elsewhere in Fig. 3, and Tables 3 and 4). Under the conditions where fold change is greater than 2 (log2 scale) and p-value of less than 0.05 (5% confidence in null hypothesis), the Bio-Plex assay kit displayed significant differences in twenty three cytokines and chemokines in comparison between healthy individuals and RA patients. Beadlyte and LINCOplex kits displayed significant differences in seventeen and fourteen immunomodulators, respectively (Table 2). Decreasing the FDR adjusted p-value to 0.01 (1% confidence in null hypothesis) does not change the number of analytes measured by the Bio-Plex and LINCOplex kits, but the number of analytes in the Beadlyte kit was reduced from seventeen to fifteen. A Venn diagram (Fig. 2) shows the number of analytes shared between the various kits at the statistical selection criteria of log2 (FC) > 2 and p-value < 0.05. Six analytes (IL-12p70, IL-5, MCP-1, Eotaxin, IL-8 and IP-10) are shared between all the three kits, 5 analytes are shared between Bio-Plex and Beadlyte (IL-7, IL-4, IL-13, IL-10 and RANTES), and 2 between Beadlyte and LINCOplex (GM-CSF and TNFα)
Figure 2.
Analysis of analytes measured by different multiplex kits. Venn diagram of elevated analytes selected with fold change (log2 scale) > 2 and using FDR adjusted p-value < 0.05, as shown in Table 2.
Table 2.
Summary of the statistical analysis for the various panels
| Panels | Number of analytes with FDR adjusted p.value for multiple comparison for Fold Change > 2 (log2 scale) |
Total Cytokines |
|||
|---|---|---|---|---|---|
|
p.val < 0.0001 |
p.val < 0.001 |
p.val < 0.01 |
p.val < 0.05 |
||
| BioPlex | 20 | 23 | 23 | 23 | 27 |
| LINCOplex | 11 | 11 | 14 | 14 | 29 |
| Beadlyte | 14 | 15 | 15 | 17 | 22 |
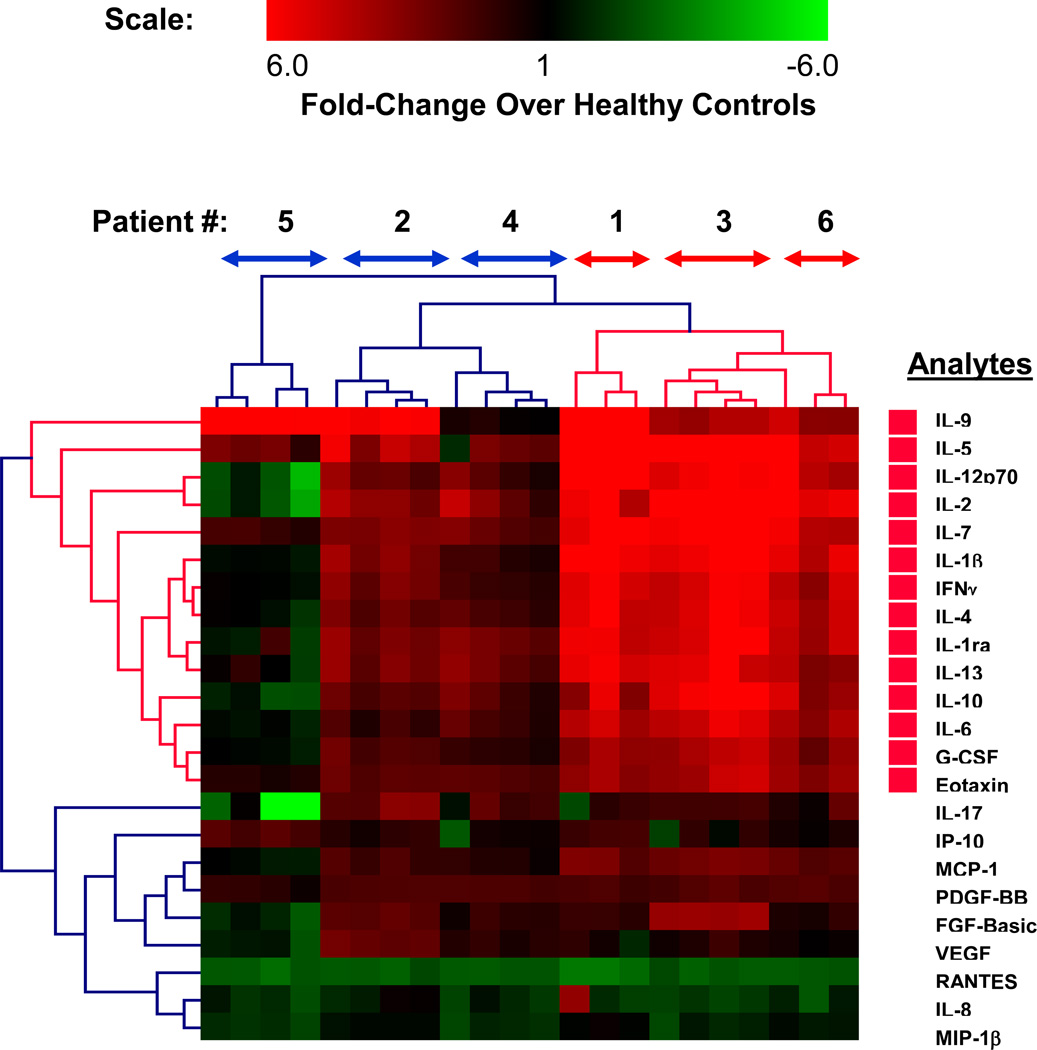
Figure 3.
Cluster analysis of analytes measured by Bioplex. Fold changes of the analytes measured using Bioplex kit (log2(FC) > 2 and p-value < 0.05) are classified by hierarchical cluster analysis (Eucledian distance metric). The clustering approach groups the patients into two major groups denoted by bidirectional arrows (red (elevated analytes) and blue (healthy control levels) along the top horizontal direction) and the respective analytes (red bars along the right vertical axis denote significantly elevated analytes). The heat-map scale at the top indicates analyte levels in patient plasma as compared to healthy controls (n=14).
Cluster analysis to identify individual cytokines and chemokines significantly elevated in each RA patient sample
To identify the patients as well as the immunomodulators that contributed maximally to the observed elevation in the levels of different analytes in plasma samples described above (Fig. 1 and 2; Table 2), a hierarchical cluster analysis was performed. All the analytes selected in Table 2 were used for the analysis. As an example, cluster analysis of RA patient data obtained by the Bio-Plex assay kit is shown in Fig.3. Out of twenty three analytes, fourteen were significantly elevated (p-value <0.05) in patients 1, 3 and 6 while elevation of analytes in patients 2 and 4 was not significant (Fig. 3). Out of the fourteen analytes that were significantly elevated, thirteen are cytokines (IL-9, IL-5, IL-12p70, IL-2, IL-7, IL-1b, IFNg, IL-4, IL-1ra, IL-13, IL-10, IL-6, GCSF) and one is a chemokine (Eotaxin). Similar cluster analysis was also performed on data obtained by the Beadlyte (14 analytes) and LINCOplex (17 analytes) assay kits. Results for patients 1, 3 and 6 by all three kits in terms of fold-changes (log2) are summarized in Table 3 and the actual values of their respective concentrations (pg/ml) are listed in Table 4. Elevation of analytes in patients 2 and 4 was not significant by any of the three multiplex kits and were therefore not included in Tables 3 ad 4. Seven of fourteen selected Beadlyte analytes and ten of the seventeen selected LINCOplex analytes were significantly elevated (p-value <0.05) in the same set of patients. Data based on the cluster analysis of all three assay kits: Bio-Plex, Beadlyte and LINCOplex are summarized in Table 3. The cluster analysis revealed three analytes common between BioPlex, Beadlyte, and Lincoplex kits that were significantly elevated in comparison to healthy samples: IL-12p70, Eotaxin and IL-4. In addition, five analytes were common between BioPlex and LINCOplex assay kits (Il-9, IL-1β, IFNγ, IL-10 and IL-6), and one analyte was common between BioPlex and Beadlyte (IL-13). There was also one analyte common between Beadlyte and LINCOplex kits (TNFα). Results from all three kits, taken together, identified analytes that were elevated by at least two different multiplex kits and include IL-12p70, Eotaxin, IL-4, Il-9, IL-1β, IFNγ, IL-10, IL-6, IL-13 and TNFα.
Discussion
In this study, large-panel multiplex microbead immunoassay kits from four manufacturers were compared by measuring cytokines and chemokines in serial plasma samples from RA patients on rituximab therapy. Such a comparative analysis is likely to more accurately identify cytokine/chemokine profiles in RA patients. This is because there are differences in the kits (see below) and therefore, analytes that are found to be elevated in common between different kits are more likely to reflect the actual increases. Comparison of different Luminex based multiplex kits for a small set of analytes (5 cytokines) has been previously reported (24).
Cluster analysis (computer modeling) of the immunomodulator concentration data from patients was performed to identify significantly elevated (p-value <0.05) immunomodulators and the patients in which these analytes were elevated (e.g., by BioPlex kit as shown in Fig. 3). Fourteen analytes were found to be elevated at different time points in three of six patients (patients 1, 3 and 6). Although patients 2 and 4 displayed elevation in composite levels of analytes as analyzed by the BioPlex kit (Fig. 1), cluster analysis did not identify individual analytes to be significantly elevated (p-value <0.05) (Fig. 3). Therefore, only the analytes classified by cluster analysis to be significantly elevated by all three multiplex kits in patients 1, 3 and 6 are summarized in Table 3. These differences in the detection of elevated cytokines highlight the differences in multiplex kits from different manufacturers. There may be a variety of reasons for variation in the performance of multiplex kits used in this study with a large number of cytokines and chemokines. In a previous study, for a limited set of five analytes, multiplex kits from different manufacturers (LINCOplex, Bio-Plex, Flourokine and BioSource multiplex kits) provided similar patterns of analytes. It is likely that the chances of differences between kits increase with the number of analytes in the multiplex assays. All three kits (Bio-Plex, LINCOplex and Beadlyte) were user friendly. We speculate that differences in antibody pairs used by different manufacturers for capture and detection of individual analytes are likely to be a key factor. In addition, differences in purified antigens (analytes) used for generating standard curves and compositions of sample diluents and assay buffers supplied by the manufacturers may also contribute to variation in results. In part, the differences may be due to interference by RF in the RA patient plasma samples. In this regard, the multiplex kit from LINCO contains a sample diluent that minimizes the effects of RF. Therefore, the data from LINCOplex kit may represent more accurate profiling of different analytes. Despite the differences in the kit compositions, it is reassuring to see that common analytes elevated between two of the kits, Bio-Plex and LINCOPlex (Eotaxin, IFN-γ, IL-10, IL12p70, IL-1β, IL-4, IL-6, and IL-9), were measured in similar concentrations in all samples (Table 4). We propose that for screening patient samples for biomarker profiling, selection of analytes by consensus between at least two kits would be more informative. Accordingly, analytes found to be elevated in patients, in comparison to healthy individuals, by at least two kits are as follows: IL-12p70, Eotaxin, IL-4, TNFα, Il-9, IL-1β, IFNγ, IL-10, IL-6, and IL-13 (Table 3).
Two key observations were made in this study: 1) Three of six patients (#1, 3 and 6), who benefited from rituximab therapy, displayed elevated plasma cytokines (IL-12p70, Eotaxin, IL-4, Il-9, IL-1β, IFNγ, IL-10, IL-6, IL-13 and TNFα), and 2) Rituximab therapy did not lead to reduction in the amounts of elevated cytokines within the nine month period of observation. The simplest explanation for these results is that because rituximab targets B-cells and because most of these elevated cytokines are macrophage and T-cell products, the levels of these cytokines were not affected by B-cell reductive therapy (25). Furthermore, because rituximab treatment was effective in patients (17) that were found to contain elevated immunomodulators in this study, the results may suggest that the observed elevation in cytokine levels mediated or supported B-cell activation and survival, which in turn could lead to a disease state that is more responsive to B-cell reductive therapy. However, it is possible that if the study was continued over a longer period of time and if B-cells were contributing to production of these cytokines (indirectly through activation by macrophages and T-cells), a decline in concentrations of these immunomodulators may have been observed. In RA patients, macrophages and fibroblasts in the synovium are thought to be responsible for elevation of some of the key cytokines, such as IL-12p70, TNFα, IL-1, GMCSF, IL-6, IL-15, IL-18 etc. (3,26). TNFα can lead to activation and enhanced survival of B-cells and fibroblasts exposed to TNFα and IFNγ (3).
The findings reported here are for a small number of patients. However, taken together with the results of previous studies that demonstrated efficacy of rituximab therapy in patients with RA (17,27), our findings may suggest that it is not the elevation of cytokines per se that drives the disease but rather B-cells that are activated, better survived and which produce auto-antibodies in this specific cytokine milieu presumably contribute to the disease pathogenesis. Because rituximab targets B-cells, an overall reduction in the number of B-cells (17), including those that are presumably activated due to elevation in certain cytokines as discussed above, would lead to a reduction in the severity of disease. If true, this implies that measurements of a subset (or all) of the above cytokines in patient plasma may identify patients who are likely to benefit from B-cell reductive therapy. These results indicate that further studies are needed to clarify the relationship of elevation in cytokines and chemokines and efficacy of B-cell reductive therapy.
Acknowledgements
We thank Dr. Resmi Ravindran for her expert help in multiplex analysis. VVK is supported by the NIH Research Infrastructure in Minority Institution grant (NIH, P20-MD002732). KJ is supported by a K30 Award from the NCRR and the UC Davis Clinical and Translational Sciences Center. This work was also in part supported by Genentech and the Schwedler foundation. This publication was also made possible by Grant Number UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Footnotes
Disclaimer: The companies who provided multiplex kits for this study (see Materials and Methods) had no influence on the study design and gathering of results. The authors do not own stock or have any other financial interests in these companies.
References
- 1.Colobran R, Pujol-Borrell R, Armengol MP, Juan M. The chemokine network. II. On how polymorphisms and alternative splicing increase the number of molecular species and configure intricate patterns of disease susceptibility. Clin Exp Immunol. 2007;150(1):1–12. doi: 10.1111/j.1365-2249.2007.03489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colobran R, Pujol-Borrell R, Armengol MP, Juan M. The chemokine network. I. How the genomic organization of chemokines contains clues for deciphering their functional complexity. Clin Exp Immunol. 2007;148(2):208–217. doi: 10.1111/j.1365-2249.2007.03344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7(6):429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 4.Sachdeva N, Asthana D. Cytokine quantitation: technologies and applications. Front Biosci. 2007;12:4682–4695. doi: 10.2741/2418. [DOI] [PubMed] [Google Scholar]
- 5.Kettman JR, Davies T, Chandler D, Oliver KG, Fulton RJ. Classification and properties of 64 multiplexed microsphere sets. Cytometry. 1998;33(2):234–243. [PubMed] [Google Scholar]
- 6.Prabhakar U, Eirikis E, Davis HM. Simultaneous quantification of proinflammatory cytokines in human plasma using the LabMAP assay. J Immunol Methods. 2002;260(1–2):207–218. doi: 10.1016/s0022-1759(01)00543-9. [DOI] [PubMed] [Google Scholar]
- 7.Bobrowski WF, McDuffie JE, Sobocinski G, Chupka J, Olle E, Bowman A, Albassam M. Comparative methods for multiplex analysis of cytokine protein expression in plasma of lipopolysaccharide-treated mice. Cytokine. 2005;32(5):194–198. doi: 10.1016/j.cyto.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Lash GE, Scaife PJ, Innes BA, Otun HA, Robson SC, Searle RF, Bulmer JN. Comparison of three multiplex cytokine analysis systems: Luminex, SearchLight and FAST Quant. J Immunol Methods. 2006;309(1–2):205–208. doi: 10.1016/j.jim.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72(11):1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 10.O'Hayre M, Salanga CL, Handel TM, Allen SJ. Chemokines and cancer: migration, intracellular signalling and intercellular communication in the microenvironment. Biochem J. 2008;409(3):635–649. doi: 10.1042/BJ20071493. [DOI] [PubMed] [Google Scholar]
- 11.Aukrust P, Yndestad A, Smith C, Ueland T, Gullestad L, Damas JK. Chemokines in cardiovascular risk prediction. Thromb Haemost. 2007;97(5):748–754. [PubMed] [Google Scholar]
- 12.Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 13.Jankovic D, Liu Z, Gause WC. Th1- and Th2-cell commitment during infectious disease: asymmetry in divergent pathways. Trends Immunol. 2001;22(8):450–457. doi: 10.1016/s1471-4906(01)01975-5. [DOI] [PubMed] [Google Scholar]
- 14.Jankovic D, Sher A, Yap G. Th1/Th2 effector choice in parasitic infection: decision making by committee. Curr Opin Immunol. 2001;13(4):403–409. doi: 10.1016/s0952-7915(00)00234-x. [DOI] [PubMed] [Google Scholar]
- 15.Szekanecz Z, Koch AE. Mechanisms of Disease: angiogenesis in inflammatory diseases. Nat Clin Pract Rheumatol. 2007;3(11):635–643. doi: 10.1038/ncprheum0647. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Zepeda EA, Rojas-Lopez A, Esquivel-Velazquez M, Ostoa-Saloma P. Regulation of the inflammatory immune response by the cytokine/chemokine network in amoebiasis. Parasite Immunol. 2007;29(12):679–684. doi: 10.1111/j.1365-3024.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- 17.Higashida J, Wun T, Schmidt S, Naguwa SM, Tuscano JM. Safety and efficacy of rituximab in patients with rheumatoid arthritis refractory to disease modifying antirheumatic drugs and anti-tumor necrosis factor-alpha treatment. J Rheumatol. 2005;32(11):2109–2115. [PubMed] [Google Scholar]
- 18.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19(2):185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 19.Dudoit S, Popper-Shaffer J, Boldrick JC. Multiple hypothesis testing in microarray experiments. U.C. Berkeley Division of Biostatistics Working Paper Series; 2002. [Google Scholar]
- 20.Gentleman RC. A language for data analysis and graphics. Journal of Computational Graphics. 1996;5:299–314. [Google Scholar]
- 21.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5(10):R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathworks Inc. Matlab. 12. Natick, MA 01760, USA: Mathworks Inc.; 2005. [Google Scholar]
- 23.Systat Software I. SigmaPlot. 9. San Jose, CA, USA: Systat Software, Inc.; 2005. [Google Scholar]
- 24.Khan SS, Smith MS, Reda D, Suffredini AF, McCoy JP., Jr Multiplex bead array assays for detection of soluble cytokines: comparisons of sensitivity and quantitative values among kits from multiple manufacturers. Cytometry B Clin Cytom. 2004;61(1):35–39. doi: 10.1002/cyto.b.20021. [DOI] [PubMed] [Google Scholar]
- 25.Raza K, Falciani F, Curnow SJ, Ross EJ, Lee CY, Akbar AN, Lord JM, Gordon C, Buckley CD, Salmon M. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther. 2005;7(4):R784–R795. doi: 10.1186/ar1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 27.di Comite G, Marinosci A, Di Matteo P, Manfredi A, Rovere-Querini P, Baldissera E, Aiello P, Corti A, Sabbadini MG. Neuroendocrine modulation induced by selective blockade of TNF-alpha in rheumatoid arthritis. Ann N Y Acad Sci. 2006;1069:428–437. doi: 10.1196/annals.1351.041. [DOI] [PubMed] [Google Scholar]