Abstract
A tumor-derived factor believed to cause hypercalcemia by acting on the parathyroid hormone (PTH) receptor was recently purified, cloned, and found to have NH2-terminal sequence homology with PTH. The 1-34 region of this protein was synthesized, evaluated for its postreceptor effects on the ROS 17/2.8 cell line, and its properties were compared to 1-34 PTH. Both 1-34 human humoral hypercalcemia factor (HCF) and 1-34 PTH stimulated adenylate cyclase with an effective concentration (EC)50 of approximately 1 nM. The extent of stimulation by both peptides was equally enhanced by dexamethasone. They both had a pronounced inhibitory effect on growth in the presence of dexamethasone, with an EC50 of approximately 0.1 nM, reduced alkaline phosphatase (AP) activity by approximately 70% in the absence of dexamethasone and by approximately 80% in the presence of dexamethasone with an EC50 of 0.03 nM, and when present at a concentration of 10 nM, reduced AP mRNA levels (estimated by Northern analysis) by approximately 80% in the presence or absence of dexamethasone. Thus, in addition to similar dose-response curves for adenylate cyclase stimulation, both HCF and PTH produced identical postreceptor effects in ROS 17/2.8 cells. These effects of HCF are probably mediated by the interaction of the tumor-derived factor with the PTH receptor.
Full text
PDF
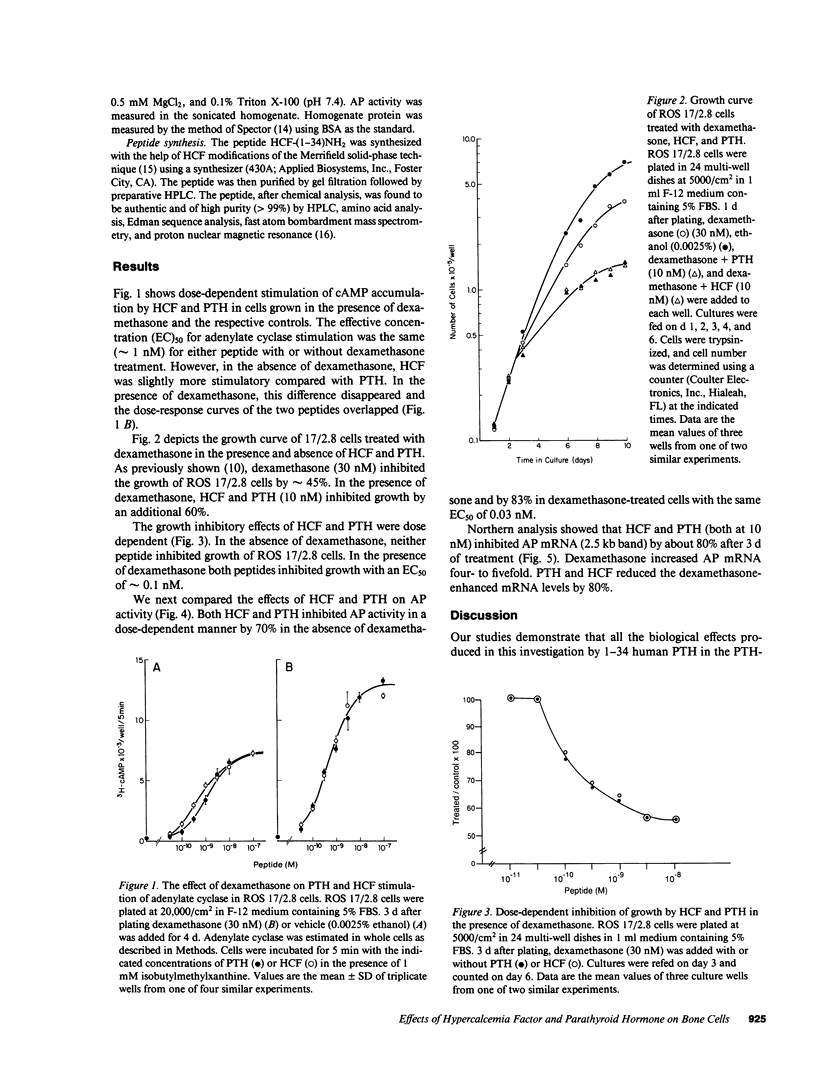
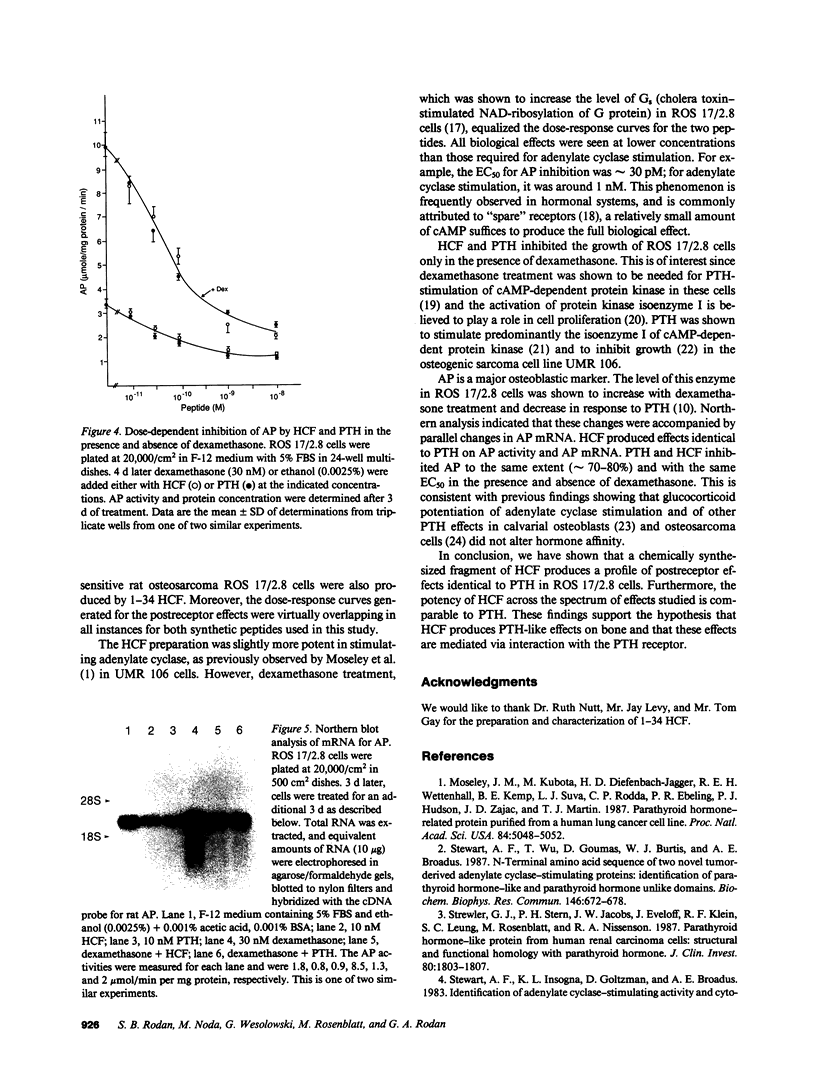

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen T. L., Feldman D. Glucocorticoid receptors and actions in subpopulations of cultured rat bone cells. Mechanism of dexamethasone potentiation of parathyroid hormone-stimulated cyclic AMP production. J Clin Invest. 1979 Apr;63(4):750–758. doi: 10.1172/JCI109359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Horiuchi N., Caulfield M. P., Fisher J. E., Goldman M. E., McKee R. L., Reagan J. E., Levy J. J., Nutt R. F., Rodan S. B., Schofield T. L. Similarity of synthetic peptide from human tumor to parathyroid hormone in vivo and in vitro. Science. 1987 Dec 11;238(4833):1566–1568. doi: 10.1126/science.3685994. [DOI] [PubMed] [Google Scholar]
- Livesey S. A., Kemp B. E., Re C. A., Partridge N. C., Martin T. J. Selective hormonal activation of cyclic AMP-dependent protein kinase isoenzymes in normal and malignant osteoblasts. J Biol Chem. 1982 Dec 25;257(24):14983–14987. [PubMed] [Google Scholar]
- Löwik C. W., van Leeuwen J. P., van der Meer J. M., van Zeeland J. K., Scheven B. A., Herrmann-Erlee M. P. A two-receptor model for the action of parathyroid hormone on osteoblasts: a role for intracellular free calcium and cAMP. Cell Calcium. 1985 Aug;6(4):311–326. doi: 10.1016/0143-4160(85)90002-8. [DOI] [PubMed] [Google Scholar]
- Majeska R. J., Nair B. C., Rodan G. A. Glucocorticoid regulation of alkaline phosphatase in the osteoblastic osteosarcoma cell line ROS 17/2.8. Endocrinology. 1985 Jan;116(1):170–179. doi: 10.1210/endo-116-1-170. [DOI] [PubMed] [Google Scholar]
- Merrifield R. B. Solid-phase peptide synthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:221–296. doi: 10.1002/9780470122778.ch6. [DOI] [PubMed] [Google Scholar]
- Moseley J. M., Kubota M., Diefenbach-Jagger H., Wettenhall R. E., Kemp B. E., Suva L. J., Rodda C. P., Ebeling P. R., Hudson P. J., Zajac J. D. Parathyroid hormone-related protein purified from a human lung cancer cell line. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5048–5052. doi: 10.1073/pnas.84.14.5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K. W., Livesey S. A., Larkins R. G., Martin T. J. Calcitonin effects on growth and on selective activation of type II isoenzyme of cyclic adenosine 3':5'-monophosphate-dependent protein kinase in T 47D human breast cancer cells. Cancer Res. 1983 Feb;43(2):794–800. [PubMed] [Google Scholar]
- Noda M., Yoon K., Thiede M., Buenaga R., Weiss M., Henthorn P., Harris H., Rodan G. A. cDNA cloning of alkaline phosphatase from rat osteosarcoma (ROS 17/2.8) cells. J Bone Miner Res. 1987 Apr;2(2):161–164. doi: 10.1002/jbmr.5650020212. [DOI] [PubMed] [Google Scholar]
- Partridge N. C., Opie A. L., Opie R. T., Martin T. J. Inhibitory effects of parathyroid hormone on growth of osteogenic sarcoma cells. Calcif Tissue Int. 1985 Sep;37(5):519–525. doi: 10.1007/BF02557835. [DOI] [PubMed] [Google Scholar]
- Rodan G. A., Martin T. J. Role of osteoblasts in hormonal control of bone resorption--a hypothesis. Calcif Tissue Int. 1981;33(4):349–351. doi: 10.1007/BF02409454. [DOI] [PubMed] [Google Scholar]
- Rodan S. B., Fischer M. K., Egan J. J., Epstein P. M., Rodan G. A. The effect of dexamethasone on parathyroid hormone stimulation of adenylate cyclase in ROS 17/2.8 cells. Endocrinology. 1984 Sep;115(3):951–958. doi: 10.1210/endo-115-3-951. [DOI] [PubMed] [Google Scholar]
- Rodan S. B., Insogna K. L., Vignery A. M., Stewart A. F., Broadus A. E., D'Souza S. M., Bertolini D. R., Mundy G. R., Rodan G. A. Factors associated with humoral hypercalcemia of malignancy stimulate adenylate cyclase in osteoblastic cells. J Clin Invest. 1983 Oct;72(4):1511–1515. doi: 10.1172/JCI111108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan S. B., Rodan G. A. Dexamethasone effects on beta-adrenergic receptors and adenylate cyclase regulatory proteins Gs and Gi in ROS 17/2.8 cells. Endocrinology. 1986 Jun;118(6):2510–2518. doi: 10.1210/endo-118-6-2510. [DOI] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Stewart A. F., Insogna K. L., Goltzman D., Broadus A. E. Identification of adenylate cyclase-stimulating activity and cytochemical glucose-6-phosphate dehydrogenase-stimulating activity in extracts of tumors from patients with humoral hypercalcemia of malignancy. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1454–1458. doi: 10.1073/pnas.80.5.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A. F., Wu T., Goumas D., Burtis W. J., Broadus A. E. N-terminal amino acid sequence of two novel tumor-derived adenylate cyclase-stimulating proteins: identification of parathyroid hormone-like and parathyroid hormone-unlike domains. Biochem Biophys Res Commun. 1987 Jul 31;146(2):672–678. doi: 10.1016/0006-291x(87)90581-x. [DOI] [PubMed] [Google Scholar]
- Strewler G. J., Stern P. H., Jacobs J. W., Eveloff J., Klein R. F., Leung S. C., Rosenblatt M., Nissenson R. A. Parathyroid hormonelike protein from human renal carcinoma cells. Structural and functional homology with parathyroid hormone. J Clin Invest. 1987 Dec;80(6):1803–1807. doi: 10.1172/JCI113275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suva L. J., Winslow G. A., Wettenhall R. E., Hammonds R. G., Moseley J. M., Diefenbach-Jagger H., Rodda C. P., Kemp B. E., Rodriguez H., Chen E. Y. A parathyroid hormone-related protein implicated in malignant hypercalcemia: cloning and expression. Science. 1987 Aug 21;237(4817):893–896. doi: 10.1126/science.3616618. [DOI] [PubMed] [Google Scholar]
- Terasaki W. L., Linden J., Brooker G. Quantitative relationship between beta-adrenergic receptor number and physiologic responses as studied with a long-lasting beta-adrenergic antagonist. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6401–6405. doi: 10.1073/pnas.76.12.6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi D. T., Hahn T. J., Iida-Klein A., Kleeman C. R., Muallem S. Parathyroid hormone-activated calcium channels in an osteoblast-like clonal osteosarcoma cell line. cAMP-dependent and cAMP-independent calcium channels. J Biol Chem. 1987 Jun 5;262(16):7711–7718. [PubMed] [Google Scholar]
- Zajac J. D., Livesey S. A., Michelangeli V. P., Rodan S. B., Rodan G. A., Martin T. J. Glucocorticoid treatment facilitates cyclic adenosine 3',5'-monophosphate-dependent protein kinase response in parathyroid hormone-responsive osteogenic sarcoma cells. Endocrinology. 1986 May;118(5):2059–2064. doi: 10.1210/endo-118-5-2059. [DOI] [PubMed] [Google Scholar]