Summary
Nuclear pore complexes (NPCs) are selective transport channels embedded in the nuclear envelope. The cylindrical NPC core forms a protein coat lining a highly curved membrane opening and has a basket-like structure appended to the nucleoplasmic side. How NPCs interact with lipids, promoting membrane bending and NPC integrity, is poorly understood. Here we show that the NPC basket proteins Nup1 and Nup60 directly induce membrane curvature by amphipathic helix insertion into the lipid bilayer. In a cell-free system, both Nup1 and Nup60 transform spherical liposomes into highly curved membrane structures. In vivo, high levels of the Nup1/Nup60 amphipathic helices cause deformation of the yeast nuclear membrane, whereas adjacent helical regions contribute to anchoring the basket to the NPC core. Basket amphipathic helices are functionally linked to distinct transmembrane nucleoporins of the NPC core, suggesting a key contribution to the membrane remodeling events that underlie NPC assembly.
Graphical Abstract
Highlights
-
•
The nuclear pore basket is tethered to the nuclear envelope
-
•
Amphipathic helices within Nup1 and Nup60 bind and bend membranes
-
•
Basket-lipid interactions contribute to NPC integrity
How nuclear pore complexes (NPCs) assemble and insert into the nuclear envelope is still unclear. Mészáros and Cibulka et al. find that nuclear pore basket proteins Nup1 and Nup60 can directly bind and remodel membranes via amphipathic helices, thereby promoting NPC and nuclear envelope integrity.
Introduction
Nuclear pore complexes (NPCs) are large protein channels that span the nuclear envelope (NE) through a pore formed by the fusion of the inner (INM) and outer nuclear membranes (ONM) (D’Angelo and Hetzer, 2008; Hoelz et al., 2011). The NPC scaffold resembles eight spokes with a radially symmetrical arrangement along the channel axis. These spokes are subdivided into three main rings surrounding a central selectivity filter through which nucleocytoplasmic transport occurs. The inner ring (or spoke ring) has the smallest diameter and is sandwiched between two outer rings with each ring closely following the curved surface of the pore membrane. Each NPC is composed of ∼30 different proteins (nucleoporins or Nups) present in multiple copies, adding up to ∼500 proteins (Alber et al., 2007). Nucleoporins can be categorized into four classes: transmembrane, core scaffold, linker, and Phe-Gly (FG) nucleoporins, with the latter constituting the NPC permeability barrier. Transport across the NPC involves transport factors such as karyopherins, which bind specific import (NLS) or export (NES) signals in their cargo.
NPC assembly can follow two different pathways (Rothballer and Kutay, 2013). During open mitosis in metazoa, the NE breaks down and is reassembled following the recruitment of nucleoporins and membrane vesicles to chromatin. During interphase, in contrast, NPCs are inserted de novo into the intact NE. This pathway is essential for organisms undergoing a closed mitosis like Saccharomyces cerevisiae. Interphase NPC assembly appears to proceed from both sides of the nuclear envelope (D’Angelo et al., 2006) and involves bending and apposition of INM and ONM, which ultimately leads to a membrane fusion event. The resulting pore membrane has a high positive (convex) curvature at the edge. Given that lipid bilayers tend to resist both bending and remodeling, forces have to be applied to curve, fuse, and subsequently stabilize the pore membrane. The cellular mechanisms of curvature generation, studied extensively in endomembrane traffic systems, fall into two groups: (1) hydrophobic insertion mechanisms, based on the wedge-like penetration of hydrophobic or amphipathic proteins into the lipid bilayer; and (2) scaffolding mechanisms, where intrinsically curved proteins adhere to the lipid bilayer and impress their shape (McMahon and Gallop, 2005). Although a similar architecture and common ancestry between NPCs and vesicle coating machineries was proposed (Brohawn et al., 2008; Debler et al., 2008; Devos et al., 2004), a unifying mechanism for membrane curvature generation by NPCs has not yet emerged. Four transmembrane Nups (Pom152, Pom34, Pom33, and Ndc1) anchor the NPC scaffold in the equatorial plane of the membrane (Chadrin et al., 2010; Miao et al., 2006; Onischenko et al., 2009; Wozniak et al., 1994). Ndc1 binds to Nup53, which serves as a linker toward the inner ring nucleoporin Nup170 (Onischenko et al., 2009). Nup53 contains an amphipathic helix that deforms membranes (Marelli et al., 2001; Vollmer et al., 2012). Another type of amphipathic helix, the ALPS motif (ArfGAP1 lipid packing sensor), was identified in different yeast nucleoporins such as Nup170, Nup188, and members of the Y-shaped Nup84 subcomplex (Doucet et al., 2010; Drin et al., 2007). ALPS-containing nucleoporins are thought to specifically sense membrane curvature by hydrophobic insertion. In addition, non-nucleoporin factors such as the Ran GTPase and ER-resident members of the reticulon and Yop1/DP1 family are implicated in NPC assembly and membrane remodeling (Dawson et al., 2009; Ryan et al., 2003). Despite these insights, key questions about how NPCs promote membrane curvature remain. It is unclear whether the known inventory of lipid-interacting nucleoporins is complete and where exactly the membrane contact points of the NPC scaffold are located. How lipid-interacting Nups cooperate mechanistically during NPC assembly is poorly understood. At present, all putative membrane-deforming activities of the NPC have been mapped to symmetric components of the NPC core scaffold. By contrast, the asymmetric appendages of the NPC, the cytoplasmic filaments and the nuclear basket, show no obvious membrane contacts in cryo-EM or computational models of the NPC (Alber et al., 2007; Beck et al., 2007).
The NPC basket is an assembly of eight flexible protein filaments that emanate from the nuclear face of the NPC core and converge in a distal ring-like structure. The basket has numerous functions including transport and chromatin regulation (Strambio-De-Castillia et al., 2010). It is composed of Nup1, Nup2, Nup60, Mlp1, and Mlp2 in yeast, whereas the metazoan basket harbors only three nucleoporins, NUP153 (an ortholog of yNup1/yNup60), NUP50 (yNup2), and TPR (yMlp1/2). The structural backbone of each basket filament is likely formed by the large coiled-coil proteins Mlp1/Mlp2 (metazoan TPR) (Niepel et al., 2013), whereas the FG portion of the other basket nucleoporins is thought to be unstructured. Compared to the NPC core scaffold, the functional architecture of the nuclear basket is perhaps the least understood part of the NPC. Approximately two decades ago, overexpression of the human basket protein NUP153 was found to cause membrane proliferation (Bastos et al., 1996). Moreover, an earlier screen suggested some affinity between Nup60 and lipids (Patel and Rexach, 2008); however, no direct link to membrane curvature induction was demonstrated. Our study now provides evidence that NPC basket proteins directly bend membranes and suggests that this function may be important for shaping the pore membrane curvature in cells.
Results
Nup1 and Nup60 Are Tethered to the NPC via Their N Termini
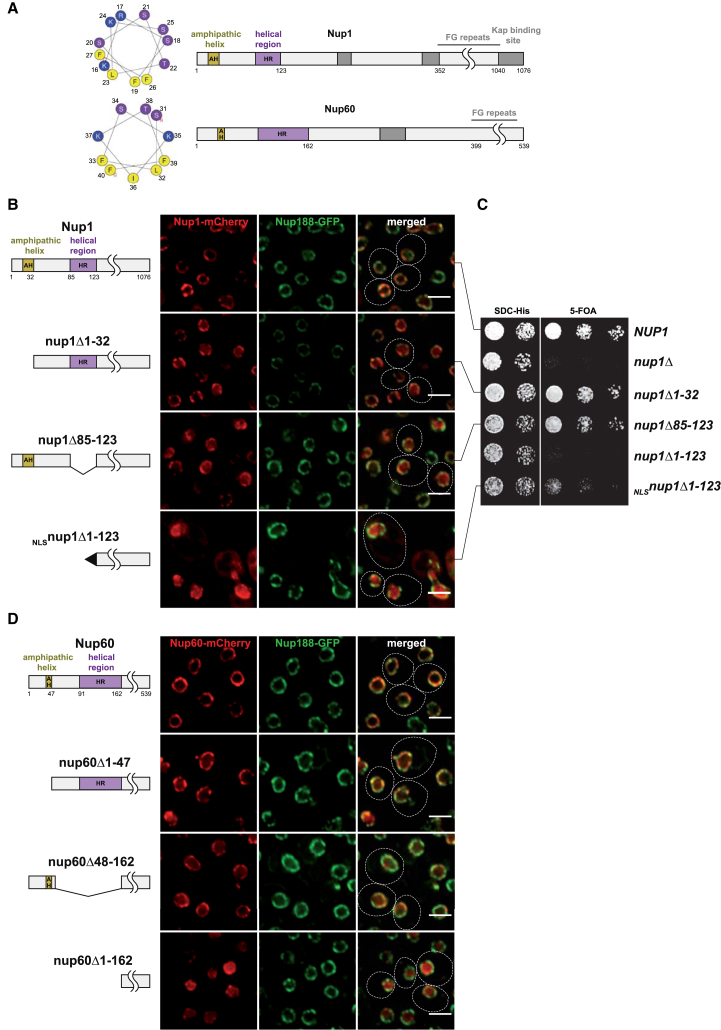
To explore how the basket interacts with the NPC core, we examined the FG nucleoporins Nup1 and Nup60 for cryptic secondary structure elements. We noticed a conserved bipartite motif comprising a predicted amphipathic helix (AH) and adjacent α-helical region (HR) at the very N terminus of both Nup1 and Nup60 (Figures 1A and S1A). To assess its function, we deleted these regions from Nup1 and Nup60 either alone or in combination. Both Nups were C-terminally tagged with the monomeric fluorophore mCherry and expressed from their endogenous promoters in the respective null mutant background. Cells containing plasmid-borne wild-type NUP1 exhibited a punctate fluorescent staining at the NE that is characteristic for NPCs (Figure 1B). Deletion of the Nup1 AH (nup1Δ1-32) led to a slight mislocalization into the nucleoplasm. This effect was more pronounced when removing the HR (nup1Δ85-123). Thus, both the AH and HR contribute to proper Nup1 localization at the NPC. Whereas the mutant Nup1 proteins were expressed at similar levels as wild-type, the abundance of the full N-terminal deletion (nup1Δ1-123) was reduced. However, appending an artificial NLS to this mutant (NLS-SV40nup1Δ1-123) restored its level (Figure S1D). Yet, localization of this truncation mutant to the NE was strongly perturbed (Figure 1B). To correlate Nup1 mislocalization with NPC function, we examined the different mutant phenotypes. The essential function of Nup1 maps to a high-affinity karyopherin-binding site at the very C terminus, which promotes disassembly of karyopherin-cargo complexes after nuclear import (Bogerd et al., 1994; Pyhtila and Rexach, 2003). When analyzed in the BY strain background, nup1Δ was lethal (Figure 1C). Deleting the AH or HR regions of Nup1 had mild growth defects, whereas deletion of the entire N terminus (nup1Δ1-123) caused a severe reduction of cell fitness, even when intranuclear localization and normal nup1Δ1-123 protein levels were maintained by appending an artificial NLS. Similar to Nup1, removing the predicted Nup60 AH (nup60Δ1-47) or HR (nup60Δ48-162) led to a partial detachment of Nup60 from the basket, whereas their combined deletion (nup60Δ1-162) caused a near complete mislocalization of Nup60 (Figures 1D and S1E for expression levels). As Nup60 forms a complex with Nup2 and plays a role in recruiting Mlp1/Mlp2 (Denning et al., 2001; Feuerbach et al., 2002), we examined the integrity of the Mlp part of the basket. Both GFP-tagged Mlp proteins formed abnormal clusters at the NE and partially mislocalized to the nucleoplasm when the Nup60 N terminus was removed (Figure S2A). Taken together, the N termini of Nup1 and Nup60 are required for a proper interaction with the NPC scaffold.
Figure 1.
Basket Tethering to the NPC via the Nup1 and Nup60 N Termini
(A) Nup1 and Nup60 motif organization based on secondary structure predictions and sequence conservation (https://www.predictprotein.org/). Predicted amphipathic helices (AH; yellow) and α-helical regions (HR; purple) are highlighted. Helical wheel projections (http://heliquest.ipmc.cnrs.fr/) of the Nup1 (16-27) and Nup60 (31-40) AHs. Boundaries are drawn to scale, gray boxes indicate other motifs.
(B) Double-fluorescence microscopy of live nup1Δ cells expressing genomically tagged Nup188-GFP and plasmid-borne NUP1 alleles (endogenous promoter) with a C-terminal mCherry tag. Note that NLSnup1Δ1-123 cells are frequently enlarged. NLS, SV40 large T-antigen NLS. Dashed lines mark the contour of cells. Scale bar represents 3 μm.
(C) Growth analysis of the NUP1 alleles used in (B). A nup1Δ strain harboring wild-type NUP1 (URA plasmid) was transformed with wild-type NUP1, empty vector, or the indicated nup1 alleles (HIS plasmids). Growth was followed on SDC-His (loading control) and on SDC+5-fluoroorotic acid (5-FOA) plates to shuffle out the URA cover plasmid. Cells were spotted onto plates in 10-fold serial dilutions and incubated for 2 (SDC-His) or 3 days (5-FOA) at 30°C.
(D) Double-fluorescence microscopy of live nup60Δ cells expressing genomically tagged Nup188-GFP and plasmid-borne NUP60 alleles with a C-terminal mCherry tag (endogenous promoter). Scale bar represents 3 μm.
The Nup1/Nup60 N Termini Interact with Karyopherins and Are Sufficient to Associate with NPCs
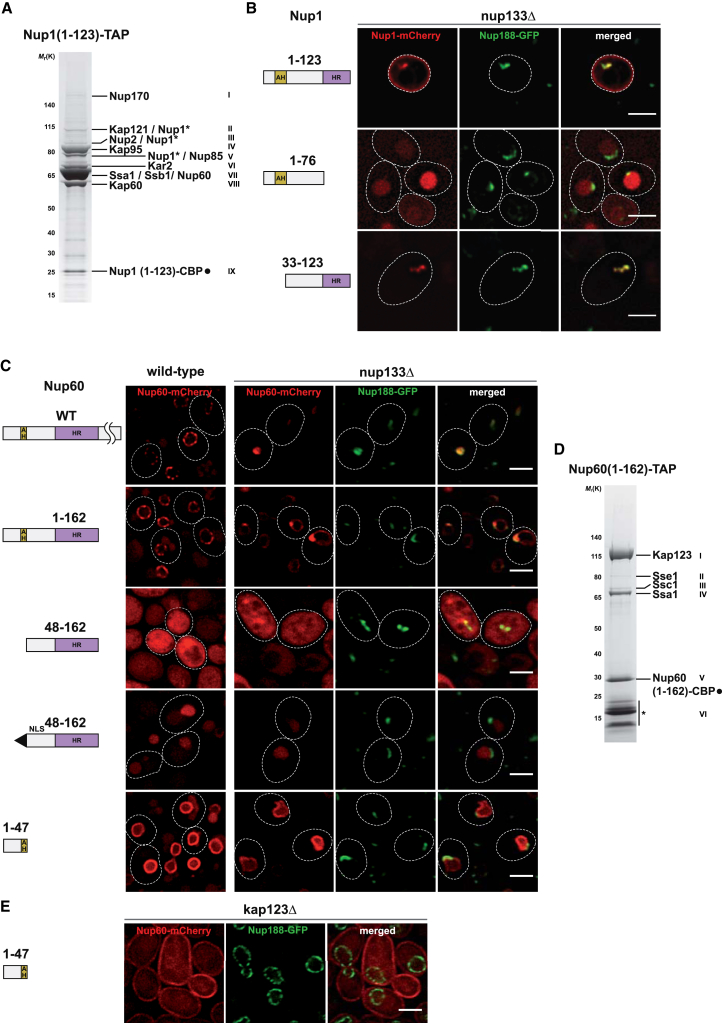
Because the Nup1/Nup60 AH and HR regions are necessary for proper basket recruitment to the NPC, we asked whether these fragments are sufficient to interact with other Nups. As the Nup1 N terminus (Nup1 aa1-123) was poorly expressed from its endogenous promoter, we tandem-affinity purified the fragment after GAL promoter-driven overexpression. Notably, the Nup1 N terminus co-enriched the Kap60/Kap95 complex, a major importer for NLS-containing cargo, in roughly stoichiometric amounts relative to the bait when analyzed by SDS-PAGE and mass spectrometry (Figures 2A and S2B). Additionally, the basket nucleoporins Nup60, Nup2, the endogenous Nup1 protein, and the scaffold nucleoporins Nup170 and Nup85 were detected at substoichiometric levels, suggesting an extended network of protein interactions. The direct NPC binding site of the Nup1 N terminus remains to be established; however, Nup170 was previously proposed to associate with Nup1 (Kenna et al., 1996). The robust co-purification of the Kap60/Kap95 complex raised the possibility of a direct interaction with Kap60 as the Nup1 N terminus contains several predicted NLS sequences (Figure S1C). Thus, we tested for direct Kap60 binding by co-expressing recombinant Kap60 together with various GST-tagged fragments of the Nup1 N terminus in Escherichia coli. Kap60 interacted efficiently with the Nup1 N terminus independently of the HR (Figure S2C), indicating that the Nup1 N terminus harbors a functional NLS to promote Nup1 import. To further dissect the contribution of the Nup1/Nup60 N termini to NPC targeting, we analyzed their subcellular distribution. We took advantage of a low-level expression of the mCherry-tagged fragments from a GAL promoter, when cells were grown in raffinose medium. To facilitate co-localization with NPCs, the Nup1 fragments were expressed in a nup133Δ background where NPCs cluster together. Notably, the Nup1 N terminus (Nup1 aa1-123) was efficiently imported into the nucleus and co-clustered with NPCs (Figure 2B). Moreover, we observed Nup1 staining of the cellular perimeter, an observation that we explored further below. Deleting the HR mislocalized the fragment (Nup1 aa1-76) mostly into the nucleoplasm. In contrast, a fragment comprising just the HR and NLS (Nup1 aa33-123) was sufficient to associate with NPCs. This indicates that the HR aids in targeting the AH to NPCs. Similarly, we found that the Nup60 N terminus (Nup60 aa1-162) associated efficiently with NPCs as evidenced by the characteristic punctate staining of the NE, which was indistinguishable from the localization pattern of full-length Nup60 (Figure 2C). An HR fragment lacking the AH (Nup60 aa48-162) was only imported into the nucleus after adding an artificial NLS, however, without being properly targeted to NPCs. Unexpectedly, the Nup60 AH fragment (Nup60 aa1-47) was imported into the nucleus and smoothly labeled the entire NE rather than individual NPC puncta, which suggested a membrane affinity. Expressing the Nup60 fragments in nup133Δ cells substantiated the above results since the Nup60 N terminus (Nup60 aa1-162) co-clustered with NPCs, whereas the AH and HR fragments alone did not. Thus, proper NPC targeting is a composite function of the Nup60 HR and AH as neither element alone was sufficient. Moreover, import likely depends on an NLS present in the first 47 residues of Nup60 (Figure S1C). To identify potential interactors of the Nup60 N terminus, we purified the overexpressed Nup60 (1-162) fragment. Notably, the karyopherin Kap123 was strongly co-enriched, while other Nups were not efficiently retained (Figures 2D and S2D). To test whether Kap123 plays a role in importing the Nup60 AH (Nup60 aa1-47), we localized the protein in kap123Δ cells. Strikingly, the Nup60 AH no longer labeled the NE but became mistargeted to the plasma membrane (Figure 2E). In contrast, import occurred normally in kap114Δ, kap120Δ, or kap142Δ cells (Figure S2E). This finding underscores a lipid affinity of the Nup60 amphipathic helix in cells and suggests a specific role for Kap123 in directing it to its proper location at the INM.
Figure 2.
The Nup1/Nup60 N Termini Are Sufficient to Associate with NPCs
(A) A plasmid-borne NUP1 (1-123)-TAP construct (GAL promoter) was transformed into wild-type cells and tandem-affinity-purified following galactose-induced overexpression (4 hr). The calmodulin eluate was analyzed by SDS-PAGE and Coomassie staining. Assigned subunits were determined by mass spectrometry (roman numerals refer to cut-out bands, see Figure S2B). Asterisks indicate degradation products of endogenous full-length Nup1. Ssa1/Ssb1 are Hsp70-type chaperones, Kar2 is an ATPase involved in ER protein import.
(B) Live imaging of NUP188-GFP nup133Δ cells expressing the indicated plasmid-borne NUP1 fragments (GAL promoter). Cells were grown in raffinose media to obtain low levels of Nup1 expression sufficient for depiction. Scale bar represents 2 μm.
(C) Live imaging of wild-type and nup133Δ cells expressing the indicated NUP60 constructs (GAL promoter). As in (B), low level protein expression was achieved by growth in raffinose medium. Abnormally shaped nuclei were observed in nup133Δ cells expressing Nup60 (1-47). NLS, SV40 large T-antigen NLS. Scale bar represents 3 μm.
(D) Coomassie-stained gel of a Nup60 (1-162) fragment purified and analyzed as in (A). Assigned subunits were determined by mass spectrometry (roman numerals refer to cut out bands, see Figure S2D). Asterisk indicates degradation products of the bait protein. Sse1/Ssc1/Ssa1 are chaperones.
(E) Analysis of Nup60 (1-47) localization in kap123Δ NUP188-GFP cells. Cells were grown in raffinose as in (B) and (C). Also see Figure S2E. Scale bar represents 3 μm.
Nup1 Can Induce Nuclear Membrane Deformation at High Levels In Vivo
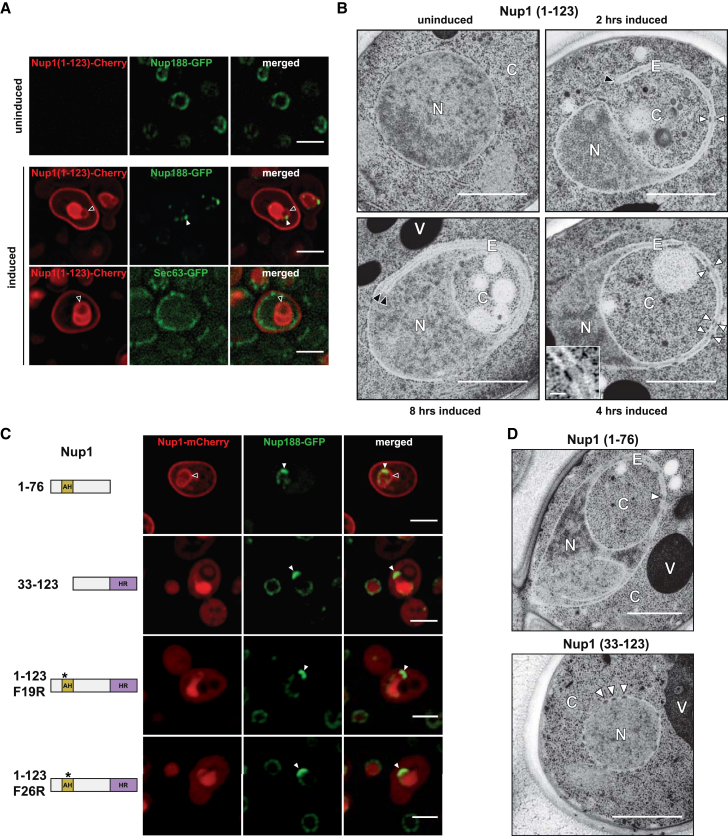
Given the known ability of amphipathic helices to remodel membranes (McMahon and Gallop, 2005), we hypothesized that overexpressing basket AHs might alter the shape of the NE. Hence, we overproduced the bipartite Nup1 N terminus (Nup1 aa1-123) with the inducible GAL promoter in a wild-type yeast background (4 hr of galactose induction). This elicited an interesting nuclear pathology: Nup1 (1-123)-mCherry was targeted to the nucleus, preferentially to the NE, and induced formation of membrane protrusions on the nuclear surface, which we termed “expansion membranes” (Figure 3A). Additionally, we observed a clustering and segregation of NPCs mainly to the outer pole of the expansion membrane when detecting Nup188-GFP. NPC clustering was seen for various other Nups, suggesting that these structures likely represent intact NPCs rather than subassemblies (Figure S3A). Overproduced Nup1 (1-123)-mCherry associated with the yeast plasma membrane (PM) but not with the ER, which we labeled with Sec63-GFP (Figure 3A, lower panel). This unexpected finding suggests that the Nup1 AH may recognize lipid determinants that are present on the NE and PM but absent from the ER. Cells were generally enlarged and frequently contained empty buds indicating mitotic defects (Figure S3A). We then characterized the Nup1-induced nuclear changes at the ultrastructural level by transmission electron microscopy (TEM). After 2 hr of Nup1 (1-123) overproduction, we observed some nuclei with a distinctive focal outgrowth of the NE that penetrated deep into the cytoplasm (Figure 3B). This expansion membrane is contiguous with the nuclear membrane and corresponds to two closely apposed sheets of NE. The leading edge of the expansion membrane had a high curvature and appeared to re-approach the nucleus at a site that was located away from the original site of outgrowth. After 4 hr of induction, the expansion membrane had apparently collapsed and fused with the remainder of the nucleus (Figure 3B) in the majority of cells. While the expansion membrane looks like a thin tube in EM cross-section, it likely forms a three-dimensional membrane cup that wraps around a portion of the cytosol. Similar nuclear pathologies were observed when overproducing larger Nup1 fragments (e.g., Nup1 aa1-1040; see Figure S3C). In response to continued Nup1 overproduction (8 hr), lamellar stacks of new membrane sheets closely associated with the INM emerged (Figure 3B). Consistent with the segregation of GFP-tagged Nups to the expansion membrane in Nup1 (1-123)-overproducing cells (Figures 3A and S3A), we readily detected NPC structures by TEM. Notably, NPCs in one sheet of the expansion membrane often vertically aligned with NPCs in the other sheet (Figure 3B, white arrows and inset). Importantly, the activity of the Nup1 N terminus is distinct from a predicted lipid-interacting ALPS motif at the Nup1 C terminus (aa987-1004) (Drin et al., 2007). Overexpressing this α-helical motif (or a predicted Nup170 ALPS motif), as specificity controls, did not result in NE alterations or NPC clustering (Figure S4A).
Figure 3.
Nup1 Overproduction Induces Nuclear Membrane Deformation
(A) Fluorescence imaging of cells expressing Nup188-GFP or Sec63-GFP (ER marker) and plasmid-borne Nup1 (1-123)-mCherry (GAL promoter). Induced refers to 4 hr of protein overproduction in galactose-containing medium. Black arrows indicate the site of nuclear membrane expansion; white arrows label NPC clusters. For protein expression levels, see Figure S3B. Scale bar represents 2 μm.
(B) Transmission electron microscopy (TEM) of representative cells, which overproduced Nup1 (1-123)-mCherry for 2, 4, or 8 hr, respectively. N, nucleus; C, cytoplasm; E, expansion membrane; V, vacuole. White arrows label single NPCs; black arrows mark the tip of the expansion membrane (2 hr) and newly formed intranuclear membrane sheets (8 hr). Scale bar represents 1 μm. Inset shows higher magnification of aligned NPCs; scale bar represents 100 nm.
(C) Live imaging of cells overexpressing the indicated Nup1 constructs for 4 hr in a NUP188-GFP background. See Figure S4B for Nup1 expression levels. Black arrows indicate expansion membrane; white arrows label NPC clusters. Note that Nup1 (1-76) overexpression in nup133Δ cells results in a similar NE phenotype, albeit with a more pronounced NPC clustering (Figure S3D). Scale bar represents 2 μm.
(D) TEM analysis of the indicated Nup1 fragments after 4 hr of overproduction. White arrows label single NPCs, same nomenclature as in (B). Scale bar represents 1 μm.
We then assessed which Nup1 motif was responsible for generating the NE defects. A fragment comprising the AH and its predicted endogenous NLS sequence (Nup1 aa1-76) was efficiently imported into the nucleus and created an overall localization pattern similar to the Nup1 (1-123) fragment (Figure 3C). Notably, this fragment was sufficient to produce the distinctive expansion membrane, lateral segregation of NPCs and PM staining as shown by fluorescent and electron microscopy (Figures 3C and 3D). In contrast, an overexpressed fragment comprising the NLS and HR alone (Nup1 aa33-123) was imported to the nucleus, clustered NPCs but did not label the PM. This fragment was highly toxic when overproduced and we frequently observed vacuolar engulfment of portions of the nucleus, an unusual “nucleophagy-like” phenotype (Figures S4B–S4D). A key prediction is that a reduction of Nup1 lipid affinity would diminish nuclear membrane deformation. Indeed, we found that mutations on the Nup1 AH hydrophobic face (F19R and F26R) (see Figure 1A), although properly imported, did not generate expansion membranes, showed strongly reduced membrane affinity (taking the PM staining as a proxy) and consequently reduced the overexpression toxicity (Figures 3C and S4B). The NPC clustering seen in these mutants may derive from the effects of the Nup1 HR (compare to Nup1 aa33-123).
In summary, high cellular levels of the Nup1 AH elicit a dramatic reshaping of the NE, which is characterized by NE membrane deformation in the initial stages and membrane hyperproliferation at later time points. The deformation, which is most clearly seen at the highly curved growth edge of the expansion membrane, is consistent with lipid binding of the Nup1 AH (further explored below). The de novo membrane synthesis might be a secondary response to the physical stress that is imposed on the NE.
The Nup60 Helical Region Modulates the Degree of Membrane Curvature Induction
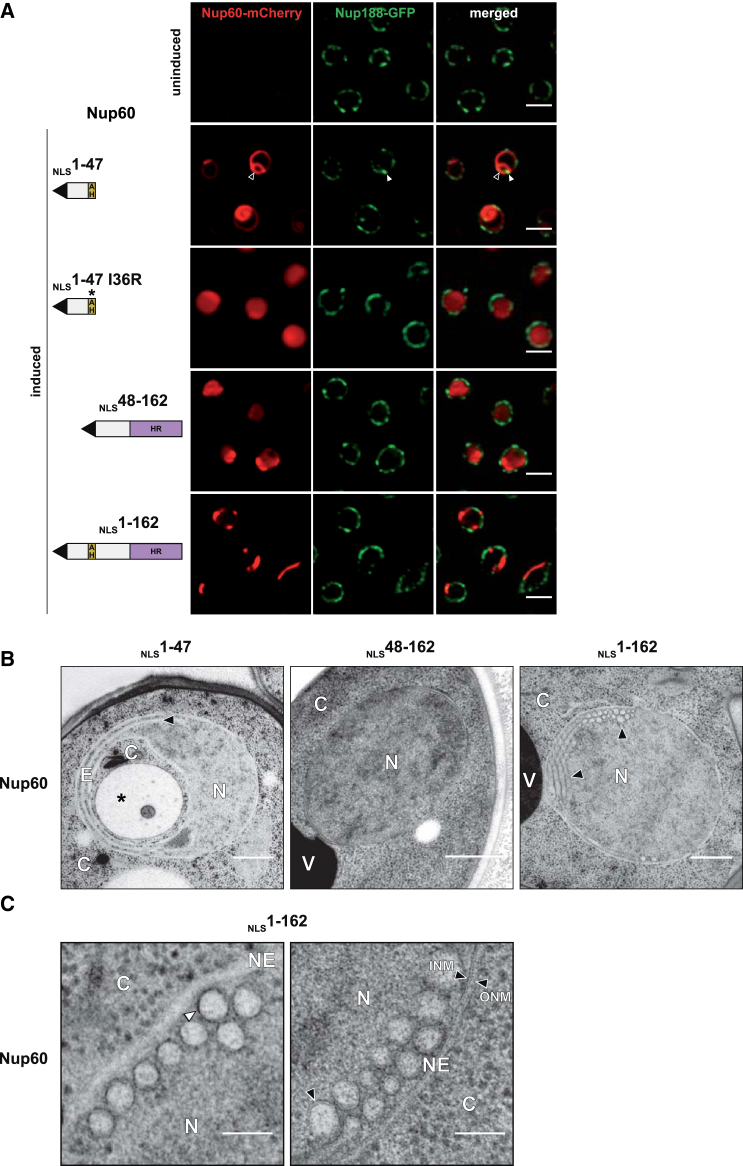
Despite a similar bipartite structure, the Nup1 and Nup60 N termini differ in sequence. We therefore tested whether Nup60 overproduction would also promote NE deformation. Upon 4 hr of overproduction, the Nup60 AH (NLSNup60 aa1-47) was targeted to the NE and induced membrane phenotypes characterized by a distinct nuclear expansion membrane and NPC clustering (Figure 4A), which resembled the Nup1-induced effects. Significantly, replacing Nup60 Ile36, a central residue on the hydrophobic side of the AH (Figure 1A), with a positively charged Arg residue abolished NE targeting and deformation and lowered the overexpression toxicity in cells (Figures 4A and S5B). Ultrastructural analysis of cells overproducing the NLSNup60 (1-47) fragment confirmed the existence of an expansion membrane that engulfed cytosol and organelles and the formation of intranuclear membrane sheets (Figures 4B and S5A; see Figures S5B and S5C for expression/toxicity). In contrast, the Nup60 HR fragment (NLSNup60 aa48-162) could not produce recognizable membrane defects, even when it was imported to the nucleus by an artificial NLS (Figures 4A and 4B). Because NPC targeting of Nup60 is a composite effect of the AH and HR, we tested whether the HR might influence the membrane deforming capacity of the AH. Significantly, NLSNup60 (1-162)-mCherry no longer generated an expansion membrane, but instead accumulated in crescent-shaped foci at the NE (Figure 4A, bottom). NPCs were largely excluded from these foci when judged by Nup188-GFP staining. TEM analysis showed that numerous highly curved membrane tubules inside the nucleus had emerged (Figure 4B). These tubules had an average diameter of 57 nm (±15 nm SD) and were tightly packed in regular arrays beneath the INM. When viewed at higher magnification, the tubules appeared to be decorated by an electron-dense protein coat on the outside (Figure 4C, left image). A modification of the EM stain allowed us to determine that the tubules were made of a single lipid bilayer (Figure 4C, right image). Overproduction of full-length Nup60 also caused the formation of intranuclear membrane tubules (Figure S5D). However, these were generally more heterogeneous in size and loosely packed against each other. This could reflect a coating of tubules with Mlp1 filaments, which co-cluster with Nup60 under this condition (Figure S5E). Consistent with a specific role of Kap123 in importing Nup60, the NE was not deformed in kap123Δ cells (Figure S5F). Taken together, the Nup60 amphipathic helix can remodel the NE membrane at high protein concentration. The adjacent α-helical region (HR), which is predicted to form a coiled coil (Figure S1B), may polymerize into a scaffold that augments the degree of membrane curvature imparted by the Nup60 amphipathic helix.
Figure 4.
High-Curvature Membrane Induction by Overexpressed Nup60
(A) Fluorescent imaging of cells expressing Nup188-GFP and plasmid-borne Nup60-mCherry constructs (GAL promoter). Induced refers to 4 hr of protein overproduction in galactose-containing medium. NLSNup60 (1-47): black arrow indicates expansion membrane; white arrow indicates NPC clusters. For expression levels and toxicity, see Figure S5B and (C). Because Nup60 (48-162) was not imported into the nucleus, we added an SV40 NLS to all Nup60 constructs for comparability. Accelerated import also reduces targeting of Nup60 (1-47) to the plasma membrane (compare Figure 4A with Figure S5F left). Scale bar represents 2 μm.
(B) TEM analysis of cells overproducing Nup60-mCherry constructs. NLSNup60 (1-47): asterisk marks an intranuclear organelle of unknown origin, black arrow labels newly formed membrane sheet. NLSNup60 (1-162): black arrows label membrane tubules in longitudinal and cross-section. N, nucleus; C, cytoplasm; E, expansion membrane; V, vacuole. Scale bar represents 500 nm.
(C) Electron micrographs of NLSNup60 (1-162) overproducing cells. Left and right images are TEM samples optimized for staining proteins or membranes, respectively. Left image shows membrane tubules in cross-section with a surrounding electron-dense coat (white arrow). Right image shows that tubules are made of a single lipid bilayer (black arrow, compare to INM/ONM). INM, inner nuclear membrane; ONM, outer nuclear membrane. Scale bar represents 100 nm.
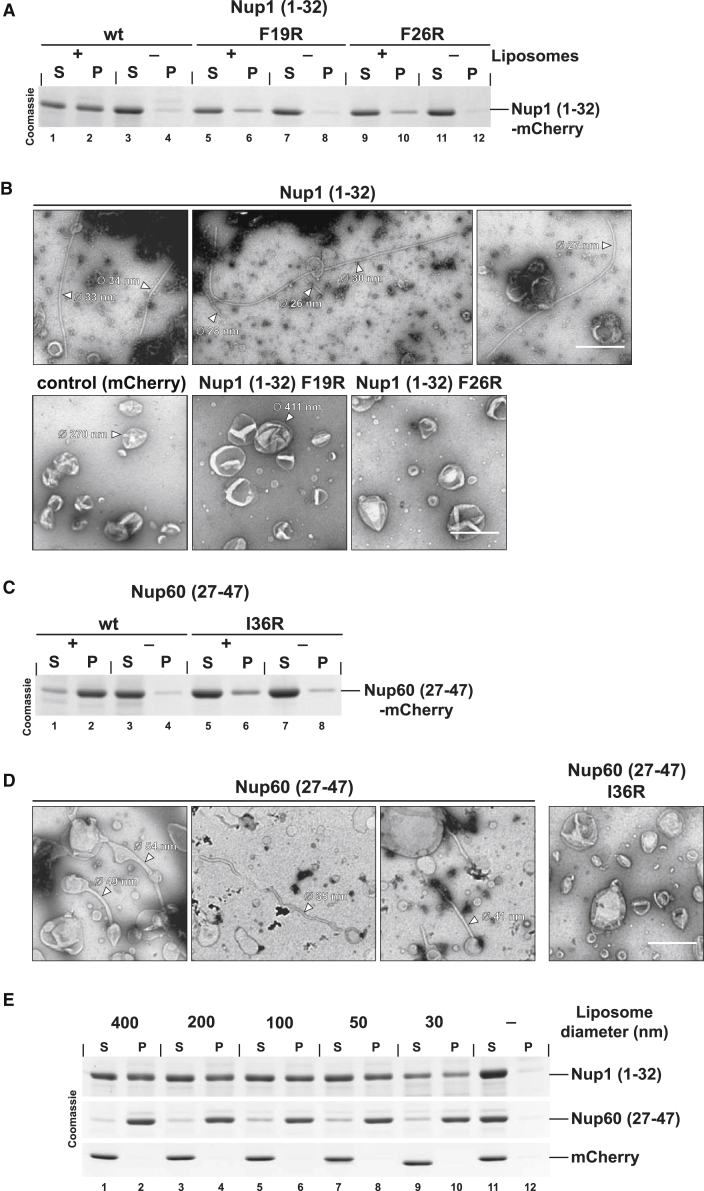
The Nup1 and Nup60 AHs Directly Bind and Deform Membranes In Vitro
Lipid-interacting amphipathic helices typically adopt an orientation parallel to the membrane plane with hydrophobic residues inserting between fatty acyl-chains and polar residues facing the polar lipid heads. An asymmetric α-helix insertion into one leaflet of the lipid bilayer can bend membranes like a wedge (McMahon and Gallop, 2005). To find out whether the Nup1/Nup60 N termini directly interact with membranes, we carried out in vitro liposome co-sedimentation assays. The Nup1 AH (Nup1 aa1-32) was C-terminally tagged with mCherry to facilitate its detection. Following purification from E. coli via a short N-terminal Strep-tag, Nup1 binding to Folch liposomes was tested. In this assay, proteins that bind to liposomes generally pellet (P) with the liposomes whereas soluble proteins that do not bind remain in the supernatant (S). Notably, a fraction of Nup1 co-sedimented with liposomes indicating a direct lipid interaction (Figure 5A, compare lanes 2 and 4). In contrast, the Nup1 F19R and F26R mutant proteins exhibited a reduced affinity for liposomes. This result suggests an impaired hydrophobic insertion into the lipid bilayer and could explain the lack of NE binding and deformation seen at high protein levels in vivo (Figure 3C). To directly assess whether Nup1 can induce membrane curvature, we incubated liposomes with recombinant Nup1 and analyzed these preparations by negative staining EM. Notably, the Nup1 AH (2.5 μM protein) could evaginate liposomes (average size: 200-300 nm) into narrow, relatively straight tubules with an average outer diameter of 30 nm (±2.7 nm SD) (Figure 5B). By comparison, the Nup1 AHs with a disrupted hydrophobic face or recombinant mCherry alone failed to tubulate membranes. These findings indicate that Nup1 directly binds to lipids and is sufficient to induce a high membrane curvature. Membrane binding by a recombinant fragment comprising the entire bipartite N terminus (Nup1 aa1-123) was not tested due to protein insolubility; however, an AH with a longer extension (Nup1 aa1-84) generated tubules of similar morphology (Figure S6B).
Figure 5.
Membrane Curvature Induction by Nup1 and Nup60 in a Reconstituted System
(A) Coomassie-stained gel of a liposome co-sedimentation assay using Folch liposomes (0.5 mg/ml, size-filtered to 400 nm) and Strep-tagged recombinant Nup1 proteins. Liposome bound protein is pelleted upon centrifugation. S, supernatant; P, pellet.
(B) In vitro liposome deformation assay showing a gallery of representative tubule shapes. Folch liposomes were mixed with purified wild-type or mutant Strep-Nup1 (1-32)-mCherry or Strep-mCherry (control) (final protein: 2.5 μM) and incubated at 22°C for 30 min prior to negative staining EM. For a protein control without liposomes, see Figure S6A. Outer diameters of liposomes and tubules are indicated. Scale bar represents 500 nm.
(C) Liposome co-sedimentation assay as in (A). Recombinant wild-type and mutant 6His-Nup60 (27-47)-mCherry protein was used.
(D) In vitro liposome deformation assay with 6His-Nup60 (27-47)-mCherry (final protein: 2.5 μM, 30 min incubation at 22°C) showing different types of tubules that are commonly observed by EM. For a Nup60 protein control without liposomes and further examples of tubule morphologies, see Figure S6C. Tubule diameters are indicated. Scale bar represents 500 nm.
(E) Liposome co-sedimentation assay with recombinant Nup1, Nup60, and mCherry (control) proteins. Liposomes of varying sizes (0.5 mg/ml) were made by extrusion through membranes of different pore diameters (as indicated). The actual liposome diameters were determined by dynamic light scattering (Figure S6D). Pellet and supernatant fractions were analyzed by SDS-PAGE and Coomassie staining.
We next characterized the properties of Nup60. When tested in the liposome co-sedimentation assay, the recombinant Nup60 AH (Nup60 aa27-47) bound to liposomes with high affinity and this interaction was reduced upon mutating the hydrophobic face of the helix (i.e., Nup60 I36R) (Figure 5C). Notably, Nup60 (2.5 μM protein) could also deform liposomes into membrane tubules, albeit with a different morphology than Nup1. The Nup60 tubules had a wider average outer diameter of 43.5 nm (± 9.5 nm SD), bulges were frequently observed and the tubules were more undulated (Figures 5D and S6C). Decreasing the protein concentration in the reconstitution reaction resulted in fewer Nup60 tubules (data not shown). The Nup60 I36R mutant protein predictably failed to generate tubules (Figure 5D). As for Nup1, this observation correlates with a lack of NE deformation of the overproduced mutant Nup60 AH in vivo (Figure 4A). We could not test whether the Nup60 HR potentiates curvature induction in vitro due to insolubility of the predicted coiled-coil domain. Nevertheless, both recombinant Nup60 and Nup1 generate tubule curvatures of a similar range as the positively curved edge of the pore membrane (ONM-INM distance: ∼30 nm). Given that some Nups such as human NUP133 are considered bona fide membrane curvature sensors, we asked whether Nup1 and Nup60 exhibit similar properties. To this end, we generated and characterized liposomes of different diameters by extrusion through membranes with varying pore sizes (Figure S6D). Small liposomes have a more highly curved surface than larger ones. Consistent with earlier reports (Drin et al., 2007), the ALPS-motif containing β-propeller of human NUP133 preferentially co-sedimented with small liposomes (Figure S6E). In contrast, Nup1 and Nup60 binding to liposomes appeared to be insensitive to the underlying membrane curvature under the conditions of the assay (Figure 5E). We conclude that the N-terminal amphipathic helices of Nup1 and Nup60 can directly bind and remodel lipid bilayers into high-curvature membrane structures.
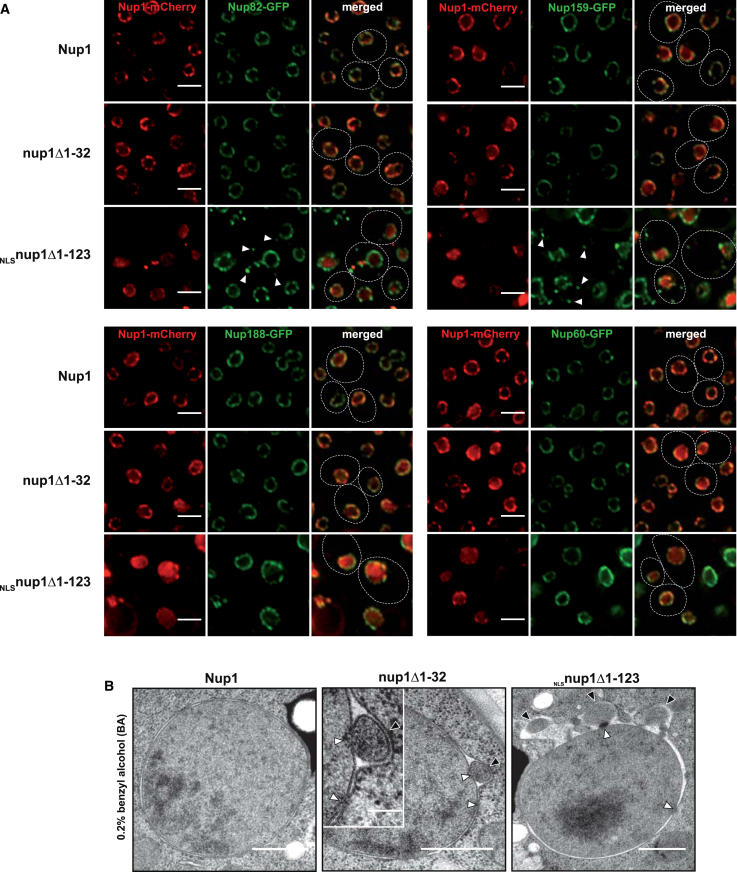
The N Terminus of Nup1 Contributes to NPC and NE Integrity
To test whether basket-induced membrane remodeling is required for the overall integrity of the NPC, we analyzed the localization of representative core scaffold (Nup188), basket (Nup60), and cytoplasmic filament Nups (Nup82/Nup159) in Nup1 mutants lacking the AH (nup1Δ1-32) or entire N terminus (NLSnup1Δ1-123). The localization of these marker Nups was largely unaffected in the nup1Δ1-32 mutant. In contrast, Nup159-GFP and Nup82-GFP, which are located on the cytoplasmic face of the NPC, partially mislocalized to the cytoplasm as multiple foci in the NLSnup1Δ1-123 mutant (Figure 6A). Because the Nup1 N terminus is capable of remodeling membranes, we reasoned that a loss of NPC integrity could be linked to an altered pore membrane structure. The NLSnup1Δ1-123 and nup1Δ1-32 mutants had no obvious ultrastructural defects at the NE when examined by TEM (data not shown). We therefore tested the influence of increased membrane stress by treating yeast cells with the membrane fluidizer benzyl alcohol (BA). The NE of wild-type cells appeared unaffected when incubated with 0.2% BA (Figure 6B). In contrast, cells lacking the Nup1 AH (nup1Δ1-32) or the entire N terminus (NLSnup1Δ1-123) exhibited distinct NE “herniations,” which were contiguous with the INM and ONM and created membrane seals over the cytoplasmic face of NPCs. These structures are reminiscent of NE seals over NPCs resulting from deletion of the cytoplasmic Nup116, which forms a complex with Nup82/Nup159 (Wente and Blobel, 1993). BA treatment did not exacerbate the mutant growth defects (data not shown), which could indicate that the membrane alterations are short-lived or compensated by the remaining intact NPCs. The mislocalization of Nup159/Nup82 may secondarily enhance the formation of NE herniations under membrane stress; however, we already observed such structures upon deleting the Nup1 AH alone (i.e., nup1Δ1-32), a condition in which the cytoplasmic Nups were correctly localized. In summary, Nup1 contributes to NPC and NE integrity possibly in conjunction with other membrane-interacting Nups.
Figure 6.
The N Terminus of Nup1 Is Required for NPC and NE Integrity
(A) Double-fluorescence microscopy of cells expressing the indicated Nup1 mCherry constructs (endogenous promoter) in nup1Δ cells harboring different genomically GFP-tagged Nups. White arrows label cytoplasmically mislocalized Nup82/159. Note that some cells also contain cytoplasmic NLSnup1Δ1-123 foci. Scale bar represents 2 μm. Appending an NLS to full-length Nup1 does not result in a similar mislocalization pattern (see Figure S7A).
(B) TEM analysis of exponentially growing cells after 4hr treatment with 0.2% benzyl alcohol. The indicated Nup1 constructs (endogenous promoter) were expressed in nup1Δ cells. Inset shows higher magnification of a membrane herniation (black arrow). White arrows indicate NPCs. Scale bar represents 500 nm (100 nm for inset).
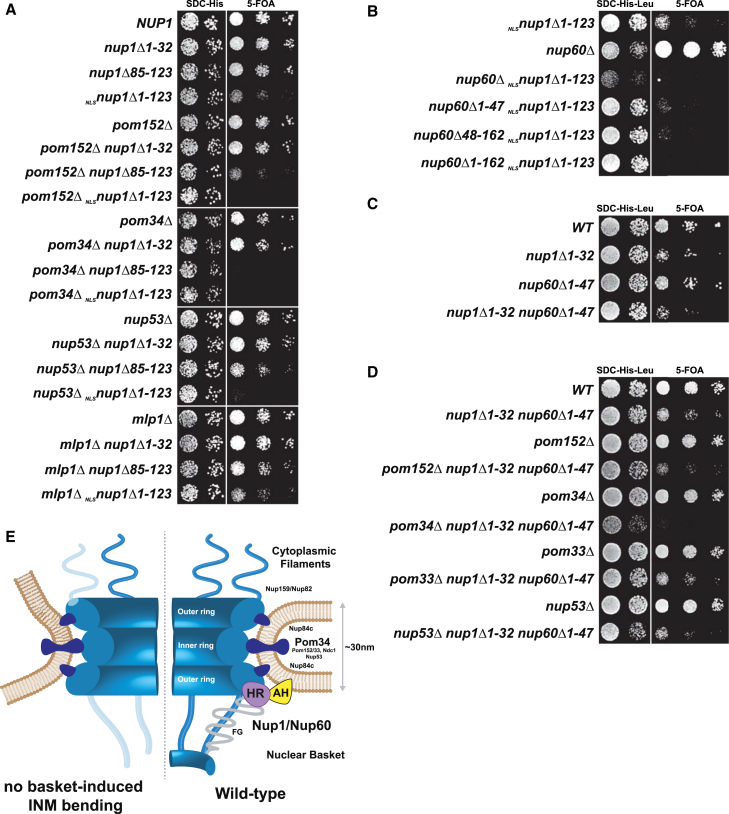
Membrane Remodeling by Nup1 and Nup60 Is Functionally Linked to Distinct Transmembrane Nucleoporins
Finally, we explored the genetic interactions of the membrane-shaping basket Nups. The NPC-lipid network is extensively buffered genetically. For example, three transmembrane Nups (POM152/34/33), NUP53, and NUP60 are all not essential in S. cerevisiae. Thus, we tested whether any of these factors would become critical for cell survival in the NLSnup1Δ1-123 mutant, which exhibited NPC and NE aberrations. Deleting POM152, POM34, or NUP53 together with the NUP1 N terminus caused synthetic lethality, whereas the basket Nup MLP1 showed no genetic interaction (Figure 7A). POM34 was more sensitive to NUP1 malfunction as synthetic lethality was already observed by deleting the NUP1 HR alone (i.e., nup1Δ85-123). The NUP1 N terminus further exhibits a synthetic lethal interaction with the NUP60 N terminus (Figure 7B). To precisely separate the basket AH function from the adjacent HRs, we generated a mutant, in which only the NUP1 and NUP60 amphipathic helices were deleted. This double mutant (nup1Δ1-32/nup60Δ1-47) exhibited no major genetic interaction under the conditions tested (Figure 7C). Strikingly, however, the NUP1/NUP60 AH function becomes essential for cell viability in the absence of POM34, a non-essential transmembrane Nup (Figure 7D). Because neither the NUP1 AH nor NUP60 (Figures 7A and S7B) exhibit an SL interaction with POM34, the observed SL interaction of the triple mutant likely reflects a synthetic genetic link in response to the combined deletion of the two AHs. The POM152 deleted triple mutant exhibited a synthetically enhanced phenotype while POM33 and NUP53 had no major genetic link to the NUP1/NUP60 amphipathic helices. Thus, Nup1 and Nup60 likely act synergistically to shape the pore membrane and display important functional links with distinct transmembrane Nups that underlie the NPC inner ring structure.
Figure 7.
Functional Links between Basket and Other Lipid-Interacting Nups
(A) Genetic interaction analysis. Double mutant strains harboring a wild-type NUP1 cover plasmid (URA marker) were co-transformed with the indicated NUP1 plasmids (HIS marker). Growth was followed on SDC-His (loading control) and on SDC+5-fluoroorotic acid (5-FOA) plates to shuffle out the URA cover plasmid. Cells were spotted in 10-fold serial dilutions and incubated for 2 (SDC-His) or 3 days (5-FOA) at 30°C.
(B and C) A nup60Δnup1Δ mutant strain harboring a wild-type NUP1 cover plasmid (URA marker) was co-transformed with different NUP1 (HIS marker) and NUP60 plasmids (LEU marker) to yield the indicated genotypes. Growth was followed on SDC-His-Leu (loading control) and on 5-FOA plates to shuffle out the URA cover plasmid.
(D) Genetic interaction analysis of the nup1Δ1-32 (HIS plasmid) / nup60Δ1-47 (LEU plasmid) mutant with the indicated Nups. Cells also contain a wild-type NUP1 cover plasmid (URA marker), which was shuffled out on 5-FOA plates (30°C).
(E) Model depicting the hypothetical changes of pore membrane and NPC structure in cells lacking INM bending by Nup1/Nup60 (AH and HR). Destabilization of cytoplasmic Nups (Nup159/Nup82) and basket Nups (Nup1/Nup60/Mlps) is indicated by light colors. Membrane destabilization may result in sealed NPCs according to a mechanism proposed by (Wente and Blobel, 1993). Known lipid-interacting parts of the NPC are indicated in dark blue. For simplicity, only one bipartite N terminus representing Nup1/Nup60 is shown.
Discussion
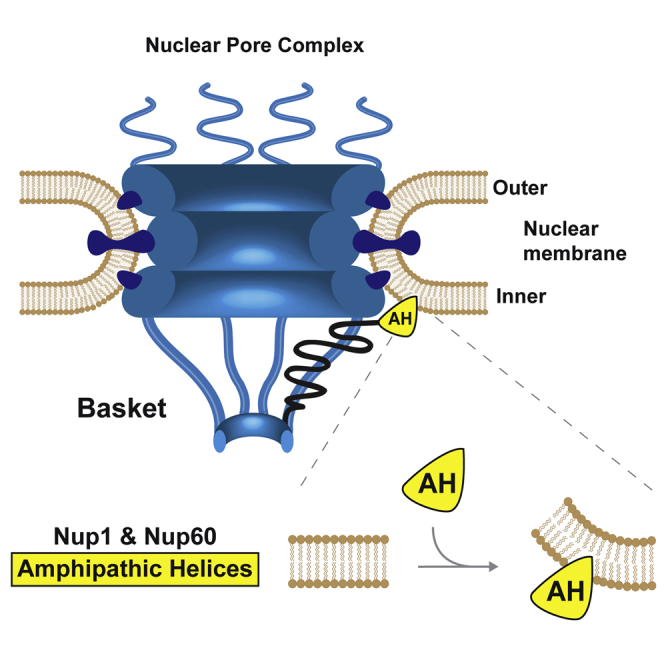
This study has revealed a connection between the NPC basket and the inner nuclear membrane, which extends the inventory of membrane-bending Nups to peripheral parts of the NPC. Our key findings are that basket amphipathic helices (1) directly bend membranes in vitro, (2) reshape the nuclear envelope at high levels in vivo, (3) play a role in maintaining NPC/NE integrity, and (4) functionally interact with other membrane Nups implicated in NPC biogenesis. We propose the existence of a lipid-interacting “belt” of basket Nups, which abuts the outer NPC ring and is important to shape the NE membrane around the “waist” of NPCs (Figure 7E). The bipartite N termini of Nup1/Nup60 probably induce high curvature by combining both hydrophobic insertion and scaffolding. The amphipathic helices may form a wedge in the lipid bilayer that occupies more space in the outer than in the inner leaflet. The adjacent HRs contribute to connecting the amphipathic helices to the rigid NPC scaffold, which has a concave outer surface complementary to the positively curved pore membrane. Additionally, the predicted coiled-coil region (HR) of Nup60 could play a role in oligomerizing Nup60 into higher-order structures that augment the degree of membrane curvature induction by the Nup60 amphipathic helix. Thus, although similar in their bipartite secondary structure, the Nup1 and Nup60 N termini may still differ in specific functional aspects.
How NPCs assemble into an intact NE is still a largely enigmatic process, but several lines of evidence suggest that it initiates from both sides of the NE, proceeds through membrane bending and INM-ONM fusion and culminates in the joining of two apposing halves of NPC precursors (Rexach, 2009). This raises the question as to how a new NPC assembly site is defined on the NE. Given that the basket is an asymmetric lipid-interacting part of the NPC, our observations suggest that basket nucleoporins might play a role in initiating NPC biogenesis on the INM side. Although speculative, the curvature-independent binding of Nup1 and Nup60 to membranes in vitro possibly reflects an ability to deform a flat piece of NE during the initial steps of NPC biogenesis. Interestingly, we found specific functional interactions between the basket amphipathic helices and Pom34, a member of a distinct protein complex of transmembrane proteins including Ndc1 and Pom152. Pom34 depletion in cells that lack Nup53 and Nup59 inhibits NPC assembly and leads to accumulation of Nup82 and other newly synthesized Nups in cytoplasmic foci (Onischenko et al., 2009). Cytoplasmic Nup82 mislocalization is also seen upon depleting Nup170 and Nup157, a condition that produces NPC-like assembly intermediates at the INM and at cytoplasmic foci (Makio et al., 2009). The mislocalization of Nup82 in cells lacking the Nup1 N terminus could reflect similar NPC assembly problems. Given the genetic links of NUP1/NUP60 to the network of transmembrane Nups, it is likely that the basket cooperates with these factors during NPC assembly.
Nup1/Nup60 insertion into the INM probably requires specialized mechanisms since an unspecific interaction with other cellular membranes prior to nuclear import must be prevented. Notably, the basket amphipathic helices lie in vicinity of NLSs and the binding of karyopherins (Nup1/Kap60 and Nup60/Kap123) may not only contribute to rapid nuclear import but also mask their hydrophobicity. The Nup60-associated Kap123 is an interesting case, as it also plays a role in importing the transmembrane Nup Pom33 (Floch et al., 2014), and the spindle pole body (SPB) component Nbp1 (Kupke et al., 2011). Nbp1 interacts with the transmembrane protein Ndc1 (a shared component of SPBs and NPCs) and binds the INM via an amphipathic helix required for SPB biogenesis. Thus, Kap123 may exhibit special properties for escorting lipid-interacting cargo to its INM destination. Ran-GTP mediated dissociation of karyopherins would expose their amphipathic helices and promote the timely interaction with the INM. Whereas the yeast basket uses both Nup1 and Nup60 to interact and shape membranes, these functions might be combined in NUP153 in higher eukaryotes, which also contains a conspicuous amphipathic helix at its N terminus.
Taken together, basket nucleoporins induce high curvature membranes, an otherwise energetically unfavorable state. The basket is part of a network of membrane shaping forces within the NPC, which must act in spatial and temporal synergy during NPC assembly and maintenance.
Experimental Procedures
Yeast Strains and Plasmids
Yeast strains and plasmids are listed in the Supplemental Experimental Procedures. Microbiological techniques were performed according to standard procedures (http://www.currentprotocols.com). Genes in yeast were tagged/deleted by a standard one-step PCR-based technique. The nup1Δ strain was created by sporulation and tetrad dissection of a diploid NUP1/ nup1Δ strain.
Live-Cell Imaging of Yeast
Exponentially growing cells were immobilized on microscope slides with agarose pads and imaged on a DeltaVision Elite microscope (GE Healthcare) at 30°C. Images were acquired with 60× or 100× oil immersion objectives and recorded with a CoolSNAP HQ2 CCD camera (Photometrics). Deconvolution was carried out using softWoRx software (GE Healthcare).
GAL-Induced Protein Overexpression
Cells were grown in selective media containing 2% raffinose (SRC-X). Expression was induced by adding galactose to a final concentration of 2%.
Immunoblotting
The following antibodies were used: anti-mCherry (Abcam), anti-Pgk1 (Abcam), anti-Kap60 (yD-18) (Santa Cruz Biotechnology), and anti-Strep (QIAGEN).
Electron Microscopy
Processing of yeast cells for EM analysis is described in the Supplemental Experimental Procedures. Samples for liposome deformation assays were adsorbed on glow discharged EM grids coated with carbon film and stained with 2% uranyl acetate. All EM samples were examined on a FEI Morgagni 268D TEM operated at 80 kV. Digital images were acquired using an 11-megapixel Morada CCD camera from Olympus-SIS. Tubule sizes were quantified with ImageJ using the average of 45 diameter measurements for each Nup1 and Nup60.
Protein Expression and Purification
Proteins were expressed in E. coli BL21 CodonPlus (DE3) RIL cells and purified by Strep-, His- or GST-affinity chromatography or via TAP-affinity purification from yeast (see Supplemental Experimental Procedures for details).
Liposome Co-Sedimentation and Deformation Assay
Brain-derived Folch lipids (Sigma) were dried under argon gas, dessicated, and rehydrated in buffer (150 mM NaCl, 50 mM Tris-HCl pH 7.5, 0.5 mM DTT) to a final concentration of 1 mg/ml. To obtain liposomes with different diameters, the lipid mixture was extruded through polycarbon membranes with pore sizes of 0.4, 0.2, 0.1, 0.05, or 0.03 μm (Nucleopore). For liposome co-sedimentation assays, 4 μg of protein was incubated with 0.5 mg/ml liposomes for 30 min at 22°C in a total volume of 50 μl. The reaction was pelleted at 180,000 × g at 22°C for 10 min. Equal volumes of pellet and supernatant fractions were analyzed by SDS-PAGE using NuPAGE Novex Bis-Tris gels (Invitrogen). For in vitro liposome deformation, assay proteins (2.5 μM final concentration) were mixed with 0.125 mg/ml liposomes and incubated for 30 min prior to negative staining EM. Assays were performed two to three times for each protein and with different batches of Folch lipids.
Author Contributions
N.M., J.C., M.J.M., and A.R. performed fluorescence microscopy, cloning, and immunoblotting; N.M. carried out the in vitro biochemistry; and M.S. performed TAP-purifications and yeast genetics. Electron microscopy was done by J.C.; A.K. designed and supervised the project and wrote the manuscript. All authors contributed to the analysis and interpretation of data.
Acknowledgments
We thank H. Kotisch (CSF EM facility) for excellent assistance with TEM, the Martens lab for reagents and advice on liposome assays, and G. Warren and S. Martens for critical comments on the manuscript. The Köhler laboratory is funded by an ERC grant (281354; NPC GENEXPRESS) and a START grant from the Austrian Science Fund (FWF; Y557-B11). M.J.M. was funded by an EMBO Long-Term Fellowship and by a Human Frontier Science Program Fellowship.
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information
References
- Alber F., Dokudovskaya S., Veenhoff L.M., Zhang W., Kipper J., Devos D., Suprapto A., Karni-Schmidt O., Williams R., Chait B.T. The molecular architecture of the nuclear pore complex. Nature. 2007;450:695–701. doi: 10.1038/nature06405. [DOI] [PubMed] [Google Scholar]
- Bastos R., Lin A., Enarson M., Burke B. Targeting and function in mRNA export of nuclear pore complex protein Nup153. J. Cell Biol. 1996;134:1141–1156. doi: 10.1083/jcb.134.5.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck M., Lucić V., Förster F., Baumeister W., Medalia O. Snapshots of nuclear pore complexes in action captured by cryo-electron tomography. Nature. 2007;449:611–615. doi: 10.1038/nature06170. [DOI] [PubMed] [Google Scholar]
- Bogerd A.M., Hoffman J.A., Amberg D.C., Fink G.R., Davis L.I. nup1 mutants exhibit pleiotropic defects in nuclear pore complex function. J. Cell Biol. 1994;127:319–332. doi: 10.1083/jcb.127.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn S.G., Leksa N.C., Spear E.D., Rajashankar K.R., Schwartz T.U. Structural evidence for common ancestry of the nuclear pore complex and vesicle coats. Science. 2008;322:1369–1373. doi: 10.1126/science.1165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadrin A., Hess B., San Roman M., Gatti X., Lombard B., Loew D., Barral Y., Palancade B., Doye V. Pom33, a novel transmembrane nucleoporin required for proper nuclear pore complex distribution. J. Cell Biol. 2010;189:795–811. doi: 10.1083/jcb.200910043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo M.A., Hetzer M.W. Structure, dynamics and function of nuclear pore complexes. Trends Cell Biol. 2008;18:456–466. doi: 10.1016/j.tcb.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo M.A., Anderson D.J., Richard E., Hetzer M.W. Nuclear pores form de novo from both sides of the nuclear envelope. Science. 2006;312:440–443. doi: 10.1126/science.1124196. [DOI] [PubMed] [Google Scholar]
- Dawson T.R., Lazarus M.D., Hetzer M.W., Wente S.R. ER membrane-bending proteins are necessary for de novo nuclear pore formation. J. Cell Biol. 2009;184:659–675. doi: 10.1083/jcb.200806174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debler E.W., Ma Y., Seo H.S., Hsia K.C., Noriega T.R., Blobel G., Hoelz A. A fence-like coat for the nuclear pore membrane. Mol. Cell. 2008;32:815–826. doi: 10.1016/j.molcel.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Denning D., Mykytka B., Allen N.P., Huang L., Al Burlingame, Rexach M. The nucleoporin Nup60p functions as a Gsp1p-GTP-sensitive tether for Nup2p at the nuclear pore complex. J. Cell Biol. 2001;154:937–950. doi: 10.1083/jcb.200101007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos D., Dokudovskaya S., Alber F., Williams R., Chait B.T., Sali A., Rout M.P. Components of coated vesicles and nuclear pore complexes share a common molecular architecture. PLoS Biol. 2004;2:e380. doi: 10.1371/journal.pbio.0020380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet C.M., Talamas J.A., Hetzer M.W. Cell cycle-dependent differences in nuclear pore complex assembly in metazoa. Cell. 2010;141:1030–1041. doi: 10.1016/j.cell.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drin G., Casella J.F., Gautier R., Boehmer T., Schwartz T.U., Antonny B. A general amphipathic α-helical motif for sensing membrane curvature. Nat. Struct. Mol. Biol. 2007;14:138–146. doi: 10.1038/nsmb1194. [DOI] [PubMed] [Google Scholar]
- Feuerbach F., Galy V., Trelles-Sticken E., Fromont-Racine M., Jacquier A., Gilson E., Olivo-Marin J.C., Scherthan H., Nehrbass U. Nuclear architecture and spatial positioning help establish transcriptional states of telomeres in yeast. Nat. Cell Biol. 2002;4:214–221. doi: 10.1038/ncb756. [DOI] [PubMed] [Google Scholar]
- Floch A.G., Tareste D., Fuchs P., Chadrin A., Naciri I., Leger T., Schlenstedt G., Palancade B., Doye V. Nuclear pore targeting of the yeast Pom33 nucleoporin depends on karyopherin- and lipid-binding. J. Cell Sci. 2014 doi: 10.1242/jcs.158915. [DOI] [PubMed] [Google Scholar]
- Hoelz A., Debler E.W., Blobel G. The structure of the nuclear pore complex. Annu. Rev. Biochem. 2011;80:613–643. doi: 10.1146/annurev-biochem-060109-151030. [DOI] [PubMed] [Google Scholar]
- Kenna M.A., Petranka J.G., Reilly J.L., Davis L.I. Yeast N1e3p/Nup170p is required for normal stoichiometry of FG nucleoporins within the nuclear pore complex. Mol. Cell. Biol. 1996;16:2025–2036. doi: 10.1128/mcb.16.5.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupke T., Di Cecco L., Müller H.M., Neuner A., Adolf F., Wieland F., Nickel W., Schiebel E. Targeting of Nbp1 to the inner nuclear membrane is essential for spindle pole body duplication. EMBO J. 2011;30:3337–3352. doi: 10.1038/emboj.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makio T., Stanton L.H., Lin C.C., Goldfarb D.S., Weis K., Wozniak R.W. The nucleoporins Nup170p and Nup157p are essential for nuclear pore complex assembly. J. Cell Biol. 2009;185:459–473. doi: 10.1083/jcb.200810029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marelli M., Lusk C.P., Chan H., Aitchison J.D., Wozniak R.W. A link between the synthesis of nucleoporins and the biogenesis of the nuclear envelope. J. Cell Biol. 2001;153:709–724. doi: 10.1083/jcb.153.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon H.T., Gallop J.L. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–596. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- Miao M., Ryan K.J., Wente S.R. The integral membrane protein Pom34p functionally links nucleoporin subcomplexes. Genetics. 2006;172:1441–1457. doi: 10.1534/genetics.105.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niepel M., Molloy K.R., Williams R., Farr J.C., Meinema A.C., Vecchietti N., Cristea I.M., Chait B.T., Rout M.P., Strambio-De-Castillia C. The nuclear basket proteins Mlp1p and Mlp2p are part of a dynamic interactome including Esc1p and the proteasome. Mol. Biol. Cell. 2013;24:3920–3938. doi: 10.1091/mbc.E13-07-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onischenko E., Stanton L.H., Madrid A.S., Kieselbach T., Weis K. Role of the Ndc1 interaction network in yeast nuclear pore complex assembly and maintenance. J. Cell Biol. 2009;185:475–491. doi: 10.1083/jcb.200810030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S.S., Rexach M.F. Discovering novel interactions at the nuclear pore complex using bead halo: a rapid method for detecting molecular interactions of high and low affinity at equilibrium. Mol. Cell. Proteomics. 2008;7:121–131. doi: 10.1074/mcp.M700407-MCP200. [DOI] [PubMed] [Google Scholar]
- Pyhtila B., Rexach M. A gradient of affinity for the karyopherin Kap95p along the yeast nuclear pore complex. J. Biol. Chem. 2003;278:42699–42709. doi: 10.1074/jbc.M307135200. [DOI] [PubMed] [Google Scholar]
- Rexach M. Piecing together nuclear pore complex assembly during interphase. J. Cell Biol. 2009;185:377–379. doi: 10.1083/jcb.200904022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothballer A., Kutay U. Poring over pores: nuclear pore complex insertion into the nuclear envelope. Trends Biochem. Sci. 2013;38:292–301. doi: 10.1016/j.tibs.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Ryan K.J., McCaffery J.M., Wente S.R. The Ran GTPase cycle is required for yeast nuclear pore complex assembly. J. Cell Biol. 2003;160:1041–1053. doi: 10.1083/jcb.200209116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strambio-De-Castillia C., Niepel M., Rout M.P. The nuclear pore complex: bridging nuclear transport and gene regulation. Nat. Rev. Mol. Cell Biol. 2010;11:490–501. doi: 10.1038/nrm2928. [DOI] [PubMed] [Google Scholar]
- Vollmer B., Schooley A., Sachdev R., Eisenhardt N., Schneider A.M., Sieverding C., Madlung J., Gerken U., Macek B., Antonin W. Dimerization and direct membrane interaction of Nup53 contribute to nuclear pore complex assembly. EMBO J. 2012;31:4072–4084. doi: 10.1038/emboj.2012.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wente S.R., Blobel G. A temperature-sensitive NUP116 null mutant forms a nuclear envelope seal over the yeast nuclear pore complex thereby blocking nucleocytoplasmic traffic. J. Cell Biol. 1993;123:275–284. doi: 10.1083/jcb.123.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak R.W., Blobel G., Rout M.P. POM152 is an integral protein of the pore membrane domain of the yeast nuclear envelope. J. Cell Biol. 1994;125:31–42. doi: 10.1083/jcb.125.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.