Abstract
Objectives
This purpose of this study was to examine clinical-pathologic factors – particularly smoking and brain metastases – in EGFR mutation positive (M+) lung adenocarcinoma (ADC) to determine their impact on survival in patients treated with first line EGFR TKI.
Methods
A retrospective review of EGFR mutation reflex testing experience for all ADC diagnosed at a tertiary Asian cancer centre from January 2009 to April 2013. Amongst this cohort, patients with advanced EGFR M+ ADC treated with first line EGFR TKI were identified to determine factors that influence progression free and overall survival.
Results
444/742 (59.8%) ADC reflex tested for EGFR mutations were EGFR M+. Amongst never-smokers (n=468), EGFR M+ were found in 74.5% of females and 76.3% of males, and amongst ever smokers (n=283), in 53.3% of females and 35.6% of males. Exon 20 mutations were found more commonly amongst heavy smokers (> 50 pack years and > 20 pack years, Pearson’s chi square p=0.044, and p=0.038 respectively). 211 patients treated with palliative first line TKI had a median PFS and OS of 9.2 and 19.6 months respectively. 26% of patients had brain metastasis at diagnosis. This was significantly detrimental to overall survival (HR 1.85, CI 1.09-3.16, p=0.024) on multivariate analysis. There was no evidence that smoking status had a significant impact on survival.
Conclusions
The high prevalence of EGFR M+ in our patient population warrants reflex testing regardless of gender and smoking status. Smoking status and dosage did not impact progression free or overall survival in patients treated with first line EGFR TKI. The presence of brain metastasis at diagnosis negatively impacts overall survival.
Introduction
EGFR tyrosine kinase inhibitors (TKI) such as gefitinib and erlotinib, are now established first line treatment options for EGFR mutation positive (EGFR M+) lung adenocarcinoma (ADC), demonstrating significant improvement in progression free survival (PFS) over platinum-based doublet chemotherapy [1–7]. Previous studies examining the impact of smoking history on TKI response often reflect surrogacy for EGFR mutations and majority of phase III studies were enriched for never smokers. A recent retrospective study suggested that smoking history and smoking dosage may be associated with significantly poorer response rates and survival outcomes in EGFR mutation positive non-small cell lung cancer (NSCLC) [8]. However, this finding is confounded by the fact that a greater proportion of smokers had received EGFR TKI beyond the second and third line setting, and the impact of smoking on survival in EGFR mutation positive NSCLC patients receiving first line EGFR TKI remains unclear [9].
Due to the high incidence of EGFR mutations in Asian ADC compared to the West [10–11], many academic hospitals, including our centre, have adopted reflex testing for EGFR mutations. As cost effectiveness of EGFR TKI is driven by patient selection based on EGFR mutation status [12], it is important to define the prevalence of the mutation in both smokers (current and ex-smokers) as well as never smokers through systematic testing of consecutive cases. Clinical pathologic factors such as smoking status [8], location of EGFR mutation [13], and presence of brain metastases [14] may impact on treatment outcomes. Of particular interest, brain metastasis in EGFR mutation positive NSCLC is a common site of involvement at diagnosis and treatment failure—occurring in up to 23% of newly diagnosed patients [15]. Elucidating prognostic factors in EGFR mutant ADC treated with first line TKI will facilitate improved stratification and identify therapeutically challenging patient subgroups.
In this study, we report our reflex EGFR testing experience on consecutive lung adenocarcinomas seen in an Asian tertiary cancer centre and determine the prevalence of EGFR mutations by gender and smoking status. Relationships between mutation spectra and clinical characteristics such as age, gender, ethnicity and smoking status were also explored. Further, in those who had received first line treatment with an EGFR TKI, we examined clinical pathologic characteristics that had an impact on survival.
Materials and Methods
Study Population
Prior to 1st June 2010, EGFR mutation testing in our centre for patients with newly diagnosed ADC was ordered as per physician discretion. From 1st June 2010 all ADC samples identified by the pathologists were reflex tested for EGFR mutations, regardless of stage and smoking status.
Smoking status for patients was obtained from electronic medical records and Lung Cancer Consortium Singapore, where patients’ lifestyle factors were captured through interviews by research coordinators. Patients were classified as never smokers (NS), and ever smokers (ex-smokers [quit ≥ 1 year] and current smokers) (ES). NS were defined as those who had smoked less than 100 sticks in their lifetime.
Patients with Stage 4 EGFR M+ disease who had received TKI therapy were evaluated. Patients who had already been commenced on TKI based on their phenotypic traits (Asian, non-smoker), but subsequently underwent confirmatory EGFR mutation testing were also included. Baseline imaging was evaluated for presence or absence of brain metastases, and response evaluation scans were performed according to physician discretion, ranging from within 6 weeks from start of TKI for the first scan, to 8–12 weekly for subsequent scans. First line TKI treated EGFR M+ ADC patients were analysed for progression free survival (PFS) and overall survival (OS), and an exploratory analysis was done for factors that influenced survival.
EGFR Mutational Analysis
EGFR mutation analysis was carried out by Sanger sequencing on genomic DNA that had been extracted from formalin fixed paraffin embedded (FFPE) tissue samples using Qiagen FFPE DNA extraction kit. Extracted DNA was then subject to polymerase chain reaction (PCR) amplification of exons 18, 19, 20 and 21, and products were analysed by direct Sanger sequencing.
Statistical Analysis
Continuous clinical variables were summarized using median and range, while categorical variables were summarized by frequency and percentage. Pearson’s chi-squared test (or Fisher’s exact test if there were expected cell frequencies less than 5) was used to test for associations between patient characteristics and mutation type, smoking status and presence of brain metastasis at diagnosis. The strength of the association was estimated using Cramer’s V.
PFS was calculated as the duration from start of TKI to the PFS date defined by clinico-radiological progression of disease on CT imaging as per RECIST 1.1. OS was calculated as the duration of time from start of TKI to date of demise. The Kaplan-Meier method was used to estimate survival functions. The log-rank test was used to determine if there was a difference in survival curves between different groups. A 2-sided p-value of less than 0.05 was taken as statistically significant. Univariate and multivariate analysis was performed using the Cox proportional hazards model. All analyses were performed in Stata (Version 12.1; StataCorp, Texas, USA).
Ethics
This research was approved by SingHealth CIRB (CIRB 2010/516/B) and all clinical investigation was conducted according to the principles expressed in the Declaration of Helsinki. The data was collected and analysed anonymously then reported.
Results
Reflex Testing Experience
A total of 994 cases of ADC were seen in our centre between 1st January 2009 and 18th April 2013. Reflex testing was adopted from 1st June 2010. A 6 monthly review from 1st June 2010 to 19th April 2013 revealed that 87–94% of all ADC cases were tested, with an EGFR M+ rate of 50–68% (S1 Table). A total of 742 patients were tested for EGFR mutations. 444 (59.8%) were positive and 289 (38.9%) negative for mutations. 9 cases were unsuccessfully profiled due to incomplete sequencing (n = 5: unsuccessful for Exon 18, n = 1; Exon 20, n = 2; Exons 20/21, n = 1 and Exons 19/20/21, n = 1), tumor content of less than 15% raising the possibility of a false negative result (n = 1) or insufficient tissue for any analysis (n = 4). None of these patients underwent a repeat biopsy and they were treated as for ADC that was EGFR mutant negative. Hence the ascertainment rate with EGFR sequencing in our centre was 98.8%.
Smoking Status, Sex and EGFR Mutation Status of 762 ADC
Amongst the 762 cases evaluated for EGFR mutations, there were 464 NS (334, 72.0% females; 130, 28.0% males), 282 ES (30, 10.6% females; 252, 89.4% males) and 11 patients with unknown smoking status (6 females and 5 males).
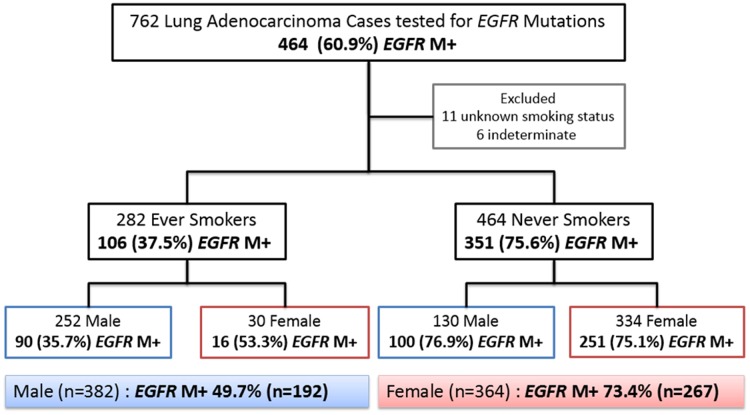
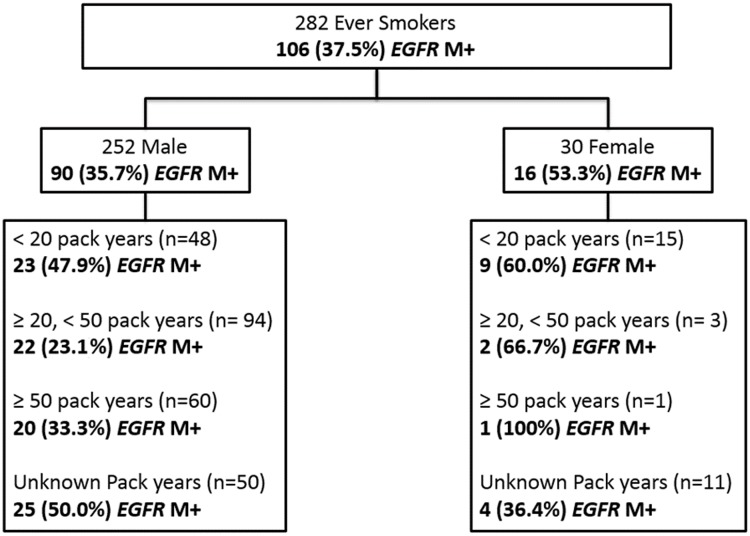
The respective EGFR M+ rates amongst female NS, female ES, male NS and male ES were 75.1%, 53.3%, 76.9%, and 35.7% (Fig 1). ES were further stratified by pack years based on available information regarding their smoking histories. EGFR M+ rates were at least 20 percent in both male and female heavy smokers but further interpretation was limited by the small numbers in these sub-groups (Fig 2).
Fig 1. Clinical characteristics and EGFR mutation status rates categorised by smoking status and sex.
11 patients with unknown smoking status, and 6 who had samples indeterminate for EGFR mutational status were excluded. 464/762 (60.9%) tested positive for EGFR mutations (EGFR M+). The number of patients needed to test in order to pick up 1 EGFR mutant lung adenocarcinoma in any sub-population stratified by sex and smoking status, was less than 3 patients (male ES; 1/0.357 = 2.8).
Fig 2. EGFR mutation rates amongst ever smokers classified by pack years.
EGFR mutations, Age, Sex, Ethnicity, Smoking Status
A total of 464 EGFR M+ patients were seen at our centre. 444 of the 464 EGFR M+ patients were tested at our centre and 17 were tested in another College of American Pathologist (CAP) accredited centres with details of mutational testing available. The remaining 3 were documented in clinical notes to have tested positive for exon 19 deletions in other external laboratory but further details of analysis were not available.
Of 461 EGFR M+, 414 (89.8%) harboured TKI sensitising mutations and 47 (10.1%) harboured mutations known to confer resistance to EGFR TKI. Single mutations in EGFR were detected in exon 18 (n = 12, 2.6%), exon 19 (n = 239, 51.8%), exon 20 (n = 29, 6.2%), and exon 21 (n = 166, 36.0%) Exon 18 mutations comprised 11 G719X mutations (8 G719A, 2 G719S, and 1 G719C), and 1 Exon 18 del (p.E709_T710>D). Exon 19 mutations comprised deletions of between 10 to 24 base pairs with or without 1) additional insertion of between 1 to 3 base pairs, or 2) substitution of 1 to 2 base pairs. The most prevalent mutations were deletions c.2235_2249del15 (n = 95, 39.7% of all Exon 19 mutations), followed by c2236_2250del15 (n = 41, 17.1% of all Exon 19 mutations). Exon 20 mutations comprised 13 insertion (51.7% of Exon 20 mutations), 11 duplications (37.9% of Exon 20 mutations) 2 T790M point mutations, 1 S768I point mutation, and 2 sensitising A763_Y764insFQEA insertion mutations. Exon 21 mutations comprised L858R (n = 159, 95.8% of all Exon 21 mutations), and L861Q (n = 6, 3.6% of all Exon 21 mutations) (Fig 3).
Fig 3. Sites of EGFR mutations amongst 461 patients.
15 (3.2%) had dual EGFR mutations: 10 with Exon 20 mutations (5 T790M & Exon 19 del; 2 T790M & L858R; 3 S768I & G719Q), 3 with G719Q & L858R, 1 Exon 19 del & L833V (Exon 21 mutation), and 1 Exon 19 del & L858R.
Exon 19 deletions were seen in younger (<65 years) patients while exon 21 mutations were relatively more common in old (≥65 years) patients (WHO criteria) (59.1% vs. 42.3%, p = 0.002, Cramer’s V = 0.181; weak association). There was no evidence of any association between sex and type of mutation (p = 0.756) or exon(s) involved (p = 0.136), or between ethnicity (Chinese vs. others) and type of mutation (p = 0.784) or exon(s) involved (p = 0.579). There was a trend towards never smokers harbouring mutations in exon 19, while more ever-smokers had mutations in exon 20 but this association was not significant (p = 0.119).
Impact of smoking dosage and EGFR mutations
Heavy smokers with more than 50 pack years appeared to have more mutations in exon 20 than those with less pack years or who had never smoked (p = 0.044, Cramer’s V = 0.145; weak association). A less stringent definition for heavy smokers (>20 pack years) still showed a significant association (18% vs. 6%, p = 0.038, Cramer’s V = 0.155; weak association). There was no significant association between type of mutation (indel vs. point) and heavy smoking status.
Use of EGFR TKI amongst EGFR M+ ADC
461 patients with EGFR M+ ADC comprised 57 (12.3%) Stage I, 20 (4.3%) Stage II, 49 (10.7%) Stage IIIA/B and 335 (72.7%) Stage IV disease. 121 were treated with curative intent. 294 patients received palliative systemic therapy of which 211 (71.8%) received first line TKI, 55 (18.7%) received 1st line chemotherapy, 28 (9.5%) received a combination of TKI and other agents including chemotherapy and combination targeted therapy in a phase I setting. 46 patients were censored as they were either lost to follow up, on best supportive care, declined treatment or passed away prior to commencing TKI. Of the 55 patients who did not receive TKI in the first line, 33 went on to receive TKI as second line therapy, 5 as third line, and 2 as fourth line. The remaining 15 never received EGFR TKIs in their lifetime. In total, 279 out of 294 (94.9%) EGFR M+ ADC received systemic therapy with a TKI in their lifetime.
Clinical characteristics of EGFR TKI treated Stage IV NSCLC
A homogenous population of 211 patients with Stage IV disease treated with first line TKI were evaluated for survival on TKI and smoking history and other clinical characteristics were analysed for impact on survival. The median age of the cohort was 62 (33–84). 128 (60.7%) were females. 166 (78.7%) had never smoked, 32(15.1%) were ex-smokers and 13 (6.2%) were smokers. Amongst ever smokers with accurate smoking histories (n = 32), the median number of pack years was 30 pack years (ranging from 0.9 to 102). 26% had proven brain metastasis at time of diagnosis. Other key characteristics are summarised in Table 1.
Table 1. Demographics of 211 patients treated with 1st line TKI.
| Variable | Number | % |
|---|---|---|
| Total number of patients | 211 | 100 |
| Age at diagnosis, years | ||
| Median (range) | 62 (33–84) | |
| ≤ 65 | 128 | 60.7 |
| > 65 | 83 | 39.3 |
| Sex | ||
| Female | 128 | 60.7 |
| Male | 83 | 39.3 |
| Ethnicity | ||
| Chinese | 179 | 84.8 |
| Malay | 19 | 9.0 |
| Indian | 4 | 1.9 |
| Others | 9 | 4.3 |
| Smoking status | ||
| Never | 166 | 78.7 |
| Ex | 32 | 15.2 |
| Current | 13 | 6.2 |
| Smoking pack-years (amongst ever-smokers only) | ||
| Median (range) | 30 (0.9–102) | |
| Unknown | 13 | 28.9 |
| Smoking pack-years (including never smokers) | ||
| < 50 | 188 | 89.1 |
| ≥ 50 | 10 | 4.7 |
| Unknown | 13 | 6.2 |
| Smoking pack-years (including never smokers) | ||
| < 20 | 178 | 84.4 |
| ≥ 20 | 20 | 9.5 |
| Unknown | 13 | 6.2 |
| ECOG at diagnosis | ||
| 0 | 78 | 37.0 |
| 1 | 116 | 55.0 |
| 2 | 11 | 5.2 |
| 3 | 5 | 2.4 |
| 4 | 1 | 0.5 |
| Brain metastasis at diagnosis | ||
| No | 156 | 73.9 |
| Yes | 55 | 26.1 |
| Type of mutation | ||
| Exon 21 L858R mutation | 72 | 34.1 |
| Exon 19 deletions | 114 | 54.0 |
| Exon 18 mutations (G719X) | 2 | 1.0 |
| Exon 21 L861Q | 4 | 1.9 |
| T790M mutation | 5 | 2.4 |
| Exon 20 mutations (other than T790M) | 7 | 3.3 |
| Double mutations (other than those containing T790M) | 2 | 1.0 |
| Others | 1 | 0.5 |
| Unknown | 4 | 1.9 |
| First line TKI | ||
| Gefitinib | 192 | 91.0 |
| Erlotinib | 10 | 4.7 |
| Afatinib | 9 | 4.3 |
| Follow-up duration, months | ||
| Median (range) | 10.2 (0–78.0) | |
Baseline characteristics were compared between patients who were never smokers vs. ever smokers and those without brain metastasis at diagnosis vs. those with brain metastasis at diagnosis (S2 and S3 Tables). There was a strong correlation between sex and smoking status (p<0.001, Cramer’s V = 0.576). 93.3% of ever smokers were male. Significant but weak associations were also observed between age and smoking status (p = 0.012, Cramer’s V = 0.173), type of mutation and smoking status (p = 0.044, Cramer’s V = 0.173), age and presence of brain metastasis (p = 0.014, Cramer’s V = 0.169), and ECOG performance status and presence of brain metastasis (p = 0.017, Cramer’s V = 0.181).
The progression free and overall survival of this specific population follows (Tables 2 and 3). Since the ever smokers were mostly male, smoking status was combined with sex into a single variable for multivariate analysis. Males were sub-categorised into never smokers and ever smokers.
Table 2. Univariate analysis of progression free survival and overall survival.
| Variable | No. of events / No. of patients | Median PFS, months (95% CI) | P-value | No. of events / No. of patients | Median OS, months (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Progression Free Survival | Overall Survival | |||||
| All patients | 114 / 210 | 9.2 (8.1–11.2) | 74 / 210 | 19.6 (16.4–23.3) | ||
| Age at diagnosis, years | ||||||
| ≤ 65 | 68 / 128 | 9.1 (6.7–10.8) | 45 / 128 | 19.6 (16.0–26.0) | ||
| > 65 | 46 / 82 | 10.6 (8.2–13.3) | 0.390 | 29 / 82 | 19.0 (14.4–UND) | 0.840 |
| Sex | ||||||
| Female | 72 / 128 | 9.4 (7.6–11.4) | 43 / 128 | 22.0 (17.6–UND) | ||
| Male | 42 / 82 | 9.2 (5.4–14.2) | 0.522 | 31 / 82 | 16.0 (13.7–22.1) | 0.237 |
| Ethnicity | ||||||
| Chinese | 103 / 178 | 8.9 (7.2–10.6) | 66 / 178 | 19.6 (16.0–23.3) | ||
| Others | 11 / 32 | 11.7 (7.4–UND) | 0.093 | 8 / 32 | 28.8 (11.1–UND) | 0.682 |
| Smoking status | ||||||
| Never | 95 / 165 | 9.2 (7.6–11.2) | 59 / 165 | 21.1 (17.1–26.0) | ||
| Ever | 19 / 45 | 11.4 (4.6–16.5) | 0.844 | 15 / 45 | 16.0 (10.2–UND) | 0.452 |
| Smoking status | ||||||
| Never | 95 / 165 | 9.2 (7.6–11.2) | 59 / 165 | 21.1 (17.1–26.0) | ||
| Ex | 15 / 32 | 10.6 (3.7–UND) | 10 / 32 | 22.1 (10.2–UND) | ||
| Current | 4 / 13 | 16.5 (1.4–UND) | 0.792 | 5 / 13 | 16.0 (2.8–UND) | 0.380 |
| Smoking pack-years | ||||||
| < 50 | 102 / 187 | 9.4 (8.2–11.2) | 64 / 187 | 21.1 (17.1–26.0) | ||
| ≥ 50 | 6 / 10 | 4.6 (0.4–UND) | 0.510 | 4 / 10 | 16.0 (6.0–UND) | 0.916 |
| Smoking pack-years | ||||||
| < 20 | 98 / 177 | 9.2 (8.1–11.4) | 61 / 177 | 21.1 (17.1–26.0) | ||
| ≥ 20 | 10 / 20 | 10.6 (3.4–UND) | 0.895 | 7 / 20 | 16.5 (9.3–UND) | 0.906 |
| ECOG at diagnosis | ||||||
| 0–1 | 101 / 193 | 9.7 (8.2–13.1) | 63 / 193 | 22.0 (17.6–26.0) | ||
| 2–4 | 13 / 17 | 5.8 (3.8–10.6) | 0.007 | 11 / 17 | 14.4 (5.8–15.8) | < 0.001 |
| Brain mets at diagnosis | ||||||
| No | 81 / 155 | 10.4 (8.2–12.8) | 47 / 155 | 22.1 (16.4–UND) | ||
| Yes | 33 / 55 | 8.2 (5.7–9.7) | 0.053 | 27 / 55 | 17.9 (10.1–23.3) | 0.029 |
| Location of mutation | ||||||
| Exon 19 | 58 / 115 | 9.7 (8.2–14.5) | 43 / 115 | 17.9 (16.0–22.1) | ||
| Exon 21 | 45 / 77 | 8.3 (5.9–11.6) | 24 / 77 | 28.8 (14.4–UND) | ||
| Others | 10 / 16 | 2.1 (1.4–11.2) | 0.013 | 7 / 16 | 22.0 (5.5–UND) | 0.583 |
| Type of mutation | ||||||
| Exon 19 deletion | 58 / 114 | 9.7 (8.2–14.5) | 43 / 114 | 17.9 (16.0–22.1) | ||
| Exon 21 L858R mutation | 40 / 71 | 9.2 (7.0–11.7) | 20 / 71 | 28.8 (14.7–UND) | ||
| Others | 13 / 21 | 4.6 (1.7–10.6) | 0.019 | 10 / 21 | 15.6 (8.1–UND) | 0.381 |
Table 3. Multivariate analysis of progression free survival and overall survival.
| Variable | No. of events/ No. of patients | Hazard ratio (95% CI) | P-value | No. of events / No. of patients | Hazard ratio (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Progression Free Survival | Overall Survival | |||||
| Overall | 111 / 206 | 0.009 | 73 / 206 | 0.032 | ||
| Age | ||||||
| ≤ 65 | 1 | 1 | ||||
| > 65 | 0.75 (0.49–1.14) | 0.173 | 0.98 (0.59–1.64) | 0.936 | ||
| Gender and smoking status | ||||||
| Female | 1 | 1 | ||||
| Male—never smoked | 1.16 (0.72–1.86) | 0.552 | 1.25 (0.68–2.28) | 0.476 | ||
| Male—ever smoked | 0.74 (0.41–1.34) | 0.327 | 1.11 (0.58–2.14) | 0.756 | ||
| Type of mutation | ||||||
| Exon 19 deletion | 1 | 1 | ||||
| Exon 21 L858R mutation | 1.27 (0.84–1.92) | 0.258 | 0.69 (0.40–1.19) | 0.186 | ||
| Others | 2.94 (1.44–5.99) | 0.003 | 1.03 (0.48–2.22) | 0.940 | ||
| Brain metastasis at diagnosis | ||||||
| No | 1 | 1 | ||||
| Yes | 1.56 (0.99–2.45) | 0.053 | 1.82 (1.07–3.11) | 0.028 | ||
| ECOG at diagnosis | ||||||
| 0–1 | 1 | 1 | ||||
| 2–4 | 2.77 (1.31–5.87) | 0.008 | 4.40 (1.87–10.35) | 0.001 | ||
| Brain metastasis and ECOG interaction | 0.49 (0.14–1.70) | 0.264 | 0.36 (0.09–1.44) | 0.147 | ||
Progression Free Survival of EGFR TKI treated Stage IV Disease
The median PFS of all patients was 9.2 months (95% CI 8.1–11.2 months). Patients with single non-exon 19/21 mutations and double mutations had inferior PFS (p = 0.013) as compared to those with exon 19/21 mutations.
There was no significant difference in the PFS of ever (current and ex-), and never smokers (p = 0.844) nor between heavy smokers and others (p = 0.510 for ≥ 50 pack years, and p = 0.895 for ≥ 20 pack years) (Table 2).
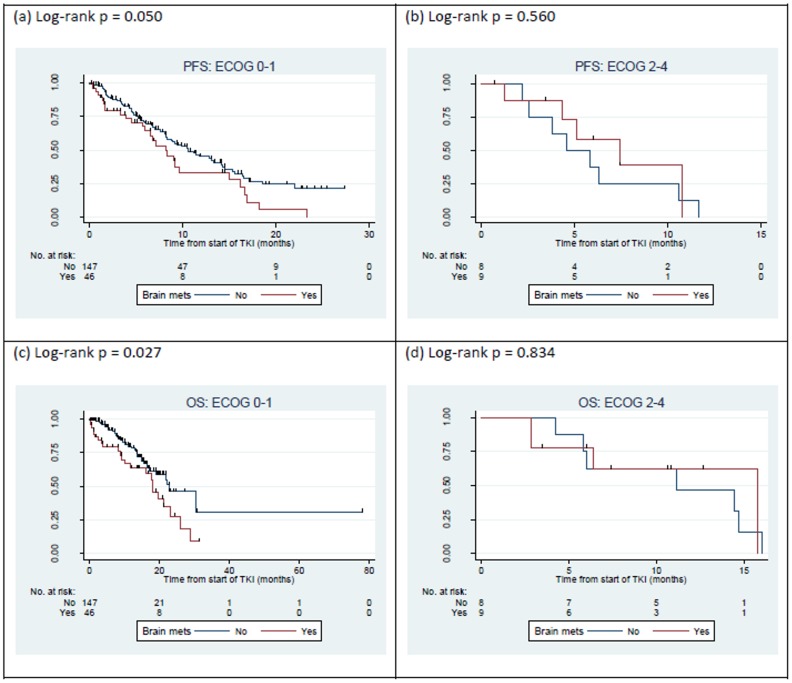
On multivariate analysis, there was a trend for an inferior PFS in patients with brain metastasis at diagnosis (HR 1.56, CI 0.99–2.45, p = 0.053) (Table 3). Kaplan-Meier plots suggested that the presence of brain metastasis did not worsen progression free survival in patients who were ECOG 2–4 (Fig 4). This interaction between brain metastasis and ECOG status was incorporated into the multivariate model.
Fig 4. Kaplan-Meier plots of cohort of 211 patients treated with 1st line EGFR TKI; (a) PFS by brain metastasis in ECOG 0–1 patients, (b) PFS by brain metastasis in ECOG 2–4 patients, (c) OS by brain metastasis in ECOG 0–1 patients, and (d) OS by brain metastasis in ECOG 2–4 patients.
Overall Survival of EGFR TKI treated Stage IV Disease
The overall median OS for all patients was 19.6 months (95% CI 16.4–23.3). The presence of brain metastasis at diagnosis was associated with a significantly worse OS in both univariate (p = 0.029), and multivariate (HR 1.82, CI 1.07–3.11, p = 0.028) analysis. Similar to findings on analysis for progression free survival, Kaplan-Meier plots suggested that the presence of brain metastasis did not worsen overall survival in patients who were ECOG 2–4, and this was incorporated into the multivariate model. The median OS of patients with no brain metastasis at diagnosis was 22.1 months while that of patients with brain metastasis at diagnosis was 17.9 months. Age, sex, ethnicity, smoking status (never vs. ever; never vs ex vs current; pack year < 50 vs pack year ≥ 50; pack year < 20 vs pack year ≥ 20), functional status, location and type of mutations had no significant impact on overall survival (Table 2). Multivariate analysis showed that smoking had no significant impact on overall survival (Table 3).
Subset analyses in defined patient cohorts
Multivariate analysis of progression free survival and overall survival was performed amongst 2 defined patient cohorts to further investigate the effect of confounding factors. The first subset comprised 82 males (40 never smokers and 42 ever smokers) and was examined for the possible impact of smoking status on survival. Due to small sample size, the analysis was adjusted for ECOG status only. There was no evidence that smoking status was related to survival in this analysis (Table 4). A second subset comprised 70 female never smokers aged ≤ 65 with ECOG 0–1 at diagnosis (48 had no brain metastasis at diagnosis and 22 had brain metastasis at diagnosis) and was studied for the impact brain metastasis at diagnosis had on survival. After adjusting for type of mutation, brain metastasis at diagnosis had a significant impact on both progression free survival (HR 2.06, CI 1.03–4.11, p = 0.041) and overall survival (HR 2.86, CI 1.20–6.84, p = 0.018) (Table 5) in this cohort.
Table 4. Multivariate analysis of progression free survival and overall survival in males only.
| Variable | No. of events / No. of patients | Hazard ratio (95% CI) | P-value | No. of events / No. of patients | Hazard ratio (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Progression Free Survival | Overall Survival | |||||
| Overall | 42 / 82 | 0.166 | 31 / 82 | 0.073 | ||
| Smoking status | ||||||
| Never | 1 | 1 | ||||
| Ever | 0.77 (0.41–1.43) | 0.401 | 1.09 (0.54–2.22) | 0.811 | ||
| ECOG at diagnosis | ||||||
| 0–1 | 1 | 1 | ||||
| 2–4 | 2.31 (0.95–5.59) | 0.064 | 3.05 (1.26–7.36) | 0.013 | ||
Table 5. Multivariate analysis of progression free survival and overall survival in female never-smokers aged ≤ 65 with ECOG PS 0–1 at diagnosis.
| Variable | No. of events / No. of patients | Hazard ratio (95% CI) | P-value | No. of events / No. of patients | Hazard ratio (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Progression Free Survival | Overall Survival | |||||
| Overall | 37 / 70 | 0.087 | 24 / 70 | 0.006 | ||
| Type of mutation | ||||||
| Exon 19 deletion | 1 | 1 | ||||
| Exon 21 L858R mutation | 0.98 (0.47–2.03) | 0.958 | 0.28 (0.08–0.98) | 0.047 | ||
| Others | 6.65 (0.74–59.45) | 0.090 | 2.66 (0.73–9.69) | 0.138 | ||
| Brain metastasis at diagnosis | ||||||
| No | 1 | 1 | ||||
| Yes | 2.06 (1.03–4.11) | 0.041 | 2.86 (1.20–6.84) | 0.018 | ||
Discussion
In our study, systematic testing of 762 lung adenocarcinoma for EGFR mutations revealed a prevalence of 61%—regardless of smoking status. Notably, we found that as high as 36% of male and 53% of female patients with a smoking history harboured EGFR mutations, and similar prevalence in males and females amongst never smokers (76.9% vs 75.1%)—underscoring the inadequacy of EGFR mutation testing based on clinical phenotype alone. This is especially relevant as cost-effectiveness of EGFR TKI is driven by patient selection based on mutation status, supporting reflex testing in our patient population regardless of smoking status and gender.
Our cohort of 211 EGFR mutant patients with stage IV NSCLC treated with first line EGFR TKI represents one of the largest to date examining prognostic factors. On multivariate analysis, taking into account EGFR mutations (exon 19 deletions versus L858R) and smoking status, the presence of brain metastasis at diagnosis—comprising 26% of our cohort—was an independent risk factor for worse overall survival, corroborating a recently reported retrospective series [16]. Although clinical activity of EGFR TKIs against intracranial disease has been previously described [17–20], most studies have comprised of modest-sized, heterogeneous and selected patient cohorts. Perhaps the most representative depiction of central nervous system (CNS) activity to date has been a retrospective series of 155 patients, where Heon et al. reported a lower 2-year cumulative risk of brain metastases in patients treated in the first line setting with an EGFR TKI (21%)—majority of whom (89%) received erlotinib—compared to chemotherapy (32%) [21]. In contrast, majority of patients in our study (91%) received gefitinib. It remains to be elucidated if erlotinib might exhibit superior control of intracranial disease due to higher CNS penetration and drug concentrations achieved compared to gefitinib [22]. Additionally, alternate treatment approaches such as pulsed high-dose strategies are actively being explored [23]. Further prospective studies addressing CNS disease are warranted, and should focus on improved delineation of leptomeningeal and cerebral metastases, quantity and quality of CNS disease (extent and symptoms), as well as optimal sequencing of EGFR TKI and radiation. Nevertheless, in the era of TKIs, EGFR M+ ADC patients with brain metastasis at diagnosis achieve a median survival of 17.9 months.
To date, there is limited data on the impact of smoking on progression free survival in patients treated with first line EGFR TKI. In three phase III trials that included up to 38% of ever smokers, subgroup analysis suggested no PFS benefit with TKI over conventional chemotherapy in contrast to those who had never smoked [5–7]. Retrospective studies evaluating the impact of smoking on the survival in EGFR M+ ADC [8, 24–26] have also yielded mixed results with only 1 study suggesting that smoking dosage has an impact on survival (Table 6). In our study of a homogenous cohort of patients with newly diagnosed metastatic EGFR mutation harbouring lung adenocarcinoma treated with EGFR TKI in the 1st line, we found no evidence that smoking status or heavy smoking had a significant impact on PFS and OS, contrary to the recent report by Kim et al [8]. We acknowledge that our sample size was limited and that smoking status was confounded with gender, which we have tried to address. It is noteworthy that majority of smokers in the Korean study received EGFR TKI in the setting of second, third line or beyond, where cumulative toxicities and poor functional status may confound outcomes. The presence or absence of brain metastasis was also not reported in that study.
Table 6. Hazards for survival from univariate analysis of populations with smoking characteristics as indicated across 3 studies do not show any significant differences in survival outcomes, while those from a more recent Korean study show that smoking has a significant impact, especially smoking dosage greater than 30 pack years.
| Study | Smoking Characteristics | Progression Free Survival | Overall Survival | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Rosell et al. [24] | Former vs. Never | 0.72 | 0.39–1.32 | 0.29 | 0.70 | 0.32–1.54 | 0.38 |
| Current vs. Never | 1.65 | 0.69–3.96 | 0.26 | 1.37 | 0.45–4.22 | 0.58 | |
| Paik et al. [23] | Smoker vs. Never | - | - | - | - | - | 0.33 |
| Morita et al. [25] | Smoker vs. Never | 1.08 | 0.60–1.96 | 0.794 | 1.22 | 0.62–2.38 | 0.570 |
| Kim et al. [8] | Ever vs. Never | 1.47 | 1.07–2.03 | 0.018 | 1.73 | 1.20–2.48 | 0.003 |
| <30 pack years vs. Never | 1.15 | 0.77–1.74 | 0.497 | 1.51 | 0.95–2.40 | 0.800 | |
| > 30 pack years vs. Never | 2.03 | 1.35–3.06 | 0.001 | 2.00 | 1.27–3.16 | 0.003 | |
The retrospective nature of our study limits our ability to evaluate on-treatment CNS activity for EGFR TKI, due to irregular frequency and thoroughness of follow-up radiological evaluation. In addition, there may yet be unaccounted bias between those with and without brain metastases, although the commonly reported factors such as performance status, smoking status and type of EGFR mutation were addressed. To our knowledge, this is the first study examining these clinical parameters in a large EGFR M+ patient cohort predominantly treated with first line gefitinib.
In conclusion, our study highlights the importance of reflex molecular testing, where as high as 36% of ever smoker males were found to harbour an EGFR mutation. Furthermore, despite the higher mutational burden in smokers [27], the observation that smoking status did not impact on PFS or OS in patients treated with first line EGFR TKI, suggests a hierarchical relationship in genetic alterations, where dominant truncal events—such as EGFR mutations—remain therapeutically tractable. On the other hand, the presence of brain metastases emerged as an independent negative prognostic factor, and continues to be a therapeutic challenge in EGFR M+ NSCLC.
Supporting Information
EGFR mutations were found in 50–68% of cases. Prior to the implementation of reflex testing, patients were tested for EGFR mutations based on physician discretion. A total of 742 patients underwent EGFR mutation testing. 444 (59.8%) were positive and 289 (38.9%) negative for mutations. 9 cases were unsuccessfully profiled. Hence the ascertainment rate with EGFR sequencing in our centre was 98.8%.
(DOCX)
(DOCX)
(DOCX)
Data Availability
All the data underlying the findings in this study are available on request to the corresponding author and subject to approval for release by the local institutional review board (National Cancer Centre Singapore Institution Representative for SingHealth Centralised Institutional Review Board). Fully anonymised information will be released in accordance to ethical guidelines spelt out by the local institution review board, and according the legal requirements of the Personal Data Protection Act 2012.
Funding Statement
This work is supported in part by a National Medical Research Council Grant (NMRC/1224/2009), National Cancer Centre Research Fund (NRFMP10111-10112). We are also grateful to the Trailblazer Foundation Ltd. and Singapore Millennium Foundation Ltd for their instrumental support to the Lung Cancer Consortium Singapore (NRFTB11122). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mok TS, Wu Y-L, Thongprasert S, Yang C-H, Chu D-T, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009. September 3;361(10):947–57. 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 2. Fukuoka M, Wu Y-L, Thongprasert S, Sunpaweravong P, Leong S-S, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). Journal of Clinical Oncology. 2011. July 20;29(21):2866–74. 10.1200/JCO.2010.33.4235 [DOI] [PubMed] [Google Scholar]
- 3. Zhou C, Wu Y-L, Chen G, Feng J, Liu X-Q, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011. August;12(8):735–42. 10.1016/S1470-2045(11)70184-X [DOI] [PubMed] [Google Scholar]
- 4. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010. June 24;362(25):2380–8. 10.1056/NEJMoa0909530 [DOI] [PubMed] [Google Scholar]
- 5. Mitsudomi T. Erlotinib, gefitinib, or chemotherapy for EGFR mutation-positive lung cancer? Lancet Oncol. 2011. August;12(8):710–1. 10.1016/S1470-2045(11)70194-2 [DOI] [PubMed] [Google Scholar]
- 6. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012. March;13(3):239–46. 10.1016/S1470-2045(11)70393-X [DOI] [PubMed] [Google Scholar]
- 7. Sequist LV, Yang JCH, Yamamoto N, O'Byrne K, Hirsh V, Mok T, et al. Phase III Study of Afatinib or Cisplatin Plus Pemetrexed in Patients With Metastatic Lung Adenocarcinoma With EGFR Mutations. Journal of Clinical Oncology. 2013. July 1. [DOI] [PubMed] [Google Scholar]
- 8. Kim MH, Kim HR, Cho BC, Bae MK, Kim EY, Lee CY, et al. Accepted Manuscript. Lung Cancer. Elsevier Ireland Ltd; 2014. February 3;:1–28. [Google Scholar]
- 9. Mitchell P, Mok T, Barraclough H, Strizek A, Lew R, van Kooten M. Smoking history as a predictive factor of treatment response in advanced non-small-cell lung cancer: a systematic review. Clin Lung Cancer. 2012. July;13(4):239–51. 10.1016/j.cllc.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 10. Dearden S, Stevens J, Wu Y-L, Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Annals of Oncology. 2013. September;24(9):2371–6. 10.1093/annonc/mdt205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tan DSW, Camilleri-Broët S, Tan EH, Alifano M, Lim W-T, Bobbio A, et al. Intertumor heterogeneity of non-small-cell lung carcinomas revealed by multiplexed mutation profiling and integrative genomics. Int J Cancer. 2014. January 31. [DOI] [PubMed] [Google Scholar]
- 12. de Lima Lopes G, Segel JE, Tan DSW, Do YK, Mok T, Finkelstein EA. Cost-effectiveness of epidermal growth factor receptor mutation testing and first-line treatment with gefitinib for patients with advanced adenocarcinoma of the lung. Cancer. 2012. February 15;118(4):1032–9. 10.1002/cncr.26372 [DOI] [PubMed] [Google Scholar]
- 13. Lee VHF, Tin VPC, Choy T-S, Lam K-O, Choi C-W, Chung L-P, et al. Association of exon 19 and 21 EGFR mutation patterns with treatment outcome after first-line tyrosine kinase inhibitor in metastatic non-small-cell lung cancer. J Thorac Oncol. 2013. September;8(9):1148–55. 10.1097/JTO.0b013e31829f684a [DOI] [PubMed] [Google Scholar]
- 14. Shin D-Y, Na II, Kim CH, Park S, Baek H, Yang SH. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol. 2014. February;9(2):195–9. 10.1097/JTO.0000000000000069 [DOI] [PubMed] [Google Scholar]
- 15. Stanic K, Zwitter M, Hitiji NT, Kern I et al. Brain metastasis in lung adenocarcinoma: impact of EGFR mutations status on incidence and survival. Radiol Oncol. 2014. June;48(2):173–183 10.2478/raon-2014-0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lim SH, Lee JY, Sun J-M, Ahn JS, Park K, Ahn M-J. Comparison of Clinical Outcomes Following Gefitinib and Erlotinib Treatment in Non—Small-Cell Lung Cancer Patients Harboring an Epidermal Growth Factor Receptor Mutation in Either Exon 19 or 21. J Thorac Oncol. 2014;9:506–11. 10.1097/JTO.0000000000000095 [DOI] [PubMed] [Google Scholar]
- 17. Porta R, Sánchez-Torres JM, Paz-Ares L, Massuti B, Reguart N, Mayo C, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. European Respiratory Journal. 2011. March;37(3):624–31. 10.1183/09031936.00195609 [DOI] [PubMed] [Google Scholar]
- 18. Jamal-Hanjani M, Spicer J. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of epidermal growth factor receptor-mutant non-small cell lung cancer metastatic to the brain. Clin Cancer Res. 2012. February 15;18(4):938–44. 10.1158/1078-0432.CCR-11-2529 [DOI] [PubMed] [Google Scholar]
- 19. Park SJ, Kim HT, Lee DH, Kim KP, Kim SW, Suh C, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer. 2012. September;77(3):556–60. 10.1016/j.lungcan.2012.05.092 [DOI] [PubMed] [Google Scholar]
- 20. Wu Y-L, Zhou C, Cheng Y, Lu S, Chen GY, Huang C, et al. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG-0803). Annals of Oncology. 2013. April;24(4):993–9. 10.1093/annonc/mds529 [DOI] [PubMed] [Google Scholar]
- 21. Heon S, Yeap BY, Lindeman NI, Joshi VA, Butaney M, Britt GJ, et al. The Impact of Initial Gefitinib or Erlotinib versus Chemotherapy on Central Nervous System Progression in Advanced Non-Small Cell Lung Cancer with EGFR Mutations. Clin Cancer Res. 2012. August 15;18(16):4406–14. 10.1158/1078-0432.CCR-12-0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Togashi Y, Masago K, Masuda S, Mizuno T, Fukudo M, Ikemi Y, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2012. September;70(3):399–405. 10.1007/s00280-012-1929-4 [DOI] [PubMed] [Google Scholar]
- 23. Grommes C, Oxnard GR, Kris MG, Miller VA, Pao W, Holodny AI, et al. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro-oncology. 2011. December;13(12):1364–9. 10.1093/neuonc/nor121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paik PK, Johnson ML, D'Angelo SP, Sima CS, Ang D, Dogan S, et al. Driver mutations determine survival in smokers and never-smokers with stage IIIB/IV lung adenocarcinomas. Cancer. 2012. December 1;118(23):5840–7. 10.1002/cncr.27637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009. September 3;361(10):958–67. 10.1056/NEJMoa0904554 [DOI] [PubMed] [Google Scholar]
- 26. Morita S, Okamoto I, Kobayashi K, Yamazaki K, Asahina H, Inoue A, et al. Combined survival analysis of prospective clinical trials of gefitinib for non-small cell lung cancer with EGFR mutations. Clin Cancer Res. 2009. July 1;15(13):4493–8. 10.1158/1078-0432.CCR-09-0391 [DOI] [PubMed] [Google Scholar]
- 27. Govindan R, Ding L, Griffith M, Subramanian J, Dees ND, Kanchi KL, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012. September 14;150(6):1121–34. 10.1016/j.cell.2012.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
EGFR mutations were found in 50–68% of cases. Prior to the implementation of reflex testing, patients were tested for EGFR mutations based on physician discretion. A total of 742 patients underwent EGFR mutation testing. 444 (59.8%) were positive and 289 (38.9%) negative for mutations. 9 cases were unsuccessfully profiled. Hence the ascertainment rate with EGFR sequencing in our centre was 98.8%.
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All the data underlying the findings in this study are available on request to the corresponding author and subject to approval for release by the local institutional review board (National Cancer Centre Singapore Institution Representative for SingHealth Centralised Institutional Review Board). Fully anonymised information will be released in accordance to ethical guidelines spelt out by the local institution review board, and according the legal requirements of the Personal Data Protection Act 2012.