Abstract
Reported here is a new concept and its practical implementation that involves the novel utilization of open metal sites (OMS) for architectural pore design. Specifically, it is shown here that OMS can be used to run extended hooks (isonicotinate in this work) from the framework wall to channel centers to effect the capture of single metal ions or clusters, with the concurrent partition of the large channel space into multiple domains, alteration of host-guest charge relationship and associated guest-exchange properties, as well as the transfer of OMS from the wall to the channel centers. The concept of the extended hook, demonstrated here in the multi-component dual-metal and dual-ligand system, should be generally applicable to a range of framework types.
The success in the development of crystalline porous materials (CPM) is attributable to the ingenious use of both inorganic and organic building blocks to establish the extended frameworks with various compositions and topologies.1–5 At an even higher level of the materials design, the embedment of metal ions within polymeric matrices or supramolecular assemblies makes it possible to introduce functional metal sites whose activity can be intricately regulated by the host. There has long been an interest in introducing secondary metal sites into porous metal-organic frameworks.6–9 In the past decade, in addition to metalloporphyrin,8 other metalloligands such as Salen complexes have been used as the crosslinking ligands in the construction of metal-organic frameworks (MOFs).9 Such ligands have peripheral donor atoms for the framework formation, as well as interior donor sites for metal capture. In general, only a single metal ion is trapped within the core of metalloligands, as clearly shown by porphyrin complexes.
Recently, we revealed a strategy for encapsulating both single metal ions and di- or trimeric clusters into MOFs. With this approach, a trifunctional ligand, 1,3,5-benzenetricarboxylate (BTC) in particular, uses two -COO groups to form the 3D framework while resorting to the third -COO group (called the hook) for metal capture.10 The BTC method is fundamentally different from the metalloligand method, because it is the cooperative action of multiple ligands (instead of multiple donor atoms from a single ligand) with 2, 3, or 4 hooks (one hook per ligand) that results in the capture of metal ions/clusters. Still, both the BTC method and the metalloligand method rely on specific features of framework organic ligands for metal capture (i.e., they have more functional groups than needed for the framework formation). As such, the strategies based on BTC and metalloligands are unusable for many common ligands (e.g., bifunctional ligands used in the synthesis of well-known MOFs such as MOF-5, MIL-88, and MIL-101) that are devoid of spare functional group after the framework formation.
Here we propose a new strategy (called the extended hook method) that makes it possible to construct MOFs with hooks from bifunctional ligands and thereby removes the intrinsic limitations of either the BTC or metallologand method, in terms of the need for polyfunctional ligands. The essence of our new method is to have hooks coming from the inorganic nodes, in contrast with those located on framework crosslinking ligands such as BTC or porphyrin. As a result, this method for the hook incorporation is in principle independent of the framework organic ligands. Clearly, it is possible to devise various ways for the anchoring of hooks onto inorganic nodes in the framework wall, considering the diversity of inorganic nodes. In this work, we take advantage of a commonly observed feature in MOFs, open metal sites, to add an auxiliary ligand (called the extended hook here, because it is much longer than the –COO hook on BTC) whose pyridal end is anchored to the open metal sites on the framework, while its carboxyl end serves as the lengthened hook to capture metal ions or clusters at the centers of channels. We anticipate that this extended hook method can be tailored to different MOFs by matching the length of the hook with the radius of cages or channels in MOFs.
Here, we introduce four new hexagonal-channel-based porous materials CPM-4, CPM-30, CPM-31, and CPM-32 (Table 1) to illustrate our proposed concept and demonstrate its feasibility in new materials design. We start our discussion with CPM-4 (made with the BTC method) which serves to contrast the new method with our previously reported BTC method. Especially highlighted here by CPM-4 is the conceptual transition from the “short hook” in CPM-4 into the “long extended hook” in CPM-31/32 which necessitates the drastic revision in the synthesis strategy and broadens the application of the hook concept to more ligands and framework types. Furthermore, CPM-4 is significant in its own right because it is the very first member of an infinite series of hexagonal MOFs that can capture metal clusters within their size-tunable channels. The entire series can be geometrically derived by progressively adding one 4-ring between two adjacent 6-rings (Figure S1). The next two members after CPM-4 are based on 12-ring AlPO4-5 (Zeolite-type: AFI) and 18-ring VPI-5 (Zeolite-type: VFI), two of the best-known zeolite-type topologies.
Table 1.
A Summary of Crystal Data and Refinement Results.
| Name | Formula[a] | Sp. Gr. | a/b(Å) | c (Å) | α/β (°) | γ (°) | R(F) |
|---|---|---|---|---|---|---|---|
| CPM-4 | [NH2(CH3)2]2[In3(BTC)5][Co2(DMF)6]·solvent | P-62c | 18.1997(8) | 20.0204(10) | 90 | 120 | 0.0380 |
| CPM-30 | [In3O(BBDC)3(H2O)3]·NO3·solvent | P-31c | 20.8131(10) | 23.1850(2) | 90 | 120 | 0.0296 |
| CPM-31 | [In3O(BBDC)3(INT)3][Zn(H2O)]·solvent | P-31c | 18.7843(8) | 25.0267(11) | 90 | 120 | 0.0381 |
| CPM-32 | [In3O(BBDC)3(INT)3][Co2(OH)(H2O)2]·NO3·solvent | P-31c | 18.8658(4) | 24.6975(13) | 90 | 120 | 0.0576 |
H3BTC =1,3,5-benzenetricarboxylic acid; H2BBDC = 4,4′-biphenyl-dicarboxylic acid; HINT = isonicotic acid.
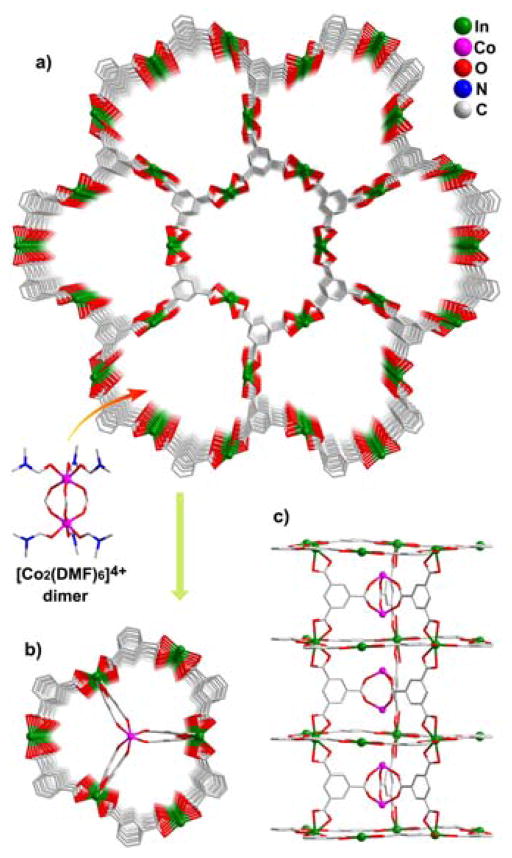
CPM-4 was synthesized by following the BTC method in the heterometallic system. It has a 3D indium-BTC framework with 1D hexagonal channels along the c-axis. Its framework can be described as 2D 3-connected honeycomb (graphite-like) indium-BTC layers pillared by interlayer BTC linkers (Figure 1). Its most fascinating feature is the unique bonding feature of each and every interlayer BTC which only uses two -COO groups for pillaring, leaving the third -COO group free to serve as “hook” for capturing cobalt dimer [Co2(RCO2)3(DMF)6]+ (Figure 1b,1c).
Figure 1.
An illustration of CPM-4. a) View of 3D Indium-BTC framework with 1D hexagonal channels along the c-axis. b) and c)
In CPM-4, metal clusters are captured at the centers of hexagonal channels, because the radius of the channel in CPM-4 matches well with the short –COO hook. It is easy to visualize that when the channel size becomes bigger, the -COO group from BTC wouldn’t be long enough to reach the centers of channels, in which case, metal clusters are captured near the wall of the channels as reported in CPM-16.10b However, as indicated in the introduction, the mode of capture shown by CPM-16 does not apply to MOFs made from bifunctional ligands that lack “spare” functional groups. For large channels, the extended hook method, reported here, offers a new and versatile mechanism for the metal capture.
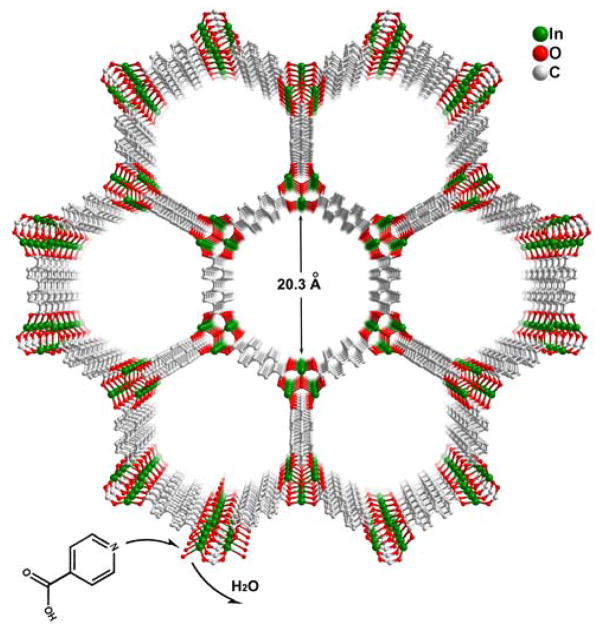
Next we present CPM-30 which is a porous host framework with open metal sites, but prior to the introduction of the extend hooks. CPM-30, made from 4,4′-biphenyldicarboxylic acid (H2bpdc), is a new indium MOF adopting the MIL-88-type framework. It exhibits a 3D framework with uniform 1D nano-sized hexagonal channels along the c-axis with the maximal window size of 20.3 Å (atom to atom distance) (Figure 2). In addition to its highly porous framework, a key feature of CPM-30 is that each trimeric [In3O(RCO2)6(H2O)3]+ node has three open metal sites coordinated by a total of three water molecules. Most important for this work is that these open metal sites point towards the centers of channels at the same height along the channels. Such alignment of open metal sites hinted at the possibility of introducing extended hooks for trapping metal ions and clusters at the centers of large channels.
Figure 2.
a) View of 3D structure of CPM-30 along the c-axis. Terminal water ligands on the indium trimers are omitted for clarity.
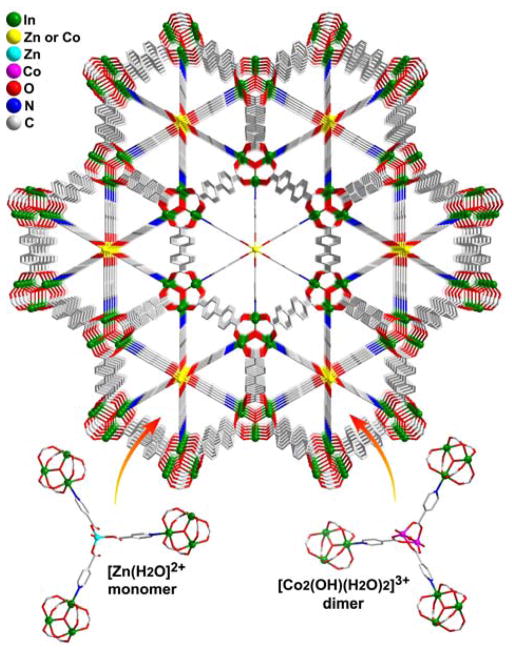
Indeed, with the introduction of both secondary metal ions (Zn2+ as well as Co2+) and the auxiliary ligand (isonicotinic acid, Hint), the linear int ligands were successfully anchored onto the open metal sites of In3O trimeric nodes through its N end, while its carboxyl end stretches all the way towards the centers of channels to grab metal ions/clusters. In this work, either single zinc ions or cobalt dimeric clusters could be encapsulated into channels, giving rise to two new compounds CPM-31 and CPM-32 (Figure 3). Such capture mode by ligands leaves open the metal coordination sites (on Zn2+ and Co2+) pointed along the direction of channels. These sites are occupied by solvent molecules.
Figure 3.
View of 3D structure of CPM-31/32 along the c-axis. Terminal water ligands on the indium trimers are omitted for clarity.
Within the hexagonal channels of CPM-31, a total of three -COO hooks (one per int ligand), positioned adjacent to the channel center on three sides, conspire to immobilize a Zn2+ ion. The fourth site on the tetrahedrally bonded Zn2+ ion is occupied by one terminal water ligand. If cobalt is used in place of zinc, a paddlewheel [Co2(OH)]3+ dimer is captured within the channels of CPM-32, reflecting the usually greater tendency of Co2+ to be non-tetrahedral. Each cobalt ion adopts trigonal bipyramidal configuration with equatorial plane occupied by three carboxylic oxygen atoms and two apical positions occupied by one OH group and one water terminal ligand (Figure 3).
The above results with CPM-30/31/32 show that the extended hook method is capable of inserting secondary inorganic nodes with different configurations, nuclearity, and charge (e.g., Zn2+ vs. [Co2(OH)]3+) into channels of MOFs. This has a profound impact on both geometrical and chemical features. First, the ability to alter framework charge properties by inserting charge-tunable metal ions/clusters in a crystallographically ordered fashion is of special interest, but still remains a great challenge in crystal engineering. The demonstrated capability of the extended hook method to introduce metal ions/clusters with different overall charge offers a feasible route for adjusting framework charge properties. For example, through the incorporation of anionic [Zn(RCO2)3(H2O)]− monomer, the original cationic framework of CPM-30 can be rendered into a neutral framework of CPM-31. Conversely, the replacement of anionic [Zn(RCO2)3(H2O)]− monomer with neutral [Co2(OH)(RCO2)3(H2O)2] dimer returns the framework back into the cationic one in CPM-32.
Furthermore, the pore space partition of highly porous MOFs into multiple domains is of increasing interest, especially for applications involving small guest molecules or ions (e.g., H2 and CO2).11 In this work, we demonstrate that the pore space partition through the extended hook method can introduce size-selective ion-exchange property. Small inorganic anion MnO4-, medium-sized anionic dye AO7− (Acid Orange 7) and large organic dye MB2− (Methyl Blue) (Figure S17) were chosen as anionic guests for size-selective ion-exchange studies, which were performed in a closed system by immersing CPM-30 or CPM-32 in organic solutions of different anions with a MOF:dye molar ratio of 10:1. During ion-exchange, all solutions were kept still for 24 hours. The results show that CPM-30 with large nano-sized hexagonal channels can readily undergo anion-exchange with different-sized anions ranging from small MnO4− to medium-sized AO7− and to large MB2−. Both UV-vis data and the color changes of the solutions before and after ion-exchange indicate that these different-sized anions can be completely exchanged from their solutions into the framework of CPM-30 (Figure S19-S21). On the other hand, while CPM-30 exhibits excellent ion-exchange ability, it lacks size-selectivity in the size range represented by these three anions due to its large channel size. In comparison, the large hexagonal channels in CPM-30 are divided into multiple smaller domains in CPM-32 while retaining the same cationic framework. The ion exchange with CPM-32 indicated that the small inorganic anion MnO4− can be completely exchanged into the framework of CPM-32. However, even after CPM-32 was immersed in either AO7− or MB2− solution for one week, no color changes of these solutions were observed. Further UV-vis measurements confirmed that even medium sized AO7− anion could not be exchanged into the framework of CPM-32. The above experiments showed the feasibility to tune the pore size in this series of cationic materials so that they may be selectively responsive to anions of different size.
In summary, a new concept called the extended hook is introduced here and it has formed the basis of a synthetic method capable of creating novel chemical and topological features in porous framework materials. Specifically, the apparent complex multi-component systems involving dual metals and dual ligands undergo hierarchical crystallization in which one metal-ligand combination forms the primary porous framework while the auxiliary ligand serves as long hooks to immobilize select metal ions or clusters. It is well-known that multi-component systems have a great potential in new materials design. However, to make better and more effective use of such complex systems, new synthetic and structural concepts are needed. Given multiple competing crystallization processes and more or less unpredictable nature of the multi-component systems, the delegation of a different role to each component, by employing metal ions and ligands with complementarity in charge, size and shape, and coordination geometry, and so on, is among the most feasible routes to make complex systems more manageable and to create new materials with previously unseen features. The work reported here is a demonstration of this type of synthesis strategies.
Supplementary Material
Acknowledgments
We thank the support of this work by the National Science Foundation under Award No. DMR-0846958. Acknowledgement is made to the donors of the ACS Petroleum Research Fund for partial support of a portion of the synthetic studies (X. B. 50635-UR10). X. B is a Henry Dreyfus Teacher Scholar.
Footnotes
Supporting Information. Experimental details, TGA, IR, XRD, cif files, additional tables and figures. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.a) Férey G, Serre C. Chem Soc Rev. 2009;38:1380. doi: 10.1039/b804302g. [DOI] [PubMed] [Google Scholar]; b) Farha OK, Hupp JT. Acc Chem Res. 2010;43:1166. doi: 10.1021/ar1000617. [DOI] [PubMed] [Google Scholar]; c) Kreno LE, Leong K, Farha OK, Allendorf M, Duyne RPV, Hupp JT. Chem Rev. 2012;112:1105. doi: 10.1021/cr200324t. [DOI] [PubMed] [Google Scholar]; d) O’Keeffe M, Yaghi OM. Chem Rev. 2012;112:675. doi: 10.1021/cr200205j. [DOI] [PubMed] [Google Scholar]; e) Cohen SM. Chem Rev. 2012;112:970. doi: 10.1021/cr200179u. [DOI] [PubMed] [Google Scholar]
- 2.a) O’Keeffe M, Peskov MA, Ramsden SJ, Yaghi OM. Acc Chem Res. 2008;41:1782. doi: 10.1021/ar800124u. [DOI] [PubMed] [Google Scholar]; b) Caskey SR, Wong-Foy AG, Matzger AJ. J Am Chem Soc. 2008;130:10870. doi: 10.1021/ja8036096. [DOI] [PubMed] [Google Scholar]; c) Perry JJ, IV, Perman JA, Zaworotko MJ. Chem Soc Rev. 2009;38:1400. doi: 10.1039/b807086p. [DOI] [PubMed] [Google Scholar]; d) Park TH, Hickman AJ, Koh K, Martin S, Wong-Foy AG, Sanford MS, Matzger AJ. J Am Chem Soc. 2011;133:20138. doi: 10.1021/ja2094316. [DOI] [PubMed] [Google Scholar]; e) Wu H, Gong Q, Olson DH, Li J. Chem Rev. 2012;112:836. doi: 10.1021/cr200216x. [DOI] [PubMed] [Google Scholar]
- 3.a) Hwang YK, Hong DY, Chang JS, Jhung SH, Seo YK, Kim J, Vimont A, Daturi M, Serre C, Férey G. Angew Chem Int Ed. 2008;47:4144. doi: 10.1002/anie.200705998. [DOI] [PubMed] [Google Scholar]; b) Morris RE, Wheatley PS. Angew Chem Int Ed. 2008;47:4966. doi: 10.1002/anie.200703934. [DOI] [PubMed] [Google Scholar]; c) Morris RE. Nat Chem. 2011;3:347. doi: 10.1038/nchem.1036. [DOI] [PubMed] [Google Scholar]; d) Mohideen MIH, Xiao B, Wheatley PS, McKinlay AC, Li Y, Slawin AMZ, Aldous DW, Cessford NF, Dueren T, Zhao X, Gill R, Thomas KM, Griffin JM, Ashbrook SE, Morris RE. Nat Chem. 2011;3:304. doi: 10.1038/nchem.1003. [DOI] [PubMed] [Google Scholar]; e) Sumida K, Rogow DL, Mason JA, McDonald TM, Bloch ED, Herm ZR, Bae TH, Long JR. Chem Rev. 2012;112:724. doi: 10.1021/cr2003272. [DOI] [PubMed] [Google Scholar]
- 4.a) Yang S, Sun J, Ramirez-Cuesta AJ, Callear SK, David WIF, Anderson DP, Newby R, Blake AJ, Parker JE, Tang CC, Schröder M. Nat Chem. 2012;4:887. doi: 10.1038/nchem.1457. [DOI] [PubMed] [Google Scholar]; b) Wriedt M, Yakovenko AA, Halder GJ, Prosvirin AV, Dunbar KR, Zhou HC. J Am Chem Soc. 2013;135:4040. doi: 10.1021/ja312347p. [DOI] [PubMed] [Google Scholar]; c) Li JR, Sculley J, Zhou HC. Chem Rev. 2012;112:869. doi: 10.1021/cr200190s. [DOI] [PubMed] [Google Scholar]; d) Dau PV, Kim M, Cohen SM. Chem Sci. 2013;4:601. [Google Scholar]
- 5.a) Wong-Foy AG, Lebel O, Matzger AJ. J Am Chem Soc. 2007;129:15740–15741. doi: 10.1021/ja0753952. [DOI] [PubMed] [Google Scholar]; b) Zheng ST, Zhang J, Yang GY. Angew Chem Int Ed. 2008;47:3909. doi: 10.1002/anie.200705709. [DOI] [PubMed] [Google Scholar]; c) Xiong WW, Athersh EU, Ng YT, Ding J, Wu T, Zhang Q. J Am Chem Soc. 2013;135:1256. doi: 10.1021/ja3116179. [DOI] [PubMed] [Google Scholar]; d) Kim M, Cahill JF, Fei H, Prather KA, Cohen SM. J Am Chem Soc. 2012;134:18082. doi: 10.1021/ja3079219. [DOI] [PubMed] [Google Scholar]; e) Lin HY, Chin CY, Huang HL, Huang WY, Sie MJ, Huang LH, Lee YH, Lin CH, Lii KH, Bu X, Wang SL. Science. 2013;339:811. doi: 10.1126/science.1232097. [DOI] [PubMed] [Google Scholar]
- 6.a) Deng H, Doonan CJ, Furukawa H, Ferreira RB, Towne J, Knobler CB, Wang B, Yaghi OM. Science. 2010;327:846. doi: 10.1126/science.1181761. [DOI] [PubMed] [Google Scholar]; b) Kent CA, Mehl BP, Ma L, Papanikolas JM, Meyer TJ, Lin W. J Am Chem Soc. 2010;132:12767. doi: 10.1021/ja102804s. [DOI] [PubMed] [Google Scholar]; c) Coskun A, Hmadeh M, Barin G, Gándara F, Li Q, Choi E, Strutt NL, Cordes DB, Slawin AMZ, Stoddart JF, Sauvage JP, Yaghi OM. Angew Chem Int Ed. 2012;51:2160. doi: 10.1002/anie.201107873. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Horike S, Kamitsubo Y, Inukai M, Fukushima T, Umeyama D, Itakura T, Kitagawa S. J Am Chem Soc. 2013;135:4612. doi: 10.1021/ja3122872. [DOI] [PubMed] [Google Scholar]
- 7.a) Yang S, Lin X, Blake1 AJ, Walker GS, Hubberstey P, Champness NR, Schröder M. Nat Chem. 2009;1:487. doi: 10.1038/nchem.333. [DOI] [PubMed] [Google Scholar]; b) Xie Z, Ma L, DeKrafft KE, Jin A, Lin W. J Am Chem Soc. 2010;132:922. doi: 10.1021/ja909629f. [DOI] [PubMed] [Google Scholar]; c) Zhang Z, Zhang L, Wojtas L, Eddaoudi M, Zaworotko MJ. J Am Chem Soc. 2012;134:11936. doi: 10.1021/ja208256u. [DOI] [PubMed] [Google Scholar]; d) Yang XL, Xie MH, Zou C, He Y, Chen B, O’Keeffe M, Wu CD. J Am Chem Soc. 2012;134:10638. doi: 10.1021/ja303728c. [DOI] [PubMed] [Google Scholar]; e) Zhu C, Yuan G, Chen X, Yang Z, Cui Y. J Am Chem Soc. 2012;134:8058. doi: 10.1021/ja302340b. [DOI] [PubMed] [Google Scholar]
- 8.a) Kahn O. Acc Chem Res. 2000;33:647. doi: 10.1021/ar9703138. [DOI] [PubMed] [Google Scholar]; b) Meng W, Breiner B, Rissanen K, Thoburn JD, Clegg JK, Nitschke JR. Angew Chem Int Ed. 2011;50:3479. doi: 10.1002/anie.201100193. [DOI] [PubMed] [Google Scholar]; c) Wang XS, Chrzanowski M, Wojtas L, Chen YS, Ma S. Chem Eur J. 2013;19:3297. doi: 10.1002/chem.201204358. [DOI] [PubMed] [Google Scholar]
- 9.a) Cho SH, Ma B, Nguyen ST, Hupp JT, Albrecht-Schmitt TE. Chem Commun. 2006:2563. doi: 10.1039/b600408c. [DOI] [PubMed] [Google Scholar]; b) Song F, Wang C, Falkowski JM, Ma L, Lin W. J Am Chem Soc. 2010;132:15390. doi: 10.1021/ja1069773. [DOI] [PubMed] [Google Scholar]; c) Das MC, Xiang S, Zhang Z, Chen B. Angew Chem Int Ed. 2011;50:10510. doi: 10.1002/anie.201101534. [DOI] [PubMed] [Google Scholar]
- 10.a) Zheng ST, Wu T, Zuo F, Chou C, Feng P, Bu X. J Am Chem Soc. 2012;134:1934. doi: 10.1021/ja209800x. [DOI] [PubMed] [Google Scholar]; b) Zheng ST, Mao C, Wu T, Lee S, Feng P, Bu X. J Am Chem Soc. 2012;134:11936. doi: 10.1021/ja305181y. [DOI] [PubMed] [Google Scholar]
- 11.a) Ma L, Lin W. Angew Chem Int Ed. 2009;48:3637. doi: 10.1002/anie.200806227. [DOI] [PubMed] [Google Scholar]; b) Zheng ST, Bu JT, Li Y, Wu T, Zuo F, Feng P, Bu X. J Am Chem Soc. 2010;132:17062. doi: 10.1021/ja106903p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.