Abstract
Preeclampsia is a leading cause of maternal and fetal morbidity and mortality. Bb, the active fragment of complement factor B (fB), has been reported to be a predictor of preeclampsia. However, conflicting results have been found by some investigators. We hypothesized that the disagreement in findings may be due to the racial/ethnic differences among various study groups, and that fB activation is significant in women of an ethnic minority with preeclampsia. We investigated the maternal and fetal levels of Bb (the activated fB fragment) in pregnant women of an ethnic minority with or without preeclampsia. We enrolled 291 pregnant women (96% of an ethnic minority, including 78% African–American). Thirteen percent of these were diagnosed with preeclampsia. Maternal venous blood was collected from all participants together with fetal umbilical cord blood samples from 154 deliveries in the 291 women. The results were analyzed using the Mann–Whitney U test and multivariate analyses. Maternal Bb levels were significantly higher in the preeclamptic group than in the nonpreeclamptic group. Levels of Bb in fetal cord blood were similar in both groups. Subgroup analyses of African–American patients’ results confirmed the study hypothesis that there would be a significant increase in Bb in the maternal blood of the preeclamptic group and no increase in Bb in the fetal cord blood of this group. These results suggest that a maternal immune response through complement fB might play a role in the development of preeclampsia, particularly in African–American patients.
Keywords: complement, preeclampsia, pregnancy
1. Introduction
Preeclampsia is a leading cause of maternal and fetal morbidity and mortality. Its cause remains poorly understood and is likely multifactorial (reviewed in depth elsewhere) (Ahn et al. 2011, Robillard et al. 2014). Briefly, deficient trophoblastic invasion of the uterine spiral arteries results in inadequate placentation, leading to placental hypoxia and ischemia (Roberts and Bell 2013, Saito and Nakashima 2014). These events, in turn, alter the maternal immune system, including activation of immune cells (e.g., NK cells, dendritic cells, neutrophils, B and T lymphocytes), releasing lipid peroxidation by-products, anti-angiogenic, autoimmune, and proinflammatory factors (e.g., sFlt-1, s-endoglin, AT1-AA, aPL, TNF-α, IL-1β, IL-6, IL-10, IL-17, IL-18, and microRNA) (Ahn et al. 2011, Robillard et al. 2014). The above factors are associated with maternal endothelial dysfunction and produce a persistent condition of systemic inflammation that eventually manifests as the renal dysfunction and hypertension of preeclampsia.
In addition to the factors described above, there is accumulating evidence that complement components are involved in the local and systemic inflammation of preeclampsia (Lynch and Salmon 2010, Girardi et al. 2011, Denny et al. 2013b).
Complement activation in general can be initiated through the classical pathway (antibodies, C1q, C2, C4), the alternative pathway (spontaneous C3 hydrolysis, factor B [fB]), and the lectin pathway (MBL, ficolins). The three pathways converge at the formation of C3 convertase. Amplification by C3 convertases occurs only via the fB-dependent alternative pathway. C3 convertases yield C3a and additional C3b, the latter forming C5a convertases that cleave C5 to C5a and C5b. C5b initiates formation of the cell membrane attack complex (C5b–C9).
Earlier studies have indicated that systemic activation of C3 in the common pathway is associated with preeclampsia. Activated C3 fragments, namely C3a and C3d, have been detected in the circulation of preeclamptic patients (Haeger et al. 1989, Boij et al. 2012), although some investigators have found no significant change in C3a levels (Soto et al. 2010, Burwick et al. 2013, Denny et al. 2013a). A recent study found that elevated circulating C3a in the first trimester of pregnancy is an independent predictive factor for preeclampsia (Lynch et al. 2011). Local C3 deposition in the placentas of preeclamptic patients is significantly elevated compared with normotensive controls (Wang et al. 2012). Treatment with C3 inhibitors (CR2-Crry and sCR1) decreased levels of indicators of preeclampsia in rodent models (Qing et al. 2011, Lillegard et al. 2013).
Systemic activation of C5, the factor immediately downstream of C3, occurs unequivocally, as demonstrated by an increase in C5a in the circulation and urine in preeclamptic patients that returns to normal one week after delivery (Haeger et al. 1989, Soto et al. 2010, Burwick et al. 2013, Denny et al. 2013a). Local deposition of the C5-9 complex, found in placentas from preeclamptic patients (Rampersad et al. 2008), may directly lead to laminar necrosis of placental membranes (Stanek and Al-Ahmadie 2005). A recent case report, with potentially far-reaching clinical implications, found that anti-C5 treatment of a patient with severe preeclampsia was able to prolong pregnancy for 17 days with no apparent untoward consequences (Burwick and Feinberg 2013).
The involvement of a particular complement pathway upstream of the C3 common cascade in preeclampsia is less clear. An early study in the 1990s suggested that the alternative pathway is involved in preeclampsia in patients with systemic lupus erythematosus (Abramson and Buyon 1992). More recently, Lynch et al. studied a large cohort of pregnant women and showed that maternal blood levels of Bb, the active fragment of complement fB, may be a predictor of preeclampsia in early pregnancy (Lynch et al. 2008, 2010). Consistent with this, colleagues of Dr. Lynch recently reported, in a cross-sectional study of 24 severe preeclampsia patients and 20 controls, that the levels of Bb increased in the maternal and cord blood of the preeclampsia patients (Hoffman et al. 2014). However, conflicting results were obtained in another crosssectional study, indicating that there was no significant difference in Bb levels among preeclamptic patients, healthy pregnant women, and healthy nonpregnant women (Derzsy et al. 2010). Derzsy’s group further suggested that lectin complement factors were not involved in preeclampsia, but that the classical complement pathway might play a role (Csuka et al. 2010).
One possible explanation for these discrepancies is that racial/ethnic differences in the various study groups affect the predisposition for preeclampsia. It is well established that among all racial groups, pregnant African–American women are at the highest risk of preeclampsia (Zhang et al. 2013). The subjects in Lynch’s study were non-Hispanic Caucasian, 65%; Hispanic Caucasian, 21%; African–American, 7% (Lynch et al. 2008, 2010). In Hoffman’s study (with Dr. Lynch) (Hoffman et al. 2014), which also showed that that the levels of Bb increased in the maternal and cord blood of the preeclampsia patients, 15 of the 24 preeclampsia patients were from ethnic minorities (2 African–American and 9 Hispanic), and 11 of the 20 controls were from an ethnic minority (4 African–American and 7 Hispanic). In contrast, the subjects in Derzsy’s study (Derzsy et al. 2010) were all Caucasian (personal communication from the corresponding author, Dr. Molvarec).
We thus hypothesized that fB activation might be significant in pregnant women of an ethnic minority with preeclampsia, particularly African–Americans. It has been reported that African– American pregnant women in New York City have the highest rate of preeclampsia in New York State (Tanaka et al. 2007). We investigated the activation of fB by measuring levels of its activated species, Bb, in a cohort of mostly African–American pregnant women in New York City and in the cord blood of the neonates of these women. The relationship between these measurements and preeclampsia was evaluated.
2. Materials and Methods
2.1. Patient enrollment and blood sampling
This cross-sectional study was approved by the Institutional Review Boards of SUNY Downstate Medical Center, University Hospital of Brooklyn at Long Island College Hospital and Lutheran Medical Center. A total of 291 pregnant women consented to participate in the study (written consent was obtained for each patient) and were enrolled consecutively. All women aged 16 years and older who were admitted to labor and delivery were eligible for the study. The exclusion criterion was to be less than 16 years of age. Maternal venous blood was collected from participants upon admission to hospital for delivery. Cord blood samples were collected from the neonates of 154 participants after delivery of the placentas (cord blood samples were not collected from the neonates of the remaining patients, mostly because of emergency or late night delivery when research team members were unavailable). Relevant demographic information was obtained (ethnicity/race was self-reported; Table 1). Preeclampsia was diagnosed and treated according to the guidelines of The American College of Obstetricians and Gynecologists (2013) by obstetricians during admission for delivery. From the time of inclusion in the study, relevant clinical data were entered into a secure database. The accuracy of the diagnoses and treatment information of the enrolled patients was reviewed by at least two investigators of the study.
Table 1.
Demographics and characteristics of normal pregnancies and pregnancies complicated by preeclampsia
| Total | Normal | Preeclamptic | |
|---|---|---|---|
| Numbers of patients | 291 | 253 | 38 |
| Race/ethnicity | |||
| African–American | 228 (78%) | 194 (77%) | 34 (89%) |
| Caucasian | 12 (4%) | 11 (4%) | 1 (3%) |
| Hispanic | 29 (10%) | 26 (10%) | 3 (8%) |
| Other minorities | 22 (8%) | 22 (9%) | 0 |
| Age ≥ 35 years | 69 (24%) | 62 (32%) | 7 (19%) |
| Primiparity | 68 (23%) | 57 (22%) | 11 (30%) |
| Mean gestational age (weeks)* | 38 ± 3 | 38 ± 3 | 37 ± 4 |
| Preterm birth (<37 weeks) | 49 (17%) | 33 (13%) | 16 (42%) |
| Caesarean section delivery | 148 (51%) | 130 (51%) | 18 (47%) |
| BMI (kg/m2) | |||
| Mean BMI* | 32 ± 3 | 32 ± 7 | 33 ± 8 |
| <25 | 34 (11%) | 28 (11%) | 6 (16%) |
| 25–30 | 92 (32%) | 83 (33%) | 7 (18%) |
| >30 | 167 (57%) | 142 (56%) | 25 (66%) |
| Medical history | |||
| HIV | 3 (1%) | 3 (1%) | 0 |
| Pregestational diabetes | 2 (<1%) | 2 (<1%) | 0 |
| Gestational diabetes | 33 (11%) | 30 (12%) | 3 (8%) |
| Chronic hypertension | 17 (6%) | 10 (4%) | 7 (18%) |
| Proteinuria | 113 (39%) | 77 (30%) | 33 (87%) |
| Previous history of preeclampsia in preeclamptic patients | 6 (16%) | N/A | 6 (16%) |
| Cigarette smoking at conception | 13 (5%) | 9 (4%) | 4 (11%) |
| Perinatal use of nonsteroidal anti-inflammatory drugs | 11 (4%) | 9 (4%) | 2 (5%) |
Mean ± standard deviation
2.2. Quantification of Bb in maternal and fetal cord blood
Bb was measured using ELISA kits from Quidel (San Diego, CA, USA).
2.3. Statistical analysis
The laboratory tests results and corresponding clinical information from each patient were entered into a Microsoft Excel database. Statistical analyses were carried out using SPSS software version 20 (IBM, New York, NY, USA) and Sigmaplot version 11 (Systat Software, San Jose, CA, USA). All data were expressed as means ± standard errors of means. Univariate analyses were performed using the two-tailed Mann–Whitney U test and unequal variances to analyze the statistical differences between the preeclamptic and the nonpreeclamptic groups. A value of P < 0.05 was considered statistically significant. For multivariate analyses, a general linear model was constructed, with Bb levels as the dependent variable and preeclampsia status as the predictor of interest. Potential nuisance confounders were included in the multivariate analyses. These included chronic hypertension, primiparity, gestational age at blood draw, patient age, BMI, and gestational diabetes. The Box–Cox analysis was used to identify a suitable power transformation of the dependent variable. Model residuals were inspected for skew and for outliers.
3. Results
The ethnicity of the 291 enrolled pregnant women was as follows: African–American, 78%; Caucasian, 4%; Hispanic, 10%; other ethnic minorities, 8% (Table 1). Overall, 96% of the participants were from racial minorities. Of the total number of enrollees, 13% were diagnosed with preeclampsia, a percentage more than double the national average (3–6%).
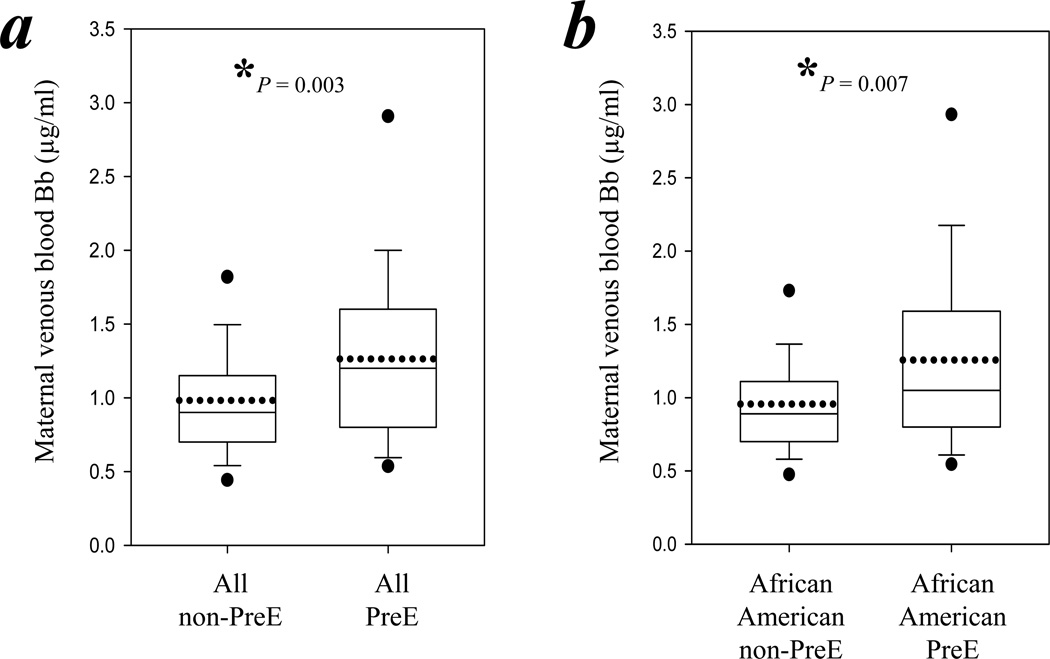
Maternal blood Bb levels were significantly higher (29%) in the preeclamptic women compared with the nonpreeclamptic women (1.26 ± 0.60 μg/ml versus 0.98 ± 0.45 μg/ml respectively, P = 0.003; Figure 1a). The post hoc power analysis revealed 83% power for this significant difference. Among the 228 African–American patients, 34 (15%) were preeclamptic. The maternal Bb levels of the preeclamptic African–American women were also significantly higher (31%) than those of nonpreeclamptic African–American women (1.26 ± 0.63 μg/ml versus 0.96 ± 0.41 μg/ml, P = 0.007; Figure 1b). The post hoc power analysis revealed 82% power for this increase.
Figure 1.
Maternal blood Bb levels were significantly higher in preeclamptic women than in nonpreeclamptic women. Maternal venous blood levels of Bb were determined by ELISA. The boundary of the box closest to zero indicates the 25th percentile, while the boundary of the box farthest from zero indicates the 75th percentile. Error bars indicate the 90th and 10th percentiles. The two filled circles indicate the 95th and 5th percentiles. The solid line within the box marks the median and the dotted line marks the mean. The asterisk indicates statistical significance (P<0.05). (a) Box chart summarizes the levels of Bb in the maternal venous blood of all nonpreeclamptic women compared with all preeclamptic women. (b) Box chart summarizes the levels of Bb in the maternal venous blood of all African–American nonpreeclamptic women compared with all African–American preeclamptic women.
In addition, multivariate analyses were performed with the maternal Bb level as the dependent variable and preeclampsia status as the predictor of interest. The unadjusted median (25th, 75th percentiles) of Bb levels for the 38 total preeclampsia cases was 0.98 μg/ml (0.56, 1.40) and for the 253 nonpreeclamptic patients, 0.80 μg/ml (0.59, 1.10). After controlling for confounders (including chronic hypertension, primiparity, gestational age at blood draw, patient ages, BMI, and gestational diabetes), the adjusted median (95% confidence interval) Bb level for the preeclamptic group was 0.91 μg/ml (0.75, 1.11) and for the nonpreeclamptic group, 0.76 μg/ml (0.67, 0.88). The adjusted means of the two groups differed significantly (P = 0.036).
Among the preeclamptic patients, 42% had preterm pregnancies, compared with 13% of nonpreeclamptic patients. There was no significant difference between the Bb levels of preterm patients and that of term patients (1.0 ± 0.6 μg/ml versus 0.9 ± 0.5 μg/ml respectively; P > 0.05).
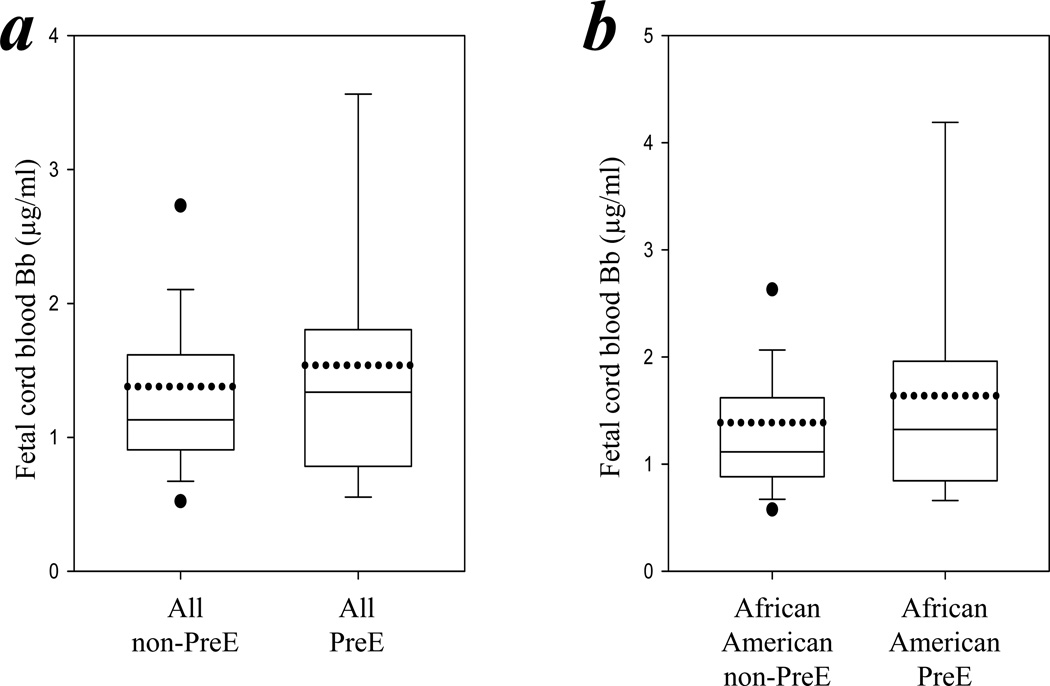
The levels of Bb, analyzed in the fetal cord blood of the neonates of 154 patients, were similar in those of the preeclamptic and nonpreeclamptic groups (1.54 ± 1.13 μg/ml versus 1.38 ± 1.03 μg/ml; P > 0.05; Figure 2a). Subgroup analyses of fetal cord blood of the neonates of African–American patients also showed no significant difference between those whose mothers were preeclamptic and those whose mothers were nonpreeclamptic (1.64 ± 1.25 μg/ml versus 1.39 ± 1.16 μg/ml respectively; P > 0.05; Figure 2b).
Figure 2.
Umbilical cord blood Bb levels were similar in neonates delivered from the preeclamptic group mothers and the nonpreeclamptic group mothers. Fetal umbilical cord blood levels of Bb were determined by ELISA. (a) Box chart summarizes the levels of Bb in the fetal cord blood of the total nonpreeclamptic group compared with all in the preeclamptic group. (b) Box chart summarizes the levels of Bb in the fetal cord blood of all African–Americans in the nonpreeclamptic group compared with all African–American preeclamptic women.
4. Discussion
Preeclampsia is a multisystem disease of pregnancy with significant maternal/neonatal mortality and morbidity. After a preeclamptic pregnancy, patients are at an increased long-term risk of cardiovascular diseases, including chronic hypertension and coronary artery disease, as well as diabetes mellitus and kidney disease (Mannisto et al. 2013). Children born after preeclamptic pregnancies are at an increased risk of hypertension and stroke (Davis et al. 2012). Preeclampsia has increased 25% in the United States in the past two decades and is a serious issue worldwide (Wallis et al. 2008). Currently, the only cure is delivery of the fetus to alleviate preeclampsia, which is less than ideal if delivery is premature.
Women with African ancestry are at a greater risk of preeclampsia than other racial groups (Zhang et al. 2013). This susceptibility, which is independent of socioeconomic status, has been speculated to be due to biological or genetic factors (Nakimuli et al. 2014). The present study of a cohort of mostly African–American pregnant women found that plasma Bb is significantly increased in preeclamptic patients compared with nonpreeclamptic patients (Figure 1). This confirms previous speculation that specific factors are involved in the preeclampsia of women with African ancestry and supports our hypothesis that Bb is such a factor.
Our observation is consistent with the reports of Lynch et al. of a significant presentation of preeclampsia in a cohort of pregnant women in Colorado (Lynch et al. 2008, 2010), 34% of whom were of an ethnic minority (21% Hispanic Caucasian, 7% African–American, 6% Asian and other minorities). However, the increase in plasma Bb in the preeclamptic patients in our study (29%) compared with that in control patients was nearly two-fold higher than that in the Lynch et al. studies (18%).
The work of Derzsy et al. showed no increase in Bb levels in patients with preeclampsia in Hungary (Derzsy et al. 2010). All patients in this study were Caucasians. Our view is that fB activation is not significant among Caucasian women with preeclampsia. As our hospitals serve predominantly African–American patients, our study could not enroll a sufficient number of Caucasian pregnant women to address this issue. Future large-scale multicenter studies are necessary to make a direct comparison of fB activation at different stages of pregnancy among significant numbers of both Caucasian women and those of an ethnic minority with preeclampsia. The results of such investigations early in pregnancy will determine the usefulness of Bb as a predictor of preeclampsia in certain sub-groups of patients.
The origin of the elevated fB activation in African–American patients is unknown. One possibility is that the regulatory proteins of complement activation are abnormal. A recent study of 40 patients with systemic lupus erythematosus who developed preeclampsia found that 18% had genetic mutations in the complement regulatory proteins MCP and CFI, but not in fH (Salmon et al. 2011). Consistent with this, a genetic variant of fH, Y402H, has been ruled out as a risk factor for preeclampsia in a study of 110 preeclamptic women (Kaare et al. 2008), although another single-nucleotide polymorphism in fH (rs1061170) was reported to be associated with an increased risk of preeclampsia (Lenhart et al. 2014). It is of note that serum fH levels were increased in one study of five preeclamptic patients (Liu et al. 2011). Our interpretation is that mutations in some complement regulatory proteins may render them dysfunctional and thus unable to control the initial activation of one or more of the three complement pathways. Once a pathway is activated, C3b will be generated, activating the amplification loop. Thus, fB will be activated, producing Bb.
The literature is unclear as to whether the classical or lectin complement pathway is involved in the pathogenesis of preeclampsia. With regard to the classical pathway (i.e., immunoglobulins, C1q, C4), autoantibodies to the angiotensin II type 1 receptor or to the basement membranes of the placenta and kidney have been associated with preeclampsia in both an animal model (Wang et al. 2012) and in humans (Foidart et al. 1986). An earlier clinical study showed that C1qbinding immune complexes were present in preeclamptic patients (Stirrat et al. 1978). A recent animal study showed that the symptoms of pregnant C1q-deficient mice mimicked the key features of human preeclampsia (Singh et al. 2011). However, another study in humans found that C1q-binding circulating immune complexes did not play a significant role in the pathogenesis of preeclampsia (Balasch et al. 1981). In addition, C1q was not significantly deposited in the placenta of preeclamptic patients (Buurma et al. 2012). Clearly, further studies are needed regarding the involvement of C1q in preeclampsia.
Clinical studies of the involvement of the lectin complement pathway in preeclampsia also generated mixed results. In some studies, plasma MBL levels were increased in preeclamptic patients (Celik and Ozan 2008, Than et al. 2008, Chaouat et al. 2009). However, others found that MBL–MASP2 activities did not correlate with the incidence of preeclampsia (Csuka et al. 2010). In agreement with this, work by Buurma et al. showed that MBL was not significantly deposited in the placenta of preeclamptic patients (Buurma et al. 2012). Ficolins have also been investigated in preeclampsia. A study of 11 preeclamptic patients found that ficolin-H and -L were present in low concentrations in the plasma but in high concentrations in the placenta, particularly in syncytiotrophoblasts undergoing apoptosis (Wang et al. 2007). Additional studies are needed to confirm the role of ficolins in preeclampsia.
Our study found no significant difference in Bb levels in the fetal cord blood in the African–American preeclamptic and nonpreeclamptic groups. This conflicts with the report by Hoffman et al., which found that cord blood Bb levels were significantly increased in 24 severely preeclamptic women compared with those of 20 normotensive control pregnant women (Hoffman et al. 2014). Our view is that the different results are due to differences in the sample size and the racial composition of the patients. Hoffman’s study contained two African– American patients in a preeclampsia group of 24 patients, while ours had 34 African–American patients in a preeclampsia group of 38. The smaller sample size and fewer African–American patients may have skewed the results in Hoffman’s study. Even though our study contained 154 cord blood samples, we acknowledge that there may still be a possible limitation of insufficient power in our cord blood analysis. Further studies with larger numbers of patients in each racial group may help to resolve this issue.
Anti-complement therapy may play a role in the management of preeclampsia, as suggested in a recent case report demonstrating that anti-C5 treatment was able to prolong pregnancy for 17 days in a patient with severe preeclampsia (Burwick and Feinberg 2013). However, in a different medical field, large clinical trials found no benefit for anti-C5 therapy in improving outcomes in cardiac patients undergoing coronary artery bypass surgery or urgent reperfusion therapy for ST elevation in myocardial infarction (Testa et al. 2008). Thus, enthusiasm for the clinical usefulness of anti-C5 for heart diseases has dampened.
Our results have two implications for potential anti-complement therapy in preeclampsia: i) complement fB may be a better drug target than C5, as it functions upstream of C5; ii) anti-fB intervention may be effective in a select population of patients, such as African–American women.
In conclusion, complement fB was activated in the maternal circulation of pregnant women of racial minorities with preeclampsia, but not in the cord blood of their neonates. The involvement of fB provides an important insight into the pathogenesis of preeclampsia. fB may have value as a therapeutic target in the management.
Supplementary Material
Highlights.
Activated complement fB, Bb, is increased in preeclamptic women, especially in African–American patients
Fetal cord blood levels of Bb were similar in patients with or without preeclampsia
A maternal immune response through fB may contribute to the pathogenesis of preeclampsia
Acknowledgements
The authors would like to thank Dr. James Cottrell for continuous support, Dr. Julie Rushbrook for editing the manuscript, and Dr. John Hartung for helpful comments. The work was supported in part by NIH grant 1R21HL088527 (MZ) and a SUNY-Downstate Dean’s Award (MZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: none declared.
References
- Abramson SB, Buyon JP. Activation of the complement pathway: comparison of normal pregnancy, preeclampsia, and systemic lupus erythematosus during pregnancy. Am J Reprod Immunol. 1992;28:183–187. doi: 10.1111/j.1600-0897.1992.tb00787.x. [DOI] [PubMed] [Google Scholar]
- Ahn H, et al. Immunologic characteristics of preeclampsia, a comprehensive review. Am J Reprod Immunol. 2011;65:377–394. doi: 10.1111/j.1600-0897.2010.00913.x. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' task force on hypertension in pregnancy. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- Balasch J, et al. Further evidence against preeclampsia as an immune complex disease. Obstet Gynecol. 1981;58:435–437. [PubMed] [Google Scholar]
- Boij R, et al. Biomarkers of coagulation, inflammation, and angiogenesis are independently associated with preeclampsia. Am J Reprod Immunol. 2012;68:258–270. doi: 10.1111/j.1600-0897.2012.01158.x. [DOI] [PubMed] [Google Scholar]
- Burwick RM, Feinberg BB. Eculizumab for the treatment of preeclampsia/HELLP syndrome. Placenta. 2013;34:201–203. doi: 10.1016/j.placenta.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Burwick RM, et al. Urinary excretion of c5b-9 in severe preeclampsia: tipping the balance of complement activation in pregnancy. Hypertension. 2013;62:1040–1045. doi: 10.1161/HYPERTENSIONAHA.113.01420. [DOI] [PubMed] [Google Scholar]
- Buurma A, et al. Preeclampsia is characterized by placental complement dysregulation. Hypertension. 2012;60:1332–1337. doi: 10.1161/HYPERTENSIONAHA.112.194324. [DOI] [PubMed] [Google Scholar]
- Celik N, Ozan H. Maternal serum mannose-binding lectin in severe preeclampsia. Clin Exp Obstet Gynecol. 2008;35:179–182. [PubMed] [Google Scholar]
- Chaouat G, et al. Early regulators in abortion and implications for a preeclampsia model. J Reprod Immunol. 2009;82:131–140. doi: 10.1016/j.jri.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Csuka D, et al. Functional analysis of the mannose-binding lectin complement pathway in normal pregnancy and preeclampsia. J Reprod Immunol. 2010;87:90–96. doi: 10.1016/j.jri.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Davis EF, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129:e1552–e1561. doi: 10.1542/peds.2011-3093. [DOI] [PubMed] [Google Scholar]
- Denny KJ, et al. Elevated complement factor c5a in maternal and umbilical cord plasma in preeclampsia. J Reprod Immunol. 2013a;97:211–216. doi: 10.1016/j.jri.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Denny KJ, et al. Complement in pregnancy:aA delicate balance. Am J Reprod Immunol. 2013b;69:3–11. doi: 10.1111/aji.12000. [DOI] [PubMed] [Google Scholar]
- Derzsy Z, et al. Activation of the complement system in normal pregnancy and preeclampsia. Mol Immunol. 2010;47:1500–1506. doi: 10.1016/j.molimm.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Foidart JM, et al. Antibodies to laminin in preeclampsia. Kidney Int. 1986;29:1050–1057. doi: 10.1038/ki.1986.106. [DOI] [PubMed] [Google Scholar]
- Girardi G, et al. Complement activation in animal and human pregnancies as a model for immunological recognition. Mol Immunol. 2011;48:1621–1630. doi: 10.1016/j.molimm.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Haeger M, et al. Complement activation and anaphylatoxin (c3a and c5a) formation in preeclampsia and by amniotic fluid. Obstet Gynecol. 1989;73:551–556. [PubMed] [Google Scholar]
- Hoffman MC, et al. Maternal and fetal alternative complement pathway activation in early severe preeclampsia. Am J Reprod Immunol. 2014;71:55–60. doi: 10.1111/aji.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaare M, et al. Complement factor H variant Y402H is not a risk factor for preeclampsia in the Finnish population. Hypertens Pregnancy. 2008;27:328–336. doi: 10.1080/10641950801955691. [DOI] [PubMed] [Google Scholar]
- Lenhart PM, et al. Adrenomedullin signaling pathway polymorphisms and adverse pregnancy outcomes. Am J Perinatol. 2014;31:327–334. doi: 10.1055/s-0033-1349345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillegard KE, et al. Complement activation is critical for placental ischemia-induced hypertension in the rat. Mol Immunol. 2013;56:91–97. doi: 10.1016/j.molimm.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, et al. Proteomic analysis of human serum for finding pathogenic factors and potential biomarkers in preeclampsia. Placenta. 2011;32:168–174. doi: 10.1016/j.placenta.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch AM, Salmon JE. Dysregulated complement activation as a common pathway of injury in preeclampsia and other pregnancy complications. Placenta. 2010;31:561–567. doi: 10.1016/j.placenta.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch AM, et al. Alternative complement pathway activation fragment bb in early pregnancy as a predictor of preeclampsia. Am J Obstet Gynecol. 2008;198:385, e1–e9. doi: 10.1016/j.ajog.2007.10.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch AM, et al. The interrelationship of complement-activation fragments and angiogenesis-related factors in early pregnancy and their association with pre-eclampsia. BJOG. 2010;117:456–462. doi: 10.1111/j.1471-0528.2009.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch AM, et al. Early elevations of the complement activation fragment c3a and adverse pregnancy outcomes. Obstet Gynecol. 2011;117:75–83. doi: 10.1097/AOG.0b013e3181fc3afa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannisto T, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127:681–690. doi: 10.1161/CIRCULATIONAHA.112.128751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakimuli A, et al. Pregnancy, parturition and preeclampsia in women of African ancestry. Am J Obstet Gynecol. 2014;210:510–520. e1. doi: 10.1016/j.ajog.2013.10.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing X, et al. Targeted inhibition of complement activation prevents features of preeclampsia in mice. Kidney Int. 2011;79:331–339. doi: 10.1038/ki.2010.393. [DOI] [PubMed] [Google Scholar]
- Rampersad R, et al. The C5b-9 membrane attack complex of complement activation localizes to villous trophoblast injury in vivo and modulates human trophoblast function in vitro. Placenta. 2008;29:855–861. doi: 10.1016/j.placenta.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JM, Bell MJ. If we know so much about preeclampsia, why haven't we cured the disease? J Reprod Immunol. 2013;99:1–9. doi: 10.1016/j.jri.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard PY, et al. Fourteen years of debate and workshops on the immunology of preeclampsia. Where are we now after the 2012 workshop? J Reprod Immunol. 2014;101–102:62–69. doi: 10.1016/j.jri.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Saito S, Nakashima A. A review of the mechanism for poor placentation in early-onset preeclampsia: the role of autophagy in trophoblast invasion and vascular remodeling. J Reprod Immunol. 2014;101–102:80–88. doi: 10.1016/j.jri.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Salmon JE, et al. Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLoS Med. 2011;8:e1001013. doi: 10.1371/journal.pmed.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, et al. Role of complement component C1q in the onset of preeclampsia in mice. Hypertension. 2011;8:716–724. doi: 10.1161/HYPERTENSIONAHA.111.175919. [DOI] [PubMed] [Google Scholar]
- Soto E, et al. Preeclampsia and pregnancies with small-for-gestational age neonates have different profiles of complement split products. J Matern Fetal Neonatal Med. 2010;23:646–657. doi: 10.3109/14767050903301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek J, Al-Ahmadie HA. Laminar necrosis of placental membranes: a histologic sign of uteroplacental hypoxia. Pediatr Dev Pathol. 2005;8:34–42. doi: 10.1007/s10024-004-8092-9. [DOI] [PubMed] [Google Scholar]
- Stirrat GM, et al. Circulating immune complexes in pre-eclampsia. Br Med J. 1978;1:1450–1451. doi: 10.1136/bmj.1.6125.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, et al. Racial disparity in hypertensive disorders of pregnancy in New York state: a 10-year longitudinal population-based study. Am J Public Health. 2007;97:163–170. doi: 10.2105/AJPH.2005.068577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa L, et al. Pexelizumab in ischemic heart disease: a systematic review and metaanalysis on 15,196 patients. J Thorac Cardiovasc Surg. 2008;136:884–893. doi: 10.1016/j.jtcvs.2007.12.062. [DOI] [PubMed] [Google Scholar]
- Than NG, et al. A role for mannose-binding lectin, a component of the innate immune system in pre-eclampsia. Am J Reprod Immunol. 2008;60:333–345. doi: 10.1111/j.1600-0897.2008.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis AB, et al. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens. 2008;21:521–526. doi: 10.1038/ajh.2008.20. [DOI] [PubMed] [Google Scholar]
- Wang CC, et al. Innate immune response by ficolin binding in apoptotic placenta is associated with the clinical syndrome of preeclampsia. Clin Chem. 2007;53:42–52. doi: 10.1373/clinchem.2007.074401. [DOI] [PubMed] [Google Scholar]
- Wang W, et al. Autoantibody-mediated complement c3a receptor activation contributes to the pathogenesis of preeclampsia. Hypertension. 2012;60:712–721. doi: 10.1161/HYPERTENSIONAHA.112.191817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, et al. Racial disparities in economic and clinical outcomes of pregnancy among medicaid recipients. Matern Child Health J. 2013;17:1518–1525. doi: 10.1007/s10995-012-1162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.