Abstract
Erythropoietin (EPO) is critical for red blood cell production and is also an effective neuroprotective agent. However, it may also contribute to pathological angiogenesis. Here we investigate the angiogenic potential of EPO and a mutant form with attenuated erythropoietic activity, EPO-R76E, on primary human retinal microvascular endothelial cells (HRMEC) and in the adult retina. Assays of death, proliferation, and tube-formation were performed on HRMECs exposed to EPO, EPO-R76E, or media alone. Postnatal day 9 wild-type mice were injected intramuscularly with adeno-associated virus vectors expressing either enhanced green fluorescent protein or EpoR76E. At 3 months, levels of EPO-R76E in the eye were quantified, and the health of the retinal vasculature was assessed by fluorescein angiography and isolectin immunolabeling. Immunohistochemistry, histology, and electroretinogram assessments were performed as measures of retinal health. Neither EPO nor EPO-R76E induced proliferation or tube-formation in HRMEC under the conditions used. EPO-R76E decreased HRMEC death in a dose-dependent manner. Long-term systemic gene delivery of EPO-R76E was safe in terms of retinal vasculature, histology, and the electroretinogram in vivo. Our results show that EPO-R76E can block HRMEC death, consistent with its role in erythropoiesis and neuroprotection. In addition, long-term gene delivery of EPO-R76E is safe in the adult retina.
Keywords: erythropoietin, retina, angiogenesis, primary human retinal microvascular endothelial cells
Introduction
Erythropoietin (EPO) is a heavily glycosylated cytokine that regulates erythropoiesis by promoting the survival of erythroid progenitor cells in the bone marrow via activation of the EPO receptor (EPOR) homodimer.1 EPO and its receptors are also expressed at low levels in non-hematopoietic tissues, including the retina.1, 2 Three receptors have been identified: EPOR; soluble EPO-R (sEPOR), which sequesters EPO from binding to the cell surface receptors; and the common β chain of the GM-CSF, IL3 and IL5 receptors (CD131).3–5 In neuronal tissue, EPO activates the PI3K/Akt pathway.1
EPO protects retinal neurons from death in models of induced or inherited retinal degeneration.6–17 However, repeated treatment with systemic EPO may be associated with negative side effects such as polycythemia, that could lead to thrombotic complications, and increases in blood pressure. One approach to minimize these side-effects is the introduction of point mutations in Epo to retain the neuroprotective activity while attenuating the erythropoietic activity.13–15, 18–21 We developed EPO-R76E, which protects neurons in models of Parkinson’s disease, retinal degeneration, and glaucoma, while maintaining the hematocrit within normal limits.8, 13–15, 21
Another concern of long-term systemic treatment with EPO is the potential for neovascularization. Studies in non-retinal developmental systems or tumor-derived cells suggest that EPO is angiogenic.22 However, the results are controversial and clinical evidence is lacking.23 There has been one study on the effect of EPO on primary human retinal microvascular endothelial cells (HRMEC). In that study, EPO increased proliferation, but not migration or tube formation.24 Exogenous EPO contributes to neovascularization in development as demonstrated in patients with retinopathy of prematurity and in animal models of oxygen induced retinopathy.25,26, 27 The current understanding of the role of EPO in retinal angiogenesis is described in a recent review.28 In this study we tested the safety of long-term systemic gene delivery of EpoR76E on the adult retina, and compared the angiogenic effects of EPO and EPO-R76E in primary HRMECs using assays of migration, proliferation, and cell death.
Results
Production and quantification of EPO-R76E
In order to compare EPO and EPO-R76E each protein needed to be produced and quantified. We utilized chinese hamster ovary (CHO) cells, which are used to produce commercial EPO, resulting in production of EPO and EPO-R76E at a comparable size as commercial EPO (Figure 1A). The concentration of EPO and EPO-R76E was obtained by performing linear regression of the average of the bands at the expected molecular weight of 35kD in three replicate lanes in a quantitative Coomassie gel of fractionated media from the stably expressing CHO cell lines (Figure 1B). The concentration of EPO and EPO-R76E in the media using this approach was 26.0±1.3ng/ml (sd) and 11.7±4.5ng/ml, respectively. To determine the sensitivity of the commercial ELISA in detecting EPO-R76E, known concentrations of EPO or EPO-R76E (based on the quantitative Coomassie) were added to the ELISA. The ELISA accurately detected EPO, but not EPO-R76E (Table 1). The ELISA detected 70% of the EPO-R76E that was present, so for all quantifications, a correction of 1.43 was applied to assure treatment with equal amounts of EPO and EPO-R76E (Table 1).
Figure 1.
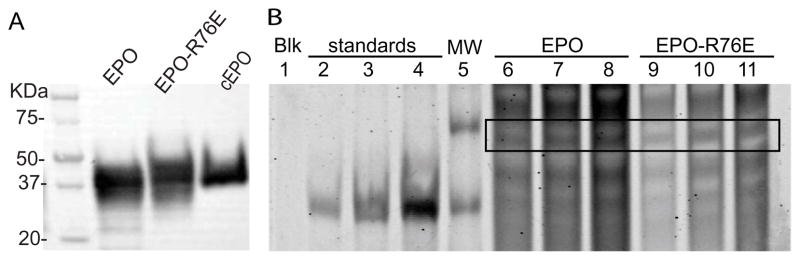
Production and quantification of EPO and EPO-R76E from CHO cells. A) Western blot showing that EPO and EPO-R76E produced from CHO cells are comparable in size to commercial EPO. B) Quantitative Coomassie gel. Equal volumes were loaded, alongside three increasing concentrations of standards, and a molecular weight marker. Lanes are as follows: 1) blank, 2) 200ng of standard protein, 3) 500ng of standard protein, 4) 1000ng of standard protein, 5) MW ladder, 6) 10μl fractionated media from EPO expressing CHO cells, 7) 15μl fractionated media from EPO expressing CHO cells, 8) 20μl fractionated media from EPO expressing CHO cells, 9) 10μl fractionated media from EPO-R76E expressing CHO cells, 10) 15μl fractionated media from EPO-R76E expressing CHO cells, 11) 20μl fractionated media from EPO-R76E expressing CHO cells. Box indicates location of EPO or EPO-R76E based on size.
Table 1.
Quantification of EPO-R76E
| Quantitative Coomassie (mU/ml) | ELISA (mU/ml) | Correction factor | |
|---|---|---|---|
|
|
|||
| EPO | 100 | 112.62 ± 0.67 | - |
| EPO-R76E | 50 | 35.97 ± 0.95 | 1.39 |
| EPO-R76E | 100 | 68.72 ± 0.38 | 1.45 |
| EPO-R76E | 200 | 140.49 ± 2.63 | 1.43 |
EPO-R76E activates the EPOR and downstream signaling in HRMECs
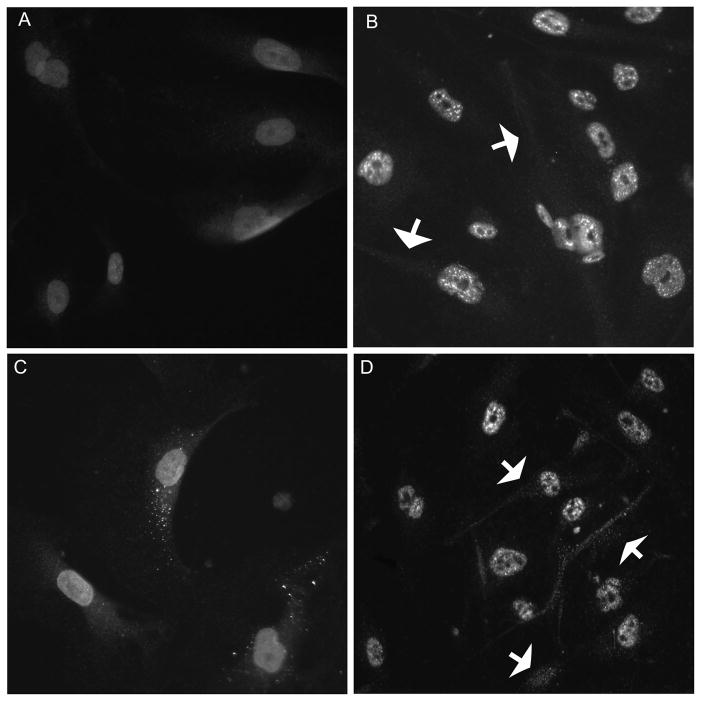
Both the EPOR and CD131 were detected on HRMECs (Figure 2). In untreated HRMECs, both receptors were present diffusely throughout the cells (Figure 2A,B). The localization of each receptor changed from a diffuse to a punctate pattern upon stimulation with 20U/ml of commercial EPO (Figure 2C,D).
Figure 2.
HRMECs contain both CD131 and EPOR. Epifluorescence micrographs of HRMECs immunolabeled with anti-CD131 (A,C) or anti-EPOR (B,D). Localization of the receptors shifts upon stimulation with EPO (C,D; arrows).
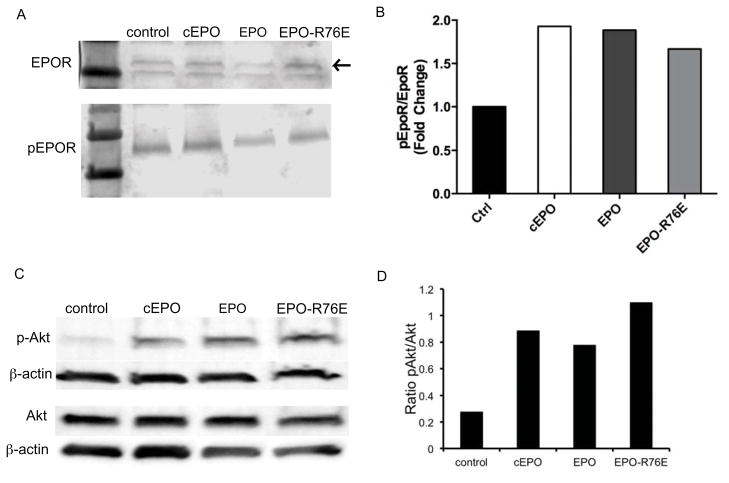
The ability of EPO-R76E to activate the EPOR was assessed by detection of phosphorylation of EPOR and the downstream signaling molecule, Akt. Treatment with EPO, EPO-R76E, and commercial EPO all induced phosphorylation of the EPOR (Figure 3A, B) and Akt (Figure 3C, D) comparably. Several concentrations of EPO and EPO-R76E were tested and the level of phosphorylation of EPOR and Akt was comparable between EPO and EPO-R76E at each concentration tested (data not shown).
Figure 3.
EPO, EPO-R76E, and commercial EPO activate the EPOR and downstream signaling. A) Western blot of phosphorylation of the EPOR (pEPOR) in HRMECs treated with EPO, EPO-R76E, and commercial EPO. Arrow indicates EPOR. B) Bar graph of quantification of Western blot with pEPOR normalized to βactin. C) Western blot of Akt and phosphoAkt after treatment with EPO, EPO-R76E, or commercial EPO. D) Bar graph of quantification of Western blot showing the ratio of pAkt to Akt after normalization of each to βactin.
Neither EPO nor EPO-R76E induce HRMEC proliferation
Proliferation of the HRMECs was induced by treatment with media containing 10% serum, but not with 1% serum (Figure 4). Since EPO has a bell-shaped dose curve,16 a wide range of EPO concentrations were tested. Doses of 5 to 20U/ml of commercial EPO, EPO, and EPO-R76E had no effect on HRMEC proliferation (data not shown). Increasing the concentrations to 30, 60, or 120U/ml still had no effect (Figure 4). This is in contrast to the efficacy of vascular endothelial growth factor to induce proliferation in HRMEC under the same conditions.29
Figure 4.
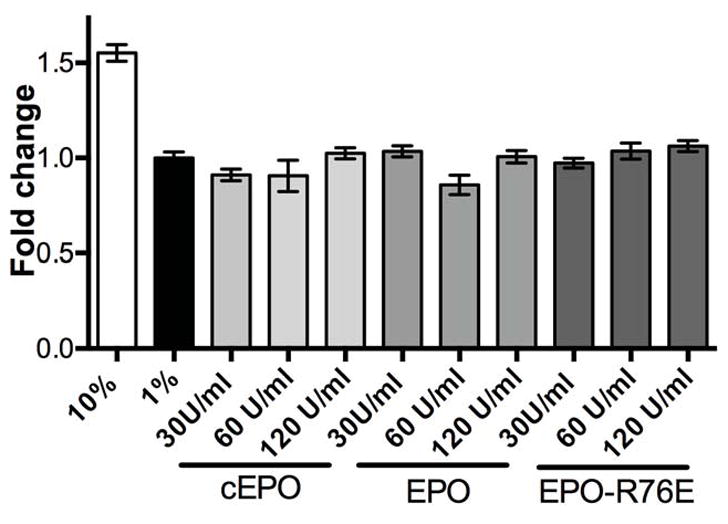
Neither EPO nor EPO-R76E induce HRMEC proliferation. Bar graph showing quantification of proliferation, normalized to baseline (1% serum). Proliferation was induced by 10% serum, but not by treatment with EPO, EPO-R76E, or commercial EPO at three different doses of each in 1% serum. Error bars indicate SEM.
Neither EPO nor EPO-R76E induce HRMEC tube formation
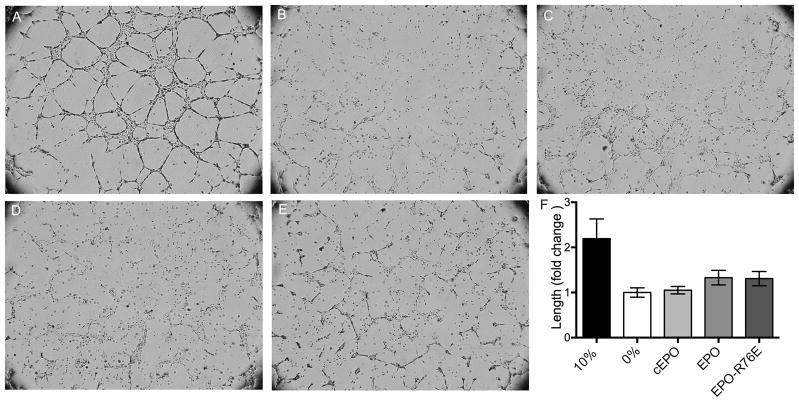
In the presence of 10% serum containing media, the HRMECs form tubes through the Matrigel (Figure 5A). No tube formation was detected in the absence of serum (Figure 5B) regardless of treatment with 40U/ml of commercial EPO, EPO, or EPO-R76E (Figure 5C–E). The length of each tube wall was quantified (Figure 5F). There was no statistically significant difference between the serum-free negative control and those cells treated with EPO, EPO-R76E, or commercial EPO. Treatment with 20U/ml of commercial EPO, EPO, and EPO-R76E was also ineffective (data not shown). This is in contrast to the efficacy of vascular endothelial growth factor to induce proliferation in HRMEC under the same conditions.29
Figure 5.
Neither EPO nor EPO-R76E induce tube formation in HRMECs. A–E) Brightfield micrographs of HRMECs treated with A) 10% serum, B) 0% serum, or C) commercial EPO, D) EPO, or E) EPO-R76E in 0% serum. F) Bar graph quantification of lengths of tube walls in each condition. Error bars indicate SEM.
EPO-R76E blocks HRMEC death
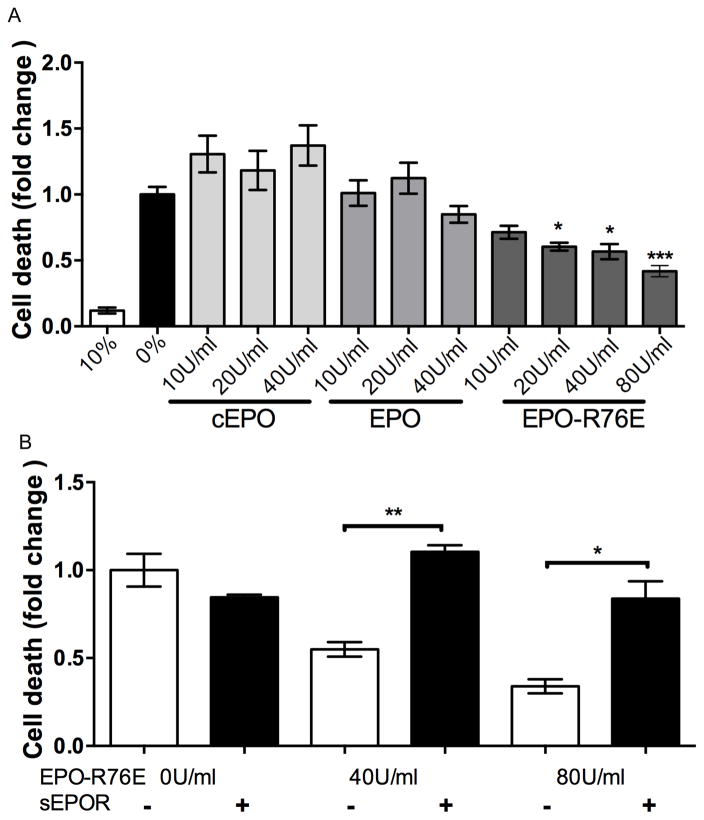
Apoptosis was detected in serum-deprived cells, but not in cells maintained in 10% serum (Figure 6A). Treatment with 10, 20, or 40U/ml commercial EPO or EPO in serum-free media caused a similar amount of apoptosis as in the no serum control. Treatment with EPO-R76E caused a dose-dependent decrease in HRMEC apoptosis, with 80U/ml being most effective, but all doses showing statistically significant protection (p<0.001). Additional cells were co-incubated with EPO-R76E and sEPOR to determine if the effect was due to the activity of EPO-R76E (Figure 6B). Treatment with sEPOR completely blocked the anti-apoptotic effect of EPO-R76E at 40 and 80U/ml (p<0.001).
Figure 6.
EPO-R76E protects the HRMECs from apoptosis. A) Bar graph of quantification of cell death in cultures treated with 10% serum or with 10, 20, 40, or 80U/ml commercial EPO, EPO, or EPO-R76E in 0% serum, normalized to 0% serum. B) Bar graph of cell death after treatment with 0, 40, or 80U/ml EPO-R76E in 0% serum with or without 2μg/ml sEPOR. Error bars indicate SEM, *p<0.05, **p<0.01, ***p<0.001.
EPO-R76E enters the eye after systemic gene delivery
Hematocrit was in the normal range for the rAAV.eGFP treated mice, 38 ± 4.7 (sd), and was elevated in mice that received rAAV.EpoR76E, 51 ± 5.7, p<0.01. However, since the mice were never phlebotomized, this increase is slight in physiological terms and had no effect on the health of the mice. Western blot analysis was performed to detect and quantify EPO-R76E in the eye after IM injection of rAAV.EpoR76E or rAAV.eGFP (Figure 7). Low levels of EPO are present endogenously in the retina, as seen in the light band from retinas of mice treated with rAAV.eGFP. However, there was an 8.7 fold increase in retina EPO levels in mice injected with rAAV.EpoR76E showing that EPO-R76E was able to enter the eye (Figure 7).
Figure 7.
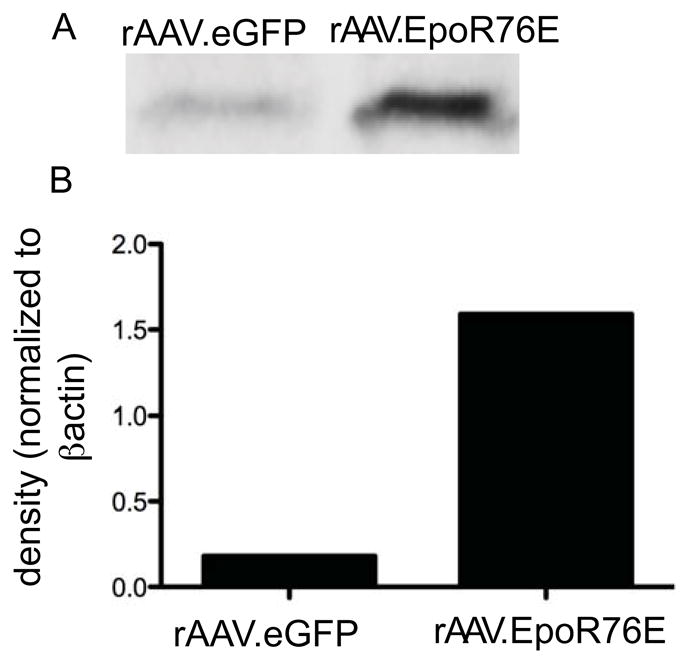
EPO-R76E enters the eye after systemic gene delivery. A) Western blot of EPO-R76E in the eye after IM injection of rAAV.eGFP or rAAV.EpoR76E. B) Bar graph of EPO levels in eyes from mice injected IM with rAAV.eGFP or rAAV.EpoR76E after normalizing to βactin.
Gene delivery of EPO-R76E does not alter the retinal vasculature in wild-type mice
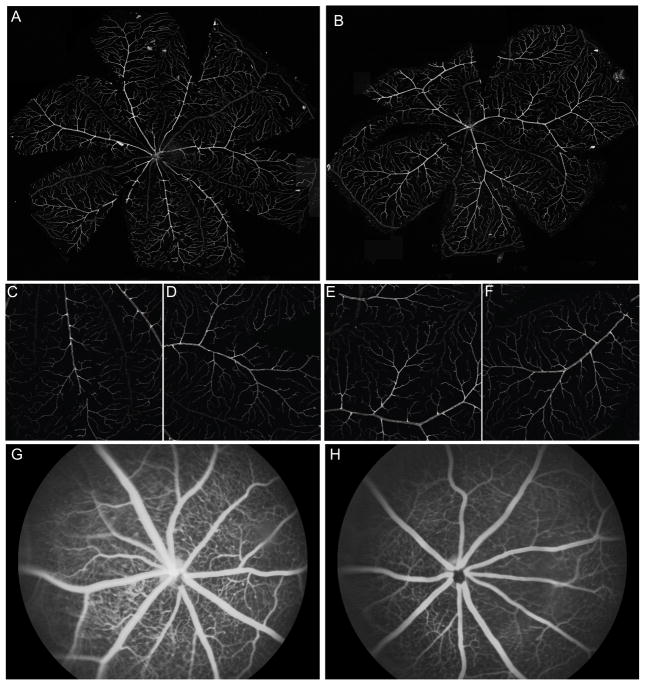
The retinal vasculature of wild-type mice had a normal branching pattern three months after IM injection with rAAV.EpoR76E (n=5) or rAAV.eGFP (n=5) based on labeling of the whole-mount retina with isolectin B4 (Figure 8A–F) and fluorescein angiography (Figure 8G,H). The vessels also retained their integrity, as demonstrated by lack of fluorescein leakage (Figure 8G,H).
Figure 8.
Long-term delivery of EPO-R76E has no deleterious effect on the retinal vasculature or histology. A,B) Montage of confocal micrographs of representative retina flatmounts from mice treated IM with rAAV.eGFP (A) or rAAV.EpoR76E (B) and labeled with anti-isolectin 1. C–F) Higher magnification images of isolectin labeling from rAAV.eGFP (C,D) or rAAV.EpoR76E (E,F) injected mice. G,H) Representative fluorescein angiography images from mice treated IM with rAAV.eGFP (G) or rAAV.EpoR76E (H).
Long-term treatment with EPO-R76E is safe in the wild-type retina
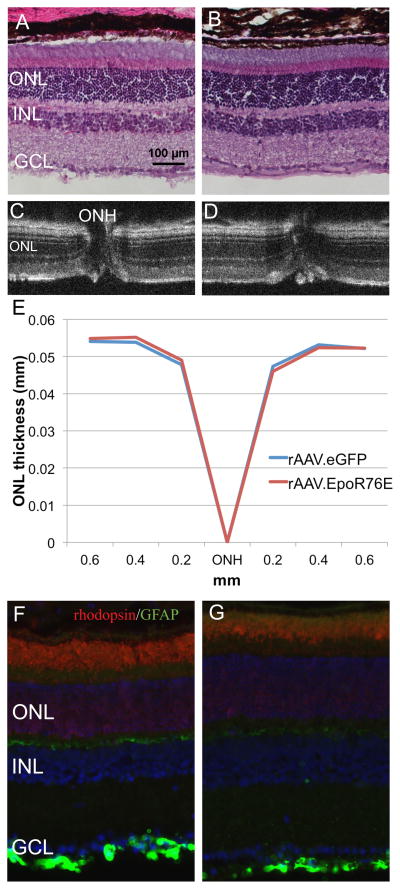
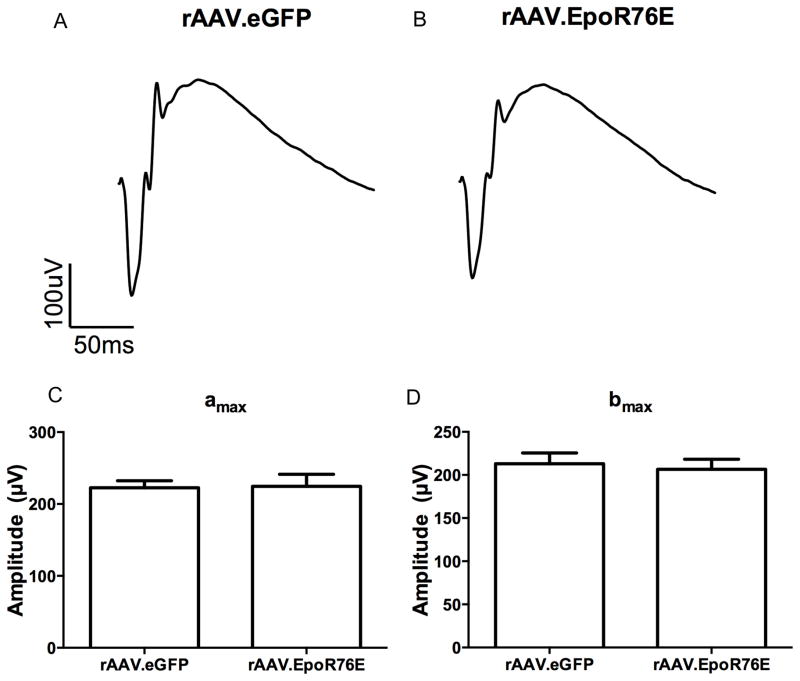
The retinal histology appears normal in mice 3 and 7 months after injection with rAAV.eGFP (n=5) or rAAV.EpoR76E (n=5) based on both standard histology (Figure 9A,B) and OCT imaging (Figure 9C,D). ONL thickness was quantified using calibrated calipers in the OCT software, there was complete overlap between the rAAV.eGFP and rAAV.EpoR76E treated groups (Figure 9E). Localization of rhodopsin and GFAP also remained normal regardless of treatment (Figure 9F, G). Rhodopsin labeling remained primarily in the outer segments, and GFAP remained restricted to the astrocytes in the ganglion cell layer. In addition, the ERG retained a normal waveform and a and b wave amplitudes in the rAAV.EpoR76E treated mice as compared to mice treated with rAAV.eGFP (Figure 10). No difference was detected between the 3 and 7 month time-points, so representative waveforms (Figure 10A, B) and amplitudes (Figure 10C,D) at 2 log*cd*s/m2 from both time-points are shown.
Figure 9.
Long-term delivery of EPO-R76E has no deleterious effect on retina structure. A, B) Light micrographs of retinas from mice treated IM with rAAV.eGFP (A) or rAAV.EpoR76E (B). C, D) OCT B-line scan images through the optic nerve head (ONH) of retinas from a mouse injected with rAAV.eGFP (C) or rAAV.EpoR76E (D). E, F) Epifluorescence micrographs of retinas from mice treated IM with rAAV.eGFP (E) or rAAV.EpoR76E (F) and immunolabeled with anti-rhodopsin (red), anti-GFAP (green) and DAPI (blue). ONL=outer nuclear layer; INL=inner nuclear layer; GCL=ganglion cell layer
Figure 10.
Long-term delivery of EPO-R76E has no deleterious effect on retina electrophysiology. A,B) Average waveforms of flash ERGs from mice treated IM with rAAV.eGFP (A) or rAAV.EpoR76E (B). C,D) Quantification of amax (C) and bmax (D) at 2 log*cd*s/m2.
Discussion
Short-term treatment with EPO was protective in patients with optic neuritis and another trial is ongoing for patients with traumatic optic neuropathy (clinicaltrials.gov).30 Animal studies have also shown that long-term gene delivery of EPO is effective in blocking degeneration in progressive retinal or optic nerve degenerations.6, 7, 11 However, long-term treatment with EPO is not safe thus limiting the clinical utility of EPO as a neuroprotective therapy. In contrast, EPO-R76E has attenuated erythropoietic activity, making it safer for long-term systemic treatment than EPO.13–15 In addition, continuous delivery of EPO via rAAV-mediated gene therapy leads to protection from neurodegenerative diseases at lower doses than are needed by repeat bolus protein injections, further increasing the safety of treatment.13–15 The goal of this study was to determine if long-term treatment with EPO-R76E caused toxicity to the normal adult retina and if EPO-R76E has a different angiogenic profile from EPO.
We did not detect increased HMREC proliferation by treatment with EPO, in contrast to a previous report using a similar dose of EPO.24 The different results could be due to higher serum conditions, higher passage cells, or the particular assay used in the other study. Other studies suggest that EPO may interact with VEGF, which is a common component in serum, to enhance angiogenesis.31 In agreement with the same previous report, we did not detect induction of tube formation.24 Surprisingly, EPO-R76E blocked HMREC death while commercial EPO and EPO had no effect at the same concentrations. The anti-apoptotic effect of EPO-R76E was blocked by co-incubation with sEPOR indicating that the effect was due to the activity of EPO-R76E. While the reason for this result is not clear, a potential explanation might be that cell death was blocked by EPO-R76E through an interaction with an EPOR, CD131 heterodimer. Since EPO-R76E does not bind to the EPOR homodimer efficiently, it may preferentially bind to the heterodimer, while the opposite could be true of commercial EPO or EPO. In this case we would expect to detect a protective effect by commercial EPO and EPO at higher doses. This will be explored in future studies.
We show that in a healthy, adult retina, EPO-R76E does not induce neovascularization. However, EPO or EPO-R76E may exacerbate pathological angiogenesis as has been reported in the hypoxic and diabetic retina.27, 28, 32 A future avenue of investigation will be to compare the effects of EPO and EPO-R76E on retinal neovascularization in the oxygen-induced retinopathy model. The results of this study provide evidence of a good safety profile for EPO-R76E by demonstrating a lack of retinal neovascularization or toxicity in primary HRMECs or the adult mouse retina at doses we commonly use to induce neuroprotection. These results are consistent with other reports showing a lack of angiogenesis by EPO.23 We also demonstrate that the lack of effect is not due to the inability of EPO-R76E to enter the eye. We show that systemic gene delivery of EPO-R76E causes an over 8-fold increase in EPO levels in the eye. This supports studies showing that EPO can cross the blood brain barrier.33
Materials and Methods
Epo and EpoR76E expression plasmids
Human Epo cDNA was cloned into pCMV6-XL4 from pCMV6.EPO (Origene, Rockville, MD). To generate EpoR76E, site directed mutagenesis was performed with a Quick Change Multi Site kit according to manufacturer instructions (Agilent Technologies, Santa Clara, CA). Primers were: sense 5′-ggaagctgtcctggagggccaggccctg-3′ and antisense 5′-cagggcctggccctccaggacagcttcc-3′. Epo and EpoR76E were sub-cloned into pBluescript with NotI and XbaI restriction enzymes and transferred to pcDNA3.1 (Life Technologies, Carlsbad, CA) in NotI and BamHI sites.
Generation of stable, high-expressing cell lines
Chinese hamster ovary cells (provided by the Vanderbilt Antibody and Protein Resource, VAPR) were grown in DMEM containing 10% fetal bovine serum, 20 mM HEPES, 1mM pyruvate, 2mM L-glutamine, 20 μg/ml proline and antibiotic-antimicotic (Life Technologies). Cultures were maintained at 37°C, 5% CO2 and 95% relative humidity. The CHO cells were transfected with the expression plasmids and high expressing colonies were identified and selected on the ClonePix FL by the VAPR (Life Technologies) using a fluorophore labeled mouse anti-EPO (MAB2871, R&D systems, Minneapolis, MN).
Production of EPO and EPO-R76E for in vitro experiments
Stably expressing CHO cells lines were grown in full DMEM media to confluency then washed and grown in media without serum for 3 days. Media was concentrated with Amicon Ultra 10K devices (Millipore, Billerica, MA) centrifuged at 5000g for 20 minutes. EPO and EPO-R76E were quantified using the Human EPO Quantikine IVD ELISA Kit per manufacturer’s instructions (R&D Systems).
Quantification of EPO-R76E
Stable lines were cultured in FreeStyle CHO Expression Medium (Life Technologies) for 72 hrs. The media was concentrated to 2ml using Amicon ultra filtration units with a MWCO of 3K (Millipore), filtered and injected on to a HiPrep 26/60 Sephacryl S-100 HR column connected to an AKTAxpress unit (GE Healthcare Life Sciences, Piscataway, NJ). Standard column procedures outlined in the Unicorn 5.11 software wizard were followed by the VAPR. Fractions were analyzed with the EPO ELISA kit.
To determine the sensitivity of the ELISA kit for EPO-R76E, a quantitative Coomassie was performed by the VAPR. Three volumes of media fractions from EPO or EPO-R76E expressing CHO cells were loaded into a Bis/Tris gel and infrared-Coomassie stained. Known concentrations of His-tagged GFP were used as standards. The amount of stained protein was quantified using an Odyssey scanner system (LI-COR, Lincoln, NE). Linear regression analysis was performed to identify the concentrations of EPO and EPO-R76E (identified by size on the gel). Then, equal concentrations of EPO and EPO-R76E were used in the EPO ELISA. Since the ELISA reports in mU/ml, a conversion was performed based on the datasheet for commercial EPO: 50μg/ml equals 7500U/ml.
Primary HRMEC cell culture
Passage 3 primary HRMEC (Cell Systems, Kirkland, WA) were cultured in plates coated with Attachment Factor (AF) in 131 Medium supplemented with Microvascular Growth Supplement (MVGS) and antibiotic-antimycotic solution (Life Technologies).
Western blot analysis of HRMECs
When 90% confluent, the HRMECs were MVGS deprived for 4 hrs and treated with 10U/ml commercial EPO (ProSpec, East Brunswick, NJ; or gift of Amgen, Thousand Oaks, CA), 10U/ml EPO or EPO-R76E in media from CHO cells, or media from untransfected cells as a control, for 20 minutes. Cells were washed and scraped in TBS containing 0.5 % Triton-X-100 and protease and phosphatase inhibitor cocktails (P8340 and P5726, Sigma, St. Louis, MO). Lysates were passed through a 27G needle, sonicated, centrifuged at 12000 rpm for 20 minutes and protein quantification was determined using a BCA protein assay kit (Pierce Biotechnology, Inc., Rockford, IL).
Equal amounts of protein were resolved by electrophoresis on 0.1% SDS in a 4–20 % Mini-protean TGX gel (Bio-Rad, Hercules, CA) and transferred to a 0.45μm PVDF membrane. For detection of Akt and pAkt, the membrane was blocked for 3 hrs in TBS containing 0.1% Tween20 (v/v), 5% (w/v) nonfat dry milk, or 5% (w/v) BSA to detect phospho-proteins. The membrane was incubated in anti-Akt (pan, 11E7, Cell Signaling, Danvers, MA), p-Akt (Ser473, Cell Signaling); or β-actin (AM4302, Life Technologies). After washing, the membrane was incubated in secondary antibodies, goat anti-rabbit (H+L, W4011) and goat anti-mouse (H+L, HRP conjugate, Promega, Madison, WI); or donkey anti-goat IgG-HRP (sc2020, Santa Cruz). The bands were developed in Western Lightning Plus-ECL (PerkinElmer, Waltham, MA), imaged on a Bio-Rad imaging system and Image Lab software and quantified with Quantity One software (Bio-Rad).
For detection of EPOR and pEPOR, the membrane was blocked for 1 hr in Odyssey Blocking Buffer TBS (LI-COR) with 0.1% Tween20. The membrane was incubated with anti-p-EpoR (Tyr456; sc-20236R, Santa Cruz, Dallas, TX); anti-EpoR (M-20; sc-697, Santa Cruz); or β-actin. This antibody was shown to be specific for EpoR in a comparative study.34 After washing, the membrane was incubated in secondary antibodies donkey anti-rabbit (H+L IRDye-680LT conjugate; 926-68023) and donkey anti-mouse (H+L IRDye-800CW conjugate; 926-32212, LI-COR). Bands were imaged on an Odyssey Scanner system and quantified using ImageJ software.
Proliferation assay
A 96-well plate was coated with AF and seeded with 1.0×103 HRMEC cells/well overnight. Cells were incubated in 1% MVGS for 12h and then treated with commercial EPO, EPO or EPO-R76E in 131 media containing 1% MVGS according to previously published protocol.29 At 48h proliferation was assessed by incubating in tetrazolium salt WST-1 (Millipore, Billerica, MA) for 4h and measuring absorbance at 450nm with a reference of 600nm (Spectramax M5, Molecular Devices, Sunnyvale, CA). The assay was repeated twice with 6 replicates per assay. A student’s t-test was performed.
Tube forming assay
A 24-well plate was coated with growth factor reduced Matrigel Basement Membrane Matrix (BD Biosciences, Franklin Lakes, NJ) and incubated at room temperature for 30min and at 37°C for 1h. Primary HRMECs were serum-starved overnight and then re-suspended in 131 media with 0.5% serum according to previously published protocol.29 Each Matrigel-coated well was plated with 12,000 cells and incubated for 15min at room temperature and 30min at 37°C and then switched to serum-free 131 media containing 40U/ml of commercial EPO, EPO, or EPO-R76E. Images were taken 24h later with an AZ Plan Apo 0.5x objective on a Nikon AZ100 microscope (Melville, NY). The assay was performed twice with 3 replicates per assay. A student’s t-test was performed.
Cell death assay
A 96-well plate was coated with AF and seeded with 7.0×103 HRMEC cells/well and attached overnight. The next day, cells were serum deprived and treated with commercial EPO, EPO, or EPO-R76E with or without 2μg/ml sEPOR. After 18 hrs, levels of DNA in the cell cytoplasm were quantified using the Cell Death Detection ELISA assay following the manufacturer’s instructions (Roche, Indianapolis, IN). Absorbance was measured at 409nm, with a 490nm reference. The assay was performed twice with three replicates per assay. A student’s t-test was performed.
Immunocytochemistry
Sterile 12 mm coverslips were placed in the wells of a 24-well plate and coated with AF. Primary HRMEC were seeded at 2.5 × 103 cells per well and incubated for 24 hrs. The cells were preserved with 4% paraformaldehyde (PFA) for 10 minutes, rinsed with PBS, and incubated in blocking buffer (0.5% Tween-20 and 5% BSA in TBS) for 1 hr. Primary antibody (1:100; anti-EPOR, sc-697; or anti-CD131, sc-678; Santa Cruz) was added to the blocking solution and cells were incubated overnight at 4°C. The cells were then rinsed with PBS and incubated in secondary antibody in the blocking solution at room temperature for 1 hr, washed with PBS and mounted in Vectashield-DAPI.
Mice
Wild type C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were maintained on a 12hr light/dark cycle in the Vanderbilt University animal facility with food and water provided ad libitum. The protocol was approved by the Animal Care and Use Committee of Vanderbilt University and was in accordance with the ARVO statement for the use of animals in vision research.
Intramuscular (IM) injection of viral vectors
The recombinant adeno-associated viral vectors (rAAV) were produced and purified by the University of Pennsylvania Vector Core. Using a beveled Hamilton syringe, 5×109 genome copies (gc) of rAAV2/8.CMV.rhesusEpoR76E or rAAV2/8.CMV.eGFP was delivered into the quadriceps of postnatal day 7 mice.
Electroretinograms (ERG)
Flash ERG recordings were performed 3 (n=15 mice per group) and 7 (n=10 mice per group) months after injection of viral vectors using the Diagnosys Espion System (Diagnosys LLC, Lowell, MA). Briefly, mice were dark-adapted overnight, anesthetized with an intraperitoneal injection of ketamine and xylazine and their pupils dilated with 1% tropicamide. Eyes were kept moist with Systane ultra eye drops. The reference needle electrode was placed in the middle of the forehead and the ground electrode was placed in the rump, both intradermally. A gold wire loop corneal electrode was used. Seven light intensities were tested (−4, −3, −2, −1, 0, 1, and 2 log cd s/m2). A Student’s ttest was performed to compare the two treatment groups.
Optical Coherence Tomography (OCT)
Immediately after performing ERG, anesthetized mice were imaged on a Bioptigen ultra-high resolution OCT system (Bioptigen, Durham, NC). The retinas were imaged with a mouse retina bore and measurements of outer nuclear layer thickness were made using digital, calibrated calipers.
Fluorescein angiography
Three months after vector injection, mice (n=9 per group) were anesthetized with 2.5% isoflurane, the eyes were dilated with 1% tropicamide and 2.5% phenylephrine and kept moist with 2.5% methylcellulose eye drops (Goniovisc Eye Care and Cure, Inc.). The cornea was positioned in contact with the Micron III lens (Phoenix Research Laboratories, Pleasanton, CA). Fluorescein (0.1ml of AK-Fluor-10%; Akorn, Inc, Lake Forest, IL) was injected intraperitoneally and images were collected using appropriate filters on the Micron III microscope.
Western blot analysis of mouse eyes
Mice (n=5 per group) were euthanized and enucleated and eyecups were fast frozen in liquid nitrogen. The same lysis buffer used for cells was added to the eyecups and tissue was mechanically disrupted and the sclera was removed. Remaining lysate was sonicated and incubated with 0.1% SDS on ice for 30 minutes followed by 20 minutes centrifugation at 14000 rpm. Protein quantification was performed as mentioned above.
Equal amounts of protein were resolved by electrophoresis on 0.1% SDS in a 4–20 % Mini-protean TGX gel (Bio-Rad) and transferred to 0.45μm PVDF membrane. The membrane was blocked for 3 hrs in TBS containing 0.1% Tween20 (v/v), 5% (w/v) nonfat dry milk, or 5% (w/v) BSA to detect phospho-proteins. The membrane was incubated in anti-EPO and β-actin. After washing, the membrane was incubated in goat anti-rabbit and goat anti-mouse, followed by washing and incubation in Western Lightning Plus-ECL. Imaging was performed on a Bio-Rad imaging system and Image Lab software and quantification was performed with Quantity One software.
Whole-mount Labeling
For analysis of retinal vasculature, mice were analyzed 3 months post-injection (n=2 per group). Enucleated eyes were preserved in 4% PFA at 4°C for at least 15 min and transferred to cold 2xPBS on ice for 10 min. After that, retinas were dissected, rinsed with PBS, and radial incisions were performed to flatten the retina. The retinas were incubated in cold (−20°C) methanol for 20 min and then blocked in phosphate buffered saline (PBS) containing 0.3% Triton and 0.2% BSA for 1 hr. The retinas were incubated overnight at 4°C with isolectin B4 (isolectin GS-IB4-alexa 488, Invitrogen, Carlsbad, CA) diluted in blocking solution. The next day, retinas were washed with PBS + 0.3% Triton and mounted in Prolong mounting media and imaged on an Olympus confocal microscope (Center Valley, PA).
Immunohistochemistry
Retinal histology and immunohistochemistry was performed at 3 and 7 months after vector injection (n=15 per treatment group). Enucleated eyes were preserved in 4% PFA for 2 hr, cryo-protected in 30% sucrose at 4°C overnight, and embedded in tissue freezing media (Triangle Biomedical, Durham, NC). Ten-micron thick sections were collected on a cryostat (Fisher, Pittsburgh, PA). Slides were rinsed with PBS and incubated at room temperature for 2 hr in normal donkey serum (1:20) in 0.1 M phosphate buffer with 0.5% bovine serum albumin and 0.1% Triton X 100 (PBT). The slides were incubated overnight at 4°C in anti-rhodopsin (1:50, Abcam, Cambridge, MA) or anti-glial fibrillary acidic protein (GFAP, 1:400, Dako, Carpinteria, CA) in PBT, then rinsed with PBS and incubated for 2 hr at room temperature in the appropriate fluorophore-conjugated secondary antibody (donkey anti-rabbit or mouse -Alexa Fluor 488 or 568, Life Technologies, Carlsbad, CA). Slides were rinsed with PBS, mounted in Vectashield Mounting medium with DAPI (Vector Laboratories, Burlingame, CA), and imaged on a Nikon Eclipse epifluorescence microscope (Nikon, Melville, NY). Images were collected at the same magnification, gain, and exposure settings.
Histology
Additional cryo-sections were stained with hematoxylin and eosin (Fisher) and brightfield microscopy was performed on an Olympus Provis AX70 microscope (n=15 per treatment group). Images were collected near the optic nerve head using a Nikon DS-Fi2 camera and NIS elements Advanced Research software (Nikon).
Acknowledgments
The authors thank Megan Capozzi in Dr. John Penn’s laboratory for assistance with the tube formation assay, Dr. Ashwath Jayagopal for assistance with fluorescein angiography, and Kristi Wynn for technical assistance. Stable cell lines were generated and EPO and EPO-R76E were quantified by the VAPR, which is supported by the Vanderbilt Institute of Chemical Biology and the Vanderbilt Ingram Cancer Center (P30 CA68485).
Grant support:
Research to Prevent Blindness Career Development Award; Research to Prevent Blindness Unrestricted Funds (P. Sternberg); CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences; Department of Defense W81XW-10-1-0528; P30-EY008126; NEI EY022349; Fight for Sight
Footnotes
DoD Non Endorsement Disclaimer:
The views, opinions and/or findings contained in this research paper are those of the authors and do not necessarily reflect the views of the Department of Defense and should not be construed as an official DoD/Army position, policy or decision unless so designated by other documentation. No official endorsement should be made.
Conflict of Interest
TSR is a co-inventor on a patent application (U.S. No. 13/979,451) regarding neuroprotective action of EPO-R76E. No licensing has occurred.
References
- 1.Ogunshola O, Bogdanova A. Epo and non-hematopoietic cells: what do we know? Meth Mol Biol. 2013;982:13–41. doi: 10.1007/978-1-62703-308-4_2. [DOI] [PubMed] [Google Scholar]
- 2.García-Ramírez M, Hernández C, Simó R. Expression of erythropoietin and its receptor in the human retina: a comparative study of diabetic and nondiabetic subjects. Diabet Care. 2008;31:1189–1194. doi: 10.2337/dc07-2075. [DOI] [PubMed] [Google Scholar]
- 3.Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- 4.Mennini T, De Paola M, Bigini P, Mastrotto C, Fumagalli E, Barbera S, et al. Nonhematopoietic erythropoietin derivatives prevent motoneuron degeneration in vitro and in vivo. Mol Med. 2006;12:153–160. doi: 10.2119/2006-00045.Mennini. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brines M, Cerami A. The receptor that tames the innate immune response. Mol Med. 2012;18:486–496. doi: 10.2119/molmed.2011.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grimm C, Wenzel A, Groszer M, Mayser H, Seeliger M, Samardzija M, et al. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med. 2002;8:718–724. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- 7.Grimm C, Wenzel A, Stanescu D, Samardzija M, Hotop S, Groszer M, et al. Constitutive overexpression of human erythropoietin protects the mouse retina against induced but not inherited retinal degeneration. J Neurosci. 2004;24:5651–5658. doi: 10.1523/JNEUROSCI.1288-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hines-Beard J, Desai S, Haag R, Esumi N, D’Surney L, Parker S, et al. Identification of a therapeutic dose of continuously delivered erythropoietin in the eye using an inducible promoter system. Curr Gene Ther. 2013;13:275–281. doi: 10.2174/15665232113139990024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilic U, Kilic E, Soliz J, Bassetti C, Gassmann M, Hermann D. Erythropoietin protects from axotomy-induced degeneration of retinal ganglion cells by activating ERK-1/-2. FASEB J. 2005;19:249–251. doi: 10.1096/fj.04-2493fje. [DOI] [PubMed] [Google Scholar]
- 10.King C, Rodger J, Bartlett C, Esmaili T, Dunlop S, Beazley L. Erythropoietin is both neuroprotective and neuroregenerative following optic nerve transection. Exp Neurol. 2007;205:48–55. doi: 10.1016/j.expneurol.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Rex T, Allocca M, Domenici L, Surace E, Maguire A, Lyubarsky A, et al. Systemic but not intraocular Epo gene transfer protects the retina from light-and genetic-induced degeneration. Mol Ther. 2004;10:855–861. doi: 10.1016/j.ymthe.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 12.Rex T, Wong Y, Kodali K, Merry S. Neuroprotection of photoreceptors by direct delivery of erythropoietin to the retina of the retinal degeneration slow mouse. Exp Eye Res. 2009;89:735–740. doi: 10.1016/j.exer.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan T, Geisert E, Hines-Beard J, Rex T. Systemic AAV-mediated gene therapy preserves retinal ganglion cells and visual function in DBA/2J glaucomatous mice. Hum Gene Ther. 2011;22:1191–1200. doi: 10.1089/hum.2011.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan T, Geisert E, Templeton J, Rex T. Dose-dependent treatment of optic nerve crush by exogenous systemic mutant erythropoietin. Exp Eye Res. 2012 doi: 10.1016/j.exer.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan T, Kodali K, Rex T. Systemic gene delivery protects the photoreceptors in the retinal degeneration slow mouse. Neurochem Res. 2011;36:613–618. doi: 10.1007/s11064-010-0272-6. [DOI] [PubMed] [Google Scholar]
- 16.Weishaupt J, Rohde G, Polking E, Siren A-L, Ehrenreich H, Bahr M. Effect of erythropoietin on axotomy-induced apoptosis in rat retinal ganglion cells. Invest Ophthalmol Vis Sci. 2004;45:1514–1522. doi: 10.1167/iovs.03-1039. [DOI] [PubMed] [Google Scholar]
- 17.Zhong L, Bradley J, Schubert W, Ahmed E, Adamis A, Shima D, et al. Erythropoietin promotes survival of retinal ganglion cells in DBA/2J glaucoma mice. Invest Ophthalmol Vis Sci. 2007;48:1212–1218. doi: 10.1167/iovs.06-0757. [DOI] [PubMed] [Google Scholar]
- 18.Villa P, van Beek J, Larsen A, Gerwien J, Christensen S, Cerami A, et al. Reduced functional deficits, neuroinflammation, and secondary tissue damage after treatment of stroke by nonerythropoietic erythropoietin derivatives. J Cereb Blood Flow Metab. 2007;27:552–563. doi: 10.1038/sj.jcbfm.9600370. [DOI] [PubMed] [Google Scholar]
- 19.Gan Y, Xing J, Jing Z, Stetler R, Zhang F, Luo Y, et al. Mutant erythropoietin without erythropoietic activity is neuroprotective against ischemic brain injury. Stroke. 2012;43:3071–3077. doi: 10.1161/STROKEAHA.112.663120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colella P, Iodici C, Di Vicino U, Annunziata I, Surace E, Auricchio A. Non-erythropoietic erythropoietin derivatives protect from light-induced and genetic photoreceptor degeneration. Hum Mol Genet. 2011;20:2251–2262. doi: 10.1093/hmg/ddr115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhanushkodi A, Akano E, Roguski E, Xue Y, Rao S, Matta S, et al. A single intramuscular injection of rAAV-mediated mutant erythropoietin protects against MPTP-induced parkinsonism. Genes Brain and Behavior. 2013;12:224–233. doi: 10.1111/gbb.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribatti D. Angiogenic activity of classical hematopoietic cytokines. Leuk Res. 2012;36:537–543. doi: 10.1016/j.leukres.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Jelkmann W, Elliott S. Erythropoietin and the vascular wall: the controversy continues. Nutr Metab Cardiovasc Dis. 2013;23:S37–43. doi: 10.1016/j.numecd.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida T, Gong J, Xu Z, Wei Y, Duh E. Inhibition of pathological retinal angiogenesis by the integrin αvβ3 antagonist tetraiodothyroacetic acid (tetrac) Exp Eye Res. 2012;94:41–48. doi: 10.1016/j.exer.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kandasamy Y, Kumar P, Hartley L. The effect of erythropoietin on the severity of retinopathy of prematurity. Eye. 2014 doi: 10.1038/eye.2014.95. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heidary G, Vanderveen D, Smith L. Retinopathy of prematurity: current concepts in molecular pathogenesis. Semin Ophthalmol. 2009;24:77–81. doi: 10.1080/08820530902800314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morita M, Ohneda O, Yamashita T, Takahashi S, Suzuki N, Nakajima O, et al. HLF/HIF-2alpha is a key factor in retinopathy of prematurity in association with erythropoietin. EMBO J. 2003;22:1134–1146. doi: 10.1093/emboj/cdg117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caprara C, Grimm C. From oxygen to erythropoietin: relevance of hypoxia for retinal development, health and disease. Progr Ret Eye Res. 2012;31:89–119. doi: 10.1016/j.preteyeres.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Capozzi M, McCollum G, Penn J. The role of cytochrome P450 epoxygenases in retinal angiogenesis. Invest Ophthalmol Vis Sci. 2014;55:4253–4260. doi: 10.1167/iovs.14-14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sühs K, Hein K, Sättler M, Görlitz A, Ciupka C, Scholz K, et al. A randomized, double-blind, phase 2 study of erythropoietin in optic neuritis. Ann Neurol. 2012;72:199–210. doi: 10.1002/ana.23573. [DOI] [PubMed] [Google Scholar]
- 31.Yang Z, Wang H, Jiang Y, Hartnett M. VEGFA activates erythropoietin receptor and enhances VEGFR2-mediated pathological angiogenesis. Am J Pathol. 2014;184:1230–1239. doi: 10.1016/j.ajpath.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe D, Suzuma K, Matsui S, Kurimoto M, Kiryu J, Kita M, et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. New Engl J Med. 2005;353:782–792. doi: 10.1056/NEJMoa041773. [DOI] [PubMed] [Google Scholar]
- 33.Banks W, Jumbe N, Farrell C, Niehoff M, Heatherington A. Passage of erythropoietic agents across the blood-brain barrier: a comparison of human and murine erythropoietin and the analog darbepoetin alfa. Eur J Pharmacol. 2004;505:93–101. doi: 10.1016/j.ejphar.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 34.Elliott S, Busse L, Bass M, Lu H, Sarosi I, Sinclair A, et al. Anti-Epo receptor antibodies do not predict Epo receptor expression. Blood. 2006;107:3454. doi: 10.1182/blood-2005-10-4066. [DOI] [PubMed] [Google Scholar]