Abstract
HIV/AIDS in Latin America is concentrated among men who have sex with men (MSM). However, accurate estimates of engagement in HIV care in this population can be difficult to ascertain because many do not self-identify as MSM. Given evidence of decreased HIV transmissibility in the context of antiretroviral therapy (ART) adherence, identifying individuals not in care who are engaging in HIV transmission risk behavior is crucial for secondary prevention. Primary aims of this study were to examine engagement in care from testing to ART adherence among MSM using online social/sexual networking across Latin America, and whether individuals not in care at each step reported greater sexual transmission risk behavior than those in care. In the overall sample (n=28,779), approximately 75% reported ever being tested for HIV and 9% reported having received an HIV diagnosis. Among known HIV-infected individuals, 20% reported not being in care, 30% reported not taking ART, and 55% reported less than 100% ART adherence. Over one-third of HIV-infected individuals reported sexual HIV transmission risk behavior, defined as unprotected anal intercourse (UAI) with a male partner of different/unknown HIV serostatus in the past three months. HIV-infected individuals not engaged in care more often reported UAI compared to those in care (OR=1.29; 95%CI=1.01–1.66). Although not statistically significant, HIV-infected individuals not on ART more often reported UAI compared to those on ART (OR=1.18; 95%CI=0.94–1.47). Individuals who reported less than 100% ART adherence more often reported UAI compared to individuals with 100% adherence (OR=1.55; 95%CI=1.26–1.90). Findings demonstrate that a substantial portion of HIV-infected MSM in Latin America who are likely not virologically suppressed from lack of ART use or adherence report sexual HIV transmission risk. Tailoring secondary HIV prevention for MSM in Latin America who are not in HIV care or adherent to ART may be warranted.
Keywords: Men who have sex with men, Latin America, Antiretroviral therapy, Adherence, Secondary prevention
Introduction
The HIV epidemic in Latin America is concentrated among men who have sex with men (MSM) (Bastos, Cáceres, Galvao, Veras, & Castilho, 2008). Almost half of all HIV infections in this region have resulted from unprotected anal intercourse between men, and the likelihood of HIV infection among MSM is higher in Latin America and the Caribbean than any other area of the world (Pan American Health Organization, 2010). Despite this, there has been scant research examining engagement in HIV care in this population, which is crucial for reducing HIV/AIDS-related mortality (Gonzalez, Martin, Munoz, & Jacobson, 2011) and transmission of unsuppressed virus to sexual partners (Cohen et al., 2011).
The HIV treatment cascade is one heuristic developed to track population-levels of HIV testing, diagnosis, linkage to care, ART use, ART adherence, and virologic suppression in order to gauge the effectiveness of public health interventions (Gardner, McLees, Steiner, del Rio, & Burman, 2011; Hall et al., 2013). Prior research has examined parts of the HIV treatment cascade among MSM in select countries, including Argentina (Carballo-Dieguez et al., 2014), Peru (Sanchez et al. 2007), and Brazil (Kerr et al., 2013). These studies have found low HIV testing rates, with less than half of MSM participants having been tested for HIV in Argentina (Carballo-Dieguez et al., 2014) and Brazil (Kerr et al., 2013), high HIV prevalence (14–17%; Carballo-Dieguez et al., 2014; Kerr et al., 2013), and lower rates of ART initiation among MSM (i.e., compared to non-MSM counterparts in Argentina; Zala et al., 2008).
However, it has been challenging to estimate engagement in care in this population given that many men may not self-identify as gay or homosexual (Cáceres, 2002; Cáceres, Konda, Pecheny, Chatterjee, & Lyerla, 2006). Yet, it is estimated that 3 to 20% of adult males have had some sexual contact with other males in Latin America (PAHO, 2010). Culturally prevalent stigma and social exclusion may create additional barriers for MSM to acknowledge their HIV risk and to access health care (Cáceres & Stall, 2003). Given these barriers, recruitment of MSM in the community or in HIV care settings may be challenging and exclude certain subsets of MSM. Although it cannot provide population-level estimates, online social/sexual networking sites may be a useful alternative to venue or community-based recruitment to reach an important and often hidden group of MSM in Latin America (Curioso et al., 2007; Marcus et al., 2009; Young et al., 2015). As internet access has expanded, there is widespread use of online social/sexual networking worldwide. It is estimated that 2.5 to 6.2 million MSM use the internet to meet sexual partners (Curioso et al., 2007; Liau, Millett, & Marks, 2006).
In addition to better characterizing engagement in care among high risk HIV-infected MSM in Latin America, it is also important to examine whether individuals not in care are engaging in potential HIV transmission risk behaviors. Individuals not on ART or adherent to ART are biologically most likely to transmit HIV due to unsuppressed viral load (Cohen et al., 2011). Identifying the behavioral HIV risk profiles of individuals not engaged in care may inform targeted secondary prevention efforts.
The current study recruited from a large online social/sexual networking website throughout Latin America to assess HIV transmission risk behavior alongside HIV care engagement. Primary aims of the current study were to examine: 1) the continuum of engagement in HIV care among a large sample of MSM recruited via online social/sexual networking across all of Latin America and 2) whether individuals not engaged in care at each step of the continuum were engaging in greater sexual risk behavior than individuals in care. Secondary aims were to identify sociodemographic correlates of individuals who may be most likely to transmit HIV and regional and country-level differences in the continuum of HIV care.
Methods
Participants and Procedures
Participants were recruited from an online social and sexual networking website that caters towards gay, bisexual and other MSM, and has members in all countries across the globe. The website allows users to create individual profiles and interact with other users using an internal messaging system. For purposes of this study, participants were only included in analyses if they indicated their home location was in one of the designated countries of interest (i.e. Latin America). Between October and November 2012, an electronic message was sent to all adult (≥18 years of age) active site users (i.e., had logged on in the past 90 days) whose profile indicated that they lived in one of the Spanish- or Portuguese-speaking countries/territories in Latin America/Caribbean, or in Spain or Portugal. The message was generated by the site and provided a brief description of the study purpose with a link to the study website. Upon visiting the study website, individuals could read a more detailed description of the study procedures. If interested, they could proceed to the online study consent form. If the individual decided to participate, they moved directly from the online consent form to the study survey. Individuals were not compensated for participation. To minimize duplicate responses, the survey was programmed to only allow access from a unique IP address on a single occasion. The e-mail remained in each individual’s electronic mailbox for a period of 30 days, after which time any unopened e-mails were removed.
The initial e-mail was sent to approximately 643,000 individuals. In total, 246,620 emails were opened and 56,584 clicked the provided link to the survey. Among these, 36,447 (64%) initiated the survey. For this study focusing on Latin America specifically, we excluded individuals not living in Latin America, and those who did not report being male sex at birth. The total sample after excluding these groups was 28,779 (79.8% of total respondents). All procedures were approved by the Fenway Institute Institutional Review Board.
Assessments
Sociodemographic characteristics
Age, sexual orientation, education, income, and type of living area (urban vs. rural) were assessed. Additionally, respondents indicated the country in which they currently lived.
HIV testing
All respondents were asked whether they had ever been tested for HIV.
Self-reported HIV
All respondents were asked to report whether they had ever been told by a health care provider that they had HIV-infection.
Receipt of HIV medical care and ART
Respondents who self-reported being HIV-infected were asked to report whether they were currently receiving medical care for HIV, and whether they were currently taking ART to treat HIV.
ART adherence
Respondents who were HIV-infected and on ART were asked to report their best estimate of how much of their prescribed ART they had taken in the last month. As recommended in the field when assessing self-reported ART adherence (Simoni, Kurth, Pearson, Pantalone, Merrill, & Frick, 2006), the instructions normalized the difficulties of adherence (i.e., “many people find it difficult to take all of their HIV medications exactly as prescribed by their doctor…We would be surprised if this were 100% for most people”). Adherence rates were dichotomized at 100% vs. less than 100% to account for overestimation of self-reported adherence levels.
Country-level ART coverage
For the seven countries with the largest sample sizes (>500; Argentina, Brazil, Chile, Columbia, Mexico, Peru, Venezuela), the percentage of the country’s HIV-infected population estimated to be receiving ART was assessed. This was derived from the World Health Organization (WHO) Global HIV/AIDS Response Report (WHO, 2011).
Sexual risk behavior was defined as any unprotected anal intercourse (UAI) with at least one male or transgender partner of different/unknown HIV serostatus in the past three months.
Statistical Analysis
To examine the continuum of engagement in HIV care in this sample, percentages were calculated for self-reported HIV testing, HIV diagnosis, engagement in HIV care, ART use, and ART adherence for all of Latin America and by region and country of residence (for the seven countries with the largest sample sizes; >500). Chi square tests were performed to examine regional and country-level differences. Additionally, the association between country-level ART coverage in those seven countries and self-reported ART use in this sample was examined using logistic regression.
To compare rates of UAI among HIV-infected individuals based on engagement in care, multivariable logistic regressions were performed to examine differences in rates of sexual risk behavior at each point in the HIV care continuum: in HIV care (yes vs. no), on ART (yes vs. no) and adherent to ART (100% vs. not 100%) adjusting for sociodemographic characteristics. To identify sociodemographic correlates of individuals who may be most likely to transmit HIV, multivariable logistic regressions were used; individuals were categorized as ‘potential HIV transmitters’ if they were HIV-infected, not on ART, and reported UAI with at least one partner with different or unknown HIV serostatus in the past three months. For all analyses, a complete-case analysis was conducted. All analyses were done in SAS v9.3.
Results
Participants
Among the 28,779 respondents, the median age was 28 (IQR=23–25). Three-quarters (75.7%) identified as homosexual or gay, 20.0% as bisexual, 3.6% as unsure/questioning and 0.7% as straight. Seventy-eight percent had at least a university education and 74.4% reported being middle income. Most (95.9%) respondents reported living in an urban area; 62.5% lived in South America, 32.9% in Mexico and 4.6% in Central America. See Table i for sociodemographic characteristics by country of residence (for countries with sample sizes >500).
Table i.
Sociodemographic characteristics, overall and by country of residence1
| Overall2 (N=28,779) |
Argentina (N=2,848) |
Brazil (N=4,592) |
Chile (N=1,912) |
Colombia (N=3,705) |
Mexico (N=9,465) |
Peru (N=869) |
Venezuela (N=3,066) |
|
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| SOCIODEMOGRAPHIC CHARACTERISTICS | Median (IQR) | |||||||
| Age | 28 (23–25) | 31 (25–39) | 29 (24–37) | 28 (23–35) | 26 (22–33) | 28 (23–35) | 28 (23–35) | 28 (23–36) |
| Sexual Orientation | % (N) | |||||||
| Heterosexual/Straight | 0.7 (198) | 0.7 (20) | 1.0 (43) | 0.9 (17) | 0.4 (16) | 0.6 (52) | 0.9 (8) | 0.8 (23) |
| Bisexual | 20.0 (5624) | 17.5 (485) | 20.4 (915) | 14.7 (273) | 18.8 (678) | 20.1 (1857) | 26.3 (224) | 24.3 (725) |
| Unsure/Questioning/Other | 3.6 (998) | 3.4 (94) | 4.0 (177) | 3.2 (59) | 3.5 (126) | 3.1 (289) | 4.6 (39) | 3.4 (101) |
| Homosexual/Gay | 75.7 (21259) | 78.4 (2181) | 74.6 (3339) | 81.2 (1511) | 77.3 (2791) | 76.2 (7053) | 68.2 (580) | 71.5 (2129) |
| Education | ||||||||
| University/Post-Graduate | 78.2 (21910) | 61.1 (1699) | 73.8 (3284) | 70.7 (1316) | 74.1 (2674) | 88.3 (8130) | 80.6 (686) | 80.1 (2394) |
| Less than University | 21.8 (6109) | 38.9 (1083) | 26.2 (1164) | 29.3 (546) | 25.9 (935) | 11.7 (1078) | 19.4 (165) | 19.9 (594) |
| Income | ||||||||
| No income | 8.1 (2193) | 7.6 (203) | 4.6 (201) | 13.9 (249) | 11.1 (382) | 8.1 (722) | 9.2 (76) | 6.4 (184) |
| Low income/lower class | 8.7 (2362) | 6.6 (177) | 11.4 (496) | 7.4 (133) | 10.8 (370) | 7.2 (647) | 10.4 (86) | 9.9 (284) |
| Middle income/middle class | 74.4 (20101) | 79.0 (2105) | 74.1 (3217) | 66.5 (1189) | 68.2 (2341) | 76.0 (6781) | 73.4 (605) | 78.8 (2259) |
| High income/upper class | 8.8 (2373) | 6.8 (181) | 9.9 (429) | 12.1 (217) | 9.9 (339) | 8.7 (775) | 6.9 (57) | 4.8 (139) |
| Urban/Rural | ||||||||
| Urban | 95.9 (27605) | 96.8 (2757) | 97.8 (4489) | 95.8 (1831) | 96.6 (3579) | 95.3 (9023) | 98.2 (853) | 94.3 (2892) |
| Rural | 4.1 (1174) | 3.2 (91) | 2.2 (103) | 4.2 (81) | 3.4 (126) | 4.7 (442) | 1.8 (16) | 5.7 (174) |
Only countries with large sample sizes (at least 500) were included in country-specific analyses
Includes Argentina, Bolivia, Brazil, Chile, Colombia, Costa Rica, Ecuador, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama, Paraguay, Peru, Uruguay, Venezuela
Engagement in HIV Care
Figure i depicts the responses at each step of the continuum of HIV care for all participants in Latin America: 74.1% reported ever being tested for HIV, and 9.0% reported HIV diagnosis. Among those who were HIV-infected, 20.0% were not in medical care, 29.1% not receiving ART, and 55.3% had less than 100% ART adherence.
Figure i.
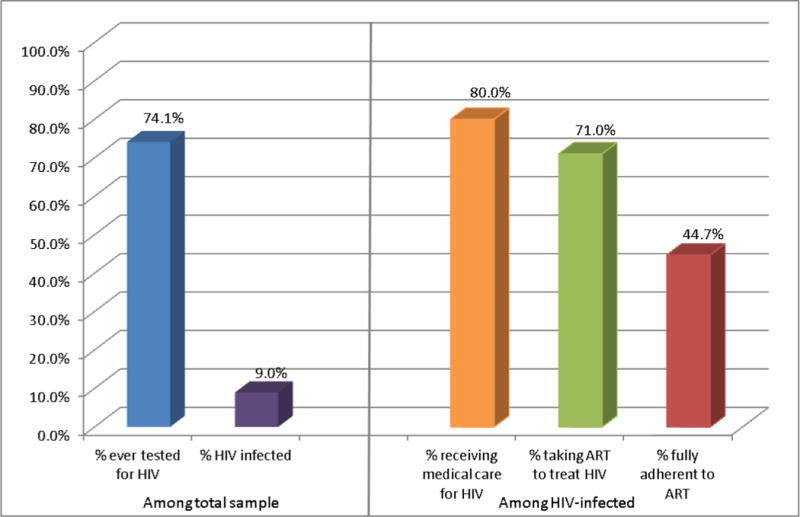
Engagement in HIV care from testing to ART adherence among all individuals surveyed across Latin America
Note: Total sample n = 28,779; HIV-infected sample n = 2,350
Table ii lists the regional and country-specific differences for rates of HIV testing, HIV diagnosis, as well as engagement in HIV care, ART use, and ART adherence (among HIV-infected individuals). There were significant regional differences in testing rates and rates of HIV diagnoses, with South America having the highest rates of both testing and self-reported HIV diagnosis compared to Mexico and Central America. There were no significant regional differences in rates of medical care receipt, ART receipt, or ART adherence. There were significant country-level differences in rates of HIV testing, HIV diagnosis, engagement in medical care and ART adherence (see Table ii). The highest rates of HIV testing were reported in Venezuela, and the highest rates of HIV infection were in Chile. Among HIV-infected individuals, the highest rates of engagement in care were reported in Brazil, and ART adherence was highest in Brazil and Argentina. There were no significant country-level differences in rates of ART use. Further, country-level ART coverage (i.e., the WHO estimates of the percentage of people living with HIV on ART in each country) was not associated with self-reported ART use in this sample (OR=1.53, 95%CI=0.86–2.75; p=0.145).
Table ii.
Regional and country-specific differences in engagement in care from testing to ART adherence
| Ever HIV tested | p-value | Self-report HIV+ | p-value | Currently in HIV care (among HIV-infected) | p-value | Currently on ART (among HIV-infected) | p-value | 100% ART Adherent (among HIV-infected on ART) | p-value | |
|---|---|---|---|---|---|---|---|---|---|---|
| % (N) | % (N) | % (N) | % (N) | % (N) | ||||||
|
|
||||||||||
| Region | <0.0001 | <0.0001 | 0.309 | 0.142 | 0.920 | |||||
| South America1 | 76.4 (12581) | 10.0 (1637) | 80.9 (1251) | 69.9 (1085) | 44.5 (678) | |||||
| Central America2 | 74.9 (915) | 6.0 (73) | 77.1 (54) | 77.5 (55) | 43.7 (31) | |||||
| Mexico | 69.5 (6071) | 7.4 (640) | 78.2 (484) | 73.2 (454) | 45.4 (280) | |||||
| Country-specific3 | <0.0001 | <0.0001 | <0.0001 | 0.188 | <0.0001 | |||||
| Argentina | 81.5 (2117) | 11.8 (303) | 81.3 (239) | 74.5 (219) | 53.5 (155) | |||||
| Brazil | 71.5 (3005) | 11.4 (478) | 91.1 (400) | 68.0 (300) | 51.7 (223) | |||||
| Chile | 76.6 (1326) | 15.2 (259) | 75.6 (183) | 70.7 (171) | 39.7 (92) | |||||
| Colombia | 70.9 (2417) | 8.3 (282) | 77.3 (208) | 68.6 (186) | 34.9 (94) | |||||
| Mexico | 69.5 (6071) | 7.4 (640) | 78.2 (484) | 73.2 (454) | 45.4 (280) | |||||
| Peru | 74.1 (578) | 10.1 (78) | 64.9 (50) | 62.3 (48) | 29.3 (22) | |||||
| Venezuela | 85.1 (2403) | 6.4 (182) | 74.7 (130) | 72.0 (126) | 39.9 (69) | |||||
Includes Argentina (15.8%), Bolivia (0.9%), Brazil (25.5%), Chile (10.6%), Colombia (20.6%), Ecuador (2.6%), Paraguay (0.8%), Peru (4.8%), Uruguay (1.3%), Venezuela (17.0%)
Includes Costa Rica (34.8%), El Salvador (10.5%), Guatemala (18.7%), Honduras (4.5%), Nicaragua (6.3%) and Panama (25.2%)
Only countries with large sample sizes (at least 500) were included in country-specific analyses
Engagement in HIV Care and UAI
Among all HIV-infected individuals (n = 2,350), 37.3% reported they had engaged in UAI with at least one partner with a different or unknown HIV serostatus in the past three months. Table iii shows the associations between engagement in HIV care at each step of the continuum among HIV-infected individuals in the sample and UAI. HIV-infected individuals not engaged in HIV care more often reported UAI compared to HIV-infected individuals in care (42.7% vs. 36.2%; OR=1.29; p < .05). Although not statistically significant (p=.155), HIV-infected individuals not on ART (compared to those on ART) more often reported UAI (40.4% vs. 36.1%); that is, 40% of those who would likely not be virologically suppressed without ART use reported engaging in behaviors that could transmit HIV. Individuals who did not report 100% adherence to ART more often reported UAI compared to individuals who did report 100% adherence (42.1% vs. 31.8%; OR=1.55; p<.0001). See table iii for additional results.
Table iii.
Associations between engagement in HIV care and sexual risk behavior, adjusted for sociodemographic characteristics1
| UAI with at least one partner with a different or unknown HIV serostatus% | AOR (95% CI)1 | p-value | |
|---|---|---|---|
|
|
|||
| Currently receiving medical care for HIV (among HIV-infected) | |||
| Yes | 36.2 | 1.0 | |
| No | 42.7 | 1.29 (1.01–1.66) | 0.044 |
| Currently on ART (among HIV-infected) | |||
| Yes | 36.1 | 1.0 | |
| No | 40.4 | 1.18 (0.94–1.47) | 0.155 |
| 100% Adherent to ART (among HIV-infected on ART) | |||
| Yes | 31.8 | 1.0 | |
| No | 42.1 | 1.55 (1.26–1.90) | <0.0001 |
Adjusted for sociodemographic characteristics, including age, urban vs. rural place of residence, region, income, education and sexual orientation; Note: HIV-infected sample n = 2,350
In the total sample, 0.8% (n=205) were included in the category of ‘potential HIV transmitter.’ Table iv shows the association between sociodemographic characteristics and being in the ‘potential HIV transmitter’ category. Only sexual orientation was significantly associated with being a potential HIV transmitter (p < 0.0001). Specifically, participants who identified as bisexual (OR=0.31, 95%CI= 0.18–0.52) or unsure/other (OR=0.12, 95%CI=0.02–0.85) were less likely to be potential HIV transmitters than those who identified as gay. All other sociodemographic characteristics were unrelated to being in the ‘potential HIV transmitter’ category (all ps > .2; see Table iv).
Table iv.
Multivariable associations between sociodemographic factors and ‘potential HIV transmitter’ category
| Sociodemographic factors | AOR (95%CI)1 | p-value |
|---|---|---|
| Age | 1.09 (0.98–1.02) | 0.923 |
| Sexual orientation | <0.0001 | |
| Heterosexual/straight | 0.58 (0.08–4.17) | |
| Bisexual | 0.31 (0.18–0.52) | |
| Unsure/questioning/other | 0.12 (0.02–0.85) | |
| Homosexual/gay | 1.0 | |
| Education | 0.241 | |
| University/Post-graduate | 1.25 (0.86–1.83) | |
| Less than university | 1.0 | |
| Income | 0.482 | |
| No income | 1.0 | |
| Low income/lower class | 1.61 (0.76–3.38) | |
| Middle income/middle class | 1.35 (0.72–2.54) | |
| High income/upper class | 1.05 (0.47–2.34) | |
| Urban/rural residence | 0.56 | |
| Urban | 1.28 (0.56–2.89) | |
| Rural | 1.0 | |
| Region | ||
| Mexico | 0.77 (0.56–1.06) | .267 |
| Central America | 0.89 (0.45–1.75) | |
| South America | 1.0 |
Adjusted for all sociodemographic characteristics listed in table;
Note: .8% of total sample (n=205) were included in the category of ‘potential HIV transmitter’ (i.e., categorized as a ‘potential HIV transmitter’ if HIV-infected, not on ART, and reported UAI with at least one partner with different or unknown HIV serostatus in the past three months.
Discussion
Current findings describe the continuum of engagement in HIV care in a large sample of MSM in Latin America who may have been at risk for HIV and other STD transmission or acquisition given internet sex-seeking (Liau et al., 2006), and who may not have self-identified or disclosed same sex behavior. Although this sampling method may present limitations regarding generalizability, online surveys via internet-based social/sexual networking may reach an often hidden population of MSM in Latin America (Curioso et al., 2007; Marcus et al., 2009; Young et al., 2015). This is important given the concentration of the HIV/AIDS epidemic among MSM in Latin America (Bastos et al., 2008) and evidence that these individuals may delay ART initiation because of high levels of HIV stigma, resulting in poorer subsequent HIV/AIDS health outcomes (Zala et al., 2008).
Unlike attempts to depict the HIV treatment cascade with a population-based sampling method (Gardner et al., 2011), we aimed to describe patterns of engagement in HIV clinical care among internet sex-seeking MSM in Latin America. Results demonstrated that approximately one-fourth of the sample had never been tested for HIV. Among HIV-infected respondents, one-fifth was not engaged in medical care, approximately one-third were not on ART, and over half reported suboptimal ART adherence. These parameters may be underestimated since it is likely that there were individuals who were unaware of their HIV status or unwilling to disclose their status in this anonymous survey. However, they provide a useful starting point in beginning to understand gaps in HIV clinical care among this group.
There has been some speculation and concern that unprotected sex may increase in the context of ART use because of behavioral disinhibition (Venkatesh, Flanigan, & Mayer, 2011). Current findings demonstrated that in this sample, HIV-infected MSM who were not on ART were as likely to engage in UAI as those who were on ART. These findings add to the limited data on the relationship between ART use and UAI among Latin American MSM. Regarding engagement in HIV care and UAI, HIV-infected individuals not in care were more likely to report UAI compared to HIV-infected individuals in care. Although not assessed directly, this may reflect those in care having received effective, behavioral interventions to reduce sexual HIV transmission risk behavior.
HIV-infected individuals who were not fully adherent to ART, and thus more likely to have detectable viremia and greater risk of HIV transmission (Cohen et al., 2011), were more often engaging in UAI compared to individuals who were fully ART adherent. Further, approximately one-fifth of the HIV-infected sample indicated they were diagnosed with HIV within the past year, and thus may pose greater transmission risk if engaging in UAI during acute HIV infection when infectiousness may be greatest (Koopman et al., 1997). However, it is important to note that the current study did not include a measure of plasma HIV RNA, so the actual magnitude of HIV transmission risk could not be assessed.
In the region- and country-specific analyses, it was interesting to note that there were no country-specific differences in rates of ART uptake. Further, rates of ART use in this sample by country did not reflect what would be expected based upon WHO estimates of national ART coverage levels. This echoes other findings that even when universal ART access may be available, there continue to be sociocultural and individual barriers to HIV treatment and ART use (Souza, Szwarcwald, & Ayres, 2007), which may be exacerbated among MSM.
Regarding the country-level differences in engagement in HIV care, South America, and Venezuela in particular, had high rates of HIV testing. Chile had the highest percentage of reported HIV diagnoses, which is consistent with other reports showing high rates of HIV/AIDS among MSM in Chile (Bastos et al., 2008). It is important to interpret these results in the context of current MSM-tailored HIV prevention and care efforts in the specific countries and regions. Brazil, a country with one of the first and most progressive national responses to providing ART free and universally in Latin America, in this sample had the highest percentage of individuals in medical care. Among those on ART, individuals in Brazil were reporting some of the highest rates of adherence. These high rates of medical care use and ART adherence in Brazil may reflect national efforts to include MSM in initiatives related to HIV prevention, treatment and care; through partnerships with the public health sector and local LGBT NGOs, there have been efforts to expand access to voluntary testing and counseling (VCT) for MSM (Pact Brasil, 2010), and other national priorities to focus on engaging MSM in HIV/AIDS prevention and reducing stigma and homophobia. Culturally tailored HIV prevention and treatment efforts are needed for MSM across Latin America to optimize engagement in care and to decrease community viral load (Cáceres, 2002; Cáceres & Stall, 2003).
A primary limitation of this study relates to the potential lack of generalizability of this sample. A recent study (Kerr et al., 2013) of a large sample of MSM in Brazil (n =3,859) found that online sexual networking was not the most predominant mode of finding sexual partners. Individuals recruited here may represent a biased sample with internet access; this sample was skewed towards higher education and income levels with the majority dwelling in urban areas, which may bias our estimates of HIV prevalence among MSM in Latin America. Indeed, the HIV prevalence estimates found in the current study are lower than what has been documented in other studies (Baral, Sifakis, Cleghorn, & Beyrer, 2007). This sample also may have had greater access to prevention and treatment, thereby overestimating rates of engagement in care. Other limitations of this study include the cross-sectional design and use of self-report data. Rates of ART adherence reported were extremely high, as is often found in self-report adherence data; as such, we elected to use adherence at 100% or not, which has limitations regarding measurement precision and bias.
Conclusions
Given the complexities of identifying openly as MSM in Latin America and increasingly popularity of anonymous social networking sites, this sampling method may have broad reach in attracting men who do not self-identify as MSM, who may be less likely to attend sampling venues, and less likely to participate in a face-to-face research study. However, continued work in this area should consider more objective indicators of the care continuum and longitudinal assessments in order to develop a broader model of factors that may further exacerbate gaps in HIV clinical care. Despite the noted limitations, these data suggest that Latin American MSM who have been diagnosed with HIV but are not engaged in care or adhering to ART are an important population to focus on with innovative HIV prevention efforts. Recent evidence suggests that social networking sites may be an appealing and efficacious platform for delivering HIV prevention interventions to high-risk populations in Latin America (e.g., Peru; Young et al., 2015). Programs that ensure both increased and consistent use of ART and reductions in sexual risk among gay men will both be crucial to ensure a slowing of the HIV epidemic in this population (Venkatesh et al., 2011).
Acknowledgments
Dr. Magidson’s work on this manuscript was supported by NIH grant T32MH093310. Dr. Safren was supported by NIH grant K24MH094214. Dr. Mayer and Dr. Safren were also supported by the Harvard University Center for AIDS Research (CFAR) NIH P30AI060354. This manuscript was supported by consultation from other members of the HU CFAR Social and Behavioral Sciences Core.
Footnotes
All authors report no conflicts of interest.
References
- Bastos FI, Cáceres C, Galvao J, Veras MA, Castilho EA. AIDS in Latin America: assessing the current status of the epidemic and the ongoing response. International Journal of Epidemiology. 2008;37:729–737. doi: 10.1093/ije/dyn127. [DOI] [PubMed] [Google Scholar]
- Cáceres CF. HIV among gay and other men who have sex with men in Latin America and the Caribbean: a hidden epidemic? AIDS. 2002;16:S23–33. doi: 10.1097/00002030-200212003-00005. [DOI] [PubMed] [Google Scholar]
- Cáceres C, Konda K, Pecheny M, Chatterjee A, Lyerla R. Estimating the number of men who have sex with men in low and middle income countries. Sexually Transmitted Infections. 2006;82(S3):iii3–iii9. doi: 10.1136/sti.2005.019489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cáceres CF, Stall R. Commentary: The human immunodeficiency virus/AIDS epidemic among men who have sex with men in Latin America and the Caribbean: it is time to bridge the gap. International Journal of Epidemiology. 2003;32:740–743. doi: 10.1093/ije/dyg214. [DOI] [PubMed] [Google Scholar]
- Carballo-Diéguez A, Balán IC, Dolezal C, Pando MA, Marone R, Barreda V, Ávila MM. HIV testing practices among men who have sex with men in Buenos Aires, Argentina. AIDS Care. 2014;26(1):33–41. doi: 10.1080/09540121.2013.793277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesar C, Shepherd BE, Krolewiecki AJ, Fink VI, Schechter M, Tuboi SH, Cahn PE. Rates and reasons for early change of first HAART in HIV-1-infected patients in 7 sites throughout the Caribbean and Latin America. PLoS One. 2010;5(6):e10490. doi: 10.1371/journal.pone.0010490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chequer P, Cuchí P, Mazin R, Calleja JM. Access to antiretroviral treatment in Latin American countries and the Caribbean. AIDS. 2002;16:S50–S57. doi: 10.1097/00002030-200212003-00008. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Fleming TR. Prevention of HIV-1 infection with early antiretroviral therapy. New England Journal of Medicine. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curioso WH, Blas MM, Nodell B, Alva IE, Kurth AE. Opportunities for providing web-based interventions to prevent sexually transmitted infections in Peru. PLoS Medicine. 2007;4(2):e11. doi: 10.1371/journal.pmed.0040011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EM, McLees MP, Steiner JF, del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clinical Infectious Diseases. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MA, Martin L, Munoz S, Jacobson JO. Patterns, trends, and sex differences in HIV/AIDS reported mortality in Latin American countries: 1996–2007. BMC Public Health. 2011;11:605. doi: 10.1186/1471-2458-11-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall HI, Frazier EL, Rhodes P, Holtgrave DR, Furlow-Parmley C, Tang T, Skarbinski J. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Internal Medicine. 2013;173(14):1337–1344. doi: 10.1001/jamainternmed.2013.6841. [DOI] [PubMed] [Google Scholar]
- Kerr LR, Mota RS, Kendall C, Pinho ADA, Mello MB, Guimaraes MD, HIVMSM Surveillance Group HIV among MSM in a large middle-income country. AIDS. 2013;27(3):427–435. doi: 10.1097/QAD.0b013e32835ad504. [DOI] [PubMed] [Google Scholar]
- Koopman JS, Jacquez JA, Welch GW, Simon CP, Foxman B, Pollock SM, Lange K. The role of early HIV infection in the spread of HIV through populations. Journal of Acquired Immune Deficiency Syndromes. 1997;14(3):249–258. doi: 10.1097/00042560-199703010-00009. [DOI] [PubMed] [Google Scholar]
- Liau A, Millett G, Marks G. Meta-analytic examination of online sex-seeking and sexual risk behavior among men who have sex with men. Sexually Transmitted Diseases. 2006;33(9):576–584. doi: 10.1097/01.olq.0000204710.35332.c5. [DOI] [PubMed] [Google Scholar]
- Marcus U, Schmidt AJ, Hamouda O, Bochow M. Estimating the regional distribution of men who have sex with men (MSM) based on Internet surveys. BMC Public Health. 2009;9(1):180. doi: 10.1186/1471-2458-9-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACT Brasil. Increase and expand HIV testing options program. Arlington, VA: AIDSSTAR-One; 2010. [Google Scholar]
- Pan American Health Organization. Blueprint for the Provision of Comprehensive Care to Gay Men and Other Men Who Have Sex with Men (MSM) in Latin America and the Caribbean. Washington, D.C.: OPS; 2010. [Google Scholar]
- Sanchez J, Lama JR, Kusunoki L, Manrique H, Goicochea P, Lucchetti A, Peruvian HIV Sentinel Surveillance Working Group HIV-1, sexually transmitted infections, and sexual behavior trends among men who have sex with men in Lima, Peru. Journal of Acquired Immune Deficiency Syndromes. 2007;44(5):578–585. doi: 10.1097/QAI.0b013e318033ff82. [DOI] [PubMed] [Google Scholar]
- Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS and Behavior. 2006;10(3):227–245. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza PRB, Jr, Szwarcwald CL, Castilho EA. Delay in introducing antiretroviral therapy in patients infected by HIV in Brazil, 2003–2006. Clinics. 2007;62(5):579–584. [PubMed] [Google Scholar]
- Venkatesh KK, Flanigan TP, Mayer KH. Is expanded HIV treatment preventing new infections? Impact of antiretroviral therapy on sexual risk behaviors in the developing world. AIDS. 2011;25(16):1939–1949. doi: 10.1097/QAD.0b013e32834b4ced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) and UNAIDS. Global HIV/AIDS Response: Epidemic Update and Health Sector Progress Towards Universal Access: Progress Report 2011. Geneva: WHO; 2011. [Google Scholar]
- Young SD, Cumberland WG, Nianogo R, Menacho LA, Galea JT, Coates T. The HOPE social media intervention for global HIV prevention in Peru: a cluster randomised controlled trial. The Lancet HIV. 2015;2(1):e27–e32. doi: 10.1016/S2352-3018(14)00006-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zala C, Rustad CA, Chan K, Khan NI, Beltran M, Warley E, Cahn P. Determinants of treatment access in a population-based cohort of HIV-positive men and women living in Argentina. Journal of the International AIDS Society. 2008;10(4):78. doi: 10.1186/1758-2652-10-4-78. [DOI] [PMC free article] [PubMed] [Google Scholar]


