Abstract
Objective
HIV-associated deficits in verbal episodic memory are commonly associated with antiretroviral non-adherence; however, the specific aspects of memory functioning (e.g., encoding, consolidation, or retrieval) that underlie this established relationship are not well understood.
Method
This study evaluated verbal memory profiles of 202 HIV+ participants who underwent a 30-day electronic monitoring of antiretroviral adherence.
Results
At the group level, non-adherence was significantly associated with lower scores on immediate and delayed passage recall and word list learning. Retention and recognition of passages and word lists were not related to adherence. Participants were then classified as having either a normal verbal memory profile, a “subcortical” retrieval profile (i.e., impaired free recall with relatively spared recognition), or a “cortical” encoding profile (e.g., cued recall intrusions) based on the Massman et al. (1990) algorithm for the California Verbal Learning Test. HIV+ participants with a classic retrieval deficit had significantly greater odds of being non-adherent than participants with a normal or encoding profile.
Conclusions
These findings suggest that adherence to prescribed antiretroviral regimens may be particularly vulnerable to disruption in HIV+ individuals due to deficits in the complex process of efficiently accessing verbal episodic information with minimal cues. A stronger relationship between non-adherence and passage (vs. word list) recall was also found and may reflect the importance of contextual features in remembering to take medications. Targeted interventions for enhancing and supporting episodic memory retrieval processes may improve antiretroviral adherence and overall health outcomes among persons living with HIV.
Keywords: Immediate recall, long-term memory, recognition, Wechsler Scales, medication compliance
Proper adherence (90-95%) to combination antiretroviral medication therapy (cART) is critical for improving health outcomes in HIV (Maggiolo et al., 2007). However, it is estimated that less than 40% of HIV+ individuals are retained in long-term healthcare management and only about one-quarter are virally suppressed in the U.S. (Centers for Disease Control and Prevention, 2012). Further, approximately half of individuals prescribed antiretroviral medications do not fully adhere to their regimen (Howard et al., 2002). Suboptimal cART adherence is associated with increased viremia, immune suppression, increased risk of HIV transmission, and mortality (e.g., García et al., 2002). Numerous factors appear to be contributing to suboptimal cART adherence, including psychiatric comorbidities (e.g., depression and active substance use), lack of social support, severity of antiretroviral side effects, beliefs about self-efficacy (Catz, Kelly, Bogart, Benotsch, & McAuliffe, 2000) and neurocognitive dysfunction (Hinkin et al., 2002).
With regard to the latter, HIV-associated neurocognitive deficits and cART non-adherence appear to have a cyclical relationship; that is, neurocognitive deficits can interfere with medication taking behaviors (e.g., forgetting doses), which in turn can accelerate disease progression and further impair neurocognitive functioning (Ettenhofer, Foley, Castellon, & Hinkin, 2010). The historic complexity of combination antiretroviral therapies (cART) may also impose an undue burden on cognitive abilities necessary for adherence (Hinkin et al., 2002). The medications comprising a cART regimen may involve differing dosages and/or administration schedules, which could increase the risk of errors (e.g., incorrect dosing, omissions) that negatively impact overall adherence, particularly among individuals with neurocognitive difficulties. Although the pill burden of cART is on a downward trajectory, polypharmacy remains common and these challenges are important given that cART is the gold standard for HIV treatment in the United States. Additionally, the prevalence of HIV-associated neurocognitive disorders (HAND) may have even risen slightly in the cART era, with approximately 50% of the population affected (Heaton et al., 2010). The elevated rates of HAND and domain-based impairment may be due to increasing lifespans resultant from high cART efficacy, leading to longer exposure to both the presence of virus as well as potentially toxic long-term effects of the drug regimens, combined with comorbid processes such as cognitive aging (Heaton et al., 2011). While HIV-associated neurocognitive deficits can be observed in a variety of neurocognitive domains, the prevalence of impairment in higher-order neurocognitive abilities, such as episodic memory in particular, are higher (Heaton et al., 2011).
In this study we therefore focus our attention on the role of episodic memory deficits in cART non-adherence. HIV-associated neural injury in frontal and temporal systems affect multiple aspects of episodic memory (Maki et al., 2009), including traditional retrospective memory for word lists, designs, and passages, as well as prospective, source, and temporal order memory (see Woods et al., 2009 for a review). The profile of HIV-associated episodic memory deficits is heterogeneous (Murji et al., 2003), with prior studies showing evidence that numerous specific memory processes may be disrupted, including learning/acquisition, storage/consolidation, and retrieval (e.g., Woods et al., 2005). Anywhere from 20 to 40% of HIV+ individuals exhibit a primary retrieval profile of memory deficits (e.g., Delis et al., 1995) consistent with injury to fronto-striato-thalamo-cortical circuits. This retrieval profile is characterized by difficulties in bringing previously stored information into conscious awareness and is evidenced by deficits in free recall of information that are ameliorated when structured retrieval cues are provided (e.g., forced-choice recognition; Delis, Kramer, Kaplan, & Ober, 2000). A retrieval profile, which is sometimes referred to as a mixed encoding/retrieval profile, can suggest that an individual only partially encodes the target information. This is because stimuli that were processed only in part are difficult to spontaneously recall in their entirety, but may be accurately recognized when presented.
HIV-associated deficits in learning and memory have been linked to poorer everyday functioning, including medication management skills (e.g., Heaton et al., 2004) and cART non-adherence. Deficits in acquisition and delayed free recall as a global composite index have been consistently associated with poor performance on laboratory-based medication management tasks (Patton et al., 2012) across varying types of stimuli (e.g., visual vs. verbal information). Among the two studies that we found examining specific memory components in the context of adherence, measures of delayed free recall were more consistently associated with medication adherence (i.e., Woods et al., 2009; Wright et al., 2011), whereas indices of initial learning show more variable associations. Yet the specificity of such delayed recall deficits is difficult to determine, as they may be a consequence of problems with encoding, forgetting, and/or retrieval. Thus, while learning and memory are consistently associated with medication management in the laboratory and daily life, little is known about the specific profile that may be driving these relationships. Identification of such profiles at the levels of both group data and individual participants is important in order to enhance the clinical identification of persons at risk for non-adherence and develop tailored compensatory mnemonic approaches to improve adherence.
To that end, Wright and colleagues (2011) applied the item specific deficit approach (ISDA), an item analytic method, to study the association between cART adherence and word list encoding, consolidation, and retrieval as measured by the California Verbal Learning Test (2nd ed; Delis et al., 2000) in 75 HIV+ participants. The ISDA indices were developed as a novel method for categorizing encoding, consolidation and retrieval deficits. The ISDA encoding index is constructed by summing the number of items recalled in less than three of the five learning trials, the ISDA consolidation index reflects the number of items recalled during the learning trials but not recalled again during any of the recall trials (cued or free), and retrieval deficits are indexed by the number of learned items recalled inconsistently across short- and long-delay trials. To control for potential group differences in learning, the consolidation and retrieval indices are each divided by the total words recalled at least once during the learning trials. When using the ISDA Wright and colleagues (2011) found that, compared to healthy adults, HIV+ individuals demonstrated poorer performance on the CVLT regardless of their level of antiretroviral adherence. Additionally, while both HIV+ groups demonstrated similar ISDA encoding deficits, only non-adherers demonstrated ISDA retrieval deficits compared to HIV− participants. Additionally, retrieval abilities, as measured by the ISDA indices, accounted for a greater proportion of variance in long-delay free recall performance for poor adherers than for good adherers.
While the study conducted by Wright and colleagues (2011) provided initial evidence of an association between retrieval deficits and suboptimal adherence in HIV, no studies have evaluated the association between suboptimal adherence and traditional list learning profiles that also incorporate retention and recognition performance as is commonly done in clinical research and practice. At the group level, such profile-based approaches have a long tradition of utility in distinguishing between different neuropsychological disorders. For example, retrieval deficits in the context of significantly improved recognition are commonly associated with “subcortical” dementias (e.g., Huntington's disease; e.g., Massman et al., 1992). “Cortical” dementias (e.g., Alzheimer's disease), on the other hand, are sometimes differentiated by rapid forgetting, minimal improvement on recognition, and high rates of cued recall intrusions (Massman et al., 1990). Our approach here is to employ those same cognitive psychology approaches of learning and memory profile distinctions to better understand the cognitive architecture of non-adherence. To further enhance the clinical relevance of this group-level analytic approach, we also propose to classify individuals’ profiles as reflective of problems with encoding versus retrieval using an established data-based algorithm that has shown utility differentiating traditionally cortical versus subcortical diseases (Massman et al., 1990). This algorithm-based individual profile approach has proven to be a worthwhile complement to group-based analyses by allowing for clinically-relevant classification accuracy data (see Woods, Weinborn, & Lovejoy, 2003) and demonstrating the heterogeneity of profiles within these traditionally “cortical” and “subcortical” groups. To our knowledge, this would be the first study to apply this algorithm-based memory profile classification of individuals to understand an important everyday functioning outcome (i.e., cART non-adherence).
Further, no study thus far has evaluated the HIV+ memory deficit profiles associated with non-adherence while using different types of verbal stimuli (e.g., passages recall versus word lists). The format in which information is presented may have a direct impact on memory performance, particularly in the context of medication management. For example, multiple studies have reported improved medication adherence when traditional prescriptions were enhanced with more descriptive instructions about the medication regimen instead of simply listing the information (e.g., Lima, Nazarian, Charney, & Lahti, 1976).
The current study seeks to expand upon previous research (e.g., Hinkin et al., 2002; Wright et al., 2011) to determine the associations between different episodic memory profiles and antiretroviral non-adherence by utilizing traditional CVLT scoring methods with the inclusion of retention and recognition indices and a concurrent measure of passage recall (i.e., the Logical Memory subtest from the Wechsler Memory Scale, 3rd ed). In addition to standard group-level analyses of these clinical memory tasks, we also endeavored to look at individual profiles of word list learning as a risk factor for non-adherence using a previously established algorithm for classifying normal, encoding and retrieval profiles. Based on the literature reviewed above, we expect that antiretroviral non-adherence will be associated with a mixed encoding/retrieval profile. These associations may be particularly pronounced on word lists versus story memory given the former's greater reliance on executive processes (e.g., Tremont, Halpert, Javorsky, & Stern, 2000), such as strategic encoding, which is reliably impaired in HIV+ individuals (e.g., Woods et al., 2005) and has been strongly associated with non-adherence (Hinkin et al., 2002).
Method
Participants
The study sample was comprised of 202 HIV+ participants recruited from the general San Diego area (e.g., community newspaper advertisements and HIV treatment centers). The study was approved by the University of California, San Diego's Human Research Protections Program. All participants provided written informed consent prior to study participation. Participants were included in the analyses if they maintained at least one current prescription for an antiretroviral (ARV) medication and completed an approximately month-long observation of adherence using Medication Event Monitoring System (MEMS; Aprex Corporation, Union City, CA). Based on a parent project (Woods et al., 2009), exclusion from the study occurred if a participant had a (1) severe psychiatric disorder (e.g., schizophrenia), (2) major neurological disease (e.g., seizure disorders, head injury with loss of consciousness lasting longer than 15 minutes, or opportunistic infections), (3) estimated verbal IQ < 70 determined using the Wechsler Test of Adult Reading (WTAR; Psychological Corporation, 2001), (4) diagnosis of a current substance use disorder at baseline, (5) positive urine toxicology screen for illicit substances (other than marijuana) at baseline.
Adherence was tracked using MEMS, which utilizes a microchip-containing bottle cap (Trackcap ®) that records the time, date, and frequency with which the bottle is opened. All participants were provided the MEMS bottle the day of neuropsychological testing (see below) and continued use for approximately 5 weeks (M = 5.61; SD = 1.17). Participants were asked to use only the bottle provided to dispense their medication, to open the bottle only for the purpose of dispensing their medication, and to dispense only one dose of medication at a time. The ARV selected for the MEMS bottle was based on (1) whether the participant would continue the medication through the course of the study, (2) the frequency with which the medication is prescribed in the general population (e.g., Efavirenz holds the highest priority due to its widespread use), and (3) its pharmacokinetic properties. Blind reviewers used the MEMS data to categorize participants as either adherent or non-adherent. Participants were determined adherent if they followed their target antiretroviral (ARV) prescription regimen ≥ 90% of the time (accounting for doses taken on schedule, number of prescribed doses taken, and number of days the correct dose was taken).
Table 1 displays descriptive data for non-cognitive variables that differed between the Adherent (n = 103) and Non-Adherent (n = 99) groups. There were no significant between-group differences in demographics (i.e., age, education, estimated verbal IQ, sex, or ethnicity), HIV disease characteristics (i.e., estimated duration of infection, current or nadir CD4 count, plasma HIV RNA, or AIDS status), or medication regimen complexity (ps >.10). The Adherence groups were also comparable on most psychiatric characteristics, including current Major Depressive Disorder (MDD), current and lifetime Generalized Anxiety Disorder (GAD), current mood as measured by the Profile of Mood States (POMS), lifetime substance dependence, and positive urine toxicology screen for cannabis on the day of testing (ps > .10). Because a positive marijuana urine toxicology screen was allowed on the day of testing, between-group differences were evaluated to determine the potential confound of acute cannabis effects on adherence. No significant differences were found between adherent (16.9%) and non-adherent (23.1%) participants or between the normal (15.9%), encoding deficit (18.2%), and retrieval deficit (30%) algorithm-derived clinical classification profiles (ps > .10). There was a significantly higher prevalence of lifetime MDD in the Non-Adherent group; however, this variable was not associated with any of the primary memory variables of interest in the entire study cohort (ps > .10), with the sole exception of CVLT-II Total Trials 1-5 (p = .048). Follow-up logistic regressions showed that LT MDD did not affect the magnitude of the association between CVLT Total Trials 1-5 and adherence (ps > .10). Moreover, CVLT-II Total Trials 1-5 was not associated with current depression or the depression/dejection subscale of the Profile of Mood States (ps > .10). As such, we did not include LT MDD as a covariate in any of our subsequent analyses.
Table 1.
Demographic, Disease, Medication, and Psychiatric Characteristics of the Adherent and Non-Adherent Groups
| Variable | Adherent (n = 103) | Non-Adherent (n = 99) | p |
|---|---|---|---|
| Demographic | |||
| Age (years) | 48.7 (11.2) | 48.2 (12.0) | .757 |
| Education (years) | 13.7 (2.7) | 13.3 (2.5) | .228 |
| Estimated verbal IQa | 103.6 (10.7) | 101.6 (12.4) | .219 |
| Sex (% men) | 83.5 | 89.9 | .179 |
| Ethnicity (% Caucasian) | 63.1 | 65.7 | .705 |
| HIV Disease | |||
| Estimated duration of infection (years) | 14.4 (7.8) | 15.0 (7.6) | .541 |
| Current CD4 countb | 563 (387-753.5) | 515 (327-730.5) | .209 |
| Nadir CD4 countc | 180 (49-269) | 123 (67-255) | .299 |
| HIV RNA log10d | 1.7 (1.6-1.7) | 1.7 (1.6-1.7) | .839 |
| AIDS status | 63.1% | 68.7% | .403 |
| Medication Regimen | |||
| Total pill burden (number of pills per day) | 4.4 (2.8) | 4.3 (2.7) | .878 |
| Duration of antiretroviral regimen (months) | 20.6(27.0) | 25.9(32.7) | .218 |
| % Prescribed number of doses taken | 98.8 (2.8) | 74.8 (24.6) | <.001 |
| % Doses taken on schedule | 93.5 (7.3) | 52.8 (27.6) | <.001 |
| Psychiatric | |||
| Major Depressive Disorder (%) | |||
| Current | 9.7 | 12.1 | .582 |
| Lifetime | 47.6 | 62.6 | .031 |
| Generalized Anxiety Disorder (%) | |||
| Current | 1.0 | 5.1 | .078 |
| Lifetime | 9.8 | 14.1 | .342 |
| POMS total (of 200) | 53.2 (33.9) | 61.4 (38.6) | .114 |
| Lifetime substance dependencee (%) | 47.6 | 54.5 | .322 |
| % Positive drug screen for cannabis | 16.9% | 23.1% | .395 |
Note. Values reflect raw scores. IQ = intelligence quotient, CD4 = cluster of differentiation, RNA = ribonucleic acid, AIDS = acquired immune deficiency syndrome, POMS = Profile of Mood States.
Verbal intelligence quotient (M = 100, SD = 15) was derived from the Wechsler Test of Adult Reading.
Median (interquartile range)
Median (interquartile range)
Median (interquartile range)
Reflects any prior substance dependence diagnosis.
Materials and Procedures
All participants received a comprehensive neuropsychological evaluation (see Woods et al., 2009) the day prior to beginning their MEMS observation period.
Memory Assessment
California Verbal Learning Test, 2nd edition (CVLT-II)
The CVLT-II (Delis et al., 2000) is a widely used and well-validated multi-trial list learning and memory test. The CVLT-II includes 16 nouns across four semantic categories that are read aloud to the participant, who is asked to recall as many words as possible. After 5 identical learning trials, a new list of 16 nouns (a distractor list) is presented to the participant for recall. Immediately following the distractor list recall, the participant is asked to recall the original list without (short-delay free recall) and with (cued recall) semantic prompts. After a 20-minute delay, the participant is again asked to recall the words from the original list without (long-delay free recall) and with (cued recall) semantic prompts. A yes/no recognition trial is then administered in which participants are read a list of words that includes both targets (i.e., words from the original list) and distractors (i.e., new words and words from the second list).
Clinical Classification Profile Algorithm
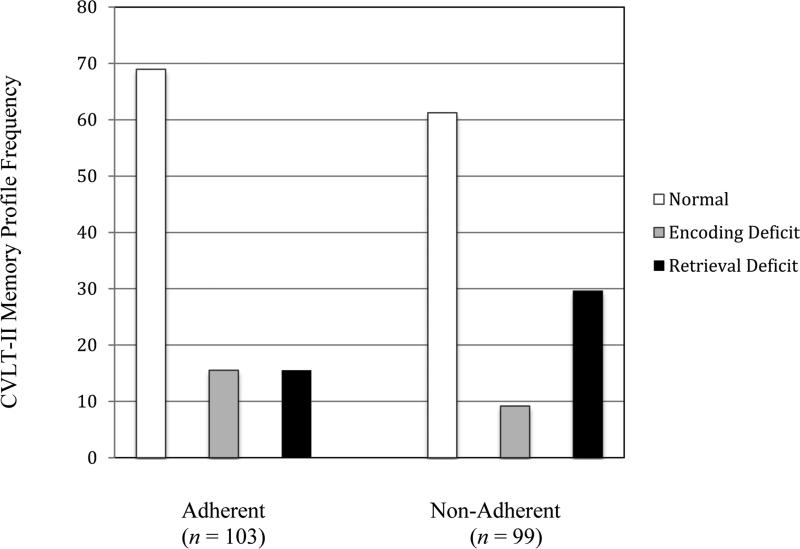
Participants were also grouped into three CVLT-II deficit profile classifications: encoding deficit (difficulty storing information in ones memory, resulting in poor performance on both free recall and recognition, in this instance operationalized by cued recall intrusion errors; Delis et al., 2000), retrieval deficit (difficulty freely recalling previously stored information, as evidenced by poor free recall performance coupled with significantly better forced-choice recognition performance; Delis et al., 2000), and normal profile (performance within normal limits on each of the CVLT-II algorithm indices; see Figure 1). These profile classifications apply a previously published algorithm (Filoteo et al., 2009; Massman et al., 1990; Massman et al., 1992) to identify which deficit is more prominent within an individual's profile of memory dysfunction. Massman and colleagues (1992) developed this algorithm by first identifying the indices that had the highest likelihood of differentiating prototypical cortical (Huntington's disease; HD) from subcortical (Alzheimer's disease; AD) dementias. These three indices included the standard scores from the total recall across all learning trials (CVLT-II trials 1-5), a standard score for the number of intrusions on two cued recall trials, and a difference score between the standard recognition discriminability score and free recall on the final CVLT-II learning trial.
Figure 1.
Frequency of the algorithm-derived clinical classification profiles in the medication adherent and non-adherent groups.
Wechsler Memory Scale, 3rd edition (WMS-III) Logical Memory
In the WMS-III Logical Memory I (LM-I) and II (LM-II) subtests (Psychological Corporation, 1997), participants are presented two narrative paragraphs (story A and story B) read aloud by the examiner, which they are asked to recall immediately following the presentation of each paragraph (LM-I). Procedures for story B immediate recall are completed twice. After a 30-minute delay (LM-II), participants are again requested to recall the information from each story. Recognition is tested immediately following the delayed recall trial with a forced-choice (yes/no) trial. The LM-I total score is derived from story A and both story B immediate free recall trials. The percent of information retained is derived by comparing the information recalled between the immediate and delayed recall conditions.
Executive Function Assessment
An executive functions composite was generated by averaging the sample-based z-scores from three clinical tasks (detailed below), in an effort to minimize type I error.
Action Fluency
This task requires participants to generate as many verbs (i.e., “things that people do”) as possible within one minute (Piatt, Fields, Paolo, & Troster, 1999).
Trail Making Test
The TMT (Army Individual Test Battery, 1944) is a paper-pencil test requiring rapid sequencing of stimuli. The relatively simpler Trial A (number sequencing) primarily measures psychomotor speed, whereas the more complex Trial B requires cognitive flexibility to rapidly switch between sequencing different types of stimuli (i.e., numbers and letters). Time to completion for Trial B was included in the Executive Function composite.
Tower of London (ToL)
The ToL (Culbertson & Zillmer, 2001) is a task that involves mental planning. Disks are placed on three pegs in a standardized order and are provided to the participant. The participant must then match the placement of their disks to those in a picture in as few moves as possible. Participants must follow specific rules (e.g., larger disks cannot be placed on top of smaller disks) while completing the task, therefore requiring mental planning prior to making physical moves. The total moves score was used in the executive composite.
Psychiatric Assessment
The Composite International Diagnostic Interview (CIDI version 2.1 World Health Organization, 1998) is a structured clinical interview used to categorize participants based on the Diagnostic and Statistical Manual of Mental Disorders (4th ed.; American Psychiatric Association, 1994) criteria. The CIDI was used to assess current and lifetime Major Depressive Disorder (MDD), Generalized Anxiety Disorder (GAD), and Substance Use Disorders. Current affective distress was assessed using the Profile of Mood States (POMS; McNair, Lorr, & Droppleman, 1981), a self-report questionnaire. Overall (Total Mood Disturbance) and subscale (Depression/Dejection, Fatigue/Inertia, Vigor/Activity, and Tension/Anxiety) scores on the POMS were derived for each participant, with higher scores indicating greater distress.
Results
Descriptive data for memory and executive scores in the adherent and non-adherent groups are displayed in Table 2. The effect of each memory variable on adherence was evaluated using t-tests. On the CVLT-II, adherence groups significantly differed on learning trials 1-5 (p < .05, F(1, 199) = 4.20, Cohen's d = .29). The differences between the adherence groups did not reach statistical significance for short-delay free recall (p < .10 , F(1, 199) = 3.60, Cohen's d = .25) or long delay free recall (p <.10 , F(1, 199) = 3.76, Cohen's d = .26), but instead fell at the level of a trend and were associated with modest effect sizes. Neither retention nor recognition showed significant main effects on adherence as measured by the CVLT-II (ps > .10).
Table 2.
Memory and Executive Performance of the Adherent and Non-Adherent Study Groups
| Cognitive Variable | Adherent (n = 103) | Non-Adherent (n = 99) | p | Cohen's d |
|---|---|---|---|---|
| California Verbal Learning Test-II | ||||
| Total trials 1-5 (of 80) | 48.9 (11.0) | 45.8 (10.5) | .042 | .29 |
| Short-delay free recall (of 16) | 10.1 (3.7) | 9.2 (3.5) | .059 | .25 |
| Long-delay free recall (of 16) | 10.3 (4.0) | 9.3 (3.7) | .054 | .26 |
| Retention (%) | −17.5 (23.7) | −20.4 (22.0) | .372 | .13 |
| Recognition (hits/false positives) | 3.0(0.9) | 2.8(0.8) | .316 | .23 |
| Wechsler Memory Scale-III | ||||
| Logical Memory I (of 75) | 42.1 (11.2) | 36.8 (12.2) | .002 | .45 |
| Logical Memory II (of 50) | 26 (9.2) | 22.7 (8.3) | .008 | .38 |
| Retention (%) | 83.5 (16.9) | 80.0 (18.5) | .166 | .20 |
| Recognition (of 30) | 25.3 (3.1) | 25.1 (3.0) | .657 | .07 |
| Executive Function | .06 (.71) | −.06 (.79) | .271 | .16 |
Note. CVLT-II and WMS-III values reflect raw scores. The executive function variable is a composite score derived from averaging the verbal fluency (actions trial), Trail Making Test trial B, and Tower of London total moves standard z-scores.
On the WMS-III LM there was an effect of memory on adherence for immediate (p < .05, F(1, 200) = 10.29, Cohen's d = .45) and delayed (p < .05, F(1, 199) = 7.23, Cohen's d = .38) story recall. Similar to the results from CVLT-II performance, no significant associations were found between adherence and story retention or recognition (ps > .10).
To ensure the relationship between memory and adherence was not an effect of regimen complexity, possible interactions between total number of daily antiretroviral pills and each memory variable was evaluated separately. No significant interactions were found on any memory variable (ps > .10).
The executive function composite score was not significantly different between the adherent (M = .06, SD = .71) and non-adherent (M = −.06, SD = .79, p = .451) groups. However, CVLT-II Total Trials 1-5 (r = .41) and long-delay free recall (r = .38), as well as Logical Memory I (r = .45) and II (r = .54), were each significantly correlated in the expected direction with the executive function composite in the non-adherent group (ps < .05).
The frequency of CVLT-II and WMS-III LM standard impairment scores are shown in Table 3. No significant differences were found between the adherent and non-adherent groups when using standardized impairment scores for all memory variables (ps > .05).
Table 3.
Frequency of Memory Deficits (z ≤ −1) in the Adherent and Non-Adherent Study Groups
| Cognitive Variable | Adherent (n = 103) | Non-Adherent (n = 99) | p |
|---|---|---|---|
| California Verbal Learning Test-II | |||
| Total trials 1-5a | .97% | 4.1% | .145 |
| Short-delay free recallb | 12.6% | 15.3% | .583 |
| Long-delay free recall | 16.5% | 22.5% | .287 |
| Retention (%) | 2.9% | 4.1% | .651 |
| Recognition (hits/false positives) | 13.6% | 12.2% | .776 |
| Wechsler Memory Scale-III | |||
| Logical Memory I (of 75) | 8.9% | 16.3% | .113 |
| Logical Memory II (of 50) | l0.9% | 10.2% | .875 |
| Retention (%) | 6.9% | 6.1% | .818 |
Note. % reflects frequency of normative standard scores less than l standard deviation below the mean. Normative scores for WMS-III recognition were not available.
Chi-square tests were conducted to evaluate differences in the frequency of CVLT algorithm-derived clinical classification profiles in the cART adherent (n = 98) and non-adherent (n = 103) groups. The overall model was significant (p = .0369, X2 = 6.60; see Figure 1 for frequency of each deficit classification), with the majority of both adherent (68.9%, n = 71) and non-adherent (61.2%, n = 60) individuals exhibiting a primary profile of normal memory functioning. The non-adherent group had fewer individuals with a primary encoding deficit profile (adherent: 15.5%, n = 16; non-adherent: 9.2%, n = 9), while a primary retrieval deficit profile was almost twice as likely in the non-adherent (29.6%, n = 29) than the adherent group (15.5%, n = 16). Non-adherent individuals were significantly more likely to have a retrieval deficit than a normal profile (odds ratio = 2.14, p = .030, 95% confidence interval = 1.06, 4.32) and among those with a primary deficit profile (encoding or retrieval), individuals categorized with retrieval difficulties were over three times more likely to be non-adherent (odds ratio = 3.22, p = .021, 95% confidence interval = 1.16, 8.93).
Discussion
Medication non-adherence is common in HIV disease, particularly among individuals with memory deficits (Hinkin et al., 2002). The current study extends our understanding of the memory profile associated with cART non-adherence. Consistent with earlier studies on this topic, our data showed that episodic verbal learning and memory have broadly medium associations with adherence in HIV+ individuals, with CVLT-II surprisingly showing smaller average effects (Cohen's d = .25-.29) than WMS-III LM (Cohen's d = .38-.45). The profile of memory findings in the non-adherent cohort was characterized by deficits in initial acquisition and delayed free recall, but not in retention or recognition. This pattern of findings is consistent with what has been described as a “retrieval” deficit, which is commonly found in persons with HAND (e.g., Delis et al., 1995) and other conditions that affect prefronto-striato-thalamo-cortical circuits (e.g., Huntington's disease; Massman et al., 1990). Of note, algorithm-derived clinical classifications of the CVLT-II (Filoteo et al., 2009; Massman et al., 1992) showed that HIV+ non-adherers have a higher prevalence of subcortical retrieval deficit profiles and that those with such retrieval profiles were three times more likely to be non-adherent than those with an encoding deficit profile. This is interpretively consistent with the work of Wright and colleagues (2011), who found that non-adherent HIV+ persons differed from healthy adults on the ISDA index of retrieval, but extends that prior paper by: (1) using traditional CVLT-II indices that include retention and recognition; (2) incorporating a measure of passage recall, (3) providing classifications of individual profiles using the algorithm-derived methods of Massman and colleagues (1992) along with traditional CVLT scoring methods and (4) demonstrating associations between the algorithm profile approach and ecologically relevant measures of everyday functioning. The significant associations between adherence and profile scores in the context of insignificant standard deficit scores (as seen in Table 3) underscores the clinical relevance of understanding individual profiles of memory deficits when evaluating everyday functioning outcomes. Future research comparing this algorithm across HIV and other well-established cortical and subcortical deficits using both neuropsychological and ecologically valid real-world tests appears warranted to better understand the true impact of these deficit profiles across disorders.
From a memory process perspective, these findings indicate that missing a medication dose is not simply a “forgetting” problem (e.g., Chesney et al., 2000), as there was no evidence of an association between adherence and memory savings scores or recognition performance. Instead, non-adherence in HIV is at least partly associated with deficits in the complex process of efficiently accessing information from episodic verbal memory with minimal retrieval cues. Such retrieval deficits may be a function of weak encoding and/or difficulties with self-initiated memory search and access to stored information. Therefore, even if an HIV+ individual understands and encodes the relevant information regarding their cART regimen, he/she may still not remember to take his/her medication as prescribed at least in part due to difficulties retrieving the proper information at the later dosing time. These memory processes did not appear to change depending on the complexity of one's regimen, instead, difficulty retrieving information was associated with non-adherence regardless of the number of pills an individual was prescribed. Importantly, these complex memory process failures may also occur in concert with failures in other aspects of cognition (e.g., deficits in prospective memory), psychiatric comorbidities, and various psychosocial factors (e.g., motivation, beliefs related to medications), which may further complicate and exacerbate the observed retrieval deficits. Indeed, there are numerous neurobehavioral aspects involved in managing one's medication regimen (e.g., attending to regimen requirements, understanding the instructions, encoding the information, retaining the information, translating it into actions, and executing the planned behaviors as scheduled; Wilson & Park, 2008).
The absence of an association between executive functions and adherence may at first appear contrary to the known pattern of deficits seen in individuals with fronto-striatal dysregulation, such as in HIV disease. Indeed, prior studies have shown that executive functions are related to cART non-adherence (e.g., Hinkin et al., 2002). However, the free recall memory variables that differentiated the adherence groups were associated with executive functions in the non-adherence group, which supports the hypothesis that a deficiency in the higher-order, organizational aspects of memory processes may play an important role in medication non-adherence. Specifically, Moscovitch (1992, 1994) described a component process theory that included a higher-order control process by which the frontal systems work “with” encoding and retrieval to properly organize information for efficient storage and recall. Poor strategic encoding and retrieval has been observed in HIV-seropositive individuals (Woods et al., 2005), and therefore the profile of deficits and the observed associations with executive dysfunction findings suggest that these strategically-driven aspects of memory processes are important for successful medication adherence in HIV.
The stronger association between non-adherence and story recall (Cohen's d = .38-.45) versus list learning (Cohen's d = .25-.29) was surprising, as we had expected the purported executive demands of list learning to be more important in the context of managing medications (see Hinkin et al., 2002). However, this unexpected finding may reflect the relevance of contextual information to understanding memory's effects on important aspects of everyday functioning, such as medication adherence. In other words, the stronger associations between story memory and adherence underscores the importance of an individual's ability to understand and independently recall medication information presented in a contextual format for proper regimen adherence. There is a long history of episodic memory research on the role of context in facilitating recall (Bartlett, 1933) and the match between the context of encoding and retrieval (e.g., transfer appropriate processing). In this instance, therefore, story recall may better match the everyday demands of memory on adherence than does list learning. Non-adherers may have greater difficulty remembering and applying regimen information presented conversationally by their healthcare providers and in the context of related information (e.g., HIV disease risks, general health practices, special instructions) as opposed to being presented with a listed dosing schedule. This provides one explanation for why the LM subtest has historically provided a more sensitive test for subtle memory deficits compared to the CVLT (e.g., Lange et al., 2002). Morgan and colleagues (2015), to this end, demonstrated that individuals with HAND evidenced difficulty with contextually-based health information. Specifically, the HAND group performed more poorly on an applied task in which health-related information was provided in the full context of a detailed scenario involving a health choice (Morgan et al., 2015). This finding is consistent with the results of the present study, suggesting that medication regimen instructions presented in the larger context of health-related information may interfere with, rather than facilitate, memory for that information and subsequently decrease adherence. These findings suggest that when complex, contextualized health information is provided to patients it should be supplemented with more focused, targeted instructions that facilitate adherence to the prescribed health plan.
Several limitations of this study are important to highlight. This study focused exclusively on verbal episodic memory. Due to a heavy reliance on reading prescription labels to obtain medication regimen information as well as visual cues when dispensing and dosing medications (e.g., accurate pillbox placement), understanding memory profiles associated with visual stimuli could also be of value. Because MEMS caps were utilized for adherence observations, the effects of commonly used compensatory behaviors, such as using pillbox dispensers (which can be an effective compensatory behavior for individuals with memory deficits; Peterson et al., 2007), on adherence were not evaluated, therefore reducing some generalizability to everyday life. Further, future studies could improve our understanding of these profiles on medication non-adherence by evaluating the efficacy of commonly used compensatory strategies (e.g., electronic organizers; Kapur, Glisky, & Wilson, 2004) among individuals with specific profiles of memory deficits. This study also examined memory associated with common words and simple stories. However, drug labels often include complex medical and numeric information (Holt, Hollon, Hughes, & Coyle, 1990), which may influence adherence. Multiple studies found that retention of medical information has been as low as 25% (Hons, 2000; Sandberg, Sharma, & Sandberg, 2012). Therefore, it would be more ecologically relevant to examine memory in the context of medical information and complex descriptions rather than the commonly understood information presented on the clinical memory tasks used in this study.
Despite these limitations, findings from this study may have implications for the development and implementation of compensatory strategies to improve cART adherence. Given that non-adherers and adherers exhibited comparable recognition abilities, compensatory adherence strategies associated with simple recognition, such as placing a pillbox by a commonly used area, may help to improve adherence in individuals with retrieval difficulties. Moreover, strategies to improve encoding may be of benefit to persons living with HIV-associated memory deficits; for example, requiring self-generation of stimulus pairing has shown to greatly improve immediate and delayed verbal recall in HIV disease (Weber, Woods, Kellogg, Grant, & Basso, 2012). Translated to a clinical setting, asking a patient to generate their own plan for remembering their medications (e.g., when to take their pills each day and what techniques they will use to remind themselves) may deepen encoding processes and facilitate later recall thereby improving adherence. Other neurorehabilitation techniques that target encoding and retrieval processes in order to deepen the memory trace may be additionally fruitful in enhancing adherence, such as visual imagery (e.g., visualizing the time and place of the planned dosing time), spaced-learning (e.g., initial encoding of the medication regimen and dosing time, allowing for increasingly longer periods across recall trials), and/or pairing medication behaviors with other daily habits that might trigger recall (e.g., brushing one's teeth). Additionally, the prominence of a primary retrieval deficit profile strengthens the subcortical dementia explanation of HAND. Because subcortical dementias are often coupled with slowed mental processes and apathy (e.g., Moretti et al., 2013) additional techniques, such as providing information at a slower rate or enhancing patient buy-in and decreasing apathy by focusing on the rationale of proper adherence, could prove helpful. Moving forward, future studies that begin to examine the utility of these techniques to improve antiretroviral adherence are certainly warranted and long overdue as the field begins to translate observations into interventions.
Acknowledgements
This work was supported by the National Institute of Health grants F31-DA035708, L30-DA032120, R01-MH073419, P30-MH62512, and T32-DA31098. This study was also supported (in part) by a Foundation for Rehabilitation Psychology Dissertation Award.
The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Terry Alexander, R.N., Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Terry Jernigan, Ph.D., Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D., Ian Everall, FRCPsych., FRCPath., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Reena Deutsch, Ph.D., Anya Umlauf, M.S.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Special thanks to Matthew Wright, Ph.D. for reviewing portions of this manuscript prior to submission.
Footnotes
Disclosure:
NIH had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Neither the authors of this paper nor the institution where the research was conducted have any relationships (financial or otherwise) that could be interpreted as a conflict of interest affecting this manuscript. Aspects of these data were presented at the 42nd annual conference of the International Neuropsychological Society in Seattle, WA. The manuscript itself has never been published either electronically or in print.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th Ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Army Individual Test Battery . Manual of Directions and Scoring. War Department, Adjutant General's Office; Washington, DC: 1944. [Google Scholar]
- Barlett FC, Burt C. Remembering: A study in experimental and social psychology. British Journal of Educational Psychology. 1933;3:187–192. doi: 10.1111/j.2044-8279.1933.tb02913.x. [Google Scholar]
- Catz SL, Kelly JA, Bogart LM, Benotsch EG, McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychology. 2000;19:124. doi: 10.1037/0278-6133.19.2.124. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) HIV in the United States: The stages of care. 2012 Retrieved from: http://www.cdc.gov/hiv/pdf/research_mmp_stagesofcare.pdf.
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, Wu AW. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. Patient Care Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Culbertson WC, Zillmer EA. The Tower of London DX (TOLDX) manual. Multi-Health Systems; North Tonawanda, NY: 2001. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test. 2nd Ed. The Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- Delis DC, Peavy G, Heaton R, Butters N, Salmon DP, Taylor M, Grant I. Do patients with HIV-associated minor cognitive/motor disorder exhibit a “subcortical” memory profile? evidence using the California Verbal Learning Test. Assessment. 1995;2:151–165. doi: 10.1177/107319119500200205. [Google Scholar]
- Ettenhofer ML, Foley J, Castellon SA, Hinkin CH. Reciprocal prediction of medication adherence and neurocognition in HIV/AIDS. Neurology. 2010;74:1217–1222. doi: 10.1212/WNL.0b013e3181d8c1ca. doi: 10.1212/WNL.0b013e3181d8c1ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filoteo JV, Salmon DP, Schiehser DM, Kane AE, Hamilton JM, Rilling LM, Galasko DR. Verbal learning and memory in patients with dementia with Lewy bodies or Parkinson's disease with dementia. Journal of Clinical and Experimental Neuropsychology. 2009;31:823–834. doi: 10.1080/13803390802572401. doi: 10.1080/13803390802572401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García DOP, Knobel H, Carmona A, Guelar A, López-Colomés JL, Caylà JA. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. Journal of Acquired Immune Deficiency Syndromes. 2002;30:105. doi: 10.1097/00042560-200205010-00014. doi: 10.1097/00042560-200205010-00014. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr., Woods SP, Ake C, Vaida F, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of NeuroVirology. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, Grant I. The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychology Society. 2004;10:317–331. doi: 10.1017/S1355617704102130. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, Stefaniak M. Medication adherence among HIV+ adults: effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59:1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. doi: 10.1212/01.WNL.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt GA, Hollon JD, Hughes SE, Coyle R. OTC labels: can consumers read and understand them?. American Pharmacy. 1990;(11):51–54. doi: 10.1016/s0160-3450(16)33630-3. [DOI] [PubMed] [Google Scholar]
- Hons YG. Do they listen? A review of information retained by patients following consent for reduction mammoplasty. British journal of plastic surgery. 2000;53:121–125. doi: 10.1054/bjps.1999.3220. doi: 0.1054/bjps.1999.3220. [DOI] [PubMed] [Google Scholar]
- Howard AA, Arnsten JH, Lo Y, Vlahov D, Rich JD, Schuman P, HER Study Group A prospective study of adherence and viral load in a large multi-center cohort of HIV-infected women. AIDS. 2002;16:2175–2182. doi: 10.1097/00002030-200211080-00010. doi: 10.1097/00002030-200211080-00010. [DOI] [PubMed] [Google Scholar]
- Kapur N, Glisky EL, Wilson BA. Technological memory aids for people with memory deficits. Neuropsychological Rehabilitation. 2004;14:41–60. doi: 10.1080/09602010343000138. [Google Scholar]
- Lange KL, Bondi MW, Salmon DP, Galasko D, Delis DC, Thomas RG, Thal LJ. Decline in verbal memory during preclinical Alzheimer's disease: Examination of the effect of APOE genotype. J Int Neuropsychol Soc. 2002;8(7):943–955. doi: 10.1017/s1355617702870096. doi: 10.1017/s1355617702870096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima J, Nazarian L, Charney E, Lahti C. Compliance with short-term antimicrobial therapy. Pediatrics. 1976;57(3):383–386. 1976. [PubMed] [Google Scholar]
- Maggiolo F, Airoldi M, Kleinloog HD, Callegaro A, Ravasio V, Arici C, Suter F. Effect of adherence to HAART on virologic outcome and on the selection of resistance-conferring mutations in NNRTI- or PI-treated patients. HIV Clinical Trials. 2007;8:282–92. doi: 10.1310/hct0805-282. doi: 10.1310/hct0805-282. [DOI] [PubMed] [Google Scholar]
- Maki PM, Cohen MH, Weber K, Little DM, Fornelli D, Rubin LH, Martin E. Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: A preliminary study. Neurology. 2009;72:1661–1668. doi: 10.1212/WNL.0b013e3181a55f65. doi: 10.1212/WNL.0b013e3181a55f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massman PJ, Delis DC, Butters N, Dupont RM, Gillin JC. The subcortical dysfunction hypothesis of memory deficits in depression: neuropsychological validation in a subgroup of patients. Journal of Clinical and Experimental Neuropsychology. 1992;14:687–706. doi: 10.1080/01688639208402856. doi: 10.1080/01688639208402856. [DOI] [PubMed] [Google Scholar]
- Massman PJ, Delis DC, Butters N, Levin BE, Salmon DP. Are all subcortical dementias alike?: Verbal learning and memory in Parkinson's and Huntington's disease patients. Journal of Clinical and Experimental Neuropsychology. 1990;12:729–744. doi: 10.1080/01688639008401015. doi: 10.1080/01688639008401015. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the profile of mood states. Educational and Industrial Testing Service; San Diego, CA: 1981. [Google Scholar]
- Moretti R, Torre P, Esposito F, Barro E, Tomietto P, Antonello RM. Apathy as a Key Symptom in Behavior Disorders: Difference Between Alzheimer's Disease and Subcortical Vascular Dementia. 2013 doi:10.5772/54264. [Google Scholar]
- Morgan EE, Iudicello JE, Cattie JE, Blackstone K, Grant I, Woods SP. Neurocognitive Impairment is Associated with Lower Health Literacy Among Persons Living with HIV Infection. AIDS and Behavior. 2015:1–12. doi: 10.1007/s10461-014-0851-7. doi: 10.1007/s10461-041-0851-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working-with-memory: A component process model based on modules and central systems. Journal of cognitive neuroscience. 1992;4:257–267. doi: 10.1162/jocn.1992.4.3.257. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working with memory: Evaluation of a component process model and comparisons with other models. In: Schacter D, Tulving E, editors. Memory systems. A Bradford Book; Cambridge, MA: 1994. pp. 369–394. [Google Scholar]
- Murji S, Rourke SB, Donders J, Carter SL, Shore D, Rourke BP. Theoretically derived CVLT subtypes in HIV-1 infection: Internal and external validation. Journal of the International Neuropsychological Society. 2003;9:1–16. doi: 10.1017/s1355617703910010. doi: 10.1017/S1355617703910010. [DOI] [PubMed] [Google Scholar]
- Patton DE, Woods SP, Franklin D, Jr., Cattie JE, Heaton RK, Grant I. Relationship of Medication Management Test-Revised (MMT-R) performance to neuropsychological functioning and antiretroviral adherence in adults with HIV. AIDS Behaviors. 2012;16:2286–2296. doi: 10.1007/s10461-012-0237-7. doi: 10.1007/s10461-012-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatt AL, Fields JA, Paolo AM, Tröster AI. Action (verb naming) fluency as an executive function measure: Convergent and divergent evidence of validity. Neuropsychologia. 1999;37:1499–1503. doi: 10.1016/s0028-3932(99)00066-4. doi: 10.1016/S0028-3932(99)00066-4. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation . WAIS-III WMS-III technical manual. Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Sandberg EH, Sharma R, Sandberg WS. Deficits in retention for verbally presented medical information. Anesthesiology. 2012;117:772–779. doi: 10.1097/ALN.0b013e31826a4b02. doi: 10.1097/01.sa.0000428894.26766.3f. [DOI] [PubMed] [Google Scholar]
- Tremont G, Halpert S, Javorsky DJ, Stern RA. Differential impact of executive dysfunction on verbal list learning and story recall. The Clinical Neuropsychologist. 2000;14(3):295–302. doi: 10.1076/1385-4046(200008)14:3;1-P;FT295. doi: 10.1076/1385-4046(200008)14:3;1-P;FT295. [DOI] [PubMed] [Google Scholar]
- Weber E, Woods SP, Kellogg E, Grant I, Basso MR. Self-generation enhances verbal recall in individuals infected with HIV. Journal of the International Neuropsychological Society. 2012;18:128–133. doi: 10.1017/S135561771100124X. doi: 10.1017/S135561771100124X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading: WTAR. Psychological Corporation; 2001. [Google Scholar]
- Wilson EAH, Park D. Prospective Memory and Health Behaviors: Context Trumps Cognition. In: Kliegel M, McDaniel MA, Einstein GO, editors. Prospective Memory. Lawrence Erlbaum Associates; New York, New York: 2008. pp. 391–407. [Google Scholar]
- Woods SP, Dawson M, Weber E, Gibson S, Grant I, Atkinson JH. Timing is everything: Antiretroviral nonadherence is associated with impairment in time-based prospective memory. Journal of the International Neuropsychological Society. 2009;15:42–52. doi: 10.1017/S1355617708090012. doi: 10.1017/S1355617708090012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Doyle KL, Morgan EE, Naar-King S, Outlaw AY, Loft S. Task Importance Affects Event-Based Prospective Memory Performance in Adults with HIV-Associated Neurocognitive Disorders and HIV-Infected Young Adults with Problematic Substance Use. Journal of the International Neuropsychological Society. 2014;20:1–11. doi: 10.1017/S1355617714000435. doi: 10.1017/S1355617714000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Scott JC, Dawson MS, Morgan EE, Carey CL, Heaton RK, Grant I. Construct validity of Hopkins Verbal Learning Test—Revised component process measures in an HIV-1 sample. Archives of Clinical Neuropsychology. 2005b;20:1061–1071. doi: 10.1016/j.acn.2005.06.007. doi: 10.1016/j.acn.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Woods SP, Weinborn M, Lovejoy DW. Are Classification Accuracy Statistics Underused in Neuropsychological Research? Journal of Clinical and Experimental Neuropsychology. 2003;25:431–439. doi: 10.1076/jcen.25.3.431.13800. doi: 10.1076/jcen.25.3.431.13800. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Composite international diagnostic interview (CIDI, version 2.1) World Health Organization; Geneva, Switzerland: 1998. [Google Scholar]
- Wright MJ, Woo E, Foley J, Ettenhofer ML, Cottingham ME, Gooding AL, Hinkin CH. Antiretroviral adherence and the nature of HIV-associated verbal memory impairment. Journal of Neuropsychiatry & Clinical Neuroscience. 2011;23:324–331. doi: 10.1176/appi.neuropsych.23.3.324. doi: 10.1176/appi.neuropsych.23.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Woo E, Schmitter-Edgecombe M, Hinkin CH, Miller EN, Gooding AL. The Item-Specific Deficit Approach to evaluating verbal memory dysfunction: Rationale, psychometrics, and application. Journal of clinical and experimental neuropsychology. 2009;31:790–802. doi: 10.1080/13803390802508918. doi: 10.1080/13803390802508918. [DOI] [PMC free article] [PubMed] [Google Scholar]