Abstract
Spontaneous and sensory-evoked cortical activity is highly state-dependent, yet relatively little is known about transitions between distinct waking states. Patterns of activity in mouse V1 differ dramatically between quiescence and locomotion, but this difference could be explained by either motor feedback or a change in arousal levels. We recorded single cells and local field potentials from area V1 in mice head-fixed on a running wheel and monitored pupil diameter to assay arousal. Using naturally occurring and induced state transitions, we dissociated arousal and locomotion effects in V1. Arousal suppressed spontaneous firing and strongly altered the temporal patterning of population activity. Moreover, heightened arousal increased the signal-to-noise ratio of visual responses and reduced noise correlations. In contrast, increased firing in anticipation of and during movement was attributable to locomotion effects. Our findings suggest complementary roles of arousal and locomotion in promoting functional flexibility in cortical circuits.
Introduction
Patterns of cortical activity differ dramatically across behavioral states, such as sleeping, anesthesia and waking (Berger, 1929; Haider et al., 2013; Steriade et al., 1993; Steriade et al., 2001). Likewise, neural responses to sensory inputs depend strongly on ongoing patterns of internally generated activity (Civillico and Contreras, 2012; Hasenstaub et al., 2007; Livingstone and Hubel, 1981). The generation of multiple activity patterns associated with sleep and anesthesia states has been examined in great detail (Berger, 1929; Contreras et al., 1996; Destexhe et al., 1999; McCormick and Bal, 1997; Steriade et al., 1993; Steriade et al., 2001). However, relatively little is known about transitions between distinct waking states, such as quiescence, arousal, and focused attention.
Recent studies in rodents have contrasted inactive vs. active behavioral states, in particular quiescent vs. whisking (Crochet and Petersen, 2006; Gentet et al., 2010; Zagha et al., 2013) or running (Bennett et al., 2013; Fu et al., 2014; Keller et al., 2012; Niell and Stryker, 2010; Polack et al., 2013; Reimer et al., 2014; Saleem et al., 2013; Schneider et al., 2014; Zhou et al., 2014), and found profound differences in cortical activity patterns that resemble the effects of focused spatial attention in primates (Cohen and Maunsell, 2009; Fries et al., 2001; Harris and Thiele, 2011; McAdams and Maunsell, 1999; Mitchell et al., 2009). In mouse primary visual cortex (V1), locomotion is accompanied by altered firing rates, a reduction in low-frequency fluctuations in the membrane potential and local field potential (LFP), and an increase in LFP gamma-band oscillations (Keller et al., 2012; Niell and Stryker, 2010; Polack et al., 2013; Reimer et al., 2014; Saleem et al., 2013). Enhanced firing rates during locomotion are particularly prominent in inhibitory interneurons (Bennett et al., 2013; Fu et al., 2014; Niell and Stryker, 2010; Polack et al., 2013; Reimer et al., 2014). Locomotion is also associated with an increase in the gain of visual responses (Bennett et al., 2013; Niell and Stryker, 2010; Polack et al., 2013; Reimer et al., 2014).
Because the most commonly studied active states involve a substantial motor component, it remains unclear whether the associated changes in cortical activity patterns are specific to motor output or more generally attributable to changes in global arousal. Recordings during manipulations of the visual environment suggest that much of the change in firing rates during locomotion is consistent with multimodal processing of visual and motor signals (Keller et al., 2012; Saleem et al., 2013). The integration of locomotor and visual signals in V1 may thus represent elements of predictive coding or play a role in spatial navigation. However, locomotion-associated changes in cortical activity have been replicated by noradrenergic and cholinergic manipulations in the absence of motor output (Fu et al., 2014; Lee et al., 2014; Polack et al., 2013). Changes in V1 activity during locomotion may therefore result from recruitment of neuromodulatory systems that regulate global arousal levels.
Wakefulness comprises states of low and high arousal, but the relationship between changes in arousal and cortical activity remains poorly understood. The functional impact of motor feedback signals to sensory cortex is likewise only beginning to be explored (Guo et al., 2014; Lee et al., 2013; Schneider et al., 2014; Zagha et al., 2013). Here we used behavioral state monitoring and manipulation to dissociate the roles of locomotion and arousal in regulating neural activity in mouse V1. We recorded from V1 in mice head-fixed on a wheel to measure locomotion and monitored pupil diameter to assess arousal (Aston-Jones and Cohen, 2005; Eldar et al., 2013; Gilzenrat et al., 2010; Reimer et al., 2014). To disentangle the effects of motor activity and arousal, we first took advantage of naturally occurring state transitions where mice initiated or finished a bout of locomotion. We examined the precise time course of changes in the LFP, single-unit firing rates, and pupil diameter around locomotion onset and offset. We further compared epochs of high and low arousal during quiescent states. In a second set of experiments, we causally manipulated behavioral state and induced a shift from low to high arousal in the absence of locomotion by delivering an air puff to the animal’s body. We find that arousal mediates most state-dependent changes in LFP activity, whereas increases in overall firing rates are attributable to locomotion. In contrast, enhancement of visual encoding during locomotion is associated with increased arousal, rather than motor activity.
RESULTS
Dissociating locomotion and arousal
To separate the contributions of locomotion and arousal to V1 activity and visual encoding, we performed simultaneous recordings of isolated single units and LFPs from multiple sites throughout layers 2–6 of V1 in awake mice (Fig. 1; n=88 sessions in 28 mice). Mice were head-fixed on a spring-mounted wheel apparatus (Fig. S1A) and recordings were made during both baseline and visual stimulation periods. Behavioral state was assessed by continuous monitoring of arousal and locomotion. To monitor arousal, we measured pupil dilation in the eye that was contralateral to the visual stimulus (Fig. 1A, Supplementary Movie 1). Transition points were defined as shifts from quiescence (used synonymously with sitting) to locomotion or from locomotion to quiescence and were identified by detecting significant changes in the statistics of the locomotion speed signal from the wheel with high temporal resolution (Fig. 1B).
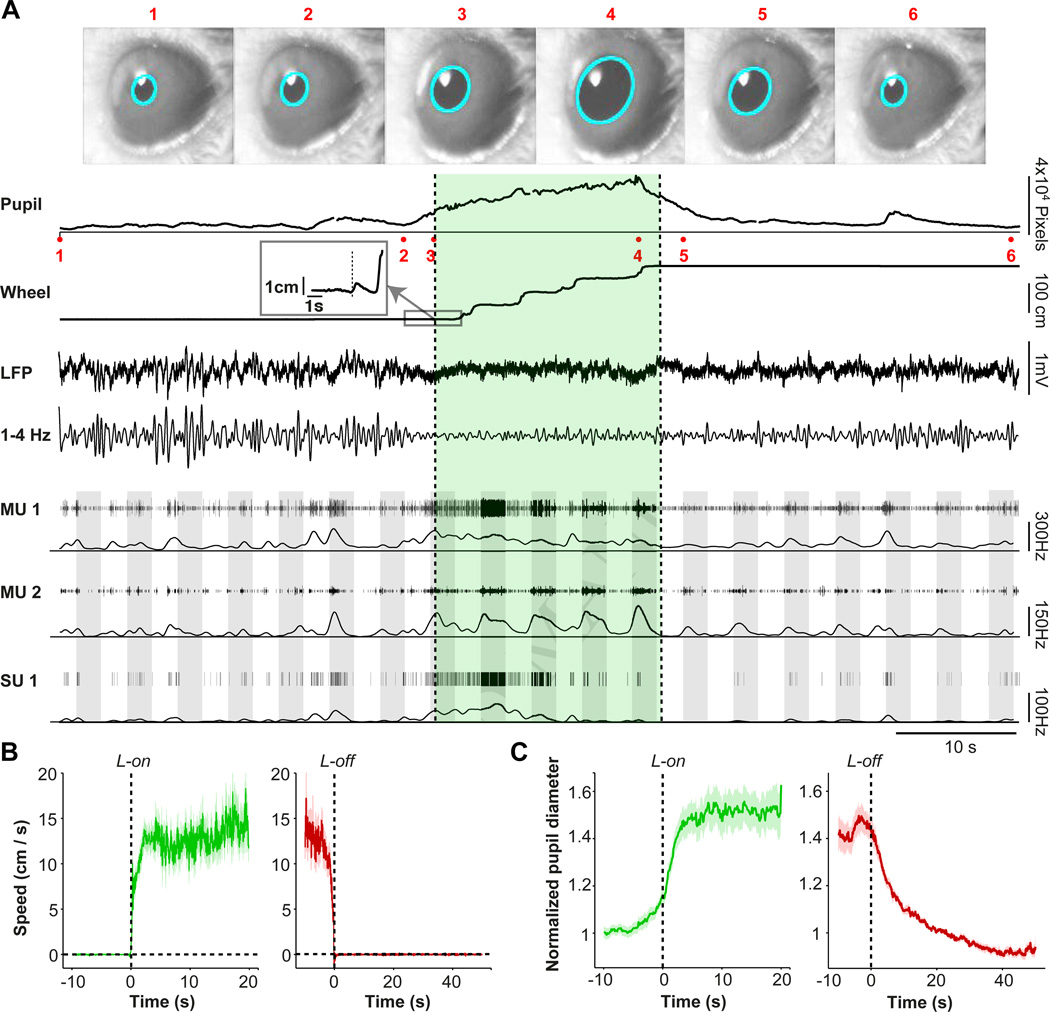
Figure 1.
Experimental paradigm to separate the contributions of arousal and locomotion to neural activity and visual encoding in V1. (A) Example data from one experiment session. Video frame images of the mouse’s eye (1–6) are shown where acquired at the times indicated in the pupil recording trace. Pupil diameter (PD) was recorded on video and extracted posthoc via a fitted ellipse (cyan). The average pupil diameter in pixel units is shown as a function of time. Locomotion is shown as a linearized version of the wheel position. Locomotion onset point is shown in the inset panel. The locomotion period is indicated by green shading. LFP recording is shown as a raw broadband LFP signal for a superficial electrode, together with the 1–4 Hz filtered signals. Thresholded multi-unit traces and spike densities (1 s Gaussian smoothing kernel with SD [Standard Deviation] of 0.25 s) are shown for a superficial (MU1) and deep (MU2) electrode, respectively, together with a single unit trace that was isolated from MU1. Grey shadings indicate visual stimuli at 100% contrast and varying orientations. (B) Locomotion speed around locomotion onset (green) and offset (red), shown as mean ± s.e.m (across sessions). (C) Population average pupil diameter, normalized to PD at 20–25 s point after locomotion offset, as a function of time around locomotion onset (green) and offset (red), shown as mean ± s.e.m.
We found complex temporal relationships between pupil diameter, locomotion, LFP dynamics, and the firing rates of V1 neurons (Fig. 1A). At locomotion onset, speed rapidly increased, reaching a plateau after about 2.5 s and remaining at ∼10–15 cm/s until just prior to locomotion offset (Fig. 1B). Locomotion speed and pupil diameter were consistently correlated at transition points. Pupil diameter increased prior to the onset of locomotion, suggesting that arousal reliably preceded movement. Pupil diameter reached a plateau after about 2.5 s, and remained elevated until locomotion offset (Fig. 1C). Locomotion offset was followed by a gradual decrease in pupil diameter that did not reach baseline values until 40 s later (Fig. 1C), indicating a substantial period of elevated arousal in the absence of locomotion. Periods of high arousal and locomotion thus occurred both together and separately, allowing us to discriminate their roles in regulating V1 activity.
LFP modulation by behavioral state transition
To dissociate LFP changes associated with locomotion and arousal, we computed time-frequency representations of LFP signals around locomotion onset and offset in the absence of visual stimuli. Locomotion onset was preceded by a sharp increase in spontaneous LFP gamma oscillations (55–65 Hz) and a decrease in low-frequency LFP fluctuations (1–4 Hz; Fig. 2A). Gamma power decreased gradually over time after locomotion onset, whereas low-frequency fluctuations remained suppressed throughout the locomotion period (Fig. 2A). In contrast, locomotion offset was followed by a gradual increase in low-frequency LFP power and a gradual decrease in LFP gamma power that lasted up to 40–50 s (Fig. 2B). The time courses of LFP low-frequency and gamma power were strongly correlated (Spearman correlation) with the time course of pupil dynamics, but not with the time course of locomotion speed (Fig. 2C). The correlations between LFP power and pupil diameter time courses were strongly linear (gamma and pupil: Pearson’s R = 0.94, p=2.4×10−6; 1–4 Hz and pupil: Pearson’s R = −0.93, p=4.1×10−5), suggesting that arousal, rather than locomotion, mediates most of the observed change in LFP patterns.
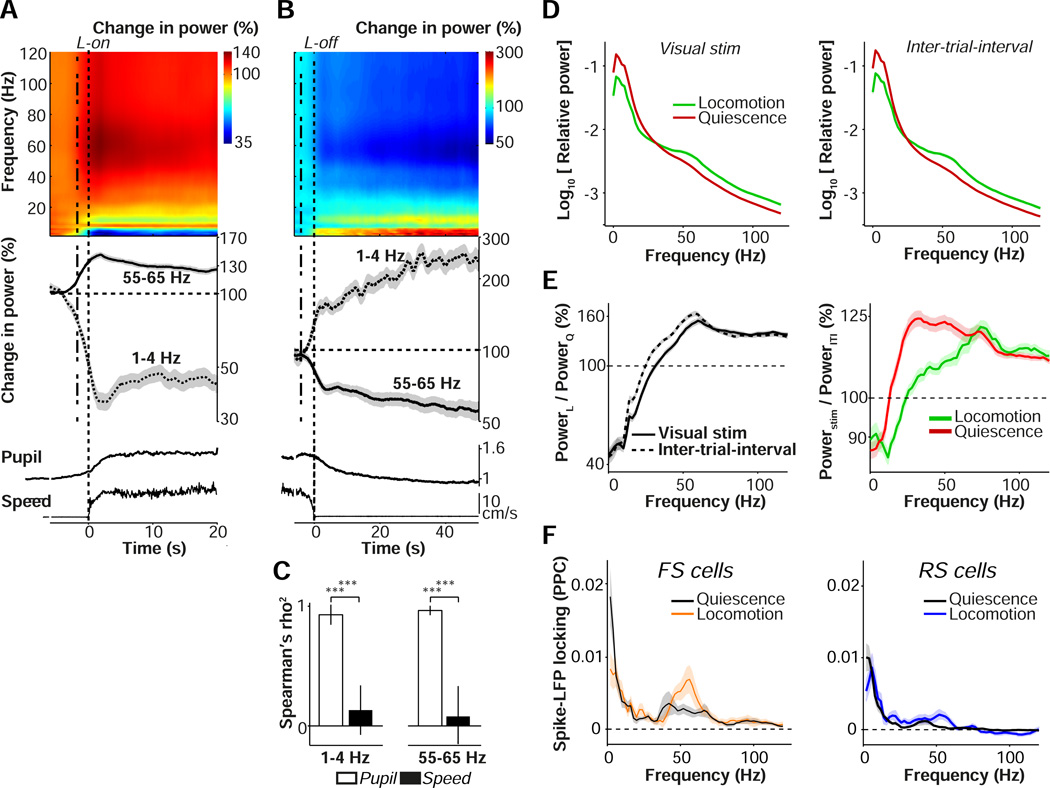
Figure 2.
Contributions of arousal and locomotion to V1 LFP rhythms. (A) Top: Time-frequency representation of LFP signals around locomotion onset (L-on), showing base-10 log-transformed relative power, i.e. power at time t divided by average power in the quiescent interval preceding L-on (−4.5 to −4 s). Power was computed in sliding windows of ±2 s using short-term Fourier Transform. Log power ratios are shown as percentages. Middle: Line plots of gamma (55–65 Hz) and low-frequency (1–4 Hz) power across n=58 sites in 11 mice, shown as mean ± s.e.m. Dashed lines at 2 s before zero represent the effective time at which data after L-on would be included in computation, revealing spectral changes before this point. Note the appearance of an 8–10 Hz theta-band that interrupts the reduction in low-frequency LFP fluctuations, presumably due to volume conduction from the hippocampus. Bottom: pupil and wheel traces, as in Fig. 1B–C. (B) As (A), but now for L-off. Relative power was computed by dividing by the average power in the epoch before locomotion offset (−3 to −2.5 s), for n=133 sites in 18 mice. (C) Spearman’s correlation (shown the square of the Spearman’s rho) between either average pupil diameter (PD) (open bars) or locomotion speed (closed bars) with average gamma and delta time courses for quiescence period, using data >−3 s before locomotion offset till 40 s after locomotion offset.***: p<0.001. Shown mean ± s.e.m. n=133 sites in 18 mice. (D) Average power spectra during visual stimulation period (left) and ITI (right), separately for locomotion and quiescent periods, for n = 196 sites in 23 mice. Power spectra were computed using multitapering of 500 ms windows, with a smoothing window of ±4 Hz. (E) Left: Base-10 log-transformed ratio of spectral power during locomotion over quiescence, separated for visual stimulation and ITI periods, shown as a percentage. Right: Base-10 log-transformed ratio of spectral power during visual stimulation over ITI period, separated for locomotion and quiescence, shown as a percentage. (F) Spike-field locking spectra (PPC) for FS (left) and RS (right) cells, separately for locomotion and quiescence periods. Shadings correspond to SEMs across cells. The difference between locomotion and quiescence was significant at both 2 Hz and 60 Hz for FS and RS cells (p<0.05, bootstrap test on mean PPC). The difference between RS and FS was significant at 60 Hz for both locomotion and quiescence (p<0.05, randomization test on means). FS: 25/12; RS: 61/18 (#cells/#mice).
We also recorded LFP activity in V1 while presenting visual stimuli. All stimuli were drifting gratings on a mean luminance background (see Material and Methods). We found that the increase in LFP gamma-band power and decrease in low-frequency power during locomotion periods were observed for both visual stimulation and inter-trial interval (ITI) epochs (Fig. 2D), in congruence with previous findings that locomotion without visual stimulation correlates with increased gamma power (Niell & Stryker, 2010). Visual stimulation during locomotion caused an increase in gamma band power at frequencies above 60 Hz, with a spectral peak around 75 Hz (Fig. 2E). During quiescent periods, visual stimulation instead caused an increase in gamma-band power at a broader range above 20 Hz, with a relatively shallow spectral peak around 30 Hz (Fig. 2E). We further found that contrast tuning of LFP gamma-band power was affected by locomotion, with increased and decreased gain at high and low gamma frequencies, respectively (Fig. S2).
To examine how LFP fluctuations related to local V1 spiking activity, we computed the strength of spike-LFP phase-locking for 34 FS (11 in L2/3, 23 in L5/6) and 157 RS (31 in L2/3, 126 in L5/6) cells that were classified based on action potential waveform characteristics (Fig. S1C–F). Locomotion increased gamma phase-locking and decreased low-frequency phase-locking for both RS and FS cells (Fig. 2F). Gamma phase-locking was stronger for FS than RS cells during periods of both quiescence and locomotion (Fig. 2F).
Cell-type specific modulation of firing rates
Next, we investigated the contributions of locomotion and arousal to V1 firing rates in the absence of visual stimuli. We compared firing rates between epochs of locomotion and quiescence and within each type of epoch to examine the temporal dynamics of changes in firing around transition points (Fig. 3A). Individual V1 neurons demonstrated a broad range of relationships between spontaneous firing rate and behavioral state (Fig. 3A, Fig. S3A). Both FS and RS cells’ spontaneous firing rates increased before locomotion onset, peaked around the time of locomotion onset, and declined over time throughout the locomotion period (Fig. 3A–B). To quantify the anticipatory effect for individual cells, we computed the Pearson cross-correlation coefficient between locomotion speed and instantaneous firing rate in the interval around locomotion onset. Most cells exhibited a strong linear correlation between locomotion speed and firing rate that was forward-shifted in time, indicating that increases in RS and FS firing rates preceded increases in locomotion speed (Fig. S3B–C).
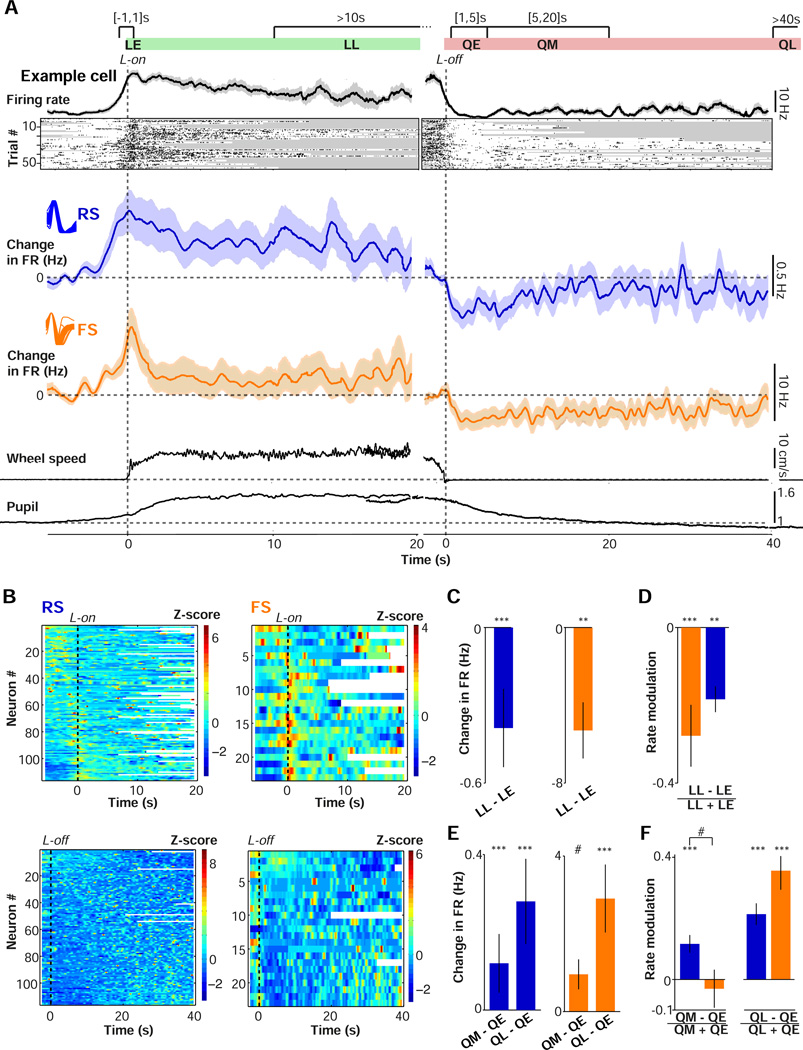
Figure 3.
Contributions of arousal and locomotion to spontaneous firing activity in V1. (A) Schematic showing division of data into epochs for analysis. LE: early locomotion period (−1 to 1 s around L-on). QE: early quiescent period (1 to 5 s after L-off). QM: middle quiescent period (5 to 20 s after L-off). QL: late quiescent period (>40 s after L-off). Plots show firing rate for an example FS cell during locomotion onset (L-on, left) and locomotion offset (L-off, right). Rasters show individual transition points for the same cell. Gray shadings indicate L-off and L-on during individual trials. Next, shown (left) the average (mean ± s.e.m.) change in RS (blue) and FS (orange) cells’ firing rates relative to a quiescent baseline period (taken −6 to −3 s before L-on) as a function of time, and (right) the change in firing rates relative to a baseline locomotion period (defined −3 to −1 seconds before L-off) as a function of time around L-off. Insets show average waveforms for RS and FS cells, as in Fig S1c. Bottom: pupil and wheel traces, as in Fig. 1b–c. (B) Upper: Overview of all recorded RS (left) and FS (right) cells, sorted by firing rate difference between quiescent baseline and the interval around L-on (−1 to 1 s). Color coding corresponds to Z-scored firing rates. For each cell, Z-scoring was performed over the mean change in firing rate in the shown interval. Lower: As in the upper panel, but as a function of time around L-off and sorted on Spearman’s correlation between time after L-off (>3 s) and quiescence. (C-D) Mean ± s.e.m. of firing rate differences (C) and firing rate modulation (D) between LE and LL epochs. Statistical testing using two-sided Rank-Wilcoxon tests. #,*,**,*** correspond to p<0.1, 0.05, 0.01, 0.001. RS: n=106/17, FS: n=18/9. (E-F) As in (C-D), but now for QE vs. QL and QM vs. QL. RS: 101/18, 101/18, FS: 21/10, 18/9 (#cells/#mice). Statistical comparison of QM vs. QL rate modulation: RS, FS: p=7.1×10−4, 8.6×10−4.
Both FS and RS firing rates were significantly higher during the early period around locomotion onset (within ±1 s of locomotion onset; LE) than during the late locomotion period (>10 s after locomotion onset; LL) (Fig. 3C, Fig. S4A). To directly compare locomotion-related changes between RS and FS cells, we computed a rate modulation value (FRb − FRa) / (FRa + FRb), which normalizes for absolute rate differences. RS and FS cells exhibited similar degrees of rate modulation during the early vs. the late locomotion period (p=0.48; Fig. 3D). We did not observe a significant difference in LE vs. LL rate modulation between cells recorded from superficial and deep layers (RS L2/3: 0.16±0.092, L5/6: 0.22±0.041, p=0.72; FS L2/3: 0.30±0.21, L5/6: 0.32±0.068, p=0.93). We found no significant difference between unclassified single (US) units (Fig. S1C–F) and RS cells (US: 0.22±0.08, p=0.96).
We next examined whether increases in firing rate in anticipation of and during locomotion might be explained by the associated change in arousal. The increase in firing rate before locomotion might be attributable to an increase in arousal, since pupil dilation precedes locomotion onset, or may alternatively reflect a preparatory phase in advance of motor execution. To separate the effects of locomotion and arousal, we therefore examined the trajectories of firing rates during quiescence following locomotion offset, when pupil diameter showed substantial variation in the absence of movement (Fig. 1B–C). For both RS and FS cells, locomotion offset was accompanied by a rapid decrease in firing rates, followed by a subsequent gradual increase over time (Fig. 3A). Thus, a state of heightened arousal, corresponding to maintained pupil dilation after the cessation of movement, is accompanied by decreased rather than increased RS and FS firing.
To directly compare firing rates across epochs within the quiescent period, we analyzed early quiescence (1–5 s after locomotion offset; QE), middle quiescence (5–20 s after locomotion offset; QM) and late quiescence (>40 s after locomotion offset; QL) (Fig. 3A). Pupil diameter was significantly decreased across the QE to QM to QL intervals after locomotion offset (Fig. S4E). In contrast, FS and RS firing rates significantly increased across the QE to QM to QL intervals after locomotion offset (Fig. 3E–F). We found no significant difference in rate modulation between FS and RS cells across quiescent epochs (Fig. 3E–F). We did not observe a significant difference in rate modulation between QE and QL intervals when comparing cells recorded from superficial and deep layers (RS L2/3: −0.23±0.10, L5/6: −0.21±0.037, p=0.64; FS L2/3: −0.41±0.13, L5/6: −0.33±0.079, p=1). No significant difference was observed between RS and US cells (US: −0.10±0.06, p=0.12).
To further quantify the trajectory of V1 firing rates over time, we computed Spearman correlation coefficients between firing rate and time after locomotion offset (using only data >3 s after locomotion offset; Material and Methods). Many cells showed a significant association between firing rate and time (FS: 10/23, RS: 37/80, at p<0.05), and the average correlation was higher than zero (Fig. S4B, mean ± s.e.m. of Spearman’s rho=0.04±0.024, p=0.034; Rank-Wilcoxon test). However, we also found subpopulations of cells whose firing rate either decreased or increased over time (Fig. 3B, Fig. S4B, FS positive: 6/23, negative: 4/23; RS positive: 20/80, negative: 17/80, p<0.05), suggesting diverse firing rate trajectories across the V1 population.
Fig. 3B shows a subset of RS and FS cells whose firing rates were lower during locomotion than during quiescence. This suppression effect was also observed when we excluded the 20s of data immediately before locomotion onset, indicating that it was not an artifact of anticipatory firing (Fig. S4C–D). To investigate whether this suppressive effect was due to arousal or locomotion, we compared the locomotion period with either the entire quiescent period, which is overall associated with low arousal levels, or with the early quiescent period, which is characterized by high arousal levels. Despite showing suppression during locomotion relative to the entire quiescent period, this subset of cells showed no suppression when we restricted the comparison to the early quiescent period (Fig. S4C–D). This suggests that the locomotion-associated firing reduction in this subset of cells is likely due to the suppressive effect of arousal, rather than locomotion. Overall, our results indicate that both RS and FS cells in V1 demonstrate increased spontaneous activity in anticipation of and during locomotion. However, a state of heightened arousal, as indicated by maintained pupil dilation after the cessation of movement, is accompanied by a decrease in spontaneous RS and FS firing. In contrast to the spectral changes in LFP signals, elevated firing during movement is thus specific to locomotion periods and unlikely to result from associated changes in global arousal.
Modulation of visual encoding by state transition
To determine the impact of locomotion and arousal on visual encoding, we recorded unit activity in V1 while presenting visual stimuli. The robust differences in visually evoked LFP dynamics between periods of locomotion and quiescence (Fig. 2, Fig. S2) suggest that visual stimulation may likewise differentially affect firing rates during these states. We therefore computed the mean peak firing rate of cells in response to stimuli presented during locomotion and quiescence (Fig. 4A–B, Fig. S5A). To separate the effects of locomotion and arousal, we divided the quiescence period into separate epochs as in Fig. 3. The exact durations of the epochs were adjusted to account for the duration and timing of stimulus presentation. We analyzed early quiescence (3–20 s after locomotion offset; QE), when the pupil was relatively dilated, and late quiescence (>40 s after locomotion offset; QL), when the pupil was relatively constricted. We used a signal-to-noise (SNR) ratio index (SNRI), defined as (FRstim - FRITI) / (FRstim + FRITI), to assess visual encoding by V1 neurons during each epoch (Material and Methods; as shown in the Material and Methods, the SNRI corresponds to a monotonic sigmoid transformation of the SNR = FRstim / FRITI, mapping the SNR onto the −1 to 1 interval). We found an overall increase in the SNRI of visual encoding during locomotion in comparison to quiescence for RS, but not FS, cells (Fig. 4C). A finer analysis of time periods found that the SNRI in early quiescence (QE), during high arousal, was larger than in late quiescence (QL), during low arousal, for both RS and FS cells (Fig. 4C). The SNRI in the quiescent period 20–40s after locomotion offset reached intermediate values for both FS and RS cells (RS: 0.16±0.044, FS: 0.35±0.054). We found that spontaneous, but not visually evoked, RS firing rates were significantly higher in the late than in the early quiescence period (Fig. S5B–D). The observed increase in signal-to-noise ratio during high arousal states thus largely resulted from the differential effects of arousal on spontaneous and evoked firing rates.
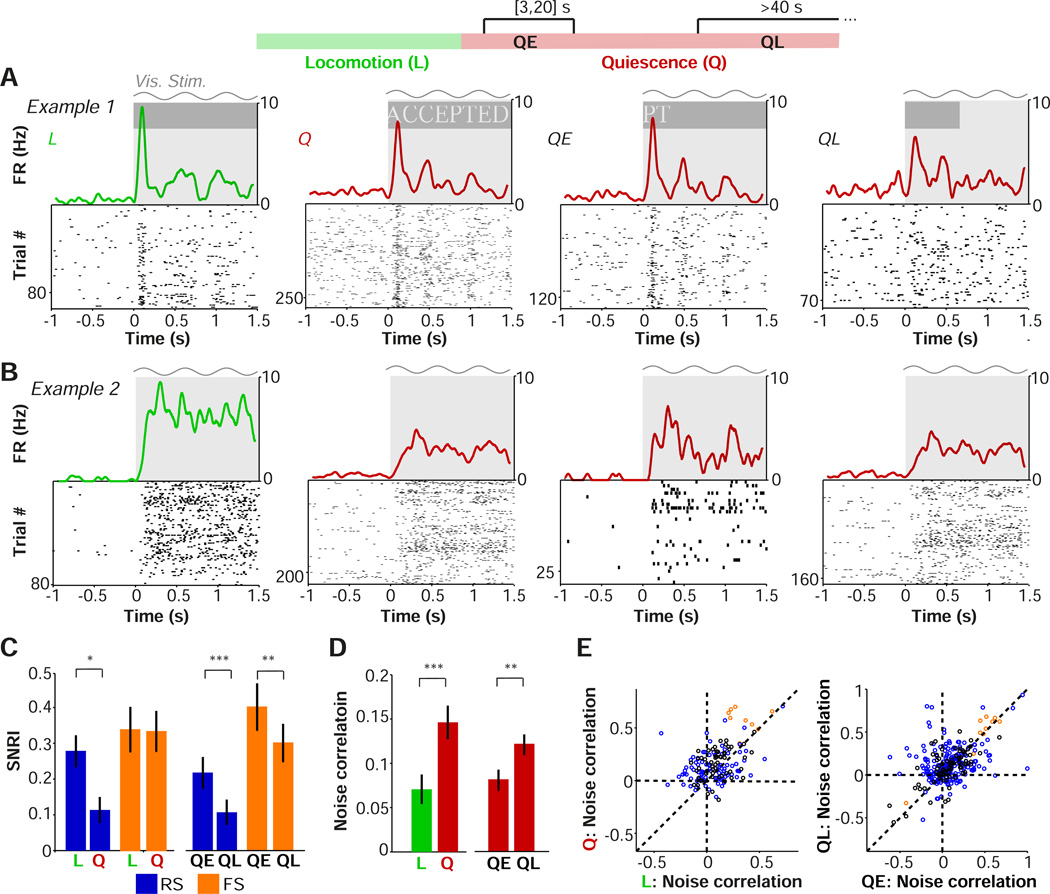
Figure 4.
Contributions of locomotion and arousal to visual encoding and noise correlations in V1. (A-B) Raster plots of the visual responses of two example RS cells with associated firing rate density (computed using ±0.025 s Gaussian kernels with SD of 0.0125 s) for all locomotion (L), all quiescence (Q), early quiescence (QE; 3 to 20 s after locomotion offset) and late quiescence (QL; >40 s after locomotion offset). Gray shading and sinusoid indicate visual stimulation. (C) Mean ± s.e.m. of signal-to-noise ratio (SNRI), defined as (FRstim−FRITI) / (FRstim+FRITI), for each period. #,*,**,*** correspond to p<0.1, 0.05, 0.01, 0.001, two-sided Rank-Wilcoxon test. RS: n=88/20 (Q-L), 101/20 (QE, QL), FS: n=23/10, 24/11(#cells/#mice). Note that values for Q are closer to those for QL because more stimulus repetitions were available for QL. (D) Mean ± s.e.m of noise correlation of firing rates during different behavioral periods. n=155/9, 289/11 (#pairs/#mice). *,**,***: p<0.05, p<0.01, p<0.001, two-sided Rank-Wilcoxon test. (E) Left: Noise correlation for locomotion vs quiescence. Circles correspond to cell pairs. FS-FS: n=9/3, RS-RS: 90/7, FS-RS (black): n=56/5. Right: as left, but for QE vs. QL. FS-FS: 10/4, RS-RS: 206/7, FS-RS: n=73/7.
The SNRI is a measure of the encoding of visual stimuli by individual neurons. However, visual stimuli are likely to be encoded by patterns of V1 population activity (Olshausen and Field, 1996; Pouget et al., 2000). We therefore calculated noise correlations, which may determine the efficiency of population coding (Cohen and Kohn, 2011; Ecker et al., 2010), for each epoch (Material and Methods). Overall, noise correlations were significantly lower during periods of locomotion than during quiescence (Fig. 4D–E). Because low-frequency and gamma-band LFP power showed gradual changes over time after locomotion offset, we hypothesized that noise correlations would increase with time after locomotion offset. Indeed, noise correlations were significantly elevated during late quiescent periods, when arousal was low, as compared to early quiescent periods (Fig. 4D–E).
These results suggest that visual encoding is enhanced during locomotion as a result of increased signal-to-noise ratio and decorrelation of visually evoked activity. However, these changes are mainly attributable to arousal, rather than locomotion, as comparable changes in signal-to-noise ratio and noise correlations were observed during periods of heightened arousal in the absence of movement.
Isolating the cortical effects of arousal
The analyses of naturally occurring state transitions described above suggest separable effects of locomotion and arousal on firing rates, LFP activity, and visual encoding in V1. However, it is possible that the period of heightened arousal following locomotion offset represents an unusual form of arousal or is affected by long-lasting modulation of V1 activity by motor signals. To more causally test the role of arousal in regulating neural activity, we induced a state of enhanced arousal by puffing air on the back of the mouse (Fig. S1B, Material and Methods). In a subset of sessions (n=20 sessions in 10 mice), we administered brief air puffs (mean±s.e.m. of rate = 0.0089±0.0018 Hz, mean±s.e.m. of air puff number= 42.23±5.63) to the mouse while monitoring pupil diameter and performing simultaneous recordings of V1 LFPs and isolated single cells (Fig. 5; n = 61 cells in 10 mice).
Figure 5.
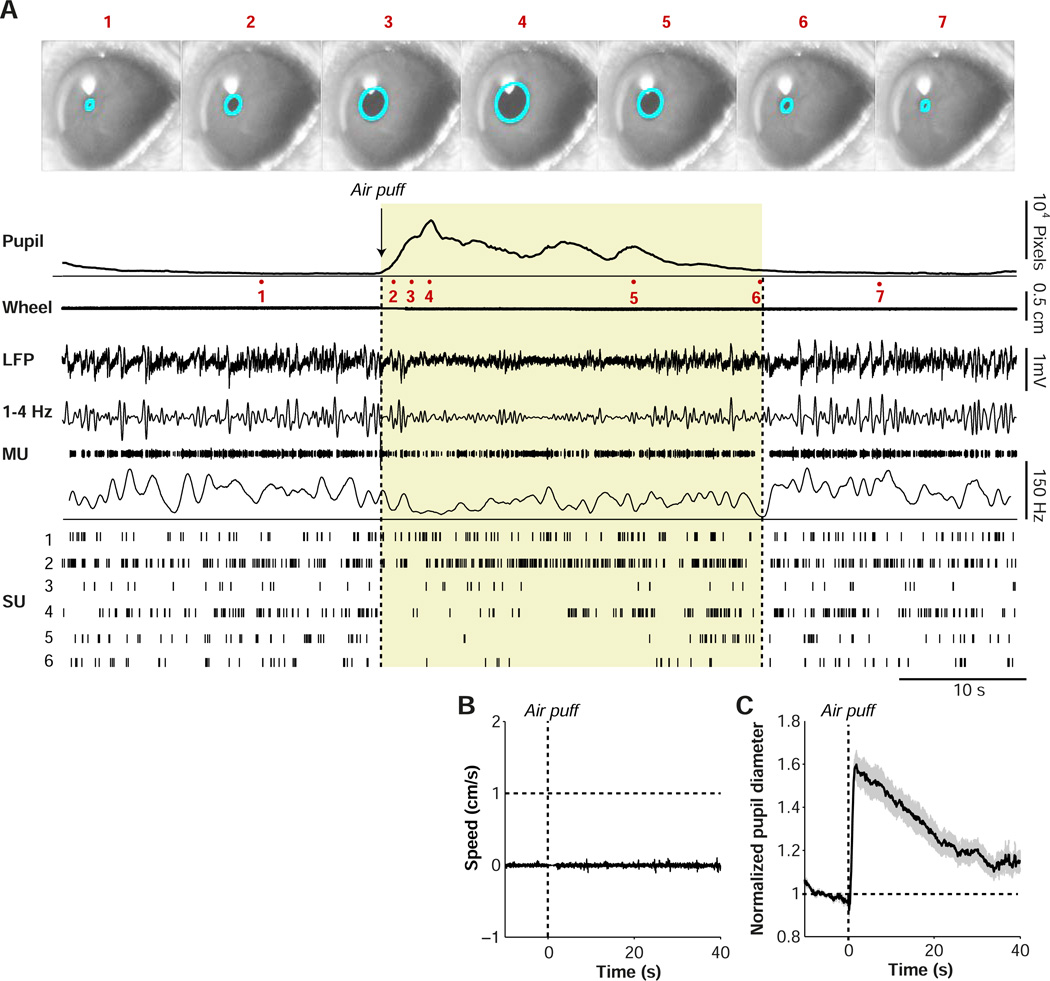
Causal induction of arousal without locomotion. (A) Example data from one experiment session. Video images captured of eye are shown for several time points (1–7). Average pupil diameter trace in pixels is shown as a function of time around air puff onset. Locomotion is shown as a linearized version of the wheel position. LFP recording is shown for one electrode, together with the 1–4 Hz filtered signal. A multiunit trace (MU) and associated spike density plot are shown for the same electrode. Single-unit traces (SU1-6) were isolated from a total of three electrodes in L5/6 during this recording session. Shaded yellow area indicates period during which pupil was dilated, for visualization purposes. (B) Average locomotion speed as a function of time around the air puff. (C) Average pupil diameter, normalized to pre-air-puff interval (−10 to 1 s before air puff), as a function of time. The s.e.m.’s were computed across sessions (n=20 in 10 mice).
Delivery of the air puff reliably induced both arousal, as measured by pupil dilation, and changes in unit activity in the absence of locomotion (Fig. 5A, Supplementary Movie 2). Across sessions (n=20 sessions in 10 mice), air puffs caused an average 1.6-fold increase in pupil diameter in the absence of locomotion (Fig. 5B–C). This increase was similar in magnitude to the average increase in pupil diameter observed during locomotion (Fig. 1C), suggesting a comparable level of arousal. We did not find a significant correlation between trial number and the normalized pupil diameter (computed in the interval 1–4 s after the air puff; Spearman’s rho=−0.05±0.07), or a difference between the first and last five trials (p=0.63, Rank-Wilcoxon test), indicating little adaptation to air puffs within sessions.
LFP modulation by induced arousal
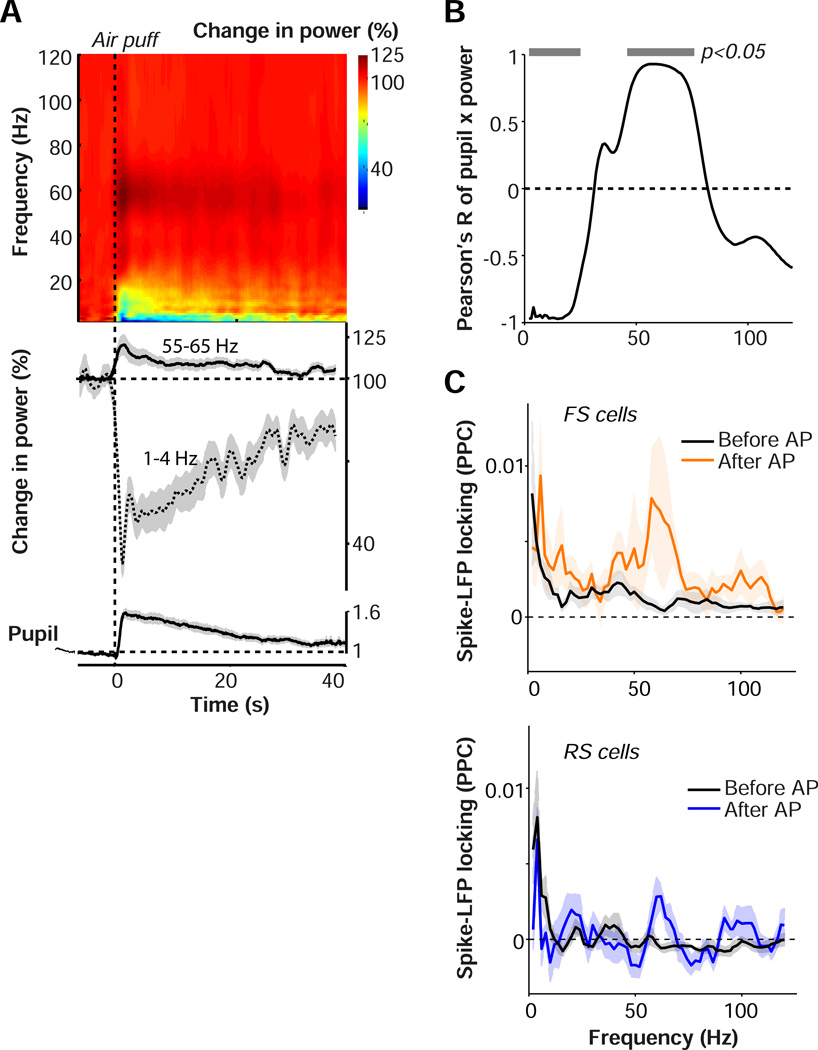
Our analysis of arousal periods following locomotion offset (Fig. 2) predicts that a shift to an aroused state in the absence of locomotion should be accompanied by a decrease in low-frequency LFP power and an increase in gamma-band power. Indeed, we found that after the air puff the LFP showed a long-lasting, 2.5 fold decrease in low-frequency fluctuations comparable in magnitude to the locomotion effect (Fig. 6A). The induced arousal also caused a 1.25-fold increase in gamma-band power. The pupil diameter time course showed a strong positive correlation with gamma-band power, and a negative correlation with low-frequency power (Fig. 6B). The increase in gamma power was accompanied by a significant increase in the spike-LFP gamma phase-locking of both RS and FS cells and by a significant decrease in low-frequency RS locking (difference between FS and RS cells for before vs. after air puff comparison n.s.; Fig. 6C). Finally, we found that air puffs during locomotion periods did not induce a significant change in either low-frequency or gamma-band power, indicating that air puffs did not yield additional effects when the mouse was already in a state of heightened arousal (Fig. S6). Together, these findings indicate that a shift to a state of high arousal without locomotion causes a decrease in low-frequency fluctuations and an increase in gamma-band fluctuations in V1.
Figure 6.
Arousal causes a frequency shift in V1 LFP activity. (A) Top: time-frequency representations of LFP signals around the air puff (AP). Base-10 log-transformed relative power, i.e. power at time t divided by average power in the interval preceding the air puff (−6 to −1 s). n = 45 sites/10 mice. Middle: Line plots of gamma and delta power, with shading representing s.e.m. across sites, around time of air puff. Bottom: pupil diameter trace, as in Fig. 5C. (B) Correlation of pupil diameter time course with LFP time course at various frequencies. Gray horizontal bars indicate significance at p<0.05, linear regression analysis. (C) Spike-field locking spectra (PPC) for FS (upper) and RS (lower) cells, separately for baseline before air puff (10 s) and period immediately after air puff (1 to 4 s). Shadings correspond to SEMs across cells. FS: 8/5; RS: 11/5 (#cells/#mice).
Rate modulation by induced arousal
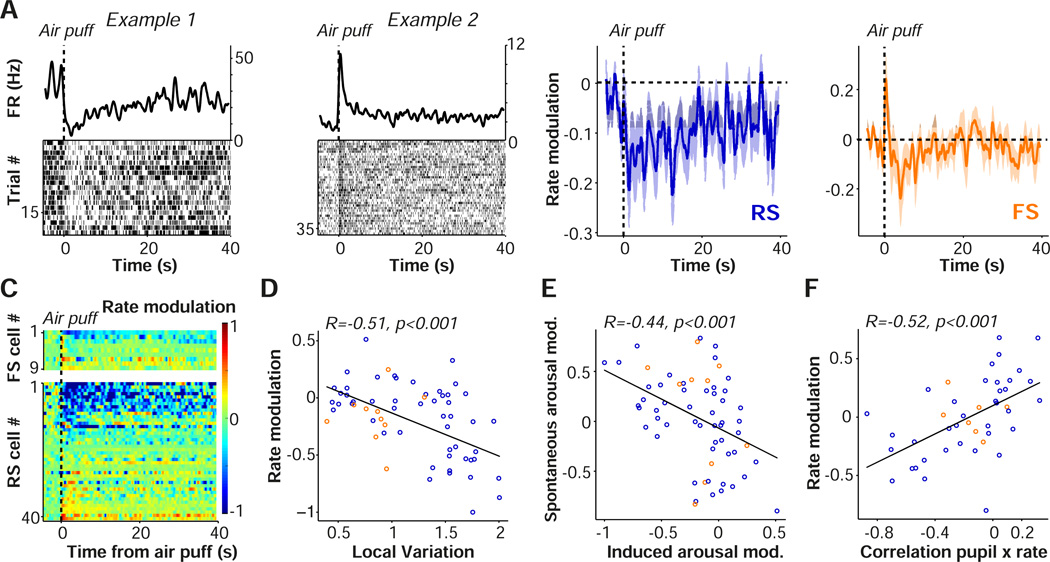
We next investigated how firing rates were affected by the causal manipulation of arousal levels. Overall, we found that the air puff caused a significant, long-lasting decrease in spontaneous firing rates (Fig. 7A–B). However, a small subpopulation of RS and FS cells showed elevated firing rates after the air puff (Fig. 7A,C). Air puffs were particularly suppressive for RS cells if they had a high propensity to engage in irregular burst firing (Fig. 7D; Material and Methods), but a similar relationship was not observed for FS cells (Fig. 7D).
Figure 7.
Arousal suppresses firing rates in V1. (A) Raster plots and spike density traces (1 s windows with Gaussian kernel and SD of 250 ms) from two example (left: FS, right: RS) cells are shown as a function of time. (B) Average modulation of firing rate, defined as [FRpost - FRpre] / [FRpost - FRpre] induced by the air puff for RS (blue) and FS (orange) cells, relative to 6 s prior to air puff. Mean ± s.e.m of modulation in the interval after the air puff (1 to 4 s) for RS = −0.19±0.046, p=4.6×10−4, n=51/9 (#cells/#mice). FS: −0.14±0.074, p=0.084, n=10/6. The difference between FS and RS cells was not significant (Rank-Wilcoxon test; p=0.87). (C) Rate modulation scores for all FS (upper) and RS (lower) cells as a function of time, relative to 6 s prior to air puff. (D) Rate modulation index, comparing 1 to 4 s post air puff to 6 s prior to air puff, as a function of local variation coefficient, a measure of firing regularity. High local variation values correspond to bursty cells and a local variation coefficient of 1 indicates Poisson-like firing (Material and Methods). RS cells: R = −0.57, p=1×10−5, n=51/9, FS cells: R=0.13, p=0.71, n=10/6 (#cells/#mice). (E) Values on the x-axis indicate induced-arousal rate modulation index (as in D). Values on the y-axis indicate spontaneous-arousal modulation, calculated as the Spearman correlation of time after locomotion (>3 s) and firing rate. R = −0.47, p = 4.15×10−4, NS: R = −0.32, p=0.36, difference RS and FS n.s., Fisher Z-test. (F) Rate modulation index (as in D) vs. correlation of pupil diameter and firing rate across trials. Correlation of pupil and firing rate was defined over trials using the interval after the air puff (1 to 4 s). Pearson’s R = 0.52, p=3.3×10−4, n=45/7.
Individual neurons should show similar effects in response to naturally occurring and induced arousal. We therefore predicted that cells whose firing rates were suppressed by the induced arousal would likewise show suppression after locomotion offset, followed by gradual increases in firing rate over time as arousal levels spontaneously decreased (Fig. 3). For this analysis, we selected only locomotion epochs that did not occur within 50 s of an air puff. We first defined a spontaneous-arousal modulation as the correlation between time after locomotion offset and firing, with positive values indicating that a cell was suppressed by arousal. We also defined the induced-arousal modulation in the interval after the air puff. We found that the spontaneous-arousal and induced-arousal modulations were significantly negatively correlated, demonstrating consistent effects of arousal on firing rate (Fig. 7E).
The coordinated effects of induced arousal on firing rate and pupil diameter also predict that fluctuations in arousal should correlate on a trial-by-trial basis with firing rates. We predicted (more) negative correlations between pupil diameter and firing rates across trials for cells whose firing was on average suppressed by the air puff. Likewise, we predicted (more) positive correlations for cells whose firing was enhanced by the air puff. These predictions were supported by trial-by-trial correlation analysis (Fig. 7F). Air puffs during locomotion periods did not induce a significant change in average firing rate, suggesting that air puffs did not have an additional suppressive effect when the mouse was already in a state of heightened arousal (Fig. S6). Together, these results suggest that global arousal is associated with decreased spontaneous firing rates.
Arousal and visual encoding
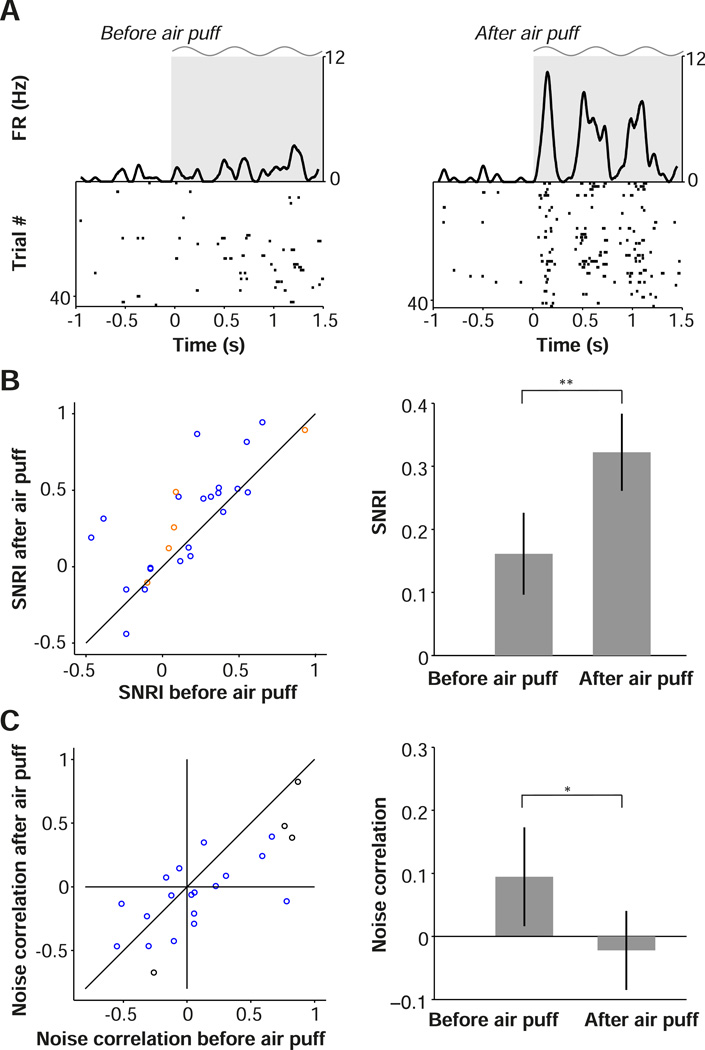
To isolate the role of arousal in modulating single cell and population visual encoding, we administered air puffs randomly in combination with presentation of visual stimuli in a subset of experiments (Material and Methods). We then compared visual responses in the 10 s before the air puff with those in the 10 s after the air puff. Across the population of cells, we found a significant increase in SNRI following the induced arousal (Fig. 8A–B). We also observed that induced arousal significantly decreased noise correlations (Fig. 8A,C). These results indicate that arousal alone replicates the effects of locomotion on visual encoding, leading to an increase in the salience of visual responses by individual cells and decorrelating activity across the population.
Figure 8.
Arousal enhances visual encoding and decreases noise correlations in V1. (A) Raster plots of the visual responses of an example cell with associated firing rate density (computed using ±0.025 s Gaussian kernels with SD of 0.0125) before (left) and after (right) air puff. Gray shading and sinusoid indicate visual stimulation. (B) Plot of signal-to-noise ratio index (SNRI) for the 10 s interval before air puff (y-axis) compared to 10 s interval after air puff (x-axis). Population SNRI is shown at right, as mean ± s.e.m (n=25/8, #cells/#pairs). (C) Noise correlation in the 10 s after air puff compared to the 10 s before air puff. Circles correspond to cell pairs (red = FS, blue = RS, black = FS/RS). Population average noise correlation is shown at right. (B-C) *, **: p<0.05, p<0.01, n=22/4 (#pairs/#mice), two-sided Rank-Wilcoxon test.
Discussion
We examined the distinct contributions of arousal and locomotion to V1 activity and visual encoding using extracellular recording in combination with behavioral state monitoring and manipulation. Our data revealed that much of the change in V1 LFP power, spike-LFP phase-locking, and unit activity associated with locomotion is the result of increased arousal. We found that heightened arousal in the absence of locomotion has several cortical impacts: 1) a change in the temporal patterning of neural activity, comprising a reduction in low-frequency oscillations and an increase in gamma synchronization, 2) a net suppression of spontaneous firing rates, and 3) a change in visual encoding at both the single-cell and population levels. In contrast, locomotion specifically contributes to an increase in RS and FS cell firing rates in anticipation of and during movement. Arousal state and motor activity thus have distinct roles in regulating activity in primary visual cortical circuits.
We found substantial differences in V1 activity between periods of quiescence and locomotion. This is consistent with several studies that used electrophysiology or calcium imaging in awake head-fixed rodents (Bennett et al., 2013; Fu et al., 2014; Keller et al., 2012; Lee et al., 2014; Niell and Stryker, 2010; Polack et al., 2013; Reimer, 2014; Saleem et al., 2013). However, previous results suggested that elevated RS cell firing during locomotion periods was not associated with locomotion, but was rather a modulation of visual responses (Bennett et al., 2013; Niell and Stryker, 2010). By focusing on state transition points, we were able to show that RS firing was strongly increased in anticipation of locomotion and during movement, even in the absence of visual stimulation. In contrast, causal induction of a state of high arousal caused a suppression of spontaneous firing rates, indicating that motor and arousal signals may have opposing effects on spontaneous spiking activity in V1. Studies in auditory cortex have found that firing rates of both RS and FS cells are suppressed by locomotion (Schneider et al., 2014; Zhou et al., 2014), whereas studies in barrel cortex have shown that firing rates of RS and FS cells are unaffected and suppressed by whisking, respectively (Gentet et al., 2010; Gentet et al., 2012; Schneider et al., 2014; Zhou et al., 2014). Thus, increased RS and FS firing during locomotion is likely specific to visual cortex and may be related to the necessity for integration of motor and visual signals in order to faithfully represent the outside world with respect to the animal’s position (Keller et al., 2012; Saleem et al., 2013).
Firing rates and LFP power in the low (1–4Hz) and gamma (55–65Hz) ranges showed changes well in advance of the onset of locomotion, as did pupil diameter. These anticipatory changes indicate that increased arousal levels and motor-related activity in V1 reliably precede the execution of motor output. Increased firing in anticipation of locomotion, consistent with motor planning or predictive coding (Keller et al., 2012; Saleem et al., 2013), could rely on top-down inputs from fronto-parietal circuits, in which many cells fire in anticipation of saccades and other movements (Bruce and Goldberg, 1985; Snyder et al., 1997). Indeed, recent work in mice has highlighted the existence of long-range inputs from frontal to striate cortex (Zhang et al., 2014) and motor cortical areas may also project to V1 or other visual areas (Miller and Vogt, 1984; Wang et al., 2011).
Distinct V1 cell populations exhibited different modulation around transition points. We observed enhanced signal-to-noise during locomotion for visual responses only in RS, putative excitatory cells but not in FS, putative inhibitory cells, potentially indicating cell-type specific regulation of visual encoding. Recent work has highlighted distinct patterns of state-dependent recruitment of different interneuron classes (Fu et al., 2014; Gentet et al., 2012; Polack et al., 2013; Reimer, 2014; Zhou et al., 2014). Notably, we found diversity within both the RS and FS cell populations in the trajectory of state-dependent changes in firing patterns at behavioral state transition points. Whereas most RS and FS cells were suppressed by arousal, a subset of both cell types instead showed enhanced firing. Firing rate suppression with arousal was particularly evident in bursting RS cells. Despite an overall reduction in firing rates, arousal was associated with enhanced phase-locking of V1 neurons to gamma rhythms. FS cells were more strongly locked to LFP gamma oscillations than RS cells, in agreement with the proposed role of FS interneurons in generating these oscillations (Cardin et al., 2009; Csicsvari et al., 2003; Sohal et al., 2009; Vinck et al., 2013; Whittington et al., 1995)
To robustly interpret the effect of arousal on firing rates, we focused on periods that were independent of motor anticipation or changes in velocity. An alternative approach would have been to regress out the influence of locomotion speed in a multivariate model to isolate the relationship between firing rate and pupil diameter, as the activity of a cell at any moment in time might potentially be well predicted by a model using both locomotion speed and pupil diameter. However, the relationship between speed and firing rate is not captured well by a linear model in the form of Activity(t) = a1*speed(t) + a2*pupil_diameter(t), because units fire in anticipation of speed changes during locomotion onsets, creating a non-linear relationship between locomotion speed and firing rate (Fig. S8). An expression of the form Activity(t) = ∑s a1(s)*speed(t-s) + a2(s)*pupil_diameter(t-s), which sums across various lags s and thereby coarsens the time resolution, is minimally required. Furthermore, although cells fire in anticipation of locomotion, their firing rates lag the offset in locomotion, indicating that the influence of locomotion speed at various time lags cannot be linearly summed. In addition, Saleem et al. (2013) found that the firing of many cells is nonlinearly related to concurrent locomotion speed. Regressing out the influence of locomotion speed in a multivariate model to isolate the relationship with pupil diameter would thus not necessarily ensure adequate regression of correlations between firing rate and locomotion. This would be problematic, given the relationship between pupil diameter and locomotion (Fig. 1).
We took advantage of direct manipulations of behavioral state to probe the dynamic range of behavioral state-dependent cortical activity. Causal induction of arousal with the air puff stimulus initiated a shift from low to high frequencies in the cortical LFP. We found that induced arousal replicated the 2.5 fold reduction in 1–4 Hz fluctuations observed during locomotion periods. Low frequency fluctuations increased only gradually after locomotion offset, with a strong linear relationship to the pupil diameter. Arousal alone therefore appears to account for the reduction in 1–4 Hz LFP power during locomotion periods. The observed linear relationship between pupil diameter and 1–4 Hz LFP power differs from recent work in which pupil diameter did not correlate with low-frequency membrane potential power, likely because only very small and short-lasting pupil fluctuations were considered (Reimer et al., 2014). LFP and firing rate changes were dissociated from one another during arousal periods, as the gradual increase in LFP low-frequency power and decrease in LFP gamma-band power were accompanied by a net increase in firing rates over the same time period.
The effect of arousal on gamma-band LFP power in the absence of locomotion (∼20% increase) was smaller than the increase in gamma-band LFP power during locomotion periods (∼40% increase). Gamma-band power showed a similar adaptation during the locomotion period as firing rates, in contrast to 1–4 Hz power. A change in arousal might therefore not exclusively account for the increase in gamma-band power during locomotion periods, suggesting that locomotion might further amplify gamma-band oscillations through the increased drive of local RS and FS cells (Cardin et al., 2009; Csicsvari et al., 2003; Sohal et al., 2009; Whittington et al., 1995). Previous work has linked noise correlations to intrinsic low-frequency fluctuations and a decrease in gamma-band oscillations (Harris and Thiele, 2011; Herrero et al., 2013; Mitchell et al., 2009; Womelsdorf et al., 2012). The effects of arousal observed here thus overlap with those of focused spatial attention in primates, where attention is associated with increased temporal patterning in the gamma band and decreased noise correlations (Cohen and Maunsell, 2009; Fries et al., 2001; Harris and Thiele, 2011; Mitchell et al., 2009).
State-dependent changes in visual encoding were highly correlated with arousal level across a wide range, suggesting extensive flexibility in the sensory processing operations of cortical circuits. An increase in the signal-to-noise ratio of visual responses in V1 has previously been reported for waking vs. sleeping states (Livingstone and Hubel, 1981; Steriade et al., 2001), indicating possible regulation of visual sensitivity by overall arousal levels. In agreement with this idea, we found that states of high arousal were associated with increased signal-to-noise ratios and decreased noise correlations, indicating enhanced encoding of visual stimuli at both the single-cell and population levels. We found a strong trial-by-trial correlation between arousal, as measured by pupil diameter, and both measures of visual encoding, suggesting a dynamic system in which changes in arousal fine-tune the gain of visual responses in V1 on a moment-to-moment basis.
Lesion and stimulation studies have supported causal roles for several major neuromodulatory systems in controlling sleep-wake transitions and the temporal pattern of cortical activity, including norepinephrine (NE) and acetylcholine (ACh) (Buzsaki et al., 1988; Carter et al., 2010; Constantinople and Bruno, 2011; Lee et al., 2014; Metherate et al., 1992; Munk et al., 1996; Pinto et al., 2013). Like arousal, neuromodulatory action desynchronizes the cortex, promotes gamma oscillations, and changes sensory encoding (Carter et al., 2010; Fu et al., 2014; Harris and Thiele, 2011; Munk et al., 1996; Pinto et al., 2013; Steriade et al., 1993). Noradrenergic blockade of awake mouse V1 eliminates the depolarization and elevated firing associated with locomotion (Polack et al., 2013). Recent evidence also points to the involvement of the mesencephalic locomotor region, a cholinergic brainstem nucleus, in the control of locomotion and regulation of V1 activity patterns and visual responses (Lee et al., 2014). In addition, cholinergic afferents from the basal forebrain nucleus of the diagonal band of Broca selectively target V1 interneurons involved in locomotion-related changes in firing rates (Fu et al., 2014). Arousal effects observed in V1 during waking state transitions are thus likely involve complex interactions between multiple neuromodulatory systems.
In summary, our data show that activity in mouse visual cortex during wakefulness is differentially regulated by arousal and motor signals. We find a complex interaction between internally generated cortical states and visual inputs. Arousal restructures spontaneous cortical activity and promotes fast gamma-band oscillations, which may be optimal for synchronized, bottom-up routing of sensory signals (Busse et al., 2011; Fries et al., 2001; van Kerkoerle et al., 2014; Womelsdorf et al., 2012). This shift in the mode of cortical operation also strongly increases the signal-to-noise ratio of visual representations. The interplay between arousal level, motor activity, and sensory input may contribute to the functional flexibility of cortical circuits.
EXPERIMENTAL PROCEDURES
Animals, headpost surgery and wheel training
We handled 4–6 month old male wild type mice for 5–10 min/day in the 5 days prior to the headpost surgery. Following surgery, mice were allowed to recover for 3–5 days before wheel training commenced. Mice were head-fixed on the wheel for increasing intervals on each successive day, and were trained on the wheel for up to 7 days or until they exhibited robust bouts of running activity during each session.
Electrophysiology
Simultaneous recordings of isolated single units and local field potentials (LFPs) were made from multiple sites throughout layers 2–6 of primary visual cortex (V1) in awake mice. Recordings were made both during baseline periods, in which the LCD monitor displayed an isoluminant gray screen, and during visual stimulation periods, in which drifting gratings were presented for 1.5–2 s, interspersed with the presentation of an isoluminant gray screen in the inter-trial interval (ITI). Infrared camera recordings were made of the eye ipsilateral to the craniotomy in order to measure pupil diameter. In a first set of experiments, we analyzed spontaneous transitions in locomotion, arousal and cortical activity. In a second set of experiments, a small tube was positioned behind the mice’s head and air puffs were delivered to the body of the mouse during quiescent periods.
Locomotion and pupil diameter analysis
A change-point detection algorithm was used to detect statistical differences in the distribution of locomotion velocities across time in order to identify locomotion on- and offset. Pupil diameter was extracted from gray-scale video frames of 800 × 600 pixels at 10 Hz. 1). Fuzzy c-means clustering was used to identify a cluster of pixels corresponding to the pupil, and the pupil was extracted using edge detection and ellipsoid fitting
Spectral LFP and spike-LFP analyses
LFP power spectral density was estimated at each time point using 7 cycles of LFP data per frequency and a Hann taper. We then performed smoothing of the spectra with rectangular box car windows such that always 4 s of data was used to estimate power at a certain time point. For computing LFP spectra during visual stimulation, we divided the data in 500 ms segments and used multitapering with ±4 Hz smoothing.
Spike-field locking was computed using the Pairwise Phase Consistency (Vinck et al., 2011, 2013), a measure of phase consistency that is not biased by the number of spikes. Spike-LFP phases were computed for each spike and frequency separately by using Discrete Fourier Transform with Hanning taper of an LFP segment of length 9/f, where f is the frequency of interest.
Noise correlations
For each unique visual stimulus, firing rates were computed for the entire stimulus period, and z-scored across (at least 3) presentations of that stimulus. We then concatenated the Z-scored firing rates across the different unique stimuli, and computed the Pearson’s correlation coefficient.
Computation of modulation and SNRI
Computation of firing rate modulation and SNRI (signal-to-noise ratio index) was always performed as y = [FR1 − FR2] / [FR1 + FR2].
Quantification of burstiness
The propensity to engage in burst firing was quantified using the coefficient of Local Variation (LV; (Shinomoto et al., 2009)), which is robust against non-stationarities in firing rates. This measure correlates strongly with the log fraction of ISIs between 2–10 ms over the fraction of ISIs between 10 and 100 ms, i.e. Log(ISIshort / ISIlong) (Pearson’s R = 0.84, p=7×10−15, Fig. S7).
Spike densities
Instantaneous firing rate was computed by convolving the spike trains either with a rectangular kernel or a Gaussian smoothing kernel. For tracking longer-lasting changes in firing rate around state transition points (Fig. 1, Fig. 3, Fig. 7), relatively long smoothing kernels (±500 ms Gaussian kernels, with SD of 250 ms) were used. For visualizing neuronal responses to visual stimuli (Fig. 4, Fig. 8), short smoothing kernels (±25ms Gaussian kernels, with SD of 12.5ms) were used.
Supplementary Material
Acknowledgements
This research was supported by a NARSAD Young Investigator award, an Alfred P. Sloan Fellowship award, a Whitehall grant, a Klingenstein fellowship award, a McKnight Scholar award, and NIH/NEI grants R00 EY018407 and R01 EY022951 to JAC, a Rubicon Grant (Netherlands Organization for Science) to MV, a Jane Coffin Childs Fund fellowship award to RBB, and a NARSAD Young Investigator award to UK. We thank James Mossner and Hyun Lee for technical support. We also thank the main developers of M-Clust (D. Redish), KlustaKwik (K. Harris) and FieldTrip (R. Oostenveld) for free use of their software. We thank M.J. Higley for comments on the manuscript.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
MV, RBB, UK and JAC designed research. RBB, MV and JAC conducted experiments. UK and JAC developed hardware. MV analyzed data. MV and JAC wrote the manuscript.
References
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Bennett C, Arroyo S, Hestrin S. Subthreshold mechanisms underlying state-dependent modulation of visual responses. Neuron. 2013;80:350–357. doi: 10.1016/j.neuron.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H. Über das elektrenkephalogramm des menschen. European Archives of Psychiatry and Clinical Neuroscience. 1929;87:527–570. [Google Scholar]
- Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Busse L, Ayaz A, Dhruv NT, Katzner S, Saleem AB, Scholvinck ML, Zaharia AD, Carandini M. The detection of visual contrast in the behaving mouse. J Neurosci. 2011;31:11351–11361. doi: 10.1523/JNEUROSCI.6689-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988;8:4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civillico EF, Contreras D. Spatiotemporal properties of sensory responses in vivo are strongly dependent on network context. Front Syst Neurosci. 2012;6:25. doi: 10.3389/fnsys.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Kohn A. Measuring and interpreting neuronal correlations. Nat Neurosci. 2011;14:811–819. doi: 10.1038/nn.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JH. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci. 2009;12:1594–1600. doi: 10.1038/nn.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinople CM, Bruno RM. Effects and mechanisms of wakefulness on local cortical networks. Neuron. 2011;69:1061–1068. doi: 10.1016/j.neuron.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D, Timofeev I, Steriade M. Mechanisms of long-lasting hyperpolarizations underlying slow sleep oscillations in cat corticothalamic networks. J Physiol. 1996;494(Pt 1):251–264. doi: 10.1113/jphysiol.1996.sp021488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crochet S, Petersen CC. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat Neurosci. 2006;9:608–610. doi: 10.1038/nn1690. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsaki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Contreras D, Steriade M. Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J Neurosci. 1999;19:4595–4608. doi: 10.1523/JNEUROSCI.19-11-04595.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker AS, Berens P, Keliris GA, Bethge M, Logothetis NK, Tolias AS. Decorrelated neuronal firing in cortical microcircuits. Science. 2010;327:584–587. doi: 10.1126/science.1179867. [DOI] [PubMed] [Google Scholar]
- Eldar E, Cohen JD, Niv Y. The effects of neural gain on attention and learning. Nat Neurosci. 2013;16:1146–1153. doi: 10.1038/nn.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, Huang ZJ, Stryker MP. A cortical circuit for gain control by behavioral state. Cell. 2014;156:1139–1152. doi: 10.1016/j.cell.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentet LJ, Avermann M, Matyas F, Staiger JF, Petersen CC. Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice. Neuron. 2010;65:422–435. doi: 10.1016/j.neuron.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Gentet LJ, Kremer Y, Taniguchi H, Huang ZJ, Staiger JF, Petersen CC. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat Neurosci. 2012;15:607–612. doi: 10.1038/nn.3051. [DOI] [PubMed] [Google Scholar]
- Gilzenrat MS, Nieuwenhuis S, Jepma M, Cohen JD. Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cogn Affect Behav Neurosci. 2010;10:252–269. doi: 10.3758/CABN.10.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo ZV, Li N, Huber D, Ophir E, Gutnisky D, Ting JT, Feng G, Svoboda K. Flow of cortical activity underlying a tactile decision in mice. Neuron. 2014;81:179–194. doi: 10.1016/j.neuron.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider B, Hausser M, Carandini M. Inhibition dominates sensory responses in the awake cortex. Nature. 2013;493:97–100. doi: 10.1038/nature11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Thiele A. Cortical state and attention. Nat Rev Neurosci. 2011;12:509–523. doi: 10.1038/nrn3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstaub A, Sachdev RN, McCormick DA. State changes rapidly modulate cortical neuronal responsiveness. J Neurosci. 2007;27:9607–9622. doi: 10.1523/JNEUROSCI.2184-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero JL, Gieselmann MA, Sanayei M, Thiele A. Attention-induced variance and noise correlation reduction in macaque V1 is mediated by NMDA receptors. Neuron. 2013;78:729–739. doi: 10.1016/j.neuron.2013.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller GB, Bonhoeffer T, Hubener M. Sensorimotor mismatch signals in primary visual cortex of the behaving mouse. Neuron. 2012;74:809–815. doi: 10.1016/j.neuron.2012.03.040. [DOI] [PubMed] [Google Scholar]
- Lee AM, Hoy JL, Bonci A, Wilbrecht L, Stryker MP, Niell CM. Identification of a brainstem circuit regulating visual cortical state in parallel with locomotion. Neuron. 2014;83:455–466. doi: 10.1016/j.neuron.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci. 2013;16:1662–1670. doi: 10.1038/nn.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Effects of sleep and arousal on the processing of visual information in the cat. Nature. 1981;291:554–561. doi: 10.1038/291554a0. [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JH. Effects of attention on the reliability of individual neurons in monkey visual cortex. Neuron. 1999;23:765–773. doi: 10.1016/s0896-6273(01)80034-9. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci. 1992;12:4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Vogt BA. Direct connections of rat visual cortex with sensory, motor, and association cortices. J Comp Neurol. 1984;226:184–202. doi: 10.1002/cne.902260204. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron. 2009;63:879–888. doi: 10.1016/j.neuron.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk MH, Roelfsema PR, Konig P, Engel AK, Singer W. Role of reticular activation in the modulation of intracortical synchronization. Science. 1996;272:271–274. doi: 10.1126/science.272.5259.271. [DOI] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65:472–479. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshausen BA, Field DJ. Emergence of simple-cell receptive field properties by learning a sparse code for natural images. Nature. 1996;381:607–609. doi: 10.1038/381607a0. [DOI] [PubMed] [Google Scholar]
- Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee SH, Harrison TC, Feng G, Dan Y. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat Neurosci. 2013;16:1857–1863. doi: 10.1038/nn.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack PO, Friedman J, Golshani P. Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat Neurosci. 2013;16:1331–1339. doi: 10.1038/nn.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouget A, Dayan P, Zemel R. Information processing with population codes. Nat Rev Neurosci. 2000;1:125–132. doi: 10.1038/35039062. [DOI] [PubMed] [Google Scholar]
- Reimer J, Froudarakis E, Cadwel CR, Yatsenko D, Denfield GH, Tolias AS. Pupil Fluctuations Track Fast Switching of Cortical States during Quiet Wakefulness. Neuron. 2014:84. doi: 10.1016/j.neuron.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem AB, Ayaz A, Jeffery KJ, Harris KD, Carandini M. Integration of visual motion and locomotion in mouse visual cortex. Nat Neurosci. 2013;16:1864–1869. doi: 10.1038/nn.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DM, Nelson A, Mooney R. A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature. 2014;513:189–194. doi: 10.1038/nature13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomoto S, Kim H, Shimokawa T, Matsuno N, Funahashi S, Shima K, Fujita I, Tamura H, Doi T, Kawano K, et al. Relating neuronal firing patterns to functional differentiation of cerebral cortex. PLoS Comput Biol. 2009;5:e1000433. doi: 10.1371/journal.pcbi.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature. 1997;386:167–170. doi: 10.1038/386167a0. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- van Kerkoerle T, Self MW, Dagnino B, Gariel-Mathis MA, Poort J, van der Togt C, Roelfsema PR. Alpha and gamma oscillations characterize feedback and feedforward processing in monkey visual cortex. Proc Natl Acad Sci U S A. 2014;111:14332–14341. doi: 10.1073/pnas.1402773111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M, Battaglia FP, Womelsdorf T, Pennartz C. Improved measures of phase-coupling between spikes and the Local Field Potential. J Comput Neurosci. 2012;33:53–75. doi: 10.1007/s10827-011-0374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M, Womelsdorf T, Buffalo EA, Desimone R, Fries P. Attentional modulation of cell-class-specific gamma-band synchronization in awake monkey area v4. Neuron. 2013;80:1077–1089. doi: 10.1016/j.neuron.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Gao E, Burkhalter A. Gateways of ventral and dorsal streams in mouse visual cortex. J Neurosci. 2011;31:1905–1918. doi: 10.1523/JNEUROSCI.3488-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Lima B, Vinck M, Oostenveld R, Singer W, Neuenschwander S, Fries P. Orientation selectivity and noise correlation in awake monkey area V1 are modulated by the gamma cycle. Proc Natl Acad Sci U S A. 2012;109:4302–4307. doi: 10.1073/pnas.1114223109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagha E, Casale AE, Sachdev RN, McGinley MJ, McCormick DA. Motor cortex feedback influences sensory processing by modulating network state. Neuron. 2013;79:567–578. doi: 10.1016/j.neuron.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Xu M, Kamigaki T, Hoang Do JP, Chang WC, Jenvay S, Miyamichi K, Luo L, Dan Y. Selective attention. Long-range and local circuits for top-down modulation of visual cortex processing. Science. 2014;345:660–665. doi: 10.1126/science.1254126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Liang F, Xiong XR, Li L, Li H, Xiao Z, Tao HW, Zhang LI. Scaling down of balanced excitation and inhibition by active behavioral states in auditory cortex. Nat Neurosci. 2014;17:841–850. doi: 10.1038/nn.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.