Abstract
There is controversy regarding whether strict blood pressure control is indicated in chronic kidney disease (CKD) since the primary results of randomized controlled trials failed to show any impact on progression of kidney disease with this strategy. However, strict blood pressure control may have other beneficial effects beyond reducing risk of end-stage renal disease (ESRD), such as lowering mortality after ESRD onset. The Modification of Diet in Renal Disease (MDRD) trial randomized 840 patients with CKD to strict (mean arterial pressure under 92 mm Hg) versus usual (mean arterial pressure under 107 mm Hg) blood pressure control between 1989–1993. Here we extended follow-up of study enrollees by linkage with United States Renal Data System and National Death Index to ascertain ESRD and vital status through 2010. Overall, 627 patients developed ESRD through 2010 with median follow-up of 19.3 years. After ESRD onset, there were 142 deaths in the strict blood pressure arm and 182 deaths in the usual blood pressure arm (significant unadjusted hazard ratio for death 0.72 (95% CI 0.58–0.89)). Overall, there were 212 deaths in the strict blood pressure control arm and 233 deaths in the usual arm (significant unadjusted hazard ratio for death 0.82 (95% CI 0.68–0.98)). Thus, although strict blood pressure control did not delay progression of CKD to ESRD, this strategy was associated with a lower risk of death after ESRD. Hence, long-term post-ESRD outcomes should be considered when formulating blood pressure targets for CKD.
Introduction
The tremendous morbidity and mortality suffered by end-stage renal disease (ESRD) patients is well known, and the prevalence of ESRD is increasing worldwide.1, 2 Given the high mortality rate of patients with ESRD, it is disappointing that numerous interventions--such as normalization of hematocrit,3 delivery of higher doses of dialysis,4, 5 use of high flux hemodialysis membranes,4 or use of calcimimetics6--have not improved mortality in this population. The lack of demonstrated benefit from these interventions could be because the underlying disease contributing to high mortality is already established by the time patients reach ESRD. For example, three-quarters of incident dialysis patients are reported to have left ventricular hypertrophy, a known independent risk factor for death in this population.7
Even though CKD and ESRD are a continuum of the same disease, studies of CKD have mostly tested the efficacy of various interventions in retarding progression towards ESRD and not examined the potential long-term impact of CKD interventions on outcomes after ESRD onset. Given the chronic and progressive nature of kidney disease, early interventions could conceivably improve the overall health status and survival of patients with ESRD, even if such early interventions fail to mitigate progression to ESRD.
In this study, we hypothesized that there would be lower risk of mortality after ESRD onset among patients whose blood pressure (BP) was more strictly controlled during the CKD phase of disease. To test this hypothesis, we extended follow-up of participants previously enrolled in Modification of Diet in Renal Disease (MDRD), the first large randomized trial of strict BP control in CKD, and focused on the effect of strict BP control on risk of death after the onset of ESRD.8, 9
Results
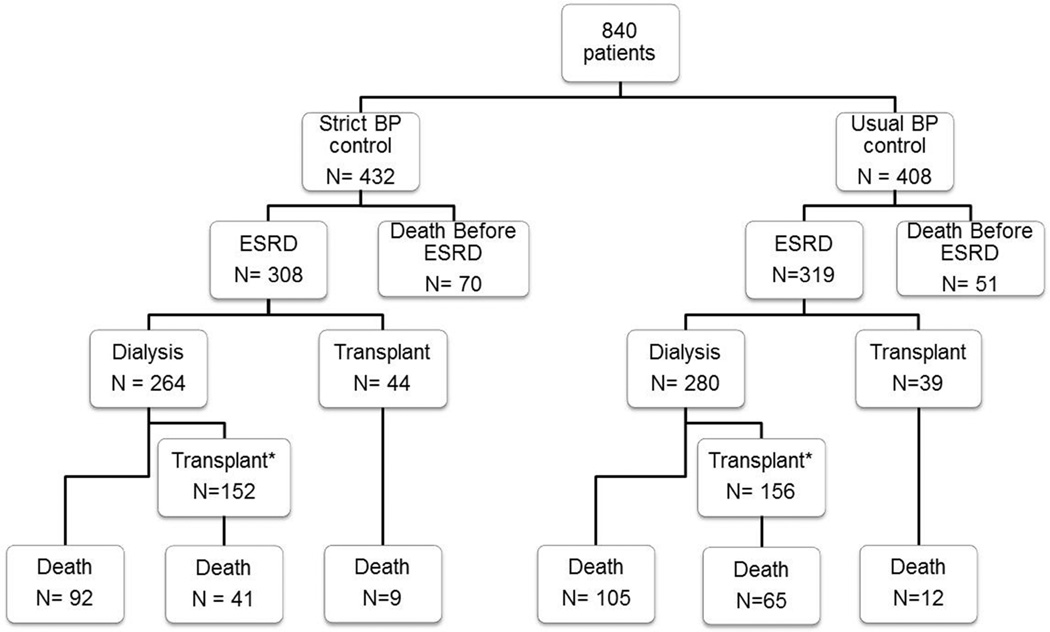
Long-term outcomes of study participants are shown in Figure 1. Median follow-up starting from the time of randomization was 19.3 years. Of the 840 enrollees, 627 developed ESRD on or before Dec 31, 2010 (Table 1 and Figure 1). There were 319 cases of ESRD in the usual BP target arm (incidence 9.9 per 100 person-years) and 308 cases of ESRD in the strict BP target arm (incidence 8.5 per 100 person-years). There was a tendency for patients in the strict BP arm to have lower hazard of ESRD, but this was not statistically significant (unadjusted Cox model hazard ratio [HR] of 0.86 [95% CI 0.73–1.001], p = 0.053).
Figure 1.
Distribution of ESRD cases and death by BP study arm assignment.
* Number with one or more kidney transplant
Table 1.
Characteristics of MDRD at baseline and at ESRD onset
| Characteristics | Strict Blood Pressure N = 432 |
Usual Blood Pressure N = 408 |
|
|---|---|---|---|
|
At time of randomization |
|||
| Age (y) (± SD) | 51.5 ± 12.6 | 52.0 ± 12.2 | |
| Men | 267 (61.8%) | 241 (59.1%) | |
| African American | 34 (7.9%) | 32 (7.8%) | |
| Diabetes | 23 (5.3%) | 20 (4.9%) | |
| Glomerular filtration rate (mL/min/1.73 m2) |
32.7 ± 12.1 | 32.3 ± 11.9 | |
| Median proteinuria (g/d) (interquartile range) |
0.33 (0.07, 1.5) | 0.32 (0.07,1.5) | |
| Cause of CKD | |||
| Polycystic kidney disease |
106 (24.5%) | 94 (23.0%) | |
| Glomerulonephritis | 134 (31.0%) | 130 (31.9%) | |
| Other | 192 (44.4%) | 184 (45.1%) | |
| Angiotensin-converting enzyme inhibitor use |
163 (37.7%) | 139 (34.1%) | |
| At ESRD onset | N = 308 | N = 319 | P-value |
| Age (y) | 55.8 ± 13.9 | 56.8 ± 13.0 | 0.33 |
| Male | 189 (61.4%) | 191 (59.9%) | 0.70 |
| African American | 23 (7.5%) | 28 (8.8%) | 0.55 |
| Preemptive Transplant | 44 (14.3%) | 39 (12.2%) | 0.45 |
| Cause of CKD | |||
| Polycystic kidney disease |
93 (30.2%) | 94 (29.5%) | 0.96 |
| Glomerulonephritis | 99 (32.1%) | 106 (33.2%) | |
| Other | 116 (37.7%) | 119 (37.3%) |
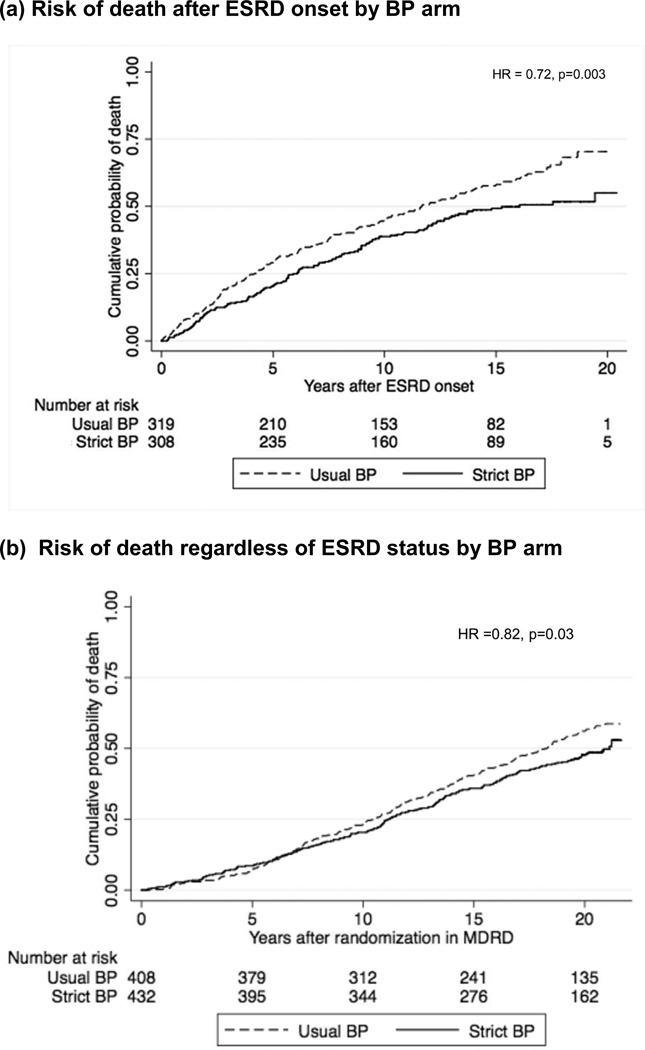
Median follow-up time post-ESRD was 10.0 years. There were 182 deaths after ESRD onset in the usual BP arm (6.1 deaths per 100 person-years) and 142 deaths after ESRD onset in the strict BP arm (4.4 deaths per 100 person-years) (Figure 2a). In our primary analysis, the patients randomized to strict BP during CKD had a lower risk of death after onset of ESRD with unadjusted HR of 0.72 (95% CI 0.58–0.89, p = 0.003). Similar results were seen in our confirmatory analysis starting the survival analysis at the time of ESRD onset (HR 0.74 [95% CI 0.59–0.92], p=0.008), adjusting for gender, race, baseline polycystic kidney disease, baseline diabetes, and age at ESRD onset. The risk of death prior to ESRD onset was not statistically significantly different comparing strict to usual BP arms (HR 1.21 [95% CI 0.85–1.74], p = 0.29).
Figure 2.
Risk of death during long-term extended follow-up of MDRD trial participants
When deaths were analyzed regardless of ESRD status, risk of death was lower among participants randomized to strict BP control (HR 0.82 [95% CI 0.68–0.98], p=0.03, Figure 2b). In sensitivity analysis, this association persisted even with adjustment for baseline demographic characteristics, diabetes, and polycystic kidney disease (HR 0.80 [95% CI 0.66–0.96], p =0.02). Similar trends were observed for all pre-specified subgroup analyses (data not shown). Tests for interaction between BP arm and proteinuria (p = 0.57) or for interaction between BP arm and GFR by study group (p = 0.60) failed to achieve statistical significance. We also tested for interaction between low protein diet and strict BP interventions (given 2×2 factorial design in MDRD) and found no evidence of interaction in either Study A (p=0.47) or Study B (p=0.12), which is consistent with results of the parent MDRD trial.
In exploratory analysis, our results were not affected by controlling for baseline ACE inhibitor use (adjusted HR 0.73 [95% CI 0.59–0.91], p = 0.005). Transplants were evenly distributed in the two blood pressure arms (Figure 1). Adjusting for transplant status as a time-varying covariate did not attenuate the association between BP arm and risk of death after ESRD (adjusted HR 0.70 [95% CI 0.56–0.87], p = 0.002). After correcting for potential lead-time differences in ESRD onset, the HR of death post-ESRD remained lower in the strict BP arm (HR 0.76 [95% CI 0.60–0.95], p = 0.02).
In the subset of patients who developed ESRD after 1995 with available data (N = 324, 52%), more patients assigned to the usual BP arm had heart failure (13.1% versus 5.3%, p = 0.02) and coronary artery disease (19.0% versus 8.2%, p=0.004) as registered on the CMS-2728 form at the time of ESRD than patients in the strict BP arm (Table 2). No differences were noted between the two arms in terms of other co-morbidities or laboratory data. In this subset of patients, the HR for death comparing those randomized to strict versus usual BP during CKD was 0.82 (95% CI 0.60–1.13, p = 0.23). After adjustment for coronary artery disease and congestive heart failure, the HR was 1.02 (95% CI 0.74–1.41, p = 0.91).
Table 2.
Characteristics at ESRD onset in the subset of patients with available data according to the Medical Evidence form
| Characteristics | Strict Blood Pressure N= 171 |
Usual Blood Pressure N= 153 |
P-value |
|---|---|---|---|
| Co-morbidities | |||
| Congestive heart failure | 9 (5.3%) | 20 (13.1%) | 0.02 |
| Coronary artery disease | 14 (8.2%) | 29 (19.0%) | 0.004 |
| Diabetes | 19 (11.1%) | 19 (12.4%) | 0.72 |
| Cerebrovascular Disease | 4 (2.3%) | 7 (4.6%) | 0.36 |
| Hypertension | 139 (81.3%) | 131 (85.6%) | 0.30 |
| Laboratory data | |||
| Albumin1 (g/dL) (± SD) | 3.7 ± 0.6 | 3.7 ± 0.6 | 0.78 |
| Hemoglobin2 (g/dL) (±SD) | 10.6 ± 1.5 | 10.3 ± 1.8 | 0.21 |
For albumin, N = 133 for strict blood pressure arm and N = 122 for usual blood pressure arm
For hemoglobin, N = 145 for strict blood pressure arm and N = 133 for usual blood pressure arm
DISCUSSION
In this extended follow-up analysis of a randomized controlled trial, we found that strict BP control during the CKD phase of disease was strongly associated with lower risk of all-cause mortality after ESRD onset, even though we did not show that strict BP control delayed progression toward ESRD. To our knowledge, few studies have examined the impact of CKD interventions (including BP control) on post-ESRD outcomes. Virtually all studies of incident ESRD patients to date--such as Dialysis Outcomes and Practice Patterns Study (DOPPS) or Comprehensive Dialysis Study -- begin data collection at or after initiation of dialysis and do not examine what transpired in the years prior to dialysis initiation.10–12 Conversely, studies enrolling patients with CKD (such as the African American Study of Kidney Disease and Hypertension [AASK] or the original MDRD study) have generally ended follow-up when ESRD develops, which precludes examination of post-ESRD outcomes.9, 13 To our knowledge, relating post-ESRD outcomes to interventions received during CKD is a relatively novel paradigm in the study of kidney disease that may shed new light on disease pathogenesis.
Our long-term follow-up results regarding reno-protection are consistent with the results of the original MDRD trial and other randomized controlled trials of strict BP control in CKD, including AASK and REIN-2, all of which showed no benefit of strict BP control in retarding progression towards ESRD.13,14 We also found no difference in deaths prior to ESRD onset, which is in agreement with the results of previous studies.13, 15 Our results differ from those of a previous analysis of risk of ESRD and death (prior to ESRD) among MDRD participants during extended follow-up through the end of 2000, but our current study incorporates significantly more follow-up time.15 The main feature that distinguishes the current analysis from prior literature is the innovative focus on risk of death post-ESRD.
Based largely on the results of trials such as MDRD, AASK, and REIN-2, the Joint National Committee (JNC) guideline panel recently concluded that there is “no benefit in slowing the progression of kidney disease from treatment with antihypertensive drug therapy to a lower BP goal (below 130/80)” and “the evidence is insufficient to determine if there is a benefit in mortality, or cardiovascular or cerebro-vascular health outcomes … to a lower BP goal.” 1616 Accordingly, the updated guidelines liberalized the BP target from 130/80 mm Hg to 140/90 mmHg among patients with CKD.16 However, perhaps end-points other than retarding CKD progression should be taken into account when formulating guidelines for BP targets for patients with CKD. Other outcomes such as post-ESRD mortality should be considered, as strict BP control may offer a mortality benefit after ESRD onset, even if it does not delay CKD progression.
The strength of our study lies in the large number of “hard outcomes,” including both ESRD and deaths during our extended follow-up period. We believe that our approach to studying the long-term impact of an intervention delivered during the CKD phase of disease is relatively novel. Prior studies that have extended follow-up of CKD patients enrolled in interventional trials have not focused on post-ESRD outcomes.15 Another strength of this study is the use of a randomized controlled trial cohort to study the effect of strict BP control, which reduces bias from unmeasured confounders. Demonstrating a similar association between strict BP control during the CKD phase of disease and reduced mortality post-ESRD in the context of observational studies would be less powerful, given the potential for confounding by imbalances in factors such as differential access to health care, health literacy, and adherence.
A limitation in our study is the lack of data on BP control post-trial closure. However, BP separation between the two arms would likely have diminished after the trial ended, which would have biased our results towards the null. The duration of active intervention was relatively short in the parent MDRD trial compared with the duration of our extended follow-up period. However, the long-term impact of trial interventions has been demonstrated in other contexts.8, 15, 17–20
We explored whether the benefit of strict BP control during CKD may be mediated by a reduction in cardiovascular disease at the time of ESRD onset. We found a significantly higher prevalence of coronary artery disease (CAD) and congestive heart failure (CHF) reported at the time of ESRD onset among patients randomized to the usual BP arm as compared to the strict BP arm. The lower rate of reported CAD and CHF in the subset of patients with available data who were previously exposed to strict BP control suggests that reduction in clinical and subclinical cardiovascular disease may be a potential mediator of the lower mortality rates seen post-ESRD. In the subset of patients with available co-morbidities, adjustment for CAD and CHF at the time of ESRD onset appeared to attenuate the association between strict BP control and risk of death post-ESRD, although our ability to evaluate mediation was limited by wide confidence intervals. We were underpowered due to missing data prior to 1995. The CMS-2728 form has been shown to have variable sensitivity but good specificity for the identification of co-morbidities,21, 22 but this lack of sensitivity should be non-differential with respect to previous MDRD BP arm assignment.
In conclusion, we found a long-term post-ESRD mortality benefit to a strict (≤130/80 mm Hg) BP target in patients with CKD during extended follow up of previous MDRD enrollees. This finding, and whether lower post-ESRD mortality stems from improved overall cardiovascular health at incident ESRD, will require further study and confirmation in other settings such as AASK. Our results may not generalize to the entire CKD population, given the unique characteristics of the MDRD cohort, which included primarily Caucasian patients with a high prevalence of polycystic kidney disease and glomerulonephritis as the cause of kidney disease and high rates of transplant and survival post-ESRD. Nevertheless, these data suggest that trials with longer follow-up are needed to understand and assess the potential impact and benefit of interventions implemented during CKD phase of disease.
Methods
Blood pressure intervention in MDRD
MDRD was a large 2×2 factorial design randomized controlled trial of the effect of strict BP control and dietary protein restriction on the progression of CKD. Details of the study design and results have been previously published.9
Briefly, between 1989 and 1993, CKD patients between 18–70 years of age with GFR 13–55 mL/min/1.73 m2 were randomized to either strict or usual BP control. Study A included 585 patients with GFR between 13 and < 24.5 mL/min/1.73 m2; Study B included 255 patients with GFR between 24.5–55 mL/min/1.73 m2. The baseline characteristics of patients in the MDRD study have been previously described and were balanced in terms of demographic characteristics and co-morbidities (Table 1).9, 15 Strict BP control was defined as a target mean arterial pressure (MAP) ≤ 92 mm Hg (corresponds to 125/75 mm Hg) for participants less than 61 years of age and a target MAP ≤ 98 mm Hg (corresponds to 135/80 mm Hg) for participants 61 years or older. Usual BP control was defined as a target MAP ≤107 mm Hg (corresponds to 140/90 mm Hg) for participants less than 61 years of age and a target MAP ≤113 mm Hg (corresponds to 160/90 mm Hg) for participants 61 years or older.9 Angiotensin-converting enzyme (ACE) inhibitors with or without diuretics were encouraged as first-line anti-hypertensive agents. The mean difference in systolic, diastolic, and mean arterial pressures between groups was 7.6 mm Hg, 3.8 mm Hg, and 5.1 mm Hg, respectively, between month four and the end of follow-up (median follow-up 2.2 years).15 At trial closure, no specific BP targets were recommended, and data on long-term BP control after trial closure are not available.
Study A participants were also randomized to a usual (1 g/kg) versus low protein diet (0.6 g/kg) and Study B participants were randomized to a low protein diet (0.6 g/kg) versus very-low protein diet (0.28 g/kg), with keto-acid and amino acid supplementation during the parent trial.
Outcome
Our primary outcome of interest was all-cause mortality after ESRD onset in patients previously randomized to strict versus usual BP control. The parent MDRD trial ended in January of 1993. We performed linkage with the United States Renal Data System (USRDS), the national ESRD registry, and the National Death Index (NDI), which compiles death certificate data, to extend ascertainment of ESRD and vital status, respectively, through December 31, 2010. ESRD was defined as receipt of chronic dialysis or kidney transplant. Patients were administratively censored if they were alive as of December 31, 2010, the most recent year of USRDS data available at the time of study performance. The USRDS and NDI have been validated previously as accurate data sources for ESRD onset and death dates, respectively.15, 21, 23–25 Institutional review board approval was obtained for data linkage and analysis.
Ascertainment of cardiovascular disease status at ESRD onset
One plausible patho-physiologic mechanism for the long-term benefit of strict BP control during CKD on long-term outcomes is reduction of cardiovascular disease burden at incident ESRD. Thus we compared patient co-morbidities recorded in the USRDS database at the time of ESRD onset using the Centers for Medicare and Medicaid (CMS) Medical Evidence (2728) form between the two arms. The CMS-2728 form is typically signed by an attending nephrologist attesting patients’ ESRD status. Data on co-morbidities were available only for the subset of MDRD enrollees who developed ESRD after 1995 (N=324), as the version of the Medical Evidence form used before 1995 did not capture data on co-morbidities at incident ESRD. Co-morbid conditions tend to be reported with high specificity but intermediate sensitivity for cardiovascular disease on the CMS-2728 form.22
Statistical analysis
We tested for differences between patient characteristics at the time of randomization and at ESRD onset using Student’s t-test, χ2, or Fisher’s exact test as appropriate. To preserve the original randomization scheme, all primary analyses were conducted in an intention-to-treat fashion. The primary outcome--all-cause mortality post-ESRD--was assessed by BP arm assignment using ESRD as a time-dependent covariate in an unadjusted Cox model of death starting at the time of randomization. In sensitivity analysis, we also adjusted for baseline age, gender, race, baseline polycystic kidney disease and baseline diabetes in analyzing the effect of BP control on death using a Cox model starting at the time of randomization.
We confirmed the results of our primary analysis by assessing all-cause mortality post-ESRD with a Cox model starting the analysis at the time of ESRD onset. Given the potential for imbalances in characteristics at the time of ESRD onset, we adjusted this analysis for gender, race, baseline polycystic kidney disease, baseline diabetes, and age at ESRD onset. Additionally, we examined risk of death over the entire follow-up period regardless of ESRD status. We pre-specified two subgroup analyses, including stratification by GFR above or below 24.5 mL/min/1.73 m2 (Study A and B cutoffs from parent trial) and by proteinuria as a continuous variable as defined at the time of randomization.9, 26 We tested for interactions between BP arm assignment and GFR or proteinuria. Given that MDRD was a 2×2 factorial study that tested the effect of strict BP control and low protein diet, we also tested for interaction between BP and dietary intervention by study group in an unadjusted Cox model.
Finally, we conducted a series of exploratory analyses. Given the slight imbalance in ACE inhibitor use in the two randomized arms at baseline,9 we repeated our analysis with adjustment for baseline ACE inhibitor use. To explore whether differences in rates of transplant accounted for any differences in post-ESRD survival, we adjusted for transplant status as a time-varying covariate. We also explored whether differences in post-ESRD survival were mediated by cardiovascular disease burden at the time of ESRD onset in the subset of patients with co-morbidities available on the CMS-2728 form by adjusting for the presence of cardiovascular disease.
We noted a trend towards earlier ESRD onset in the usual BP arm that did not reach statistical significance. To assess whether our results remained robust after correction for any potential lead-time differences in ESRD onset, we performed an analysis in which we separately ranked patients in the strict and usual BP arms from shortest to longest time to ESRD onset. We then added the difference between ESRD onset time for equally ranked participants in the two arms to the actual observed ESRD date for participants in the usual BP arm in order to correct for the potential lead-time in ESRD onset. Thus, among participants in the usual BP arm, we reclassified some post-ESRD deaths and person-years of follow up as pre-ESRD deaths and person-years of follow-up. We then repeated our primary analysis using the lead-time corrected data. All analyses were performed using Stata 13.
Acknowledgments
Sources of support:
Dr. Ku was funded by the American Kidney Fund Clinical Scientist in Nephrology Program. This work was also supported by the National Institutes of Health (K24 DK92291 to Dr. Hsu, Contract N01-DK-7-005 and K24 DK85153 to Dr. Johansen, and K24 DK78204 to Dr. Sarnak). The interpretation and reporting of the data presented here are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Footnotes
Disclosures: None.
REFERENCES
- 1.Eknoyan G, Lameire N, Barsoum R, et al. The burden of kidney disease: improving global outcomes. Kidney Int. 2004;66:1310–1314. doi: 10.1111/j.1523-1755.2004.00894.x. [DOI] [PubMed] [Google Scholar]
- 2.USRDS Annual Data Report 2011. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011. [Google Scholar]
- 3.Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 4.Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347:2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 5.Paniagua R, Amato D, Vonesh E, et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002;13:1307–1320. doi: 10.1681/ASN.V1351307. [DOI] [PubMed] [Google Scholar]
- 6.Investigators ET, Chertow GM, Block GA, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482–2494. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 7.Foley RN, Parfrey PS, Harnett JD, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186–192. doi: 10.1038/ki.1995.22. [DOI] [PubMed] [Google Scholar]
- 8.Mortality rates after 10.5 years for participants in the Multiple Risk Factor Intervention Trial. Findings related to a priori hypotheses of the trial. The Multiple Risk Factor Intervention Trial Research Group. JAMA. 1990;263:1795–1801. doi: 10.1001/jama.1990.03440130083030. [DOI] [PubMed] [Google Scholar]
- 9.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994;330:877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 10.Plantinga LC, Fink NE, Levin NW, et al. Early, intermediate, and long-term risk factors for mortality in incident dialysis patients: the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. Am J Kidney Dis. 2007;49:831–840. doi: 10.1053/j.ajkd.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Abbott KC, Glanton CW, Trespalacios FC, et al. Body mass index, dialysis modality, and survival: analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II Study. Kidney Int. 2004;65:597–605. doi: 10.1111/j.1523-1755.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 12.Bao Y, Dalrymple L, Chertow GM, et al. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172:1071–1077. doi: 10.1001/archinternmed.2012.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright JT, Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 14.Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939–946. doi: 10.1016/S0140-6736(05)71082-5. [DOI] [PubMed] [Google Scholar]
- 15.Sarnak MJ, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med. 2005;142:342–351. doi: 10.7326/0003-4819-142-5-200503010-00009. [DOI] [PubMed] [Google Scholar]
- 16.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 17.Lachin JM, Orchard TJ, Nathan DM, et al. Update on cardiovascular outcomes at 30 years of the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37:39–43. doi: 10.2337/dc13-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Writing Team for the Diabetes C, Complications Trial/Epidemiology of Diabetes I, Complications Research G. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290:2159–2167. doi: 10.1001/jama.290.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Group DER, de Boer IH, Sun W, et al. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med. 2011;365:2366–2376. doi: 10.1056/NEJMoa1111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.How good are the data? USRDS data validation special study. Am J Kidney Dis. 1992;20:68–83. [PubMed] [Google Scholar]
- 22.Longenecker JC, Coresh J, Klag MJ, et al. Validation of comorbid conditions on the end-stage renal disease medical evidence report: the CHOICE study. Choices for Healthy Outcomes in Caring for ESRD. J Am Soc Nephrol. 2000;11:520–529. doi: 10.1681/ASN.V113520. [DOI] [PubMed] [Google Scholar]
- 23.Fillenbaum GG, Burchett BM, Blazer DG. Identifying a national death index match. Am J Epidemiol. 2009;170:515–518. doi: 10.1093/aje/kwp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitsnefes MM, Laskin BL, Dahhou M, et al. Mortality risk among children initially treated with dialysis for end-stage kidney disease, 1990–2010. JAMA. 2013;309:1921–1929. doi: 10.1001/jama.2013.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Hare AM, Rodriguez RA, Hailpern SM, et al. Regional variation in health care intensity and treatment practices for end-stage renal disease in older adults. JAMA. 2010;304:180–186. doi: 10.1001/jama.2010.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med. 1995;123:754–762. doi: 10.7326/0003-4819-123-10-199511150-00003. [DOI] [PubMed] [Google Scholar]