Abstract
Purpose
Monocarboxylate transporter (MCT) inhibition represents a potential treatment strategy for γ-hydroxybutyric acid (GHB) overdose by blocking its renal reabsorption in the kidney. This study further evaluated the effects of a novel, highly potent MCT inhibitor, AR-C155858, on GHB toxicokinetics/toxicodynamics (TK/TD).
Methods
Rats were administered GHB (200, 600 or 1500 mg/kg i.v. or 1500 mg/kg po) with and without AR-C155858. Breathing frequency was continuously monitored using whole-body plethysmography. Plasma and urine samples were collected up to 8 hours. The effect of AR-C155858 on GHB brain/plasma partitioning was also assessed.
Results
AR-C155858 treatment significantly increased GHB renal and total clearance after intravenous GHB administration at all the GHB doses used in this study. GHB-induced respiratory depression was significantly improved by AR-C155858 as demonstrated by an improvement in the respiratory rate. AR-C155858 treatment also resulted in a significant reduction in brain/plasma partitioning of GHB (0.1 ± 0.03) when compared to GHB alone (0.25 ± 0.02). GHB CLR and CLoral (CL/F) following oral administration were also significantly increased following AR-C155858 treatment (from 1.82 ± 0.63 to 5.74 ± 0.86 and 6.52 ± 0.88 to 10.2 ± 0.75 ml/min/kg, respectively).
Conclusion
The novel and highly potent MCT inhibitor represents a potential treatment option for GHB overdose.
Keywords: MCT inhibitor, GHB, AR-C155858, toxicity, respiratory depression
Introduction
γ-hydroxybutyric acid (GHB) is an endogenous short chain fatty acid found in the mammalian brain. It is also present in mammalian tissues such as liver, kidney, heart, and skeletal muscle (1). GHB is approved in the United States under the trade name of Xyrem® (Jazz Pharmaceuticals, Palo Alto, CA) for the treatment of narcolepsy associated with catalepsy. However, GHB is widely abused as a recreational drug due to its euphoric effects and as a means of drug-facilitated sexual assault for its hypnotic effects thereby limiting its therapeutic potential (2). GHB overdose can result in severe adverse effects such as sedation, coma, hypothermia, respiratory depression, and death (3, 4). According to a recent report, cardiorespiratory depression was one of the most common causes of GHB related lethality (5). There is currently no approved treatment for GHB overdose and treatment is limited to supportive care.
GHB exhibits dose-dependent pharmacokinetics both in rats and humans (6, 7). The nonlinearity in pharmacokinetics has been attributed to its saturable metabolism, saturable oral absorption and saturable renal reabsorption (7–9). Renal clearance is a minor pathway of GHB elimination at low doses but becomes the predominant route for its elimination at higher doses (9, 10). This nonlinearity in renal clearance of GHB is due to the saturation of its active renal reabsorption in the renal proximal tubule, a process mediated by a family of transporters known as monocarboxylate transporters (MCTs) (9, 11). MCTs are proton dependent transporters that are responsible for the transport of short chain monocarboxylic acids including GHB (12). MCT1 (SLC16A1), the most extensively characterized isoform is ubiquitously distributed throughout the body including kidney, intestine and brain, all of which are important for the disposition of GHB in the body (12). Another transporter family involved in the transport of endogenous monocarboxylates is the sodium coupled MCTs (SMCTs), which contains only two members SMCT1 (SLC5A8) and SMCT2 (SLC5A12). SMCTs share similar substrate specificity but are more limited in their distribution when compared to MCTs, with SMCT1 protein being detected in kidney, intestine, salivary gland, thyroid gland, brain, and retina (13). These act as a symporter and are dependent on a sodium gradient for their functional activity (13, 14). Previous work from our laboratory has demonstrated that GHB is a substrate for MCT 1, 2, and 4 (11, 15) and SMCT1 (16). Our laboratory has further evaluated MCT inhibition as a potential treatment strategy for GHB overdose by inhibiting its active renal reabsorption mediated by MCTs (9). Administration of MCT inhibitors such as L-lactate (inhibits proton- and sodium-dependent transporters) and luteolin (inhibits proton-dependent transporters) results in an increase in GHB renal and total clearance in rats (17, 18). Additionally, the administration of L-lactate, in combination with osmotic diuretic mannitol, increases the renal clearance of GHB in healthy human volunteers (19).
GHB is known to bind to both GHB and GABAB receptors in the brain, with its pharmacological effects such as sedation, hypothermia and respiratory depression mediated by binding to GABAB receptors (20–22). Treatment with GABAB receptor antagonists results in an improvement in these toxicodynamic end points. Apart from the role of MCTs in the renal reabsorption of GHB, they play an important role in the entry of GHB into the brain, which is its site of action (23–25). Recent results from our laboratory have shown that L-lactate, although not a very potent MCT inhibitor, when administered at higher doses (plasma steady state L-lactate concentrations ≈ 4–5 mM) can result in decreased extracellular fluid concentrations of GHB in the frontal cortex in rats; lower doses of L-lactate (plasma steady state L-lactate concentrations ≈ 1–2 mM) have no significant effect (26). These data suggest that MCT inhibition at the blood-brain barrier can serve as an additional mechanism of action in treating GHB overdose.
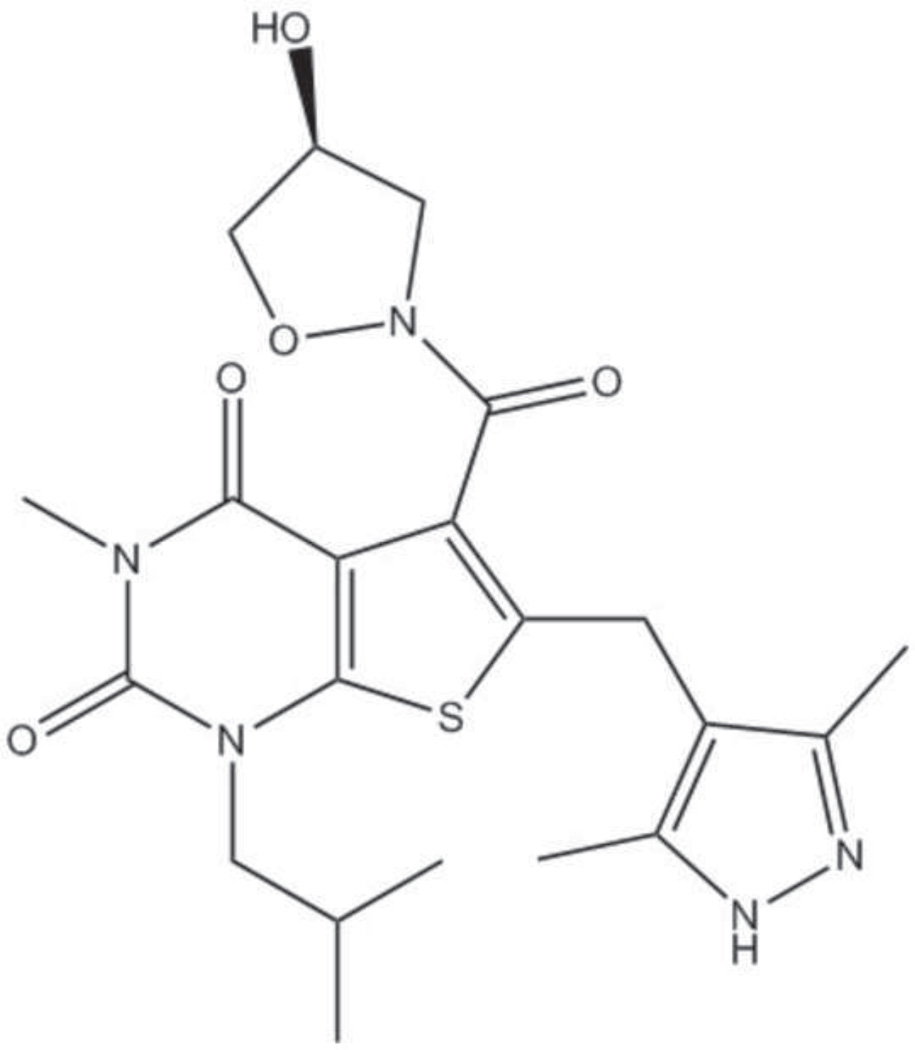
Recently, a class of immunosuppressive compounds has been identified as potent MCT inhibitors and has been shown to inhibit both rat and human lymphocyte proliferation (27, 28). Additionally, these compounds showed activity in an in vivo model of graft-versus-host response and high- and low-responder cardiac transplant models in rats (27). One of the compounds of this class, AR-C155858 (Figure 1), has been shown to be a tight-binding non-competitive MCT1 inhibitor with a Ki value of approximately 2.3 nM in rat erythrocytes which express only MCT1 (29). AR-C155858 can also inhibit MCT2 but does not inhibit MCT4.
Figure 1.
Structure of AR-C155858.
The objective of this work was to evaluate the effects of the novel and highly potent MCT inhibitor, AR-C155858, on intravenous GHB toxicokinetics and toxicodynamics, using respiratory depression as an end point. We also assessed the effects of AR-C155858 on the brain distribution of GHB. We further explored the use of this inhibitor as a potential treatment strategy for overdose following oral GHB administration which is the common mode of GHB ingestion in a recreational setting. Finally, preliminary studies to evaluate the mechanism of AR-C155858 inhibition of GHB uptake were performed in vitro by characterizing its effect on GHB uptake in KNRK cells, a rat kidney cell line.
Materials and Methods
Chemicals and Reagents
Sodium GHB was provided by the National Institute on Drug Abuse. Deuterated GHB (GHB-d6) was purchased from Cerilliant Corporation (Round Rock, TX). High-performance liquid chromatography grade acetonitrile and acetic acid were purchased from Honeywell Burdick & Jackson (Muskegon, MI). 6-[(3,5-Dimethyl-1H-pyrazol-4-yl)methyl]-5-[[(4S)-4-hydroxy-2-isoxazolidinyl]carbonyl]-3-methyl-1-(2-methylpropyl)thieno[2,3-d]pyrimidine-2,4(1H,3H)-dione (AR-C155858) was purchased from Chemscene (Monmouth Junction, NJ). KNRK cells were purchased from American Type Culture Collection (Manassas, VA).
Animals and Surgery
Male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 270 to 330 g were used for all the experiments. Animals were housed under controlled temperature and humidity with an artificial 12 h light/dark cycle, and food was available ad libitum. Animals were allowed to acclimate to their environment for a minimum of 1 week before surgery. The jugular and femoral vein cannulae were surgically implanted under anesthesia with ketamine/xylazine. Cannulae were flushed daily with 40 IU/ml heparinized saline to maintain patency. Rats were allowed a minimum of 72 h for recovery from surgery before conducting experiments. All animal procedures were approved by University at Buffalo Institutional Animal Care and Use Committee.
Toxicokinetic/Toxicodynamic studies
Effect of AR-C155858 on toxicokinetics and respiratory depression of intravenous GHB
The effect of AR-C155858 on GHB-induced respiratory depression was studied using whole-body plethysmography (model PLY4213; Buxco Research Systems, Wilmington, NC) similar to our previously published studies (22). Animals were allowed to acclimate to the plethysmography chambers for 45 minutes followed by collection of five baseline measurements of respiratory parameters over 15 minutes. GHB was administered intravenously as 200, 600 or 1500 mg/kg bolus with or without AR-C155858 (1 or 5 mg/kg i.v. bolus). In the GHB 600 mg/kg group, a lower dose of AR-C155858 (0.1 mg/kg i.v. bolus) was also administered. In all the treatment groups, AR-C155858 was administered 5 minutes after GHB administration. This experiment was performed at a similar time and in a similar manner to our previous study assessing respiratory effects of GHB alone (22); therefore, data from rats administered GHB 200, 600, and 1500 mg/kg alone were used from the previous publication for comparison purposes. The time of GHB administration was considered as time 0. Blood and urine samples were collected at intervals up to 8 hours after GHB administration. The respiratory parameters, breathing frequency, tidal volume, and minute volume (breathing frequency x tidal volume) were recorded at 2.5, 5, 7.5, 10, 15, 20, 25, and 30 minutes and every 15 minutes thereafter until 8 hours. In all groups of animals, GHB was administered as a 300 mg/ml solution in sterile water via the jugular vein cannula. The AR-C155858 bolus was administered as a 0.1, 1 or 2.5 mg/ml solution in 10 % cyclodextrin in normal saline via the jugular vein cannula. All the treatment groups included 3–6 animals and were compared with their respective GHB alone group to determine the effects of AR-C155858 on GHB-induced respiratory depression. A separate group of animals received AR-C155858 alone (1 mg/kg i.v. bolus) to study the effect of this inhibitor itself on respiration.
Effect of AR-C155858 on GHB blood-brain partitioning at steady state
To assess the effect of AR-C155858 on the transport of GHB into the brain, GHB (400 mg/kg i.v. bolus followed by 208 mg/kg/hr i.v. infusion) was administered alone or in combination with AR-C155858 (5 mg/kg i.v. bolus) (n=4). The GHB dose was selected to produce steady-state GHB plasma concentrations of 800 µg/ml, similar to the high concentrations of GHB observed in rats after 600 mg/kg GHB i.v. used in the toxicokinetic study above. In addition this GHB concentration is similar to those seen in clinical cases of GHB overdose (5). The animals were euthanized at 4 h post GHB administration under isoflurane anesthesia followed by collection of blood and brain samples at steady state. Brain samples were immediately frozen in liquid nitrogen and stored at −80°C until analysis.
Effect of AR-C155858 on oral GHB toxicokinetics
Because GHB is commonly abused orally, the effect AR-C155858 was assessed on GHB toxicokinetics after oral administration in rats. Animals were administered GHB by oral gavage with or without AR-C155858 (5 mg/kg i.v. bolus). AR-C155858 was either administered 5 minutes or 1 hour post GHB administration (n = 4–6). In another group of animals, both AR-C155858 (10 mg/kg) and GHB (1500 mg/kg) were administered at the same time by oral gavage. Rats were fasted overnight before drug administration. Blood and urine samples were collected at intervals up to 15 hours after GHB administration. GHB alone was administered as a 300 mg/ml solution in water and AR-C155858 as a 2.5 mg/ml solution in 10% cyclodextrin in normal saline.
Sample Analysis
GHB plasma, urine and brain concentrations were measured using previously validated liquid chromatography coupled to tandem mass spectrometry (LC/MS/MS) assay (22, 30). Briefly, plasma samples were prepared by adding 5 µl of internal standard solution containing GHB-d6 (125 µg/ml) to 50 µl of sample. Plasma standards and quality controls were prepared by adding 5 µl of internal standard solution containing GHB-d6 (125 µg/ml) and 5 µl of stock solution containing GHB to 45 µl of blank plasma, and 800 µl of 0.1% formic acid in acetonitrile was added to precipitate the plasma proteins. The samples were vortexed, followed by centrifugation at 10,000g for 20 min at 4°C. An aliquot (750 µl) of the supernatant was aspirated and evaporated under a stream of nitrogen gas. The samples were then reconstituted in 250 µl of aqueous mobile phase.
AR-C155858 plasma concentrations were determined using a newly developed and validated LC/MS/MS method. Samples were prepared by adding 800 µl of 0.1% formic acid in acetonitrile to 50 µl of plasma sample. Plasma standards and quality controls were prepared by adding 800 µl of 0.1% formic acid in acetonitrile to 45 µl of blank plasma plus 5 µl of AR-C155858 stock solution. The samples were vortexed followed by centrifugation at 10,000g for 20 minutes at 4°C. An aliquot (750 µl) of the supernatant was collected and then evaporated under a stream of nitrogen gas, followed by reconstitution in 250 µl of aqueous mobile phase.
The LC/MS/MS assay was performed on Agilent 1100 series HPLC with binary pump and autosampler (Agilent Technologies, Santa Clara, CA) connected to a Perkin Elmer Sciex API 3000 triple-quadruple tandem mass spectrometer with a turbo ion spray (Applied Biosystems, Foster City, CA). Chromatographic separation was achieved by injecting 7 µl of sample on an Xterra MS C18 column (250 × 2.1 mm i.d., 5-µm particle size; Waters, Milford, MA). Mobile phase A consisted of 5/95 acetonitrile/water with 0.1% acetic acid and mobile phase B was 95/5 acetonitrile/water with 0.1% acetic acid. The flow rate was 200 µl/min with a gradient elution profile and a total run time of 20 min. The mass spectrometer was operated in a positive ionization mode with multiple reaction monitoring. Q1/Q3 m/z ratio for the parent/product ion of AR-C155858 was 462.3/373.2. The mass spectrometer parameters were optimized at a declustering potential of 35 V, focusing potential of 100 V, collision energy of 25 V, entrance potential of 10 V, and collision cell exit potential of 5 V. The ion spray voltage was set at 5500 V with temperature at 350°C. Nebulizer and curtain gas flow were set at 7 ml/min and 8 ml/min, respectively. The retention time for AR-C155858 was 5.65 minutes. The data was analyzed using Analyst software version 1.4.2 (Applied Biosystems, Foster City, CA).
Regression analysis of AR-C155858 peak areas to its concentrations was utilized to assess linearity of the curve. The intra-day and inter-day precision and accuracy were determined using quality control samples at 1 ng/ml (low QC), 25 ng/ml (medium QC) and 40 ng/ml (high QC). For determination of the intra-day precision and accuracy, quality control samples were analyzed in triplicate on each day whereas for the inter-day precision and accuracy, quality control samples were analyzed on three different days. A calibration curve was run on each analysis day along with the quality controls. The precision was determined by the coefficient of variation (CV%) and accuracy was measured by comparing the calculated concentration to the known concentration.
Data/Statistical Analysis
Noncompartmental analysis was performed using Phoenix WinNonlin software (Pharsight, Palo Alto, CA) to determine GHB toxicokinetic parameters. Area under the plasma concentration-time curve (AUC) was determined using the trapezoidal method, with AUC values extrapolate to time infinity. The extrapolated AUC accounted for 2% or less of the total AUC after IV administration. Total clearance (CL) or oral clearance (CL/F) was determined as dose/AUC. Renal clearance (CLR) was determined as Ae/AUC, where Ae is the total amount of GHB excreted in the urine. Urine samples were collected over time and the urinary amounts of GHB plateaued to a constant value, demonstrating complete collection. Metabolic or nonrenal clearance (CLM) was determined as CL - CLR. To assess the effect of AR-C155858 on GHB-induced respiratory depression, the toxicodynamic descriptors of area under the effect curve (AUEC), maximum effect (Emax) and duration of effect (Td) were used. AUEC was determined using Phoenix WinNonlin software. In all studies, mean values were compared using Student t-test or one-way analysis of variance followed by Tukey’s post hoc test for the detection of statistically significant differences in toxicokinetic/toxicodynamic parameters. Differences resulting in a P-value < 0.05 were considered statistically significant.
AR-C155858 Cell Uptake Studies
KNRK cells (a normal rat kidney cell line transformed by Kirsten murine sarcoma virus) were grown in Dulbecco’s modified Eagle’s medium supplemented with 5% fetal bovine serum, 100 units of penicillin, and 100 µg/ml streptomycin. Cells were incubated at 37°C in a humidified atmosphere with 5% CO2/95% air. Culture medium was changed every 2 to 3 days, and cells were passaged biweekly using 0.25% trypsin-EDTA. For the experiments, passage numbers between 7 and 9 were used. Cells were seeded in 35 mm (diameter) plastic dishes 2–3 days before the uptake study at a density of 2 × 105 cells/well. On the day of the experiment, the culture medium was removed, and cells were washed three times with uptake buffer (137 mM NaCl, 5.4 mM KCl, 2.8 mM CaCl2, 1.2 mM MgCl2.6H2O, and 10 mM HEPES, pH 6.5). To study the time dependent inhibition of AR-C155858 on GHB uptake, the cells were pre-incubated with 100 nM of AR-C155858 in uptake buffer for 0, 5, 15, 30, 45 and 60 min at room temperature. The AR-C155858 solution was aspirated and one ml of uptake buffer containing 10 µM [3H] GHB was added to the dishes for 1 minute. For the concentration dependent inhibition, cells were pre-incubated with varying concentrations of AR-C155858 (1, 5, 10, 25, 50, 75, 100 and 1000 nM) at room temperature followed by addition of a solution containing10 µM [3H] GHB and AR-C155858 for 1 minute at pH 6.5. The reversibility of inhibition by AR-C155858 was also studied by washing the cells three times with room temperature uptake buffer after pre-incubation with the inhibitor followed by addition of a solution containing GHB alone over 1 minute. Previous work indicated that 1 minute is within the time of linear uptake of GHB in KNRK cells. The uptake was stopped by aspirating the buffer and washing three times with ice-cold uptake buffer. The cells were lysed in 0.5 ml of lysis buffer (1N NaOH) for 1 h. After cell lysis, NaOH was neutralized by the addition of 0.5 ml 1.0 N HCl. Radioactivity was determined by adding 3 ml of scintillation fluid to 400 µl of cell lysate and counting with a liquid scintillation counter (1900 CA, Tri-Carb liquid scintillation analyzer; PerkinElmer Life and Analytical Sciences, Waltham, MA). Protein concentrations were determined by the bicinchoninic acid protein assay with bovine serum albumin as the protein standard. The results were normalized for the protein content of the cell lysate.
Results
Plasma AR-C155858 LC/MS/MS Assay
The lower limit of quantification for AR-C155858 in rat plasma was found to be 0.5 ng/ml with acceptable error in precision and accuracy of less than 20%. The standard curve range for AR-C155858 was 0.5 to 50 ng/ml based on regression analysis of peak areas to AR-C155858 concentrations with a correlation coefficient (r2 > 0.999). The intra-day and inter- day precision and accuracy of the quality control samples are summarized in Table 1.
Table 1.
LCMS/MS assay intra-day and inter-day accuracy and precision for AR-C155858 in rat plasma
| Nominal concentration (ng/ml) |
Measured concentration (ng/ml) |
SD | Precision (CV%) |
Accuracy (%) |
|
|---|---|---|---|---|---|
| 1 | 1.09 | 0.02 | 1.40 | 108.7 | |
| Intra-day | 25 | 26.3 | 0.76 | 2.87 | 105.3 |
| 40 | 40.6 | 1.15 | 2.84 | 101.6 | |
| 1 | 1.06 | 0.03 | 2.55 | 105.9 | |
| Inter-day | 25 | 26.1 | 0.54 | 2.07 | 104.7 |
| 40 | 40.9 | 0.60 | 1.46 | 102.3 |
Each individual value is the mean of triplicate measurements. The analysis was performed over 3 days.
Effect of AR-C155858 on intravenous GHB Toxicokinetics/Toxicodynamics
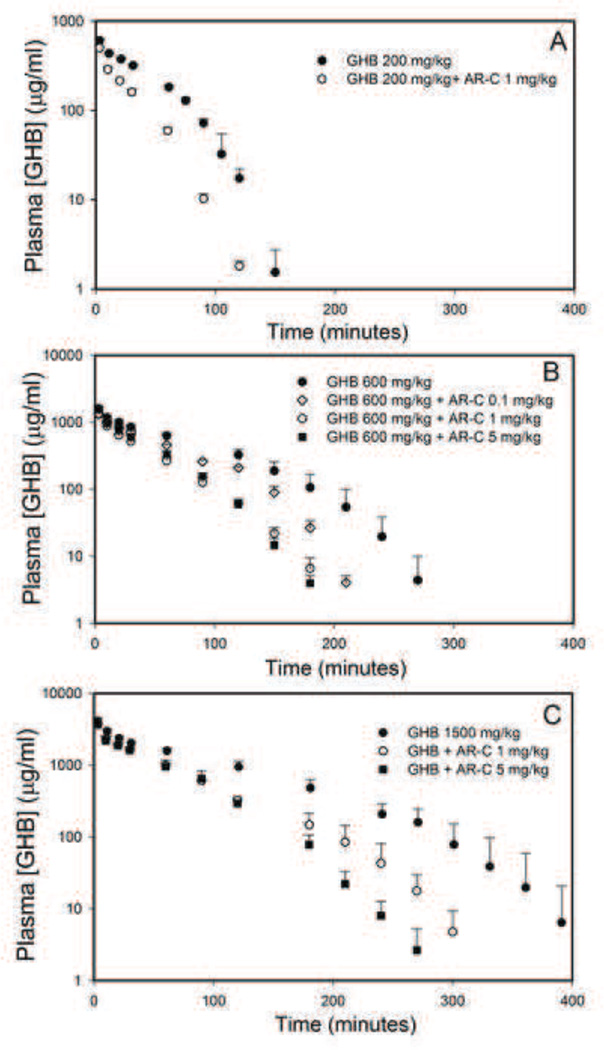
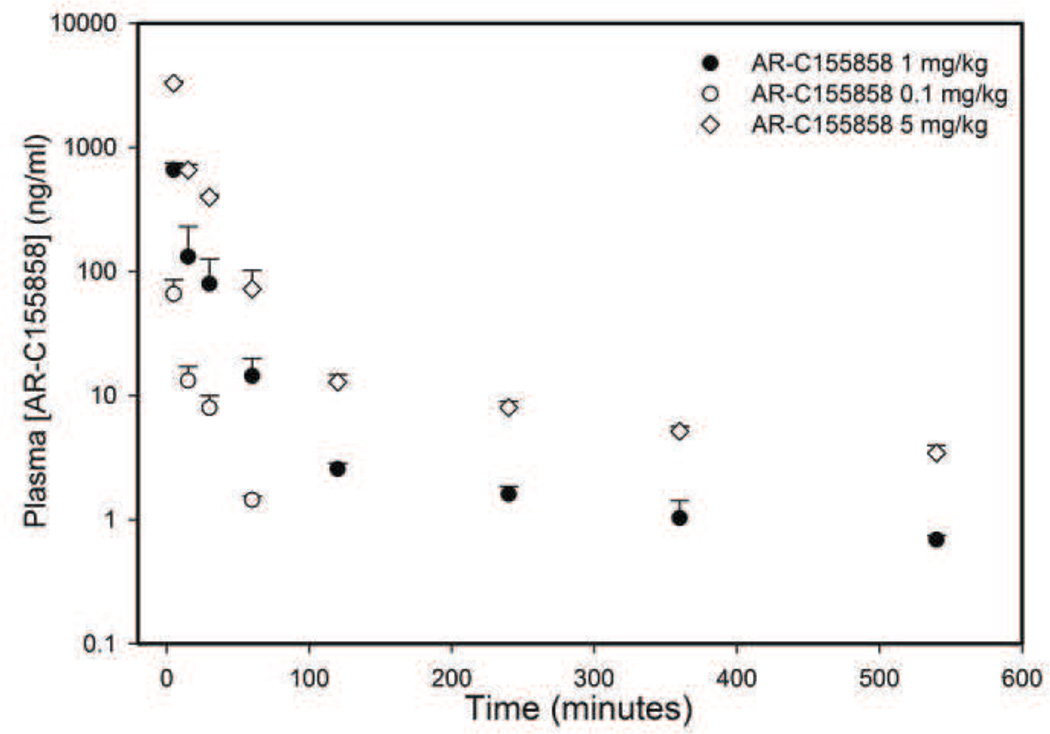
Plasma concentrations of GHB (200, 600 and 1500 mg/kg) in the presence of different doses of AR-C155858 (0.1, 1 or 5 mg/kg) is displayed in Figure 2. AR-C155858 administration resulted in a significant decrease in GHB plasma exposure. Noncompartmental analysis of GHB plasma concentration-time profile showed that there was a significant increase in the time-averaged GHB renal and total clearance following AR-C155858 treatment at all the doses of GHB used in this study as shown in Table 2. Interestingly, AR-C155858 (1 mg/kg) administration also resulted in a significant increase in GHB non-renal (metabolic) clearance at GHB 200 and 600 mg/kg. AR-C155858 plasma concentrations following 0.1, 1, and 5 mg/kg i.v. bolus exhibit a biexponential decline and dose-proportional pharmacokinetics as shown in Figure 3.
Figure 2.
Effect of AR-C155858 on intravenous GHB toxicokinetics. GHB (A) 200 mg/kg, (B) 600 mg/kg, and (C) 1500 mg/kg was administered as an i.v. bolus with or without AR-C155858 (0.1, 1 or 5 mg/kg). AR-C155858 was administered 5 minutes after GHB administration. Data presented as mean ± SD, n=4–6.
Table 2.
Toxicokinetics of intravenous GHB with and without AR-C155858 treatment
| GHB 200 mg/kg |
GHB 200 mg/kg + AR-C 1 mg/kg |
GHB 600 mg/kg |
GHB 600 mg/kg + AR-C 0.1 mg/kg |
GHB 600 mg/kg + AR-C 1 mg/kg |
GHB 600 mg/kg + AR-C 5 mg/kg |
GHB 1500 mg/kg |
GHB 1500 mg/kg + AR-C 1 mg/kg |
GHB 1500 mg/kg + AR-C 5 mg/kg |
|
|---|---|---|---|---|---|---|---|---|---|
| AUC (mg.min/ml) |
26.2 (0.90) |
13.3* (0.52) |
102 (12.4) |
71.3* (3.23) |
47.8* (6.58) |
52.8* (2.85) |
288 (39.8) |
170* (13.3) |
160* (17.4) |
| CLT (ml/min/kg) |
7.60 (0.29) |
15.0* (0.61) |
6.00 (0.74) | 8.42* (0.38) |
13.4* (1.98) |
11.4* (0.60) |
5.16 (0.70) |
9.25* (0.75) |
9.42* (0.98) |
| CLR (ml/min/kg) |
0.44 (0.20) |
4.41* (0.68) |
1.68 (0.75) |
3.87* (0.29) |
6.58* (0.41) |
6.38* (0.79) |
3.18 (0.66) |
7.09* (0.94) |
7.44* (1.07) |
| CLM (ml/min/kg) |
7.15 (0.45) |
10.6* (0.68) |
4.31 (0.34) |
4.56 (0.31) |
6.82* (1.58) |
5.00 (1.04) |
1.99 (0.16) |
2.16 (0.29) |
1.98 (0.22) |
| Urinary recovery (%) |
5.90 (2.86) |
29.4* (4.17) |
27.3 (9.77) |
45.9* (2.25) |
49.5* (4.00) |
56.2* (7.55) |
61.1 (5.02) |
76.4* (4.26) |
78.8* (3.98) |
GHB (200 mg/kg, 600 mg/kg, and 1500 mg/kg) was administered as an i.v. bolus with or without AR-C155858 (0.1, 1 or 5 mg/kg). AR-C155858 was administered 5 minutes after GHB administration. Data presented as mean (SD), n=4–6. One way analysis of variance followed by Tukey’s post-hoc test (GHB 600 and 1500 mg/kg) or Student’s t-test (GHB 200 mg/kg) was used to determine statistically significant difference between treatment groups.
P < 0.05 significantly different from their respective GHB alone group.
Figure 3.
Pharmacokinetics of AR-C155858 in rats following intravenous administration. AR-C155858 was administered at doses of 0.1, 1, or 5 mg/kg as an i.v. bolus. Data presented as mean ± SD, n=3–4.
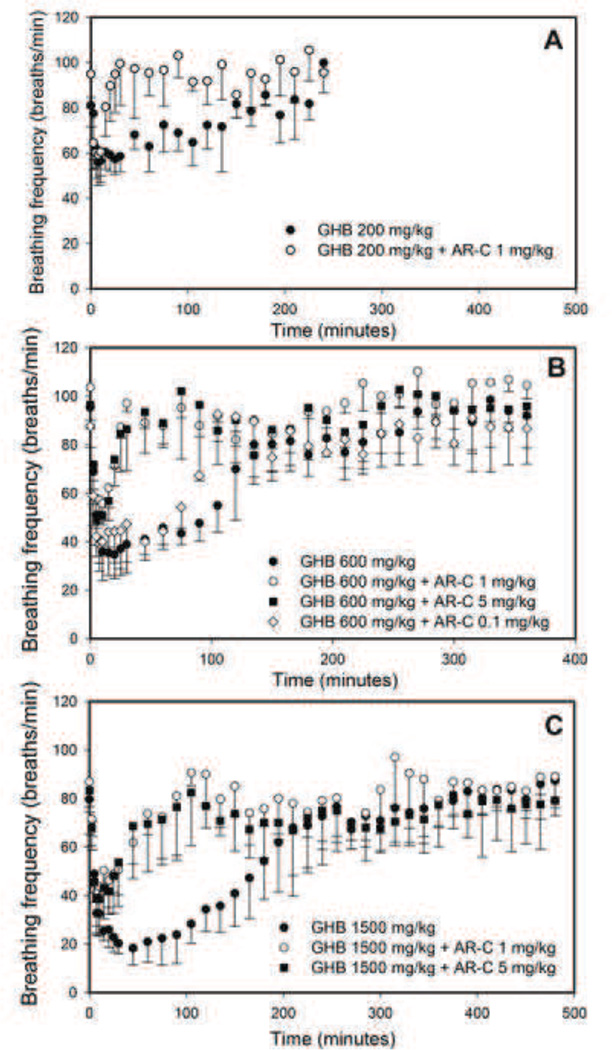
The effects of AR-C155858 on GHB-induced respiratory depression are displayed in Figure 4. Following a dose of GHB 200 mg/kg, AR-C155858 treatment resulted in an improvement in GHB-induced respiratory depression, measured by a decline in the breathing frequency. This is shown by a significant decrease in the AUEC for breathing frequency and also a decrease in the duration of response (Td) as displayed in Table 3. There was no change in Emax for breathing frequency by AR-C155858 at this lowest dose of GHB. Following a dose of GHB 600 mg/kg, AR-C155858 (1 and 5 mg/kg) administration significantly improved all the toxicodynamic parameters of breathing frequency (AUEC, Emax, and Td). A lower dose of AR-C155858 0.1 mg/kg, however, only led to improvement in the parameters of AUEC and Td with no significant improvement in Emax. Similar improvement in respiratory depression following AR-C155858 treatment was observed at a higher dose of GHB (1500 mg/kg) as demonstrated by an improvement in all the toxicodynamic parameters (AUEC, Emax, and Td). AR-C155858 had no effects on respiration by itself.
Figure 4.
Effect of AR-C on GHB-induced respiratory depression. GHB (A) 200 mg/kg, (B) 600 mg/kg, and (C) 1500 mg/kg was administered as an i.v. bolus with or without AR-C155858 (0.1, 1 or 5 mg/kg). Data presented as mean ± SD, n=4–6. AR-C155858 was administered 5 minutes after GHB administration. Data presented as mean ± SD, n=4–6.
Table 3.
Effect of AR-C155858 on GHB-induced respiratory depression
| GHB 200 mg/kg |
GHB 200 mg/kg + AR-C 1 mg/kg |
GHB 600 mg/kg alone |
GHB 600 mg/kg + AR-C 0.1 mg/kg |
GHB 600 mg/kg + AR-C 1 mg/kg |
GHB 600 mg/kg + AR-C 5 mg/kg |
GHB 1500 mg/kg alone |
GHB 1500 mg/kg + AR-C 1 mg/kg |
GHB 1500 mg/kg + AR-C 5 mg/kg |
|
|---|---|---|---|---|---|---|---|---|---|
| AUEC (breaths) |
2243 (1508) |
327* (3.71) |
7987 (1228) |
3697* (555) |
1520* (633) |
1347* (537) |
12505 (1995) |
2318* (883) |
1957* (1438) |
| Emax (breaths/ min) |
51.9 (8.51) |
58.8 (9.92) |
29.5 (6.94) |
31.6 (5.56) |
54.9* (6.62) |
47.9* (2.59) |
15.6 (7.05) |
38.1* (8.70) |
37.2* (13.7) |
| Td (min) | 113 (60.6) |
22.5* (5.00) |
153 (12.5) |
100* (8.66) |
28.3* (2.89) |
43.3* (17.5) |
281 (72.8) |
98.3* (7.64) |
93.3* (25.3) |
GHB 200 mg/kg, 600 mg/kg, and 1500 mg/kg was administered as an i.v. bolus with or without AR-C155858 (0.1, 1 or 5 mg/kg). Data presented as mean (SD), n=3–6. AR-C155858 was administered 5 minutes after GHB administration. One-way analysis of variance followed by Tukey’s post hoc test (GHB 600 and 1500 mg/kg) or Student’s t-test (GHB 200 mg/kg) was used to determine statistically significant differences in mean toxicodynamic parameters between groups.
P < 0.05 significantly different from respective GHB alone group.
Effect of AR-C155858 on GHB blood-brain partitioning
The effect of AR-C155858 5 mg/kg on GHB brain and plasma concentrations at steady-state are displayed in Table 4. AR-C155858 administration resulted in a significant decrease in GHB brain and plasma concentrations at steady state. This resulted in a significant decrease in GHB brain/plasma ratio as shown in Table 4.
Table 4.
Effect of AR-C155858 treatment on GHB blood-brain partitioning
| Cplasma (mg/ml) | Cbrain (mg/g) | GHB brain/plasma ratio |
|
|---|---|---|---|
| GHB alone | 0.87 ± 0.05 | 0.22 ± 0.03 | 0.25 ± 0.02 |
| GHB + AR-C155858 | 0.30 ± 0.03* | 0.03 ± 0.01* | 0.10 ± 0.03* |
Cbrain, brain GHB concentration at steady-state; Cplasma, plasma GHB concentration at steady-state.
GHB 400 mg/kg i.v. bolus + 208 mg/kg/h i.v. infusion was administered alone or with AR-C155858 (5 mg/kg i.v. bolus). AR-C155858 was administered 5 minutes after GHB administration. Brain and plasma samples were obtained at 4 h. Student’s t-test was used to determine statistically significant differences. Data presented as mean ± SD, n=4–5.
P < 0.05 significantly different from GHB alone.
Effect of AR-C155858 on oral GHB toxicokinetics
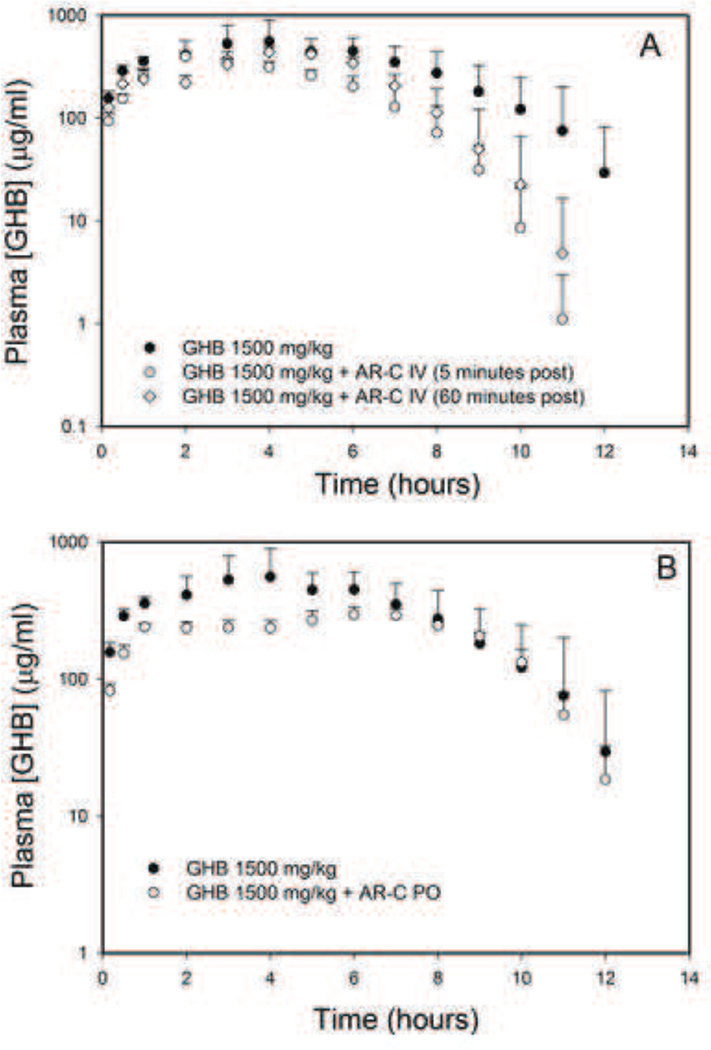
AR-C155858 treatment, either 5 min or 1 h post GHB administration, resulted in a significant increase in GHB renal, as well as oral clearance, with no effects on GHB metabolic clearance (Table 5). The Cmax of GHB was not affected by intravenous AR-C155858 suggesting minimal effects on its MCT-mediated absorption by this inhibitor (Figure 5A). The administration of AR-C155858 orally in combination with oral GHB resulted in a decrease in GHB Cmax (Figure 5B) and increase in its renal and oral clearance with no changes in metabolic clearance as shown in Table 5.
Table 5.
Effect of AR-C155858 on the oral toxicokinetics of GHB 1500 mg/kg
| GHB 1500 mg/kg oral |
GHB 1500 mg/kg oral + AR-C 5 mg/kg IV (60 min post) |
GHB 1500 mg/kg oral + AR-C 5 mg/kg IV (5 min post) |
GHB 1500 mg/kg oral + AR-C 10 mg/kg PO |
|
|---|---|---|---|---|
| AUC (mg.min/ml) |
233 (30.0) |
148* (10.7) |
124* (20.79) |
150* (5.71) |
| CL/F (ml/min/kg) |
6.52 (0.88) |
10.2* (0.75) |
12.32* (2.24) |
10.0* (0.38) |
| CLR (ml/min/kg) |
1.82 (0.63) |
5.74* (0.86) |
5.38* (0.54) |
4.95* (0.45) |
| Urinary excretion (%) |
28.2 (10.1) |
56.2* (6.84) |
44.97* (10.8) |
49.5* (5.41) |
| CLM (ml/min/kg) |
4.70 (0.99) |
4.44 (0.63) |
6.94 (2.69) |
5.07 (0.66) |
| Cmax (µg/ml) |
659 (259) |
451 (37.2) |
399 (41.4) |
300* (34.0) |
CL/F, GHB oral clearance (total clearance/bioavailability); CLR, GHB renal clearance; Cmax, maximum GHB plasma concentration; CLM, GHB non renal clearance.
GHB was administered by oral gavage. AR-C155858 (5 mg/kg) was administered as an i.v. bolus either 5 minutes or 1 hour post GHB administration. AR-C155858 (10 mg/kg PO) was also administered together with GHB by oral gavage. Data are presented as mean ± S.D., n = 4–8. One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was used to compare toxicokinetic parameters between treatment groups and GHB alone.
P < 0.05 compared with GHB alone.
Figure 5.
Plasma concentrations of GHB after oral administration of GHB (1500 mg/kg) with and without AR-C155858 (A) Intravenous AR-C155858 administration, and (B) Oral AR-C155858 administration. In the intravenous treatment group, AR-C155858 was administered as 5 mg/kg i.v. bolus and GHB was administered by oral gavage. AR-C155858 was administered either 5 minutes or 1 hour post GHB dose. In the oral treatment group, AR-C155858 and GHB were simultaneously administered by oral gavage. Data are presented as mean ± S.D., n = 4–8 in each group.
Effect of AR-C155858 on GHB uptake in KNRK cells
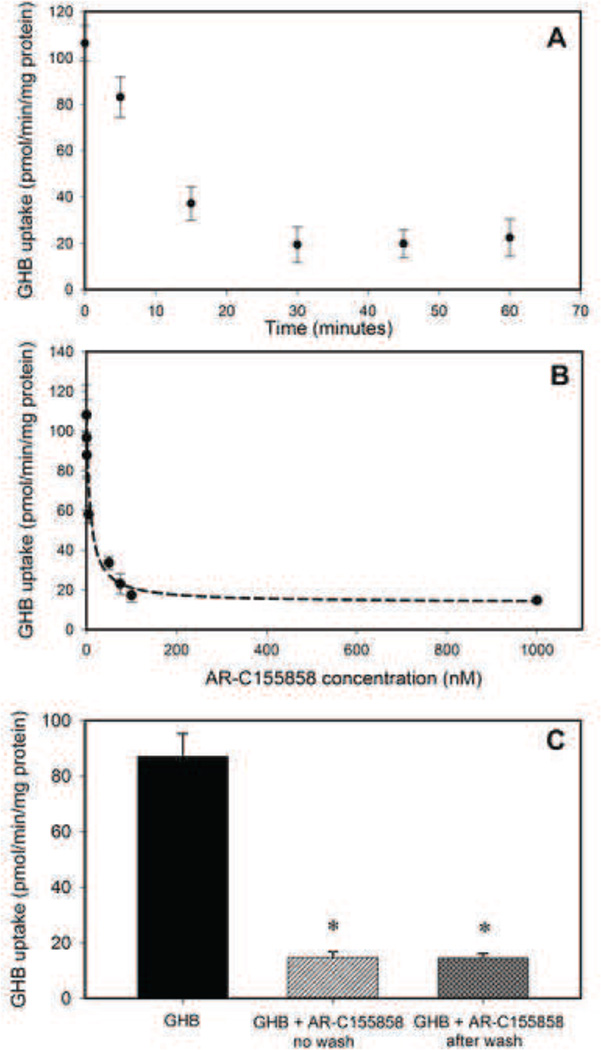
Since we observed an increase in renal clearance of GHB when animals were treated with GHB in combination with AR-C155858 treatment, we further characterized the inhibition by AR-C155858 in KNRK cells which is a rat kidney cell line. AR-C155858 demonstrated a time-dependent inhibition (Figure 6A) of GHB uptake with maximal inhibition observed following 30 minutes of pre-incubation with this inhibitor. Therefore, 30 minutes pre-incubation was chosen for concentration-dependent inhibition studies. AR-C155858 displayed a linear trend in inhibition (70% inhibition) up to 30 nM with maximal inhibition at 100 nM (Figure 6B). The IC50 of AR-C155858 for inhibition of GHB uptake was found to be 6.50 nM. Interestingly, this inhibition of uptake by AR-C155858 was found not to be rapidly reversible as observed by continued inhibition of GHB uptake when uptake was studied after washing the cells to remove the inhibitor (Figure 6C).
Figure 6.
Inhibition of GHB uptake by AR-C155858 in rat KNRK cells. (A) Time-dependent inhibition, (B) Concentration-dependent inhibition, and (C) Effect of washing on inhibition by AR-C155858. For time-dependence of inhibition, KNRK cells were pre-incubated with 100 nM AR-C155858 for 0–60 min at 37°C followed by 1 minute incubation with 10 µM [3H] GHB. For concentration-dependent inhibition, cells were pre-incubated for 30 minutes at 37°C with varying concentrations of AR-C155858. For reversibility of inhibition, GHB uptake was studied after 30 minutes pre-incubation with 100 nM AR-C155858 with or without a wash step (3 times) in between. Data represented as mean ± SD of three sets of studies conducted in triplicate. One-way analysis of variance followed by Tukey’s post-hoc test was used to determine statistically significant differences in GHB uptake after AR-C155858 treatment. * P < 0.05 significantly from GHB alone.
Discussion
GHB is widely abused as a recreational drug at nightclubs and raves (31). There is currently no approved treatment strategy for GHB overdose. GHB is a substrate for MCT1, a transporter with ubiquitous distribution in the body and responsible for its oral absorption, renal reabsorption and transport across the BBB (8, 9, 11, 24). GHB has also been shown to be a substrate for SMCT1 in rat thyroid follicular cells (16), however, the importance of SMCT1 in the renal reabsorption of GHB is not yet completely understood. Previous studies have demonstrated that MCT inhibitors such as L-lactate and luteolin increase GHB renal and total clearance and improve GHB-induced respiratory depression and decrease sleep time (17, 18, 22). In addition, L-lactate administration results in decreased GHB frontal cortex ECF concentrations with higher doses resulting in greater reductions (26). In this study, we evaluated the effects of a novel and highly potent MCT inhibitor, AR-C155858, on the toxicokinetics/toxicodynamics of GHB following both intravenous and oral administration in rats.
AR-C155858 is a potent MCT1 inhibitor recently developed as an immunosuppressive compound (27). Ovens et al. recently characterized the properties of this inhibitor and determined a Ki value of 2.3 nM for inhibition of lactate transport in rat erythrocytes (expresses only MCT1) (29). In addition, when expressed in Xenopus laevis oocytes, both MCT1 and MCT2 were potently inhibited by AR-C155858, with no inhibition of MCT4. We performed studies with AR-C155858 in rat KNRK cells and found time- and concentration-dependent inhibition of GHB uptake with an IC50 value of 6.8 nM, consistent with reports of inhibition of L-lactate uptake by AR-C155858 in rat erythrocytes (29). Interestingly, the inhibition could not be reversed by simply washing the cells with buffer to remove the inhibitor. The binding site for AR-C155858 has been demonstrated to be present within the C-terminal half of MCT1 on the cytosolic side of the membrane and therefore, it was suggested that AR-C155858 must cross the cell membrane before binding to this intracellular site on MCT1 (29). Therefore, the time-dependence and non-reversibility of inhibition observed in our study could be due to a slow entry of AR-C155858 into the cell before its binding to MCT1, as well as a slow efflux from cells. Additionally, other possible explanations include mechanism-based inhibition of MCT1, as reported for OATP (32) . The results of our toxicokinetic study showed that treatment with AR-C155858 significantly reduces GHB plasma concentrations following intravenous administration and increases both GHB time-averaged renal and total clearances at all the doses of GHB used in this study. The GHB doses in this study were selected to obtain plasma GHB concentrations that are relevant to concentrations of GHB observed in clinical cases of overdose (5). The increase in GHB clearance by AR-C155858 was much greater when compared to other MCT inhibitors such as L-lactate and luteolin studied previously (17, 22). This is consistent with our hypothesis that a more potent MCT inhibitor may represent a more effective treatment strategy for GHB overdose. However, effects of AR-C155858 were dose-dependent as its dose was increased from 0.1 to 1 mg/kg, with no further effect seen at a higher dose (5 mg/kg) when compared to that seen at 1 mg/kg. Evaluation of the mechanism underlying inhibition of MCTs by AR-C155858 requires further investigation in order to explain these results, taking into consideration the IC50 and the potential non-reversibility of the interaction.
GHB exhibits capacity-limited metabolism and capacity-limited renal reabsorption (7, 9). At lower doses, renal clearance is a negligible route of GHB elimination with the contribution of renal clearance increasing at higher GHB doses. Recent studies have shown that the administration of MCT inhibitor, L-lactate with a lower dose of GHB (200 mg/kg) results in an increase in GHB renal clearance without any increase in total clearance due to the negligible role of renal elimination at this dose (33). Similar results were obtained in a pilot clinical study where no change in total oral clearance was observed with L-lactate (19). In addition, another MCT inhibitor, luteolin, did not result in a significant increase in GHB clearance at a low GHB dose of 400 mg/kg (17). We, therefore, studied the effects of the more potent MCT inhibitor, AR-C155858, at a lower dose of GHB, i.e. 200 mg/kg. Interestingly, we also observed a significant increase in GHB metabolic clearance following treatment with AR-C155858, in addition to an increase in its renal and total clearance. GHB is primarily metabolized by GHB dehydrogenase as well as a mitochondrial transhydrogenase (34). To our knowledge, there is no report of AR-C155858 affecting the metabolism of GHB. The increase in metabolic clearance with AR-C155858 treatment is more likely an indirect effect due to the lower plasma concentrations caused by increased renal clearance, which would increase GHB metabolism since GHB metabolism exhibits Michaelis-Menten kinetics at this and higher doses. At this low dose, plasma concentrations are likely close to the Km value and so changes in GHB concentrations will produce pronounced changes in the metabolism of GHB. However, at higher GHB doses, plasma concentrations would still remain much greater than Km values for much of the time course, so little change would be evident in the time-averaged metabolic clearance.
We have previously demonstrated that GHB causes a dose-dependent respiratory depression in rats, measured by a decline in breathing frequency. The primary effect of GHB on respiration is a decrease in breathing frequency, accompanied by a compensatory increase in tidal volume, which allows minute volume to remain constant until doses approach lethality (22). Therefore, we looked at the effects of AR-C155858 on breathing frequency as it is a more sensitive parameter to measure GHB-induced respiratory depression. Our results demonstrate a very significant improvement in GHB-induced respiratory depression following AR-C155858 treatment with a 5–6 fold reduction in the duration of respiratory depression observed at higher GHB doses. This rapid reversal of GHB-induced respiratory depression following AR-C155858 treatment can be partly explained by the increase in GHB renal and total clearance observed with this inhibitor. MCT1 is the only isoform present at the BBB and is largely responsible for GHB brain uptake (23–25, 35). Therefore, along with effects on renal clearance, AR-C155858 may alter the entry of GHB into the brain, i.e., its site of action. This is further supported by a 3-fold reduction in brain/plasma partitioning of GHB under steady state conditions (GHB plasma steady state concentrations ≈ 1000 µg/ml) with AR-C155858 administration observed in the current study. This GHB concentration was chosen to mimic GHB concentrations achieved in our toxicokinetic/toxicodynamic studies with intravenous bolus dosing. These results are also consistent with previous reports of improvement in respiratory depression by the MCT inhibitor, L-lactate (22), where the effects were 3-fold lower than that observed with AR-C155858 in the current study. This can be explained by the considerable difference in potencies of these MCT inhibitors (36). A recent report from our laboratory has shown that L-lactate, at lower doses, does not affect GHB BBB transport, with effects observed only at higher L-lactate doses (26). This could be the potential reason for significantly greater effects with AR-C155858 in the current study when compared to L-lactate. These data further suggest the importance of inhibition of the MCT-mediated brain uptake of GHB for treatment of its overdose.
Another interesting finding of the current study is that there was no significant improvement in breathing frequency by AR-C155858 at a dose of 5 mg/kg compared with a 1 mg/kg dose, similar with the effect of this inhibitor on GHB toxicokinetics. Previous reports have demonstrated that the sedative and hypnotic effects of GHB correlate with its brain concentrations (24, 30). Therefore, our results suggest that the respiratory effects of GHB also correlate with its concentration in the brain and needs to be confirmed in future studies by measuring GHB brain ECF concentrations in the presence of AR-C155858.
In a recreational setting, GHB is commonly ingested by the oral route (37). Therefore, we assessed the effects of intravenous AR-C155858 on GHB toxicokinetics after a high oral dose in rats to mimic clinically relevant conditions. It has been shown that GHB exhibits saturable oral absorption in rats illustrated by a less than proportional increase in GHB Cmax and AUC with increasing doses (7, 38). This saturable absorption of GHB has been shown to be mediated by MCTs in the intestine (8, 39). Our results show that intravenous administration of AR-C155858, one hour after GHB administration increases its renal and oral clearance to similar extents when compared to GHB alone, with no effects on non-renal clearance. In addition, there was no change in GHB plasma Cmax following AR-C155858 treatment. The GHB dose used in these studies was 1500 mg/kg po, similar to that used in earlier studies where saturable and prolonged absorption was observed (38). The effects of AR-C155858 are in contrast to what has been observed in earlier studies with L-lactate. Intravenous L-lactate resulted in an increase in non-renal and total oral clearances of GHB (1500 mg/kg po) without changes in its renal clearance. The authors suggested that L-lactate may affect MCT-mediated absorption of GHB, leading to lower plasma concentrations and potentially increasing non-renal clearance due to its concentration-dependent and nonlinear behavior, thereby increasing total oral clearance without effects on renal reabsorption (38). In our study, AR-C155858 increases total oral GHB clearance to the same extent as the increase in its renal clearance by inhibiting its MCT-mediated renal reabsorption. Both GHB and L-lactate are also substrates for sodium dependent MCTs (16). Therefore GHB can be transported through the intestine by both MCTs and SMCTs but the relative contribution of MCTs and SMCTs to GHB intestinal transport is currently unknown. In addition, L-lactate can inhibit SMCTs in addition to MCTs. Although it is known that AR-C155858 inhibits both MCT1 and MCT2, its ability to inhibit SMCT1 is not yet known. The differential inhibitory effect of these inhibitors on SMCTs could potentially be one of the reasons for the observed differences in their effects. Recent work done in our laboratory characterizing the interaction of GHB with L-lactate using mechanistic TK/TD modeling study also supports a major role for SMCTs in the effects of lactate (40). Further studies are however needed to confirm the mechanisms underlying the effects of L-lactate and AR-C155858 on high oral doses of GHB.
Due to the potential role of MCTs in GHB oral absorption, we also assessed the effects of oral administration of AR-C155858 on oral GHB toxicokinetics. The observed increase in total oral clearance can be explained by the increase in renal clearance of GHB, similar to what was observed after intravenous administration of AR-C155858. Further experiments are necessary to characterize the effects of AR-C155858 on the oral absorption of GHB, in order to assess any additional effects on absorption.
Our results demonstrate that AR-C155858 can serve as a potential treatment strategy for the treatment of GHB overdose. While AR-C155858 has been shown to have immunosuppressive effects (27), which might limit its therapeutic potential, the doses of AR-C155858 used in this study are very low compared to the doses required to achieve immunosuppression. In addition, immunosuppression is observed after chronic administration of AR-C155858, in contrast to the acute administration used as a potential overdose treatment strategy.
Conclusions
In summary, the novel and highly potent inhibitor, AR-C155858 increases renal and total clearance of GHB following both intravenous and oral administration in rats. AR-C155858 also results in significant improvement of GHB-induced respiratory depression which may be mediated by inhibition of its renal reabsorption and brain uptake, both processes mediated by MCTs. Our studies demonstrate proof-of-concept in utilizing MCT inhibition as a potential treatment strategy by improving GHB-induced respiratory depression which leads to death in cases of GHB overdose.
Acknowledgement
The authors thank Donna Ruszaj for her assistance in developing the current LC-MS/MS method. This work was supported by the National Institutes of Health National Institute on Drug Abuse [grant DA023223]. NV was funded in part by a fellowship from Pfizer Global Inc.
Abbreviations
- ABEC
area below the effect curve
- AUC
area under the plasma concentration-time curve
- Cl
clearance
- ClM
metabolic clearance
- ClR
renal clearance
- Emax
maximum pharmacodynamic effect
- GABA
γ-aminobutyric acid
- GHB
γ-hydroxybutyrate
- MCT
monocarboxylate transporter
- Td
duration of effect
- TK/TD
toxicokinetics/toxicodynamics
Footnotes
Authorship Contributions
Participated in research design: Morris, Vijay and Morse
Conducted experiments: Vijay and Morse
Contributed new reagents or analytic tools: Morris and Vijay
Performed data analysis: Vijay, Morris
Wrote or contributed to the writing of the manuscript: Morris and Vijay
References
- 1.Maitre M. The gamma-hydroxybutyrate signalling system in brain: organization and functional implications. Prog Neurobiol. 1997;51(3):337–361. doi: 10.1016/s0301-0082(96)00064-0. [DOI] [PubMed] [Google Scholar]
- 2.Wong CG, Chan KF, Gibson KM, Snead OC. Gamma-hydroxybutyric acid: neurobiology and toxicology of a recreational drug. Toxicol Rev. 2004;23(1):3–20. doi: 10.2165/00139709-200423010-00002. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Stokes SA, Woeckener A. A tale of novel intoxication: a review of the effects of gamma-hydroxybutyric acid with recommendations for management. Ann Emerg Med. 1998;31(6):729–736. [PubMed] [Google Scholar]
- 4.Mason PE, Kerns WP., 2nd Gamma hydroxybutyric acid (GHB) intoxication. Acad Emerg Med. 2002;9(7):730–739. doi: 10.1111/j.1553-2712.2002.tb02154.x. [DOI] [PubMed] [Google Scholar]
- 5.Zvosec DL, Smith SW, Porrata T, Strobl AQ, Dyer JE. Case series of 226 gamma-hydroxybutyrate-associated deaths: lethal toxicity and trauma. Am J Emerg Med. 2011;29(3):319–332. doi: 10.1016/j.ajem.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Palatini P, Tedeschi L, Frison G, Padrini R, Zordan R, Orlando R, et al. Dose-dependent absorption and elimination of gamma-hydroxybutyric acid in healthy volunteers. Eur J Clin Pharmacol. 1993;45(4):353–356. doi: 10.1007/BF00265954. [DOI] [PubMed] [Google Scholar]
- 7.Lettieri JT, Fung HL. Dose-dependent pharmacokinetics and hypnotic effects of sodium gamma-hydroxybutyrate in the rat. J Pharmacol Exp Ther. 1979;208(1):7–11. [PubMed] [Google Scholar]
- 8.Arena C, Fung HL. Absorption of sodium gamma-hydroxybutyrate and its prodrug gamma-butyrolactone: relationship between in vitro transport and in vivo absorption. J Pharm Sci. 1980;69(3):356–358. doi: 10.1002/jps.2600690331. [DOI] [PubMed] [Google Scholar]
- 9.Morris ME, Hu K, Wang Q. Renal clearance of gamma-hydroxybutyric acid in rats: increasing renal elimination as a detoxification strategy. J Pharmacol Exp Ther. 2005;313(3):1194–1202. doi: 10.1124/jpet.105.083253. [DOI] [PubMed] [Google Scholar]
- 10.Lettieri J, Fung HL. Absorption and first-pass metabolism of 14C–gamma-hydroxybutyric acid. Res Commun Chem Pathol Pharmacol. 1976;13(3):425–437. [PubMed] [Google Scholar]
- 11.Wang Q, Darling IM, Morris ME. Transport of gamma-hydroxybutyrate in rat kidney membrane vesicles: Role of monocarboxylate transporters. J Pharmacol Exp Ther. 2006;318(2):751–761. doi: 10.1124/jpet.106.105965. [DOI] [PubMed] [Google Scholar]
- 12.Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999;343(Pt 2):281–299. [PMC free article] [PubMed] [Google Scholar]
- 13.Ganapathy V, Thangaraju M, Gopal E, Martin PM, Itagaki S, Miyauchi S, et al. Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J. 2008;10(1):193–199. doi: 10.1208/s12248-008-9022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gopal E, Fei YJ, Sugawara M, Miyauchi S, Zhuang L, Martin P, et al. Expression of slc5a8 in kidney and its role in Na(+)-coupled transport of lactate. J Biol Chem. 2004;279(43):44522–44532. doi: 10.1074/jbc.M405365200. [DOI] [PubMed] [Google Scholar]
- 15.Wang Q, Lu Y, Morris ME. Monocarboxylate transporter (MCT) mediates the transport of gamma-hydroxybutyrate in human kidney HK-2 cells. Pharm Res. 2007;24(6):1067–1078. doi: 10.1007/s11095-006-9228-6. [DOI] [PubMed] [Google Scholar]
- 16.Cui D, Morris ME. The drug of abuse gamma-hydroxybutyrate is a substrate for sodium-coupled monocarboxylate transporter (SMCT) 1 (SLC5A8): characterization of SMCT-mediated uptake and inhibition. Drug Metab Dispos. 2009;37(7):1404–1410. doi: 10.1124/dmd.109.027169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Morris ME. Flavonoids modulate monocarboxylate transporter-1-mediated transport of gamma-hydroxybutyrate in vitro and in vivo. Drug Metab Dispos. 2007;35(2):201–208. doi: 10.1124/dmd.106.012369. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q, Wang X, Morris ME. Effects of L-lactate and D-mannitol on gamma-hydroxybutyrate toxicokinetics and toxicodynamics in rats. Drug Metab Dispos. 2008;36(11):2244–2251. doi: 10.1124/dmd.108.022996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris ME, Morse BL, Baciewicz GJ, Tessena MM, Acquisto NM, Hutchinson DJ, DiCenzo R. Monocarboxylate Transporter Inhibition with Osmotic Diuresis Increases γ-Hydroxybutyrate Renal Elimination in Humans: A Proof-of-Concept Study. J Clinic Toxicol. 2011:1. doi: 10.4172/2161-0495.1000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carai MA, Colombo G, Brunetti G, Melis S, Serra S, Vacca G, et al. Role of GABA(B) receptors in the sedative/hypnotic effect of gamma-hydroxybutyric acid. Eur J Pharmacol. 2001;428(3):315–321. doi: 10.1016/s0014-2999(01)01334-6. [DOI] [PubMed] [Google Scholar]
- 21.Kaupmann K, Cryan JF, Wellendorph P, Mombereau C, Sansig G, Klebs K, et al. Specific gamma-hydroxybutyrate-binding sites but loss of pharmacological effects of gamma-hydroxybutyrate in GABA(B)(1)-deficient mice. Eur J Neurosci. 2003;18(10):2722–2730. doi: 10.1111/j.1460-9568.2003.03013.x. [DOI] [PubMed] [Google Scholar]
- 22.Morse BL, Vijay N, Morris ME. gamma-Hydroxybutyrate (GHB)-induced respiratory depression: combined receptor-transporter inhibition therapy for treatment in GHB overdose. Mol Pharmacol. 2012;82(2):226–235. doi: 10.1124/mol.112.078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhattacharya I, Boje KM. GHB (gamma-hydroxybutyrate) carrier-mediated transport across the blood-brain barrier. J Pharmacol Exp Ther. 2004;311(1):92–98. doi: 10.1124/jpet.104.069682. [DOI] [PubMed] [Google Scholar]
- 24.Roiko SA, Felmlee MA, Morris ME. Brain uptake of the drug of abuse gamma-hydroxybutyric acid in rats. Drug Metab Dispos. 2012;40(1):212–218. doi: 10.1124/dmd.111.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vijay N, Morris ME. Role of Monocarboxylate Transporters in Drug Delivery to the Brain. Curr Pharm Des. 2013 doi: 10.2174/13816128113199990462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roiko SA, Vijay N, Felmlee MA, Morris ME. Brain extracellular gamma-hydroxybutyrate concentrations are decreased by L-lactate in rats: role in the treatment of overdoses. Pharm Res. 2013;30(5):1338–1348. doi: 10.1007/s11095-013-0973-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pahlman C, Qi Z, Murray CM, Ferguson D, Bundick RV, Donald DK, et al. Immunosuppressive properties of a series of novel inhibitors of the monocarboxylate transporter MCT-1. Transpl Int. 2013;26(1):22–29. doi: 10.1111/j.1432-2277.2012.01579.x. [DOI] [PubMed] [Google Scholar]
- 28.Ekberg H, Qi Z, Pahlman C, Veress B, Bundick RV, Craggs RI, et al. The specific monocarboxylate transporter-1 (MCT-1) inhibitor, AR-C117977, induces donor-specific suppression, reducing acute and chronic allograft rejection in the rat. Transplantation. 2007;84(9):1191–1199. doi: 10.1097/01.tp.0000287541.53389.be. [DOI] [PubMed] [Google Scholar]
- 29.Ovens MJ, Davies AJ, Wilson MC, Murray CM, Halestrap AP. AR-C155858 is a potent inhibitor of monocarboxylate transporters MCT1 and MCT2 that binds to an intracellular site involving transmembrane helices 7–10. Biochem J. 2010;425(3):523–530. doi: 10.1042/BJ20091515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Felmlee MA, Roiko SA, Morse BL, Morris ME. Concentration-effect relationships for the drug of abuse gamma-hydroxybutyric acid. J Pharmacol Exp Ther. 2010;333(3):764–771. doi: 10.1124/jpet.109.165381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drasbek KR, Christensen J, Jensen K. Gamma-hydroxybutyrate--a drug of abuse. Acta Neurol Scand. 2006;114(3):145–156. doi: 10.1111/j.1600-0404.2006.00712.x. [DOI] [PubMed] [Google Scholar]
- 32.Shitara Y, Nagamatsu Y, Wada S, Sugiyama Y, Horie T. Long-lasting inhibition of the transporter-mediated hepatic uptake of sulfobromophthalein by cyclosporin a in rats. Drug Metab Dispos. 2009;37(6):1172–1178. doi: 10.1124/dmd.108.025544. [DOI] [PubMed] [Google Scholar]
- 33.Morse BL, Morris ME. Effects of monocarboxylate transporter inhibition on the oral toxicokinetics/toxicodynamics of gamma-hydroxybutyrate and gamma-butyrolactone. J Pharmacol Exp Ther. 2013;345(1):102–110. doi: 10.1124/jpet.112.202796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaufman EE, Nelson T. An overview of gamma-hydroxybutyrate catabolism: the role of the cytosolic NADP(+)-dependent oxidoreductase EC 1.1.1.19 and of a mitochondrial hydroxyacid-oxoacid transhydrogenase in the initial, rate-limiting step in this pathway. Neurochem Res. 1991;16(9):965–974. doi: 10.1007/BF00965839. [DOI] [PubMed] [Google Scholar]
- 35.Gerhart DZ, Enerson BE, Zhdankina OY, Leino RL, Drewes LR. Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. Am J Physiol. 1997;273(1 Pt 1):E207–E213. doi: 10.1152/ajpendo.1997.273.1.E207. [DOI] [PubMed] [Google Scholar]
- 36.Morse BL, Felmlee MA, Morris ME. gamma-Hydroxybutyrate blood/plasma partitioning: effect of physiologic pH on transport by monocarboxylate transporters. Drug Metab Dispos. 2012;40(1):64–69. doi: 10.1124/dmd.111.041285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galicia M, Nogue S, Miro O. Liquid ecstasy intoxication: clinical features of 505 consecutive emergency department patients. Emerg Med J. 2011;28(6):462–466. doi: 10.1136/emj.2008.068403. [DOI] [PubMed] [Google Scholar]
- 38.Morse BL, Morris ME. Toxicokinetics/Toxicodynamics of gamma-Hydroxybutyrate-Ethanol Intoxication: Evaluation of Potential Treatment Strategies. J Pharmacol Exp Ther. 2013;346(3):504–513. doi: 10.1124/jpet.113.206250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lam WK, Felmlee MA, Morris ME. Monocarboxylate transporter-mediated transport of gamma-hydroxybutyric acid in human intestinal Caco-2 cells. Drug Metab Dispos. 2010;38(3):441–447. doi: 10.1124/dmd.109.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morse BA, Vijay N, Morris ME. Mechanistic modeling of Monocarboxylate Transporter-Mediated Toxicokinetic/Toxicodynamic Interactions between γ-hydroxybutyrate and L-lactate. AAPS J. 2014;16(4):756–770. doi: 10.1208/s12248-014-9593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]