Abstract
Background
Anastomotic leak is one of the most serious complications following Roux-en-Y gastric bypass (RYGB).
Objective
To examine the relationship between technical factors and incidence of clinically relevant anastomotic leak following RYGB in Longitudinal Assessment of Bariatric Surgery (LABS).
Setting
Eleven bariatric centers in the United States, University and Private Practice.
Methods
Patient characteristics, technical factors of surgery, and postoperative outcomes were assessed by trained researchers using standardized protocols. Correlation of surgical factors of patients undergoing RYGB (n=4444) with the incidence of post-operative anastomotic leak was assessed by univariate chi-squared analysis.
Results
Forty-four participants (1.0%, 95%CI 0.7% to 1.3%) experienced a clinically relevant anastomotic leak. Of these, 39 (89%) underwent abdominal re-operation and 3 (7%) died. Technical factors associated with anastomotic leak were open surgery (p<0.0001), revision surgery (p<0.0001) and use of an abdominal drain (p=0.02). Provocative leak testing, method of gastrojejunostomy and use of fibrin sealant were not associated with anastomotic leak.
Conclusions
Anastomotic leak following RYGB was rare (1.0%). Most cases required reintervention, however, the majority (93%) recovered from this event. Open surgery, revision surgery and routine drain placement were associated with increased leak rate. Some of these findings may be due to differences in pre-operative patient risk.
Introduction
The last two decades have seen a dramatic increase in the numbers of bariatric operations performed in the United States and worldwide. Reasons for this include the growing epidemic of obesity,(1, 2) the demonstrated effectiveness of bariatric surgery in improving life expectancy and serious co-morbidities,(3, 4) and the excellent safety profile of modern bariatric surgery. (5) Although multiple surgical options currently exist to promote durable weight loss, Roux-en-Y gastric bypass (RYGB) remains one of the most commonly performed operations.(6, 7)
Although RYGB is effective in promoting durable weight loss (8) it may be complicated by a number of major post-operative events. Anastomotic leak following gastric bypass is rare, however its consequences may be devastating. Reported rates of anastomotic leak vary from 0.6 to 4.4%.(9) Surgical re-exploration is usually required for anastomotic leak and hospital stay is prolonged,(10, 11) resulting in increased cost and morbidity. Anastomotic leak is also an independent risk factor for early post-operative mortality.(12)
Factors associated with anastomotic leak include clinical (or patient) factors and technical factors. Identified clinical factors associated with anastomotic leak include male sex, age and presence of sleep apnea.(12) Unfortunately, other than not offering surgery to high risk individuals, there is often little that can be done to reduce clinical risk. In contrast to clinical risk factors, technical risk factors are potentially modifiable by the operating surgeon to reduce risk of anastomotic leak. Examples of technical factors include method of constructing the anastomoses, intra-operative leak testing and routine abdominal drainage. However the rare incidence of anastomotic leak following RYGB makes it difficult for a single surgeon or center to accrue enough events to identify risk factors or investigate strategies to reduce its incidence. Consequently many of the strategies employed by surgeons are either based on basic surgical principals or extrapolated from other gastrointestinal surgery.(13, 14) A recent guideline published by the American Society of Metabolic and Bariatric Surgery found no high quality evidence to support any intervention to reduce the incidence of anastomotic leaks.(15)
The Longitudinal Assessment of Bariatric Surgery (LABS) is an 11-center consortium funded by the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) in the National Institute of Health (NIH) that conducts observational cohort studies of bariatric surgical outcomes. These involve largely prospective, standardized and comprehensive collection of clinical data. LABS-1 collected 30-day outcome data in consecutive patients, aged 18-years or older, undergoing primary bariatric surgery. LABS-2 comprises more detailed and ongoing data collection in a selected cohort of patients, restricted to those who had not had prior bariatric surgery. The purpose of the present study was to describe the incidence and outcomes of anastomotic leak following RYGB in LABS, and to examine technical factors associated with its occurrence.
Methods
Patients
Patients were recruited by LABS into either of two cohorts, designated LABS-1 and LABS-2, at one of the eleven participating centers: University of Pittsburgh Medical Center (Pennsylvania), New York-Presbyterian Hospital [Columbia-Presbyterian or Valley Hospitals, or Weill-Cornell Medical College] (New York and New Jersey), East Carolina Medical Center (North Carolina), the MeritCare Health Systems through the Neuropsychiatric Research Institute (North Dakota), Sacramento Bariatric (California), University of Washington Medical Center or Virginia Mason Medical Center (Washington), and Oregon Health and Sciences University or Legacy Good Samaritan Medical Center (Oregon). The LABS protocols and consent forms were approved by the Institutional Review Board at each institution.
Protocols
LABS-1, a study of 30 day outcomes, included all consecutive patients at least 18 years of age who consented to participate. LABS-2 involves long term follow-up and data collection. Accordingly, in contrast to LABS-1, non-consecutive patients who would be able to undertake the required follow-up were selected for recruitment, excluding anyone who had undergone bariatric surgery prior to enrolling in LABS-2. LABS inclusion criteria and data collection have previously been described in detail.(16) Data were collected for bariatric surgeries performed between March 2005 and April 2009 and sent to the Data Coordinating Center (DCC) at the University of Pittsburgh, Graduate School of Public Health.
The analysis included LABS subjects (LABS-1 and LABS-2 patients) who underwent RYGB, either open or laparoscopic, including revision of RYGB.
Data Collection
Data were collected prospectively on a large number of clinical and technical factors,(16) however the site of an identified anastomotic leak was not part of the initial LABS data collection protocol. As site of anastomotic leak was considered important to this analysis, this information was collected retrospectively by LABS site investigators by review of case notes and discussion with the treating surgeons. Method of gastrojejunostomy was recorded on the initial data collection forms as a binary response to whether each of three methods (hand sewing, linear stapling or circular stapling) was used in constructing the anastomosis. This was recoded to reflect the general use of these clinical terms by surgeons. Linear stapled with or without hand sewn is treated as predominantly linear stapled, circular stapled with or without hand sewn is classified as predominantly circular stapled, hand sewn with no staples is classified as predominantly hand sewn.
The primary outcome for this analysis is clinically relevant leak (CRL). Clinically relevant leak is defined as a patients undergoing readmission or reintervention for a suspected anastomotic leak, where the presence of a leak was subsequently confirmed by the investigator or LABS adjudication subcommittee upon review of the medical record. Anastomotic leaks diagnosed on the basis of radiology or analysis of abdominal drain contents, that did not require readmission or reintervention, were not considered CRLs for the purpose of this analysis.
Statistical Analysis
Baseline demographic characteristics are presented as frequencies and percentages for categorical variables and compared between groups (e.g. participants with and without anastomosis test) using chi-square test or the exact equivalent (e.g., Fisher’s exact test), as appropriate. Continuous characteristics such as the duration of surgery are summarized with medians and interquartile ranges and are compared between groups using Wilcoxon’s rank-sum test. Incidence of CRL in the overall sample and in the subgroups is presented as frequencies and percentages. The association between baseline demographics, co-morbidities, intra-operative factors and routine leak testing with the likelihood of clinically relevant leak was evaluated using chi-square test or its exact version (when the expected frequencies were smaller than 5), separately for each variable. Pair-wise comparisons between various methods of anastomosis were conducted using Holm’s step-down method to adjust for multiplicity.
We did not consider a multivariable adjusted analysis as the outcome CRL was rare (approximately 1%) and hence the sample size was too small to conduct a statistically powered adjusted analysis. For all statistical analyses, Statistical Analysis Systems (SAS), version 9.3 (SAS Institute, Cary, NC), was used. A cut-off of <0.05 was used to determine statistical significance.
Results
The analysis included 4444 patients who had RYGB surgery in LABS consortium (LABS-1 and LABS-2). Patient clinical characteristics are presented in Table 1.
Table 1.
Participant Demographics, overall, and by anastomosis test
| Characteristic | Total (N=4444) | Patients without anastomosis test (N=636) | Patients with anastomosis test (N=3803) | p value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Surgery performed | <.0001 | ||||||
| Laparoscopic Gastric Bypass | 3841 | 86.4 | 379 | 59.6 | 3458 | 90.9 | |
| Open Gastric Bypass | 603 | 13.6 | 257 | 40.4 | 345 | 9.1 | |
| Patient Age (years), range | 18.0–75.0 | 18.0–75.0 | 18.0–73.0 | 0.057 | |||
| Median (Q1, Q3) | 45.0(36.0,53.0) | 44.0(35.5, 52.0) | 45.0(36.0, 53.0) | ||||
| Age (years) | 0.04 | ||||||
| <30 | 431 | 9.7 | 81 | 12.7 | 349 | 9.2 | |
| 30–39 | 1126 | 25.3 | 164 | 25.8 | 962 | 25.3 | |
| 40–49 | 1302 | 29.3 | 187 | 29.4 | 1113 | 29.3 | |
| 50–59 | 1179 | 26.5 | 152 | 23.9 | 1025 | 27.0 | |
| 60+ | 406 | 9.1 | 52 | 8.2 | 354 | 9.3 | |
| BMI (kg/m2), range | 17.8–107.4 | 17.8–79.7 | 19.2–107.4 | 0.006 | |||
| Median (Q1, Q3) | 46.9(42.6,52.6) | 48.1(43.1,53.6) | 46.8(42.5,52.4) | ||||
| BMI (kg/m2) | 0.053 | ||||||
| <35 | 66 | 1.5 | 6 | 0.9 | 58 | 1.5 | |
| 35–<40 | 462 | 10.4 | 54 | 8.5 | 407 | 10.7 | |
| 40–<50 | 2346 | 52.8 | 321 | 50.5 | 2024 | 53.2 | |
| 50–<60 | 1174 | 26.4 | 191 | 30.0 | 982 | 25.8 | |
| 60+ | 396 | 8.9 | 64 | 10.1 | 332 | 8.7 | |
| Male | 0.04 | ||||||
| No | 3574 | 80.4 | 493 | 77.5 | 3078 | 80.9 | |
| Yes | 870 | 19.6 | 143 | 22.5 | 725 | 19.1 | |
| White/Caucasian (41 missing) | <.0001 | ||||||
| No | 509 | 11.6 | 40 | 6.3 | 468 | 12.4 | |
| Yes | 3894 | 88.4 | 594 | 93.7 | 3296 | 87.6 | |
| Hispanic (2 missing) | <.0001 | ||||||
| No | 4186 | 94.2 | 623 | 98.1 | 3558 | 93.6 | |
| Yes | 256 | 5.8 | 12 | 1.9 | 244 | 6.4 | |
| Current or recent smoker (13 missing) | <.0001 | ||||||
| No | 3766 | 85.0 | 503 | 79.2 | 3258 | 85.9 | |
| Yes | 665 | 15.0 | 132 | 20.8 | 533 | 14.1 | |
Clinically relevant leaks
Of the included 4444 patients, 44 suffered a CRL (1%). The most common site of leak was the gastrojejunostomy (28/44, Table 2) followed by the jejunojenostomy (7/44). Clinical characteristics of the patients with and without CRL is presented in table 3. Although not statistically adequately powered (due to low incidence rate), we explored the bivariate association (unadjusted) between patient and surgeon characteristics with incidence of CRL. In unadjusted analysis, no demographic characteristics were significantly associated with the incidence of CRL.
Table 2.
Site of Clinically Relevant Leak1
| Site of Leak# | Total (N=43*) | |
|---|---|---|
| Gastric pouch staple line | 5 | 11.4 |
| Gastrojejunostomy | 28 | 63.6 |
| Gastric remnant staple line | 2 | 4.6 |
| Jejunojejunostomy | 7 | 15.9 |
| Small intestine other | 1 | 2.3 |
| Others^ | 4 | 9.1 |
1 participant missing site of leak form
Multiple sites may apply
Clinically relevant leak is a confirmed leak at Gastric pouch staple line or Gastrojejunostomy
List of others (n=4)
Colon
Intra-abdominal abscess - Exploratory surgery negative
Gastric fundus
Esophageal leak
Table 3a.
Clinically Relevant Leak (CRL) by demographics
| Characteristic | N | CRL | p value | |
|---|---|---|---|---|
| n | % | |||
| Age (years) | 0.46 | |||
| <30 | 431 | 2 | 0.5 | |
| 30–39 | 1126 | 8 | 0.7 | |
| 40–49 | 1302 | 15 | 1.2 | |
| 50–59 | 1179 | 13 | 1.1 | |
| 60+ | 406 | 6 | 1.5 | |
| BMI (kg/m^2) | 0.07 | |||
| <35 | 66 | 2 | 3.0 | |
| 35–<40 | 462 | 7 | 1.5 | |
| 40–<50 | 2346 | 16 | 0.7 | |
| 50–<60 | 1174 | 12 | 1.0 | |
| 60+ | 396 | 7 | 1.8 | |
| Male | 0.88 | |||
| No | 3574 | 35 | 0.9 | |
| Yes | 870 | 9 | 1.0 | |
| White/Caucasian (41 missing) | 0.61 | |||
| No | 509 | 4 | 0.8 | |
| Yes | 3894 | 40 | 1.0 | |
| Hispanic (2 missing) | 0.76 | |||
| No | 4186 | 41 | 0.9 | |
| Yes | 256 | 3 | 1.2 | |
| Current or recent smoker (13 missing) | 0.13 | |||
| No | 3766 | 41 | 1.1 | |
| Yes | 665 | 3 | 0.5 | |
| Albumin (g/dl) (394 missing) | 0.65 | |||
| low: <3 g/dl | 24 | 0 | 0.0 | |
| normal: 3–6 g/dl | 4026 | 35 | 0.9 | |
Of the 44 patients with a CRL, 39 required re-operation (17 laparoscopic, 12 open). The median time between RYGB and re-intervention was 5.5 days (range 1–25 days). Median length of stay was 7.0 days in those with a CRL compared to 2.0 days for those without. Approximately 98% of those with no CRL had a length of hospital stay under 7 days, compared to 50% with a CRL.
The mortality rate was 6.8% (3/44) among those with a CRL compared to 0.3% (13/4400) among those with no CRL.
Technical Factors
A CRL was more common among those undergoing a revision RYGB compared to a primary surgery (Table 4), and after open compared to laparoscopic RYGB. Placement of an abdominal drain was also associated with an increased incidence of CRL. The use of fibrin sealant was not associated with CRL in LABS. These associations persisted when analysis was restricted to leaks occurring at the gastrojejunostomy or gastric pouch only.
Table 4a.
Clinically Relevant Leak (CRL) by technical factors
| Characteristic | N | CRL | p value | |
|---|---|---|---|---|
| n | % | |||
| Surgery type | <.0001 | |||
| Primary surgery | 4286 | 34 | 0.8 | |
| Revision surgery | 149 | 10 | 6.7 | |
| Neither primary or revision surgery | 9 | 0 | 0.0 | |
| Surgery performed | <.0001 | |||
| Laparoscopic Gastric Bypass | 3841 | 29 | 0.8 | |
| Open Gastric Bypass | 603 | 15 | 2.5 | |
| Gastrojejunostomy sealed (22 missing) | 0.36 | |||
| No | 1854 | 21 | 1.1 | |
| Yes | 2568 | 22 | 0.9 | |
| Drain placed at Gastrojejunostomy | 0.02 | |||
| No | 3711 | 31 | 0.8 | |
| Yes | 733 | 13 | 1.8 | |
| Method of Gastrojejunostomy (33 missing) | 0.02 | |||
| Predominantly linear stapled | 1594 | 9 | 0.6 | |
| Predominantly EEA | 1731 | 17 | 1.0 | |
| Predominantly hand sewn | 1086 | 18 | 1.7 | |
| Anastomosis tested (5 missing) | 0.06 | |||
| No | 636 | 2 | 0.3 | |
| Yes | 3803 | 42 | 1.1 | |
In the initial analysis CRL was observed less frequently after a linear stapled gastrojejunostomy compared to circular stapled or hand sewn gastrojejunostomy. However, as method of gastrojejunostomy is unlikely to affect CRL at sites other than the gastrojejunostomy, sensitivity analysis was conducted by restricting the analysis to leaks occurring at the gastrojejunostomy only. In this analysis the incidence of CRL at gastrojejunostomy was 0.31% versus 0.64% versus 0.92% for predominantly linear stapled versus predominantly circular stapled versus hand sewn gastrojejunal anastomoses respectively (p=0.12).
Provocative leak testing
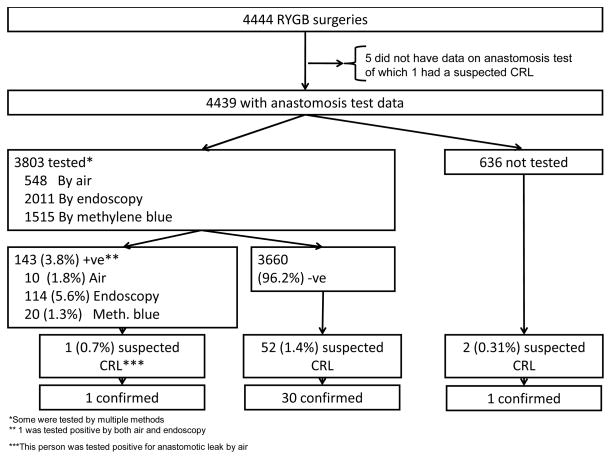
Of the 33 surgeons who had done at least 10 LABS surgeries, 29 surgeons (87.8%) routinely tested (in 90% or more cases) the gastrojejunal anastomosis during surgery. Of the 3803 (85.7%) patients who underwent provocative leak testing 143 (3.8%) had a positive test. The anastomosis test was done by three methods: 548 were tested with air of which 10 (1.8%) tested positive, 2011 were tested with endoscopy of which 114 (5.6%) tested positive, and 1515 were tested with methylene blue of which 20 (1.3%) tested positive (Figure 1).
Figure 1.
Of the 3803 with anastomosis test result, 143 (3.8%) had a positive leak test. No demographic or clinical characteristics showed any trend of association with the likelihood of a positive leak test (data not shown). No statistically significant difference in CRL rate was found between those undergoing positive, negative, or no provocative leak test.
Median operative time (skin incision to skin closed) was similar for those surgeries which included anastomosis test compared to those which did not (136 versus 138 minutes, p=0.14).
Discussion
This analysis found the incidence of CRL following RYGB in LABS is rare at 1%. Clinically relevant leaks were more common after open and revision surgery and when an abdominal drain was placed at the time of surgery. No difference in CRL at the gastrojejunostomy was observed between the different methods of constructing this anastomosis. Provocative leak testing was not associated with the incidence of gastrojejunostomy CRL in LABS. Most cases of CRL required reintervention with consequent increase in hospital stay and mortality risk, however most cases also recovered from this event.
The findings of the present study compare favorably to other large series on anastomotic leak following RYGB. A single center series examining the outcomes of 3828 patients undergoing RYGB over a 23-year period showed an anastomotic leak rate of 3.9%.(10) Anastomotic leaks were more common after revisional RYGB (8.0%), however in contrast to LABS, leaks were more common after laparoscopic RYGB (5.2%) than open RYGB (2.6%). It is important to note that this series includes period where laparoscopic RYGB was introduced, and that fewer than 1/3rd of the procedures were laparoscopic (1080 out of 3828). In this series, the overall mortality after anastomotic leak was 14.7%, although mortality was higher after leak at the jejunojeunostomy (40%) than the gastrojejunostomy (9%). Sixty-eight percent of patients in this series with anastomotic leak at the gastrojejunostomy were managed non-operatively, with none of these requiring later surgical intervention and no mortality in this group.
Another single center series examined the outcomes of 60 patients with anastomotic leak from a total of 1764 undergoing RYGB (leak rate 3.4%).(17) Again approximately one third (n=573) of patients had RYGB performed via a laparoscopic approach. The authors of this series divided leaks into localized subclinical leaks (n=12) and those where the patients exhibited clinical signs of sepsis (n=48). The overall mortality rate from anastomotic leak was 10%, one of the three patients with leak a the jejunojejunostomy died (mortality rate = 33%). None of the patients with subclinical leaks died.
A systematic review focusing specifically on patients undergoing laparoscopic RYGB identified 10 studies including 3464 patients. A total of 71 anastomotic leaks were identified to give an unadjusted anastomotic leak rate of 2%. Although this anastomotic leak rate was more in keeping with that found in LABS, the included studies may have incorporated patients receiving surgery early in the experience of laparoscopic RYGB. Due to the nature of this review, no analysis was conducted exploring the associations of clinical or technical factors with anastomotic leak.
A Medline based literature search was unable to identify any large studies exploring the relationships between multiple technical factors and anastomotic leak following RYGB. Nevertheless individual technical factors have been investigated in numerous clinical studies. One such factor is whether the gastrojejunostomy is constructed using a linear stapled, circular stapled or hand sewn technique. One of the interesting findings of the present analysis was the decreased overall anastomotic leak rate following linear stapled anastomoses in LABS, however this finding failed to persist when restricted to leaks at the gastrojejunostomy only. This corresponds with the findings of a Michigan Bariatric Surgery Collaborative survey where reported leak rates were identical for these three anastomosis techniques,(18) although circular stapled gastrojejunostomies were associated with an increased risk of anastomotic haemorrhage and wound infection. A meta-analysis of studies comparing linear stapled to circular stapled anastomoses also found no difference between anastomotic leak rates, but did show an increased stricture rate with circular stapled anastomoses.(19) As plausible explanations as to how method of gastrojejunostomy would affect leaks at other sites are lacking, this finding from LABS may have resulted from chance alone.
The use of tissue sealants was not associated with alteration of the anastomosis leak rate in LABS. This is consistent with the results of a randomized trial of fibrin sealant following RYGB where no difference in anastomotic leak rate was observed between the treatment and control groups,(20) although the use of fibrin sealant was associated with a reduced risk of anastomotic haemorrhage.
Placement of an abdominal drain was associated with an increased incidence of CRL in LABS. This study is not a randomized trial of drain usage, and data were not collected on the reason for drain placement. It is possible that drains were more likely to be placed when a surgeon was concerned about an elevated risk of CRL. These results however do not support the assumption that abdominal drainage reduces the need for reintervention for CRL. This finding is consistent with previous comparative studies showing routine drain placement to be unnecessary following RYGB.(21) Randomized trials evaluating the use of routine drainage following other major abdominal surgery have also failed to demonstrate benefit, even suggesting an increased complication rate with drain placement following cholecystectomy.(22–25)
No randomized trials could be identified of intra-operative leak testing for RYGB, however a randomized trial of pneumatic testing in colorectal anastomoses showed a decreased CRL rate in tested patients.(13) Three series’ of patients undergoing RYGB with routine endoscopic intraoperative leak testing report CRL rates of less than 1%.(26–28) Another study compared leak testing via methylene blue delivered by orogastric tube with endoscopic leak testing.(29) In this study endoscopic leak testing had a higher rate of positive leak tests intraoperatively but a lower rate of post-operative CRL. Analysis of LABS data also showed more positive leak tests following endoscopic testing compared to other methods, however since only a handful of patients (269) were tested by multiple methods, we do not have sufficient data to compare different methods of testing. Although no difference was found in CRL between participants undergoing positive, negative or no provocative leak test these findings should be interpreted with caution. Number of CRLs was small, and as with drain placement, no data were collected on intraoperative events that could have influenced a surgeon’s decision to perform leak testing. Furthermore, the presence of a positive leak test would usually result in the operating surgeon reinforcing or revising the anastomosis, preventing CRL. Unfortunately data on the management of a positive leak test were not collected in LABS. Difference in patient risk or technical factors not measured or corrected for in LABS could also partially explain the findings of the present analysis.
LABS is a large multi-center study incorporating university hospitals and private clinics. Despite the large numbers of included patients and comprehensive data collection, the present study has revealed several counterintuitive findings. One explanation for this is the design of LABS as an observational study rather than a randomized trial. Randomized trials are the only study design able to account for non-measured confounding factors, however large randomized trials are expensive, time consuming, and only able to draw strong conclusions regarding the randomized intervention. Despite this limitation, LABS data collection and risk adjustment was pre-specified to include all variables deemed potentially clinically relevant study investigators. Nevertheless it is possible that unmeasured clinical or technical factors may have contributed to some of the unexpected findings of this analysis. Participants may have undergone open RYGB because of anticipated technical difficulty; and surgeons may have chosen to use fibrin sealant, place a drain and conduct provocative leak testing when they already had concerns about the integrity of an anastomosis.
The definition of CRL used in this study is another potential weakness. Due to the pre-specified design for definition and capture of post-operative complications, it was not possible to identify anastomotic leaks that did not require readmission to hospital, or endoscopic, radiological or surgical reintervention. We hypothesize that the only likely situation where this would occur is if a drain left at the initial RYGB was completely successful in draining the leak. However the finding of increased rates of CRL in patient where a drain was placed at the initial RYGB makes this outcome unlikely.
Another potential weakness of this study is the small number of CRLs. While testament to the excellent safety of modern RYGB surgery, this limited our ability to perform adjusted analyses of associations. Also, univariable associations presented were not adequately powered. Therefore, the conclusions drawn should be taken as observations from a large prospective multi-center study. The low number of CRLs in the present study also limits the ability to extrapolate these findings to centers with a higher rate of CRL.
The results of the present study confirm the low incidence of CRL in modern RYGB surgery. Although revision surgery continues to present an increased risk of CRL, our findings do not support increased leaks rates after laparoscopic RYGB compared to the open approach. In fact the reverse now appears to be true. Although anastomotic leak remains a major complication, with prompt diagnosis and appropriate management, resulting mortality is rare.
Some of the unexpected or counterintuitive findings of the present study might be addressed by future research. The question of whether or not to test gastrojejunal anastomoses intraoperatively would be best addressed by multi-center randomized controlled trials. However, to detect a doubling of anastomotic leak rate from 1% to 2%, would require randomizing over 5000 patients. Such trials are logistically difficult and expensive to conduct. An alternative is systematic review and meta-analysis of smaller randomized trials. Universally agreed endpoints, and well designed studies would facilitate later quantitative summary of the results.
Conclusion
Anastomotic leak following RYGB in LABS was rare, limiting the power of even this large multi-center study to examine associated factors. Method of gastrojejunostomy, routine provocative leak testing, or use of fibrin sealant were not associated with incidence of CRL in LABS. In this non-randomized study, increased incidence of CRL was observed following revision or open RYGB compared to primary laparoscopic surgery. No technical factors were identified as associated with a reduced incidence of CRL.
Table 3b.
Clinically Relevant Leak (CRL) by health status
| Characteristic | N | CRL | p value | |
|---|---|---|---|---|
| n | % | |||
| Hypertension | 0.39 | |||
| No | 1897 | 16 | 0.8 | |
| Yes | 2547 | 28 | 1.1 | |
| Hypertension therapy (75 missing) | 0.77 | |||
| No medication | 263 | 4 | 1.5 | |
| Single medication | 1099 | 11 | 1.0 | |
| Multiple medication | 1110 | 13 | 1.2 | |
| Diabetes | 0.10 | |||
| No | 2849 | 23 | 0.8 | |
| Yes | 1595 | 21 | 1.3 | |
| Type of diabetes medication (4 missing) | 0.42 | |||
| No diabetes medication | 232 | 1 | 0.4 | |
| Single oral medication | 476 | 5 | 1.1 | |
| Multiple oral medication | 369 | 7 | 1.9 | |
| Insulin (with or without oral meds) | 514 | 8 | 1.6 | |
| Congestive heart failure | 0.27 | |||
| No | 4348 | 42 | 0.9 | |
| Yes | 96 | 2 | 2.1 | |
| Asthma | 0.89 | |||
| No | 3373 | 33 | 1.0 | |
| Yes | 1071 | 11 | 1.0 | |
| Inability to walk 200 ft (1 missing) | 0.19 | |||
| No | 4359 | 42 | 1.0 | |
| Yes | 84 | 2 | 2.4 | |
| History of DVT or PE | 0.07 | |||
| No | 4271 | 40 | 0.9 | |
| Yes | 173 | 4 | 2.3 | |
| Sleep apnea | 0.42 | |||
| No | 2187 | 19 | 0.9 | |
| Yes | 2257 | 25 | 1.1 | |
| CPAP | 0.23 | |||
| No | 423 | 7 | 1.7 | |
| Yes | 1834 | 18 | 1.0 | |
| Supplemental oxygen dependent (9 missing) | 0.93 | |||
| No | 2165 | 24 | 1.1 | |
| Yes | 83 | 1 | 1.2 | |
| Ischemic heart disease | 0.63 | |||
| No | 4213 | 41 | 1.0 | |
| Yes | 231 | 3 | 1.3 | |
| Pulmonary hypertension | 0.47 | |||
| No | 4394 | 43 | 1.0 | |
| Yes | 50 | 1 | 2.0 | |
| Venous edema w/ulcerations (280 missing) | 0.0003 | |||
| No | 3961 | 34 | 0.9 | |
| Yes | 203 | 7 | 3.4 | |
| Beta-blocker (61 missing) | 0.45 | |||
| No | 3558 | 33 | 0.9 | |
| Yes | 825 | 10 | 1.2 | |
| Statin/lipid-lowering agent | 0.0827 | |||
| No | 3241 | 27 | 0.8 | |
| Yes | 1203 | 17 | 1.4 | |
| Therapeutic anticoagulation | 0.06 | |||
| No | 4227 | 39 | 0.9 | |
| Yes | 217 | 5 | 2.3 | |
| Narcotic | 0.22 | |||
| No | 3645 | 33 | 0.9 | |
| Yes | 799 | 11 | 1.4 | |
| Anti-depressant (61 missing) | 0.005 | |||
| No | 2545 | 16 | 0.6 | |
| Yes | 1838 | 27 | 1.5 | |
Table 4b.
Clinically Relevant Leak (CRL) at gastrojejunostomy or gastric pouch by technical factors
| Characteristic | N | CRL at GJ | p value | |
|---|---|---|---|---|
| n | % | |||
| Surgery type | <.0001 | |||
| Primary Surgery | 4286 | 25 | 0.6 | |
| Revision surgery | 149 | 7 | 4.7 | |
| Not first time and not revision | 9 | 0 | 0.0 | |
| Surgery performed | <.0001 | |||
| Laparoscopic Gastric Bypass | 3841 | 21 | 0.5 | |
| Open Gastric Bypass | 603 | 11 | 1.8 | |
| Gastrojejunostomy sealed (22 missing) | 0.999 | |||
| No | 1854 | 13 | 0.7 | |
| Yes | 2568 | 18 | 0.7 | |
| Drain placed at Gastrojejunostomy | 0.02 | |||
| No | 3711 | 22 | 0.6 | |
| Yes | 733 | 10 | 1.4 | |
| Method of Gastrojejunostomy (33 missing) | 0.02 | |||
| Predominantly linear stapled | 1594 | 6 | 0.4 | |
| Predominantly EEA | 1731 | 12 | 0.7 | |
| Predominantly hand sewn | 1086 | 14 | 1.3 | |
| Anastomosis tested (5 missing) | 0.07 | |||
| No | 636 | 1 | 0.2 | |
| Yes | 3803 | 31 | 0.8 | |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Mokdad AH. Epidemiology of obesity in the Western Hemisphere. J Clin Endocrinol Metab. 2008;93:S1–8. doi: 10.1210/jc.2008-1356. [DOI] [PubMed] [Google Scholar]
- 3.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 4.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 5.Flum DR, Belle SH, King WC, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361:445–54. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinojosa MW, Varela JE, Parikh D, Smith BR, Nguyen XM, Nguyen NT. National trends in use and outcome of laparoscopic adjustable gastric banding. Surg Obes Relat Dis. 2009;5:150–5. doi: 10.1016/j.soard.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294:1909–17. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- 8.White S, Brooks E, Jurikova L, Stubbs RS. Long-term outcomes after gastric bypass. Obes Surg. 2005;15:155–63. doi: 10.1381/0960892053268282. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen NT, Wilson SE. Complications of antiobesity surgery. Nat Clin Pract Gastroenterol Hepatol. 2007;4:138–47. doi: 10.1038/ncpgasthep0734. [DOI] [PubMed] [Google Scholar]
- 10.Lee S, Carmody B, Wolfe L, et al. Effect of location and speed of diagnosis on anastomotic leak outcomes in 3828 gastric bypass cases. J Gastrointest Surg. 2007;11:708–13. doi: 10.1007/s11605-007-0085-3. [DOI] [PubMed] [Google Scholar]
- 11.Marshall JS, Srivastava A, Gupta SK, Rossi TR, DeBord JR. Roux-en-Y gastric bypass leak complications. Arch Surg. 2003;138:520–3. doi: 10.1001/archsurg.138.5.520. discussion 523. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez AZJ, DeMaria EJ, Tichansky DS, et al. Experience with over 3,000 open and laparoscopic bariatric procedures: multivariate analysis of factors related to leak and resultant mortality. Surg Endosc. 2004;18:193–7. doi: 10.1007/s00464-003-8926-y. [DOI] [PubMed] [Google Scholar]
- 13.Beard JD, Nicholson ML, Sayers RD, Lloyd D, Everson NW. Intraoperative air testing of colorectal anastomoses: a prospective, randomized trial. Br J Surg. 1990;77:1095–7. doi: 10.1002/bjs.1800771006. [DOI] [PubMed] [Google Scholar]
- 14.Ricciardi R, Roberts PL, Marcello PW, Hall JF, Read TE, Schoetz DJ. Anastomotic leak testing after colorectal resection: what are the data? Arch Surg. 2009;144:407–11. doi: 10.1001/archsurg.2009.43. discussion 411. [DOI] [PubMed] [Google Scholar]
- 15.ASMBS guideline on the prevention and detection of gastrointestinal leak after gastric bypass including the role of imaging and surgical exploration. Surg Obes Relat Dis. 2009;5:293–6. doi: 10.1016/j.soard.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Belle SH, Berk PD, Courcoulas AP, et al. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surg Obes Relat Dis. 2007;3:116–26. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Csendes A, Burgos AM, Braghetto I. Classification and management of leaks after gastric bypass for patients with morbid obesity: a prospective study of 60 patients. Obes Surg. 2012;22:855–62. doi: 10.1007/s11695-011-0519-6. [DOI] [PubMed] [Google Scholar]
- 18.Finks JF, Carlin A, Share D, et al. Effect of surgical techniques on clinical outcomes after laparoscopic gastric bypass--results from the Michigan Bariatric Surgery Collaborative. Surg Obes Relat Dis. 2011;7:284–9. doi: 10.1016/j.soard.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Giordano S, Salminen P, Biancari F, Victorzon M. Linear stapler technique may be safer than circular in gastrojejunal anastomosis for laparoscopic Roux-en-Y gastric bypass: a meta-analysis of comparative studies. Obes Surg. 2011;21:1958–64. doi: 10.1007/s11695-011-0520-0. [DOI] [PubMed] [Google Scholar]
- 20.Silecchia G, Boru CE, Mouiel J, et al. The use of fibrin sealant to prevent major complications following laparoscopic gastric bypass: results of a multicenter, randomized trial. Surg Endosc. 2008;22:2492–7. doi: 10.1007/s00464-008-9885-0. [DOI] [PubMed] [Google Scholar]
- 21.Kavuturu S, Rogers AM, Haluck RS. Routine drain placement in Roux-en-Y gastric bypass: an expanded retrospective comparative study of 755 patients and review of the literature. Obes Surg. 2012;22:177–81. doi: 10.1007/s11695-011-0560-5. [DOI] [PubMed] [Google Scholar]
- 22.Gurusamy KS, Samraj K. Routine abdominal drainage for uncomplicated open cholecystectomy. Cochrane Database Syst Rev. 2007:CD006003. doi: 10.1002/14651858.CD006003.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gurusamy KS, Samraj K, Davidson BR. Routine abdominal drainage for uncomplicated liver resection. Cochrane Database Syst Rev. 2007:CD006232. doi: 10.1002/14651858.CD006232.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Gurusamy KS, Samraj K, Mullerat P, Davidson BR. Routine abdominal drainage for uncomplicated laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2007:CD006004. doi: 10.1002/14651858.CD006004.pub3. [DOI] [PubMed] [Google Scholar]
- 25.Jesus EC, Karliczek A, Matos D, Castro AA, Atallah AN. Prophylactic anastomotic drainage for colorectal surgery. Cochrane Database Syst Rev. 2004:CD002100. doi: 10.1002/14651858.CD002100.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alasfar F, Chand B. Intraoperative endoscopy for laparoscopic Roux-en-Y gastric bypass: leak test and beyond. Surg Laparosc Endosc Percutan Tech. 2010;20:424–7. doi: 10.1097/SLE.0b013e3182008e2c. [DOI] [PubMed] [Google Scholar]
- 27.Kligman MD. Intraoperative endoscopic pneumatic testing for gastrojejunal anastomotic integrity during laparoscopic Roux-en-Y gastric bypass. Surg Endosc. 2007;21:1403–5. doi: 10.1007/s00464-006-9175-7. [DOI] [PubMed] [Google Scholar]
- 28.Shin RB. Intraoperative endoscopic test resulting in no postoperative leaks from the gastric pouch and gastrojejunal anastomosis in 366 laparoscopic Roux-en-Y gastric bypasses. Obes Surg. 2004;14:1067–9. doi: 10.1381/0960892041975613. [DOI] [PubMed] [Google Scholar]
- 29.Alaedeen D, Madan AK, Ro CY, Khan KA, Martinez JM, Tichansky DS. Intraoperative endoscopy and leaks after laparoscopic Roux-en-Y gastric bypass. Am Surg. 2009;75:485–8. discussion 488. [PubMed] [Google Scholar]