Abstract
Glioblastoma (GBM) is the most frequent and lethal brain cancer. The lack of early detection methods, the presence of rapidly growing tumor cells and the high levels of recurrence due to chemo- and radio-resistance make this cancer an extremely difficult disease to treat. Emerging studies have focused on inhibiting AKT activation; here we demonstrate that in primary GBM tumor samples, full-dose inhibition of AKT activity leads to differential responses among samples in the context of cell death and self- renewal, reinforcing the notion that GBM is a heterogeneous disease. In contrast, low-dose AKT inhibition when combined with fractionation of radiation doses leads to a significant apoptosis-mediated cell death of primary patient-derived GBM cells. Therefore, low-dose targeted therapies might be better for radiosensitization of primary GBM cells and further allow for reducing the clinical toxicities often associated with targeting the AKT/PI3K/mTOR pathway. This work emphasizes the discrepancies between cell lines and primary tumors in drug testing, and indicates that there are salient differences between patients, highlighting the need for personalized medicine in treating high-grade glioma.
Keywords: Glioblastoma stem cells, AKT, radiation therapy, primary cells
Introduction
Glioblastoma (GBM) is the most frequent and lethal brain cancer. About 22,000 Americans are diagnosed with GBM annually (1). Lack of early detection methods and rapid growth kinetics, with most patients dying within 2 years; make this cancer especially deadly (2). Currently, the standard of care is safe maximal surgical resection followed by radiation therapy and concurrent and post-concurrent chemotherapy with the oral alkylating agent, temozolamide. However, recurrence is virtually universal and represents a serious impediment to patient survival. Even with the post-operative combination regimen, median survival is generally only extended by a few months. In the past twenty-five years, this cancer has seen only modest improvements in patient survival.
While the concept of cancer stem-like cells (CSCs) exists in leukemias, the evidence for the same phenomenon in solid tumors and especially in GBM has only been recently solidified with a lineage tracing study (3). CSCs recapitulate the properties of normal component lineages, and are characterized by three defining hallmarks: self-renewal, ability to differentiate into multiple lineages and maintenance of multipotency. GBM contains cellular niches with phenotypic properties supporting self-renewal (3-5), survival under hypoxic conditions (6) and resistance to radiation-induced DNA damage (7, 8). Accordingly, the importance of CSCs is derived from their potentially critical role(s) in tumor initiation and response to therapy. CSCs can be modeled in vitro using the sphere-forming potential with cells derived from these spheres that potently induce tumors in mice (4, 9). The frequent GBM recurrence is derived in large by the marked radio- and chemo-resistance. Therapeutic resistance is likely due to multiple factors within the GBM tumor, but several studies suggested that subpopulations of cancer cells in GBM (i.e. Brain cancer stem-like cells or BCSCs) are highly resistant to radiation and chemotherapies (2, 10).
Since GBMs are generally poorly differentiated and contain morphologically distinct cells, it appears to fit with the model of BCSCs (3, 11, 12). Furthermore, a classification scheme established by The Cancer Genome Atlas (TCGA) demonstrated that GBMs can be transcriptionally clustered into one of 4 subtypes; proneural, neural, classical and mesenchymal subtypes. Therefore suggesting that malignant lineages can potentially be derived from both phenotypically-diverse tumor-initiating cells (13) including adult neural stem cells (NSCs) (14), progenitor cells (15), or even dedifferentiated neurons (16), and distinct signaling axes with core defects primarily in tyrosine kinase receptor, anti-apoptotic, and cell cycle regulatory pathways (17). Most recently, single cell RNA-sequencing of a number of GBM tumors demonstrated the presence of multiple subtypes of single tumor cells within each tumor suggesting that while population studies detect dominant transcriptional programs in GBM, diverse intratumor subtype heterogeneity is may be a key biological feature of GBM (18).
The study of BCSCs is of high clinical importance due to their roles in radio- and chemo-resistance. It was suggested that the subfraction of CD133+ putative BCSCs survive radiation treatment better than their CD133- counterpart mostly due to enhanced DNA repair capabilities (7). The ability of CSCs to self-protect from radiation-induced cell death has been further attributed to upregulation of genes that scavenge free radicals and reduce the levels of oxidative stress–induced damage, a common consequence of radiation (19, 20). As radiation remains the primary post-operative therapy for GBM patients, it is important that we focus on potentially resistant BCSCs to reduce post-therapy recurrence, despite of BCSCs being phenotypically and molecular may be a moving target.
The AKT serine/threonine kinase family, consisting of AKT-1, AKT-2 and AKT-3, is an integral part of the PI3K growth and apoptosis pathway. Aberrant AKT activation and signaling is common in GBM (21) and was linked to GBM progression as demonstrated by conversion of grade III anaplastic astrocytoma to grade IV GBM in an in vitro model (22). Similarly, hyperactivation of AKT signaling was associated with worse progression-free and overall survival in GBM patients (23, 24). It is therefore critical to evaluate AKT inhibitors in the context of BCSCs in GBM. Indeed, several reports have demonstrated that inhibition of AKT is an effective radiosensitizing mechanism (25, 26) that also reduces the CSC population in the non-heterogeneous GBM cell lines by increasing their rates of apoptosis and reducing sphere formation (27, 28). In the present study, we examined the effects of a pharmacological AKT inhibitor in combination with radiation on primary GBM samples grown under serum-free conditions that promote BCSC sphere phenotype (4, 9, 18), or expanded in adherent monolayers in differentiation conditions (9, 29). The combination of AKT inhibition and radiation was moderately effective in inducing cell death and inhibiting tumorigenesis in a number of the primary tumors forced to differentiate and in reducing levels of NESTIN, a NSC marker, but was not efficacious in reducing another surrogate marker of stemness, secondary neurospheres. These studies highlight the importance of tailoring targeted therapies against BCSCs through utilizing precision cancer medicine approaches.
Materials and Methods
Culture of human GBM cells
De-identified primary human tumor samples were obtained from GBM patients undergoing craniotomy resection at Robert Wood Johnson University Hospital under an IRB approved protocol. Cells were obtained through mechanical dissociation of the tumor tissue using a blade and plated in DMEM/F12 medium in the presence of B-27 supplement, 20 ng/ml of both human recombinant EGF and human recombinant FGF. The following day, the culture was collected, incubated with Accutase at 37°C and passed through a needle to obtain a single cell suspension and re-plated in the same supplemented medium. Upon reaching confluency, half of the neurospheres were plated into EMEM with serum to create an adherent monolayer population.
Immunohistochemistry (IHC)
Primary antibodies were first optimized on control tissues using Ventana Medical Systems Discovery XT automated immunostainer. Slides were deparaffinized and antigen retrieval was performed using CC1 (Cell Conditioning Solution, Ventana Medical Systems, Cat# 950-124). Primary antibodies were applied at the indicated dilutions and slides were incubated at 37°C, then slides were incubated with the secondary antibodies followed by chromogenic detection kit DABMap (Ventana Medical Systems). Slides were counterstained with hematoxylin, dehydrated and cleared before cover slipping. Antibodies used, dilutions and incubation times are provided in Supplementary Table 1.
Treatment of GBM cells
The following treatment scheme was used in all experiments unless stated otherwise: cells were either untreated (UT) as controls, or treated with 1μM of AKT inhibitor (AKT Inhibitor VIII, isosyme selective, AKT1/2, Calbiochem) (AKT-i), were irradiated one hour later at 3 Gy in a Gamma-cell 40 Exactor (MDS Nordion), or received the combination of AKT-i and radiation. Treatment was repeated for 5 consecutive days; fresh drug was added to new medium each day.
MTT cell viability assay
Cells were seeded in 96 well plates and exposed to the drug and radiation the following day for 5 consecutive days. After 48 hours of end of treatment, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma) was added to wells and incubated at 37°C; crystals were dissolved in DMSO and absorbance was measured on a fluorescent plate reader at 570 nm to determine the inhibitory concentrations that kill 50% of the cells (IC50).
Clonogenic survival assay
Cells were seeded at various dilutions into 10 cm2 tissue culture dishes and allowed to attach overnight. One μM of AKT-i was added one hour prior to irradiation at various doses. Colonies were allowed to grow for 14 days before staining with Methylene Blue and were then counted. Colonies were defined as clusters of greater than 50 cells. Survival fraction is defined as the total number of clones in irradiated cells divided by the total number of clones in identical, nonirradiated cells and reported in log scale.
Soft agar colony formation assays
Cells at a density of 1.5 × 104 growing as a monolayer were seeded in a 0.35% soft agar layer plated on top of a 0.75% hard agar base. The following day, cells were subjected to the 5 day treatment scheme, after which, cells were washed and fresh, plain media was added every other day for 10 days before the cells were imaged.
Immunofluorescence
Chamber slides were coated with poly-ornithine for one hour at 37°C and subsequently coated with laminin for one hour at 37°C. Cells growing in neurospheres media were plated and allowed to attach overnight before starting a 5-day treatment scheme. At the specified time point, immunofluorescence staining was performed; briefly: cells were fixed in Formaldehyde - Fresh (Fischer Scientific), washed and permeabilized in a Triton-X-100 PBS solution followed by an hour blocking period in a PBS solution containing 4% BSA and 1% FBS. Primary antibodies were incubated overnight at 4°C in blocking solution followed by incubation with secondary antibodies for one hour at room temperature. Slides were mounted with hard set mounting media with DAPI (Vector Shield) before imaging with a fluorescent microscope (Zeiss AxioObserver).
Secondary neurosphere formation assay
Cells from primary tumors maintained as neurospheres were grown in 6 well plates and treated for 5 days. After 48 hours, spheres were dissected into a single cell suspension using Accutase (Gibco) and a syringe-needle. Cells were seeded at a clonogenic density (30) (20 cells per well) into a 96 well plate; and number of secondary spheres formed per well was counted after 14 days.
Western blot
Cells or primary tumor tissues were suspended in cell lyses buffer (Cell Signaling) supplemented with protease inhibitor cocktail (Sigma) followed by a brief sonication. Western blotting was performed according manufacturer’s protocol (NuPAGE system, Invitrogen) using 25 μg of protein on 4-12% Bis-Tris gradient gels. Primary antibodies were incubated overnight at 4°C. Membranes were incubated with secondary antibody for one hour at room temperature; and all washes were performed in the SNAP i.d. system. Protein signals were detected using Pierce chemiluminescence method. Antibodies and dilutions used for western blotting are provided in Supplementary Table 1.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 6 (GraphPad Software Inc., www.graphpad.com). Data are presented as mean ± standard deviation (SD). Statistical significance was determined by student’s t-test or ANOVA (one-way or two-way) with Bonferroni post-hoc test, unless otherwise indicated. A p-value of <0.05 is considered statistically significant and represented by a single asterisk.
Results
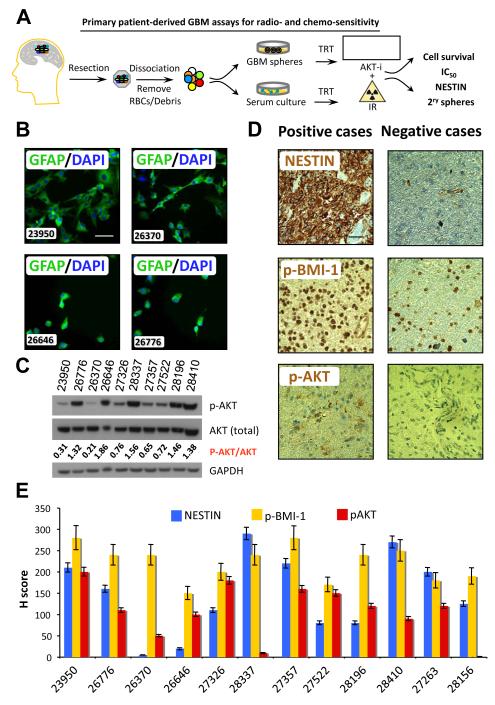
Fresh primary samples were received from patients undergoing surgical resection of WHO grade IV gliomas (GBM) at Robert Wood Johnson University Hospital. Cells were maintained under serum-free conditions that promote BCSC sphere phenotype (4, 9, 18), or in adherent monolayers in differentiation conditions (9, 29) (Fig. 1A). Primary cells maintained in adherent monolayers in the presence of serum were positive for GFAP confirming the retention of a hallmark glial protein in these cultures (Fig. 1B). This observation supported our assumption that even when cultured in adherent monolayers, these tumor-derived cells retained characteristic markers of high-grade GBM.
Figure 1.
Characterization of primary GBM samples. (A) Model for examining of treatment responses among patient-derived GBM cells cultured either in neurosphere or monolayer conditions and treated with AKT-i and/or IR. TRT, treatment. (B) GFAP expression (green) in cells cultured from primary tumors (marker of GBM) grown in serum and differentiated; cells were counterstained with DAPI. (C) Protein lysates derived from primary GBM were probed for phosphorylated AKT and total AKT by western blotting. (D) Tumors were fixed, sectioned and stained for NESTIN, phospho-BMI-1 (p-BMI-1), and phospho-AKT (p-AKT). Images were quantified for amount of positive staining, as well as for intensity of staining. Representative positive and negative cases are shown. (E) For quantitative analyses of IHC, H score is calculated as (% at 0) * 0 + (% at 1+) * 1 + (% at 2+) * 2 + (% at 3+) * 3 = Range 0 – 300 based on analyses of at least 10 fields per slide averaged by two qualified examiners.
AKT is a downstream serine/threonine kinase hub in the receptor tyrosine kinase (RTK)/PTEN/PI3K pathway that amplifies growth signals, regulate mTOR signaling and phosphorylates BMI-1 therefore enhancing their oncogenic activities (31-33). Constitutively active and phosphorylated AKT in GBM cells, usually resulting from mutated or lost PTEN, leads to uncontrolled growth, evasion of apoptosis and GBM tumor invasion (21). Therefore, inhibition of AKT is an attractive target for GBM therapy (33). We first investigated the levels of active p-AKT in our GBM samples. The majority of tumors had a detectable; yet culture condition-dependent, p-AKT expression (Fig. 1C), providing a strong rationale for targeting PI3K/AKT in high-grade GBM. To examine the expression of NSC markers that are associated with GBM stem-like cell self-renewal and tumor initiation (3), sequential sections of fresh tumors were subjected to immunostaining for NESTIN, p-BMI-1 and p-AKT by immunohistochemistry (IHC) (Fig. 1D). The number of positive cells as well as the intensity of the staining of NESTIN, p-BMI-1 and p-AKT were scored (Table 1 and Fig. 1E). From IHC analysis, 10 out of 12 GBM tumors collected expressed p-AKT with varying degrees of intensity (amount of protein present). BMI-1 expression was associated with GBM stem-like cell self-renewal (34, 35), radiation resistance (36), apoptotic resistance (37), has previously been shown to correlate with higher grade GBM and worse prognosis (37, 38), and activated p-BMI-1 was indeed expressed in all samples. NESTIN, which was utilized as a marker of NSCs that may have aggressive BCSC features (3, 39), was expressed in over 80% of GBM tumors, and when expressed was present in very high quantities. Additionally, patient characteristics derived from pathologic reports included age, gender, the expression of glial acidic fibrillary protein (GFAP), the proliferation marker Ki67, the presence of the R132H IDH-1 mutation by IHC, amplification of EGFR and the loss of the tumor suppressor PTEN as measured by FISH probes (Table 1).
Table 1.
Primary GBM patient characteristics.
| Patient code |
Age | M/F | GFAP +ve |
Ki67 % | IDH1 +ve |
EGFR amp. |
PTEN loss |
p-AKT score |
p-AKT | BMI-1 score |
BMI-1 intensity |
NESTIN score |
NESTIN intensity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 23950 | 75 | F | Yes | 40% | ND | No | 84% | 1 | 3 | 3 | 3 | 2 | 3 |
| 26370 | 39 | M | Yes | <5% | ND | ND | ND | 1 | 3 | 3 | 3 | 0 | 0 |
| 26646 | 46 | M | Yes | <10% | No | ND | 60% | 0-1 | 3 | 3 | 2-3 | <1% | 3 |
| 26776 | 77 | F | Yes | 8% | ND | ND | ND | 0 | 0 | 3 | 3 | 2 | 3 |
| 27263 | 45 | M | Yes | 35% | No | Yes | 85% | 1 | 1 | 1 | 2 | 1 | 3 |
| 27326 | 66 | M | Yes | ~50% | No | Yes | 42% | <1% | 0 | 3 | 3 | 1 | 3 |
| 27357 | 55 | F | Yes | 50% | No | No | 57% | 0 | 0 | 3 | 3 | 2 | 3 |
| 27522 | 61 | M | Yes | ~20% | No | ND | ND | 1 | 2 | 2 | 2 | 1 | 3 |
| 28156 | 56 | M | Yes | <10% | No | ND | ND | 0 | 0 | 3 | 2 | 1 | 3 |
| 28196 | 59 | F | Yes | 10-40% | No | Yes | 60% | 1 | 3 | 3 | 3 | 1 | 3 |
| 28337 | 57 | M | Yes | 20% | No | ND | ND | 0 | 0 | 2 | 2-3 | 3 | 3 |
| 28410 | 76 | M | Yes | ~10% | No | No | 73% | 1 | 2-3 | 3 | 3 | 3 | 3 |
Patient ID codes are indicated in the left column. Analyses for IDH1 positive GBM cells, EGFR amplification (amp.) and PTEN loss were performed based on routine clinical diagnostic tests on samples from GBM biopsies including Fluorescent In Situ Hybridization (FISH) analyses when possible based on the amount of tissues available. ND, not done. The expressions of BMI-1, NESTIN and p-AKT were evaluated by IHC analyses.
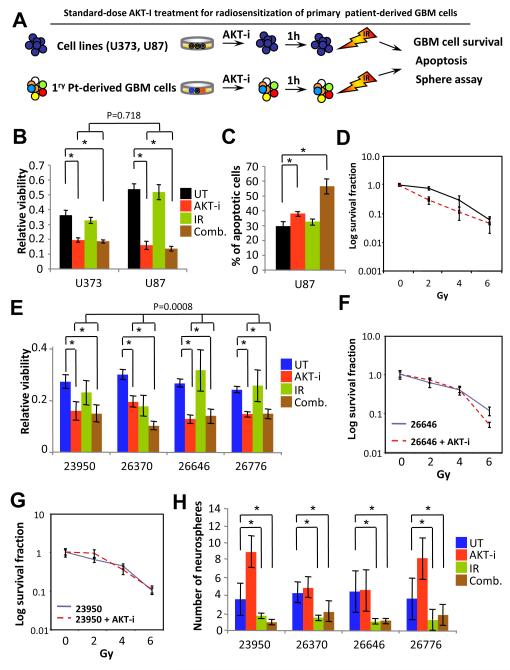
In order to assess the effects of the AKT inhibitor on patient-derived primary GBM cells, we performed MTT assays to measure cell survival. The IC50 after 24-hour of treatment with the AKT inhibitor was 5 μM and a time course analyses with protein lysates revealed that p-AKT was most inhibited one hour post-treatment (data not shown). The current standard of care for newly diagnosed GBM is surgical resection followed by radiotherapy plus Temozolomide (40), however, resistance to therapy and recurrence is virtually universal. Given the extreme genetic and biological heterogeneity of GBM cells (18), it is likely that monotherapy would not be effective, let alone its potential to select for resistant clones driving recurrence (3), and that combination of targeted therapies would be essential to eradicate GBM cells. To develop a combination therapy strategy taking advantage of the findings that AKT may be playing a role in cyto-protection and DNA damage repair (41), thus conferring a survival advantage, we utilized a clinically relevant strategy (Fig. 2A) by treating either established GBM cell lines or patient-derived primary GBM cells with 3 Gy of radiation at one hour post-AKT-i treatment when lower levels of p-AKT would render cells more vulnerable to DNA damage and double-strand breaks. Cell survival was measured by MTT assays at 24-hour post radiation treatment. Interestingly, with a single dose, the combination treatment was not more effective than treating the cells with AKT-i alone (Fig. 2B), but resulted in greater number of apoptotic cells (Fig. 2C), suggesting that AKT-i might render the radioresistant GBM cells more apoptotic. Furthermore, to assess the effects of AKT-i on the ability of cells from GBM cell lines in the presence of radiation, a clonogenic survival assay was performed with and without the inhibitor in combination with increasing single fractions of radiation (Fig. 2D). In this clonogenic survival assay, long-term survival of cells from GBM cell lines was significantly impaired in the combination treatment when compared to radiation alone (Supplementary Table 2) but treatment effects were not different between U373 and U87 cells (p= 0.17). To examine the effects of this therapeutic strategy in primary GBM cells, primary tumor cells were subjected to the same treatment parameters as cell lines and evaluated for cell survival (Fig. 2E). In contrast to the effects of AKT-i on the homogeneous cells from GBM cell lines, overall analyses for difference in treatment effects among primary GBM cells was highly significant (p=0.0008) (Supplementary Table 3-4). While radiation alone failed, the combination of the AKT-i and radiation significantly enhanced killing of primary patient-derived cells from GBM tumors 23950, 26370, 26646 and 26776 (Fig. 2E). Furthermore, clonogenic survival assays of cells from two of these tumors that formed colonies were then used to perform clonogenic assay. Notably, sensitivity to radiation when the AKT–i was used was seen in long-term survival assays only when the AKT-i was combined with higher radiation doses at 6 Gy, and significantly enhanced killing of primary patient-derived cells from GBM tumor 26646. In contrast, the combination of AKT-i and lower radiation doses (e.g. 2 Gy) was significantly radioprotective of primary patient-derived cells from GBM tumors 23950 and 26646 (Fig. 2F-G). Moreover, the combination of AKT-i and radiation significantly reduced the number of neurospheres derived from primary patient-derived GBM cells, an effect that might be attributed to IR, when compared to untreated or AKT-i treatment only (Fig. 2H). These findings reveal inherited radiosensitivity features in GBM cells that may explain treatment failures among primary GBM cells, but not when treatment effects among established cells lines are considered, highlighting the biological differences between cell lines and primary GBM tumors that underscore the needs for better in vitro drug discovery models.
Figure 2.
Differential treatment responses of primary GBM samples. (A) Model for examining standard AKT-i treatment responses among established cell lines and patient-derived GBM cells cultured and treated with AKT-i and/or IR. (B) MTT assay with U87 and U373 cells to measure short-term viability. (C) Apoptosis analysis of Annexin V and PI positive U87 cells after treatment analyzed with flow cytometry. (D) Log survival curve demonstrating the long-term clonogenic survival of U373 cells treated with 5 μM of AKT-i and increasing doses of radiation. (E) MTT assays with patient-derived GBM cells to measure short-term viability. (F) Comparison of long-term clonogenic survival of 26646 patient-derived GBM cells treated with 5 μM of AKT-i and increasing doses of radiation. (G) Comparison of long-term clonogenic survival of 26646 patient-derived GBM cells treated with 5 μM of AKT-i and increasing doses of radiation. Note the radioprotective effects at low radiation doses. (H). Number of clonally derived neurospheres derived from treated patient-derived GBM cells. While AKT-i treatment might have increased the number of neurospheres derived from 23950 and 26776 patient-derived GBM cells, the combination of AKT-i and radiation significantly reduced the number of neurospheres from all 4 patient-derived GBM cells when compared to AKT-i treatment (p<0.001).
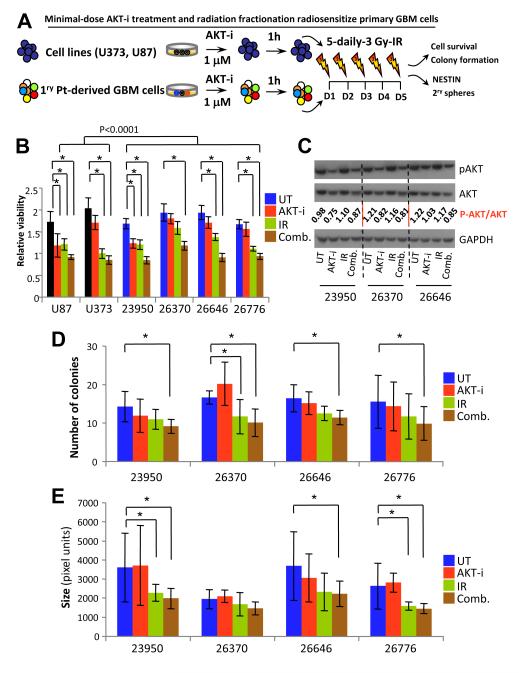
Since most GBM patients receive a regimen involving multiple doses of chemotherapy and radiation fractionation, we attempted to simulate the clinical settings by modulating the doses and frequency of AKT-i use. Given the shallow dose-response curves of drugs targeting the AKT/PI3K/mTOR pathway that have recently been revealed (42), and that single-cell analyses correlated these responses with significant and heritable cell-to-cell variability (42) and subtype heterogeneity (18), we revised a clinically-feasible regimen where cells derived from primary GBM cultures were treated with a minimal 1 μM of AKT-i followed by 3 Gy IR one hour later, every day for 5 days (Fig. 3A). At 48 hour after this 5-day treatment regimen, relative cell survival was determined using an MTT assay (Fig. 3B). In cells derived from established cell lines and primary patient GBM samples, treatment with this low-dose AKT-i by itself did not cause appreciable amounts of cell death compared to the untreated control (Fig. 3B). In the cells treated with 3 Gy IR only for all 5 days, most of cells derived from primary patient GBM samples, but not those from established cell lines, showed a significant amount of cell death (Fig.3B). However, the combination IR and AKT-i resulted in a more significant decrease in cellular viability compared to the untreated control from all GBM cells (p<0.0001) (Supplementary Table 4). These data suggest the intriguing possibility that lower doses of certain targeted therapies such as those targeting the AKT/PI3K/mTOR pathway might be better for radiosensitization. Lysates were collected from these samples and were probed for levels of total and p-AKT (Fig. 3C). These analyses demonstrated that low-dose AKT-i resulted in inhibition of p-AKT, without an appreciable cell killing, IR had no effects on p-AKT, while combination therapy that resulted in a significant inhibition of GBM cell survival was associated with a reduction of p-AKT, however, levels of pan-AKT also appeared to be slightly affected (Fig. 3C). By nature, the clonogenic survival assay measures the effect of a single dose of radiation on cell survival and growth; we instead utilized the soft agar colony formation assay as alternative method of assessing long-term growth from an initially low number of cells (43). Cells were seeded in soft agar and were treated daily with 1 μM Akt inhibitor and 3 Gy of IR for 5 days; colony formation was assayed 10-15 days after the last treatment. The numbers of colonies formed in single treatment groups, as well as with combination treatments were counted. In most cells derived from primary patient GBM samples, there was a significant reduction in colonies derived from cells treated with combination of AKT-i and IR (1, 3 and 5 Gys) compared to untreated controls (p<0.0001) (Fig. 3D and Supplementary Table 5). There was also a statistically significant difference between cells treated with IR alone (3 and 5 Gys) compared to cells treated with combination therapy (p<0.017) (Supplementary Table 5). In addition, we examined the size of the colonies derived from different treatments. Combination therapy was effective in significantly reducing the size of colonies formed in soft agar (p<0.0001) (Fig. 3E), at levels more significant than the colony size reduction achieved with IR only (Supplementary Table 6). These data suggest that a repeated low-dose AKT-i may be more effective in inducing death of cancer cells when combined with IR, but importantly this regimen might reduce the toxicity often associated with chemotherapy and targeting the AKT/PI3K/mTOR pathway. In addition, it appears that the combination therapy is effective in reducing the number of cells capable of initiating colonies in soft agar tumorigenicity assay, while IR by itself is effective in decreasing the bulk (size) of colonies formed by GBM cells.
Figure 3.
Treatment responses of minimal-dose AKT-i in primary GBM cells. (A) Model for examining minimal-dose AKT-i treatment responses among established cell lines and patient-derived GBM cells cultured and treated with 1 μM AKT-i and/or IR. (B) Tumor cells were treated with AKT-i followed by 3 Gy irradiation daily for 5 days, relative survival was measured with an MTT assay, n = 36 wells per treatment, each experiment was repeated thrice. MTT assays with U87 and U373 cells and patient-derived GBM cells were used to measure short-term viability. (C) Protein lysates derived from primary GBM cells treated with minimal-dose AKT-i were probed for phosphorylated AKT and total AKT in tumors cells treated with the 5 day scheme by western blotting. (D) Clonal numbers of tumor cells were seeded as single cells in soft agar and were treated with the 5 day regimen; images were collected 15 days later and number of colonies was counted; each treatment was repeated in triplicate wells and experiment was repeated three times. (E) Graph depicts average size of the colonies formed growing in soft agar.
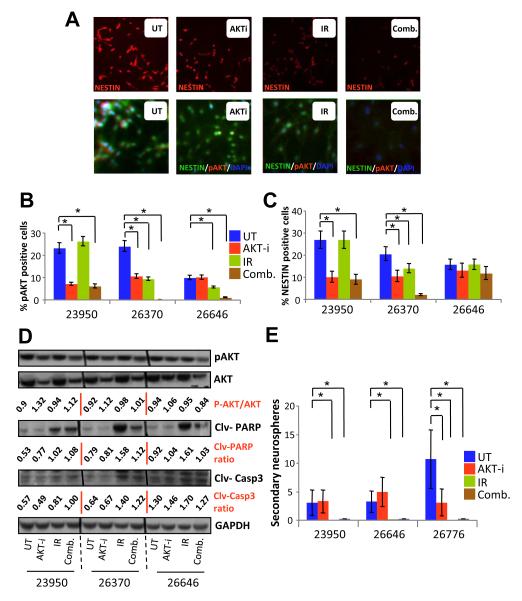
Since AKT is involved in the maintenance of a de-differentiated “stem-like” state (44), cells derived from primary patient GBM samples were plated onto chamber slides and subjected to the 5-day treatment regimen. At 48-hour after the last “dose”, cells were fixed, stained and analyzed for NESTIN and p-AKT expression (Fig. 4A-C). IR alone did not affect the levels of NESTIN. In contrast, combination treatment was effective in reducing the levels of NESTIN in all three primary patient GBM samples examined when compared to untreated cells (p<0.041) (Supplementary Table 7). Moreover, combination treatment was significantly more effective than AKT-i alone for GBM tumor 26370 (Fig. 4C). These treatment effects were associated with apoptotic changes, an effect that might be attributed to IR, as demonstrated by increased cleaved PARP and cleaved CASPASE-3 in lysates from treated cells (Fig. 4D). To assess the stem cell-like potential of treated versus untreated cells, we utilized the secondary re-population assay as a functional assay rather than selecting for phenotypic BCSC surface markers that are dependent on their niche and culture conditions. Tumor cells were again treated for 5 days and were then re-plated in fresh media at clonogenic concentration of 20 cells/well in 96-well plates (Fig. 4E). Interestingly, cells which had initially only been treated with IR or combination therapy lost their ability to form secondary spheres (Fig 4E). These studies reveal that combining AKT inhibition with IR effectively decreases the stemness of BCSCs as suggested by reducing NESTIN expression and secondary neurosphere formation. Collectively, our data suggest that lower doses of certain targeted therapies such as AKT-i might be better for radiosensitization of primary GBM cells and afford the opportunity to reduce the toxicities often associated with chemotherapy and targeting the AKT/PI3K/mTOR pathway.
Figure 4.
Expression markers and neurosphere responses of minimal-dose AKT-i in primary GBM cells. (A) Representative images of cells plated on chamber slides were treated every day for 5 days with AKT-i and radiation; 48 hours later, cells were fixed and stained for NESTIN, p-AKT and DAPI for nuclear staining. (B) Quantitation of p-AKT expression per 100 cells per treatment from experiments that were repeated thrice. (C) Quantitation of NESTIN expression per 100 cells per treatment from experiments that were repeated thrice. (D) Protein lysates derived from untreated and treated primary GBM cells were probed for p-AKT, total AKT, cleaved PARP and cleaved caspase 3 against GAPDH as a control by western blotting. (E) Self-renewal of GBMs as represented by number of secondary neurosphere formation from cells treated for 5 days.
Discussion
Our study highlights some of the myriad biological differences between cell lines and primary tumor specimens as well as the genetic differences between tumors from different patients. A study utilizing data from The Cancer Genome Atlas categorized GBM tumors into four main subtypes based on molecular profiling (13), however other studies asserted that there could be distinct variability within a single tumor and even the presence of multiple subtypes of single GBM tumor cells (18, 45, 46). Intratumor heterogeneity may arise from the divergent phenotypic and genetic profiles of BCSCs (3, 11, 47, 48), furthermore, multiple stem cell clones can maintain distinct populations within the same tumor (intra-tumor heterogeneity) (49) that interact with each other, providing support and complex signaling (46). These nuances make an already challenging cancer to manage even more of a complex signaling maze to target. Therefore, the predicted heterogeneity of chemoresistance requires better in vitro models before investing in more time- and resource-consuming drug screening assays and mouse models, but it is further clear that the distinctions between established cell lines and cells isolated from primary tumor samples can be profound (50). Molecular and/or pharmacological studies done in cell lines could potentially lead to increased failures in the clinic and time lost in finding effective therapeutic strategies. Here, we were able to establish several primary tumors as cells in culture and were able to characterize them for key traits of GBM; they all expressed GFAP, a classic marker of GBM, albeit at different levels. Cells derived from primary GBM tumors predominantly expressed high levels of NESTIN (10/12), reiterating the highly undifferentiated nature of grade IV glioma and corroborating other reports of increased NESTIN expression in higher tumor grades (39, 51). The same was true for the expression of BMI-1, a critical BCSC self renewal regulator; all of our samples highly expressed BMI-1 (12/12) corroborating data correlating increasing BMI-1 levels with higher tumor grades (37). We also found that levels of phosphorylated/active AKT vary between GBM tumors. Differing expressions of p-AKT have been reported, with fifty percent of tumors expressed this activated form (52). Yet another group reported dissimilar levels within a single tumor (21). Fifty percent of our samples displayed at least some p-AKT (6/12), validating that p-AKT is indeed an important target in GBM. AKT is associated with oncogenic activities in many cancers types including GBM (27, 53-56). It has been a therapeutic target with attempts to utilize AKT inhibitors in clinical trials (57). Yet another reason to block AKT in GBM is related to its role in DNA damage repair (41, 58), making it a rational target for combination with radiation therapy.
Noting that radiation is the main treatment modality for GBM, we investigated the effects of combining a pan inhibitor of AKT with radiation therapy. Similar to other reports (25-28), we were able to show that GBM cell lines, U373 and U87, were sensitive to a single treatment of 5 μM of a commercially available AKT inhibitor. Moreover, they were not affected by a single fraction of 3 Gy of radiation or radiosensitized with the AKT inhibitor. However, in long-term clonogenic survival assays, there was a modest but significant decrease in survival in cells treated with the combination. When examining the more clinically relevant primary GBM samples, only one out of 4 of GBM tumors had 50% cell death with the same concentration of 5 μM of the inhibitor but all had modest decrease in survival when treated with the combination compared to the untreated. Interestingly, long term clonogenic survival showed no difference between 2 different tumors treated with the inhibitor and radiation compared to just increasing fractions of radiation and there was no difference in secondary neurosphere formation in any of tumors when AKT was inhibited. In fact, two of the tumor cells had on average, double the number of spheres. Radiation treatment was more effective in reducing the self-renewal potential compared to the untreated control. This hints that the levels of activated AKT might be higher in tumors than in cell lines and also that there are probably more complex signaling pathways and feedback loops in GBM tumors than cell lines. These data are in contrast with some of the work demonstrating the utility of inhibiting AKT in combination with radiation (25, 41, 59). However, all of these studies have used monolayer culture maintained cell lines that are not as accurate of a representation of patient tumors. Another caveat that might explain the difference is the fact that established cell lines are grown in media with serum, whereas our GBM tumor cells were grown in serum-free media. It is plausible that cells growing in serum, which are necessarily more differentiated, are susceptible to ionizing radiation that targets rapidly diving cells. Indeed, a key study supports these findings by demonstrating dynamic parameters related to AKT inhibitor response and variability between individual cells (42).
Since single dose AKT inhibition and radiation did impact cell survival and BCSCs are traditionally radiation resistant (7, 60), we provide evidence for the first time, to the best of our knowledge, that a repeated dose scheme may be used to target BCSCs and may hint that these particular cells could be susceptible to repeated radiation fractions. This finding warrants further investigation in clinically relevant clonal assays. After reducing the daily dose of the AKT inhibitor to only a fifth of the IC50 concentration, we found a radiosensitization effect in all 4 tumors, and decreases in both number and size of colonies with combination treatment. It was interesting to us that while the levels of p-AKT was indeed diminished in those treated every day for 5 days with the inhibitor as expected, total AKT decreased as well. Speculatively, it is possible that inhibiting activated AKT feeds back as a negative regulator of the total levels of protein as well, either directly or through interacting proteins such as BMI-1.
We have chosen to investigate two “stemness” parameters, NESTIN expression and secondary neurosphere formation, rather than using any specific cell surface marker for BCSCs. In 3 out of the 4 tumors tested, the AKT inhibitor significantly decreased the number of cells expressing NESTIN, however, a synergistic effect with radiation was only present in one tumor, 26370. To assess the effects of this treatment on BCSCs, we employed two different measures of “stemness” that are not specific to any single marker; however, although both 23950 and 26370 had a significant decrease in NESTIN expression, there was no effect on their ability to form secondary neurospheres. Although aggregates were removed and cells were re-plated for growth as secondary spheres at a clonal density, other factors such as the ability to detect quiescent stem cells might limit the interpretation of this assay (61). In addition, there is a possibility that culture conditions might modulate the proportion of stem-like cells and may not be an accurate reflection of BCSCs in vivo (61). These data are leading us to believe that a more stringent and consistent functional parameters of stem-cell properties need to be developed.
For better management of GBM, it is critical to examine additional combination therapeutic strategies based on differences in survival pathways. Given that GBM is a heterogeneous disease (13), it is plausible to classify GBM tumors into subtypes based on their corresponding in vitro chemo- and radio-sensitivity profiles in order to develop tailored mono- and combination targeted therapies using precision cancer medicine approaches (49). The establishment of multiple primary GBM patient derived cells here bring us closer towards achieving the goal of developing new therapeutic strategies to improve survival of GBM patients.
Supplementary Material
Acknowledgements
We thank Drs. Vassiliki Karantza and Shridar Ganesan (Rutgers Cancer Institute of New Jersey) for the helpful discussion. We thank Devora Schiff for technical assistance with primary samples, Biospecimen Repository Service (BRS) at Rutgers Cancer Institute of New Jersey and RWJUH for help with GBM sample acquisition and Dr. Sinai Kim (Rutgers Cancer Institute of New Jersey Biometric Services) for statistical analyses. We are grateful to S. Nimer (University of Florida) for providing the p-BMI-1 antibody.
Grant support: This project has been supported by National Science Foundation IGERT program (Predoctoral fellowship to M. Mehta), Cancer Center Support Grant from the National Cancer Institute (P30 CA072720), Rutgers Cancer Institute of New Jersey (New Investigator Award to H.E. Sabaawy), and in part by Breast Cancer Foundation (Grant to B.G. Haffty).
Footnotes
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
“The authors disclose no potential conflicts of interest.”
References
- 1.Dunn GP, Rinne ML, Wykosky J, Genovese G, Quayle SN, Dunn IF, et al. Emerging insights into the molecular and cellular basis of glioblastoma. Genes Dev. 2012;26:756–84. doi: 10.1101/gad.187922.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–6. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 5.Chen R, Nishimura MC, Bumbaca SM, Kharbanda S, Forrest WF, Kasman IM, et al. A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer Cell. 2010;17:362–75. doi: 10.1016/j.ccr.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–13. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 8.Bhat KP, Balasubramaniyan V, Vaillant B, Ezhilarasan R, Hummelink K, Hollingsworth F, et al. Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer Cell. 2013;24:331–46. doi: 10.1016/j.ccr.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 10.Sakariassen PO, Immervoll H, Chekenya M. Cancer stem cells as mediators of treatment resistance in brain tumors: status and controversies. Neoplasia. 2007;9:882–92. doi: 10.1593/neo.07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 12.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–21. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 13.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zong H, Verhaak RG, Canoll P. The cellular origin for malignant glioma and prospects for clinical advancements. Expert Rev Mol Diagn. 2012;12:383–94. doi: 10.1586/erm.12.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugiarto S, Persson AI, Munoz EG, Waldhuber M, Lamagna C, Andor N, et al. Asymmetry-defective oligodendrocyte progenitors are glioma precursors. Cancer Cell. 2011;20:328–40. doi: 10.1016/j.ccr.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedmann-Morvinski D, Bushong EA, Ke E, Soda Y, Marumoto T, Singer O, et al. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science. 2012;338:1080–4. doi: 10.1126/science.1226929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, McKay RM, Parada LF. Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell. 2012;149:36–47. doi: 10.1016/j.cell.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamal M, Rath BH, Williams ES, Camphausen K, Tofilon PJ. Microenvironmental regulation of glioblastoma radioresponse. Clin Cancer Res. 2010;16:6049–59. doi: 10.1158/1078-0432.CCR-10-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–3. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riemenschneider MJ, Betensky RA, Pasedag SM, Louis DN. AKT activation in human glioblastomas enhances proliferation via TSC2 and S6 kinase signaling. Cancer Res. 2006;66:5618–23. doi: 10.1158/0008-5472.CAN-06-0364. [DOI] [PubMed] [Google Scholar]
- 22.Sonoda Y, Ozawa T, Aldape KD, Deen DF, Berger MS, Pieper RO. Akt pathway activation converts anaplastic astrocytoma to glioblastoma multiforme in a human astrocyte model of glioma. Cancer research. 2001;61:6674–8. [PubMed] [Google Scholar]
- 23.Chakravarti A, Zhai G, Suzuki Y, Sarkesh S, Black PM, Muzikansky A, et al. The prognostic significance of phosphatidylinositol 3-kinase pathway activation in human gliomas. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:1926–33. doi: 10.1200/JCO.2004.07.193. [DOI] [PubMed] [Google Scholar]
- 24.Matsutani T, Nagai Y, Mine S, Murai H, Saeki N, Iwadate Y. Akt/protein kinase B overexpression as an accurate prognostic marker in adult diffuse astrocytoma. Acta Neurochir (Wien) 2009;151:263–8. doi: 10.1007/s00701-009-0199-3. discussion 8. [DOI] [PubMed] [Google Scholar]
- 25.Li HF, Kim JS, Waldman T. Radiation-induced Akt activation modulates radioresistance in human glioblastoma cells. Radiat Oncol. 2009;4:43. doi: 10.1186/1748-717X-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujiwara K, Iwado E, Mills GB, Sawaya R, Kondo S, Kondo Y. Akt inhibitor shows anticancer and radiosensitizing effects in malignant glioma cells by inducing autophagy. Int J Oncol. 2007;31:753–60. [PubMed] [Google Scholar]
- 27.Gallia GL, Tyler BM, Hann CL, Siu IM, Giranda VL, Vescovi AL, et al. Inhibition of Akt inhibits growth of glioblastoma and glioblastoma stem-like cells. Mol Cancer Ther. 2009;8:386–93. doi: 10.1158/1535-7163.MCT-08-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eyler CE, Foo WC, LaFiura KM, McLendon RE, Hjelmeland AB, Rich JN. Brain cancer stem cells display preferential sensitivity to Akt inhibition. Stem Cells. 2008;26:3027–36. doi: 10.1634/stemcells.2007-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–33. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 30.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–9. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 31.Nacerddine K, Beaudry JB, Ginjala V, Westerman B, Mattiroli F, Song JY, et al. Akt-mediated phosphorylation of Bmi1 modulates its oncogenic potential, E3 ligase activity, and DNA damage repair activity in mouse prostate cancer. J Clin Invest. 2012;122:1920–32. doi: 10.1172/JCI57477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Liu F, Yu H, Zhao X, Sashida G, Deblasio A, et al. Akt phosphorylates the transcriptional repressor bmi1 to block its effects on the tumor-suppressing ink4a-arf locus. Sci Signal. 2012;5:ra77. doi: 10.1126/scisignal.2003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDowell KA, Riggins GJ, Gallia GL. Targeting the AKT pathway in glioblastoma. Curr Pharm Des. 2011;17:2411–20. doi: 10.2174/138161211797249224. [DOI] [PubMed] [Google Scholar]
- 34.Zencak D, Lingbeek M, Kostic C, Tekaya M, Tanger E, Hornfeld D, et al. Bmi1 loss produces an increase in astroglial cells and a decrease in neural stem cell population and proliferation. J Neurosci. 2005;25:5774–83. doi: 10.1523/JNEUROSCI.3452-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdouh M, Facchino S, Chatoo W, Balasingam V, Ferreira J, Bernier G. BMI1 sustains human glioblastoma multiforme stem cell renewal. J Neurosci. 2009;29:8884–96. doi: 10.1523/JNEUROSCI.0968-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Facchino S, Abdouh M, Chatoo W, Bernier G. BMI1 confers radioresistance to normal and cancerous neural stem cells through recruitment of the DNA damage response machinery. J Neurosci. 2010;30:10096–111. doi: 10.1523/JNEUROSCI.1634-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Gong LY, Song LB, Jiang LL, Liu LP, Wu J, et al. Oncoprotein Bmi-1 renders apoptotic resistance to glioma cells through activation of the IKK-nuclear factor-kappaB Pathway. Am J Pathol. 2010;176:699–709. doi: 10.2353/ajpath.2010.090502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayry V, Tanner M, Blom T, Tynninen O, Roselli A, Ollikainen M, et al. Copy number alterations of the polycomb gene BMI1 in gliomas. Acta Neuropathol. 2008;116:97–102. doi: 10.1007/s00401-008-0376-0. [DOI] [PubMed] [Google Scholar]
- 39.Chinnaiyan P, Wang M, Rojiani AM, Tofilon PJ, Chakravarti A, Ang KK, et al. The prognostic value of nestin expression in newly diagnosed glioblastoma: report from the Radiation Therapy Oncology Group. Radiat Oncol. 2008;3:32. doi: 10.1186/1748-717X-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 41.Kao GD, Jiang Z, Fernandes AM, Gupta AK, Maity A. Inhibition of phosphatidylinositol-3-OH kinase/Akt signaling impairs DNA repair in glioblastoma cells following ionizing radiation. J Biol Chem. 2007;282:21206–12. doi: 10.1074/jbc.M703042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fallahi-Sichani M, Honarnejad S, Heiser LM, Gray JW, Sorger PK. Metrics other than potency reveal systematic variation in responses to cancer drugs. Nat Chem Biol. 2013;9:708–14. doi: 10.1038/nchembio.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Louis SA, Rietze RL, Deleyrolle L, Wagey RE, Thomas TE, Eaves AC, et al. Enumeration of neural stem and progenitor cells in the neural colony-forming cell assay. Stem Cells. 2008;26:988–96. doi: 10.1634/stemcells.2007-0867. [DOI] [PubMed] [Google Scholar]
- 44.Hambardzumyan D, Squatrito M, Carbajal E, Holland EC. Glioma formation, cancer stem cells, and akt signaling. Stem cell reviews. 2008;4:203–10. doi: 10.1007/s12015-008-9021-5. [DOI] [PubMed] [Google Scholar]
- 45.Sottoriva A, Spiteri I, Piccirillo SG, Touloumis A, Collins VP, Marioni JC, et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci U S A. 2013;110:4009–14. doi: 10.1073/pnas.1219747110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonavia R, Inda MM, Cavenee WK, Furnari FB. Heterogeneity maintenance in glioblastoma: a social network. Cancer Res. 2011;71:4055–60. doi: 10.1158/0008-5472.CAN-11-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, et al. CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–5. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 48.Zarkoob H, Taube JH, Singh SK, Mani SA, Kohandel M. Investigating the link between molecular subtypes of glioblastoma, epithelial-mesenchymal transition, and CD133 cell surface protein. PLoS One. 2013;8:e64169. doi: 10.1371/journal.pone.0064169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sabaawy HE. Genetic Heterogeneity and Clonal Evolution of Tumor Cells and their Impact on Precision Cancer Medicine. J Leuk (Los Angel) 2013;1:1000124. doi: 10.4172/2329-6917.1000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun. 2013;4:2126. doi: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang M, Song T, Yang L, Chen R, Wu L, Yang Z, et al. Nestin and CD133: valuable stem cell-specific markers for determining clinical outcome of glioma patients. J Exp Clin Cancer Res. 2008;27:85. doi: 10.1186/1756-9966-27-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haas-Kogan DA, Prados MD, Tihan T, Eberhard DA, Jelluma N, Arvold ND, et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005;97:880–7. doi: 10.1093/jnci/dji161. [DOI] [PubMed] [Google Scholar]
- 53.Baeza N, Weller M, Yonekawa Y, Kleihues P, Ohgaki H. PTEN methylation and expression in glioblastomas. Acta Neuropathol. 2003;106:479–85. doi: 10.1007/s00401-003-0748-4. [DOI] [PubMed] [Google Scholar]
- 54.Knobbe CB, Reifenberger G. Genetic alterations and aberrant expression of genes related to the phosphatidyl-inositol-3′-kinase/protein kinase B (Akt) signal transduction pathway in glioblastomas. Brain Pathol. 2003;13:507–18. doi: 10.1111/j.1750-3639.2003.tb00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molina JR, Hayashi Y, Stephens C, Georgescu MM. Invasive glioblastoma cells acquire stemness and increased Akt activation. Neoplasia. 2010;12:453–63. doi: 10.1593/neo.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954–63. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 57.Ihle NT, Powis G. Take your PIK: phosphatidylinositol 3-kinase inhibitors race through the clinic and toward cancer therapy. Mol Cancer Ther. 2009;8:1–9. doi: 10.1158/1535-7163.MCT-08-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Golding SE, Morgan RN, Adams BR, Hawkins AJ, Povirk LF, Valerie K. Pro-survival AKT and ERK signaling from EGFR and mutant EGFRvIII enhances DNA double-strand break repair in human glioma cells. Cancer Biol Ther. 2009;8:730–8. doi: 10.4161/cbt.8.8.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chautard E, Loubeau G, Tchirkov A, Chassagne J, Vermot-Desroches C, Morel L, et al. Akt signaling pathway: a target for radiosensitizing human malignant glioma. Neuro Oncol. 2010;12:434–43. doi: 10.1093/neuonc/nop059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rich JN. Cancer stem cells in radiation resistance. Cancer Res. 2007;67:8980–4. doi: 10.1158/0008-5472.CAN-07-0895. [DOI] [PubMed] [Google Scholar]
- 61.Pastrana E, Silva-Vargas V, Doetsch F. Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell Stem Cell. 2011;8:486–98. doi: 10.1016/j.stem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.