Abstract
Many abused drugs lead to changes in endogenous brain-derived neurotrophic factor (BDNF) expression in neural circuits responsible for addictive behaviors. BDNF is a known molecular mediator of memory consolidation processes, evident at both behavioral and neurophysiological levels. Specific neural circuits are responsible for storing and executing drug-procuring motor programs, whereas other neural circuits are responsible for the active suppression of these “seeking” systems. These seeking-circuits are established as associations are formed between drug-associated cues and the conditioned responses they elicit. Such conditioned responses (e.g. drug seeking) can be diminished either through a passive weakening of seeking-circuits or an active suppression of those circuits through extinction. Extinction learning occurs when the association between cues and drug are violated, for example, by cue exposure without the drug present. Cue exposure therapy has been proposed as a therapeutic avenue for the treatment of addictions. Here we explore the role of BDNF in extinction circuits, compared to seeking-circuits that “incubate” over prolonged withdrawal periods. We begin by discussing the role of BDNF in extinction memory for fear and cocaine-seeking behaviors, where extinction circuits overlap in infralimbic prefrontal cortex (PFC). We highlight the ability of estrogen to promote BDNF-like effects in hippocampal–prefrontal circuits and consider the role of sex differences in extinction and incubation of drug-seeking behaviors. Finally, we examine how opiates and alcohol “break the mold” in terms of BDNF function in extinction circuits.
Keywords: Cocaine, Alcohol, Opiates, Brain-derived neurotrophic factor, Extinction memory, Hippocampal–prefrontal systems
1. Introduction
It has long been recognized that addiction is a disorder of learning and memory (Berke and Hyman, 2000; Di Chiara et al., 1999; Torregrossa et al., 2011; White, 1996). Repetitive drug use strengthens the learned associations between the interoceptive (e.g. rewarding) effects of a drug and various cues that predict drug availability and/or responses that lead to drug reward. The rewarding properties of abused drugs are thought to arise from the release of dopamine in the ventral tegmental area (VTA) projections to the nucleus accumbens, particularly the shell (Roberts et al., 1977; Wise and Bozarth, 1985). Given that dopamine plays an integral role in learning and memory, it can be difficult to distinguish dopamine’s effects on the experience of drug reward or the hedonic aspects of drug taking, versus the strengthening of the behavior through reinforcement learning, whereby learned associations between cues and the drug they predict come to drive drug-seeking behavior. Indeed, once a cue becomes predictive of reward, dopamine neurons shift their firing from reward onset to cue onset (Hollerman and Schultz, 1999; Schultz et al., 1997). The incentive sensitization theory of addiction suggests that over time, the incentive salience, or motivational significance, of drug-related cues becomes pathologically amplified (Robinson and Berridge, 1993, 2001). This may account, in part, for the “incubation,” or progressive amplification, of drug seeking that has been reported with longer periods of protracted abstinence (Grimm et al., 2001; Tran-Nguyen et al., 1998).
Brain-derived neurotrophic factor (BDNF) has a well-known role not only in neurodevelopment, where it has been shown to support synaptogenesis (Lu and Figurov, 1997; Lu et al., 2009; Shen and Cowan, 2010), but also in memory formation, where it promotes synaptic restructuring (Lu and Figurov, 1997; Lu et al., 2007; Rex et al., 2007; Schjetnan and Escobar, 2010; Yamada et al., 2002). Both of these processes likely contribute to the storage and retrieval of memories, an area of growing interest in the treatment of neuropsychiatric disorders with an etiological basis in learning and memory mechanisms. One of the hallmarks of novel learning is the activation of N-methyl-D-aspartate (NMDA) ionotropic glutamate receptors, which promote Hebbian learning by acting as coincidence detectors in the central nervous system (Tsien, 2001). At the cellular level, BDNF appears to support memory, at least in part, by enhancing NMDA receptor currents in neurons via activation of its tyrosine kinase receptor TrkB and subsequent intermediary molecules (e.g. the protein Fyn) (Xu et al., 2006; Yamada and Nabeshima, 2004). BDNF can be presynaptically released from neurons in an activity-dependent manner, and this typically occurs during neuronal bursting (Balkowiec and Katz, 2002), a type of high-frequency activity pattern that has been observed during episodes of memory consolidation (Burgos-Robles et al., 2007; Cooper, 2002). In the postsynaptic neuron, BDNF promotes NMDA receptor-dependent bursting (Levine et al., 1998; Madara and Levine, 2008; Rosas-Vidal et al., 2014) and thus, at a systems level, may work to strengthen neural circuits responsible for memory storage and retrieval.
Memory manipulation tactics are an area of active investigation for the treatment of addiction and other disorders involving a hyper reactivity to cues, including posttraumatic stress disorder (PTSD) (Lee et al., 2006; Milton, 2012; Monfils et al., 2009; Schiller et al., 2012; Spiers and Bendor, 2014). There are two main options for treatment of maladaptive memories: (1) erasure of the pathological memory, or (2) creation of a new, inhibitory memory that opposes the pathological associations (Kiefer and Dinter, 2011; Merlo et al., 2014; Nic Dhonnchadha and Kantak, 2011). Memory erasure has been achieved through a process termed reconsolidation blockade, which interferes with the “re-storing” of memories once they have been retrieved (Milton and Everitt, 2010; Nader et al., 2000; Schwabe et al., 2014). In contrast, extinction training has been used to suppress pathological responses and is known to involve consolidation of a new inhibitory memory trace (Bouton, 1993; Bouton et al., 2006; Pavlov, 1927). The inhibitory extinction memory is thought to then compete with the original memory trace for control over the expression of conditioned behavior (Quirk et al., 2006). This occurs through the recruitment of inhibitory brain structures that are situated to inhibit expression centers that drive the prepotent conditioned response (Peters et al., 2009; Quirk et al., 2006).
Extinction and incubation have been argued to be opposing mnemonic processes, and BDNF plays a role in both, through its actions in distinct neural circuits. Here we compare BDNF effects on each of these processes, across different classes of abused drugs, focusing primarily on heroin and alcohol (cf. McGinty et al., 2014, for further comparison with cocaine). As estrogen has been shown to produce similar effects to BDNF on learning and memory, we also consider the impact of sex differences on these processes. We begin with a general discussion on the role of BDNF in memory, drawing from a literature on fear learning and memory.
2. BDNF and extinction memory
Extinction memory has particular therapeutic potential as it can be used to bring conditioned behaviors under cognitive and emotional control (Kantak and Nic Dhonnchadha, 2011; Kaplan et al., 2010). Extinction training is analogous to cue exposure therapy, where drug-related cues are presented in the absence of the outcome they predict (e.g. in the absence of the abused drug) (Davis et al., 2006; Myers and Carlezon, 2012). Over time, the conditioned responses triggered by the cues are diminished, or extinguished, through this new learning that the cues are now meaningless. The inhibitory learning that occurs during cue exposure therapy can be enhanced by pretreatment with the NMDA receptor co-agonist, D-cycloserine (Myers and Carlezon, 2012), and analogous effects have been observed in animal models of PTSD and drug addiction that incorporate an extinction learning phase (Kelamangalath et al., 2009; Ledgerwood et al., 2005; Thanos et al., 2011; Torregrossa et al., 2010). We know from these animal models that extinction of both fear and cocaine seeking rely on the prefrontal cortex (PFC), specifically the ventromedial portion (e.g. rodent infralimbic PFC) (Peters et al., 2008, 2009; Quirk and Mueller, 2007).
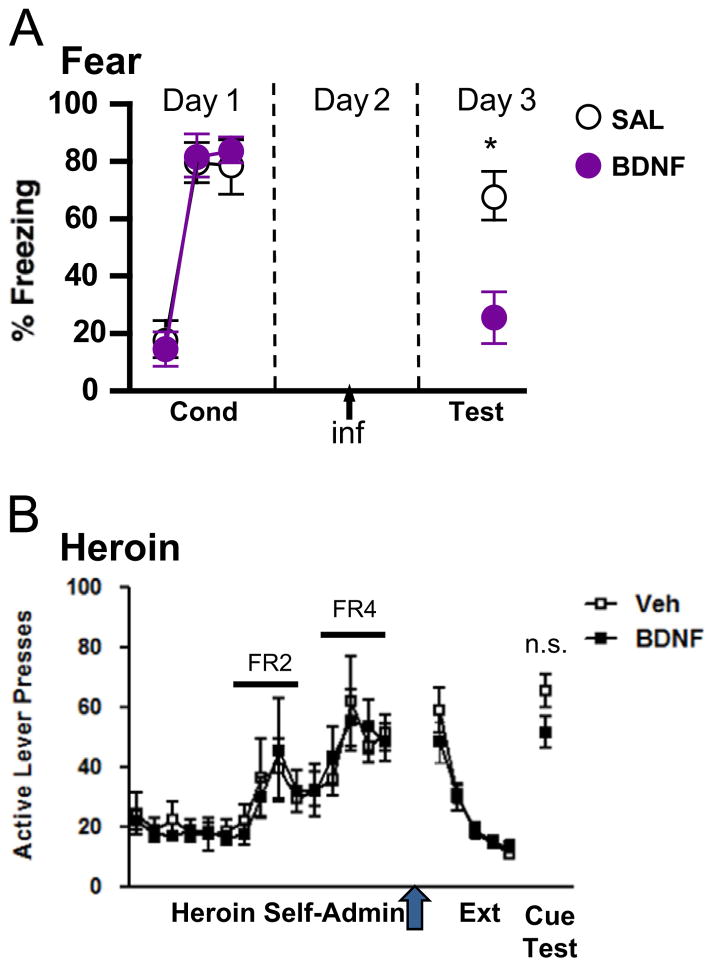
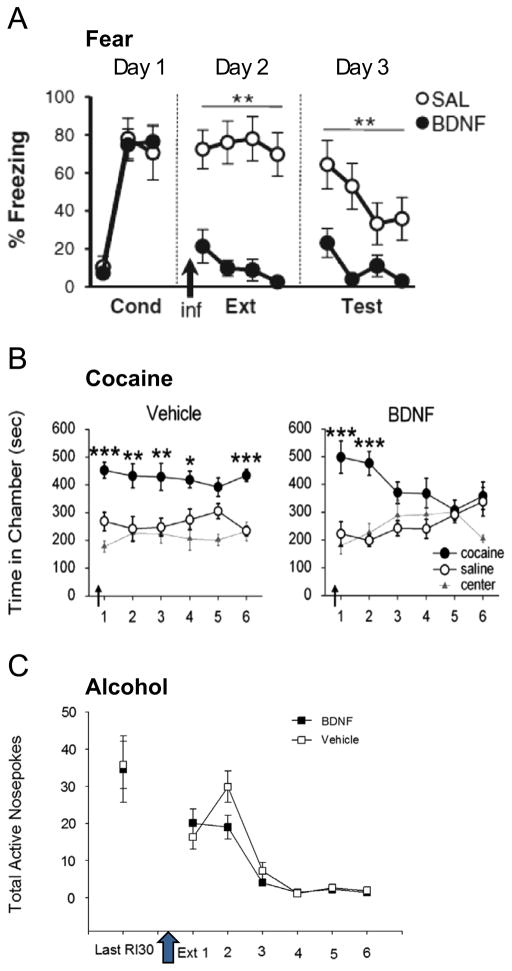
For conditioned fear behavior, BDNF produces therapeutic reductions in cue-induced fear, particularly BDNF in the hippocampal–infralimbic pathway (Peters et al., 2010; Rosas-Vidal et al., 2014). Exogenous BDNF applied to either brain site reduces fear in a manner that is reminiscent of extinction-induced reductions in fear (Figs. 1 and 2A). The importance of the hippocampal to infralimbic projection is underscored by the observation that BDNF applied directly to hippocampus can reduce fear in an infralimbic BDNF-dependent fashion (Peters et al., 2010). Furthermore, exogenous application of hippocampal BDNF promotes neuronal bursting in infralimbic neurons, a known correlate of fear extinction memory consolidation (Rosas-Vidal et al., 2014). Long-term potentiation (LTP) also develops in the hippocampal-prefrontal pathway after extinction learning occurs (Farinelli et al., 2006), and disrupting this potentiation disrupts fear extinction memory retrieval (Farinelli et al., 2006; Inoue et al., 2013; Judo et al., 2010). Given that BDNF promotes LTP in cortical neurons (Abidin et al., 2007; Cabezas and Buno, 2010; Lu et al., 2010, 2007; Messaoudi et al., 2002), it may simulate fear extinction memory by emulating this neurophysiological plasticity.
Fig. 1.
Hippocampal BDNF substitutes for fear extinction, but not extinction of heroin seeking. (A) BDNF was infused into hippocampus on the day between conditioning and test in an auditory conditioned fear paradigm. BDNF reduced fear at test, as though it substituted for extinction training (Republished with permission from Peters et al., 2010). (B) BDNF was infused into hippocampus the day prior to initiating extinction training in a heroin self-administration model. This treatment did not alter extinction learning and memory, nor did it alter cue-induced reinstatement of heroin seeking one week later (see text for additional experimental details).
Fig. 2.
BDNF has extinction-like effects on fear and cocaine memories, but not alcohol seeking. (A) BDNF was infused into infralimbic PFC just prior to the initial extinction training session in an auditory conditioned fear paradigm. Immediate reductions in fear were observed that persisted the following day (Republished with permission from Peters et al., 2010). (B) BDNF was infused into infralimbic PFC prior to the first of several repeated extinction trials in a cocaine conditioned place preference model. Though no immediate effects of BDNF were evident, BDNF facilitated extinction learning over trials (Republished with permission of Journal of Neuroscience, from “Infralimbic BDNF/TrkB enhancement of GluN2B currents facilitates extinction of a cocaine-conditioned place preference,” Otis et al., 2014; permission conveyed through Copyright Clearance Center, Inc.). (C) Adult male CD1 mice were trained to self-administer 10% ethanol. Mice were food restricted to approximately 95% of free feeding weights, but had ad libitum access to water throughout training. Testing was performed in the light cycle. Mice were trained to lever press on a fixed ratio schedule, and then were graduated to a random interval 30 s schedule in which each response after a randomly generated interval was reinforced. Reinforcer delivery was paired with presentation of a tone+light compound cue. BDNF was infused into the infralimbic PFC at AP+1.9, ML +/−0.4, DV −2.2 prior to testing in extinction. During test sessions, levers were extended into the chambers, but responses did not produce reinforcer delivery or cue presentation.
3. The estrogen–BDNF connection
Estrogen effects on neuronal plasticity and cognition are similar to those of BDNF (Aguirre and Baudry, 2009; Luine and Frankfurt, 2013; Sato et al., 2007; Scharfman and Maclusky, 2005; Sherwin, 2002, 2005; Smith and McMahon, 2006). The most abundant estrogen in the brain, 17β-estradiol, exerts its effects through receptors (ERα and ERβ) on both the cell membrane and the nucleus (Levin, 2002). The gene for BDNF has an estrogen response element, which may mediate the ability of estradiol to increase BDNF protein levels in the PFC and hippocampus (Harte-Hargrove et al., 2013; Liu et al., 2001; but see Murphy et al., 1998), although a transsynaptic mechanism has also been suggested (Blurton-Jones et al., 2003). Estradiol-induced increases in BDNF levels are seen in ovariectomized rats (Scharfman et al., 2007) and in freely cycling rats at the start of proestrus and into estrus, when estradiol levels are high (Harte-Hargrove et al., 2013; Scharfman et al., 2003). These observed increases in hippocampal-prefrontal BDNF may account for estradiol’s ability to enhance both spatial (hippocampal) and non-spatial (prefrontal) memories (Luine and Frankfurt, 2013).
Importantly, estradiol has also been shown to enhance fear extinction memory in female rats when administered during memory consolidation, and this effect can be emulated using an ERβ, but not an ERα receptor agonist (Chang et al., 2009; Galvin and Ninan, 2014; Zeidan et al., 2011). In female humans, fear extinction memory recall is better when extinction is conducted during high-estradiol states (Milad et al., 2009, 2010). In both female rats and humans, these effects are associated with enhanced activation and synaptic plasticity in ventromedial PFC (Galvin and Ninan, 2014; Zeidan et al., 2011), as well as downstream activation of NMDA receptors in this region (Galvin and Ninan, 2014). These studies are consistent with observations that low estrogen is associated with extinction failure in females with PTSD (Glover et al., 2012). Following trauma, women are more prone than men to develop anxiety disorders, including PTSD, and low estrogen, including the blockade of estrogen effects by birth control (Graham and Milad, 2012), may in part explain this vulnerability (Lebron-Milad et al., 2012). Even in male rats, estradiol synthesis is required for successful fear extinction formation (Graham and Milad, 2014), suggesting that these effects of estrogen are relevant to both sexes. Though it remains to be shown whether the extinction-enhancing effects of estradiol are BDNF-dependent, they are congruent with the ability of estradiol to increase BDNF in hippocampal-prefrontal circuits where it promotes extinction.
4. BDNF and cocaine seeking
The PFC is an integral part of the extinction circuit for both fear and cocaine memories, but is BDNF and/or TrkB signaling a common mechanism for extinction? A recent study suggests this may be the case; Otis et al. (2014) observed an extinction-enhancing effect of infralimbic-applied exogenous BDNF on cocaine conditioned place preference (CPP) memory (Fig. 2B). However, this BDNF effect only emerged after extinction training, whereas infralimbic BDNF is capable of inducing fear extinction independent of training (Otis et al., 2014; Peters et al., 2010). In both cases, NMDA receptor activation is a requisite downstream target of BDNF (Otis et al., 2014; Peters et al., 2010). Furthermore, BDNF may be a downstream mechanism by which AMPAkines facilitate extinction of cocaine seeking (Jourdi et al., 2009; LaLumiere et al., 2010). Whether or not the hippocampus is the source of infralimbic BDNF for cocaine extinction remains an open question. One study revealed increased hippocampal BDNF levels associated with the ability of environmental enrichment to reduce cocaine seeking after abstinence (Thiel et al., 2011). Notably, however, this effect did not preclude the incubation of cocaine seeking.
One theory of addiction postulates that an increase in the incentive salience of drug-associated cues increases relapse vulnerability (Robinson and Berridge, 2008). Sign trackers, animals that track a reward-predictive cue instead of the reward itself, are thought to be more addiction prone compared to their goal-tracking counterparts that track the reward location. Sign trackers have reduced levels of prefrontal BDNF, are more prone to the incubation of cue-elicited fear, and exhibit increased cue-induced reinstatement of cocaine seeking after extinction (Morrow et al., 2014; Saunders and Robinson, 2010; Yager and Robinson, 2012). This suggests a protective role of prefrontal BDNF in reducing the salience of conditioned cues and their propensity to trigger relapse. In light of recent evidence that suggests goal-trackers may be more prone to relapse triggered by contextual cues (Saunders et al., 2014), the types of cues that trigger relapse could be a critically important factor when considering addiction therapeutics that increase prefrontal BDNF.
Prefrontal-applied BDNF has been shown to reduce cocaine seeking, at least in part, by normalizing accumbens glutamate homeostasis (Berglind et al., 2007, 2009). Interestingly, however, this effect is attributed to BDNF actions in prelimbic, not infralimbic, PFC. These dorsal and ventral subregions of medial PFC are known for their opposing influences on behavior, and this dichotomy of function is preserved for both fear and cocaine-seeking behaviors (Peters et al., 2009). The timing of the prelimbic BDNF infusion (immediately after the last cocaine self-administration session) may be of crucial importance for these observed effects on cocaine seeking, and cocaine may need to be “on board” at the time of treatment (Berglind et al., 2009; McGinty et al., 2009). Such a profile suggests that prelimbic BDNF may disrupt reconsolidation of the cocaine memory and consequent action control (Gourley et al., 2012), which is conceptually quite different from the extinction-enhancing effects of infralimbic BDNF (Milton, 2012; Torregrossa and Taylor, 2012). From a treatment perspective, however, both of these mechanisms are viable therapeutic pursuits.
The incubation of cocaine seeking that occurs after prolonged withdrawal has been linked to mesolimbic BDNF (Graham et al., 2007; Grimm et al., 2003; Li et al., 2013; Lu et al.,2004a, 2004b). Local infusion of BDNF into the VTA immediately after the last cocaine self-administration session produces a persistent increase in cocaine seeking (Lu et al., 2004a), opposite the decreases reported for PFC BDNF discussed above (Berglind et al., 2007, 2009; McGinty et al., 2009; Otis et al., 2014). Similarly, BDNF in the nucleus accumbens appears to promote cocaine seeking and delay extinction, whereas accumbens BDNF knockdown reduces cocaine seeking (Bahi et al., 2008; Graham et al., 2007). These effects may be primarily localized to the nucleus accumbens shell, as BDNF in the accumbens core has been shown to both reduce cocaine seeking (Li et al., 2013) and enhance cocaine cue-mediated behaviors (Horger et al., 1999). Thus, in contrast with the therapeutic effects of BDNF in the PFC, the effects of BDNF in the VTA-accumbens pathway tend to enhance cocaine seeking. This underscores the importance of neural circuitry considerations when considering BDNF-based addiction therapies.
5. BDNF and opiate seeking
The neurobiological and anatomical substrates of opiate seeking appear to be distinct from those identified for cocaine seeking (see Badiani et al., 2011 for review). For example, in contrast to cocaine seeking, heroin seeking appears to incubate independently of accumbens BDNF (Theberge et al., 2012). The role of BDNF in the VTA-accumbens pathway is an area of growing interest and dispute for opiate reward and memory. A recent study from Koo and colleagues (2012) indicates that VTA BDNF opposes the rewarding effects of morphine by reducing activity in presumed accumbens-projecting dopamine neurons (Koo et al., 2012). However, these data stand in stark opposition with earlier work from Vargas-Perez et al. (2009), who found that VTA BDNF neither opposes nor facilitates morphine reward, but rather switches the mechanism for opiate reward from a dopamine-independent to a dopamine-dependent one. This occurs through BDNF’s reversal of GABA currents in VTA neurons from inhibitory to excitatory, and this effect is thought to underlie the negative aversive state associated with opiate withdrawal (Vargas-Perez et al., 2014).
The reasons for these discrepancies are unclear, but they point to a need for further investigation of the role of BDNF within the VTA-accumbens pathway. Koo et al. (2014) have also observed a negative relationship between accumbens BDNF and morphine CPP memory, but in a cell-type specific manner. That is, selective ablation of the TrkB BDNF receptor on dopamine D1 receptor-containing medium spiny neurons resulted in reduced GABA-A receptor currents in these neurons, ultimately promoting morphine reward (Koo et al., 2014). Given that morphine exposure led to a similar cell-type specific downregulation of TrkB in D1-neurons, the authors hypothesized that this neuroadaptation contributes to morphine addiction. Notably, however, direct infusion of BDNF into the accumbens did not alter morphine CPP (Koo et al., 2012). Thus, while the precise role of BDNF in the mesoaccumbens system must still be resolved, it is clear that it differs from that reported for cocaine.
Similarly, data are lacking regarding the functional impact of cortical BDNF on opiate memories. However, some evidence supports a potential link between cortical BDNF and the inhibition of opiate behaviors. For example, suppression of the receptor for activated C kinase 1 (RACK1) using a short hairpin strategy blocks morphine CPP memory formation, while simultaneously increasing BDNF transcription in PFC and hippocampus (Wan et al., 2011). However, it is difficult to directly relate fluctuations in mRNA to functional alterations in BDNF protein, as these measures often do not always correlate (see Table 1). Memantine, a drug with mixed pharmacology, including antagonism of NMDA receptors, also attenuates morphine reward-memory in a CPP paradigm and prevents morphine-induced reductions in BDNF protein within the PFC and nucleus accumbens (Chen et al., 2011). While these studies suggest an association between reduced morphine reward and increased prefrontal (and hippocampal or accumbal) BDNF, another study implicates prefrontal BDNF in extinction of morphine conditioned place aversion (Wang et al., 2012). Extinction of this aversive memory was also dependent on NMDA receptor and activation of an ERK-CREB pathway, consistent with mechanisms of extinction for fear and cocaine. This raises the intriguing possibility that the aversive and rewarding components of opiate memories may have distinct neural mechanisms of extinction.
Table 1.
Effects of ethanol and opiate exposure on BDNF mRNA and protein expression. While BDNF and opiates appear to impact BDNF expression, the direction and magnitude of these effects depends on the duration and method of exposure as well as where changes are measured. A greater understanding of the role of BDNF in opiate and alcohol seeking behavior requires a more thorough analysis of the time course and loci of these effects.
| Species | Drug exposure | Measure | Change | Reference |
|---|---|---|---|---|
| Ethanol | ||||
| Human (dependent alcoholics) | Self-administration | Serum BDNF protein | ↓in serum | Zanardini et al. (2011) |
| Human (abstinent alcoholics) | Self-administration | protein | ↑in serum =in plasma |
D’Sa et al. (2012) |
| Human (30 day abstinent alcoholics) | Self-administration | protein | ↓in plasma | Joe et al. (2007) |
| Mouse | Acute injection (2 mg/kg) Self-admin, 4 weeks |
mRNA | ↑in hippocampus and dorsal striatum =in PFC ↑in dorsal striatum =in PFC and hippocampus |
McGough et al. (2004) |
| Mouse | Vapor chamber, 2 weeks | mRNA | ↓in PFC =in accumbens and hippocampus |
Melendez et al., (2012) |
| Mouse | Self-admin Acute Chronic, 6 weeks | mRNA | ↑in dorsal striatum =in accumbens and cortex =in dorsal striatum and accumbens ↓in ventral PFC, frontal and posterior cortex |
Logrip et al. (2009) |
| Rat | Liquid diet, 28 weeks | mRNA | ↓in hippocampus | MacLennan et al. (1995) |
| Rat | Vapor chamber, 10 days | Protein | ↓in hippocampus | Hauser et al. (2011) |
| Rat | Vapor chamber, 4 weeks 12 hours withdrawal |
mRNA | ↓in CA1 and dentate gyrus, supraoptic nucleus of hypothalamus ↓in CA1, dentate gyrus at control levels. ↑in CA3 and supraoptic nucleus |
Tapia-Arancibia et al. (2001) |
| Rat | Vapor chamber, 7 weeks | mRNA | ↓in medial PFC | Tapocik et al. (2012, 2014) |
| Opiate | ||||
| Rat | Heroin injection, 5 days | Protein | ↑in prelimbic PFC ↓in dorsal hippocampus =in ventral hippocampus and nucleus accumbens |
Zilkha et al. (2014) |
| Rat | Self-administration, 1, 11, or 30 days abstinent | mRNA and protein | =in nucleus accumbens and dorsal striatum | Theberge et al. (2012) |
| Human (dependent) | Self-administration | Protein | ↑in serum | Heberlein et al. (2011) |
| Human (dependent) | Self-administration | Protein | ↓in serum | Angelucci et al. (2007) |
| Human (dependent) | Self-administration In withdrawal | Protein | ↓in serum No change |
Zhang et al. (2014) |
In humans, the Val66Met polymorphism in the BDNF gene has been linked to incidence and age of onset of heroin abuse (Cheng et al., 2005; Hou et al., 2010), as well as willingness to invest more time and money in obtaining heroin (Greenwald et al., 2012). Given that this polymorphism has been associated with deficits in hippocampal release of BDNF and impaired extinction (Soliman et al., 2010; Yu et al., 2009), one might expect heroin use to be perpetuated by such extinction deficits, provided hippocampal BDNF is a shared mechanism for extinction memory between fear and opiates. Opiate exposure disrupts hippocampal long-term potentiation (LTP) (Salmanzadeh et al., 2003), a proposed neurophysiological correlate of memory, and re-exposure to opiates during withdrawal can restore this hippocampal LTP (Pu et al., 2002), but notably, extinction does not (Portugal et al., 2014). At least one study has suggested the hippocampus is involved in extinction of morphine CPP (Billa et al., 2008), but while molecular changes in hippocampus were observed after extinction, no functional manipulation of hippocampus was performed to test its role in extinction behavior.
We performed an experiment to test the ability of exogenous, hippocampal-applied BDNF to alter heroin extinction and reinstatement in a self-administration model of relapse. BDNF (0.75 μg/side) was infused into hippocampus the day after the last heroin self-administration session. Extinction training commenced the day after this infusion. This design is analogous to the study by Peters et al. (2010), where hippocampal infusions of BDNF the day after fear conditioning reduced fear on the first extinction session the next day. Interestingly, there were no significant effects of hippocampal-applied BDNF on extinction or reinstatement of heroin seeking (Fig. 1B). BDNF can induce LTP when applied exogenously to the hippocampus of naïve animals (Messaoudi et al., 2002), and this may account for its therapeutic, extinction-like effects on fear, particularly given that synaptic potentiation in hippocampus has been associated with extinction memory (Saito et al., 2012). These negative findings in the heroin model suggest that BDNF may not induce LTP in the opiate-exposed hippocampus, that hippocampal LTP may not mediate extinction of heroin seeking, or that conditions are not optimal to detect an effect in our model.
Although hippocampal-applied BDNF did not alter extinction or reinstatement of heroin seeking in our hands, it is notable that animals extinguished their heroin-seeking behavior rapidly, over just five 1-hour sessions. Thus, they do not appear to be extinction-impaired per se. As recently reviewed by Peters et al. (2013), the infralimbic PFC may play a fundamentally different role in cocaine- versus heroin-seeking behavior. Whereas the infralimbic PFC promotes extinction of cocaine seeking (LaLumiere et al., 2010; Peters et al., 2008), it appears to promote relapse for heroin (Bossert et al., 2011, 2012). While this may be due to the existence of separate neural ensembles for extinction and relapse within the structure that complicate interpretations of whole-structure manipulations, the lack of involvement of infra-limbic PFC in extinction of heroin seeking, coupled with the lack of hippocampal BDNF involvement, supports the notion that extinction of heroin seeking may rely on fundamentally different neural mechanisms than those for fear and cocaine.
6. BDNF and alcohol seeking
A growing, though mixed, literature has implicated alterations in BDNF in risk for both the development of alcohol use disorders and in propensity to relapse. Serum BDNF levels appear to be decreased in alcohol dependent individuals (Zanardini et al., 2011), but these serum levels may rebound during abstinence (Huang et al., 2010), reaching levels that are in some cases higher than those of control populations (D’Sa et al., 2012). It is unclear, however, how these peripheral findings relate to changes within the central nervous system in humans. In addition to changes in peripheral BDNF levels, considerable evidence points toward a relationship between genetic differences in the BDNF system and alcohol use disorders. Though findings are inconsistent (Nedic et al., 2012), data suggest that the Val66Met BDNF polymorphism may predict the development of alcohol use disorders. Beyond general alterations in BDNF signaling in the alcoholic population, the Val66Met polymorphism has been specifically associated with increased propensity to relapse after shorter abstinent periods (Wojnar et al., 2009). Interestingly, the BDNF Val66Met polymorphism has also been associated with executive function in individuals at risk for alcohol use disorders. In non-alcoholic adults with alcoholic parents, the polymorphism was predictive of cognitive performance on a number of measures (Benzerouk et al., 2013) suggesting that differences in BDNF signaling may promote alcohol use disorders, and potentially relapse, through a more general effect on executive function.
Animal models have added substantially to this literature, with a number of studies showing that alcohol exposure differentially impacts BDNF expression in limbic corticostriatal circuits that mediate reward-seeking and -taking behaviors (Jeanblanc et al., 2009, 2012; Logrip et al., 2009). While some animal models suggest correlations between serum levels and brain BDNF levels (Klein et al., 2010), changes in BDNF after alcohol exposure do not appear to be uniform across the brain, suggesting difficulty in relating peripheral changes to changes within specific brain regions. In particular, chronic ethanol exposure increases BDNF mRNA in the striatum in a RACK1-dependent manner (Leggio et al., 2014; McGough et al., 2004). In contrast, decreases in BDNF mRNA in the hippocampus, hypothalamus and cortex have been reported (Logrip et al., 2009; MacLennan et al., 1995; Tapia-Arancibia et al., 2001). Subchronic ethanol exposure increases TrkB mRNA expression in the basal forebrain and cortex (Miki et al., 2013), and increases in BDNF mRNA have been observed in the hippocampus and hypothalamus after acute withdrawal (Tapia-Arancibia et al., 2001). Thus, the precise timing and extent of ethanol exposure is likely to be critical in interpreting ethanol effects on BDNF expression. These changes in mRNA expression are likely downstream to ethanol-induced changes in chromatin remodeling, suggesting epigenetic regulation of BDNF expression as a result of ethanol exposure (Stragier et al., 2014).
Together, the clinical and preclinical data implicate innate and alcohol-induced alterations in BDNF in alcohol use disorders. A number of studies have shown that manipulating BDNF or its signaling partners can alter ethanol consumption. For example, BDNF heterozygotes exhibit reduced ethanol consumption (McGough et al., 2004), similar to that observed with TrkB antagonism, which also opposes ethanol-induced changes in the dopamine system (Leggio et al., 2014). Interestingly, BDNF infusions directly into the VTA do not alter expression of ethanol CPP, but do convert the underlying mechanism for this CPP from a dopamine-dependent to a dopamine-independent one (Ting-A-Kee et al., 2013). As with opiates, this simulates the endogenous switch that occurs during withdrawal, despite the fact these dopamine contingencies are opposite for ethanol and opiates (Ting-A-Kee et al., 2013; Vargas-Perez et al., 2009, 2014; but see Koo et al., 2012). By contrast, loss of BDNF in the dorsolateral striatum impairs both ethanol CPP memory and free consumption (Bahi and Dreyer, 2013), as well as intake in a self-administration model (Jeanblanc et al., 2009). The mechanism by which BDNF signaling reduces ethanol consumption, preference and self-administration is unclear. Consistent with other drugs of abuse, uncontrolled drug seeking characterizes alcohol use disorders. Chronic ethanol exposure has been shown to result in impairments in behavioral flexibility (Kroener et al., 2012; Trantham-Davidson et al., 2014), and at least adolescent ethanol exposure impairs extinction learning (Gass et al., 2014). Notably, chronic ethanol exposure can also impair extinction of fear, suggesting that ethanol exposure itself can produce changes in extinction neural circuits (Holmes et al., 2012), though a role for ethanol-induced changes in BDNF has not to our knowledge been implicated in these effects.
Like other reinforcers, extinction of ethanol seeking appears to involve the infralimbic PFC. Ethanol extinction can be facilitated by modulation of mGluR5 signaling that is infralimbic-dependent (Gass et al., 2014). In addition, individual differences in resistance to ethanol extinction are associated with innate differences in the expression of PSA-NCAM, a proplasticity molecule, in the infralimbic PFC (Barker et al., 2012). Loss of PSA-NCAM selectively within the infra-limbic, but not prelimbic PFC results in the inability to extinguish ethanol seeking. Given this role for infralimbic PFC in the extinction of ethanol seeking, we investigated whether infralimbic BDNF infusions would facilitate extinction. Surprisingly, we saw no facilitation of extinction learning (Fig. 2C) after infusion of BDNF (0.4 μg/μl, 0.2 μl/side) into the infralimbic PFC. Importantly, while animals in this study self-administered ethanol, these animals were not ethanol dependent. Because ethanol dependence itself alters extinction learning (Gass et al., 2014; Holmes et al., 2012), it remains possible that BDNF administration within the infralimbic PFC may rescue dependence-induced impairments in extinction learning.
These data suggest that BDNF administration within the infralimbic PFC is not sufficient to mimic extinction memory, in contrast with fear extinction (Peters et al., 2010). Interestingly, the role of infralimbic PFC in ethanol extinction is less clearly established relative to cocaine. Inactivation of the infralimbic PFC does not prevent expression of extinction of ethanol seeking as it does for cocaine and fear (Peters et al., 2009; Willcocks and McNally, 2013). Though infralimbic PFC plasticity appears to be involved in ethanol extinction (Barker et al., 2012; Gass et al., 2014) and extinction of fear in ethanol-dependent animals (Holmes et al., 2012), inactivation after the acquisition of extinction does not impact subsequent expression of that extinction. This may in part be because ethanol engages different subcortical structures than cocaine that may mediate extinction despite the loss of infralimbic activity. Because alcohol is a caloric reinforcer – as opposed to other drugs of abuse – it is important to consider that there may be a greater involvement of satiety circuitry. As with cocaine, inactivation of the nucleus accumbens shell has been shown to reinstate extinguished ethanol seeking (Millan et al., 2010; Peters et al., 2008). How interactions between the shell and infralimbic PFC are involved in the extinction of ethanol seeking is as yet unknown. Interestingly, these authors expanded upon this finding by demonstrating that nucleus accumbens shell mediates ethanol extinction by inhibiting hypothalamic neurons that are largely involved in signaling satiety. Indeed, the context-induced reinstatement of ethanol seeking is associated with activation of the nucleus accumbens shell to lateral hypothalamus pathway, and inactivation of the lateral hypothalamus prevent reinstatement (Marchant et al., 2009). Together with findings implicating infralimbic projections to the dorsomedial hypothalamus in the expression of ethanol extinction (Marchant et al., 2010), these data suggest a larger network through which infralimbic PFC may interact with hypothalamic targets to drive the extinction and reinstatement of ethanol seeking. This interaction suggests that the profile of ethanol extinction may share overlapping features with food or other caloric reinforcers, rather than other drugs of abuse, due to the engagement of hypothalamic satiety circuits.
7. Sex differences, BDNF, and drug seeking
Importantly, the data described above were collected primarily in male animals. Notable sex differences exist in a number of components of addictive behavior, in both animal models and in human populations (Becker and Hu, 2008; Carroll and Anker, 2010; Lynch et al., 2002; Yu et al., 2007). Female rats tend to show greater resistance to cocaine extinction (Fuchs et al., 2005; Hilderbrand and Lasek, 2013; Kippin et al., 2005; Kosten and Zhang, 2008), as well as heightened reinstatement, cue reactivity (Bobzean et al., 2010; Feltenstein et al., 2011; Lynch and Carroll, 2000), and incubation of cocaine seeking (Kerstetter et al., 2008). In female rats, 17β-estradiol is required for extinction of cocaine CPP, and chronic treatment with 17β-estradiol can facilitate extinction (Larson and Carroll, 2007). It is possible that these effects are mediated through estrogen interactions with BDNF signaling, as with fear (Lebron-Milad and Milad, 2012; Milad et al., 2009, 2010), though direct evidence for this is lacking with regards to cocaine.
There are significant sex differences in ethanol seeking behaviors that appear to be both sex chromosome and gonadal hormone related. Female mice have been shown repeatedly to self-administer higher volumes of ethanol than male animals (Barker et al., 2010), though chromosomal male mice developed ethanol-seeking habits more rapidly. While to our knowledge, sex differences in extinction of ethanol seeking have not been reported, as described above, sex drives differential roles for BDNF in learning and memory. Importantly, sex also appears to mediate the expression of hippocampal BDNF during acute alcohol withdrawal, with females showing extensive increases in BDNF, and males showing a more restricted increase (Alele and Devaud, 2013). Interestingly, these changes were independent of current gonadal status. Since early withdrawal is a known stressor, it is difficult to determine whether these effects were due to ethanol withdrawal, stress, or a combination thereof.
While female rats have been shown to acquire heroin self-administration more rapidly than males (Lynch and Carroll, 1999) and appear to be more motivated to seek heroin (Carroll et al., 2002), to our knowledge no data exist showing sex differences in the extinction of heroin seeking. Given that craving for opioids is significantly higher in women (Back et al., 2011), and susceptibility to cue-induced craving and relapse may be greater in women than men with comorbid cocaine and heroin addiction (Kennedy et al., 2013), it will be important to consider sex differences in the ability to extinguish conditioned responses to drug-associated cues.
8. Conclusions
The available literature implicates a cyclical interaction between BDNF and drug seeking behavior for all drugs of abuse. Indeed, chronic drug exposure appears to impact BDNF levels in both human and rodents (see Table 1), and innate differences in BDNF have been associated with the development of addiction for multiple substances of abuse, including cocaine, heroin and ethanol. BDNF signaling appears to be involved in multiple facets of addiction, including reward and motivation, but critically its expression and function is known to mediate many of the learning and memory processes that are dysregulated in addiction. The loss of the ability to acquire and express extinction may be involved in the initial development of addiction as well as the chronic relapse that defines the disorder.
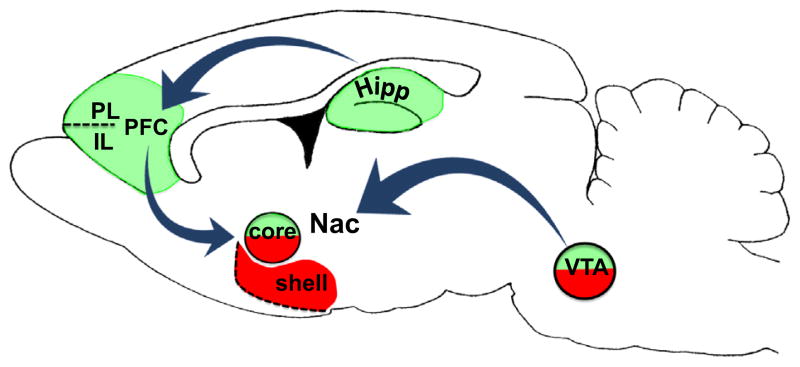
The particular brain sites and neural pathways where BDNF is expressed and released are critically important for determining whether BDNF actions will be pro-relapse or pro-extinction (Fig. 3). The incubation effect, wherein relapse is amplified after longer periods of abstinence, involves the VTA-accumbens pathway (Bahi et al., 2008; Graham et al., 2007; Grimm et al., 2003; Li et al., 2013; Lu et al., 2004a), at least for cocaine and perhaps also opiates, though there are conflicting reports (Koo et al., 2012; Vargas-Perez et al., 2009, 2014). Some evidence supports the importance of accumbens subregions in BDNF’s effects, with the shell being pro-relapse, but the core being capable of both pro-relapse and pro-extinction functions (Horger et al., 1999; Li et al., 2013). Perhaps this relates to differential sources of BDNF input to the accumbens core. It is possible that accumbal-BDNF derived from the PFC produces therapeutic reductions in drug seeking (McGinty et al., 2009), whereas accumbal-BDNF derived from the VTA may promote drug seeking. The hippocampal-prefrontal pathway, by contrast, promotes fear extinction (Peters et al., 2010; Rosas-Vidal et al., 2014), but data is notably lacking from drug addiction models. At present, negative data suggest that infralimbic PFC may not be required for the extinction of alcohol and heroin seeking (Peters et al., 2013; Willcocks and McNally, 2013; Fig. 2C), and that hippocampal BDNF does not enhance extinction of heroin seeking (Fig. 1B).
Fig. 3.
BDNF produces therapeutic versus pathological effects on drug and fear conditioned behaviors depending on its locus of action within mesolimbic and corticostriatal circuits. Therapeutic (green) reductions in fear and drug seeking have been linked to elevated BDNF protein in the hippocampus (Peters et al., 2010, fear), prelimbic (PL; Berglind et al., 2007, 2009; McGinty et al., 2009, cocaine) and infralimbic (IL; Otis et al., 2014, cocaine; Peters et al., 2010, Rosas-Vidal et al., 2014, fear) prefrontal cortices, and nucleus accumbens (Nac) core (Li et al., 2013, cocaine), although for the core, a pathological (red) enhancement of drug seeking has also been observed (Horger et al., 1999). Enhancement of drug-seeking behavior has also been associated with elevated BDNF protein in the Nac shell (Graham et al., 2007; Bahi et al., 2008; Li et al., 2013, cocaine) and ventral tegmental area (VTA; Grimm et al., 2003; Lu et al., 2004a, cocaine; Vargas-Perez et al. 2009, 2014, opiates; Ting-A-Kee et al., 2013, alcohol). Conflicting evidence suggests that BDNF in the VTA may be therapeutic for opiate memories (Koo et al., 2012).
The reason for this apparent incongruence between extinction neural circuits across different drug classes requires further investigation. One of the hallmarks of addiction is decreased behavioral flexibility and tendency to perseverate in drug-seeking behavior, and the hippocampal-prefrontal BDNF projection is critical for flexible behavior, including extinction (Sakata et al., 2013). In particular, CA1 neurons project to infralimbic PFC (Hoover and Vertes, 2007), and these are the putative cells that supply BDNF to infralimbic neurons, thus enhancing bursting and inducing extinction memory (Peters et al., 2010; Rosas-Vidal et al., 2014). Sakata et al. (2013) recently demonstrated that BDNF-dependent LTP in CA1 neurons is critical for flexible behaviors including fear extinction. These data suggest that BDNF-LTP in the CA1 projection neurons may be a critical substrate for behavioral flexibility, raising the intriguing possibility that exogenous-applied BDNF in this pathway may be capable of inducing LTP (Messaoudi et al., 2002) and reducing drug seeking, at least for drugs that require the hippocampal–prefrontal projection for extinction.
Importantly, while there are notable sex differences in drug seeking and taking, sex differences in the relationship between BDNF signaling and drug seeking are only beginning to be understood. Data indicate that estrogens interact with BDNF to impact the learning and memory processes that are thought to go awry in addictive behavior, suggesting a likely mechanism by which BDNF would differentially impact drug-seeking behavior in males and females, and perhaps across estrus cycle. Low estrogen leads to a loss of hippocampal LTP (Kramar et al., 2009) and cognitive deficits (Sherwin, 2005), both of which can be ameliorated by replacing estrogen (Kramar et al., 2009; Sherwin, 2002; Smith and McMahon, 2006). Hence, the therapeutic potential of BDNF to enhance the extinction of drug seeking requires a careful consideration of sex differences, and especially the role of BDNF-estrogen interactions.
Acknowledgments
This research was supported by grants NIH grants AA023141 and AA020135 to JMB, P50 AA012870 to JRT, and DA038235 to JP, as well as a grant from the Netherlands Organization for Scientific Research (ZonMW/NWO 91611035) to JP.
References
- Abidin I, Kohler T, Weiler E, Zoidl G, Eysel UT, Lessmann V, Mittmann T. Reduced presynaptic efficiency of excitatory synaptic transmission impairs LTP in the visual cortex of BDNF-heterozygous mice. Eur J Neurosci. 2007;24:3519–3531. doi: 10.1111/j.1460-9568.2006.05242.x. [DOI] [PubMed] [Google Scholar]
- Aguirre CC, Baudry M. Progesterone reverses 17beta-estradiol-mediated neuroprotection and BDNF induction in cultured hippocampal slices. Eur J Neurosci. 2009;29:447–454. doi: 10.1111/j.1460-9568.2008.06591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alele PE, Devaud LL. Expression of cFos and brain-derived neurotrophic factor in cortex and hippocampus of ethanol-withdrawn male and female rats. J Pharmacol Pharmacother. 2013;4:265–274. doi: 10.4103/0976-500X.119712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelucci F, Ricci V, Pomponi M, Conte G, Mathe AA, Attilio Tonali P, Bria P. Chronic heroin and cocaine abuse is associated with decreased serum concentrations of the nerve growth factor and brain-derived neurotrophic factor. J Psychopharmacol. 2007;21:820–825. doi: 10.1177/0269881107078491. [DOI] [PubMed] [Google Scholar]
- Back SE, Payne RL, Wahlquist AH, Carter RE, Stroud Z, Haynes L, Hillhouse M, Brady KT, Ling W. Comparative profiles of men and women with opioid dependence: results from a national multisite effectiveness trial. Am J Drug Alcohol Abuse. 2011;37:313–323. doi: 10.3109/00952990.2011.596982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi A, Boyer F, Chandrasekar V, Dreyer JL. Role of accumbens BDNF and TrkB in cocaine-induced psychomotor sensitization, conditioned-place preference, and reinstatement in rats. Psychopharmacology (Berlin) 2008;199:169–182. doi: 10.1007/s00213-008-1164-1. [DOI] [PubMed] [Google Scholar]
- Bahi A, Dreyer JL. Striatal modulation of BDNF expression using microRNA124a-expressing lentiviral vectors impairs ethanol-induced conditioned-place preference and voluntary alcohol consumption. Eur J Neurosci. 2013;38:2328–2337. doi: 10.1111/ejn.12228. [DOI] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci. 2002;22:10399–10407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Torregrossa MM, Arnold AP, Taylor JR. Dissociation of genetic and hormonal influences on sex differences in alcoholism-related behaviors. J Neurosci. 2010;30:9140–9144. doi: 10.1523/JNEUROSCI.0548-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Torregrossa MM, Taylor JR. Low prefrontal PSA-NCAM confers risk for alcoholism-related behavior. Nat Neurosci. 2012;15:1356–1358. doi: 10.1038/nn.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzerouk F, Gierski F, Gorwood P, Ramoz N, Stefaniak N, Hubsch B, Kaladjian A, Limosin F. Brain-derived neurotrophic factor (BDNF) Val66Met polymorphism and its implication in executive functions in adult offspring of alcohol-dependent probands. Alcohol. 2013;47:271–274. doi: 10.1016/j.alcohol.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr, Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW, Jr, LaLumiere RT, Kalivas PW, McGinty JF. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci. 2009;29:3715–3719. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Billa SK, Sinha N, Rudrabhatla SR, Moron JA. Extinction of morphine-dependent conditioned behavior is associated with increased phosphorylation of the GluR1 subunit of AMPA receptors at hippocampal synapses. Eur J Neurosci. 2008;29:55–64. doi: 10.1111/j.1460-9568.2008.06560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blurton-Jones M, Kuan PN, Tuszynski MH. Anatomical evidence for transsynaptic influences of estrogen on brain-derived neurotrophic factor expression. J Comp Neurol. 2003;468:347–360. doi: 10.1002/cne.10989. [DOI] [PubMed] [Google Scholar]
- Bobzean SA, Dennis TS, Addison BD, Perrotti LI. Influence of sex on reinstatement of cocaine-conditioned place preference. Brain Res Bull. 2010;83:331–336. doi: 10.1016/j.brainresbull.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, Shaham Y. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci. 2011;14:420–422. doi: 10.1038/nn.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M, Shaham Y. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci. 2012;32:4982–4991. doi: 10.1523/JNEUROSCI.0005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Cabezas C, Buno W. BDNF is required for the induction of a presynaptic component of the functional conversion of silent synapses. Hippocampus. 2010;21:374–385. doi: 10.1002/hipo.20754. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berl) 2002;161:304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Chang YJ, Yang CH, Liang YC, Yeh CM, Huang CC, Hsu KS. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor beta. Hippocampus. 2009;19:1142–1150. doi: 10.1002/hipo.20581. [DOI] [PubMed] [Google Scholar]
- Chen SL, Tao PL, Chu CH, Chen SH, Wu HE, Tseng LF, Hong JS, Lu RB. Low-dose memantine attenuated morphine addictive behavior through its anti-inflammation and neurotrophic effects in rats. J Neuroimmune Pharmacol. 2011;7:444–453. doi: 10.1007/s11481-011-9337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Hong CJ, Yu YW, Chen TJ, Wu HC, Tsai SJ. Brain-derived neurotrophic factor (Val66Met) genetic polymorphism is associated with substance abuse in males. Brain Res Mol Brain Res. 2005;140:86–90. doi: 10.1016/j.molbrainres.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Cooper DC. The significance of action potential bursting in the brain reward circuit. Neurochem Int. 2002;41:333–340. doi: 10.1016/s0197-0186(02)00068-2. [DOI] [PubMed] [Google Scholar]
- Dileone RJ, Anderson GM, Sinha R. Serum and plasma brain-derived neurotrophic factor (BDNF) in abstinent alcoholics and social drinkers. Alcohol. 2012;46:253–259. doi: 10.1016/j.alcohol.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Cadoni C, Carboni E. Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Ann NY Acad Sci. 1999;877:461–485. doi: 10.1111/j.1749-6632.1999.tb09283.x. [DOI] [PubMed] [Google Scholar]
- Farinelli M, Deschaux O, Hugues S, Thevenet A, Garcia R. Hippocampal train stimulation modulates recall of fear extinction independently of prefrontal cortex synaptic plasticity and lesions. Learn Mem. 2006;13:329–334. doi: 10.1101/lm.204806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, See RE. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology (Berlin) 2011;216:53–62. doi: 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berlin) 2005;179:662–672. doi: 10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- Galvin C, Ninan I. Regulation of the mouse medial prefrontal cortical synapses by endogenous estradiol. Neuropsychopharmacology. 2014;39:2086–2094. doi: 10.1038/npp.2014.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Trantham-Davidson H, Kassab AS, Glen WB, Olive MF, Chandler LJ. Enhancement of extinction learning attenuates ethanol-seeking behavior and alters plasticity in the prefrontal cortex. J Neurosci. 2014;34:7562–7574. doi: 10.1523/JNEUROSCI.5616-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, Norrholm SD. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol Psychiatry. 2012;72:19–24. doi: 10.1016/j.biopsych.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Swanson AM, Jacobs AM, Howell JL, Mo M, Dileone RJ, Koleske AJ, Taylor JR. Action control is mediated by prefrontal BDNF and glucocorticoid receptor binding. Proc Natl Acad Sci USA. 2012;109:20714–20719. doi: 10.1073/pnas.1208342109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Milad MR. Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biol Psychiatry. 2012;73:371–378. doi: 10.1016/j.biopsych.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Milad MR. Inhibition of estradiol synthesis impairs fear extinction in male rats. Learn Mem. 2014;21:347–350. doi: 10.1101/lm.034926.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Steinmiller CL, Sliwerska E, Lundahl L, Burmeister M. BDNF Val(66)Met genotype is associated with drug-seeking phenotypes in heroin-dependent individuals: a pilot study. Addict Biol. 2012;18:836–845. doi: 10.1111/j.1369-1600.2011.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte-Hargrove LC, MacLusky NJ, Scharfman HE. Brain-derived neurotrophic factor–estrogen interactions in the hippocampal mossy fiber pathway: implications for normal brain function and disease. Neuroscience. 2013;239:46–66. doi: 10.1016/j.neuroscience.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Getachew B, Taylor RE, Tizabi Y. Alcohol induced depressive-like behavior is associated with a reduction in hippocampal BDNF. Pharmacol Biochem Behav. 2011;100:253–258. doi: 10.1016/j.pbb.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein A, Dursteler-MacFarland KM, Lenz B, Frieling H, Grosch M, Bonsch D, Kornhuber J, Wiesbeck GA, Bleich S, Hillemacher T. Serum levels of BDNF are associated with craving in opiate-dependent patients. J Psychopharmacol. 2011;25:1480–1484. doi: 10.1177/0269881111411332. [DOI] [PubMed] [Google Scholar]
- Hilderbrand ER, Lasek AW. Sex differences in cocaine conditioned place preference in C57BL/6J mice. Neuroreport. 2013;25:105–109. doi: 10.1097/WNR.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1999;1:304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, Macpherson KP, Debrouse L, Colacicco G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA, Kash TL, Gunduz-Cinar O, Camp M. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat Neurosci. 2012;15:1359–1361. doi: 10.1038/nn.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou H, Qing Z, Jia S, Zhang X, Hu S, Hu J. Influence of brain-derived neurotrophic factor (val66met) genetic polymorphism on the ages of onset for heroin abuse in males. Brain Res. 2010;1353:245–248. doi: 10.1016/j.brainres.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Huang MC, Chen CH, Liu HC, Chen CC, Ho CC, Leu SJ. Differential patterns of serum brain-derived neurotrophic factor levels in alcoholic patients with and without delirium tremens during acute withdrawal. Alcohol Clin Exp Res. 2010;35:126–131. doi: 10.1111/j.1530-0277.2010.01329.x. [DOI] [PubMed] [Google Scholar]
- Inoue S, Kamiyama H, Matsumoto M, Yanagawa Y, Hiraide S, Saito Y, Shimamura K, Togashi H. Synaptic modulation via basolateral amygdala on the rat hippocampus-medial prefrontal cortex pathway in fear extinction. J Pharmacol Sci. 2013;123:267–278. doi: 10.1254/jphs.13123fp. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, He DY, Carnicella S, Kharazia V, Janak PH, Ron D. Endogenous BDNF in the dorsolateral striatum gates alcohol drinking. J Neurosci. 2009;29:13494–13502. doi: 10.1523/JNEUROSCI.2243-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanblanc J, Logrip ML, Janak PH, Ron D. BDNF-mediated regulation of ethanol consumption requires the activation of the MAP kinase pathway and protein synthesis. Eur J Neurosci. 2012;37:607–612. doi: 10.1111/ejn.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe KH, Kim YK, Kim TS, Roh SW, Choi SW, Kim YB, Lee HJ, Kim DJ. Decreased plasma brain-derived neurotrophic factor levels in patients with alcohol dependence. Alcohol Clin Exp Res. 2007;31:1833–1838. doi: 10.1111/j.1530-0277.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- Jourdi H, Hsu YT, Zhou M, Qin Q, Bi X, Baudry M. Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation. J Neurosci. 2009;29:8688–8697. doi: 10.1523/JNEUROSCI.6078-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judo C, Matsumoto M, Yamazaki D, Hiraide S, Yanagawa Y, Kimura S, Shimamura K, Togashi H. Early stress exposure impairs synaptic potentiation in the rat medial prefrontal cortex underlying contextual fear extinction. Neuroscience. 2010;169:1705–1714. doi: 10.1016/j.neuroscience.2010.06.035. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Nic Dhonnchadha BA. Pharmacological enhancement of drug cue extinction learning: translational challenges. Ann NY Acad Sci. 2011;1216:122–137. doi: 10.1111/j.1749-6632.2010.05899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GB, Heinrichs SC, Carey RJ. Treatment of addiction and anxiety using extinction approaches: neural mechanisms and their treatment implications. Pharmacol Biochem Behav. 2010;97:619–625. doi: 10.1016/j.pbb.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Kelamangalath L, Seymour CM, Wagner JJ. D-serine facilitates the effects of extinction to reduce cocaine-primed reinstatement of drug-seeking behavior. Neurobiol Learn Mem. 2009;92:544–551. doi: 10.1016/j.nlm.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AP, Epstein DH, Phillips KA, Preston KL. Sex differences in cocaine/heroin users: drug-use triggers and craving in daily life. Drug Alcohol Depend. 2013;132:29–37. doi: 10.1016/j.drugalcdep.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology (Berlin) 2008;198:63–75. doi: 10.1007/s00213-008-1089-8. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Dinter C. New approaches to addiction treatment based on learning and memory. Curr Top Behav Neurosci. 2011;13:671–684. doi: 10.1007/7854_2011_147. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE. Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology (Berlin) 2005;182:245–252. doi: 10.1007/s00213-005-0071-y. [DOI] [PubMed] [Google Scholar]
- Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, Knudsen GM, Aznar S. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol. 2010;14:347–353. doi: 10.1017/S1461145710000738. [DOI] [PubMed] [Google Scholar]
- Koo JW, Mazei-Robison MS, Chaudhury D, Juarez B, LaPlant Q, Ferguson D, Feng J, Sun H, Scobie KN, Damez-Werno D, Crumiller M, Ohnishi YN, Ohnishi YH, Mouzon E, Dietz DM, Lobo MK, Neve RL, Russo SJ, Han MH, Nestler EJ. BDNF is a negative modulator of morphine action. Science. 2012;338:124–128. doi: 10.1126/science.1222265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Lobo MK, Chaudhury D, Labonte B, Friedman A, Heller E, Pena CJ, Han MH, Nestler EJ. Loss of BDNF signaling in D1R-expressing NAc neurons enhances morphine reward by reducing GABA inhibition. Neuropsychopharmacology. 2014;39:2646–2653. doi: 10.1038/npp.2014.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY. Sex differences in non-reinforced responding for cocaine. Am J Drug Alcohol Abuse. 2008;34:473–488. doi: 10.1080/00952990802082206. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Chen LY, Rex CS, Gall CM, Lynch G. Estrogen’s place in the family of synaptic modulators. Mol Cell Pharmacol. 2009;1:258–262. [PMC free article] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One. 2012;7:e37541. doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Carroll ME. Estrogen receptor beta, but not alpha, mediates estrogen’s effect on cocaine-induced reinstatement of extinguished cocaine-seeking behavior in ovariectomized female rats. Neuropsychopharmacology. 2007;32:1334–1345. doi: 10.1038/sj.npp.1301249. [DOI] [PubMed] [Google Scholar]
- Lebron-Milad K, Graham BM, Milad MR. Low estradiol levels: a vulnerability factor for the development of posttraumatic stress disorder. Biol Psychiatry. 2012;72:6–7. doi: 10.1016/j.biopsych.2012.04.029. [DOI] [PubMed] [Google Scholar]
- Lebron-Milad K, Milad MR. Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biol Mood Anxiety Disord. 2012;2:3. doi: 10.1186/2045-5380-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine facilitates extinction of learned fear: effects on reacquisition and generalized extinction. Biol Psychiatry. 2005;57:841–847. doi: 10.1016/j.biopsych.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio GM, Camillieri G, Platania CB, Castorina A, Marrazzo G, Torrisi SA, Nona CN, D’Agata V, Nobrega J, Stark H, Bucolo C, Le Foll B, Drago F, Salomone S. Dopamine D3 receptor is necessary for ethanol consumption: an approach with buspirone. Neuropsychopharmacology. 2014;39:2017–2028. doi: 10.1038/npp.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER. Cellular functions of plasma membrane estrogen receptors. Steroids. 2002;67:471–475. doi: 10.1016/s0039-128x(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Levine ES, Crozier RA, Black IB, Plummer MR. Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-D-aspartic acid receptor activity. Proc Natl Acad Sci USA. 1998;95:10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, DeJoseph MR, Urban JH, Bahi A, Dreyer JL, Meredith GE, Ford KA, Ferrario CR, Loweth JA, Wolf ME. Different roles of BDNF in nucleus accumbens core versus shell during the incubation of cue-induced cocaine craving and its long-term maintenance. J Neurosci. 2013;33:1130–1142. doi: 10.1523/JNEUROSCI.3082-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Fowler CD, Young LJ, Yan Q, Insel TR, Wang Z. Expression and estrogen regulation of brain-derived neurotrophic factor gene and protein in the forebrain of female prairie voles. J Comp Neurol. 2001;433:499–514. doi: 10.1002/cne.1156. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Escalating ethanol intake is associated with altered corticostriatal BDNF expression. J Neurochem. 2009;109:1459–1468. doi: 10.1111/j.1471-4159.2009.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Figurov A. Role of neurotrophins in synapse development and plasticity. Rev Neurosci. 1997;8:1–12. doi: 10.1515/revneuro.1997.8.1.1. [DOI] [PubMed] [Google Scholar]
- Lu B, Wang KH, Nose A. Molecular mechanisms underlying neural circuit formation. Curr Opin Neurobiol. 2009;19:162–167. doi: 10.1016/j.conb.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Cheng PL, Lim BK, Khoshnevisrad N, Poo MM. Elevated BDNF after cocaine withdrawal facilitates LTP in medial prefrontal cortex by suppressing GABA inhibition. Neuron. 2010;67:821–833. doi: 10.1016/j.neuron.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004a;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004b;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2007;89:312–323. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Frankfurt M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience. 2013;239:34–45. doi: 10.1016/j.neuroscience.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berlin) 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology (Berlin) 2000;148:196–200. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berlin) 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- MacLennan AJ, Lee N, Walker DW. Chronic ethanol administration decreases brain-derived neurotrophic factor gene expression in the rat hippocampus. Neurosci Lett. 1995;197:105–108. doi: 10.1016/0304-3940(95)11922-j. [DOI] [PubMed] [Google Scholar]
- Madara JC, Levine ES. Presynaptic and postsynaptic NMDA receptors mediate distinct effects of brain-derived neurotrophic factor on synaptic transmission. J Neurophysiol. 2008;100:3175–3184. doi: 10.1152/jn.90880.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Hamlin AS, McNally GP. Lateral hypothalamus is required for context-induced reinstatement of extinguished reward seeking. J Neurosci. 2009;29:1331–1342. doi: 10.1523/JNEUROSCI.5194-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Furlong TM, McNally GP. Medial dorsal hypothalamus mediates the inhibition of reward seeking after extinction. J Neurosci. 2010;30:14102–14115. doi: 10.1523/JNEUROSCI.4079-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty JF, Zelek-Molik A, Sun WL. Cocaine self-administration causes signaling deficits in corticostriatal circuitry that are reversed by BDNF in early withdrawal. Brain Res. 2014 doi: 10.1016/j.brainres.2014.09.050. http://dx.doi.org/10.1016/j.brainres.2014.09.050. [DOI] [PMC free article] [PubMed]
- McGinty JF, Whitfield TW, Jr, Berglind WJ. Brain-derived neurotrophic factor and cocaine addiction. Brain Res. 2009;1314:183–193. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough NN, He DY, Logrip ML, Jeanblanc J, Phamluong K, Luong K, Kharazia V, Janak PH, Ron D. RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. J Neurosci. 2004;24:10542–10552. doi: 10.1523/JNEUROSCI.3714-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, McGinty JF, Kalivas PW, Becker HC. Brain region-specific gene expression changes after chronic intermittent ethanol exposure and early withdrawal in C57BL/6J mice. Addict Biol. 2012;17:351–364. doi: 10.1111/j.1369-1600.2011.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo E, Milton AL, Goozee ZY, Theobald DE, Everitt BJ. Reconsolidation and extinction are dissociable and mutually exclusive processes: behavioral and molecular evidence. J Neurosci. 2014;34:2422–2431. doi: 10.1523/JNEUROSCI.4001-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi E, Ying SW, Kanhema T, Croll SD, Bramham CR. Brain-derived neurotrophic factor triggers transcription-dependent, late phase long-term potentiation in vivo. J Neurosci. 2002;22:7453–7461. doi: 10.1523/JNEUROSCI.22-17-07453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Kusaka T, Yokoyama T, Ohta K, Suzuki S, Warita K, Jamal M, Wang ZY, Ueki M, Liu JQ, Yakura T, Tamai M, Sumitani K, Hosomi N, Takeuchi Y. Short-term ethanol exposure causes imbalanced neurotrophic factor allocation in the basal forebrain cholinergic system: a novel insight into understanding the initial processes of alcohol addiction. J Neural Transm. 2013;121:201–210. doi: 10.1007/s00702-013-1085-y. [DOI] [PubMed] [Google Scholar]
- Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164:887–895. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, Goldstein JM. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–658. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, Furlong TM, McNally GP. Accumbens shell-hypothalamus interactions mediate extinction of alcohol seeking. J Neurosci. 2010;30:4626–4635. doi: 10.1523/JNEUROSCI.4933-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ. The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. Eur J Neurosci. 2010;31:2308–2319. doi: 10.1111/j.1460-9568.2010.07249.x. [DOI] [PubMed] [Google Scholar]
- Milton AL. Drink, drugs and disruption: memory manipulation for the treatment of addiction. Curr Opin Neurobiol. 2012;23:706–712. doi: 10.1016/j.conb.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction–reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Saunders BT, Maren S, Robinson TE. Sign-tracking to an appetitive cue predicts incubation of conditioned fear in rats. Behav Brain Res. 2014;S0166–4328:00220–00224. doi: 10.1016/j.bbr.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Segal M. Brain-derived neurotrophic factor mediates estradiol-induced dendritic spine formation in hippocampal neurons. Proc Natl Acad Sci USA. 1998;95:11412–11417. doi: 10.1073/pnas.95.19.11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA., Jr D-Cycloserine effects on extinction of conditioned responses to drug-related cues. Biol Psychiatry. 2012;71:947–955. doi: 10.1016/j.biopsych.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nedic G, Perkovic MN, Sviglin KN, Muck-Seler D, Borovecki F, Pivac N. Brain-derived neurotrophic factor Val66Met polymorphism and alcohol-related phenotypes. Prog Neuropsychopharmacol Biol Psychiatry. 2012;40:193–198. doi: 10.1016/j.pnpbp.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Kantak KM. Cognitive enhancers for facilitating drug cue extinction: insights from animal models. Pharmacol Biochem Behav. 2011;99:229–244. doi: 10.1016/j.pbb.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JM, Fitzgerald MK, Mueller D. Infralimbic BDNF/TrkB enhancement of GluN2B currents facilitates extinction of a cocaine-conditioned place preference. J Neurosci. 2014;34:6057–6064. doi: 10.1523/JNEUROSCI.4980-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov I. Conditioned Reflexes. Oxford University Press; London: 1927. [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328:1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Pattij T, De Vries TJ. Targeting cocaine versus heroin memories: divergent roles within ventromedial prefrontal cortex. Trends Pharmacol Sci. 2013;34:689–695. doi: 10.1016/j.tips.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Portugal GS, Al-Hasani R, Fakira AK, Gonzalez Romero JL, Melyan Z, McCall JG, Bruchas MR, Moron JA. Hippocampal long-term potentiation is disrupted during expression and extinction but is restored after reinstatement of morphine place preference. J Neurosci. 2014;34:527–538. doi: 10.1523/JNEUROSCI.2838-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu L, Bao GB, Xu NJ, Ma L, Pei G. Hippocampal long-term potentiation is reduced by chronic opiate treatment and can be restored by re-exposure to opiates. J Neurosci. 2002;22:1914–1921. doi: 10.1523/JNEUROSCI.22-05-01914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2007;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Lin CY, Kramar EA, Chen LY, Gall CM, Lynch G. Brain-derived neurotrophic factor promotes long-term potentiation-related cytoskeletal changes in adult hippocampus. J Neurosci. 2007;27:3017–3029. doi: 10.1523/JNEUROSCI.4037-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav. 1977;6:615–620. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc London B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Vidal LE, Do-Monte FH, Sotres-Bayon F, Quirk GJ. Hippocampal-prefrontal BDNF and memory for fear extinction. Neuropsychopharmacology. 2014;39:2161–2169. doi: 10.1038/npp.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Matsumoto M, Otani S, Yanagawa Y, Hiraide S, Ishikawa S, Kimura S, Shimamura K, Togashi H. Phase-dependent synaptic changes in the hippocampal CA1 field underlying extinction processes in freely moving rats. Neurobiol Learn Mem. 2012;97:361–369. doi: 10.1016/j.nlm.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Sakata K, Martinowich K, Woo NH, Schloesser RJ, Jimenez DV, Ji Y, Shen L, Lu B. Role of activity-dependent BDNF expression in hippocampal-prefrontal cortical regulation of behavioral perseverance. Proc Natl Acad Sci USA. 2013;110:15103–15108. doi: 10.1073/pnas.1222872110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmanzadeh F, Fathollahi Y, Semnanian S, Shafizadeh M. Dependence on morphine impairs the induction of long-term potentiation in the CA1 region of rat hippocampal slices. Brain Res. 2003;965:108–113. doi: 10.1016/s0006-8993(02)04144-6. [DOI] [PubMed] [Google Scholar]
- Sato K, Akaishi T, Matsuki N, Ohno Y, Nakazawa K. Beta-estradiol induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain Res. 2007;1150:108–120. doi: 10.1016/j.brainres.2007.02.093. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol Psychiatry. 2010;67:730–736. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, O’Donnell EG, Aurbach EL, Robinson TE. A cocaine context renews drug seeking preferentially in a subset of individuals. Neuropsychopharmacology. 2014;39:2816–2823. doi: 10.1038/npp.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Maclusky NJ. Similarities between actions of estrogen and BDNF in the hippocampus: coincidence or clue? Trends Neurosci. 2005;28:79–85. doi: 10.1016/j.tins.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Hintz TM, Gomez J, Stormes KA, Barouk S, Malthankar-Phatak GH, McCloskey DP, Luine VN, Maclusky NJ. Changes in hippocampal function of ovariectomized rats after sequential low doses of estradiol to simulate the preovulatory estrogen surge. Eur J Neurosci. 2007;26:2595–2612. doi: 10.1111/j.1460-9568.2007.05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Raio CM, Phelps EA. Extinction training during the reconsolidation window prevents recovery of fear. J Vis Exp. 2012:e3893. doi: 10.3791/3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schjetnan AG, Escobar ML. In vivo BDNF modulation of hippocampal mossy fiber plasticity induced by high frequency stimulation. Hippocampus. 2010;22:1–8. doi: 10.1002/hipo.20866. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Nader K, Pruessner JC. Reconsolidation of human memory: brain mechanisms and clinical relevance. Biol Psychiatry. 2014;76:274–280. doi: 10.1016/j.biopsych.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Shen K, Cowan CW. Guidance molecules in synapse formation and plasticity. Cold Spring Harb Perspect Biol. 2010;2:a001842. doi: 10.1101/cshperspect.a001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Trends Pharmacol Sci. 2002;23:527–534. doi: 10.1016/s0165-6147(02)02093-x. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Surgical menopause, estrogen, and cognitive function in women: what do the findings tell us? Ann NY Acad Sci. 2005;1052:3–10. doi: 10.1196/annals.1347.001. [DOI] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. J Neurosci. 2006;26:8517–8522. doi: 10.1523/JNEUROSCI.5279-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH, Voss HU, Glover G, Ballon DJ, Liston C, Teslovich T, Van Kempen T, Lee FS, Casey BJ. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers HJ, Bendor D. Enhance, delete, incept: manipulating hippocampus-dependent memories. Brain Res Bull. 2014;105:2–7. doi: 10.1016/j.brainresbull.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stragier E, Massart R, Salery M, Hamon M, Geny D, Martin V, Boulle F, Lanfumey L. Ethanol-induced epigenetic regulations at the Bdnf gene in C57BL/6J mice. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.38. [DOI] [PubMed] [Google Scholar]
- Tapia-Arancibia L, Rage F, Givalois L, Dingeon P, Arancibia S, Beauge F. Effects of alcohol on brain-derived neurotrophic factor mRNA expression in discrete regions of the rat hippocampus and hypothalamus. J Neurosci Res. 2001;63:200–208. doi: 10.1002/1097-4547(20010115)63:2<200::AID-JNR1012>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Tapocik JD, Solomon M, Flanigan M, Meinhardt M, Barbier E, Schank JR, Schwandt M, Sommer WH, Heilig M. Coordinated dysregulation of mRNAs and microRNAs in the rat medial prefrontal cortex following a history of alcohol dependence. Pharmacogenomics J. 2012;13:286–296. doi: 10.1038/tpj.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapocik JD, Barbier E, Flanigan M, Solomon M, Pincus A, Pilling A, Sun H, Schank JR, King C, Heilig M. microRNA-206 in rat medial prefrontal cortex regulates BDNF expression and alcohol drinking. J Neurosci. 2014;34:4581–4588. doi: 10.1523/JNEUROSCI.0445-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Bermeo C, Wang GJ, Volkow ND. D-cycloserine facilitates extinction of cocaine self-administration in rats. Synapse. 2011;65:938–944. doi: 10.1002/syn.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theberge FR, Pickens CL, Goldart E, Fanous S, Hope BT, Liu QR, Shaham Y. Association of time-dependent changes in mu opioid receptor mRNA, but not BDNF, TrkB, or MeCP2 mRNA and protein expression in the rat nucleus accumbens with incubation of heroin craving. Psychopharmacology (Berl) 2012;224:559–571. doi: 10.1007/s00213-012-2784-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Painter MR, Pentkowski NS, Mitroi D, Crawford CA, Neisewander JL. Environmental enrichment counters cocaine abstinence-induced stress and brain reactivity to cocaine cues but fails to prevent the incubation effect. Addict Biol. 2011;17:365–377. doi: 10.1111/j.1369-1600.2011.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting-A-Kee R, Vargas-Perez H, Bufalino MR, Bahi A, Dreyer JL, Tyndale RF, van der Kooy D. Infusion of brain-derived neurotrophic factor into the ventral tegmental area switches the substrates mediating ethanol motivation. Eur J Neurosci. 2013;37:996–1003. doi: 10.1111/ejn.12105. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Sanchez H, Taylor JR. D-cycloserine reduces the context specificity of pavlovian extinction of cocaine cues through actions in the nucleus accumbens. J Neurosci. 2010;30:10526–10533. doi: 10.1523/JNEUROSCI.2523-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Corlett PR, Taylor JR. Aberrant learning and memory in addiction. Neurobiol Learn Mem. 2011;96:609–623. doi: 10.1016/j.nlm.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Taylor JR. Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology (Berlin) 2012;226:659–672. doi: 10.1007/s00213-012-2750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Burnett EJ, Gass JT, Lopez MF, Mulholland PJ, Centanni SW, Floresco SB, Chandler LJ. Chronic alcohol disrupts dopamine receptor activity and the cognitive function of the medial prefrontal cortex. J Neurosci. 2014;34:3706–3718. doi: 10.1523/JNEUROSCI.0623-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien JZ. Linking Hebb’s coincidence-detection to memory formation. Curr Opin Neurobiol. 2001;10:266–273. doi: 10.1016/s0959-4388(00)00070-2. [DOI] [PubMed] [Google Scholar]
- Vargas-Perez H, Ting AKR, Walton CH, Hansen DM, Razavi R, Clarke L, Bufalino MR, Allison DW, Steffensen SC, van der Kooy D. Ventral tegmental area BDNF induces an opiate-dependent-like reward state in naive rats. Science. 2009;324:1732–1734. doi: 10.1126/science.1168501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Perez H, Bahi A, Bufalino MR, Ting-A-Kee R, Maal-Bared G, Lam J, Fahmy A, Clarke L, Blanchard JK, Larsen BR, Steffensen S, Dreyer JL, van der Kooy D. BDNF signaling in the VTA links the drug-dependent state to drug withdrawal aversions. J Neurosci. 2014;34:7899–7909. doi: 10.1523/JNEUROSCI.3776-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Xie Y, Su L, Liu Y, Wang Y, Wang Z. RACK1 affects morphine reward via BDNF. Brain Res. 2011;1416:26–34. doi: 10.1016/j.brainres.2011.07.045. [DOI] [PubMed] [Google Scholar]
- Wang WS, Kang S, Liu WT, Li M, Liu Y, Yu C, Chen J, Chi ZQ, He L, Liu JG. Extinction of aversive memories associated with morphine withdrawal requires ERK-mediated epigenetic regulation of brain-derived neurotrophic factor transcription in the rat ventromedial prefrontal cortex. J Neurosci. 2012;32:13763–13775. doi: 10.1523/JNEUROSCI.1991-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM. Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction. 1996;91:921–49. (discussion 951–65) [PubMed] [Google Scholar]
- Willcocks AL, McNally GP. The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. Eur J Neurosci. 2013;37:259–268. doi: 10.1111/ejn.12031. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. Brain mechanisms of drug reward and euphoria. Psychiatr Med. 1985;3:445–460. [PubMed] [Google Scholar]
- Wojnar M, Brower KJ, Strobbe S, Ilgen M, Matsumoto H, Nowosad I, Sliwerska E, Burmeister M. Association between Val66Met brain-derived neurotrophic factor (BDNF) gene polymorphism and post-treatment relapse in alcohol dependence. Alcohol Clin Exp Res. 2009;33:693–702. doi: 10.1111/j.1530-0277.2008.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Plummer MR, Len GW, Nakazawa T, Yamamoto T, Black IB, Wu K. Brain-derived neurotrophic factor rapidly increases NMDA receptor channel activity through Fyn-mediated phosphorylation. Brain Res. 2006;1121:22–34. doi: 10.1016/j.brainres.2006.08.129. [DOI] [PubMed] [Google Scholar]
- Yager LM, Robinson TE. A classically conditioned cocaine cue acquires greater control over motivated behavior in rats prone to attribute incentive salience to a food cue. Psychopharmacology (Berl) 2012;226:217–228. doi: 10.1007/s00213-012-2890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Mizuno M, Nabeshima T. Role for brain-derived neurotrophic factor in learning and memory. Life Sci. 2002;70:735–744. doi: 10.1016/s0024-3205(01)01461-8. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nabeshima T. Interaction of BDNF/TrkB signaling with NMDA receptor in learning and memory. Drug News Perspect. 2004;17:435–438. doi: 10.1358/dnp.2004.17.7.863702. [DOI] [PubMed] [Google Scholar]
- Yu H, Wang Y, Pattwell S, Jing D, Liu T, Zhang Y, Bath KG, Lee FS, Chen ZY. Variant BDNF Val66Met polymorphism affects extinction of conditioned aversive memory. J Neurosci. 2009;29:4056–4064. doi: 10.1523/JNEUROSCI.5539-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhang S, Epstein DH, Fang Y, Shi J, Qin H, Yao S, Le Foll B, Lu L. Gender and stimulus difference in cue-induced responses in abstinent heroin users. Pharmacol Biochem Behav. 2007;86:485–492. doi: 10.1016/j.pbb.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Zanardini R, Fontana A, Pagano R, Mazzaro E, Bergamasco F, Romagnosi G, Gennarelli M, Bocchio-Chiavetto L. Alterations of brain-derived neurotrophic factor serum levels in patients with alcohol dependence. Alcohol Clin Exp Res. 2011;35:1529–1533. doi: 10.1111/j.1530-0277.2011.01489.x. [DOI] [PubMed] [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Goldstein JM, Milad MR. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry. 2011;70:920–927. doi: 10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang X, Su H, Tao J, Xie Y, Han B, Lu Y, Wei Y, Sun H, Wang Y, Wu W, Zou S, Liang H, Zoghbi AW, Tang W, He J. Increased serum brain-derived neurotrophic factor levels during opiate withdrawal. Neurosci Lett. 2014;571:61–65. doi: 10.1016/j.neulet.2014.04.048. [DOI] [PubMed] [Google Scholar]
- Zilkha N, Feigin E, Barnea-Ygael N, Zangen A. Induction of depressive-like effects by subchronic exposure to cocaine or heroin in laboratory rats. J Neurochem. 2014;130:575–582. doi: 10.1111/jnc.12753. [DOI] [PubMed] [Google Scholar]