Abstract
Iron, copper and zinc are required nutrients for many organisms but also potent toxins if misappropriated. An overload of any of these metals can be cytotoxic and ultimately lead to organ failure, whereas deficiencies can result in anemia, weakened immune system function, and other medical conditions. Cellular metal imbalances have been implicated in neurodegenerative diseases, cancer and infection. It is therefore critical for living organisms to maintain careful control of both the total levels and subcellular distributions of these metals to maintain healthy function. This perspective explores several strategies envisioned to alter the bioavailability of metal ions by using synthetic metal-binding agents targeted for diseases where misappropriated metal ions are suspected of exacerbating cellular damage. Specifically, we discuss chemical properties that influence the pharmacological outcome of a subset of metal-binding agents known as ionophores, and review several examples that have shown multiple pharmacological activities in metal-related diseases, with a specific focus on copper.
1. Introduction
Metal dyshomeostasis and oxidative stress
Essential metal ions including zinc (Zn), copper (Cu) and iron (Fe) are strictly controlled in the body and maintained within appropriate physiological levels by dedicated proteins associated with metal trafficking and homeostasis pathways. However, aberrant metal metabolism that disrupts this homeostasis can contribute to human disease. The regulation of Cu and Fe are particularly important due to their innate property of accessing multiple oxidation states. In a biological context, the common oxidation states are Cu+/2+ and Fe2+/3+, although higher oxidation numbers can be accessed during some enzymatic cycles. Under certain conditions, these redox-active metals can catalyze the Fenton reaction (Scheme 1), wherein they react with hydrogen peroxide (H2O2) and generate hydroxyl anion (OH−) and hydroxyl radical (OH•), the most reactive of the reactive oxygen species (ROS). In the presence of reductants, such as ascorbate or glutathione, this reaction becomes catalytic, as the metal is reduced and is able to react with another molecule of hydrogen peroxide. If unchecked, this reaction can produce dangerously high levels of hydroxyl radicals, leading to irreparable damage of lipids, nucleic acids, and proteins, ultimately resulting in cell death.
Scheme 1.
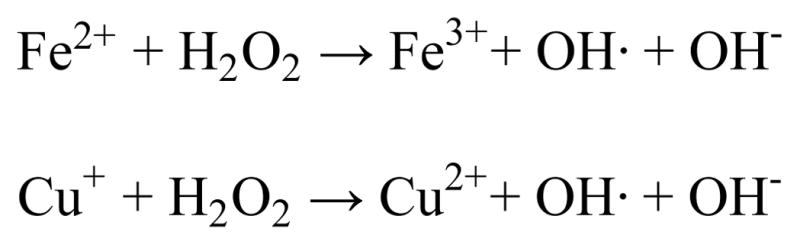
Fenton reaction
In the case of Cu, ROS formation directly from Cu redox cycling cannot be the sole mechanism of Cu toxicity to cells. Studies show that elevated levels of Cu+ remain highly toxic in both bacteria and yeast cells growing under anaerobic conditions, an environment where ROS are unable to form.1–3 Recently, Imlay et al. demonstrated that Cu rapidly inactivates the catalytic iron-sulfur (Fe-S) clusters of dehydratases in Escherichia coli.4 The study shows that Fe-S clusters are the primary targets of Cu, which is thought to displace Fe from the cluster and coordinate the sulfur donor ligands, thereby disabling enzyme activity.4 Enzyme activity could be reactivated by adding neocuproine as an intracellular Cu+ chelating agent, and subsequently supplementing with additional Fe2+.
Chemically manipulating metal status in biology: chelator vs. ionophore
Altering the levels of metal ions by using metal chelating agents is a promising strategy to minimize cellular damage in cases where labile metal ions are producing ROS. Chelating agents are also useful for studying cellular processes related to metal ion transport, storage, use and trafficking. Chelating agents are defined by their ability to bind to metal ions via multiple points of contact to form metal complexes. From a traditional pharmacological standpoint, medicinal chelating agents are used for the therapeutic treatment and removal of metals from the body. Historically, chelating agents have been used to remove toxic exogenous metals such as lead or arsenic.
While chelating agents that sequester and remove heavy metals are still used today for toxic metal poisoning, chelation therapy can also be used to reduce levels of essential metals, particularly in the case of genetic Fe and Cu overload disorders. In Wilson’s disease, as one example, mutations on the ATP7B gene lead to a defect in Cu distribution and detoxification that results in Cu overload in the liver and other organs.5 Currently, there is no curative treatment, and all chelation therapies must be taken lifelong and tend to limit Cu systemically. The first Cu overload chelation therapy was British anti-Lewisite (BAL), but its many painful side effects led to the introduction of D-penicillamine (D-pen) in 1956 and triethylenetetramine (TETA) in 1982, which improved the quality of life for patients with this disease (Fig. 1).6
Fig. 1.
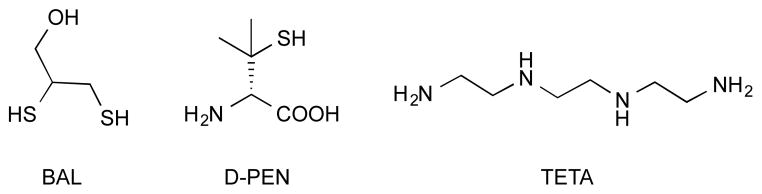
Cu chelators British anti-Lewisite (BAL), D-penicillamine (D-pen) and triethylenetetramine (TETA) for treatment of Wilson’s Disease.
Metal ionophores constitute a distinct subset of chelating agents. Similar to a chelator, an ionophore binds metal ions by multiple points of contact to form metal complexes. The key difference lies in the functional outcome of the metal complex. As described above, a traditional medicinal chelator results in excretion of the metal from the system. In contrast, ionophores typically form lipophilic metal complexes that enable intracellular access for the metal.
The term ionophore (ion bearer) was coined by Pressman in 1967 to describe several classes of antibiotics produced primarily by Streptomyces species. These ‘ionophores’ preferentially bind to sodium (Na+) and potassium (K+) ions and mediate their transport across lipid barriers.7 Monensin A is one example of over 100 naturally occurring ionophores known to form a complex with Na+ and transport it across cell membranes. Importantly, metal affinity varies depending on the solution environment, so once exposed to an aqueous phase, the ion is released from its carrier ligand and is free to exert its biological function. This class of ionophores has broad-spectrum antimicrobial activity against gram-positive bacteria (Staphylococcus, Bacillus), with no activity against gram-negative bacteria.8
While ‘ionophore’ was originally implicated with alkali metal ions, the name has been deployed more broadly to describe compounds that are attracted to any metal ion, with an implicit association as serving to shuttle the ion across a lipid barrier. Given the ambiguous nature of the term, other names given to compounds with this function include ‘metal shuttle’ and ‘metal chaperone’. For the purpose of this perspective, we will adopt the term ‘ionophore’ to mean metal-binding ligands that shuttle or chaperone d-block metals across lipid membranes.
Chemical properties that influence the pharmacological outcome of an ionophore’s activity include the coordination environment presented to the metal ion, the affinity of the ionophore to various metal ions, the redox potential of the metal complexes, and the lipophilicity of the individual and combined species. These properties will be discussed in more detail in the following sections, with a focus on Cu.
2. Chemical properties that affect ionophore activity
Coordination environment
The nature and reactivity of a metal complex is affected by the coordination environment created around the metal center, which includes the identity of the coordinating atoms, the number of coordination sites, and the geometric configuration of the whole complex. The donor atoms that bind to the metal can provide some level of selectivity or preference towards particular metal ions. The principle of “Hard and Soft Acids and Bases” (HSAB) developed by Pearson in the 1960s established a classification of metal ions and their ligands.9 The presence of sulfur, for example, will likely favor soft metal ions like Cu+ while disfavoring hard cations like Ca2+. Conversely, hard, anionic donors like oxygen in alkoxide or phenoxide ligands attract hard trivalent ions like Fe3+ while being less drawn to Cu+. Neutral oxygen and nitrogen donors, meanwhile, are commonly found as donors to metal cations of intermediate hard/soft preference, including Zn2+, Cu2+ and Fe2+.
In addition to the type of donor atom, the number of attachment points, or denticity, the ligand presents to the metal influences complex formation and stability. As denticity increases, stability of the complex usually increases as well, a consequence known as the chelate effect. The boost in affinity is especially pronounced when the size of the binding site created by one or more chelate rings matches the size of a particular ion.10 The resulting stability of a metal complex has ramifications for the biological activity of an ionophore, since stability is a factor in determining whether the ionophore retains the metal, releases it into solution, or exchanges it to other molecules (including proteins) of higher affinity.
Metal affinity
The affinity for a particular metal ion over others is a measure of an ionophore’s selectivity. However, it is important to note that while an ionophore may be ‘selective’ for a certain metal it does not mean it is limited to binding only that metal ion. The chemical similarities of many metals are close enough in their geometric preference, HSAB character, and size that it is difficult to be limited to one specific metal in the presence of a mier, S. Payton, K. A. Tseitlin, J. N. Kremsky, L. Lai, X. Li, R. ve a thermodynamic preference for a specific metal ion, it does not mean that it binds that metal at the exclusion of others.
Redox potential
The ease of reduction of a given metal complex is evaluated by determining the reduction potential. Complexes with negative reduction potentials favor the oxidized form, for example Fe3+ and Cu2+. On the contrary, complexes with positive reduction potentials favor the reduced state, Fe2+ and Cu+. Given the fact that metals in different oxidation states have different ionic radii and different donor atom preferences, changing the oxidation state of a metal influences the thermodynamic stability of the complex. A stable complex, for example, that is reduced to a lower oxidation state could lose its metal because of diminished affinity of the ligand for the lower oxidation state metal.
In addition to influencing complex stability, the reduction potential of a metal complex also affects its reactivity. An example of a chelator that provides a thermodynamically stable coordination environment is ethylenediaminetetraacetic acid (EDTA), which has a high binding affinity for Fe3+ (log β1 = 25.1).11 The reduction potential of the iron complex (+120 mV vs NHE), however, places it in a window of biological oxidation/reduction reactions that exacerbate ROS formation.12 While reduction potentials that are on either the positive or negative extreme prefer one oxidation state and resist redox cycling, complexes that are close to zero versus NHE are thermodynamically capable of being reduced by cellular agents like ascorbate, NADH or glutathione, and oxidized by O2 or H2O2, thereby producing OH•.
Lipophilicity
An important factor for pharmacological screening and drug development is the prediction of absorption and transport of a molecule through cellular membranes. Drugs cross biological barriers most frequently through passive transport, which strongly depends on their lipophilicity. In the case of a chelating ionophore, the active “drug” is both the free ligand and its metal complex, so taking into account the lipophilicity of the ligand with and without the metal ion is important. Once a metal ion is bound to an ionophore, there may be an increase in the lipophilic character of the complex, which may enhance its ability to permeate the lipid layers of the cell membrane. For example, structure–activity relationships of Cu complexes with various 8-hydroxyquinoline ligands revealed the importance of lipophilicity for cytotoxic activity toward cancer cells. The results showed that ligands with log P values between 1.5 and 3 resulted in the highest cytotoxic activity.13 In identifying a ligand that can chaperone metal ions through cell membranes to different compartments, the hydrophobic versus hydrophilic nature of the complex will either hinder or facilitate this flux of metals into a cellular compartment.
Chemical properties – a case study for Cu
The metal ion Cu is essential to biological processes due to its redox chemistry between the two oxidation states Cu2+ and Cu+. According to the HSAB principle, Cu2+ is classified as a borderline Lewis acid, whereas Cu+ is a soft acid.9 This difference in classification helps to explain the different coordination properties of these two oxidation states and their resulting impact on biological systems. Under an oxidizing extracellular environment, Cu is expected to exist as Cu2+. However, upon entry into the cell the metal faces a reducing environment more conducive for Cu+. In its oxidized state, Cu2+ has a geometric preference for square planar, square pyramidal, or axially distorted octahedral geometries due to Jahn-Teller distortions of its d9 electron configuration. Cu2+ also prefers to bind hard Lewis bases (carbonyl and carboxylate oxygen, amide nitrogen) and borderline bases like imidazole nitrogen. On the other hand, Cu+ preferentially binds to soft Lewis bases (thiolate or thioether sulfur) and is flexible in geometric arrangement of tetrahedral, trigonal or linear. These preferences for different coordination environments due to the redox state of Cu will be important when discussing the activity and ability of these molecules to increase cellular Cu levels.
3. Therapeutic application of ionophores for reallocating metals
While traditional chelation therapy removes excess metal ions from the body, emerging studies suggest that ionophores that correct cellular metal imbalances may be therapeutic options for treating neurodegeneration, cancer, and infection.
In Alzheimer’s Disease (AD), for example, data suggest that Cu and Zn levels are abnormally high in the hallmark extracellular amyloid beta (Aβ) plaques, but paradoxically low intracellularly.14 The hypothesis has therefore been posited that redistribution of Cu and Zn from the plaques to the intracellular environment could be a disease-modifying strategy.15
Cu has also been shown to play an important role in cancer. In particular, data suggest that high concentrations of Cu are involved in the development and proliferation of tumors.16, 17 Research in this area has focused on ways to decrease the levels of Cu using traditional Cu chelators like tetrathiomolybdate (TTM), trientine, and D-penicillamine (D-pen). Studies have shown that traditional Cu chelation with TTM and D-pen suppresses angiogenesis, the ability to form new capillary branches from existing blood vessels, in several carcinoma xenograft mouse models such as breast, head and neck, and melanoma.18–21 However, traditional Cu chelators induce systemic Cu deficiency in healthy as well as cancerous tissues, leading to off-target side effects such as axon demyelination and altered collagen production due to lysyl oxidase inhibition.22,23,24 The therapeutic window for traditional Cu chelators may therefore be difficultly narrow.25
Another potential treatment option is targeting metal complexes towards cancer cells over normal cells. This alternative treatment uses metal complexes to selectively increase intracellular metal ions and either restore the metal balance or provide an excessive amount of redox active metal leading to cellular damage of the cancer cells.
In the following sections, we will discuss metal complexes that have shown promise as selective Cu ionophores with multiple pharmacological activities in metal-related diseases. Specifically, we will highlight their ability to restore the balance of misappropriated metal ions or target redox-active metal ions to specific locations.
8-Hydroxyquinoline, Clioquinol, PBT2
8-hydroxyquinoline (8HQ) and its derivatives clioquinol (CQ) and PBT2 are planar chelating agents that bind metal ions through the oxygen and nitrogen atoms in a bidentate fashion. These donor atoms provide a preference for Cu2+ and Zn2+ with moderate binding affinity (Cu2+, log β2 = 26.2 and Zn2+ log β2 = 15.8).11, 26 However, this does not exclude the fact that other metal ions bind with 8HQ and its derivatives, although most of the therapeutic activity and research related to these agents has been done looking at their effect on Zn and Cu.
Historically, 8HQ has been used as a fungicide in agriculture and a preservative in textile, wood and paper industries.27 The antimicrobial activity of 8HQ was observed to be dependent on its complexes with divalent metal ions, notably Fe or Cu.28, 29 An 8HQ derivative, CQ was originally manufactured as a topical treatment for skin wounds but became common as an oral antiparasitic and antibacterial drug.30, 31 Its mechanism of action is likely related to its ionophore properties of increasing the intestinal absorption of Zn.30, 31 The oral use of CQ in Japan between 1950 and 1970 has been linked to cases of subacute myelo-optico-neuropathy (SMON).30, 31 Several mechanisms have been suggested for the cause of this neurological disorder, including vitamin B12 deficiency and elevated Zn levels in the CNS.32 While the exact mechanism has not been confirmed, there are indications that vitamin B12 deficiency exacerbated by CQ can be alleviated by B12 supplementation to decrease the occurrence of SMON in patients.30, 31 Derivatives of 8HQ are currently only commercially used as an antimicrobial agent in topical treatments or application of crops in agriculture due to its limited selectivity against pathogen and host tissue.
In the context of AD, it is suggested that ionophores must cross the blood brain barrier (BBB) and have a moderate metal affinity that is strong enough to extract Cu2+ and Zn2+ from extracellular Aβ aggregates but weak enough to release the metal ion intracellularly to restore the metal reserves of the neurons.15, 33 The ionophoric properties of CQ were studied in neuronal cells to compare its ability to promote the uptake of Cu2+, Zn2+, and Fe3+ ions across the cell membrane. The effect of CQ on cellular production of ROS was also measured. These in vitro studies showed that CQ inhibits metal-mediated Aβ peptide aggregation and diminishes ROS production by metal–Aβ species, while at the same time leads to concentrations of intracellular Cu that are selectively elevated compared to Zn and Fe and relative to untreated controls.34 Further studies demonstrated that treatment of transgenic mouse model with CQ showed promise by decreasing Aβ accumulation by 50%.35 A pilot phase 2 clinical trial of CQ in a small cohort of 36 patients with moderately severe AD showed a decrease in plasma Aβ42 levels.36 While there was some indication of a possible slowing in cognitive decline, the trial was not large enough to provide evidence of a positive clinical benefit for patients.36 In addition, patients showed various side effects including mutagenicity and neurotoxicity, which were hypothesized to be a result of di-iodo-8-hydroxyquinoline impurity. Due to the di-iodo impurity in CQ combined with an improved second-generation 8HQ analogue, PBT2, further clinical trials on CQ were terminated.37, 38
This second generation drug, PBT2, has increased permeability through the BBB, increased ionophoric property, and higher solubility compared to CQ.34 It was observed that PBT2 could selectively chelate Cu and Zn and form neutral soluble complexes capable of passing through cellular membranes and increasing the bioavailable Cu and Zn in neuronal cells.34 Promising preclinical phase I and IIa trials demonstrated significant reduction in Aβ levels and improvement in some aspects of cognitive function.34, 39 However, the results from the phase IIb, randomized clinical trial were not as promising.40 The clinical trial, which involved 42 patients with mild AD treated with either PBT2 or placebo over 52 weeks, showed no statistically significant reduction in levels of Aβ plaques in the brains of AD patients treated with PBT2 versus placebo, though surprisingly both groups showed a reduction in overall Aβ levels. However, PBT2 was well tolerated with no adverse effects and seemed to preserve the hippocampal brain volume better than placebo. While these data cast doubt on PBT2’s ability to modify the disease, research continues in AD with 8HQ derivatives and in other neurodegenerative diseases that have similar characteristics of protein misfolding and displacement of metal ions such as huntington’s disease (HD) and amyotrophic lateral sclerosis (ALS). PBT2 showed promising results by reducing toxicity in a C. elegans model of polyQ aggregation and improved motor performance in a mouse model of HD.41 While it is not conclusive if CQ transports metal ions to provide activity in HD or if these effects may be due to other activities such as reduction of ROS, it is still promising research to be noted.
Similar to AD, anticancer activity provided by 8HQ derivatives including CQ is related to its interaction with Cu and Zn ions. It has been reported that CQ exerts selective anticancer activity toward breast, prostate, leukemia and myeloma cancer cells compared to normal cells.42–45 Further analysis showed CQ in the presence of Cu increased cytotoxicity, thought to be due to the elevated Cu promoting oxidative stress in the cell. This result suggests that CQ can transport metal ions into cells as an ionophore instead of a traditional metal chelator that would limit the available Cu. Another aspect of how CQ can provide antitumor activity is its ability to inhibit the proteasome, which has been shown to be dependent on the presence of Cu, though extremely high concentrations of CQ were required for proteasome inhibition.44
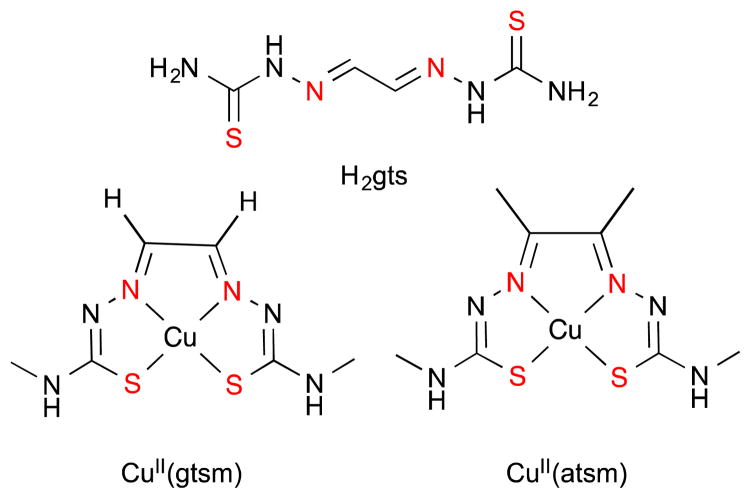
Thiosemicarbazones – ATSM and GTSM
Thiosemicarbazones and bis(thiosemicarbazones) have a wide range of pharmacological activity that is linked to their ability to chelate transitions metals such as Cu, Fe, and Zn, resulting in stable, lipophilic, and often neutral complexes. Studies relating to the anticancer activity of thiosemicarbazones began in the 1950s with observations that glyoxalbis(thiosemicarbazone) (H2gts, Fig. 3) inhibited sarcoma 180 tumor growth in Swiss mice.46 Derivatives of other bis(thiosemicarbazones) were found to have similar anticancer activity that depended on Cu or Zn, though acute hepatic toxicity and weight loss resulted after treatment in a pharmacological study of rats, mice, dogs and monkeys.47–49 This acute toxicity has led researchers to focus on finding derivatives with lower cytotoxicity by increasing selectivity for carcinoma cells over healthy cells through its ionophoric property of chaperoning Cu and releasing under certain environmental conditions.
Fig. 3.
The structure of glyoxal-bis(thiosemicarbazone) (H2gts), glyoxal-bis(4-methylthiosemicarbazonato)copper(II) (CuII(GTSM)), diacetyl-bis(4-methylthiosemicabazonato) copper(II) (CuII(ATSM). The nitrogen and oxygen metal ion coordination sites are highlighted in red and are common among all molecules of this scaffold.
Two thiosemicarbazone derivatives, diacetyl-bis(4methylthiosemicabazonato)copper(II) (CuII(ATSM)) and glyoxal-bis(4-methylthiosemicarbazonato)copper(II) (CuII(GTSM)), with subtle differences due to the substituents on the backbone of the ligand (Fig. 3), have different reactivity and pharmacological properties. The addition of methyl group substituents on the ligand backbone lowers the potential for the coordinated Cu2+ to be reduced to Cu+ (E1/2 = 0.44 mV for CuII(GTSM) compared to E1/2 = −0.60 mV for CuII(ATSM), versus AgCl/Ag).50 This difference in reduction potential results in CuII(ATSM) retaining coordinated Cu better under normal intracellular reductive environments, whereas Cu dissociates readily from CuII(GTSM), thereby increasing intracellular bioavailable Cu. An important distinction is that both metal complexes are able to pass through the membrane at comparable concentrations as measured by ICP-MS.51 This difference in redox behaviour provides ATSM and GTSM unique properties that have been exploited in research and will be discussed briefly.
Due to the elevated levels of intracellular Cu seen in cancer, researchers have looked at whether ionophoric Cu compounds, like CuII(ATSM) and CuII(GTSM) could be used to selectively treat carcinoma cells over normal cells. CuII(ATSM) and CuII(GTSM) were evaluated for their therapeutic efficacy in prostate cancer cells in vitro and in vivo.52 CuII(GTSM) was cytotoxic against cancerous prostate cells in vitro and significantly reduced prostate cancer burden in the TRAMP mouse model.52 On the other hand, CuII(ATSM), was not as cytotoxic in vitro and did not reduce the prostate cancer burden in the mouse model. This trend follows the ionophoric property discussed above, that CuII(GTSM), upon entry into the cell, releases Cu that results in toxicity. Because CuII(ATSM) has a lower reduction potential, it remains intact as the CuII complex intracellularly.
While CuII(ATSM) retains its Cu under normal cell conditions, a more forcing reducing environment like that resulting from hypoxia can lead to reduction and irreversible loss of the Cu+.50 Hypoxia refers to low oxygen environment and has been associated with aggressive tumors that are resistant to chemotherapy, as well as other health problems including heart disease and stroke. The ability to image these diseases and diagnose problems early would be beneficial in providing therapeutic intervention. Consequently, radioactive 60Cu, 62Cu, 64Cu isotopes of CuII(ATSM) have been investigated as hypoxia-selective imaging agents. The first study analyzing 62Cu–(ATSM) as a hypoxic imaging agent was on a rat heart model with either normoxic or hypoxic conditions. The results showed that treatment under either condition led to a rapid uptake of 62Cu–(ATSM), but after 15 minutes only ~20% of the bolus dose was retained in the heart under normoxic conditions, whereas ~80% of the 62Cu was retained under hypoxic conditions.53 Subsequent testing showed that treatment with CuII(ATSM) resulted in increased levels of cell-associated Cu in hypoxic SH-SY5Y neuroblastoma cells compared to control transfected cells, suggesting that Cu delivered as CuII(ATSM) preferentially accumulates in hypoxic environments.51 Due to the promising results of CuII(ATSM), a clinical trial was conducted in which 14 patients diagnosed with cervical cancer were imaged with 60Cu(ATSM). The results indicated that uptake of 60Cu(ATSM) was predictive of prognosis.54 Recently, 62Cu(ATSM) was tested as an imaging agent in a single subject with mitochondrial myopathy, encephalopathy, and lactic acidosis with stroke-like episodes (MELAS), and 15 patients with Parkinson’s Disease.55, 56 Unlike the previous studies that emphasized CuII(ATSM) as an imaging agent for hypoxic tissue, these studies described selective accumulation of Cu from 62Cu(ATSM) as a method to image regionalized oxidative stress.55, 56
Given the promising results of CuII(ATSM) as an imaging agent for neurodegenerative disease and the apparent attraction of bis(thiosemicarbazones) for oxidative stress environments, it is not surprising that studies began looking at the applicability of using these metal complexes to reallocate metal imbalances in the brain, a hallmark of several neurodegenerative diseases. Therefore, a study examined the effect of CuII(ATSM) and CuII(GTSM) on the extracellular levels of Aβ in ovary cells overexpressing the amyloid precursor protein for Aβ.50 The study found that overall levels of intracellular Cu were elevated for both CuII(ATSM) and CuII(GTSM), as determined by ICP-MS.50 However, only treatment with CuII(GTSM) resulted in a dose-dependent reduction of extracellular Aβ.50, 57 Subsequently, treatment of APP/PS1 transgenic AD mice with CuII(GTSM) showed a decrease in the abundance of Aβ along with significant cognitive improvement.58 This reduction in Aβ along with improvement of cognition of AD mice provides strong support that, similar to 8HQ derivatives, thiosemicarbazones can increase bioavailable Cu in a fashion that could provide disease-altering treatment.
In the context of antimicrobial therapeutics, the development of antimicrobial agents that have selective toxicity against pathogens over host tissue is a continued focus of researchers, especially in the use of metal complexes. CuII(ATSM) and CuII(GTSM) are promising Cu-based drugs due to their wide use in clinical experiments and relatively low cytotoxic effects to healthy tissue. The antimicrobial effects of CuII(ATSM) and CuII(GTSM) were evaluated in the human pathogen Neisseria gonorrhoeae.59 It was observed that CuII(GTSM) and CuII(ATSM) were more than 100 times more effective at killing N. gonorrhoeae than Cu(NO3)2.59 It was determined the antimicrobial activity of the of these CuII bis(thiosemicarbazones) was due to inhibition of NADH dehydrogenases in the bacterial respiratory chain. ATSM and GTSM induced Cu dependent inhibition of Mycobacterium tuberculosis with GTSM in the presence of Cu being more potent.60
Disulfiram
Disulfiram (DSF, Fig. 4) is a member of the dithiocarbamate family that was first approved by the Food and Drug Administration (FDA) for treatment of alcoholism. DSF induces adverse side effects in patients who have consumed alcohol by inhibiting aldehyde dehydrogenase, a crucial enzyme in ethanol detoxification. Upon administration of DSF, the drug is reduced by glutathione reductase to diethyldithiocarbamate (DDC), which is capable of complexing Cu (Fig. 4).61, 62 The Cu(DDC)2 complex is more acid-stable, neutral and hydrophobic than DSF itself, thereby facilitating absorption into the bloodstream and the BBB. The Cu-dependent activity of this drug is probably due to its metabolic break down in the body to DDC.
Fig. 4.
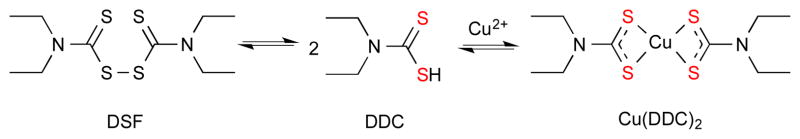
Disulfiram (DSF) in aqueous solutions is reduced to diethyldithiocarbamate (DDC) and in the presence of Cu2+ forms bis(diethyldithiocarbamate)copper(II): Cu(DDC)2. The sulfur metal ion coordination sites are highlighted in red.
DSF has anticancer activity through its ability to increase ROS, inhibit the proteasome, and induce apoptosis.63 While its activity is multifaceted, studies have shown that co-administration with Cu increases DSF’s anticancer activity and selectivity. A study in breast cancer cells demonstrated that DSF has selective anticancer activity in the presence of Cu by acting as a proteasome inhibitor and inducing apoptosis.64 Furthermore, treatment of nude mice with DSF significantly inhibited breast tumor growth.64 In addition, DSF has been shown to induce ROS and activate apoptotic or paraptotic death in melanoma cells and fibrosarcoma cells in a Cu-dependent manner and is currently undergoing evaluation in a number of cancers.65 Interestingly, prostate cancer patients have relatively elevated Cu levels that may provide a differential advantage for drugs that manipulate cellular Cu as an anticancer strategy.25 It was found that treatment of DSF supplemented with Cu enhanced the growth inhibitory activity of DSF in prostate cancer mouse model suggesting it likely inhibits these cells by overloading Cu in the cell.25 A clinical trial is on going to explore the impact of Cu supplementation of DSF efficacy in patients with advanced prostate cancer.
Recently, a promising new application for DSF has been suggested to reallocate Cu in Menkes Disease (MD). This genetic Cu metabolism disorder is caused by mutations in the ATP7A gene that controls Cu transport from the cytosol to the Golgi apparatus and Cu excretion from cells.66, 67 In patients with MD, dysfunctional ATP7A causes a failure of Cu absorption from the intestines and a resulting systemic Cu deficiency that manifests in connective tissue abnormalities, severe neurodegeneration and kinky hair.68 Injection of Cu-histidine is the standard treatment to correct the systemic Cu deficiency. This treatment decreases efficacy as patients age, and the increasingly higher doses required to correct Cu levels in the brain can lead to toxicity in the kidneys.69
Researchers have been looking for Cu ionophores to chaperone Cu through the BBB and supply the brain with sufficient Cu levels without cytotoxicity elsewhere. Toward this goal, DSF was investigated for its effect on the distribution of injected radioactive 64Cu in MD model mice imaged by positron emission tomography (PET).70 The results showed that Cu administered without DSF accumulated in the kidneys, while Cu co-administered with DSF led to increased Cu uptake in the brain and liver with a decrease in kidneys.70 This study validated earlier results showing oral DSF with injection of Cu increased cerebrum Cu levels and decreased Cu kidney levels after 8 weeks of treatment.71 In addition, this study showed that DSF enhances expression of cytochrome c oxidase, a Cu-dependent enzyme that is reduced in the MD mouse. This recovery in a key Cu-dependent enzyme shows DSF’s ability to make Cu bioavailable.71, 72 Similar results were found with the DSF metabolite, DDC in its ability to improve Cu concentrations in the brain and activity of cytochrome c oxidase, suggesting DSF and its metabolites are viable candidates to redistribute Cu to the brain.73
Elesclomol
Elesclomol (STA-4783) is being developed as an anticancer agent that selectively binds Cu2+ to form a slightly distorted square planar complex with a high binding affinity of 1024.1 M−1 (Fig. 5).74 It was identified by screening a unique compound library for cytotoxic effects on cancer cells, with further optimization of a chemically unstable hit compound.75 Preclinical studies showed that growth of human tumor xenografts of breast, lung, and lymphoma cancers in nude mice were unaffected by elesclomol alone but synergistically reduced by co-administration with paclitaxel, a commonly prescribed chemotherapeutic agent.76 While the underlying mechanism behind the synergistic effect with paclitaxel is not known, preliminary data suggest elesclomol increases the intracellular level of oxidative stress above the threshold cancer cells can handle, thereby resulting in apoptosis and cell death.77
Fig. 5.
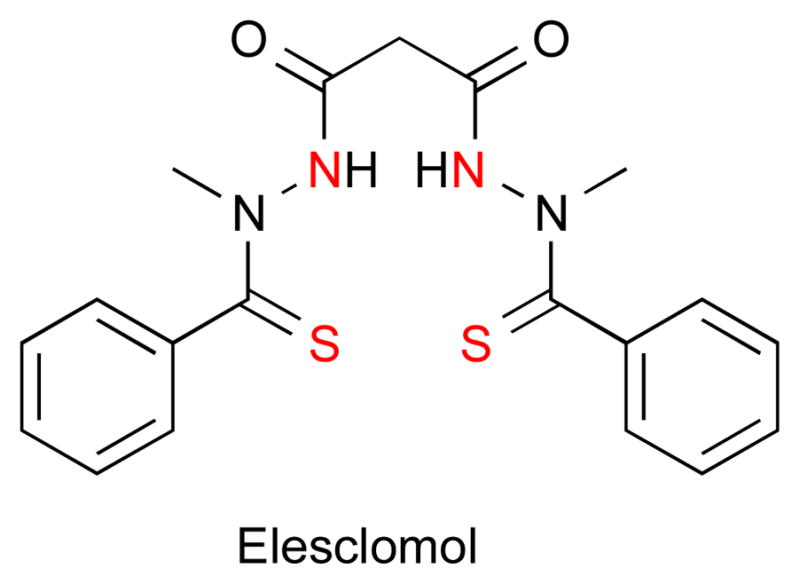
The structure of elesclomol is depicted with the nitrogen and sulfur metal ion coordination sites highlighted in red.
Recently, elesclomol-induced ROS generation has been shown to be dependent on the chelation and redox cycling of Cu. The cell viability of melanoma cancer cells along with the uptake of Cu were analyzed after treatment with elesclomol with and without addition of Cu.78 The results show that elesclomol scavenges Cu from the culture medium and enters the cell as the intact complex. The addition of bathocuproinedisulfonic acid (BCS), a Cu+ chelator that limits accessible Cu in cell culture medium, minimized the effects of Cu plus elesclomol, suggesting that the activity of elesclomol requires Cu.78 Furthermore, elesclomol-Cu accumulates specifically in the mitochondria compared to cytosol or nucleus. To better elucidate elesclomol’s cytotoxic target, researchers used a single mutation yeast strain library of Saccharomyces cerevisiae and compared the growth of mutated S. cerevisiae to wild type (WT).79 In testing this gene mutation library, there was no ‘unique’ protein target, however, mutations that were sensitive to treatment were centralized around respiring mitochondria. It is interesting to note that wild type S. cerevisiae cultures treated with elesclomol and Cu showed potent growth inhibition, while treatment with elesclomol alone had no effect on growth. Furthermore, the co-treatment of elesclomol and Cu in S. cerevisiae led to cell death, suggesting promise for elesclomol as an antimicrobial agent.79
Pyrithione
Pyrithione (PyS, Fig. 6) is an antimicrobial agent with common applications as the active ingredient zinc pyrithione (ZPT) in antidandruff shampoos and antifouling paint for ships. The crystalline complex is a dimer of Zn(PyS)2 units in which the O of one PyS unit bridges to the adjacent Zn to form trigonal bipyramidal Zn centers.80 A trans-square planar Cu(PyS)2 complex is also known.81 Despite decades of successful use to treat human scalps against the fungal pathogens Malassezia glabosa and M. restricta, the mechanism behind the antifungal activity has just recently begun to be elucidated.
Fig. 6.

The structure of pyrithione (1-hydroxypyridine-2-thione) is depicted with the sulfur and oxygen metal ion coordination sites highlighted in red.
Independent studies suggest the mechanism of action for ZPT originates from its ability to elevate Zn levels, induce an Fe starvation response, and/or depolarize the cell membrane.82, 83 Recently, a new mechanism of action has come to light in which ZPT exchanges its Zn for Cu in a biological medium. It is suggested that the Cu complex mediates the elevation of intracellular Cu levels and inhibits growth. This increase in cellular Cu content was determined by a combination of atomic absorption spectroscopy, a gene expression response indicative of excess Cu in the cell through the downregulation of Cu importers, and a requirement for environmental Cu for PyS’s antifungal activity.84, 85 The study also correlated the upregulation of the Fe regulon to the inactivation of proteins containing Fe-S clusters and not to Fe starvation.84, 86
While ZPT has been extensively used as a bactericide and fungicide, the Zn metal complex itself is highly insoluble in aqueous solutions and therefore poor bioavailability. In order to overcome this barrier, water-soluble derivatives of ZPT were developed in hopes they would elevate intracellular Zn levels in cancer to produce antiproliferative activity. After developing several water-solubilized versions of ZPT, a derivative a tri(ethyleneglycol)-methyl ether substituent at the 5-position (PCI-5003) provided an increase in intracellular Zn levels in vitro and inhibited growth of lung and prostate cancer cells grown in xenografts models.87
Responsive metal chelation by prochelators
The challenge in developing chelating agents and ionophores to manipulate biological metal ions is selecting only the misallocated, harmful metal ion without disturbing the healthy metal balance of the body. Some ionophores discussed above have shown selectivity for specific environments, such as ATSM for hypoxia or oxidative stress. An alternative approach is to prevent non-specific metal binding by adding a chemical moiety that masks one or more donor atoms on the ligand and thereby decreases the binding affinity to metal ions. Release of the chemical moiety from the ionophore by a stimulus associated with a particular disease condition in principle provides a strategy for targeting these agents at the desired site. For example, our lab has developed a prochelator strategy that takes advantage of the reactivity associated with oxidative stress to generate metal chelators that inhibit further metal-induced oxidative damage.88, 89 These prochelators have little to no affinity for metal ions due to a boronic ester that masks a latent hydroxyl group needed for metal binding. However, in the presence of high concentrations of hydrogen peroxide the boronic ester of the prochelator is converted to a phenol and the chelator strongly binds iron to prevent Fenton chemistry.89 An example is a later-generation prochelator BHAPI, which does not perturb the Fe status of non-stressed cells, unlike standard Fe chelators.90
We recently established prochelators as a class of antimicrobial compounds that synergize with the host’s response to infection and disrupt the efficacy of microbial Cu detoxification.91 As described in a previous section, 8-hydroxyquinoline (8HQ) is a privileged metal-binding scaffold with known metal-dependent biological activity, including antimicrobial activity.29 However, these agents can be toxic to healthy mammalian cells as well.13, 92–94 In order to create a conditionally active agent that would preferentially act on microbial cells over mammalian cells, we explored the antifungal activity of QBP, a prochelator form of 8HQ that contains a boronic ester masking group in place of 8HQ’s hydroxyl group (Scheme 2).89 We demonstrated that the oxidative burst generated by activated macrophages is sufficient to mediate conversion of nontoxic QBP to 8HQ. Importantly, the toxicity of released 8HQ was pronounced for the opportunistic fungal pathogen Cryptococcus neoformans but minimal for the murine RAW macrophages. We further showed that 8HQ exerts its fungicidal effects by increasing cell-associated Cu to overwhelm the Cu detoxification capacity of C. neoformans. The Cu-dependent antimicrobial activity of 8HQ against a spectrum of microbial pathogens suggests that this strategy may have broad utility.
Scheme 2.
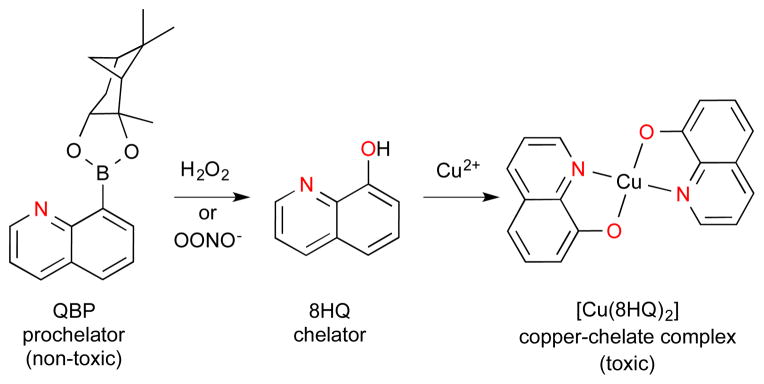
The non-toxic prochelator QBP in the presence of H2O2 or OONO− converts to form the metal chelating agent 8HQ that forms a microbicidal bis(di-8-hydroxyquinoline) copper(II) complex: Cu(8HQ)2. The nitrogen and oxygen metal ion coordination sites are highlighted in red.
4. Conclusion
Research aimed at developing metal ionophores as potential treatments for a broad range of conditions, including neurodegenerative diseases, cancer and bacterial infections is moving in a new direction that blends altering concentration of a target metal ion with additional functionality to increase selectivity to the environmental conditions of a particular disease. In this Perspective, we have highlighted a range of therapeutic applications where Cu ionophores are effective as copper binding agents that alter copper concentrations in ways that mitigate potential cellular damage while also providing some degree of specificity for disease conditions. Inorganic chemists have an important role to play in identifying and preparing compounds with molecular features that tailor metal complexes for a particular application. Some of the tailoring features explored here included those that facilitate permeability across the blood-brain barrier, target specific subcellular locations like the mitochondria, or are active under unique conditions like hypoxia or oxidative stress. The trends outlined here are likely more broadly applicable than the select examples discussed, providing plenty of room for new advances.
Fig. 2.
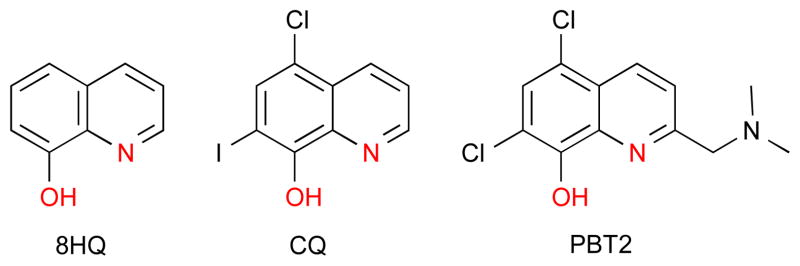
The structures of 8-hydroxyquinoline (8HQ), clioquinol (CQ) and PBT2. The oxygen, nitrogen metal ion coordination sites are highlighted in red and are in common among all molecules utilizing the 8-OH quinoline scaffold.
Acknowledgments
We thank the NIH for supporting our work in this area (GM084176) and (5T32GM007105).
Notes and references
- 1.Beswick PH, Hall GH, Hook AJ, Little K, McBrien DCH, Lott KAK. Chem Biol Interact. 1976;14:347–356. doi: 10.1016/0009-2797(76)90113-7. [DOI] [PubMed] [Google Scholar]
- 2.Strain J, Culotta VC. Molec Gen Genet. 1996;251:139–145. doi: 10.1007/BF02172911. [DOI] [PubMed] [Google Scholar]
- 3.Macomber L, Rensing C, Imlay JA. J Bacteriol. 2007;189:1616–1626. doi: 10.1128/JB.01357-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macomber L, Imlay JA. Proc Natl Acad Sci USA. 2009;106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML. Lancet. 2007;369:397–408. doi: 10.1016/S0140-6736(07)60196-2. [DOI] [PubMed] [Google Scholar]
- 6.Delangle P, Mintz E. Dalton Trans. 2012;41:6359–6370. doi: 10.1039/c2dt12188c. [DOI] [PubMed] [Google Scholar]
- 7.Pressman BC, Harris EJ, Jagger WS, Johnson JH. Proc Natl Acad Sci USA. 1967;58:1949–1956. doi: 10.1073/pnas.58.5.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowicki D, Huczynski A. Bio Med Res Int. 2013;2013:14. [Google Scholar]
- 9.Pearson RG. J Am Chem Soc. 1963;85:3533–3539. [Google Scholar]
- 10.Hancock RD, Martell AE. Chem Rev. 1989;89:1875–1914. [Google Scholar]
- 11.Smith RM, Martell AE, Motekaitis RJ. NIST critically selected stability constants of metal complexes database. Gaithersburg, MD: 2007. [Google Scholar]
- 12.Buettner JB., GR Radiat Res. 1996;145:532–541. [PubMed] [Google Scholar]
- 13.Tardito S, Barilli A, Bassanetti I, Tegoni M, Bussolati O, Franchi-Gazzola R, Mucchino C, Marchio L. J Med Chem. 2012;55:10448–10459. doi: 10.1021/jm301053a. [DOI] [PubMed] [Google Scholar]
- 14.Hung YH, Robb EL, Volitakis I, Ho M, Evin G, Li QX, Culvenor JG, Masters CL, Cherny RA, Bush AI. J Biol Chem. 2009;284:21899–21907. doi: 10.1074/jbc.M109.019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duce JA, Bush AI. Prog Neurobiol. 2010;92:1–18. doi: 10.1016/j.pneurobio.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Harris ED. Nutr Rev. 2004;62:60–64. doi: 10.1111/j.1753-4887.2004.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 17.Brewer G. Exp Biol Med. 2001;226:665–673. doi: 10.1177/153537020222600712. [DOI] [PubMed] [Google Scholar]
- 18.Pan Q, Kleer C, van Golen K, Irani J, Bottema K, Bias C, De Carvalho M, Mesri E, Robins D, Dick R. Cancer Res. 2002;62:4854–4859. [PubMed] [Google Scholar]
- 19.Samoszuk M, Nguyen V. Anticancer Res. 1996;16:1219–1223. [PubMed] [Google Scholar]
- 20.Hassouneh B, Islam M, Nagel T, Pan Q, Merajver SD, Teknos TN. Molec Cancer Therap. 2007;6:1039–1045. doi: 10.1158/1535-7163.MCT-06-0524. [DOI] [PubMed] [Google Scholar]
- 21.Brady DC, Crowe MS, Turski ML, Hobbs GA, Yao X, Chaikuad A, Knapp S, Xiao K, Campbell SL, Thiele DJ, Counter CM. Nature. 2014;509:492–496. doi: 10.1038/nature13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshii J, Yoshiji H, Kuriyama S, Ikenaka Y, Noguchi R, Okuda H, Tsujinoue H, Nakatani T, Kishida H, Nakae D, Gomez DE, De Lorenzo MS, Tejera AM, Fukui H. Int J Cancer. 2001;94:768–773. doi: 10.1002/ijc.1537. [DOI] [PubMed] [Google Scholar]
- 23.Nayak C, Dhurat R, Pereira R, Kagne R, Khatu S. Indian J Dermatol Venereol Leprol. 2011;77:55–58. doi: 10.4103/0378-6323.74982. [DOI] [PubMed] [Google Scholar]
- 24.Kaveer N, Narayan S. Neurol India. 2006;54:110–111. doi: 10.4103/0028-3886.25146. [DOI] [PubMed] [Google Scholar]
- 25.Safi R, Nelson ER, Chitneni SK, Franz KJ, George DJ, Zalutsky MR, McDonnell DP. Cancer Res. 2014;74:5819–5831. doi: 10.1158/0008-5472.CAN-13-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston WD, Freiser H. J Am Chem Soc. 1952;74:5239–5242. [Google Scholar]
- 27.Prachayasittikul V, Prachayasittikul S, Ruchirawat S, Prachayasittikul V. Drug Des Devel Ther. 2013;7:1157–1178. doi: 10.2147/DDDT.S49763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albert A, Hampton A, Selbie FR, Simon RD. Br J Exp Pathol. 1953;35:75–84. [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson BI, Swaby RJ. Aust J Sci Res B. 1951;4:275–282. doi: 10.1071/bi9510275. [DOI] [PubMed] [Google Scholar]
- 30.Arbiser JL, Kraeft SK, van Leeuwen R, Hurwitz SJ, Selig M, Dickersin GR, Flint A, Byers HR, Chen LB. Molec Med. 1998;4:665–670. [PMC free article] [PubMed] [Google Scholar]
- 31.Tateishi J. Neuropathol. 2000;20:20–24. [Google Scholar]
- 32.Schaumburg H, Herskovitz S. Neurol. 2008;71:622–623. doi: 10.1212/01.wnl.0000324620.86645.c1. [DOI] [PubMed] [Google Scholar]
- 33.Ritchie CW, Bush AI, Mackinnon A, Macfarlane S, Mastwyk M, MacGregor L, Kiers L, Cherny R, Li QX, Tammer A, Carrington D, Mavros C, Volitakis I, Xilinas M, Ames D, Davis S, Beyreuther K, Tanzi RE, Masters CL. Arch Neurol. 2003;60:1685–1691. doi: 10.1001/archneur.60.12.1685. [DOI] [PubMed] [Google Scholar]
- 34.Adlard PA, Cherny RA, Finkelstein DI, Gautier E, Robb E, Cortes M, Volitakis I, Liu X, Smith JP, Perez K, Laughton K, Li QX, Charman SA, Nicolazzo JA, Wilkins S, Deleva K, Lynch T, Kok G, Ritchie CW, Tanzi RE, Cappai R, Masters CL, Barnham KJ, Bush AI. Neuron. 2008;59:43–55. doi: 10.1016/j.neuron.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 35.Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim YS, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, Bush AI. Neuron. 2001;30:665–676. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- 36.Sampson Elizabeth L, Jenagaratnam L, McShane R. Cochrane Database of Systematic Reviews. 2014:CD005380. doi: 10.1002/14651858.CD005380.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Regland B, Lehmann W, Abedini I, Blennow K, Jonsson M, Karlsson I, Sjögren M, Wallin A, Xilinas M, Gottfries CG. Dement Geriatr Cogn Disord. 2001;12:408–414. doi: 10.1159/000051288. [DOI] [PubMed] [Google Scholar]
- 38.Prana Biotechnology. 2005 [Google Scholar]
- 39.Lannfelt L, Blennow K, Zetterberg H, Batsman S, Ames D, Harrison J, Masters CL, Targum S, Bush AI, Murdoch R, Wilson J, Ritchie CW. Lancet Neurol. 2008;7:779–786. doi: 10.1016/S1474-4422(08)70167-4. [DOI] [PubMed] [Google Scholar]
- 40.Prana Biotechnology announces top line results of Phase 2 IMAGINE trial of PBT2 in Alzheimer’s disease. Prana Biotechnology; 2014. [Google Scholar]
- 41.Cherny R, Ayton S, Finkelstein DI, Bush AIMG, Massa S. J Huntingtons Dis. 2012;1:211–219. doi: 10.3233/JHD-120029. [DOI] [PubMed] [Google Scholar]
- 42.Daniel K, Chen D, Orlu S, Cui Q, Miller F, Dou QP. Breast Cancer Res. 2005;7:R897–R908. doi: 10.1186/bcr1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding WQ, Lind SE. IUBMB Life. 2009;61:1013–1018. doi: 10.1002/iub.253. [DOI] [PubMed] [Google Scholar]
- 44.Chen D, Cui QC, Yang H, Barrea RA, Sarkar FH, Sheng S, Yan B, Reddy GPV, Dou QP. Cancer Res. 2007;67:1636–1644. doi: 10.1158/0008-5472.CAN-06-3546. [DOI] [PubMed] [Google Scholar]
- 45.Mao X, Li X, Sprangers R, Wang X, Venugopal A, Wood T, Zhang Y, Kuntz DA, Coe E, Trudel S, Rose D, Batey RA, Kay LE, Schimmer AD. Leukemia. 2008;23:585–590. doi: 10.1038/leu.2008.232. [DOI] [PubMed] [Google Scholar]
- 46.French FA, Freedlander BL. Cancer Res. 1958;18:172–175. [PubMed] [Google Scholar]
- 47.Mihich E, Simpson CL, Mulhern AI. Cancer Res. 1965;25:1417–1431. [PubMed] [Google Scholar]
- 48.Barry VC, Conalty ML, O’Sullivan JF. Cancer Res. 1966;26:2165–2168. [PubMed] [Google Scholar]
- 49.Petering HG, Buskirk HH, Underwood GE. Cancer Res. 1964;24:367–372. [PubMed] [Google Scholar]
- 50.Xiao Z, Donnelly PS, Zimmermann M, Wedd AG. Inorg Chem. 2008;47:4338–4347. doi: 10.1021/ic702440e. [DOI] [PubMed] [Google Scholar]
- 51.Donnelly PS, Liddell JR, Lim S, Paterson BM, Cater MA, Savva MS, Mot AI, James JL, Trounce IA, White AR, Crouch PJ. Proc Natl Acad Sci USA. 2012;109:47–52. doi: 10.1073/pnas.1116227108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cater MA, Pearson HB, Wolyniec K, Klaver P, Bilandzic M, Paterson BM, Bush AI, Humbert PO, La Fontaine S, Donnelly PS, Haupt Y. ACS Chem Biol. 2013;8:1621–1631. doi: 10.1021/cb400198p. [DOI] [PubMed] [Google Scholar]
- 53.Fujibayashi Y, Taniuchi H, Yonekura Y, Ohtani H, Konishi J, Yokoyama A. J, Nucl Med. 1997;38:1155–1160. [PubMed] [Google Scholar]
- 54.Dedeoglu A, Cormier K, Payton S, Tseitlin KA, Kremsky JN, Lai L, Li X, Moir RD, Tanzi RE, Bush AI, Kowall NW, Rogers JT, Huang X. Exp Geront. 2004;39:1641–1649. doi: 10.1016/j.exger.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 55.Ikawa M, Okazawa H, Kudo T, Kuriyama M, Fujibayashi Y, Yoneda M. Nucl Med Biol. 2011;38:945–951. doi: 10.1016/j.nucmedbio.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 56.Ikawa M, Okazawa H, Arakawa K, Kudo T, Kimura H, Fujibayashi Y, Kuriyama M, Yoneda M. Mitochondrion. 2009;9:144–148. doi: 10.1016/j.mito.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 57.White AR, Du T, Laughton KM, Volitakis I, Sharples RA, Xilinas ME, Hoke DE, Holsinger RMD, Evin G, Cherny RA, Hill AF, Barnham KJ, Li QX, Bush AI, Masters CL. J Biol Chem. 2006;281:17670–17680. doi: 10.1074/jbc.M602487200. [DOI] [PubMed] [Google Scholar]
- 58.Crouch PJ, Hung LW, Adlard PA, Cortes M, Lal V, Filiz G, Perez KA, Nurjono M, Caragounis A, Du T, Laughton K, Volitakis I, Bush AI, Li QX, Masters CL, Cappai R, Cherny RA, Donnelly PS, White AR, Barnham KJ. Proc Natl Acad Sci USA. 2009;106:381–386. doi: 10.1073/pnas.0809057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Djoko KY, Paterson BM, Donnelly PS, McEwan AG. Metallomics. 2014;6:854–863. doi: 10.1039/c3mt00348e. [DOI] [PubMed] [Google Scholar]
- 60.Speer A, Shrestha TB, Bossmann SH, Basaraba RJ, Harber GJ, Michalek SM, Niederweis M, Kutsch O, Wolschendorf F. Antimicrob Agents Chemother. 2013;57:1089–1091. doi: 10.1128/AAC.01781-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johansson B, Stankiewicz Z. Biochem Pharmacol. 1985;34:2989–2991. doi: 10.1016/0006-2952(85)90026-7. [DOI] [PubMed] [Google Scholar]
- 62.Aaseth J, Soli NE, Forre O. Acta Pharmacol Toxicol. 1979;45:41–44. doi: 10.1111/j.1600-0773.1979.tb02358.x. [DOI] [PubMed] [Google Scholar]
- 63.Cen D, Gonzalez RI, Buckmeier JA, Kahlon RS, Tohidian NB, Meyskens FL. Molec Cancer Ther. 2002;1:197–204. [PubMed] [Google Scholar]
- 64.Chen D, Cui QC, Yang H, Dou QP. Cancer Res. 2006;66:10425–10433. doi: 10.1158/0008-5472.CAN-06-2126. [DOI] [PubMed] [Google Scholar]
- 65.Tardito S, Bassanetti I, Bignardi C, Elviri L, Tegoni M, Mucchino C, Bussolati O, Franchi-Gazzola R, Marchio L. J Am Chem Soc. 2011;133:6235–6242. doi: 10.1021/ja109413c. [DOI] [PubMed] [Google Scholar]
- 66.Daniel K, Harbach R, Guida W, Dou Q. Front Biosci. 2004;9:2652–2662. doi: 10.2741/1424. [DOI] [PubMed] [Google Scholar]
- 67.Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowicz LM, Devoto M, Peppercorn J, Bush AI, Sternlieb I, Pirastu M, Gusella JF, Evgrafov O, Penchaszadeh GK, Honig B, Edelman IS, Soares MB, Scheinberg IH, Gilliam TC. Nat Genet. 1993;5:344–350. doi: 10.1038/ng1293-344. [DOI] [PubMed] [Google Scholar]
- 68.Kodama H, Murata Y, Kobayashi M. Pediatr Int. 1999;41:423–429. doi: 10.1046/j.1442-200x.1999.01095.x. [DOI] [PubMed] [Google Scholar]
- 69.Kreuder J, Otten A, Fuder H, Tümer Z, Tønnesen T, Horn N, Dralle D. Eur J Ped. 1993;152:828–832. doi: 10.1007/BF02073380. [DOI] [PubMed] [Google Scholar]
- 70.Nomura S, Nozaki S, Hamazaki T, Takeda T, Ninomiya E, Kudo S, Hayashinaka E, Wada Y, Hiroki T, Fujisawa C, Kodama H, Shintaku H, Watanabe Y. J Nucl Med. 2014;55:845–851. doi: 10.2967/jnumed.113.131797. [DOI] [PubMed] [Google Scholar]
- 71.Bhadhprasit W, Kodama H, Fujisawa C, Hiroki T, Ogawa E. J Trace Elem Med Biol. 2012;26:105–108. doi: 10.1016/j.jtemb.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 72.Meguro Y, Kodama H, Abe T, Kobayashi S, Kodama Y, Nishimura M. Brain Dev. 1991;13:184–186. doi: 10.1016/s0387-7604(12)80027-1. [DOI] [PubMed] [Google Scholar]
- 73.Kodama H, Sato E, Gu YH, Shiga K, Fujisawa C, Kozuma T. J Inherited Met Dis. 2005;28:971–978. doi: 10.1007/s10545-005-0150-6. [DOI] [PubMed] [Google Scholar]
- 74.Yadav AA, Patel D, Wu X, Hasinoff BB. J Inorg Biochem. 2013;126:1–6. doi: 10.1016/j.jinorgbio.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 75.Chen S, Sun L, Koya K, Tatsuta N, Xia Z, Korbut T, Du Z, Wu J, Liang G, Jiang J, Ono M, Zhou D, Sonderfan A. Bioorg Med Chem Lett. 2013;23:5070–5076. doi: 10.1016/j.bmcl.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 76.Berkenblit A, Eder JP, Ryan DP, Seiden MV, Tatsuta N, Sherman ML, Dahl TA, Dezube BJ, Supko JG. Clin Cancer Res. 2007;13:584–590. doi: 10.1158/1078-0432.CCR-06-0964. [DOI] [PubMed] [Google Scholar]
- 77.Kirshner JR, He S, Balasubramanyam V, Kepros J, Yang CY, Zhang M, Du Z, Barsoum J, Bertin J. Mol Cancer Ther. 2008;7:2319–2327. doi: 10.1158/1535-7163.MCT-08-0298. [DOI] [PubMed] [Google Scholar]
- 78.Nagai M, Vo NH, Shin Ogawa L, Chimmanamada D, Inoue T, Chu J, Beaudette-Zlatanova BC, Lu R, Blackman RK, Barsoum J, Koya K, Wada Y. Free Radical Bio Med. 2012;52:2142–2150. doi: 10.1016/j.freeradbiomed.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 79.Blackman RK, Cheung-Ong K, Gebbia M, Proia DA, He S, Kepros J, Jonneaux A, Marchetti P, Kluza J, Rao PE, Wada Y, Giaever G, Nislow C. PLoS ONE. 2012;7:1–10. doi: 10.1371/journal.pone.0029798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barnett BL, Kretschmar HC, Hartman FA. Inorg Chem. 1977;16:1834–1838. [Google Scholar]
- 81.Bond AD, Feeder N, Teat SJ, Jones W. Acta Cryst. 2001;C57:1157–1158. doi: 10.1107/s0108270101012306. [DOI] [PubMed] [Google Scholar]
- 82.Ermolayeva E, Sanders D. Appl Environ Microbiol. 1995;61:3385–3390. doi: 10.1128/aem.61.9.3385-3390.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yasokawa D, Murata S, Iwahashi Y, Kitagawa E, Kishi K, Okumura Y, Iwahashi H. J Biosci Bioeng. 2010;109:479–486. doi: 10.1016/j.jbiosc.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 84.Reeder NL, Kaplan J, Xu J, Youngquist RS, Wallace J, Hu P, Juhlin KD, Schwartz JR, Grant RA, Fieno A, Nemeth S, Reichling T, Tiesman JP, Mills T, Steinke M, Wang SL, Saunders CW. Antimicrob Agents Chemother. 2011;55:5753–5760. doi: 10.1128/AAC.00724-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reeder NL, Xu J, Youngquist RS, Schwartz JR, Rust RC, Saunders CW. Br J Derm. 2011;165:9–12. doi: 10.1111/j.1365-2133.2011.10571.x. [DOI] [PubMed] [Google Scholar]
- 86.Chillappagari S, Seubert A, Trip H, Kuipers OP, Marahiel MA, Miethke M. J Bacteriol. 2010;192:2512–2524. doi: 10.1128/JB.00058-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Magda D, Lecane P, Wang Z, Hu W, Thiemann P, Ma X, Dranchak PK, Wang X, Lynch V, Wei W, Csokai V, Hacia JG, Sessler JL. Cancer Res. 2008;68:5318–5325. doi: 10.1158/0008-5472.CAN-08-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Charkoudian LK, Pham DM, Kwon AM, Vangeloff AD, Franz KJ. Dalton Trans. 2007:5031–5042. doi: 10.1039/b705199a. [DOI] [PubMed] [Google Scholar]
- 89.Dickens MG, Franz KJ. ChemBioChem. 2010;11:59–62. doi: 10.1002/cbic.200900597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kielar F, Helsel ME, Wang Q, Franz KJ. Metallomics. 2012;4:899–909. doi: 10.1039/c2mt20069d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Festa RA, Helsel ME, Franz KJ, Thiele DJ. Chem & Biol. 2014;21:977–987. doi: 10.1016/j.chembiol.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bernstein EH, Pienta PW, Gershon H. Toxicol and Appl Pharmacol. 1963;5:599–604. doi: 10.1016/0041-008x(63)90005-x. [DOI] [PubMed] [Google Scholar]
- 93.Leanderson P, Tagesson C. Carcinogen. 1996;17:545–550. doi: 10.1093/carcin/17.3.545. [DOI] [PubMed] [Google Scholar]
- 94.Oliveri V, Giuffrida ML, Vecchio G, Aiello C, Viale M. Dalton Trans. 2012;41:4530–4535. doi: 10.1039/c2dt12371a. [DOI] [PubMed] [Google Scholar]