Abstract
Although helminth infections are characteristically associated with Th2-mediated responses that include the production of the prototypical cytokines IL-4, IL-5, and IL-13 by CD4+ cells, the production of IgE, peripheral blood eosinophilia and mucus production in localized sites, these responses are largely attenuated when helminth infections become less acute. This modulation of the immune response that occurs with chronic helminth infection is often induced by molecules secreted by helminth parasites, by non-Th2 regulatory CD4+ cells, and by non-classical B cells, macrophages and dendritic cells. This review will focus on those parasite- and host-mediated mechanisms underlying the modulated T cell response that occurs as the default in chronic helminth infections.
Parasitic helminths are complex organisms (worms), characterized by their ability to maintain chronic infections in human hosts and are a major health care concern worldwide (often in resource limited countries) infecting more than two billion people. Common helminth infections impose major social, economic, and medical burdens on those regions where these infections are most endemic. Anthelmintic therapy, despite its success under certain circumstances, still suffers from drug distribution logistics, the length of treatment for certain helminths and the potential problem of drug resistance. Therefore, the focus of this review will be on the strategies used by these parasites to establish and maintain infection, processes that likely modulate host immune responses.
Host-helminth interface
Helminths, in contrast to the single cell protozoa, are large extracellular (the exception being Trichinella spiralis) parasites that do not multiply in their vertebrate hosts. Helminths have complex life cycles often with many developmental stages that can be antigenically distinct leading to distinct immune responses that evolve differentially over the course of a helminth infection. In addition, because of differing tissue tropism of the various helminths, responses can be either localized (e.g. intestinal mucosa and draining lymph nodes in intestinal nematode infection or skin/subcutaneous tissue and draining lymph nodes in O. volvulus infection) or systemic (e.g. in schistosomiasis, toxocariasis, blood-borne filarial infections). Moreover, because helminths survive in the host for decades chronically producing larvae (or eggs), their modus operandi involves host immune modulation such that their own survival (and continued transmission from host to host) is assured.
The prototypical host immune response to all pathogenic helminths of humans (based largely on studies in easily polarized mouse models of infection) is one often characterized as Type-2 (or Th2) and involves: 1) the production of the cytokines IL-4, IL-5, IL-9, IL-10 and IL-13; 2) the induction of antigen-specific IgG1, IgG4 and IgE; and 3) the expanded populations of eosinophils and alternatively activated macrophages/immunoregulatory monocytes (1-3). This Type 2 response occurs primarily at the time of patency (when egg laying or microfilarial release from adult females occurs (1), its initiation requiring interaction with many different cell types, most notably: 1) stromal cells; 2) dendritic cell and macrophage populations; 3) eosinophils; 4) mast cells and basophils; 5) dermal cells; 6) epithelial cells; and 7) innate lymphoid cells (ILCs). These innate responses that promote Type-2 responses are most often very quickly modulated by both adaptive and natural regulatory T cells, regulatory monocytes/macrophages (Mregs) and B cells (Breg), eosinophils and likely other, heretofore, unidentified cell populations (Figure 1).
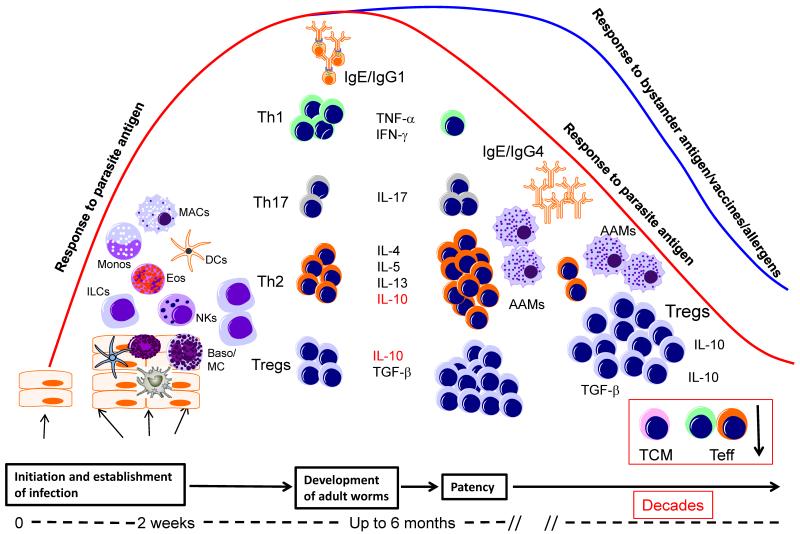
Figure 1.
Immune responses in helminth infections as a function of time following infection. Infectious stages of helminth parasites initiate infection at barrier sites and activate a variety of different cell types such as innate lymphoid cells (ILCs), macrophages (MAC), dendritic cells (DCs), natural killer cells (NK), eosinophils (Eos) and basophils/mast cells (Baso/MC). At this relatively early phase of infection the parasite induces the differentiation of effector Th1, Th17 and Th2 cells, which together with IgE antibody, may lead to attrition of some of the parasites. At the time of patency (when egg laying/microfilarial release occurs) there is a small expansion of Th2 CD4+ cells and a concomitant contraction of Th1 cells. With the evolution of chronic longstanding infection, there is an associated expansion of IL-10- and/or TGF-β-producing regulatory T cells (Tregs). The high levels of IL-10 produced induce the production of IgG4 which together with IL-4, IL-13, and/or TGF-β induce the differentiation of alternatively activated macrophages (AAM) and inhibit the function of a variety of other cells including central memory (TCM) and effector (Teff) T cells.
Mechanisms of evasion and immune modulation by helminth parasites
Helminths exert profound regulatory effects on the host with both parasite antigen-specific and more generalized levels of immune modulation. It has been shown that patients with filarial infections, schistosomiasis, or even soil transmitted gastrointestinal helminths (STHs) have markedly diminished responses to parasite antigens (3, 4); in addition, these parasites also can induce attenuated responses to non-helminth antigens (5-8) including those that are delivered as approved vaccines (9-13). The chronicity of many of these helminth parasites is presumed to reflect successful immune evasion strategies that allow these parasites to avoid elimination (14); this immune evasion is often dependent on the modulation of the parasite-specific host responses.
Parasite-dependent mechanisms of evasion and immune-modulation
As parasites enter immunologically privileged sites such as the central nervous system or the eye (e.g. Toxocara spp., Taenia solium, O. volvulus) or when, because of the complexities of their life cycle, they enter a host cell that renders them immunologically privileged, as seen in T. spiralis infection (15), they are provided a means for remaining hidden from immune attack. Encystation, another mechanism utilized by helminth parasites to avoid immune-mediated attack, occurs in infections such as Echinococcus spp. and T. solium. Loosely related to this process is a process seen in O. volvulus infection in which the adult parasites live within a nodule that is encased in host derived lymphatic endothelial-like cells (16) within which is human extracellular matrix.
Parasite gene-encoded secreted (or surface exposed) proteins, glycoproteins, glycans, and lipoproteins also appear to play an important role in host immune modulation (17). Perhaps the best studied is a phosphorylcholine (PC)-containing molecule called ES-62 (18) from filarial worms that has been shown to inhibit the proliferation of CD4+ T cells and conventional B cells, decrease IL-4 and IFN-γ production, promote IL-10 production by B1 B cells, and condition APCs to inhibit Th1 responses (19-22). ES-62 has also been shown to exhibit bystander anti-inflammatory activity in animal models of arthritis (23), skin sensitization (24) and lung airway hyper-reactivity (25).
Helminth antigens have a wide array of glycan- and lipid-containing proteins (26, 27) that are structurally related to host-derived glycans and lipids (28). The host-like glycans interact directly with mammalian C-type lectin receptors, galectins, jacalins, and mannose receptors to shape innate and adaptive immune responses (29). Similarly, helminth lipids have also been implicated in immune modulation with schistosome lyso-phosphatidyl serine by conditioning dendritic cells to induce IL-10 secreting Tregs (30).
The schistosome-secreted proteins alpha-1 (also known as IL-4 inducing principle of schistosome eggs or IPSE) and omega-1 (a ribonuclease) - both of which are secreted/excreted by schistosome eggs - have been shown to play a significant role in shaping the CD4+ effector response (31-34).
Helminth parasites also utilize mechanisms involving cytokine mimicry and/or antagonism to alter the host immune response. The first helminth-encoded cytokines were found to be homologs of TGF-β (35, 36), and many helminth genomes encode members of the TGF-β receptor superfamily. All of the filarial helminths produce homologs of macrophage migration inhibitory factors (MIF) (37-41) and SOCS-1 (42, 43), molecules known to be anti-inflammatory. T. muris was shown to express a homolog of IFN-γ that binds to the IFN-γR in vitro (44).
Helminth parasites have the ability to utilize chemokine- or chemokine receptor-like proteins to modulate host immunity. As more and more helminth genomes are being elucidated and annotated, increasingly more chemokine and chemokine receptor mimics are being discovered (e.g. in the filariae (42) or in N. americanus (45)). Specific examples with experimental data underlying this concept of chemokine mimicry include an Ascaris suum-expressed neutrophil chemoattractant with chemokine binding properties (46) and an S. mansoni chemokine receptor-like protein that binds CXCL8 and CCL3 resulting in an anti-inflammatory effect (47).
Helminths secrete two major classes of protease inhibitors - cystatins and serpins - each with proposed immunomodulatory roles (48-50). Cystatins inhibit cysteine proteases (cathepsins and aspartyl endopeptidases) required for antigen processing and presentation and thereby inhibiting T cell activation. They have also been shown to elicit the regulatory cytokine – IL-10, leading to direct impairment of T cell proliferation. The serpins are serine protease inhibitors that specifically inhibit neutrophil proteinases, cathepsin G and neutrophil elastase.
Other parasite products mediate their effect by blocking effector functions including recruitment and activation of inflammatory cells and by limiting the destructive potential of activated granulocytes or macrophages in the local extracellular milieu. Platelet activating factor (PAF) can be inactivated by a secreted N. brasiliensis-derived PAF hydrolase (51). Eotaxin-1 has been shown to be degraded by a hookworm metalloprotease (52). Other modulators, such as helminth-derived or -induced prostaglandins and other arachidonic acid family members are known regulate a variety of cellular functions (53-55). Finally, helminths (known to be susceptible to oxidation-mediated killing) express both secreted and membrane associated glutathione peroxidase, glutathione-S-transferase and superoxide dismutase that have the potential for subverting this killing mechanism (56-59).
Host –related factors in immune-mediated modulation
Cells of the innate immune system
In the process of establishing a patent infection, the infectious stages must traverse a set of barriers the cells of which form an innate interface that can serve to modulate/induce certain immune or inflammatory functions (see Figure 1). This section examines these cells in the context of their interaction with parasitic helminths.
Helminths and epithelial/innate lymphoid cells
Epithelial cells are the first barrier layer that helminth larvae encounter, and the capacity of these cells to interact with helminth parasites through pattern recognition receptors (TLRs, NLRs) and through the production of IL-25, IL-33 and thymic stromal lymphopoietin, (TSLP), considered to be “alarmins” (60), seems be important in driving an early response to these pathogens. In addition, epithelial cells in the intestine, for instance, are in constant contact with the intestinal microflora thereby being well positioned for immunological surveillance in the gastrointestinal tract. Moreover, epithelial cells (throughout the body) have been shown to induce tolerogenic signals to T cells and B cells (61).
Innate lymphoid cells (ILCs) represent a novel family of hematopoietic effectors that serve protective roles in innate immune responses to infectious microorganisms and in homeostasis of tissue stromal cells (reviewed in (62, 63)). Among these are the ILC2 cells that are known to produce IL-5, IL-13, IL-9, IL-4, and IL-10. Although they are found in abundance in the skin, in subepithelial portions of the intestine and airways among other sites, whether they play a regulatory role through the secretion of IL-10 remains an unanswered question.
Helminths and dendritic cells
Dendritic cells (DC) are antigen-presenting cells that play an essential role in presenting antigen to T cells to initiate immune responses. The role of DCs in T cell subset differentiation has been well characterized in both murine and human helminth infections (reviewed in (64-66)) and, on balance, helminth-educated DCs clearly promote both Th2 and regulatory responses (67-71). However how helminths (and their secreted/excreted products) alter DC function is less well understood, although helminth products very clearly alter the function and maturation of DC (72, 73) as well as impair in their ability to respond pattern recognition receptor stimulation (7, 69, 74, 75). By whatever mechanism, these helminth-modulated DC fail to respond appropriately to other infectious stimuli (e.g. Mycobacterium tuberculosis or P. falciparum (7, 76-78).
Helminths and macrophages
Although macrophages can function as effector cells in bacterial and protozoal infections through the production of nitric oxide (among other mediators) their interaction with helminths through the action of IL-4 and IL-13 induce a population of macrophages termed alternatively activated macrophages (AAMs) that are characterized by the expression of arginase, YM1, YM2, and RELM-α (79-82) These AAMs are known to be important in wound healing. By virtue of their expression of regulatory molecules such as IL-10, TGF-β and PDL2 these macrophages may have a predominantly regulatory role in helminth infections.
While helminth infection does induce expression of these AAMs and other regulatory monocyte/macrophage populations in humans ((83-86), the primary functional consequences of having increased frequencies of these cells is interference with full T cell activation (86, 87).
Helminths and eosinophils
Blood and tissue eosinophilia is characteristic of helminth infection and is mediated largely by IL-5. Recruitment of eosinophils to the site of infection occurs very early in experimental helminth infection and occurs by 2-3 weeks following human infection (88, 89). Although much attention has been drawn to eosinophils as effectors in killing helminth parasites, more and more evidence also points to their role in tissue remodeling, metabolic homeostasis, and their ability to act as anti-inflammatory cells through the release of pre-formed cytokines.
Helminths and basophils/mast cells
Basophils are an important component of the immune response to helminth infections (90, 91). Basophils gained prominence because of their potential role in Th2 cell differentiation as the initial source of “innate” IL-4 (92) that was capable of driving a Th2 response in murine models of helminth infection but also of inducing AAMs. However, the preponderance of evidence now suggests that in helminth infection, an adaptive Th2 response (and IgE production) precedes the basophil-induced IL-4 (93-98). Mast cells certainly contribute inflammatory responses directed toward many helminth parasites, but likely play little role in modulating responses to helminth parasites in the tissues (99).
Cells of the adaptive immune system
Helminths and T cells
As noted in the introductory paragraphs, helminth infections, at the time of patency, are associated with a marked Th2 polarization that is modulated relatively soon thereafter, through the expansion of regulatory cell populations (largely Tregs, but also Mregs and Bregs). The ontogeny of the Th2 response and its effector function is the subject of several other papers in this series and thus will not be discussed further herein.
In mouse models of filarial and schistosome infections parasite survival has been linked to the activity of Tregs. Indeed immunity to infection (when it can be induced) can be enhanced by Treg depletion. Because both natural regulatory T cells (nTregs) and adaptive regulatory Tregs (aTregs) are expanded following helminth infections they will be discussed in turn.
nTregs play an important role in the modulation of T cell responses in infectious diseases, cancer and autoimmune diseases (100-103). Characterized by the surface expression of CD25 and CD127 and by the transcription factor Foxp3, nTregs can suppress T cell responses through a contact-dependent mechanism the nature of which is still not fully understood. Evidence from mouse models and to a lesser degree from human studies argues that nTregs play a role in controlling pathology and immunity during helminth infections. In H. polygyrus-infected mice, for example, nTregs are present in greater frequencies, express higher levels of CD103, and are intrinsically more able to suppress T cell responses than Tregs from naïve mice. In murine filarial infections, parasite survival is linked to nTreg activity. Similarly, Treg cells are also instrumental in controlling Th2 responses in chronic S. mansoni infection.
aTregs, in contrast, are known to act through the production of cytokines, particularly IL-10 and TGF-β Evidence for the involvement of regulatory T cells (particularly aTregs) in helminth mediated down modulation of the immune responses has been accumulating. IL-10 and TGF-β both factors associated with regulatory T cells, are elicited in response to helminth infections and in vitro neutralization of IL-10 and TGF-β (to a lesser extent) restores T cell proliferation and cytokine production in filarial infections (lymphatic filariasis and onchocerciasis) and in schistosomiasis (104-109). In addition, T cell clones secreting IL-10 and TGF-β have been isolated from patients with onchocerciasis (110).
Helminths and B cells
Helminths interface with B cells through the T cell dependent induction of antibody production and at the cellular level by inducing B cell activation and cytokine production, IL-10 most prominently (111). Immune regulation by B cells in the context of helminth infections has been best studied in mouse models of and human studies in schistosome infection. In murine schistosome infection it was clearly shown that B cell deficiency leads to enhanced CD4-mediated pathology and that IL-10 producing B cells (Bregs) were important for prevention of pathology (112, 113]. In humans with S. haematobium infections, such IL-10 producing Bregs have also been shown not only to be expanded but also to downregulate the parasite antigen-driven T cell effector cytokine response through the production of IL-10 (114).
Modulation of T cell effector function
The hallmark of most helminth infections is their chronicity (115), a state that requires the dampening of effector responses. The signature of such a dampened immune response in helminth infection is a down-regulated parasite-specific effector T cell response (so-called T cell hyporesponsiveness). Effector T cell responses can be modulated through a variety of mechanisms that include at the T cell level: 1) the production of IL-10 (107, 109, 116) and TGF-β (110, 117, 118); 2) increased surface expression of CTLA-4 (119) and PD-1(120); 3) the modulation of the transcription factor Tbet (43); and 4) the induction of anergy (121). Finally, T cells from filarial infected individuals exhibit classical signs of anergy including diminished T cell proliferation to parasite antigens, lack of IL-2 production (122) and increased expression of E3 ubiquitin ligases (121). Similarly, anergic T cells are found in both humans and mice with schistosomiasis and, in the latter case, these T cells express high levels of GRAIL (gene related to anergy in lymphocytes) (123).
Effector T cell function can also be limited by “faulty” signals delivered by antigen presenting cells. Dendritic cells are the first antigen-presenting cells to encounter helminth parasites, and helminth modulation of DC function has been well characterized (72). Filarial parasites induce downregulation of MHC class I and class II as well as cytokines and other genes involved in antigen presentation, thereby rendering DC suboptimal in activation of CD4+ T cells (124). Schistosomes induce similar effects on DC (69) and by Nlrp3-induced IL-β production (125, 126). Finally, soluble products from several pathogenic helminths have been shown to induce apoptosis of DC (127, 128).
The role of helminth infections in modulating the activation status of macrophages has already been alluded to above. However, a heterogeneous population of immature myeloid cells termed myeloid-derived suppressor cells (MDSC) share the ability to suppress immune responses. Several helminth parasites have been associated with the expansion and accumulation of MDSC (129-131).
Putting it all together
Helminth parasites, be they systemic or localized to the GI tract, are characteristically long-lived and have evolved in such a way that they rarely induce symptomatic infections. Moreover, complete parasite elimination in immunocompetent hosts often is dictated by the lifespan of the particular parasite rather than by the host immune and inflammatory responses, responses that would be deleterious. Therefore, the disease manifestations, which are less often found in these infections, are commonly associated with immune- and not parasite-mediated pathology. The optimal response to helminth infection is one that balances parasite control at levels where the parasite can be tolerated and immune homeostasis can be maintained without significant tissue damage. This immune homeostasis requires the orchestration of myriad factors (both host- and parasite-derived [see Figure 2]) that serve to limit pathology and allow for parasite survival and continued transmission from host to host.
Figure 2.
Schematized understanding of the host response to helminth infection. Shown in blue are the characteristic mechanisms induced by the host responses (purple) or by the helminth infections/products (green) that lead to specific outcomes (red).
The consequences of the chronicity of many tissue invasive helminth parasites remain relatively unstudied, as do the consequences of the enormous antigenic load provided by the secreted/excreted products of these parasites. How high antigen load and/or chronic antigenic stimulation can have such profound functional effects on cells of both the innate and adaptive arms of the immune system will likely be addressed in the near future. What is clear, however, is that one must expand/shift the paradigm away from helminth-induced Type 2 responses to allow for an understanding of helminth-induced immune modulation.
Acknowledgements
This work was funded in part by the Division of Intramural Research (DIR), National Institute of Allergy and Infectious Diseases, NIH.
Footnotes
Disclosures: None
References
- 1.Maizels RM, Pearce EJ, Artis D, Yazdanbakhsh M, Wynn TA. Regulation of pathogenesis and immunity in helminth infections. J Exp Med. 2009;206:2059–2066. doi: 10.1084/jem.20091903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol. 2011;11:375–388. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 3.Babu S, Nutman TB. Immunology of lymphatic filariasis. Parasite Immunol. 2014;36:338–346. doi: 10.1111/pim.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harnett W, Harnett MM. Lymphocyte hyporesponsiveness during filarial nematode infection. Parasite Immunol. 2008;30:447–453. doi: 10.1111/j.1365-3024.2008.01045.x. [DOI] [PubMed] [Google Scholar]
- 5.Wammes LJ, Hamid F, Wiria AE, et al. Regulatory T cells in human geohelminth infection suppress immune responses to BCG and Plasmodium falciparum. Eur J Immunol. 2010;40:437–442. doi: 10.1002/eji.200939699. [DOI] [PubMed] [Google Scholar]
- 6.Remoli ME, Giacomini E, Petruccioli E, et al. Bystander inhibition of dendritic cell differentiation by Mycobacterium tuberculosis-induced IL-10. Immunol Cell Biol. 2011;89:437–446. doi: 10.1038/icb.2010.106. [DOI] [PubMed] [Google Scholar]
- 7.Metenou S, Kovacs M, Dembele B, Coulibaly YI, Klion AD, Nutman TB. Interferon regulatory factor modulation underlies the bystander suppression of malaria antigen-driven IL-12 and IFN-gamma in filaria-malaria co-infection. Eur J Immunol. 2012;42:641–650. doi: 10.1002/eji.201141991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schabussova I, Ul-Haq O, Hoflehner E, et al. Oesophagostomum dentatum extract modulates T cell-dependent immune responses to bystander antigens and prevents the development of allergy in mice. PLoS One. 2013;8:e67544. doi: 10.1371/journal.pone.0067544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabin EA, Araujo MI, Carvalho EM, Pearce EJ. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J Infect Dis. 1996;173:269–272. doi: 10.1093/infdis/173.1.269. [DOI] [PubMed] [Google Scholar]
- 10.Cooper PJ, Espinel I, Paredes W, Guderian RH, Nutman TB. Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: a possible role for interleukin-10. J Infect Dis. 1998;178:1133–1138. doi: 10.1086/515661. [DOI] [PubMed] [Google Scholar]
- 11.Cooper PJ, Espinel I, Wieseman M, et al. Human onchocerciasis and tetanus vaccination: impact on the postvaccination antitetanus antibody response. Infect Immun. 1999;67:5951–5957. doi: 10.1128/iai.67.11.5951-5957.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper PJ, Chico M, Sandoval C, et al. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect Immun. 2001;69:1574–1580. doi: 10.1128/IAI.69.3.1574-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nookala S, Srinivasan S, Kaliraj P, Narayanan RB, Nutman TB. Impairment of tetanus-specific cellular and humoral responses following tetanus vaccination in human lymphatic filariasis. Infect Immun. 2004;72:2598–2604. doi: 10.1128/IAI.72.5.2598-2604.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steel C, Varma S, Nutman TB. Regulation of global gene expression in human Loa loa infection is a function of chronicity. PLoS Negl Trop Dis. 2012;6:e1527. doi: 10.1371/journal.pntd.0001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashour DS. Trichinella spiralis immunomodulation: an interactive multifactorial process. Expert Rev Clin Immunol. 2013;9:669–675. doi: 10.1586/1744666X.2013.811187. [DOI] [PubMed] [Google Scholar]
- 16.Mackenzie CD, Huntington MK, Wanji S, Lovato RV, Eversole RR, Geary TG. The association of adult Onchocerca volvulus with lymphatic vessels. J Parasitol. 2010;96:219–221. doi: 10.1645/GE-2236.1. [DOI] [PubMed] [Google Scholar]
- 17.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–744. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 18.Harnett W, Harnett MM. Immunomodulatory activity and therapeutic potential of the filarial nematode secreted product, ES-62. Adv Exp Med Biol. 2009;666:88–94. doi: 10.1007/978-1-4419-1601-3_7. [DOI] [PubMed] [Google Scholar]
- 19.Harnett W, Harnett MM. Modulation of the host immune system by phosphorylcholine-containing glycoproteins secreted by parasitic filarial nematodes. Biochim Biophys Acta. 2001;1539:7–15. doi: 10.1016/s0167-4889(01)00101-x. [DOI] [PubMed] [Google Scholar]
- 20.Harnett W, Goodridge HS, Harnett MM. Subversion of immune cell signal transduction pathways by the secreted filarial nematode product, ES-62. Parasitology. 2005;130(Suppl):S63–68. doi: 10.1017/S0031182005008164. [DOI] [PubMed] [Google Scholar]
- 21.Marshall FA, Watson KA, Garside P, Harnett MM, Harnett W. Effect of activated antigen-specific B cells on ES-62-mediated modulation of effector function of heterologous antigen-specific T cells in vivo. Immunology. 2008;123:411–425. doi: 10.1111/j.1365-2567.2007.02706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Riyami L, Harnett W. Immunomodulatory properties of ES-62, a phosphorylcholine-containing glycoprotein secreted by Acanthocheilonema viteae. Endocr Metab Immune Disord Drug Targets. 2012;12:45–52. doi: 10.2174/187153012799278893. [DOI] [PubMed] [Google Scholar]
- 23.Rodgers DT, Pineda MA, McGrath MA, Al-Riyami L, Harnett W, Harnett MM. Protection against collagen-induced arthritis in mice afforded by the parasitic worm product, ES-62, is associated with restoration of the levels of interleukin-10-producing B cells and reduced plasma cell infiltration of the joints. Immunology. 2014;141:457–466. doi: 10.1111/imm.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melendez AJ, Harnett MM, Pushparaj PN, et al. Inhibition of FcepsilonRI-mediated mast cell responses by ES-62, a product of parasitic filarial nematodes. Nat Med. 2007;13:1375–1381. doi: 10.1038/nm1654. [DOI] [PubMed] [Google Scholar]
- 25.Rzepecka J, Siebeke I, Coltherd JC, et al. The helminth product, ES-62, protects against airway inflammation by resetting the Th cell phenotype. Int J Parasitol. 2013;43:211–223. doi: 10.1016/j.ijpara.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prasanphanich NS, Mickum ML, Heimburg-Molinaro J, Cummings RD. Glycoconjugates in host-helminth interactions. Front Immunol. 2013;4:240. doi: 10.3389/fimmu.2013.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tundup S, Srivastava L, Harn DA., Jr. Polarization of host immune responses by helminth-expressed glycans. Ann N Y Acad Sci. 2012;1253:E1–e13. doi: 10.1111/j.1749-6632.2012.06618.x. [DOI] [PubMed] [Google Scholar]
- 28.Harn DA, McDonald J, Atochina O, Da’dara AA. Modulation of host immune responses by helminth glycans. Immunol Rev. 2009;230:247–257. doi: 10.1111/j.1600-065X.2009.00799.x. [DOI] [PubMed] [Google Scholar]
- 29.Meevissen MH, Yazdanbakhsh M, Hokke CH. Schistosoma mansoni egg glycoproteins and C-type lectins of host immune cells: molecular partners that shape immune responses. Exp Parasitol. 2012;132:14–21. doi: 10.1016/j.exppara.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 30.van der Kleij D, Latz E, Brouwers JF, et al. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J Biol Chem. 2002;277:48122–48129. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- 31.Schramm G, Falcone FH, Gronow A, et al. Molecular characterization of an interleukin-4-inducing factor from Schistosoma mansoni eggs. J Biol Chem. 2003;278:18384–18392. doi: 10.1074/jbc.M300497200. [DOI] [PubMed] [Google Scholar]
- 32.Everts B, Hussaarts L, Driessen NN, et al. Schistosome-derived omega-1 drives Th2 polarization by suppressing protein synthesis following internalization by the mannose receptor. J Exp Med. 2012;209:1753–1767. s1751. doi: 10.1084/jem.20111381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinfelder S, Andersen JF, Cannons JL, et al. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1) J Exp Med. 2009;206:1681–1690. doi: 10.1084/jem.20082462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Everts B, Perona-Wright G, Smits HH, et al. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J Exp Med. 2009;206:1673–1680. doi: 10.1084/jem.20082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomez-Escobar N, Lewis E, Maizels RM. A novel member of the transforming growth factor-beta (TGF-beta) superfamily from the filarial nematodes Brugia malayi and B. pahangi. Exp Parasitol. 1998;88:200–209. doi: 10.1006/expr.1998.4248. [DOI] [PubMed] [Google Scholar]
- 36.Maizels RM, Gomez-Escobar N, Gregory WF, Murray J, Zang X. Immune evasion genes from filarial nematodes. Int J Parasitol. 2001;31:889–898. doi: 10.1016/s0020-7519(01)00213-2. [DOI] [PubMed] [Google Scholar]
- 37.Ajonina-Ekoti I, Kurosinski MA, Younis AE, et al. Comparative analysis of macrophage migration inhibitory factors (MIFs) from the parasitic nematode Onchocerca volvulus and the free-living nematode Caenorhabditis elegans. Parasitol Res. 2013;112:3335–3346. doi: 10.1007/s00436-013-3513-1. [DOI] [PubMed] [Google Scholar]
- 38.Prieto-Lafuente L, Gregory WF, Allen JE, Maizels RM. MIF homologues from a filarial nematode parasite synergize with IL-4 to induce alternative activation of host macrophages. J Leukoc Biol. 2009;85:844–854. doi: 10.1189/jlb.0808459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zang X, Taylor P, Wang JM, et al. Homologues of human macrophage migration inhibitory factor from a parasitic nematode. Gene cloning, protein activity, and crystal structure. J Biol Chem. 2002;277:44261–44267. doi: 10.1074/jbc.M204655200. [DOI] [PubMed] [Google Scholar]
- 40.Falcone FH, Loke P, Zang X, MacDonald AS, Maizels RM, Allen JE. A Brugia malayi homolog of macrophage migration inhibitory factor reveals an important link between macrophages and eosinophil recruitment during nematode infection. J Immunol. 2001;167:5348–5354. doi: 10.4049/jimmunol.167.9.5348. [DOI] [PubMed] [Google Scholar]
- 41.Pastrana DV, Raghavan N, FitzGerald P, et al. Filarial nematode parasites secrete a homologue of the human cytokine macrophage migration inhibitory factor. Infect Immun. 1998;66:5955–5963. doi: 10.1128/iai.66.12.5955-5963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Desjardins CA, Cerqueira GC, Goldberg JM, et al. Genomics of Loa loa, a Wolbachia-free filarial parasite of humans. Nat Genet. 2013;45:495–500. doi: 10.1038/ng.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Babu S, Kumaraswami V, Nutman TB. Transcriptional control of impaired Th1 responses in patent lymphatic filariasis by T-box expressed in T cells and suppressor of cytokine signaling genes. Infect Immun. 2005;73:3394–3401. doi: 10.1128/IAI.73.6.3394-3401.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grencis RK, Entwistle GM. Production of an interferon-gamma homologue by an intestinal nematode: functionally significant or interesting artefact? Parasitology. 1997;115(Suppl):S101–106. doi: 10.1017/s0031182097002114. [DOI] [PubMed] [Google Scholar]
- 45.Tang YT, Gao X, Rosa BA, et al. Genome of the human hookworm Necator americanus. Nat Genet. 2014;46:261–269. doi: 10.1038/ng.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Falcone FH, Rossi AG, Sharkey R, Brown AP, Pritchard DI, Maizels RM. Ascaris suum-derived products induce human neutrophil activation via a G protein-coupled receptor that interacts with the interleukin-8 receptor pathway. Infect Immun. 2001;69:4007–4018. doi: 10.1128/IAI.69.6.4007-4018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith P, Mangan NE, Walsh CM, et al. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J Immunol. 2007;178:4557–4566. doi: 10.4049/jimmunol.178.7.4557. [DOI] [PubMed] [Google Scholar]
- 48.Zang X, Maizels RM. Serine proteinase inhibitors from nematodes and the arms race between host and pathogen. Trends Biochem Sci. 2001;26:191–197. doi: 10.1016/s0968-0004(00)01761-8. [DOI] [PubMed] [Google Scholar]
- 49.Gregory WF, Maizels RM. Cystatins from filarial parasites: evolution, adaptation and function in the host-parasite relationship. Int J Biochem Cell Biol. 2008;40:1389–1398. doi: 10.1016/j.biocel.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 50.Hartmann S, Lucius R. Modulation of host immune responses by nematode cystatins. Int J Parasitol. 2003;33:1291–1302. doi: 10.1016/s0020-7519(03)00163-2. [DOI] [PubMed] [Google Scholar]
- 51.Blackburn CC, Selkirk ME. Inactivation of platelet-activating factor by a putative acetylhydrolase from the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunology. 1992;75:41–46. [PMC free article] [PubMed] [Google Scholar]
- 52.Culley FJ, Brown A, Conroy DM, Sabroe I, Pritchard DI, Williams TJ. Eotaxin is specifically cleaved by hookworm metalloproteases preventing its action in vitro and in vivo. J Immunol. 2000;165:6447–6453. doi: 10.4049/jimmunol.165.11.6447. [DOI] [PubMed] [Google Scholar]
- 53.Szkudlinski J. Occurrence of prostaglandins and other eicosanoids in parasites and their role in host-parasite interaction. Wiad Parazytol. 2000;46:439–446. [PubMed] [Google Scholar]
- 54.Kubata BK, Duszenko M, Martin KS, Urade Y. Molecular basis for prostaglandin production in hosts and parasites. Trends Parasitol. 2007;23:325–331. doi: 10.1016/j.pt.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Franchini GR, Porfido JL, Ibanez Shimabukuro M, et al. The unusual lipid binding proteins of parasitic helminths and their potential roles in parasitism and as therapeutic targets. Prostaglandins Leukot Essent Fatty Acids. 2014 doi: 10.1016/j.plefa.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 56.Callahan HL, Crouch RK, James ER. Helminth anti-oxidant enzymes: a protective mechanism against host oxidants? Parasitol Today. 1988;4:218–225. doi: 10.1016/0169-4758(88)90162-7. [DOI] [PubMed] [Google Scholar]
- 57.Gretes MC, Poole LB, Karplus PA. Peroxiredoxins in parasites. Antioxid Redox Signal. 2012;17:608–633. doi: 10.1089/ars.2011.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ishii T, Warabi E, Yanagawa T. Novel roles of peroxiredoxins in inflammation, cancer and innate immunity. J Clin Biochem Nutr. 2012;50:91–105. doi: 10.3164/jcbn.11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams DL, Bonilla M, Gladyshev VN, Salinas G. Thioredoxin glutathione reductase-dependent redox networks in platyhelminth parasites. Antioxid Redox Signal. 2013;19:735–745. doi: 10.1089/ars.2012.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perrigoue JG, Marshall FA, Artis D. On the hunt for helminths: innate immune cells in the recognition and response to helminth parasites. Cell Microbiol. 2008;10:1757–1764. doi: 10.1111/j.1462-5822.2008.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smits HH, Everts B, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections protect against allergic diseases by active regulatory processes. Curr Allergy Asthma Rep. 2010;10:3–12. doi: 10.1007/s11882-009-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spits H, Artis D, Colonna M, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 63.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 64.Hussaarts L, Yazdanbakhsh M, Guigas B. Priming dendritic cells for th2 polarization: lessons learned from helminths and implications for metabolic disorders. Front Immunol. 2014;5:499. doi: 10.3389/fimmu.2014.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pearce EJ, Kane CM, Sun J. Regulation of dendritic cell function by pathogen-derived molecules plays a key role in dictating the outcome of the adaptive immune response. Chem Immunol Allergy. 2006;90:82–90. doi: 10.1159/000088882. [DOI] [PubMed] [Google Scholar]
- 66.White RR, Artavanis-Tsakonas K. How helminths use excretory secretory fractions to modulate dendritic cells. Virulence. 2012;3:668–677. doi: 10.4161/viru.22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Connor LM, Tang SC, Camberis M, Le Gros G, Ronchese F. Helminth-conditioned dendritic cells prime CD4+ T cells to IL-4 production in vivo. J Immunol. 2014;193:2709–2717. doi: 10.4049/jimmunol.1400374. [DOI] [PubMed] [Google Scholar]
- 68.Cook PC, Jones LH, Jenkins SJ, Wynn TA, Allen JE, MacDonald AS. Alternatively activated dendritic cells regulate CD4+ T-cell polarization in vitro and in vivo. Proc Natl Acad Sci U S A. 2012;109:9977–9982. doi: 10.1073/pnas.1121231109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Everts B, Adegnika AA, Kruize YC, Smits HH, Kremsner PG, Yazdanbakhsh M. Functional impairment of human myeloid dendritic cells during Schistosoma haematobium infection. PLoS Negl Trop Dis. 2010;4:e667. doi: 10.1371/journal.pntd.0000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Everts B, Pearce EJ. Metabolic control of dendritic cell activation and function: recent advances and clinical implications. Front Immunol. 2014;5:203. doi: 10.3389/fimmu.2014.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao Y, Nish SA, Jiang R, et al. Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells. Immunity. 2013;39:722–732. doi: 10.1016/j.immuni.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Everts B, Smits HH, Hokke CH, Yazdanbakhsh M. Helminths and dendritic cells: sensing and regulating via pattern recognition receptors, Th2 and Treg responses. Eur J Immunol. 2010;40:1525–1537. doi: 10.1002/eji.200940109. [DOI] [PubMed] [Google Scholar]
- 73.van Riet E, Everts B, Retra K, et al. Combined TLR2 and TLR4 ligation in the context of bacterial or helminth extracts in human monocyte derived dendritic cells: molecular correlates for Th1/Th2 polarization. BMC Immunol. 2009;10:9. doi: 10.1186/1471-2172-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Semnani RT, Law M, Kubofcik J, Nutman TB. Filaria-induced immune evasion: suppression by the infective stage of Brugia malayi at the earliest host-parasite interface. J Immunol. 2004;172:6229–6238. doi: 10.4049/jimmunol.172.10.6229. [DOI] [PubMed] [Google Scholar]
- 75.Langelaar M, Aranzamendi C, Franssen F, et al. Suppression of dendritic cell maturation by Trichinella spiralis excretory/secretory products. Parasite Immunol. 2009;31:641–645. doi: 10.1111/j.1365-3024.2009.01136.x. [DOI] [PubMed] [Google Scholar]
- 76.van Riet E, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology. 2007;212:475–490. doi: 10.1016/j.imbio.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 77.Talaat KR, Bonawitz RE, Domenech P, Nutman TB. Preexposure to live Brugia malayi microfilariae alters the innate response of human dendritic cells to Mycobacterium tuberculosis. J Infect Dis. 2006;193:196–204. doi: 10.1086/498912. [DOI] [PubMed] [Google Scholar]
- 78.Semnani RT, Mahapatra L, Dembele B, et al. Expanded numbers of circulating myeloid dendritic cells in patent human filarial infection reflect lower CCR1 expression. J Immunol. 2010;185:6364–6372. doi: 10.4049/jimmunol.1001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Allen JE, Loke P. Divergent roles for macrophages in lymphatic filariasis. Parasite Immunol. 2001;23:345–352. doi: 10.1046/j.1365-3024.2001.00394.x. [DOI] [PubMed] [Google Scholar]
- 80.Loke P, Nair MG, Parkinson J, Guiliano D, Blaxter M, Allen JE. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 2002;3:7. doi: 10.1186/1471-2172-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nair MG, Cochrane DW, Allen JE. Macrophages in chronic type 2 inflammation have a novel phenotype characterized by the abundant expression of Ym1 and Fizz1 that can be partly replicated in vitro. Immunol Lett. 2003;85:173–180. doi: 10.1016/s0165-2478(02)00225-0. [DOI] [PubMed] [Google Scholar]
- 82.Mylonas KJ, Nair MG, Prieto-Lafuente L, Paape D, Allen JE. Alternatively activated macrophages elicited by helminth infection can be reprogrammed to enable microbial killing. J Immunol. 2009;182:3084–3094. doi: 10.4049/jimmunol.0803463. [DOI] [PubMed] [Google Scholar]
- 83.Babu S, Kumaraswami V, Nutman TB. Alternatively activated and immunoregulatory monocytes in human filarial infections. J Infect Dis. 2009;199:1827–1837. doi: 10.1086/599090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Semnani RT. The interaction between filarial parasites and human monocyte/macrophage populations. Adv Exp Med Biol. 2013;785:49–56. doi: 10.1007/978-1-4614-6217-0_6. [DOI] [PubMed] [Google Scholar]
- 85.Semnani RT, Mahapatra L, Moore V, Sanprasert V, Nutman TB. Functional and phenotypic characteristics of alternative activation induced in human monocytes by interleukin-4 or the parasitic nematode Brugia malayi. Infect Immun. 2011;79:3957–3965. doi: 10.1128/IAI.05191-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schonemeyer A, Lucius R, Sonnenburg B, et al. Modulation of human T cell responses and macrophage functions by onchocystatin, a secreted protein of the filarial nematode Onchocerca volvulus. J Immunol. 2001;167:3207–3215. doi: 10.4049/jimmunol.167.6.3207. [DOI] [PubMed] [Google Scholar]
- 87.O’Regan NL, Steinfelder S, Venugopal G, et al. Brugia malayi Microfilariae Induce a Regulatory Monocyte/Macrophage Phenotype That Suppresses Innate and Adaptive Immune Responses. PLoS Negl Trop Dis. 2014;8:e3206. doi: 10.1371/journal.pntd.0003206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nutman TB. Experimental infection of humans with filariae. Rev Infect Dis. 1991;13:1018–1022. doi: 10.1093/clinids/13.5.1018. [DOI] [PubMed] [Google Scholar]
- 89.Maxwell C, Hussain R, Nutman TB, et al. The clinical and immunologic responses of normal human volunteers to low dose hookworm (Necator americanus) infection. Am J Trop Med Hyg. 1987;37:126–134. doi: 10.4269/ajtmh.1987.37.126. [DOI] [PubMed] [Google Scholar]
- 90.Karasuyama H, Yamanishi Y. Basophils have emerged as a key player in immunity. Curr Opin Immunol. 2014;31:1–7. doi: 10.1016/j.coi.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 91.Torrero MN, Morris CP, Mitre BK, et al. Basophils help establish protective immunity induced by irradiated larval vaccination for filariasis. Vaccine. 2013;31:3675–3682. doi: 10.1016/j.vaccine.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Siracusa MC, Perrigoue JG, Comeau MR, Artis D. New paradigms in basophil development, regulation and function. Immunol Cell Biol. 2010;88:275–284. doi: 10.1038/icb.2010.1. [DOI] [PubMed] [Google Scholar]
- 93.Leon-Cabrera S, Flisser A. Are basophils important mediators for helminth-induced Th2 immune responses? A debate. J Biomed Biotechnol. 2012;2012:274150. doi: 10.1155/2012/274150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schwartz C, Turqueti-Neves A, Hartmann S, Yu P, Nimmerjahn F, Voehringer D. Basophil-mediated protection against gastrointestinal helminths requires IgE-induced cytokine secretion. Proc Natl Acad Sci U S A. 2014;111:E5169–5177. doi: 10.1073/pnas.1412663111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van Beek AA, Knol EF, de Vos P, Smelt MJ, Savelkoul HF, van Neerven RJ. Recent developments in basophil research: do basophils initiate and perpetuate type 2 T-helper cell responses? Int Arch Allergy Immunol. 2013;160:7–17. doi: 10.1159/000341633. [DOI] [PubMed] [Google Scholar]
- 96.Voehringer D. Regulation of type 2 immunity by basophils. Adv Exp Med Biol. 2013;785:37–41. doi: 10.1007/978-1-4614-6217-0_4. [DOI] [PubMed] [Google Scholar]
- 97.Mitre E, Nutman TB. IgE memory: persistence of antigen-specific IgE responses years after treatment of human filarial infections. J Allergy Clin Immunol. 2006;117:939–945. doi: 10.1016/j.jaci.2005.12.1341. [DOI] [PubMed] [Google Scholar]
- 98.Mitre E, Taylor RT, Kubofcik J, Nutman TB. Parasite antigen-driven basophils are a major source of IL-4 in human filarial infections. J Immunol. 2004;172:2439–2445. doi: 10.4049/jimmunol.172.4.2439. [DOI] [PubMed] [Google Scholar]
- 99.Tsai M, Grimbaldeston M, Galli SJ. Mast cells and immunoregulation/immunomodulation. Adv Exp Med Biol. 2011;716:186–211. doi: 10.1007/978-1-4419-9533-9_11. [DOI] [PubMed] [Google Scholar]
- 100.Belkaid Y, Blank RB, Suffia I. Natural regulatory T cells and parasites: a common quest for host homeostasis. Immunol Rev. 2006;212:287–300. doi: 10.1111/j.0105-2896.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- 101.Belkaid Y. Role of Foxp3-positive regulatory T cells during infection. Eur J Immunol. 2008;38:918–921. doi: 10.1002/eji.200738120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol. 2014;27:1–7. doi: 10.1016/j.coi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 103.Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev. 2014;259:88–102. doi: 10.1111/imr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Falcao PL, Malaquias LC, Martins-Filho OA, et al. Human Schistosomiasis mansoni: IL-10 modulates the in vitro granuloma formation. Parasite Immunol. 1998;20:447–454. doi: 10.1046/j.1365-3024.1998.00166.x. [DOI] [PubMed] [Google Scholar]
- 105.Malaquias LC, Falcao PL, Silveira AM, et al. Cytokine regulation of human immune response to Schistosoma mansoni: analysis of the role of IL-4, IL-5 and IL-10 on peripheral blood mononuclear cell responses. Scand J Immunol. 1997;46:393–398. doi: 10.1046/j.1365-3083.1997.d01-136.x. [DOI] [PubMed] [Google Scholar]
- 106.Boros DL, Whitfield JR. Endogenous IL-10 regulates IFN-gamma and IL-5 cytokine production and the granulomatous response in Schistosomiasis mansoni-infected mice. Immunology. 1998;94:481–487. doi: 10.1046/j.1365-2567.1998.00544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.King CL, Mahanty S, Kumaraswami V, et al. Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. J Clin Invest. 1993;92:1667–1673. doi: 10.1172/JCI116752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mahanty S, Luke HE, Kumaraswami V, Narayanan PR, Vijayshekaran V, Nutman TB. Stage-specific induction of cytokines regulates the immune response in lymphatic filariasis. Exp Parasitol. 1996;84:282–290. doi: 10.1006/expr.1996.0114. [DOI] [PubMed] [Google Scholar]
- 109.Mahanty S, Mollis SN, Ravichandran M, et al. High levels of spontaneous and parasite antigen-driven interleukin-10 production are associated with antigen-specific hyporesponsiveness in human lymphatic filariasis. J Infect Dis. 1996;173:769–773. doi: 10.1093/infdis/173.3.769. [DOI] [PubMed] [Google Scholar]
- 110.Doetze A, Satoguina J, Burchard G, et al. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by T(h)3/T(r)1-type cytokines IL-10 and transforming growth factor-beta but not by a T(h)1 to T(h)2 shift. Int Immunol. 2000;12:623–630. doi: 10.1093/intimm/12.5.623. [DOI] [PubMed] [Google Scholar]
- 111.Harris N, Gause WC. To B or not to B: B cells and the Th2-type immune response to helminths. Trends Immunol. 2011;32:80–88. doi: 10.1016/j.it.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mangan NE, Fallon RE, Smith P, van Rooijen N, McKenzie AN, Fallon PG. Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J Immunol. 2004;173:6346–6356. doi: 10.4049/jimmunol.173.10.6346. [DOI] [PubMed] [Google Scholar]
- 113.Smits HH, Hammad H, van Nimwegen M, et al. Protective effect of Schistosoma mansoni infection on allergic airway inflammation depends on the intensity and chronicity of infection. J Allergy Clin Immunol. 2007;120:932–940. doi: 10.1016/j.jaci.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 114.van der Vlugt LE, Zinsou JF, Ozir-Fazalalikhan A, et al. Interleukin 10 (IL-10)-producing CD1dhi regulatory B cells from Schistosoma haematobium-infected individuals induce IL-10-positive T cells and suppress effector T-cell cytokines. J Infect Dis. 2014;210:1207–1216. doi: 10.1093/infdis/jiu257. [DOI] [PubMed] [Google Scholar]
- 115.Steel C, Nutman TB. Altered T cell memory and effector cell development in chronic lymphatic filarial infection that is independent of persistent parasite antigen. PLoS One. 2011;6:e19197. doi: 10.1371/journal.pone.0019197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mahanty S, Nutman TB. Immunoregulation in human lymphatic filariasis: the role of interleukin 10. Parasite Immunol. 1995;17:385–392. doi: 10.1111/j.1365-3024.1995.tb00906.x. [DOI] [PubMed] [Google Scholar]
- 117.Korten S, Hoerauf A, Kaifi JT, Buttner DW. Low levels of transforming growth factor-beta (TGF-beta) and reduced suppression of Th2-mediated inflammation in hyperreactive human onchocerciasis. Parasitology. 2011;138:35–45. doi: 10.1017/S0031182010000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Korten S, Kaifi JT, Buttner DW, Hoerauf A. Transforming growth factor-beta expression by host cells is elicited locally by the filarial nematode Onchocerca volvulus in hyporeactive patients independently from Wolbachia. Microbes Infect. 2010;12:555–564. doi: 10.1016/j.micinf.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 119.Steel C, Nutman TB. CTLA-4 in filarial infections: implications for a role in diminished T cell reactivity. J Immunol. 2003;170:1930–1938. doi: 10.4049/jimmunol.170.4.1930. [DOI] [PubMed] [Google Scholar]
- 120.Babu S, Bhat SQ, Kumar NP, et al. Human Type 1 and 17 Responses in Latent Tuberculosis Are Modulated by Coincident Filarial Infection through Cytotoxic T Lymphocyte Antigen-4 and Programmed Death-1. J Infect Dis. 2009;200:288–298. doi: 10.1086/599797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J Immunol. 2006;176:3248–3256. doi: 10.4049/jimmunol.176.5.3248. [DOI] [PubMed] [Google Scholar]
- 122.Nutman TB, Kumaraswami V, Ottesen EA. Parasite-specific anergy in human filariasis. Insights after analysis of parasite antigen-driven lymphokine production. J Clin Invest. 1987;79:1516–1523. doi: 10.1172/JCI112982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Taylor JJ, Krawczyk CM, Mohrs M, Pearce EJ. Th2 cell hyporesponsiveness during chronic murine schistosomiasis is cell intrinsic and linked to GRAIL expression. J Clin Invest. 2009;119:1019–1028. doi: 10.1172/JCI36534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Semnani RT, Sabzevari H, Iyer R, Nutman TB. Filarial antigens impair the function of human dendritic cells during differentiation. Infect Immun. 2001;69:5813–5822. doi: 10.1128/IAI.69.9.5813-5822.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Clay GM, Sutterwala FS, Wilson ME. NLR proteins and parasitic disease. Immunol Res. 2014;59:142–152. doi: 10.1007/s12026-014-8544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ritter M, Gross O, Kays S, et al. Schistosoma mansoni triggers Dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc Natl Acad Sci U S A. 2010;107:20459–20464. doi: 10.1073/pnas.1010337107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nono JK, Pletinckx K, Lutz MB, Brehm K. Excretory/secretory-products of Echinococcus multilocularis larvae induce apoptosis and tolerogenic properties in dendritic cells in vitro. PLoS Negl Trop Dis. 2012;6:e1516. doi: 10.1371/journal.pntd.0001516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Semnani RT, Liu AY, Sabzevari H, et al. Brugia malayi microfilariae induce cell death in human dendritic cells, inhibit their ability to make IL-12 and IL-10, and reduce their capacity to activate CD4+ T cells. J Immunol. 2003;171:1950–1960. doi: 10.4049/jimmunol.171.4.1950. [DOI] [PubMed] [Google Scholar]
- 129.Pan W, Zhou HJ, Shen YJ, et al. Surveillance on the status of immune cells after Echinnococcus granulosus protoscoleces infection in Balb/c mice. PLoS One. 2013;8:e59746. doi: 10.1371/journal.pone.0059746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Peon AN, Espinoza-Jimenez A, Terrazas LI. Immunoregulation by Taenia crassiceps and its antigens. Biomed Res Int. 2013;2013:498583. doi: 10.1155/2013/498583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Van Ginderachter JA, Beschin A, De Baetselier P, Raes G. Myeloid-derived suppressor cells in parasitic infections. Eur J Immunol. 2010;40:2976–2985. doi: 10.1002/eji.201040911. [DOI] [PubMed] [Google Scholar]