Abstract
Renal ischemia-reperfusion (IR) injury (IRI) following shock states or transplantation causes tissue damage and delayed graft function, respectively. The Wnt/β-catenin signaling pathway plays a critical role in nephrogenesis. We therefore hypothesized that pharmacological activation of Wnt/β-catenin signaling by Wnt agonist, a synthetic pyrimidine, could protect kidneys from IRI. Adult male rats were subjected to bilateral clamping of the renal pedicles with microvascular clips for 60 min, followed by reperfusion. Wnt agonist (5 mg/kg BW) or vehicle (20% DMSO in saline) was administered intravenously 1 h prior to ischemia. Blood and renal tissues were collected 24 h after IR for evaluation. Renal IR caused a significant reduction of β-catenin and its downstream target gene cyclin D1 by 65% and 39%, respectively, compared to the sham, while Wnt agonist restored them to the sham levels. The number and intensity of cells staining with the proliferation marker Ki67 in ischematized kidneys were enhanced by Wnt agonist. The integrity of the renal histological architecture in the Wnt agonist group was better preserved than the vehicle group. Wnt agonist significantly lowered serum levels of creatinine, AST, and LDH, inhibited the production of IL-6 and IL-1β, and MPO activities. Lastly, Wnt agonist reduced iNOS, nitrotyrosine proteins and 4-hydroxynonenal in the kidneys by 60%, 47% and 21%, respectively, compared to the vehicle. These results indicate that Wnt agonist improves renal regeneration and function while attenuating inflammation and oxidative stress in the kidneys after IR. Thus, pharmacologic stimulation of Wnt/β-catenin signaling provides a beneficial effect on the prevention of renal IRI.
Keywords: Wnt agonist, Wnt/β-catenin, renal ischemia-reperfusion, proliferation, oxidative stress
INTRODUCTION
Ischemia-reperfusion (IR) injury (IRI) is a key contributor to acute renal failure (ARF) and carries a high mortality rate following septic shock, hemorrhagic shock, renal transplantation, and major cardiovascular procedures. Indeed, it has been observed that the ARF mortality rate can reach 60% in ICU patients recovering from septic shock, which climbs as high as 80% for post-operative IRI (1, 2). IRI is of particular concern in the renal transplant setting as it can intensify the immune response, leading to increased risk of renal allograft rejection or chronic allograft damage (3, 4).
One of the crucial events in the kidney’s natural response to ischemic or toxic insult is the activation of resident stem/progenitor cells that allows for nephron regeneration (5). The canonical Wnt/β-catenin signaling axis has been well-established as having a central role in stem and progenitor cell renewal and differentiation (6). Furthermore, Wnt/β-catenin signaling is known to strongly influence organogenesis and carries vital embryologic responsibilities, particularly in kidney development and maturation (6). The importance of canonical Wnt/β-catenin activity in the kidney is preserved postnataly, as well, for in a murine model of renal IRI, tubule-specific β-catenin deficiency mice exhibited increased cellular injury, increased creatinine, and increased mortality after IR compared to control mice (7). Together, this information suggests that there may be a role for pharmacologic strategies that exploit the Wnt/β-catenin axis to assist in renal recovery following IRI.
β-Catenin is a tightly regulated intracellular protein that is normally tagged for proteasomal degradation in the absence of Wnt stimulation (6). This is owing to a phosphorylation of β-catenin performed by a multi-protein complex consisting of glycogen synthase kinase 3β (GSK-3β), axin, and adenomatous polyposis coli (APC), collectively creating the β-catenin destruction complex (6). The canonical Wnt/β-catenin axis is activated when endogenous Wnt ligands bind frizzled receptors on the cell surface. β-catenin is then dephosphorylated to be able to accumulate in the cytoplasm and migrate to the cell nucleus, where it can interact with the transcription factors T-cell factor and lymphoid enhancer factor (Tcf/Lef) (6). These interactions ultimately catalyze the upregulation of downstream target gene expression, including those responsible for cell-cycle progression, such as c-myc and cyclin D1 (6).
One such means of potentially utilizing this pathway is through the administration of 2-amino-4-[3,4-(methylenedioxy)benzylamino]6-(3-methoxyphenyl)pyrimidine (Wnt agonist), a modified pyrimidine that was recently discovered through screening the activation of β-catenin/Tcf reporter genes in cultured cells (8). Its activity was confirmed in vivo by demonstrating its effects in models of Xenopus development, thereby establishing it as a novel small-molecule agonist of the canonical Wnt/β-catenin axis (8). We therefore hypothesized that Wnt agonist treatment would protect the kidneys from the injury induced by IR. In the present study, we used a rat model of IRI and examined the effect of Wnt agonist treatment on the activation of Wnt/β-catenin signaling and regeneration in the kidneys after IR. We then assessed the serum levels of renal injury and function makers as well as the histologic structure of the kidneys. Finally, we examined the effect of Wnt agonist on oxidative stress and inflammation in the kidneys following IR.
MATERIALS AND METHODS
Experimental animals
Male adult Sprague-Dawley rats (250–275 g; Charles River Laboratories, Wilmington, MA) were kept in a temperature-controlled facility on a 12-h light/dark cycle and fed a standard rat chow diet. Rats were fasted overnight before undergoing surgery but provided with water ad libitum. All experiments were performed in accordance with the National Institutes of Health guidelines for use of experimental animals, and this study was approved by the Institutional Animal Care and Use Committee (IACUC) of the Feinstein Institute for Medical Research.
Animal model of renal IR
Rats were anesthetized with intraperitoneal injection of sodium pentobarbital solution (40 mg/kg BW), after which the right hind limb and ventral abdomen were shaved and cleansed with a 10% povidone-iodine wash. A catheter was then inserted into the right femoral vein and rats received a 0.5 ml infusion of Wnt agonist (5 mg/kg BW, EMD Biosciences, San Diego, CA) or vehicle [20% dimethyl sulfoxide (DMSO) in normal saline] over 30 min. Thirty min after completion of infusion, a 3-cm midline laparotomy incision was made and the vascular pedicles of both kidneys were occluded with microvascular clamps. Clamps were removed after 60 min to allow for reperfusion, the abdomen was closed, and rats were returned to their cages. Sham operated animals underwent midline laparotomy alone. The dosage of Wnt agonist was based on our previous study in hepatic IR model (9). Blood and kidney samples were collected 24 h after clamp removal and stored at −80°C prior to analysis.
Determination of serum enzymes and biochemical markers
Whole blood samples were centrifuged at 4,000 rpm for 12 min to collect serum, which was then stored at −80°C prior to analysis. The activities of aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and creatinine were determined by assay kits from Pointe Scientific (Lincoln Park, MI) performed according to the manufacturer’s instructions.
Western blotting analysis
Frozen kidneys were pulverized and 100 mg samples were lysed and homogenized in 300 µl lysis buffer (10 mM Tris-HCl pH 7.5, 120 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS) containing a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) using high frequency sonication. Samples were centrifuged at 14,000 rpm for 15 min at 4°C to allow for collection of supernatant. Following determination of sample protein concentrations by Pierce BCA protein assay kit (Pierce Biotechnology, Rockford, IL), 50 µg samples were separated on 4–12% Bis-Tris gels and transferred to nitrocellulose membranes. Blot membranes were then incubated with primary antibody against β-catenin, cyclin D1, inducible nitric oxide synthase (iNOS), nitrotyrosine, 4-hydroxynonenal (4-HNE) or β-actin (Santa Cruz BioTechnologies, Santa Cruz, CA). All protein bands were detected by species-specific fluorescence-labeled secondary antibodies and analyzed by the LI-COR Odyssey Fc Imager (LI-COR, Lincoln, NE).
Histologic evaluation of renal injury
Coronal plane kidney biopsies were taken following 24 h of reperfusion and stored in 10% formalin before being fixed in paraffin. Biopsies were then sectioned to 4-µm cuts and stained with hematoxylin-eosin. Cortico-medullary junction injury was then assessed in a blinded fashion using a semi-quantitative light microscopy evaluation. The histologic injury score for each sample was expressed as the sum of the individual scores given for 5 different parameters: dilation or loss of Bowman’s space, flattening of renal tubular epithelium, loss of tubular brush border, microhemorrhage, and tubular casts. For each parameter scoring was as follows: 0, none; 1, ≤10%; 2, 11–25%; 3, 26–50%; 4, >50%. Each sample’s score was determined by averaging the findings from 10 microscopic fields.
Renal myeloperoxidase assessment
Frozen kidneys were pulverized and 100 mg of tissue was homogenized in 1 ml of KPO4 buffer containing 0.5% hexadecyltrimethylammonium bromide by using high frequency sonication and incubated at 60°C for 2 h. Samples were centrifuged and the supernatants were extracted. The reaction was carried out in a 96-well plate by adding samples to a phosphate buffer containing o-dianisidine hydrochloride and hydrogen peroxide. Light absorbance was read at 460 nm at 1-min internals over a period of 5 min. Myeloperoxidase (MPO) activity (1 unit was equal to the change in absorbance per min) was expressed as units per gram of tissue.
Immunostaining for Ki67
Paraffin-embedded kidney sections were dewaxed in xylene and rehydrated in a graded series of ethanol. Slides were incubated in 0.92% citric acid buffer (Vector Laboratories, Burlingame, CA) at 95°C for 15 min. After cooling to room temperature, the slides were incubated with 2% H2O2 in 60% methanol and blocked in 2% normal rabbit serum/TBS, after which they were incubated with goat anti-Ki67 antibody (1:50, Santa Cruz Biotechnologies) in 1% normal rabbit serum/TBS with 0.02% Triton X-100 at 4°C overnight. The detection was carried out as per the instructions provided by an immunohistochemistry kit with NovaRED substrate (Vector Laboratories).
Real-time RT-PCR analysis
Total kidney RNA was extracted using “TRIzol reagent” (Invitrogen, Carlsbad, CA). Real-time PCR was carried out on cDNA samples which were reverse transcribed from 2 µg RNA samples using murine leukemia virus reverse-transcriptase (Applied Biosystems, Foster City, CA). PCR was carried out in a 24 µl final volume containing 0.08 µmol of each forward and reverse primer, 2 µl cDNA, 9.2 µl H2O and 12 µl SYBR Green PCR Master Mix (Applied Biosystems). Amplification was conducted in an Applied Biosystems 7300 real-time PCR machine under the thermal profile of 50°C for 2 min and 95°C for 10 min, followed by 45 cycles of 95°C for 15 sec and 60°C for 1 min. The level of rat β-actin mRNA was used for normalization and each specific mRNA analysis was conducted in duplicate. Relative expression of mRNA was calculated by the 2−ΔΔCt method and results were expressed as fold change in comparison to the sham samples. The primer sequences are as follows: Rat IL-1β, 5'-GAC CTG TTC TTT GAG GCT GAC A-3' (forward) and 5'-AGT CAA GGG CTT GGA AGC AA-3' (reverse); Rat IL-6, 5'-AGG GAG ATC TTG GAA ATG AGA AAA-3' (forward) and 5'-CAT CAT CGC TGT TCA TAC AAT CAG-3' (reverse); Rat β-actin, 5'-CGT GAA AAG ATG ACC CAG ACT A-3' (forward) and 5'-TGG TAC GAC CAG AGG CAT ACA G-3' (reverse).
Statistical analysis
All data are expressed as a mean ± SEM and compared by one-way analysis of variance (ANOVA) and the Student-Newman-Keuls (SNK) test. Differences in values were considered significant if P < .05.
RESULTS
Wnt agonist preserves the Wnt/β-catenin axis following renal IR
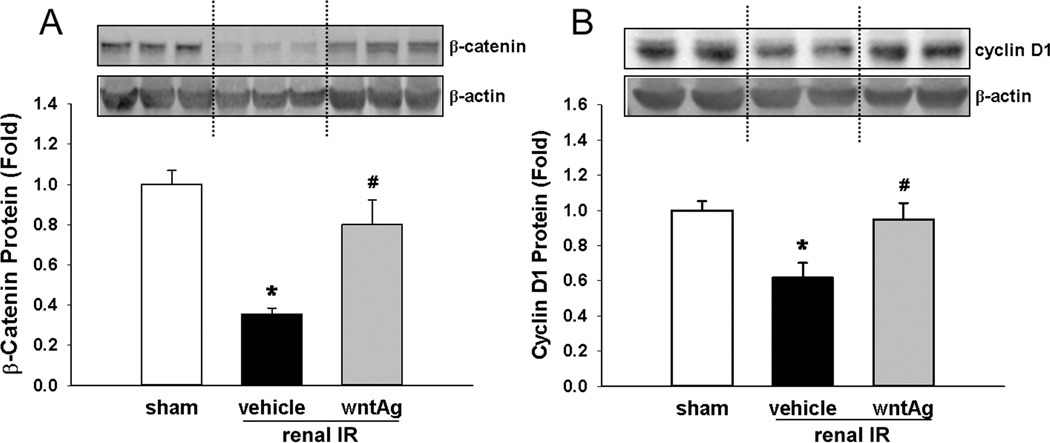
In order to investigate the effect of IR on Wnt/β-catenin signaling in the kidneys, we measured protein levels of β-catenin and its target gene 24 h after insult. Renal IRI caused a 65% decrease in the measurable levels of β-catenin, which was restored to within 80% of sham levels when Wnt agonist was administered prior to IR (Fig. 1A). In a likewise manner, we observed that renal IR led to a 39% decrease in renal protein levels of cyclin D1, a cell cycle promoter that is activated as a direct target gene of β-catenin/Tcf interaction (6). Consistent with this notion, we observed that with Wnt agonist treatment, renal protein levels of cyclin D1 were returned to 95% of the sham levels after IR (Fig. 1B). These findings demonstrated that renal IRI caused a down-regulation of signaling through the canonical Wnt/β-catenin axis, which was able to be restored with the administration of Wnt agonist.
FIG. 1. Effect of renal IR and Wnt agonist on β-catenin and downstream Wnt/β-catenin signaling.
Kidney tissues were harvested 24 h after reperfusion from the sham, vehicle, and Wnt agonist-treated (wntAg) groups. Total lysates were subjected to Western blotting, with representative blots against (A) β-catenin and β-actin, and (B) cyclin D1 and β-actin, shown. Blots were scanned and quantified by densitometry. Band intensity of β-catenin was normalized to the corresponding band intensity of β-actin. The ratio of the sham group is designated as 1 for comparison. Data presented as means ± SEM (n=4–6/group) and compared by one-way ANOVA and SNK method; *P < 0.05 vs. sham; #P < 0.05 vs. vehicle.
Wnt agonist decreases renal tissue injury
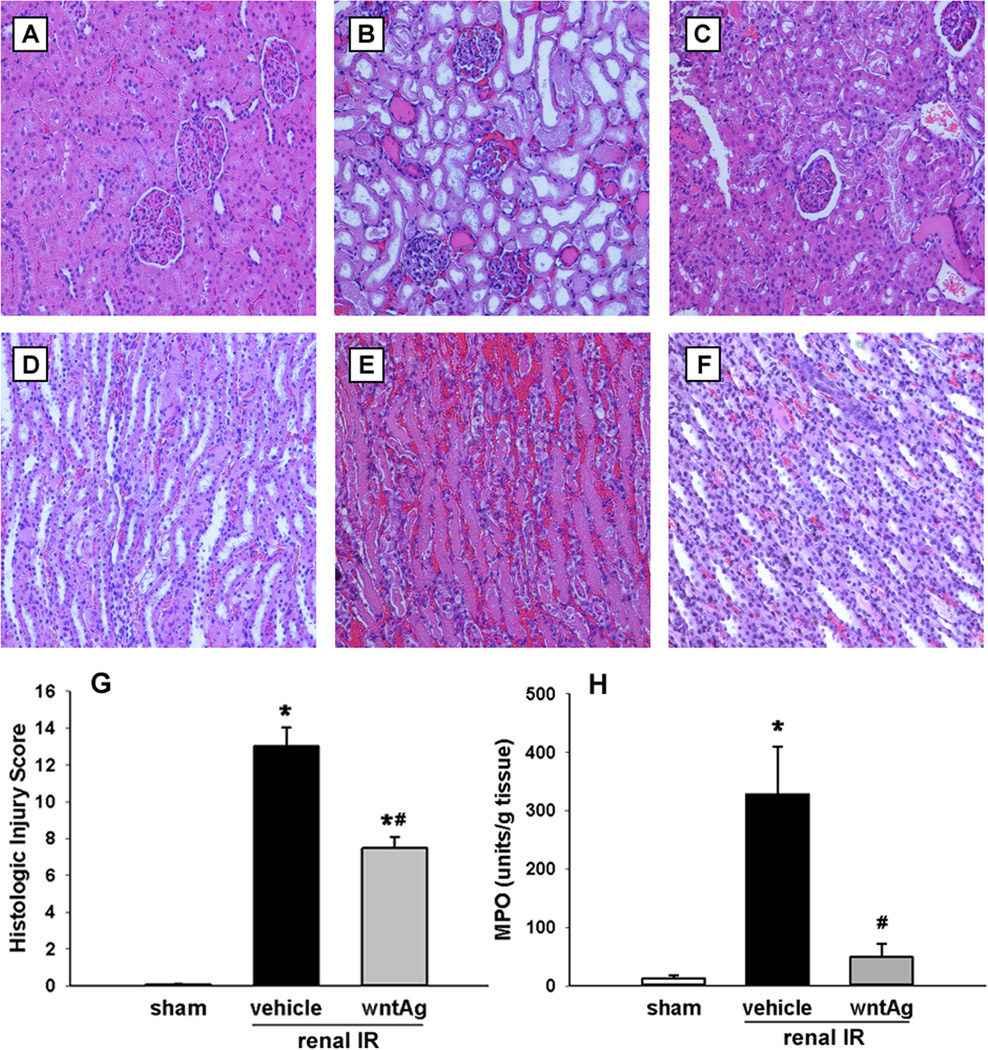
Not surprisingly, 60 min of bilateral clamping of the renal vasculature caused extensive cellular injury, measurable through increases in cellular enzymes and biochemical markers, and visible through histologic disruption of normal nephron architecture. Serum levels of creatinine, AST, and LDH raised 11.7-, 3.6-, and 10.0-fold, respectively, 24 h after renal IR. Wnt agonist treatment, however, blunted these rises by 41%, 42%, and 50%, respectively (Fig. 2). Although AST and LDH are not kidney-specific organ injury markers, they have been commonly used for indicating the severity of cellular injury in the kidney after IR (10–12). These findings correlated with the visible changes that renal IRI caused on histopathologic examination. In comparison to the sham (Fig. 3A and D), at 24 h from clamp release ischematized kidneys treated only with vehicle demonstrated abnormalities in Bowman’s capsule spacing, disrupted convoluted tubule epithelium, hemorrhage, and intratubular cast formation (Fig. 3B and E). We observed, however, an improvement in all of the previously mentioned parameters in animals treated with Wnt agonist prior to IR (Fig. 3C and F). According to our modified quantitative injury scoring system (13), the vehicle group had an average histologic injury score of 13 out of a possible 20, while Wnt agonist treatment decreased the average histologic injury score down to 7.5, a 42% improvement (Fig. 3G). Moreover, renal MPO levels were determined in order to ascertain levels of neutrophil-derived inflammation following IR. When compared to sham-operated animals, IR caused a 25-fold increase in kidney MPO levels in vehicle-treated animals, which was reduced by 89% in animals treated with Wnt agonist prior to IR (Fig. 3H).
FIG. 2. Effect of Wnt agonist on tissue injury markers after renal IR.
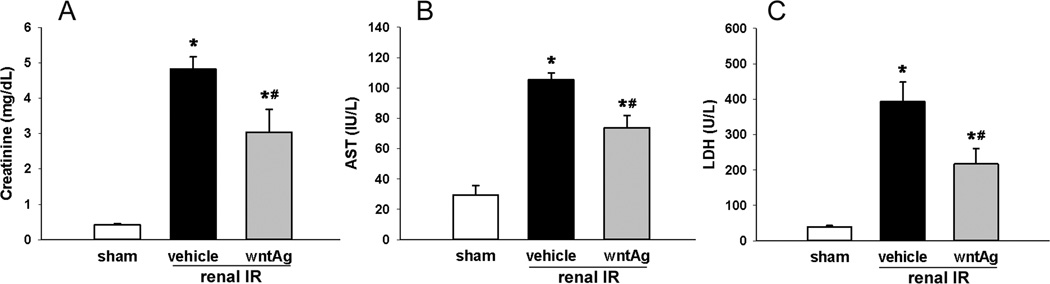
Serum samples were collected 24 h after reperfusion from the sham, vehicle, and Wnt agonist-treated (wntAg) groups for measuring (A) creatinine, (B) AST, and (C) LDH. Data presented as means ± SEM (n=4–6/group) and compared by one-way ANOVA and SNK method; *P < 0.05 vs. sham; #P < 0.05 vs. vehicle.
FIG. 3. Effect of Wnt agonist on kidney histologic architecture and MPO-induced damage after renal IR.
Kidneys were harvested 24 h after reperfusion, fixed, and stained with hematoxylin-eosin. Representative photomicrographs at 200× magnification of cortex and medulla, respectively, of (A, D) sham, (B, E) vehicle, and (C, F) Wnt agonist-treated (wntAg) groups. (G) Semi-quantitative histologic injury score measuring differences in dilation or loss of Bowman’s space, flattening of renal tubular epithelium, loss of tubular brush border, microhemorrhage, and tubular casts examined on standard hematoxylin-eosin staining as described in Materials and Methods. (H) Kidney tissue myeloperoxidase (MPO) activity was determined by spectrophotometer. Data presented as means ± SEM (n=4–6/group) and compared by one-way ANOVA and SNK method; *P < 0.05 vs. sham; #P < 0.05 vs. vehicle.
Wnt agonist aides the regenerative capability of renal tubular cells following renal IR
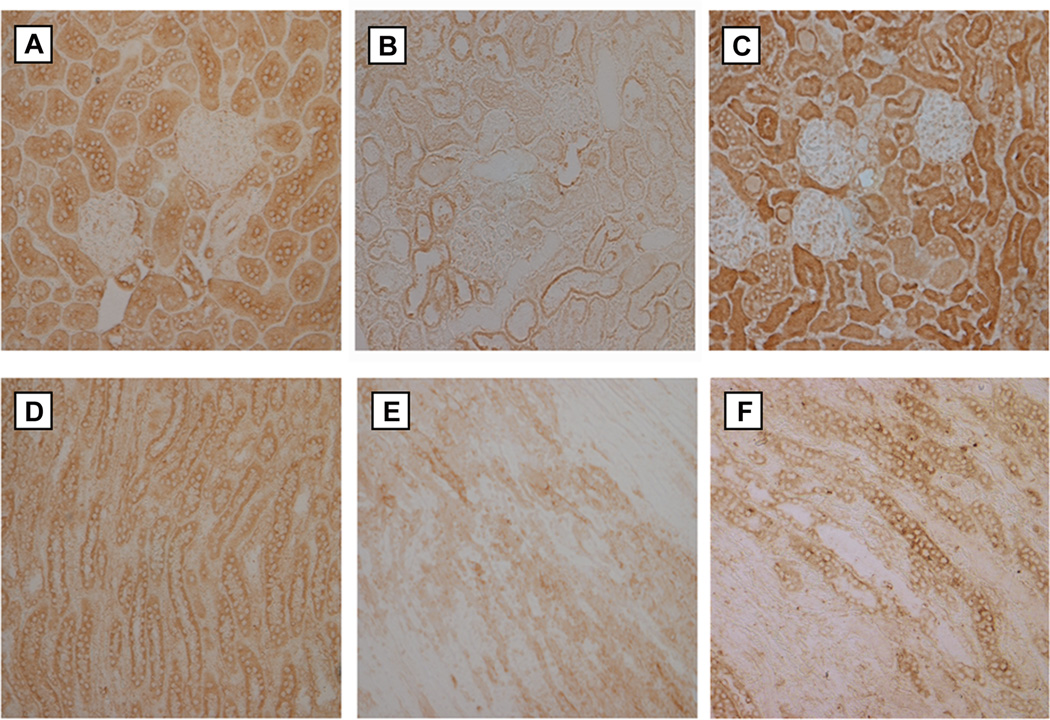
In order to ascertain the effect that Wnt agonist had on cellular regeneration and proliferation in the setting of renal IRI, we performed immunostaining for Ki67 in renal tissue. Ki67 is a nuclear protein used to determine the growth fraction of cell populations as it is necessary for proliferation and is only present in active phases of the cell cycle, and therefore absent from G0 cells (14). Compared to sham-operated animals (Fig. 4A and D), both the renal cortex and medulla of vehicle-treated IRI kidneys demonstrated a drastic decrease in both the number and intensity of cells staining positive for Ki67 (Fig. 4B and E). In contrast to the vehicle group, those animals that received Wnt agonist demonstrated a Ki67 staining pattern that much more closely resembled the sham group (Fig. 4C and F). This pattern indicates that treatment with Wnt agonist restored the proliferative ability of renal tubular cells after renal IRI.
FIG. 4. Effect of Wnt agonist on tubular cell proliferation after renal IR.
Kidneys were harvested 24 h after reperfusion, fixed, and subjected to immunohistochemical analysis against Ki67 (brown staining). Representative photomicrographs at 200× magnification of cortex and medulla, respectively, of (A, D) sham, (B, E) vehicle, and (C, F) Wnt agonist-treated groups.
Wnt agonist reduces the oxidative stress in the kidneys following renal IR
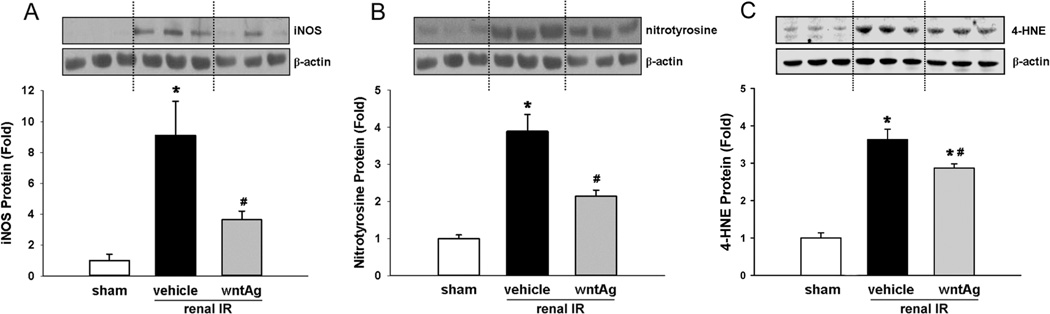
We measured the renal protein levels of iNOS and nitrotyrosine after 24 h of reperfusion as a means of measuring levels of nitrosative stress caused by IRI. In comparison to sham-operated animals, the vehicle-treated group demonstrated 9.1- and 3.9-fold rises in renal levels of iNOS and nitrotyrosine, respectively, after IR (Fig. 5A and B). Wnt agonist treatment, however, was able to mitigate the tissue damage due to nitrogen radical generation as it blunted the IRI-induced rises in iNOS and nitrotyrosine by 68% and 47%, respectively (Fig. 5A and B). We also measured the levels of 4-HNE protein adducts in the kidneys after 24 h of reperfusion. 4-HNE is one of the breakdown molecules after reactive oxygen species (ROS) target lipids and initiate the lipid peroxidation process, and has been widely accepted as an inducer of oxidative stress (15). The 4-HNE levels in the vehicle group were increased 3.6-fold in comparison with the sham, while its levels reduced by 21% after treatment with Wnt agonist (Fig. 5C).
FIG. 5. Effect of Wnt agonist on the expression levels of iNOS, nitrotyrosine and 4-HNE after renal IR.
Kidney tissues were harvested 24 h after reperfusion from the sham, vehicle, and Wnt agonist-treated (wntAg) groups. Total lysates were subjected to Western blotting. Representative blots against (A) iNOS and β-actin, (B) nitrotyrosine and β-actin, and (C) 4-HNE protein adducts and β-actin are shown. Blots were scanned and quantified by densitometry. Band intensity of iNOS, nitrotyrosine, or 4-HNE protein adducts was normalized to the corresponding band intensity of β-actin. The ratio of the sham group is designated as 1 for comparison. Data presented as means ± SEM (n=4–6/group) and compared by one-way ANOVA and SNK method; *P < 0.05 vs. sham; #P < 0.05 vs. vehicle.
Wnt agonist attenuates inflammatory cytokine generation following renal IR
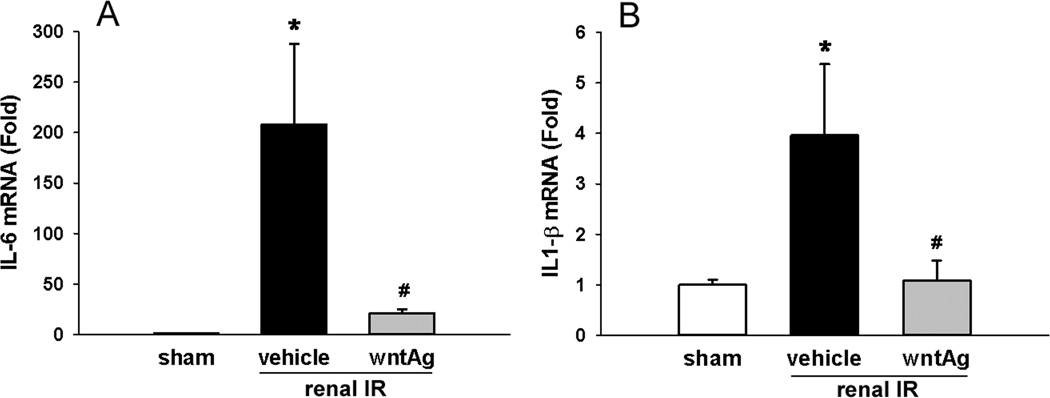
Renal IL-6 and IL-1β mRNA levels were measured as a means of determining the effect that Wnt agonist had on the activation of inflammatory cascades within the kidneys secondary to IRI. Renal IR caused a 208-fold increase in kidney tissue levels of IL-6 mRNA 24 h after ischemia (Fig. 6A). This was limited to just a 21-fold increase compared to the sham group in those animals treated with Wnt agonist; representing a near 10-fold decrease in generation of this potent inflammatory cytokine compared to vehicle-treated animals (Fig. 6A). Kidney tissue levels of IL-1β mRNA also demonstrated an increase following IR, rising 4-fold compared to the sham group gene expression (Fig. 6B). Wnt agonist treatment, however, was associated with a 96% attenuation of the rise in measureable IL-1β mRNA levels (Fig. 6B).
FIG. 6. Effect of Wnt agonist on proinflammatory cytokine in the kidneys after renal IR.
Kidney tissues were harvested 24 h after reperfusion from the sham, vehicle, and Wnt agonist-treated (wntAg) groups. Renal (A) IL-6 and (B) IL-1β mRNA expression was determined by real time RT-PCR analysis and expression levels were normalized to β-actin. The ratio of the sham group is designated as 1 for comparison. Data presented as means ± SEM (n=4–6/group) and compared by one-way ANOVA and SNK method; *P < 0.05 vs. sham; #P < 0.05 vs. vehicle.
DISCUSSION
Though it is difficult to accurately quantify the incidence of renal IRI annually, when consideration is given to the fact that over 18,000 kidney transplants are performed in the US on a yearly basis it becomes apparent that many patients are at risk for IRI (16). The number at risk grows larger still with the inclusion of an estimated 200,000 cases of septic shock occurring annually in the US, which is recognized as a leading cause of ARF (1, 17). Add to these estimates the incidence of IRI following resuscitation from hypovolemic/hemorrhagic shock or major vascular surgeries and the need for treatments that can counter renal IRI is further underscored. The canonical Wnt/β-catenin signaling axis may be well-suited for renoprotection and repair due to its importance in kidney development and homeostasis. We herein reported the effect that pharmacologic activation of the Wnt/β-catenin axis had on kidney injury in a rodent model of renal IR. In untreated animals, 60 min of bilateral renal IR caused downregulation of β-catenin activity, induced tissue injury, activated inflammatory pathways and impaired renal function. Contrasting this, administration of Wnt agonist mitigated the damage caused by renal IR and increased the regenerative capability of the kidney post-insult.
Our findings that β-catenin activity is protective in the setting of renal IRI are consistent with recent evidence suggesting that β-catenin plays a central role in the cellular response to stress. It was established by Essers et al. that β-catenin is needed for the generation of the manganese-superoxide dismutase sod-3 for anti-oxidant response in a nematode model of paraquat poisoning (18). Indeed, in C. elegans that under stress/ROS conditions, β-catenin is necessary for the transcription of factors that increase ROS clearance, stress response, and survival (19). These observations correlate with our findings that the importance of β-catenin in the stress response is preserved in a mammalian model of severe kidney injury, as we witnessed a strong correlation between diminished β-catenin levels following IR with increases in the levels of 4-HNE protein adducts, a reflect of increased generation of ROS. Canonical Wnt signaling is important in maintaining homeostasis not only against oxidative stress, however, an example of this coming from the observation by Sabbagh and colleagues that Wnt antagonists exacerbated renal osteodystrophy in mice with polycystic kidney disease (20). Given then that Wnt/β-catenin signaling is likely important in renal homeostasis and recovery from stress, an obstacle to preventing kidney injury arises from the evidence that Wnt/β-catenin signaling is actually downregulated in several injury models.
Several in vivo studies have previously established that under hypoxic conditions, cytoplasmic β-catenin stores are shunted away from the β-catenin/Tcf interactions that allow for cell replication and towards other transcription factors promoting senescence for survival (21). Whereas hypoxia in cellular models is a reasonable substitute for the ischemic phase in animal IR models, the effects of H2O2 are often substituted for the oxidative effects of the reperfusion phase of IR. Shin and colleagues demonstrate that H2O2-mediated cytotoxicity inhibits activation of β-catenin-Tcf/Lef-dependent transcriptional activity by upregulating the activity of GSK-3β, the central component of the β-catenin destruction complex (22). These findings are consistent with our observations that β-catenin and its Tcf/Lef-dependent downstream target gene, cyclin D1, are downregulated in the rat kidney following IR. Moreover, treatments that target cyclin D1 expression may provide utility in renal IRI as cyclin D1 has been shown to be downregulated in cell lines exposed to hypoxia or H2O2, whereas rodent studies have demonstrated that increased cyclin D1 expression through increased Wnt signaling is associated with recovery from ARF (23).
The importance of canonical Wnt/β-catenin signaling in recovery from renal IR is underscored by previous findings that one of the keys to renal repair following ischemic ARF is the proliferation of surviving proximal tubular cells (24). Pharmacologic treatment to aid this process might then be useful considering that normal rat kidney cells have demonstrated a lack of innate upregulation of β-catenin-Tcf signaling following ischemia (25). In this study, rats that underwent renal IRI demonstrated substantially less staining for Ki67 in the kidney compared to sham-operated animals. Wnt agonist treatment, however, resulted in renal Ki67 staining patterns that closely resembled sham animals. Our observation echoes a recent demonstration that Ki67 staining in kidney tubular cells is indicative of renal regeneration and proliferation following IR in rats (26). This suggests that treatment with Wnt agonist may represent a successful means of upregulating regenerative pathways that are crucial to recovery following severe renal insult.
Another means by which Wnt agonist treatment aided in the blunting of renal injury was through limiting inflammation and nitrosative stress, processes at the core of the pathophysiology behind IRI. Under normal circumstances, proper blood vessel tone is maintained by endothelial nitric oxide synthase (eNOS), which under stress conditions is replaced by harmful levels of iNOS (27). iNOS has been shown to be one of the key mediators of injury in renal IR and methods that seek to inhibit the production of iNOS have proven beneficial in animal models of renal IRI (27). Our model demonstrates that the production of iNOS that is characteristic of IRI can be attenuated with the administration of Wnt agonist. The tissue damage caused by reactive nitrogen species (RNS) is due to the iNOS-mediated creation of peroxynitrite (ONOO−), levels of which can be determined by its surrogate, nitrotyrosine (28). Our study confirms, as well, that nitrotyrosine protein levels were decreased in the kidneys of animals treated with Wnt agonist prior to renal IR.
Inflammatory cytokines generated within the kidneys are partly to blame for the creation of iNOS in post-ischemic renal tissue, as it has been demonstrated that renal tubular cells themselves secrete IL-6 and IL-1β following ischemic injury (24, 29). This renal-derived IL-1β can then amplify the induction of iNOS (30). We demonstrated that the gene expression of both cytokines was downregulated in the kidneys of animals treated with Wnt agonist. Ultimately, cytokines and RNS/ROS work to recruit neutrophils into kidney tissue following renal IR. This then results in drastic increases in neutrophil-derived MPO, which is considered along with ONOO− to be most culpable in causing IRI (29, 30). In our study, Wnt agonist treatment provided a near 90% reduction in renal MPO activity after IR. The importance of reducing MPO activity in IR is supported by the finding of Matthijsen et al., who observed that MPO knockout mice demonstrated significantly less renal neutrophils, less inflammation and improved renal function 24 h after renal IR (31). Moreover, it has been suggested that there is a close interplay between MPO levels and nitric oxide radicals in kidney diseases, and that reducing iNOS levels can inhibit the production and activity of MPO in renal IR (32). Wnt agonist demonstrated significant anti-inflammatory properties by drastically reducing both of these mediators compared to vehicle-treated animals.
It was previously recognized that Brown Norway rats are particularly resistant to renal IR due to congenital increased in Wnt/β-catenin signaling relative to other species of rats (33). In another study, transfection with a vector coding for the Wnt1 ligand protected human kidney cells from apoptosis after exposure to staurosporin (7). Pharmacologic attempts to manipulate Wnt signaling have also been used, but have been either limited to in vitro studies, genetic knockout studies, or have utilized GSK-3β inhibition as a means of limiting IRI (34, 35). GSK-3β is involved in multiple signaling pathways other than Wnt (6). Wnt agonist acts independently of GSK-3β to activate β-catenin/Tcf transcriptional activity although its mechanism of action is still unclear (6, 8). We recently reported the benefits of Wnt agonist administration in an animal model of hepatic IR, the first to utilize a pharmacologic Wnt/β-catenin agonist in an in vivo study of mammalian injury (9). Our observations in this study of renal IR demonstrate that Wnt agonist was able to upregulate β-catenin signaling to increase cellular proliferation while limiting inflammation and oxidative stress.
Here, we report the renoprotective properties of Wnt agonist in renal IRI under prevention setting. Further investigation is needed to enhance the clinical implication of applying Wnt agonist for the treatment, such as post-treatment for ARF and demonstration in an animal transplant model. In addition, for developing Wnt agonist in clinical use, a series of toxicity, pharmacokinetic, and dose-dependent studies need to perform. Nevertheless, this study provides another scientific aspect in finding a new way to protect renal IR injury.
ACKNOWLEDGEMENTS
We thank Zhimin Wang for her technical assistance.
Source of Funding: This study was supported by the National Institutes of Health (NIH) grant HL076179 to P.W.
ABBREVIATIONS
- 4-HNE
4-Hydroxynonenal
- ANOVA
analysis of variance
- APC
adenomatous polyposis coli
- ARF
acute renal failure
- AST
aspartate aminotransferase
- eNOS
endothelial nitric oxide synthase
- GSK
glycogen synthase kinase
- iNOS
inducible nitric oxide synthase
- IR
ischemia-reperfusion (IR)
- IRI
ischemia-reperfusion injury
- Lef
lymphoid enhancer factor
- LDH
lactate dehydrogenase
- MPO
myeloperoxidase
- ONOO−
peroxynitrite
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SNK
Student-Newman-Keuls
- Tcf
t-cell factor
Footnotes
Conflicts of Interest: All authors report no financial interests or potential conflicts of interest.
REFERENCES
- 1.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 2.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334(22):1448–1460. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 3.Nankivell BJ, Alexander SI. Rejection of the kidney allograft. N Engl J Med. 2010;363(15):1451–1462. doi: 10.1056/NEJMra0902927. [DOI] [PubMed] [Google Scholar]
- 4.Grinyo JM. Role of ischemia-reperfusion injury in the development of chronic renal allograft damage. Transplant Proc. 2001;33(7–8):3741–3742. doi: 10.1016/s0041-1345(01)02527-1. [DOI] [PubMed] [Google Scholar]
- 5.Bagul A, Frost JH, Drage M. Stem cells and their role in renal ischaemia reperfusion injury. Am J Nephrol. 2013;37(1):16–29. doi: 10.1159/000345731. [DOI] [PubMed] [Google Scholar]
- 6.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Zhou D, Li Y, Lin L, Zhou L, Igarashi P, Liu Y. Tubule-specific ablation of endogenous beta-catenin aggravates acute kidney injury in mice. Kidney Int. 2012;82(5):537–547. doi: 10.1038/ki.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Wu X, Mitchell B, Kintner C, Ding S, Schultz PG. A small-molecule agonist of the Wnt signaling pathway. Angew Chem Int Ed Engl. 2005;44(13):1987–1990. doi: 10.1002/anie.200462552. [DOI] [PubMed] [Google Scholar]
- 9.Kuncewitch M, Yang WL, Molmenti E, Nicastro J, Coppa GF, Wang P. Wnt agonist attenuates liver injury and improves survival after hepatic ischemia/reperfusion. Shock. 2013;39(1):3–10. doi: 10.1097/SHK.0b013e3182764fe8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feilleux-Duche S, Garlatti M, Aggerbeck M, Poyard M, Bouguet J, Hanoune J, Barouki R. Cell-specific regulation of cytosolic aspartate aminotransferase by glucocorticoids in the rat kidney. Am J Physiol. 1993;265(5 Pt 1):C1298–C1305. doi: 10.1152/ajpcell.1993.265.5.C1298. [DOI] [PubMed] [Google Scholar]
- 11.Heyman SN, Rosen S, Epstein FH, Spokes K, Brezis ML. Loop diuretics reduce hypoxic damage to proximal tubules of the isolated perfused rat kidney. Kidney Int. 1994;45(4):981–985. doi: 10.1038/ki.1994.132. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee PK, Patel NS, Sivarajah A, Kvale EO, Dugo L, Cuzzocrea S, Brown PA, Stewart KN, Mota-Filipe H, Britti D, Yaqoob MM, Thiemermann C. GW274150, a potent and highly selective inhibitor of iNOS, reduces experimental renal ischemia/reperfusion injury. Kidney Int. 2003;63(3):853–865. doi: 10.1046/j.1523-1755.2003.00802.x. [DOI] [PubMed] [Google Scholar]
- 13.Idrovo JP, Yang WL, Matsuda A, Nicastro J, Coppa GF, Wang P. Post-treatment with the combination of 5-aminoimidazole-4-carboxyamide ribonucleoside and carnitine improves renal function after ischemia/reperfusion injury. Shock. 2012;37(1):39–46. doi: 10.1097/SHK.0b013e31823185d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42(4):318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Department of Health and Human Services [Internet] National Kidney and Urologic Diseases Information Clearinghouse, Kidney Diseases Statistics for the United States. [updated 2012 Nov 15; cited 2014 Aug 20]; Available from: http://kidney.niddk.nih.gov/KUDiseases/pubs/kustats/index.aspx#8.
- 17.Wenzel RP, Edmond MB. Severe sepsis-national estimates. Crit Care Med. 2001;29(7):1472–1474. doi: 10.1097/00003246-200107000-00028. [DOI] [PubMed] [Google Scholar]
- 18.Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science. 2005;308(5725):1181–1184. doi: 10.1126/science.1109083. [DOI] [PubMed] [Google Scholar]
- 19.Hoogeboom D, Burgering BM. Should I stay or should I go: beta-catenin decides under stress. Biochim Biophys Acta. 2009;1796(2):63–74. doi: 10.1016/j.bbcan.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Sabbagh Y, Graciolli FG, O'Brien S, Tang W, dos Reis LM, Ryan S, Phillips L, Boulanger J, Song W, Bracken C, Liu S, Ledbetter S, Dechow P, Canziani ME, Carvalho AB, Jorgetti V, Moyses RM, Schiavi SC. Repression of osteocyte Wnt/beta-catenin signaling is an early event in the progression of renal osteodystrophy. J Bone Miner Res. 2012;27(8):1757–1772. doi: 10.1002/jbmr.1630. [DOI] [PubMed] [Google Scholar]
- 21.Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol. 2007;9(2):210–217. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]
- 22.Shin SY, Chin BR, Lee YH, Kim JH. Involvement of glycogen synthase kinase-3beta in hydrogen peroxide-induced suppression of Tcf/Lef-dependent transcriptional activity. Cell Signal. 2006;18(5):601–607. doi: 10.1016/j.cellsig.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Terada Y, Tanaka H, Okado T, Shimamura H, Inoshita S, Kuwahara M, Sasaki S. Expression and function of the developmental gene Wnt-4 during experimental acute renal failure in rats. J Am Soc Nephrol. 2003;14(5):1223–1233. doi: 10.1097/01.asn.0000060577.94532.06. [DOI] [PubMed] [Google Scholar]
- 24.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14(8):2199–2210. doi: 10.1097/01.asn.0000079785.13922.f6. [DOI] [PubMed] [Google Scholar]
- 25.Chen G, Akintola AD, Catania JM, Covington MD, Dean DD, Trzeciakowski JP, Burghardt RC, Parrish AR. Ischemia-induced cleavage of cadherins in NRK cells is not sufficient for beta-catenin transcriptional activity. Cell Commun Adhes. 2007;14(4):111–123. doi: 10.1080/15419060701556943. [DOI] [PubMed] [Google Scholar]
- 26.Feng Z, Ting J, Alfonso Z, Strem BM, Fraser JK, Rutenberg J, Kuo HC, Pinkernell K. Fresh and cryopreserved, uncultured adipose tissue-derived stem and regenerative cells ameliorate ischemia-reperfusion-induced acute kidney injury. Nephrol Dial Transplant. 2010;25(12):3874–3884. doi: 10.1093/ndt/gfq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatterjee PK, Patel NS, Kvale EO, Cuzzocrea S, Brown PA, Stewart KN, Mota-Filipe H, Thiemermann C. Inhibition of inducible nitric oxide synthase reduces renal ischemia/reperfusion injury. Kidney Int. 2002;61(3):862–871. doi: 10.1046/j.1523-1755.2002.00234.x. [DOI] [PubMed] [Google Scholar]
- 28.Walker LM, Walker PD, Imam SZ, Ali SF, Mayeux PR. Evidence for peroxynitrite formation in renal ischemia-reperfusion injury: studies with the inducible nitric oxide synthase inhibitor L-N(6)-(1-Iminoethyl)lysine. J Pharmacol Exp Ther. 2000;295(1):417–422. [PubMed] [Google Scholar]
- 29.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121(11):4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Paola R, Genovese T, Impellizzeri D, Ahmad A, Cuzzocrea S, Esposito E. The renal injury and inflammation caused by ischemia-reperfusion are reduced by genetic inhibition of TNF-alphaR1: a comparison with infliximab treatment. Eur J Pharmacol. 2013;700(1–3):134–146. doi: 10.1016/j.ejphar.2012.11.066. [DOI] [PubMed] [Google Scholar]
- 31.Matthijsen RA, Huugen D, Hoebers NT, de Vries B, Peutz-Kootstra CJ, Aratani Y, Daha MR, Tervaert JW, Buurman WA, Heeringa P. Myeloperoxidase is critically involved in the induction of organ damage after renal ischemia reperfusion. Am J Pathol. 2007;171(6):1743–1752. doi: 10.2353/ajpath.2007.070184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malle E, Buch T, Grone HJ. Myeloperoxidase in kidney disease. Kidney Int. 2003;64(6):1956–1967. doi: 10.1046/j.1523-1755.2003.00336.x. [DOI] [PubMed] [Google Scholar]
- 33.Vinas JL, Sola A, Jung M, Mastora C, Vinuesa E, Pi F, Hotter G. Inhibitory action of Wnt target gene osteopontin on mitochondrial cytochrome c release determines renal ischemic resistance. Am J Physiol Renal Physiol. 2010;299(1):F234–242. doi: 10.1152/ajprenal.00687.2009. [DOI] [PubMed] [Google Scholar]
- 34.Cho YD, Kim WJ, Yoon WJ, Woo KM, Baek JH, Lee G, Kim GS, Ryoo HM. Wnt3a stimulates Mepe, matrix extracellular phosphoglycoprotein, expression directly by the activation of the canonical Wnt signaling pathway and indirectly through the stimulation of autocrine Bmp-2 expression. J Cell Physiol. 2012;227(6):2287–2296. doi: 10.1002/jcp.24038. [DOI] [PubMed] [Google Scholar]
- 35.Dugo L, Abdelrahman M, Murch O, Mazzon E, Cuzzocrea S, Thiemermann C. Glycogen synthase kinase-3beta inhibitors protect against the organ injury and dysfunction caused by hemorrhage and resuscitation. Shock. 2006;25(5):485–491. doi: 10.1097/01.shk.0000209545.29671.31. [DOI] [PubMed] [Google Scholar]