Abstract
The genes ABCC8 and KCNJ11 have received intense focus in type 2 diabetes mellitus (T2DM) research over the past two decades. It has been hypothesized that the p.E23K (KCNJ11) mutation in the 11p15.1 region may play an important role in the development of T2DM. In 2009, Hamming et al. found that the p.1369A (ABCC8) variant may be a causal factor in the disease; therefore, in this study we performed a meta-analysis to evaluate the association between these single nucleotide polymorphisms (SNPs), including our original data on the Siberian population (1384 T2DM and 414 controls). We found rs5219 and rs757110 were not associated with T2DM in this population, and that there was linkage disequilibrium in Siberians (D’=0.766, r2= 0.5633). In addition, the haplotype rs757110[T]-rs5219[C] (p.23K/p.S1369) was associated with T2DM (OR = 1.52, 95% CI: 1.04-2.24). We included 44 original studies published by June 2014 in a meta-analysis of the p.E23K association with T2DM. The total OR was 1.14 (95% CI: 1.11-1.17) for p.E23K for a total sample size of 137,298. For p.S1369A, a meta-analysis was conducted on a total of 10 studies with a total sample size of 14,136 and pooled OR of 1.14 [95% CI (1.08-1.19); p = 2 x 10-6]. Our calculations identified causal genetic variation within the ABCC8/KCNJ11 region for T2DM with an OR of approximately 1.15 in Caucasians and Asians. Moreover, the OR value was not dependent on the frequency of p.E23K or p.S1369A in the populations.
Introduction
Type 2 diabetes mellitus (T2DM) is a pandemic that affects 6% of the adult population in developed countries [1]. Both genetic and environmental factors play a role in the development of T2DM. In particular, ABCC8(ATP-binding cassette, sub-family C (CFTR/MRP), member 8) and KCNJ11(potassium channel, inwardly rectifying subfamily J, member 11) have been the focus of T2DM research over the past two decades due to their possible role in the pathogenesis. These genes are located4.5 kb apart on chromosome 11p15.1 and encode the human Kir6.2 (KCNJ11) and SUR1 (ABCC8) subunits of plasma membrane potassium (K) ATP channels expressed in pancreatic β-cells. The promoters of both genes have been cloned [2], and polymorphisms in these promoters can lead to aberrant expression of K ATP channels, which can consequently disrupt the normal stoichiometry (4 Kir6:4 SUR1) of the two subunits that is essential for proper channel function [3]. It is well-known that mutations in these genes cause the autosomal recessive disorder familial persistent hyperinsulinemic hypoglycemia of infancy[4,5]. In 2002,Schwanstecher et al. provided evidence that ap.E23K polymorphism in KCNJ11(SNP rs5219) alters protein function by inducing the spontaneous overactivation of pancreatic β-cells. This results in an increase in the threshold ATP concentration required for insulin release [6]. SNP rs5219 has been shown to be associated with T2DM in many populations of Europe and East Asia, but not in Ashkenasi Jewish [7], Mongolian [8], or Indian [9,10] populations. It has been hypothesized that the rs5219 (p.E23K) variation in the 11p15.1 region may play an important role in the development of T2DM, thus making it a popular marker to assess inKCNJ11.However, to date the influence of ABCC8 SNPs on the susceptibility to T2DM has not been well-characterized. Additional SNPs within ABCC8have been studied for predisposition toT2DM(e.g., exon 16: c.2117-3C>T, exon 18: p.T759T (ACC->ACT, rs1801261), and exon 33: p.S1369A).It is difficult to generalize the results between the different association studies on the SNPs of ABCC8.In 2009, Hamming et al. found that the p.K23/p.1369A haplotype that resulted from a direct effect of the ABCC8p.1369A risk allele led to a decrease in ATP inhibition, which was likely due to mild increases in intrinsic K ATP channel MgATPase activity. Moreover, a strong linkage disequilibrium in the 11p15.1 region has been observed in European populations (r2 = 0.98) [11]. It would thus be necessary to genotype both polymorphisms in populations in which p.E23K and p.S1369A mutations are present at high frequency. Earlier association studies of these polymorphisms in KCNJ11 and ABCC8 were only conducted in the regions of Russia near Europe [12], but not Siberia. Therefore, the first aim of this study was to explore associations between T2DM and two SNPs, KCNJ11 (p.E23K) and ABCC8 (p.S1369A), in a Siberian population. To the best of our knowledge, this is the largest study reported to date on the association of these SNPs in Russians with T2DM.Despite the clear functional role that these two genes may have in the pathogenesis of T2DM as well as several association and meta-analysis studies, the generalizing conclusion for all races have not been done. All previous meta-analyses of p.E23K and p.S1369A included different groups of selected studies, and some studies were not included. To date, there has been no summary analysis of all previous results. Therefore, the second aim of this study was to perform a meta-analysis of these SNPs based on data taken from all available previous studies as well as our original data.
Materials and Methods
Participants
The study population was comprised of 1384 individuals with T2DM (Female = 78%, mean age ± SD of 59.7±8.6 y, mean BMI = 33.6 ± 6.5 kg/m2) and the control group included 414 healthy individuals (Female = 57%, mean age ± SD of 32.7±10.6 y, mean BMI = 23.6 ± 4.0 kg/m2). Diabetic and control individuals were recruited at the diabetes center at the Novosibirsk Regional State Hospital. All individuals participating in the study were members of the European Russian ethnic group. The protocol (#52) was approved by The Local Ethics Committee of the Novosibirsk State Medical University on March 19th, 2013. All participants signed a written informed consent. All consecutive patients were deemed eligible pending signed informed consent and meeting inclusion/exclusion criteria. A control group was included that consisted of volunteers who were 18 years older with normal fasting and normal 2-h oral glucose tolerance test (OGTT).T2DM was defined according to the WHO 1999 criteria [13]. A clinical examination was performed that included an interview, anthropometric measurements, and blood collection. Distributions of the primary phenotypes are listed in Table 1.
Table 1. Demographic summary of European Russian participants.
| Diabetes mellitus 2 type | Control | |||
|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | |
| Age (years) | 59.7 ± 8.6 | 60.0 | 32.7±10.6 | 31.0 |
| BMI (kg/m2) | 33.6 ± 6.5 | 32.9 | 23.6 ± 4.0 | 23.1 |
| HbA1c(mmol/mol) | 71.6 ± 0.6 | 69.4 | - | - |
| C-peptide (pmol/l) | 674.2 ± 414.4 | 619.0 | - | - |
| Women (%) | 78% | 57% | ||
| Subjects, n | 1384 | 414 | ||
Genotyping
DNA was isolated from venous blood using a standard procedure. Briefly, samples were collected and the blood was separated and lysed. Protein was then hydrolyzed with proteinase K and the DNA was extracted using phenol-chloroform followed by precipitation with ethanol. Genotyping of the SNPs was performed by real-time PCR using the following TaqMan probes(rs5219: forward primer 5’-ATACGTGCTGACACGCCTG-3’, reverse primer 5’-TGCCTTTCTTGGACACAAAGC-3’, 5’-R6G-ACCCTGCCGAGCCCA-BHQ-3’, 5’-FAM-ACCCTGCCAAGCCCA-BHQ-3’; rs757110: forward primer 5’-CTACGACAGCTCCCTGAAGC-3’, reverse primer 5’-TGACTGCGAAGCCATCC-3’, 5’-FAM-CCCTCATCTCCCCTGGACA-BHQ-3’, 5’-R6G-CCCTCATCGCCCCTGG-BHQ-3’).The PCR mixture contained DNA (40–100 ng), each primer (300 nM), TaqMan probes conjugated with FAM or R6G (100–200 nM each), dNTPs (200 μM), amplification buffer, and Taqpolymerase (0.5 U/reaction) in a total volume of 25 μL. Amplification was performed using aCFX96 cycler (Bio-Rad, USA) under the following conditions: initial denaturation for 3 min at 96ºC followed by40 cycles of denaturation at 96ºC for 10 s and annealing of primers and subsequent elongation at 60ºC for 40 s. The call rate for both SNPs was 100%.Case and control samples were plated together. We also included 10% duplicate pairs for both case and control samples. The concordance was >99%.
Statistical data analysis
The Hardy-Weinberg Equilibrium was evaluated using an exact test of Hardy-Weinberg Equilibrium for 2-Allele markers in the R package “genetics”. Possible associations between SNPs and disease development were found using logistic regression analysis adjusted for age and gender, as implemented in the “glm” function of the R package for statistical analysis (www.r-project.org).Meta-analysis and estimated heterogeneity were carried out using the ‘rmeta’ package for R (http://cran.r-project.org/web/packages/rmeta/rmeta.pdf). Pooled odds ratios (ORs) were computed by the fixed-effect model for data combined under no heterogeneity between studies. If there was significant heterogeneity between studies, then the random effects model was applied for combined data. Haplotype analysis was carried out using the ‘haplo.stats’ package for R (http://cran.r-project.org/web/packages/haplo.stats/haplo.stats.pdf).Results were considered statistically significant for all statistical calculations if P< 0.05.The power of the study was calculated using software available online (http://pngu.mgh.harvard.edu/~purcell/gpc/cc2.html).
Results
Association of SNPs with T2DM
In this study we assessed SNP genotypes [rs5219 (p.E23K) and rs757110 (p.S1369A)] and found that the distribution of both SNPs corresponded to a Hardy-Weinberg equilibrium (HWE) in T2DM patients and control groups (Table 2). The frequencies of occurrence of the T allele of rs5219 in KCNJ11 were 0.63 and 0.62 in control and case groups, respectively. The frequencies of occurrence of the G allele of rs757110 in ABCC8 were 0.61 and 0.62 in control and case groups, respectively. Associations between the T2DM and genotypes of SNPs were estimated using logistic regression analysis adjusted for age and gender for three inheritance models: additive, dominant, and recessive. We found that neither rs5219 nor rs757110 were associated with T2DM in this patient population.
Table 2. Odds Ratio for Three Genetic Models for SNPs: rs5219 and rs757110.
| Gene (SNP) | Control (total = 414) | T2DM (total = 1384) | OR (95% CI) co-dominant model, p-value, AIC | OR (95% CI) additive model, p-value, AIC | OR (95% CI) dominant model, p-value, AIC | OR (95% CI) recessive model, p-value, AIC |
| KCNJ11 (rs5219) | CC/CT/TT 158/204/52HWE = 0.29 RAF = 0.37 | CC/CT/TT 535/656/193HWE = 0.77 RAF = 0.38 | СС: referenceCT: 0.95 [0.75–1.20] p = 0.67TT: 1.10 [0.77–1.56] p = 0.61AIC = 1945.6 | 1.02 [0.87–1.20] p = 0.81AIC = 1944.3 | 0.98 [0.78–1.23] p = 0.86AIC = 1944.3 | 1.13 [0.81–1.57] p = 0.47AIC = 1943.8 |
| ABCC8 (rs757110) | TT/TG/GG 160/189/65HWE = 0.47RAF = 0.39 | TT/TG/GG 526/651/207HWE = 0.82RAF = 0.38 | TT: referenceTG: 1.08 [0.78–1.49] p = 0.63 GG: 1.03 [0.74–1.44] p = 0.85AIC = 1946.1 | 1.00 [0.85–1.17] p = 0.98AIC = 1944.3 | 1.03 [0.82–1.29] p = 0.81AIC = 1944.3 | 0.94 [0.75–1.18] p = 0.71AIC = 1944.2 |
AIC—Akaike Information Criterion, lower the AIC value better the model.Abbreviations: HWE—p-value of Hardy-Weinberg equilibrium, RAF—risk allele freaquency (T for KCNJ11(rs5219) and G for ABCC8 (rs757110)).
Meta-analysis of rs5219 of KCNJ11
Study selection and characteristics of included studies
All published studies that evaluated the association between rs5219 of KCNJ11 (p.E23K) and T2DM or NIDDM were collected through a PubMed search of studies published before June 2014 using the search terms “KCNJ11”, “polymorphism”, “T2DM”, and “rs5219” in different combinations. Studies included in our meta-analysis met the following criteria: 1) conducted as a case-control design; 2) evaluated the association of rs5219 and T2DM; 3) written in English or included an abstract in English with sufficient information; 4) reported sufficient data for an odds ratios (OR) calculation; and 5) reported sufficient data for obeying HWE. We also performed a manual search of references for potentially relevant articles, and missing information was requested from article authors. If a reply was not forthcoming, then the study was excluded from the meta-analysis. Allele frequencies were calculated from the corresponding genotype distributions when not provided: [Fr.p.23K = (2NKK+NEK)/2*(NEE+NEK+NKK)]. In addition, we found several meta-analyses that had been previously published. Available information on the study design, race or ethnicity of participants, first author, published year, reference, sample size of case and control, OR, 95% confidence intervals, and rare allele frequency in the control group are shown in Table 3.
Table 3. Characteristics studies of association SNP rs5219 (p.E23K) of KCNJ11 and T2DM.
| Num | Study ID | Type | Race / ethnicity | OR | 95% C.I. | p | Case * | Control * | Fr. §§ |
|---|---|---|---|---|---|---|---|---|---|
| 1 | U.K. cohort. Sakura (1996) [20]# | CCCG | Caucasian | 1.53 | 0.99–2.38 | 0.06 | 100(38+45+17) | 82(44+27+11) | 0.30 |
| 2 | Danish. Hansen (1997) [21]# | CCCG | Caucasian | 1.41 | 0.86–2.33 | 0.18 | 58 (21+26+11) | 75 (33+34+8) | 0.23 |
| 3 | U.K. cohort. Inoue (1997) [14]# | CCCG | Caucasian | 1.10 | 0.76–1.60 | 0.62 | 172(72+78+22) | 96(38+52+6) § | 0.23 |
| 4 | Utah. Inoue (1997) [14]# | CCCG | Caucasian | 0.86 | 0.55–1.33 | 0.48 | 119 (52+55+12) | 68 (21+44+3) § | 0.27 |
| 5 | French. Hani (1998) [45] | CCCG | Caucasian | 1.65 | 1.18–2.30 | 0.003 | 191 (53+87+51) | 114 (45+53+16) | 0.27 |
| 6 | Japanese. Keiko (1999) [19] # | CCCG | Asian | 1.12 | 0.62–2.04 | 0.71 | 31 (11+13+7) | 76 (22+46+8) § | 0.41 |
| 7 | U.K. cohort. Gloyn (2001) [22] | CCCG | Caucasian | 1.30 | 1.04–1.63 | 0.02 | 360(133+161+66) | 307(125+152+30) | 0.25 |
| 8 | Japanese. Yamada (2001) [49] | CCCG | Asian | 1.22 | 0.78–1.90 | 0.54 | 103 | 73 | 0.34 |
| 9 | North Zealand in Denmark. Nielsen (2003) [23] | CCCG | Caucasian | 1.11 | 0.96–1.27 | 0.15 | 803 (287+382+134) | 862 (330+408+124) | 0.28 |
| Meta-analysis 4, 5, 7, 9 by Nielsen et al. [23] | meta | Caucasian | 1.49 r | 1.20–1.83 | 0.002 | 1473 (525+685+263) | 1351 (521+657+173) | 0.27 | |
| 10 | U.K. cohort. Gloyn (2003) [50] | CCCG | Caucasian | 1.18 | 1.04–1.34 | 0.01 | 854 (308+412+134) | 1182 (491+534+157) | 0.26 |
| Meta-analysis 1–5, 7 by Gloyn (2003) [50] | meta | Caucasian | 1.30 | 1.13–1.49 | 0.0003 | 1000 | 742 | ND | |
| Meta-analysis 1–5, 7, 10 by Gloyn (2003) [50] | meta | Caucasian | 1.23 | 1.12–1.36 | 1.5 x 10 –5 | 1854 | 1924 | ND | |
| 11 | U.K. cohort. Barroso (2003)[51] | CCCG | Caucasian | 1.19 | 0.99–1.43 | 0.07 | 499 (198+220+81) | 494 (212+225+57) | 0.24 |
| Meta-analysis 3, 5–9 by Florez et al. (2004) [48] | meta | Caucasian | 1.14 | 1.06–1.22 | 0.0002 | 2879 | 3055 | ND | |
| 12 | Scandinavian. Florez (2004) [48] | CCCG | Caucasian | 1.19 | 1.00–1.43 | 0.05 | 477 (113+244+120) | 473 (129+250+94) | 0.46 |
| 13 | Canadian. Florez (2004) [48] | CCCG | Caucasian | 1.05 | 0.71–1.55 | 0.82 | 104 (27+54+23) | 98 (27+50+21) | 0.47 |
| 14 | Sweden. Florez (2004) [48] | CCCG | Caucasian | 1.23 | 1.03–1.48 | 0.02 | 496 (174+237+85) | 506 (209+229+68) | 0.36 |
| Meta-analysis 12–14 and sibships by Florez et al. (2004) [48] | meta | Caucasian | 1.17 | 1.05–1.32 | 0.003 | 1077 | 1077 | ND | |
| Meta-analysis 3, 5, 7, 9–14 and sibships by Florez et al. (2004) [48] | meta | Caucasian | 1.15 | 1.08–1.22 | < 10 –5 | 5083 | 4747 | ND | |
| 15 | Danish. Hansen (2005) [52] | CCCG | Caucasian | 1.19 | 1.09–1.31 | 0.0002 | 1187 (423+568+196) | 4791 (1955+2195+641) | 0.36 |
| 16 | Netherland. van Dam (2005) [53] | CCCG | Caucasian | 1.27 | 0.98–1.65 | 0.07 | 192(66+92+34) | 296 (119+141+36) | 0.36 |
| 17 | U.K. cohort. Weedon (2006) [54] | CCCG | Caucasian | 1.14 | 1.05–1.23 | 0.001 | 2332 | 3592 | 0.35 |
| 18 | Japanese. Yokoi (2006) [26] | CCCG | Asian | 1.08 | 0.97–1.21 | 0.15 | 1590 (610+734+246) | 1244 (503+570+171) | 0.37 |
| 19 | WTCCC. 2007 [55] | CCGWA | Caucasian | 1.15 | 1.05–1.25 | 1.3 x 10–3 | 1924 | 2938 | ND |
| 20 | Diabetes Genetics Initiative (DGI. 2007) [56] | CCGWA | Caucasian | 1.15 | 1.09–1.21 | 1.0 x 10–7 | 6529 | 7252 | 0.47 |
| 21 | Finland-United States Investigation of NIDDM Genetics (FUSION. 2007) [57] | CCGWA | Caucasian | 1.11 | 1.02–1.20 | 0.014 | 2376 | 2432 | 0.46 |
| Meta-analysis 19–21 by Saxena et al. (2007) [56] | meta | Caucasian | 1.14 | 1.10–1.19 | 6.7 x 10 –11 | 10829 | 12622 | ND | |
| 22 | Boston. Qi (2007) [58] | CCCG | Caucasian | 1.25 | 1.09–1.44 | 0.002 | 714 (245+322+115) | 1120 (446+505+127) | 0.35 |
| 23 | Japanese. Horikoshi (2007)[59] | CCCG | Asian | 1.04 | 0.90–1.19 | 0.60 | 858 (334+393+131) | 862 (332+417+113) | 0.37 |
| 24 | Korean. Koo (2007) [16]# | CCCG | Asian | 1.28 | 1.10–1.49 | 0.002 | 761 (244+364+150) | 630 (255+273+102) § | 0.38 |
| 25 | Japanese. Sakamoto (2007) [60] | CCCG | Asian | 1.21 | 1.05–1.38 | 0.007 | 906 (333+446+127) | 889 (386+396+107) | 0.34 |
| 26 | Japanese. Doi (2007) [61] | PCG+CCCG | Asian | 1.26 | 1.09–1.45 | 0.002 | 550 (202+263+85) | 1433 (617+655+161) | 0.34 |
| 27 | Czech. Cejkova (2007) [62] | CCCG | Caucasian | 1.01 | 0.71–1.43 | 0.96 | 172 (66+85+21) | 113 (48+47+18) | 0.37 |
| 28 | African-American subject. Sale (2007) [17] # | CCCG | African-American | 0.69d | 0.49–0.99 | 0.045 | 577 | 596 § | 0.07 |
| 29 | Finnish. Willer (2007) [63] | CCCG | Caucasian | 1.22 | 1.08–1.38 | 0.002 | 1114 (284+560+270) | 953 (286+486+181) | 0.44 |
| 30 | Japanese. Omori (2008) [64] | CCCG | Asian | 1.25 | 1.08–1.46 | 0.003 | 1630 | 1064 | 0.36 |
| 31 | D.E.S.I.R. cohort. Vaxillaire (2008) [65] | CCCG | Caucasian | 1.09 | 0.91–1.29 | 0.36 | 327 (101+137+49) | 2684 (994+1287+403) | 0.39 |
| 32 | MalmoPreventive Project (MPP). Lyssenko (2008)[39] | PCG | Caucasian | 1.13 | 1.06–1.21 | 3.6 x 10–4 | 15600 (2063 with T2DM past 23.5 years) | 13537 | 0.41 |
| 33 | Botnia in Finland. Lyssenko(2008) [39] | PCG | Caucasian | 0.98 | 0.75–1.26 | 0.85 | 2635 (138 with T2DM past 23.5 years) | 0.51 | |
| 34 | Saudi. Alsmadi (2008) [47] | CCCG | Arab | 1.69 | 1.30–2.20 | 0.00009 | 550 (341+187+22) | 335(252+75+8) | 0.14 |
| 35 | Ashkenazi Jewish. Bronstein (2008) [18] # | CCCG | Ashkenazi Jewish | ND | ND | ND | 1131 | 1147 § | 0.39 |
| 36 | Sikh Diabetes Study. Sanghera (2008) [9] | CCCG | Indian | 0.86 | 0.71–1.04 | 0.12 | 532 (226+247+59) | 374 (148+169+57) | 0.38 |
| 37 | France and Switzerland. Cauchi (2008) [40] | CCCG | Caucasian | 0.96 | 0.90–1.03 | 0.28 | 2734 (1112+1220+402) § | 4234 (1625+2006+603) | 0.38 |
| 38 | Japanese NIBI. Takeuchi (2009) [66] | CCGWA | Asian | 1.07 | 1.01–1.13 | 0.01 | 5461 (2182+2511+768) | 6894 (2883+3121+890) | 0.36 |
| Meta-analysis 23, 30 & 38 by Takeuchi et al (2009) [66] | meta | Japanese | 1.09 | 1.04–1.13 | 3.4 x 10 –4 | 7954 (3129+3667+1158) | 8809 (3638+4050+1121) | 0.36 | |
| 39 | Shanghai Diabetes Study. Hu (2009) [67] | CCCG | Asian | 1.14 | 1.03–1.25 | 0.008 | 1849 | 1785 | 0.39 |
| 40 | Japanese. Tabara (2009) [68] | CCCG | Asian | 1.18 | 0.97–1.43 | 0.09 | 484 (169+232+83) | 397 (152+195+50) | 0.37 |
| 41 | Chinese. Zhou (2009) [69] | CCCG | Asian | 1.09 | 0.99–1.20 | 0.09 | 1848 (656+863+329) | 1910 (692+930+288) | 0.39 |
| Meta-analysis of 8, 18, 24, 25, 26, 30, 40, 41 by Zhou et al (2009) [69] | meta | Asian | 1.15 | 1.10–1.21 | 3 x 10 –9 | 7874 | 7629 | ND | |
| 42 | Chinese Han population from Beijing. Wang (2009) [70] | CCCG | Asian | 1.40 | 1.12–1.76 | 0.004 | 400 | 400 | ND |
| 43 | Russian (Moscow). Chistakov (2009) [12]# | CCCG | Caucasian | 1.54 | 1.08–2.20 | 0.023 | 127 (28+72+29) | 117 (36+69+12) § | 0.40 |
| 44 | Tunisian population. Ezzidi (2009) [71] | CCCG | Arab | 1.15 | 0.97–1.36 | 0.12 | 805 (371+352+82) | 503 (250+213+40) | 0.29 |
| 45 | USA. Cornelis (2009)[72] | CCCG | Caucasian | 1.08 | 1.00–1.17 | 0.04 | 2709 (1055+1275+379) | 3344 (1382+1536+426) | 0.36 |
| 46 | Ashkenazi Jewish. Neuman (2010) [7] | CCCG | Ashkenazi Jewish | 1.05 | 0.90–1.23 | 0.52 | 573 (228+266+79) | 843 (339+404+100) | 0.36 |
| 47 | Han Chinesse. Wen (2010)[73] | CCCG | Asian | 1.07 | 0.95–1.21 | 0.26 | 1165 (395+587+183) | 1135 (425+517+193) | 0.40 |
| 48 | Indo-European ethnicity. Chauhan (2010) [74] | CCCG | Indo-European | 1.39 | 1.26–1.54 | 6.7 x 10–11 | 2486 | 2678 | 0.31 |
| 49 | Russian (Moscow). Chistakov (2010) [15]# | CCCG | Caucasian | 1.41 | 1.20–1.66 | 0.00003 | 588 (134+339+115) § | 597 (183+352+62) § | 0.40 |
| 50 | Chinese. Liu (2010)[75] | CCCG | Asian | 1.26 | 1.03–1.55 | 0.02 | 397 (131+180+86) | 392 (147+187+58) | 0.39 |
| 51 | UK Asian Diabetes Study (UKADS) and DiabetesGenetics in Pakistan (DGP). Rees (2011) [41] | CCCG | Asian | 0.98 | 0.88–1.08 | 0.71 | 1678 [857(UKADS) 821(DGP)] | 1584 [417(UKADS) 1167 (DGP)] | 0.38 |
| 52 | Indo-European ethnicity. Chavali (2011) [10] | CCCG | Indo-European | 1.00 | 0.88–1.13 | 0.89 | 1019 | 1006 | 0.35 |
| Meta-analysis of 30. 38 by Yang et al (2012) [76] | meta | Japanese | 1.23 | 1.13–1.35 | 2 x 10 –6 | 7091 | 7958 | ND | |
| Meta-analysis of 39. 41. 42. 47 by Yang et al (2012)[76] | meta | Chinese | 1.12 | 1.06–1.19 | 5 x 10 –5 | 5262 | 5231 | ND | |
| Meta-analysis of 24. 30. 38. 39. 41. 42. 47 by Yang et al (2012) [76] | meta | Asian | 1.16 | 1.11–1.21 | 4 x 10 –11 | 13114 | 13819 | ND | |
| 53 | Tunisians. Mtiraoui (2012) [77] | CCCG | Arab | 1.27 | 1.09–1.47 | 8 x 10–4 | 1470 | 838 | ND |
| 54 | Mongolian. Odgerel (2012) [8] | CCCG | Mongolian | 1.07 | 0.80–1.44 | 0.65 | 177 | 216 | 0.32 |
| Meta-analysis of 49 studies by Gong [78] | meta | All | 1.13 | 1.10–1.15 | 7 x 10 –8 | 64403 | 122945 | ND | |
| 55 | urban Ghana. Danquah (2013)[79]# | CCCG | Akan | NA | NA | NA | 675(674+1+0) | 377 (377+0+0) | 0.00 |
| Meta-analysis of 15 European studies by Qin (2013) [80] | meta | European | 1.16 | 1.11–1.20 | 4 x 10 –11 | 9165 | 13300 | 36.8 | |
| Meta-analysis of 13 Asian studies by Qin (2013) [80] | meta | Asian | 1.11 | 1.04–1.20 | 0.002 | 13213 | 13229 | ND | |
| Meta-analysis of 7 Chinese studies by Qin (2013) [80] | meta | Chinese | ND | ND | 0.28 | 6308 | 6213 | 0.40 | |
| Meta-analysis of 5 Japanese studies by Qin (2013) [80] | meta | Japanese | 1.16 | 1.08–1.24 | 5 x 10 –5 | 3561 | 4039 | 0.35 | |
| Meta-analysis of 33 studies by Qin (2013) [80] | meta | All | 1.15 | 1.10–1.21 | 7 x 10 –7 | 23262 | 27042 | ND | |
| Meta-analysis of 22 studies by Qiu (2014)[42] | meta | Caucasian | 1.12 | 1.08–1.16 | 10 –9 | ND | ND | 0.40 | |
| Meta-analysis of 14 studies by Qiu (2014) [42] | meta | East Asian | 1.13 | 1.08–1.17 | 10 –7 | ND | ND | 0.36 | |
| Meta-analysis of 5 studies by Qiu (2014) [42] | meta | Indians | 1.06 | 0.87–1.29 | 0.56 | ND | ND | 0.34 | |
| Meta-analysis of 7 studies by Qiu (2014) [42] | meta | Others | 1.09 | 0.97–1.23 | 0.15 | ND | ND | ND | |
| Meta-analysis of 48 studies by Qiu (2014) [42] | meta | All | 1.12 | 1.09–1.16 | 3 x 10 –16 | ND | ND | ND | |
Results of previous meta-analysis are shown in bold.
* Total number persons in groups is given. Number of people with a particular genotype is shown in brackets. EE+EK+KK respectively.
§—not in Hardy-Weinberg Equilibrium.
§§—frequency of T allele (p.23K) in the control group.
#—data was excluded from the meta-analysis (see study selection);
Abbreviations: r- under recessive model; d—dominant model; additive model is default variant; ND—no available data; CCGWA—genome-wide association study. Case-control design; CCCG—case-control design candidate gene study; meta—meta-analysis; PCG-prospective candidate gene study.
Meta-analysis results
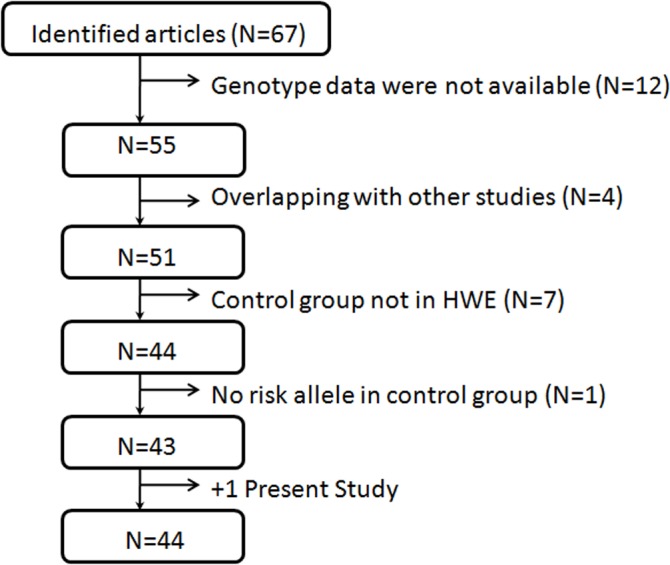
We identified a total of 67 potential articles for meta-analysis, but 24 were excluded for not meeting the required criteria (Fig 1). We did not include the Utahand U.K. samples from Inoue et al. [14] because p.E23K failed to meet HWE in the control group (p = 0.002 and p = 0.04, respectively). Results from both studies with Russian samples were excluded because in one study, rs5219 failed to obey HWE in T2DM (p = 0.0002) and control (p = 2 x 10–8) groups, and in the other study the control group failed to meet this criterion (p = 0.02) [12,15]. Results from Koo et al. [16] were excluded because control group genotypes reached borderline significance for HWE (p = 0.05), and the results of Sale et al. [17] were also excluded because rs5219 (p = 0.0009) deviated from HWE proportions in African-American patients. In that study, the minor allele for this SNP was rare (0.056) in the population, and results for only dominant models were available. The results from Bronstein et al. [18] also exhibited a significant deviation (P < 0.05) from HWE, and the results of Keiko et al. [19] were also excluded due to a HWE mismatch. We did not include data comprising 182 and 268 British patients studied by Sakura et al. [20] and Inoue et al. [14], respectively, as well as 133 Danish patients reported by Hansen et al. [21]. These were included in the UKPDS cohort [22] and Nielsen samples were addressed in Nielsen et al.[23].Results of Miyake et al. [24] were excluded because they overlapped with the patients of Horikoshi et al. [25] and Yokoi et al. [26]. Studies by Altshuler et al.[27], Love-Gregory et al. [28], Sladek et al. [29], Steinthorsdottir et al. [30], Salonen et al. [31], Hanson et al. [32], Hayes et al. [33], Rampersaud et al. [34], Yu et al. [35], Turki et al. [36], Thorsby et al. [37], and Yamauchi et al. [38] were also excluded from our meta-analysis because genotype data were not available.
Fig 1. Study selection for meta-analysis of rs5219 of KCNJ11.
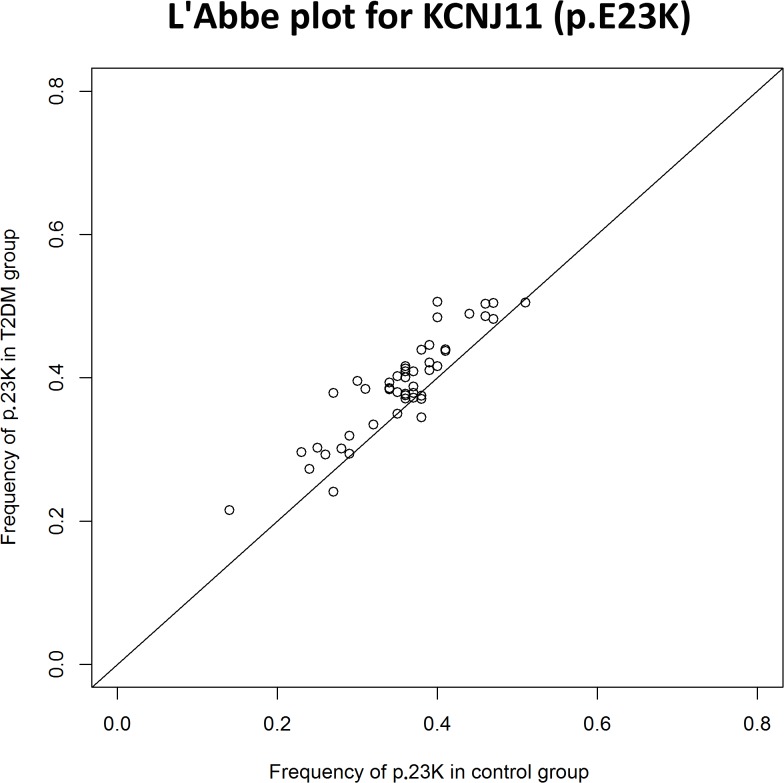
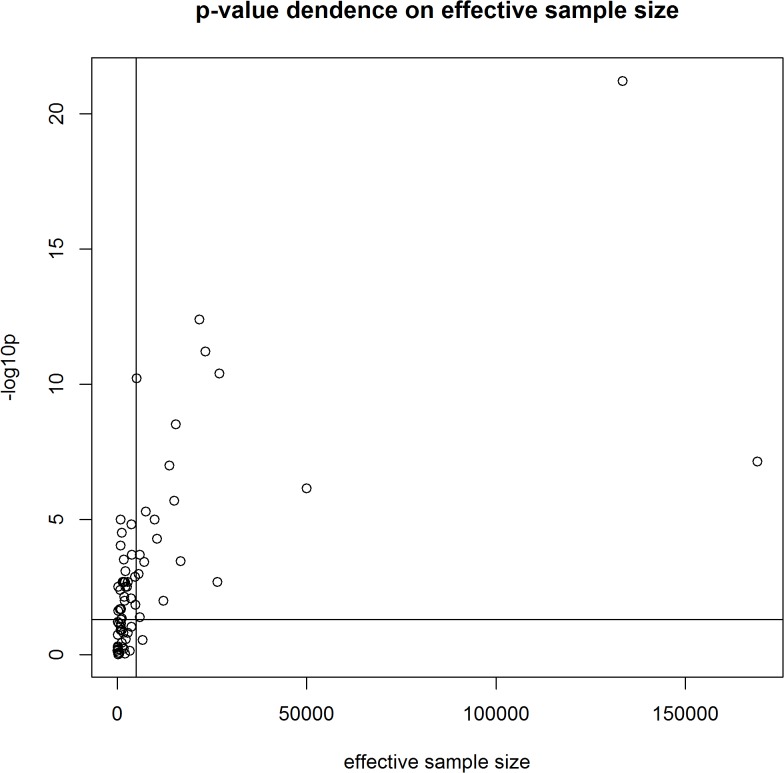
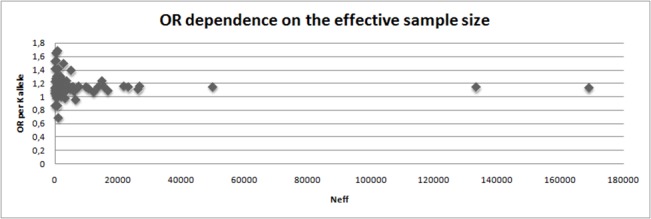
We performed a meta-analysis of our own results combined with previously published data on the association between rs5219 and T2DM (Table 4, Fig 2). A total of 56,210 T2DM patients and 81,088 control patients from 44 studies were included (total sample size was 137,298). In 41 studies, the 5219[T] allele was the risk allele; in four studies, the opposite allele was identified as the risk [9,39–41]. In the first meta-analysis, the total OR for all studies was 1.14 (95% CI: 1.11–1.17) with a statistical significance of P = 6 x 10–22, and the heterogeneity test revealed significant differences between studies (P< 0.001).No publication bias for the SNP rs5219 was found according to Begg’s correlation analysis (corrected z = 1.26 and corrected P = 0.21); however, according to the Egger there was publication bias between studies test (t = 1.90, p = 0.06) (Fig 2). We then divided all studies into six groups according to ethnicity (Caucasian, Asian, Indian, Arabian, Mongolian, and Ashkenazi Jewish) and conducted a meta-analysis for each group. Significant heterogeneity was found for the Caucasian, Indian, and Arabian subgroups. The highest OR was obtained for Arabians [OR = 1.28, 95%CI (1.15–1.42)] and the lowest OR was observed for Asians [OR = 1.11, 95%CI (1.07–1.14)].We found that rs5219 was not associated with T2DM for Indian, Mongolian, and Ashkenazi Jewish groups. We next performed a meta-analysis that included all seven studies in which the control group did not obey the HWE (S1 Fig, Table 4) [12,14–16,19–21]. Sample sizes of the T2DM and control groups increased to 57,994 and 82,733, respectively, with a total sample size of 140,727. The pooled OR was 1.15 [95% CI (1.12–1.19)] with significant heterogeneity between studies (p < 0.001).Previous meta-analyses also exhibited heterogeneity between studies [42]due to the following possible reasons: ethnicity, sample size, mean age of cases and controls, gender distribution in cases and controls, and body mass index (BMI). However, BMI alone only explained approximately 11% of the heterogeneity (p = 0.03).We plotted an L’Abbe plot [43]to visualize differences in the frequency of rs5219 between populations (Fig 3) and assessed the dependence of ORs and p values from effective sample sizes (Figs 4 and 5). We used the effective population size as given by the doubled harmonic means of case and control group sizes to overcome the differences in proportion between controls and cases.
Table 4. Results of meta-analysis.
| Group | Studies | Case | Control | OR (95%CI) | p-value | phet |
|---|---|---|---|---|---|---|
| Results of meta-analysis of p.E23K (KCNJ11) | ||||||
| Caucasian | 23 | 29679 | 54233 | 1.14 (1.10–1.17) | 6 x 10–13 | 0.004 |
| Asian | 14 | 18919 | 20062 | 1.11 (1.07–1.14) | 3 x 10–8 | 0.06 |
| Indian | 3 | 4037 | 4058 | 1.07 (0.80–1.42) | 0.65 | <0.001 |
| Arabian | 3 | 2825 | 1676 | 1.28 (1.15–1.42) | 6 x 10–6 | 0.02 |
| Ashkenazi Jewish | 1 | 573 | 843 | 1.05 (0.90–1.23) | 0.52 | 1 |
| Mongolian | 1 | 177 | 216 | 1.07 (0.80–1.44) | 0.65 | 1 |
| Total (in HWE) | 44 | 56 210 | 81 088 | 1.14 (1.11–1.17) | 6 x 10–22 | <0.001 |
| Total (+ not in HWE) | 52 | 57 994 | 82 733 | 1.15 (1.12–1.19) | 4 x 10–25 | <0.001 |
| Results of meta-analysis of p.S1369A (ABCC8) | ||||||
| Caucasian | 4 | 3794 | 2725 | 1.13 (1.04–1.22) | 0.004 | 0.47 |
| Asian | 4 | 2336 | 2908 | 1.14 (1.05–1.23) | 0.002 | 0.10 |
| Indian | 1 | 1019 | 1006 | 1.18 (1.04–1.34) | 0.01 | 1 |
| Mongolian | 1 | 177 | 216 | 1.09 (0.81–1.46) | 0.58 | 1 |
| Total (in HWE) | 10 | 5897 | 6441 | 1.14 (1.08–1.19) | 2 x 10–6 | 0.41 |
phet—p-value of heterogeneity between studies
Fig 2. Forest and funnel plots of meta-analysis of rs5219 of KCNJ11.
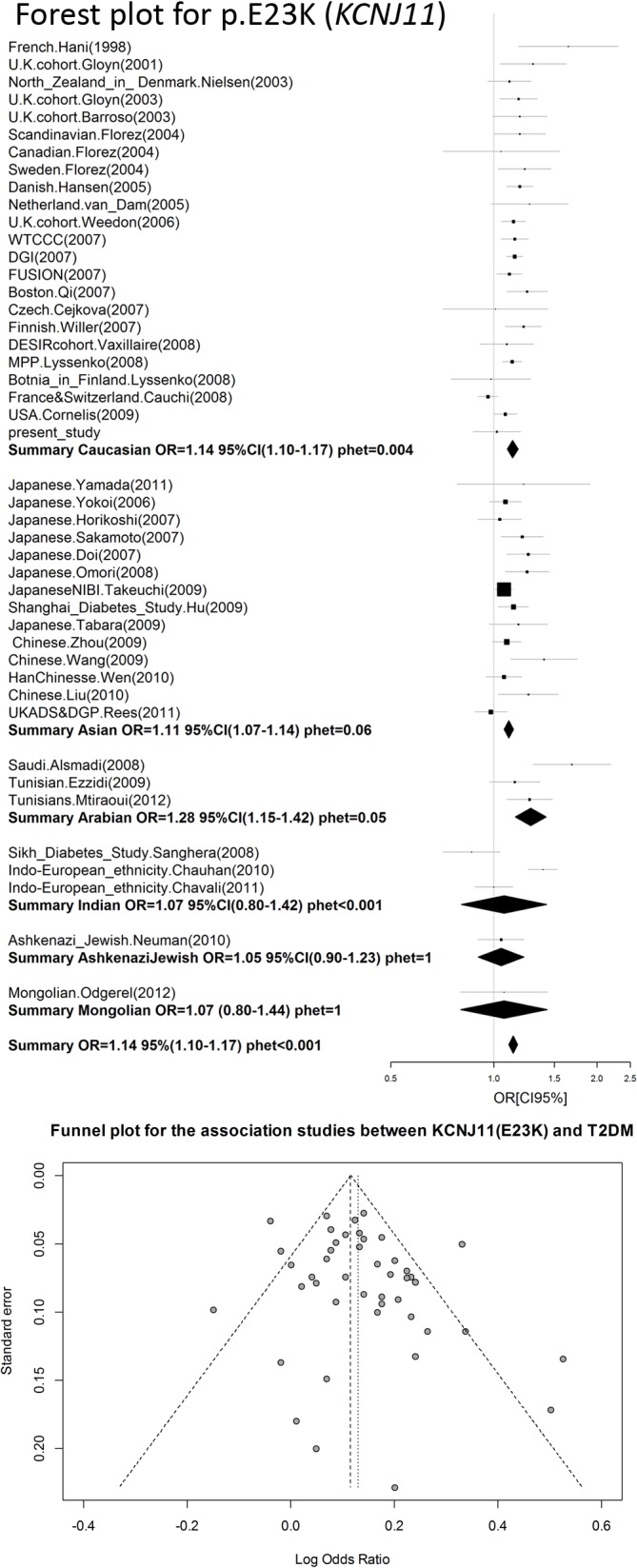
Fig 3. L’Abbe plot for KCNJ11 (p.E23K).
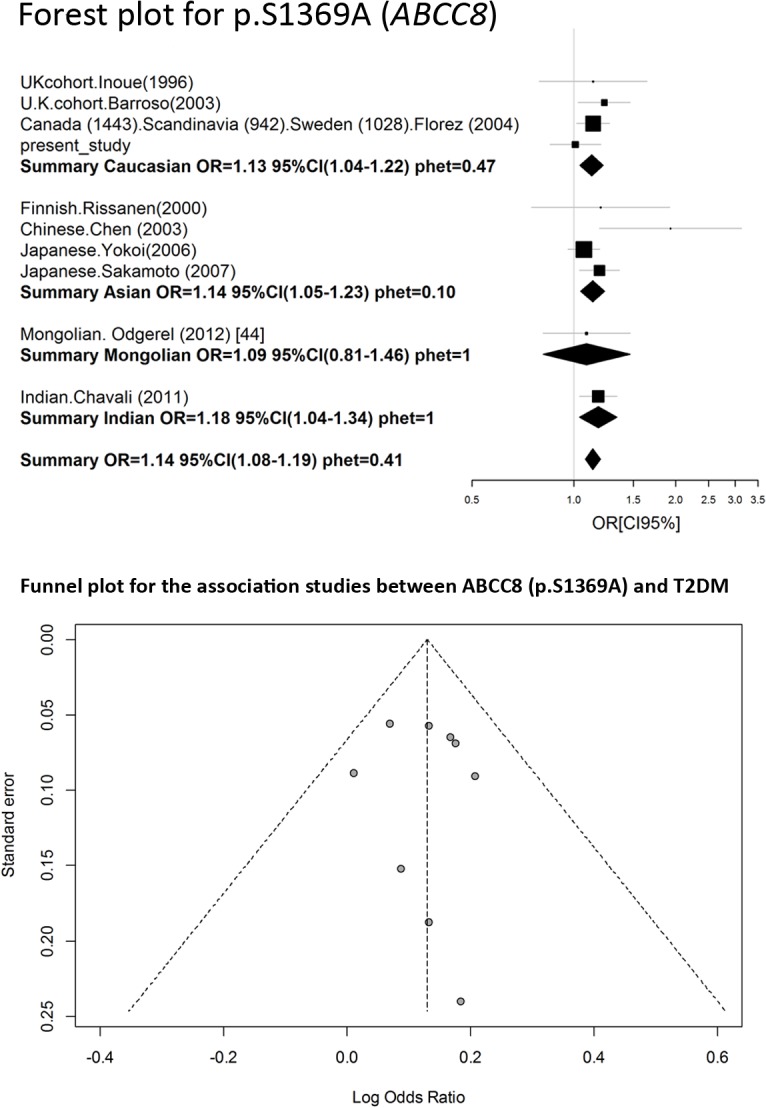
Fig 4. OR dependence on the effective sample size.
Fig 5. P-value dependence on the effective sample size.
Meta-analysis of rs757110 of ABCC8
A search for publications on the rs757110 was conducted in a similar manner as described for the rs5219 search. All of the available information identified is shown in Table 5. We exclude data of both Hani et al. and Ishiyama-Shigemoto et al. [44] that were referenced by Qin et al. We found no information in the reference manuscript [45], and some data in the Qin manuscript were incorrect. Data from Ohta et al. [46] were also excluded because the original article did not contain sufficient information. We did not include the Utah patients from Inoue et al. [14] because p.S1369A did not comply with HWE for the control group (p = 0.01).The Begg’s correlation analysis (corrected z = 1.34 and corrected P = 0.18) and the Egger test (t = 1.25, p = 0.25) found no publication bias for SNP rs5219 (Fig 6). A meta-analysis was conducted on a total of 10 studies, including our present study (Fig 5, Table 4). The total sample size was 14,136, which included 7281 cases and 6855 controls. The pooled OR was 1.14 [95% CI (1.08–1.19); p = 2 x 10–6] and there was no heterogeneity between studies (p = 0.41).
Table 5. Characteristics studies of association SNP rs757110 (p.S1396A) of ABCC8 and T2DM.
| Num | Study ID | Type | Race/Ethnicity | OR | 95% C.I. | p | Case §§§ | Control §§§ | Fr. §§ |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Utah. Inoue (1996) [81] # | CCCG | Caucasian | 0.96 | 0.66–1.41 | 0.85 | 133 (58+58+17) | 103 (37+59+7) § | 0.35 |
| 2 | UK cohort. Inoue (1996) [81] | CCCG | Caucasian | 1.14 | 0.79–1.63 | 0.49 | 187 (98+67+22) | 120 (64+47+9) | 0.27 |
| 3 | French. Hani (1998)* # | CCCG | Caucasian | ND | ND | ND | 168 | 106 | 0.34 |
| 4 | Japanese. Ohta (1998) # [46] | CCCG | Asian | ND | ND | NS | 100 (46+36+18) | 67 | ND |
| 5 | Japanese. Ishiyama (1998)* # [44] | CCCG | Asian | 1.10 | 0.85–1.43 | ND | 1590 (570+744+276) | 1244 (463+587+194) | 0.39 |
| 6 | Finnish. Rissanen (2000) [82] | CCCG | Asian | 1.20 | 0.75–1.90 | 0.45 | 40 | 377 | 0.42 |
| 7 | U.K. cohort. Barroso (2003) [51] | CCCG | Caucasian | 1.23 | 1.03–1.48 | 0.02 | 502 (189+225+88) | 499 (205+238+56) | 0.35 |
| 8 | Chinese. Chen (2003)* | CCCG | Asian | 1.93 | 1.19–3.14 | 0.008 | 105 (20+60+25) | 51 (19+27+5) | 0.36 |
| 9 | Canada (1443), Scandinavia (942), Sweden (1028). Florez (2004) [48] | CCCG | Caucasian | 1.14 | 1.02–1.28 | 0.02 | 1721 | 1692 | ND |
| 10 | Japanese. Yokoi (2006) [26] | CCCG | Asian | 1.07 | 0.96–1.19 | 0.23 | 1244 (463+587+194) | 1590 (570+744+276) | 0.41 |
| 11 | Japanese. Sakamoto (2007)* [60] | CCCG | Asian | 1.19 | 1.04–1.36 | 0.01 | 902 (310+441+151) | 890 (357+407+126) | 0.37 |
| 12 | Indian. Chavali (2011)* [10] | CCCG | Indian | 1.18 | 1.04–1.34 | 0.01 | 1019 | 1006 | 0.38 |
| 13 | Mongolian. Odgerel (2012) [8] | CCCG | Mongolian | 1.09 | 0.81–1.46 | 0.58 | 177 | 216 | 0.32 |
| Meta-analysis of 9studies by Qin (2013) [76] | meta | All | 1.12 | 1.07–1.18 | 10 –6 | 5835 | 5261 | ND |
Results of meta-analysis are shown in bold.
§—not in Hardy-Weinberg Equilibrium.
§§—frequency of G allele (p.1369G) in the control group.
§§§Total number persons in groups is given. Number of people with a particular genotype is shown in brackets. SS+SA+AA, respectively.
#—data was excluded from the meta-analysis (see text in article).
Abbreviations: ND—no available data; CCGWA—genome-wide association study. case-control design; CCCG—case-control design candidate gene study; NS—not significant; meta—meta-analysis.
*—data are given according to article Qin et al. [80]
Fig 6. Forest and funnel plots of meta-analysis of rs757110 of ABCC8.
We also performed a meta-analysis on the Caucasian, Asian, Indian, and Mongolian ethnic groups, but the Indian and Mongolian groups were presented in one study. The pooled OR was 1.13 (1.04–1.22) for Caucasians and 1.14 (1.05–1.23) for Asians.
Linkage disequilibrium between rs757110 (ABCC8 p.S1369A) and rs5219 (KCNJ11 p.E23K) and association of haplotypes
We estimated the linkage disequilibrium (LD) between SNPs rs5219 and rs757110 and found that they were in LD (D’ = 0.766, r2 = 0.5633, χ2 = 1012.81). We also performed a haplotype analysis for rs5219 andrs757110 and found that the haplotype rs757110[T]-rs5219[C] was associated with T2DM [Table 6; OR = 1.52, 95% CI (1.04–2.24); empirical p = 0.03].
Table 6. Analysis of association between T2DM and haplotypes at SNPs rs5219 and rs757110.
| Rs757110 | Rs5219 | Frequency in Cases | Frequency in Control | Sample frequency | OR | 95% C.I. | Empirical p-value |
|---|---|---|---|---|---|---|---|
| T | C | 0.555842 | 0.5777821 | 0.561038 | reference | — | — |
| T | T* | 0.059404 | 0.036913 | 0.054090 | 1.52 | 1.04–2.24 | 0.03 |
| G* | C | 0.067713 | 0.050198 | 0.063545 | 1.27 | 0.93–1.73 | 0.14 |
| G* | T* | 0.317041 | 0.335067 | 0.321327 | 0.99 | 0.83–1.17 | 0.88 |
Abbreviations: 95% CI, 95% confidence interval; OR, odds ratio; Sample frequency—haplotype frequency in MS and control groups together; empirical p-value—p-value of association haplotype with MS;
*—marked the risk allele from meta-analysis.
Analysis was performed using logistic regression.
Discussion
This study assessed whether SNPs rs5219 and rs757110 are associated with a predisposition to T2DM in Siberians and found that neither SNP was associated with the disease. The power of the study reached 28% for both SNPs, and approximately 2400 case-control pairs would be required for 80% power to detect the risk allele among Caucasians.
We also reviewed all available previous studies involving p.E23K (KCNJ11) and p.S1369A (ABCC8) (Tables 3 and 5). To date, 67 original studies and 10 meta-analyses have analyzed the association of the p.E23K variant of KCNJ11 with T2DM (Fig 1, Table 3). Cohorts of American, Danish, French, Japanese, North Zealand, Scandinavian, Canadian, Sweden, Dutch, Finland, Boston cohort, Korean, Czech, African-American, Finish, Ashkenazi Jewish, Indians, Chinese, Arabian, Mongolian, urban Ghana, and Russian patients have been studied. The OR for the p.23K allele ranged from 0.69 in African Americans [17] to 1.69 in Arabians [47]. The first pooled OR from meta-analysis was 1.49 [23] and became 1.11–1.14 when the effective sample size reached 20,000 people (Fig 4). A similar trend in the OR was observed in original studies of Asians and Caucasians. In our meta-analysis of p.E23K, the pooled OR was 1.14 and 1.11 for the Caucasian and Asian cohorts, respectively (Table 4). The frequency of the K allele ranged from 0% in urban Ghana patients to 50% in Finnish patients in previous studies. Moreover, the K allele frequency was different between the race controls: 0.40 (95% CI: 0.37–0.42) for Caucasian, 0.36(95% CI: 0.34–0.38) for Asian, and 0.34(95% CI: 0.27–0.41) for Indian [42]. Schwanstecher et al. hypothesizedthat a high frequency of polymorphisms may be a consequence of the selective advantages. Reducing glucose uptake by muscle and fat led to better glucose uptake by insulin-independent tissues, such as brain [6].We also analyzed the dependence of OR on the risk allele (p.23K) frequency using an L’abbe plot (Fig 3) and found that the OR depends more on the effective sample size than the frequency of the risk allele in the population. We also assessed if the p-values from all previous studies depended on effective sample sizes (Fig 5). The vertical line corresponds to an effective sample size of 5000, which is necessary for a predictive power of 80%. The horizontal line corresponds to p = 0.05. It is obvious that for all studies except one in which the sample size was more than 5000, the effective sample size showed statistically significant differences in p.E23K between the control and T2DM patients. However, all of the meta-analyses conducted had problems of heterogeneity between studies, and heterogeneity remained after separate analyses of individual races, including Caucasian, Indian, and Arabian populations. Only Asians trended towards homogeneity. Qiu et al. reported BMI as the reason for heterogeneity, which accounted for approximately 11% of the heterogeneity among the individuals studied (p = 0.03) [42].We hypothesize that heterogeneity may also be caused by the younger mean age of the control group, since T2DM is a disease with a relatively late onset in life. Thus, a young control group may include patients that will develop T2DM in the future. The number of such persons in the control group depends on the prevalence of T2DM in the cohort set, which in turn depends on various external factors. The higher the proportion of futureT2DM patients in the control, the more the true value OR is understated.
The association between p.S1369A (ABCC8) has been reported in nine available studies to date, and the pooled OR was 1.14 [95% CI (1.08–1.19)]. Heterogeneity between studies was absent. In contrast to p.E23K, p.S1369Awasassociated with T2DM not only in Asians and Caucasians, but also in Indians (Table 6, Fig 6), where the same OR ranged from 1.13 to 1.18. The frequency of the p.1369A allele only modestly varied between populations (27–42%).
In summary, our data support the hypothesis that genetic variation located in the ABCC8/KCNJ11 region is associated with the development of T2DM with an OR of approximately 1.15. We also found that haplotype rs757110[T]-rs5219[C] (p.23K/p.S1369) was associated with T2DM [OR = 1.52, 95% CI (1.04–2.24); empirical p = 0.03). In a previous study of ABCC8/KCNJ11, the region haplotype (p.23K/p.1369A) was also associated with T2DM [OR = 1.15 95% CI (1.03–1.29); p = 0.01] [48]. In fact, that study could not distinguish the p.23K and p.1369A alleles because the LD between SUR1 p.S1369A and Kir6.2 p.E23K was very strong in the cohort (r2 = 0.9). Moreover, each chromosome containing the K allele in p.E23K also contained the A allele in p.S1369A (frequency of p.E23/p.S1369 = 0.57, p.23K/p.1369A = 0.42, and p.23K/p.S1369 = 0.01). In our study, the LD was not as strong (r2 = 0.5633), and the rare haplotype frequency was higher (p.E23/p.S1369 = 0.56, p.23K/p.1369A = 0.32, p.23K/p.S1369 = 0.06, and p.E23/p.1369A = 0.05). Based on our results, the p.23K/p.S1369 haplotype may be a T2DM causal variant. However, the association of p.23K/p.S1369 had borderline statistical significance, which was most likely due to the small sample size. Further studies are needed in much larger sample sizes and in populations in which chromosomes recombinant for p.E23K and p.S1369Aare higher than in European populations [48]. Given the low frequency of p.23K/p.S1369 and p.E23/p.1369A haplotypes in Europeans (~1%) and the current OR of 1.15, approximately 120,000 case/control pairs are necessary to distinguish between the two [23].
Conclusions
Our calculations identified causal genetic variation within the ABCC8/KCNJ11 region for T2DM with an OR of approximately 1.15 in Caucasians and Asians. The OR value was not dependent on the frequency of p.E23K or p.S1369A in the populations.
Supporting Information
(TIF)
The table was formed in accordance with the requirements of the site http://www.prisma-statement.org/statement.htm.
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was supported by a grant from the Russian Foundation for Basic Research (13-04-00520).
References
- 1. Health at a Glance: Europe: 2012. OECD Publishing; 2012. 10.1787/9789264183896-en Available: http://www.oecd-ilibrary.org/social-issues-migration-health/health-at-a-glance-europe-2012_9789264183896-en. [DOI] [Google Scholar]
- 2. Ashfield R, Ashcroft SJ. Cloning of the promoters for the beta-cell ATP-sensitive K-channel subunits Kir6.2 and SUR1. Diabetes. 1998; 47: 1274–1280. [DOI] [PubMed] [Google Scholar]
- 3. Clement JP, Kunjilwar K, Gonzalez G, Schwanstecher M, Panten U, Aguilar-Bryan L, et al. Association and stoichiometry of K(ATP) channel subunits. Neuron. 1997; 18: 827–838. [DOI] [PubMed] [Google Scholar]
- 4. Thomas PM, Cote GJ, Wohllk N, Haddad B, Mathew PM, Rabl W, et al. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science. 1995; 268: 426–429. [DOI] [PubMed] [Google Scholar]
- 5. Thomas P, Ye Y, Lightner E. Mutation of the pancreatic islet inward rectifier Kir6.2 also leads to familial persistent hyperinsulinemic hypoglycemia of infancy. Hum Mol Genet. 1996; 5: 1809–1812. [DOI] [PubMed] [Google Scholar]
- 6. Schwanstecher C, Meyer U, Schwanstecher M. KIR6.2 Polymorphism Predisposes to Type 2 Diabetes by Inducing Overactivity of Pancreatic-Cell ATP-Sensitive K+ Channels. Diabetes. 2002; 51: 875–879. 10.2337/diabetes.51.3.875 [DOI] [PubMed] [Google Scholar]
- 7. Neuman RJ, Wasson J, Atzmon G, Wainstein J, Yerushalmi Y, Cohen J, et al. Gene-gene interactions lead to higher risk for development of type 2 diabetes in an Ashkenazi Jewish population. PLoS One. 2010; 5: e9903 10.1371/journal.pone.0009903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Odgerel Z, Lee HS, Erdenebileg N, Gandbold S, Luvsanjamba M, Sambuuqhin N, et al. Genetic variants in potassium channels are associated with type 2 diabetes in a Mongolian population. J Diabetes. 2012; 4: 238–242. 10.1111/j.1753-0407.2011.00177.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sanghera DK, Ortega L, Han S, Singh J, Ralhan SK, Wander GS, et al. Impact of nine common type 2 diabetes risk polymorphisms in Asian Indian Sikhs: PPARG2 (Pro12Ala), IGF2BP2, TCF7L2 and FTO variants confer a significant risk. BMC Med Genet. 2008; 9: 59 10.1186/1471-2350-9-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chavali S, Mahajan A, Tabassum R, Dwivedi OP, Chauhan G, Ghosh S, et al. Association of variants in genes involved in pancreatic β-cell development and function with type 2 diabetes in North Indians. J Hum Genet. 2011; 56: 695–700. 10.1038/jhg.2011.83 [DOI] [PubMed] [Google Scholar]
- 11. Hamming KSC, Soliman D, Matemisz LC, Niazi O, Lang Y, Gloyn AL, et al. Coexpression of the type 2 diabetes susceptibility gene variants KCNJ11 E23K and ABCC8 S1369A alter the ATP and sulfonylurea sensitivities of the ATP-sensitive K(+) channel. Diabetes. 2009; 58: 2419–2424. 10.2337/db09-0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chistiakov DA, Potapov VA, Khodirev DC, Shamkhalova MS, Shestakova MV, Nosikov VV.Genetic variations in the pancreatic ATP-sensitive potassium channel, beta-cell dysfunction, and susceptibility to type 2 diabetes. Acta Diabetol. 2009; 46: 43–49. 10.1007/s00592-008-0056-5 [DOI] [PubMed] [Google Scholar]
- 13. Alberti KG, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15: 539–553. [DOI] [PubMed] [Google Scholar]
- 14. Inoue H, Ferrer J, Warren-Perry M, Zhang Y, Millns H, Turner RC, et al. Sequence variants in the pancreatic islet beta-cell inwardly rectifying K+ channel Kir6.2 (Bir) gene: identification and lack of role in Caucasian patients with NIDDM. Diabetes. 1997; 46: 502–507. [DOI] [PubMed] [Google Scholar]
- 15. Chistiakov D a, Potapov V a, Khodirev DS, Shamkhalova MS, Shestakova MV, Nosikov VV Replication of association between polymorphisms of the pancreatic ATP-sensitive potassium channel and susceptibility to type 2 diabetes in two Russian urban populations. Cent Eur J Biol. 2009; 5: 67–77. [Google Scholar]
- 16. Koo BK, Cho YM, Park BL, Cheong HS, Shin HD, Jang HC, et al. Polymorphisms of KCNJ11 (Kir6.2 gene) are associated with Type 2 diabetes and hypertension in the Korean population. Diabet Med. 2007; 24: 178–186. [DOI] [PubMed] [Google Scholar]
- 17. Sale MM, Smith SG, Mychaleckyj JC, Keene KL, Langefeld CD, Leak TC, et al. Variants of the transcription factor 7-like 2 (TCF7L2) gene are associated with type 2 diabetes in an African-American population enriched for nephropathy. Diabetes. 2007; 56: 2638–2642. [DOI] [PubMed] [Google Scholar]
- 18. Bronstein M, Pisanté A, Yakir B, Darvasi A. Type 2 diabetes susceptibility loci in the Ashkenazi Jewish population. Hum Genet. 2008; 124: 101–104. 10.1007/s00439-008-0520-x [DOI] [PubMed] [Google Scholar]
- 19. Keiko K, Keiko A, Hiroto B TM. Search of Kir6.2 transgenic in Japanese non-insulin dependent diabetes mellitus. Ann Rep Kansai Coll Acupunct Med. 1999; 14: 58–61. [Google Scholar]
- 20. Sakura H, Wat N, Horton V, Millns H, Turner RC, Ashcroft FM. Sequence variations in the human Kir6.2 gene, a subunit of the beta-cell ATP-sensitive K-channel: no association with NIDDM in while Caucasian subjects or evidence of abnormal function when expressed in vitro. Diabetologia. 1996; 39: 1233–1236. [DOI] [PubMed] [Google Scholar]
- 21. Hansen L, Echwald SM, Hansen T, Urhammer SA, Clausen JO, Pedersen O. Amino acid polymorphisms in the ATP-regulatable inward rectifier Kir6.2 and their relationships to glucose- and tolbutamide-induced insulin secretion, the insulin sensitivity index, and NIDDM. Diabetes. 1997; 46: 508–512. [DOI] [PubMed] [Google Scholar]
- 22. Gloyn AL, Hashim Y, Ashcroft SJ, Ashfield R, Wiltshire S, Turner RC, et al. Association studies of variants in promoter and coding regions of beta-cell ATP-sensitive K-channel genes SUR1 and Kir6.2 with Type 2 diabetes mellitus (UKPDS 53). Diabet Med. 2001; 18: 206–212. [DOI] [PubMed] [Google Scholar]
- 23. Nielsen ED, Hansen L, Carstensen B, Echwald SM, Drivsholm T, Glumer C, et al. The E23K variant of Kir6.2 associates with impaired post-OGTT serum insulin response and increased risk of type 2 diabetes. Diabetes. 2003; 52: 573–577. [DOI] [PubMed] [Google Scholar]
- 24. Miyake K, Yang W, Hara K, Yasuda K, Horikawa Y, Osawa H, et al. Construction of a prediction model for type 2 diabetes mellitus in the Japanese population based on 11 genes with strong evidence of the association. J Hum Genet.2009; 54: 236–241. 10.1038/jhg.2009.17 [DOI] [PubMed] [Google Scholar]
- 25. Horikoshi M, Hara K, Ito C, Shojima N, Nagai R, Ueki K, et al. Variations in the HHEX gene are associated with increased risk of type 2 diabetes in the Japanese population. Diabetologia. 2007; 50: 2461–2466. [DOI] [PubMed] [Google Scholar]
- 26. Yokoi N, Kanamori M, Horikawa Y, Takeda J, Sanke T, Furuta H, et al. Association studies of variants in the genes involved in pancreatic beta-cell function in type 2 diabetes in Japanese subjects. Diabetes. 2006; 55: 2379–2386. 10.2337/db05-1203 [DOI] [PubMed] [Google Scholar]
- 27. Altshuler D, Hirschhorn JN, Klannemark M, Lindgren CM, Vohl MC, Nemesh J et al. The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet. 2000; 26: 76–80. [DOI] [PubMed] [Google Scholar]
- 28. Love-Gregory L, Wasson J, Lin J, Skolnick G, Suarez B, Permutt MA. E23K single nucleotide polymorphism in the islet ATP-sensitive potassium channel gene (Kir6.2) contributes as much to the risk of Type II diabetes in Caucasians as the PPARgamma Pro12Ala variant. Diabetologia. 2003. 46: 136–137. [DOI] [PubMed] [Google Scholar]
- 29. Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007; 445: 881–885. [DOI] [PubMed] [Google Scholar]
- 30. Steinthorsdottir V, Thorleifsson G, Reynisdottir I, Benediktsson R, Jonsdottir T, Walters GB, et al. A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet. 2007; 39: 770–775. [DOI] [PubMed] [Google Scholar]
- 31. Salonen JT, Uimari P, Aalto J- M, Pirskanen M, Kaikkonen J, Todorova B, et al. Type 2 diabetes whole-genome association study in four populations: the DiaGen consortium. Am J Hum Genet. 2007; 81: 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hanson RL, Bogardus C, Duggan D, Kobes S, Knowlton M, Infante AM, et al. A search for variants associated with young-onset type 2 diabetes in American Indians in a 100K genotyping array. Diabetes. 2007; 56: 3045–3052. [DOI] [PubMed] [Google Scholar]
- 33. Hayes MG, Pluzhnikov A, Miyake K, Sun Y, Ng MC, Roe CA, et al. Identification of type 2 diabetes genes in Mexican Americans through genome-wide association studies. Diabetes. 2007; 56: 3033–3044. [DOI] [PubMed] [Google Scholar]
- 34. Rampersaud E, Damcott CM, Fu M, Shen H, McArdle P, Shi X, et al. Identification of novel candidate genes for type 2 diabetes from a genome-wide association scan in the Old Order Amish: evidence for replication from diabetes-related quantitative traits and from independent populations. Diabetes. 2007; 56: 3053–3062. [DOI] [PubMed] [Google Scholar]
- 35. Yu M, Xu X-J, Yin J-Y, Wu J, Chen X, Gong ZC, et al. KCNJ11 Lys23Glu and TCF7L2 rs290487(C/T) polymorphisms affect therapeutic efficacy of repaglinide in Chinese patients with type 2 diabetes. Clin Pharmacol Ther. 2010; 87: 330–335. 10.1038/clpt.2009.242 [DOI] [PubMed] [Google Scholar]
- 36. Turki A, Al-Zaben GS, Khirallah M, Marmouch H, Mahjoub T, Almawi WY. Gender-dependent associations of CDKN2A/2B, KCNJ11, POLI, SLC30A8, and TCF7L2 variants with type 2 diabetes in (North African) Tunisian Arabs. Diabetes Res Clin Pract. 2014; 103: e40–3. 10.1016/j.diabres.2013.12.040 [DOI] [PubMed] [Google Scholar]
- 37. Thorsby PM, Midthjell K, Gjerlaugsen N, Holmen J, Hanssen KF, Birkeland KI, et al. Comparison of genetic risk in three candidate genes (TCF7L2, PPARG, KCNJ11) with traditional risk factors for type 2 diabetes in a population-based study—the HUNT study. Scand J Clin Lab Invest. 2009; 69: 282–287. 10.1080/00365510802538188 [DOI] [PubMed] [Google Scholar]
- 38. Yamauchi T, Hara K, Maeda S, Yasuda K, Takahashi A, Horikoshi M, et al. A genome-wide association study in the Japanese population identifies susceptibility loci for type 2 diabetes at UBE2E2 and C2CD4A-C2CD4B. Nat Genet. 2010; 42: 864–868. 10.1038/ng.660 [DOI] [PubMed] [Google Scholar]
- 39. Lyssenko V, Jonsson A, Almgren P, Pulizzi N, Isomaa B, Tuomi T, et al. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med. 2008; 359: 2220–2232. 10.1056/NEJMoa0801869 [DOI] [PubMed] [Google Scholar]
- 40. Cauchi S, Nead KT, Choquet H, Horber F, Potoczna N, Balkau B, et al. The genetic susceptibility to type 2 diabetes may be modulated by obesity status: implications for association studies. BMC Med Genet. 2008; 9: 45 10.1186/1471-2350-9-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rees SD, Hydrie MZI, Shera a S, Kumar S, O’Hare JP, Barnett AH, et al. Replication of 13 genome-wide association (GWA)-validated risk variants for type 2 diabetes in Pakistani populations. Diabetologia. 2011; 54: 1368–1374. 10.1007/s00125-011-2063-2 [DOI] [PubMed] [Google Scholar]
- 42. Qiu L, Na R, Xu R, Wang S, Sheng H, Wu W, et al. Quantitative Assessment of the Effect of KCNJ11 Gene Polymorphism on the Risk of Type 2 Diabetes. PLoS One. 2014; 9: e93961 10.1371/journal.pone.0093961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. L’Abbé KA, Detsky AS, O’Rourke K. Meta-analysis in clinical research. Ann Intern Med. 1987; 107: 224–233. [DOI] [PubMed] [Google Scholar]
- 44. Ishiyama-Shigemoto S, Yamada K, Yuan X, Koyama W, Nonaka K. Clinical characterization of polymorphisms in the sulphonylurea receptor 1 gene in Japanese subjects with Type 2 diabetes mellitus. Diabet Med. 1998; 15: 826–829. [DOI] [PubMed] [Google Scholar]
- 45. Hani EH, Boutin P, Durand E, Inoue H, Permutt MA, Velho G, et al. Missense mutations in the pancreatic islet beta cell inwardly rectifying K+ channel gene (KIR6.2/BIR): a meta-analysis suggests a role in the polygenic basis of Type II diabetes mellitus in Caucasians. Diabetologia. 1998; 41: 1511–1515. [DOI] [PubMed] [Google Scholar]
- 46. Ohta Y, Tanizawa Y, Inoue H, Hosaka T, Ueda K, Matsutani A, et al. Identification and functional analysis of sulfonylurea receptor 1 variants in Japanese patients with NIDDM. Diabetes. 1998; 47: 476–481. [DOI] [PubMed] [Google Scholar]
- 47. Alsmadi O, Al-Rubeaan K, Wakil SM, Imtiaz F, Mohamed G, Al-Saud H, et al. Genetic study of Saudi diabetes (GSSD): significant association of the KCNJ11 E23K polymorphism with type 2 diabetes. Diabetes Metab Res Rev. 2008; 24: 137–140. [DOI] [PubMed] [Google Scholar]
- 48. Florez JC, Burtt N, de Bakker PIW, Almgren P, Tuomi T, Holmkvist J, et al. Haplotype structure and genotype-phenotype correlations of the sulfonylurea receptor and the islet ATP-sensitive potassium channel gene region. Diabetes. 2004; 53: 1360–1368. [DOI] [PubMed] [Google Scholar]
- 49. Yamada Y, Kuroe A, Li Q, Someya Y, Kubota A, Ihara Y, et al. Genomic variation in pancreatic ion channel genes in Japanese type 2 diabetic patients. Diabetes Metab Res Rev 2001; 17: 213–216. [DOI] [PubMed] [Google Scholar]
- 50. Gloyn AL, Weedon MN, Owen KR, Turner MJ, Knight BA, Hitman G, et al. Large-scale association studies of variants in genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes 2003; 52: 568–572. [DOI] [PubMed] [Google Scholar]
- 51. Barroso I, Luan J, Middelberg RPS, Harding A-H, Franks PW, Jakes RW, et al. Candidate gene association study in type 2 diabetes indicates a role for genes involved in beta-cell function as well as insulin action. PLoS Biol. 2003; 1: E20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hansen SK, Nielsen E- MD, Ek J, Andersen G, Glümer C, Carstensen B, et al. Analysis of separate and combined effects of common variation in KCNJ11 and PPARG on risk of type 2 diabetes. J Clin Endocrinol Metab. 2005; 90: 3629–3637. [DOI] [PubMed] [Google Scholar]
- 53. Van Dam RM, Hoebee B, Seidell JC, Schaap MM, de Bruin TW, Feskens EJ. Common variants in the ATP-sensitive K+ channel genes KCNJ11 (Kir6.2) and ABCC8 (SUR1) in relation to glucose intolerance: population-based studies and meta-analyses. Diabet Med 2005; 22: 590–598. [DOI] [PubMed] [Google Scholar]
- 54. Weedon MN, McCarthy MI, Hitman G, Walker M, Groves CJ, Zeggini E, et al. Combining information from common type 2 diabetes risk polymorphisms improves disease prediction. PLoS Med. 2006; 3: e374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. (2007). Nature. 2007; 447: 661–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007; 316: 1331–1336. [DOI] [PubMed] [Google Scholar]
- 57. Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007; 316: 1341–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Qi L, van Dam RM, Asselbergs FW, Hu FB. Gene-gene interactions between HNF4A and KCNJ11 in predicting Type 2 diabetes in women. Diabet Med. 2007; 24: 1187–1191. [DOI] [PubMed] [Google Scholar]
- 59. Horikoshi M, Hara K, Ito C, Shojima N, Nagai R, Ueki K, et al. Variations in the HHEX gene are associated with increased risk of type 2 diabetes in the Japanese population. Diabetologia. 2007; 50: 2461–2466. [DOI] [PubMed] [Google Scholar]
- 60. Sakamoto Y, Inoue H, Keshavarz P, Miyawaki K, Yamaguchi Y, Moritani M, et al. SNPs in the KCNJ11-ABCC8 gene locus are associated with type 2 diabetes and blood pressure levels in the Japanese population. J Hum Genet. 2007; 52: 781–793. [DOI] [PubMed] [Google Scholar]
- 61. Doi Y, Kubo M, Ninomiya T, Yonemoto K, Iwase M, Arima H, et al. Impact of Kir6.2 E23K polymorphism on the development of type 2 diabetes in a general Japanese population: The Hisayama Study. Diabetes. 2007; 56: 2829–2833. [DOI] [PubMed] [Google Scholar]
- 62. Cejková P, Novota P, Cerná M, Kolostová K, Nováková D, Kucera P, et al. KCNJ11 E23K polymorphism and diabetes mellitus with adult onset in Czech patients. Folia Biol (Praha). 2007; 53: 173–175. [PubMed] [Google Scholar]
- 63. Willer CJ, Bonnycastle LL, Conneely KN, Duren WL, Jackson AU, Scott LJ, et al. Screening of 134 single nucleotide polymorphisms (SNPs) previously associated with type 2 diabetes replicates association with 12 SNPs in nine genes. Diabetes. 2007; 56: 256–264. [DOI] [PubMed] [Google Scholar]
- 64. Omori S, Tanaka Y, Takahashi A, Hirose H, Kashiwagi A, Kaku K, et al. Association of CDKAL1, IGF2BP2, CDKN2A/B, HHEX, SLC30A8, and KCNJ11 with susceptibility to type 2 diabetes in a Japanese population. Diabetes. 2008; 57: 791–795. [DOI] [PubMed] [Google Scholar]
- 65. Vaxillaire M, Veslot J, Dina C, Proença C, Cauchi S, Charpentier G, et al. Impact of common type 2 diabetes risk polymorphisms in the DESIR prospective study. Diabetes. 2008; 57: 244–254. [DOI] [PubMed] [Google Scholar]
- 66. Takeuchi F, Serizawa M, Yamamoto K, Fujisawa T, Nakashima E, Ohnaka K, et al. Confirmation of multiple risk Loci and genetic impacts by a genome-wide association study of type 2 diabetes in the Japanese population. Diabetes. 2009; 58: 1690–1699. 10.2337/db08-1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hu C, Zhang R, Wang C, Wang J, Ma X, Lu J, et al. PPARG, KCNJ11, CDKAL1, CDKN2A-CDKN2B, IDE-KIF11-HHEX, IGF2BP2 and SLC30A8 are associated with type 2 diabetes in a Chinese population. PLoS One. 2009; 4: e7643 10.1371/journal.pone.0007643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tabara Y, Osawa H, Kawamoto R, Onuma H, Shimizu I, Miki T, et al. Replication Study of Candidate Genes Associated With Type 2 Diabetes Based On Genome-Wide Screening. Diabetes. 2009; 58: 493–498. 10.2337/db07-1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhou D, Zhang D, Liu Y, Zhao T, Chen Z, Liu Z, et al. The E23K variation in the KCNJ11 gene is associated with type 2 diabetes in Chinese and East Asian population. J Hum Genet. 2009; 54: 433–435. 10.1038/jhg.2009.54 [DOI] [PubMed] [Google Scholar]
- 70. Wang F, Han X, Ren Q, Zhang X, Han L, luo YY, et al. Effect of genetic variants in KCNJ11, ABCC8, PPARG and HNF4A loci on the susceptibility of type 2 diabetes in Chinese Han population. Chin Med J (Engl). 2009; 122: 2477–2482. [PubMed] [Google Scholar]
- 71. Ezzidi I, Mtiraoui N, Cauchi S, Vaillant E, Dechaume A, Chaieb M, et al. Contribution of type 2 diabetes associated loci in the Arabic population from Tunisia: a case-control study. BMC Med Genet. 2009; 10: 33 10.1186/1471-2350-10-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cornelis MC, Qi L, Zhang C, Kraft P, Manson J, Cai T, et al. Joint effects of common genetic variants on the risk for type 2 diabetes in U.S. men and women of European ancestry. Ann Intern Med. 2009; 150: 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wen J, Rönn T, Olsson A, Yang Z, Lu B, Du Y, et al. Investigation of type 2 diabetes risk alleles support CDKN2A/B, CDKAL1, and TCF7L2 as susceptibility genes in a Han Chinese cohort. PLoS One. 2010; 5: e9153 10.1371/journal.pone.0009153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chauhan G, Spurgeon CJ, Tabassum R, Bhaskar S, Kulkarni SR, Mahajan A, et al. Impact of common variants of PPARG, KCNJ11, TCF7L2, SLC30A8, HHEX, CDKN2A, IGF2BP2, and CDKAL1 on the risk of type 2 diabetes in 5,164 Indians. Diabetes. 2010; 59: 2068–2074. 10.2337/db09-1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liu L, Lei J, Liu H, Zou Q, Sun Y, et al. Identification of susceptibility genes loci associated with type 2 diabetes. Wuhan Univ J Nat Sci. 2010; 15: 171–175. [Google Scholar]
- 76. Yang L, Zhou X, Luo Y, Sun X, Tang Y, Guo W, et al. Association between KCNJ11 gene polymorphisms and risk of type 2 diabetes mellitus in East Asian populations: a meta-analysis in 42,573 individuals. Mol Biol Rep. 2012; 39: 645–659. 10.1007/s11033-011-0782-6 [DOI] [PubMed] [Google Scholar]
- 77. Mtiraoui N, Turki A, Nemr R, Echtay A, Izzidi I, Al-Zaben GS, et al. Contribution of common variants of ENPP1, IGF2BP2, KCNJ11, MLXIPL, PPARγ, SLC30A8 and TCF7L2 to the risk of type 2 diabetes in Lebanese and Tunisian Arabs. Diabetes Metab. 2012; 38: 444–449. 10.1016/j.diabet.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 78. Gong B, Yu J, Li H, Li W, Tong X. The effect of KCNJ11 polymorphism on the risk of type 2 diabetes: a global meta-analysis based on 49 case-control studies. DNA Cell Biol. 2012; 31: 801–810. 10.1089/dna.2011.1445 [DOI] [PubMed] [Google Scholar]
- 79. Danquah I, Othmer T, Frank LK, Bedu-Addo G, Schulze MB, Mockenhaupt FP. The TCF7L2 rs7903146 (T) allele is associated with type 2 diabetes in urban Ghana: a hospital-based case-control study. BMC Med Genet. 2013; 14: 96 10.1186/1471-2350-14-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Qin LJ, Lv Y, Huang QY. Meta-analysis of association of common variants in the KCNJ11-ABCC8 region with type 2 diabetes. Genet Mol Res. 2013; 12: 2990–3002. 10.4238/2013.August.20.1 [DOI] [PubMed] [Google Scholar]
- 81. Inoue H, Ferrer J, Welling CM, Elbein SC, Hoffman M, Mayorga R, et al. Sequence variants in the sulfonylurea receptor (SUR) gene are associated with NIDDM in Caucasians. Diabetes. 1996; 45: 825–831. [DOI] [PubMed] [Google Scholar]
- 82. Rissanen J, Markkanen A, Kärkkäinen P, Pihlajamäki J, Kekäläinen P, Mykkänen L, et al. (2000) Sulfonylurea receptor 1 gene variants are associated with gestational diabetes and type 2 diabetes but not with altered secretion of insulin. Diabetes Care. 2000; 23: 70–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
The table was formed in accordance with the requirements of the site http://www.prisma-statement.org/statement.htm.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.