Abstract
The inflammatory response induced by burn injury contributes to increased incidence of infections, sepsis, organ failure, and mortality. Thus, monitoring post-burn inflammation is of paramount importance but so far there are no reliable biomarkers available to monitor and/or predict infectious complications after burn. As IL-8 is a major mediator for inflammatory responses, the aim of our study was to determine whether IL-8 expression can be used to predict post-burn sepsis, infections, and mortality other outcomes post-burn. Plasma cytokines, acute phase proteins, constitutive proteins, and hormones were analyzed during the first 60 days post injury from 468 pediatric burn patients. Demographics and clinical outcome variables (length of stay, infection, sepsis, multiorgan failure (MOF), and mortality were recorded. A cut-off level for IL-8 was determined using receiver operating characteristic (ROC) analysis. Statistical significance is set at (p<0.05). ROC analysis identified a cut-off level of 234 pg/ml for IL-8 for survival. Patients were grouped according to their average IL-8 levels relative to this cut off and stratified into high (H) (n=133) and low (L) (n=335) groups. In the L group, regression analysis revealed a significant predictive value of IL-8 to percent of total body surface area (TBSA) burned and incidence of MOF (p<0.001). In the H group IL-8 levels were able to predict sepsis (p<0.002). In the H group, elevated IL-8 was associated with increased inflammatory and acute phase responses compared to the L group (p<0.05). High levels of IL-8 correlated with increased MOF, sepsis, and mortality. These data suggest that serum levels of IL-8 may be a valid biomarker for monitoring sepsis, infections, and mortality in burn patients.
Keywords: IL-8, infection, burn, sepsis, MOF, biomarker, mortality, organ failure
INTRODUCTION
The incidence of severe infectious complications after burn injury increases mortality by 40% (1); thus, successful treatment of infections and septic episodes are paramount to decrease death due to severe burn injury (2). Early identification of infections and sepsis remain one of the major clinical challenges. The destruction of large areas of the dermal barrier leaves the severely burned patient susceptible to bacterial, fungal, and viral infections (3, 4). Treatment is often empiric, not specific, and is frequently initiated too late (4). Timely identification of invasive pathogens can allow treatments targeted to the infection. Unfortunately current methods for identifying pathologic micro-organisms such as routine wound culturing or PCR are not sensitive or accurate enough at this stage. Positive identification of infectious agents by the microbiological laboratory is delayed in most cases, increasing the potential for developing life-threatening infectious complications. The ability to detect and treat emerging infections as early as possible would enable timely initiation of specific therapies targeted against the invading microorganism, thereby reducing post-burn morbidity and mortality, and improving patient outcome. The identification of a biomarker which is sensitive and that can be quickly measured would greatly advance the care for these critically injured burn patients.
Burn injury itself leads to an acute and prolonged hyper-inflammatory state that has been well described by many (5-9). Although several studies have reported the utility of using pro-inflammatory markers such as tumor necrosis factor α (TNF-α), C-reactive protein (CRP) or interleukin 6 (IL-6) to monitor post-burn inflammation or immune dysfunction (5, 6, 10, 11), the successful establishment of these same inflammatory markers as biomarkers of infection have been less well explored. Our earlier work has shown that the ratio of IL-6 to TNF-α can be used to predict death from sepsis following burn injury (12). Furthermore, in studies of adult burn patients, we have shown that IL-4, IL-8, granulocyte macrophage colony-stimulating factor, and monocyte chemotactic protein 1 may serve as predictive biomarkers of death from sepsis and/or multiple organ failure (MOF) (13). The incidence of MOF has also been correlated with differential cytokine expression (Jeschke, Finnerty, Herndon, unpublished data). A major mediator of the acute inflammatory response to infection and injury, IL-8 acts quickly, suggesting that early monitoring of this chemokine may provide quick information regarding infection status (11, 14-19). Aside from the recruitment of neutrophils, IL-8 signaling is implicated in the mechanisms underlying angiogenesis, cell growth and tissue remodeling (20). IL-8 production is induced by cellular dysfunction, exogenous stimuli such as bacteria and viruses, and via TNF-α or nuclear transcription factor kappa B (NF-κB) (15, 21). Based on IL-8's role as a mediator inflammation and of proliferation (16, 22), we hypothesized that systemic levels of IL-8 could be used to predict infections and sepsis in severely burned patients.
PATIENTS AND METHODS
Four-hundred sixty-eight pediatric burn patients with severe burns over 30% of total body surface area (TBSA) were enrolled in this study. All patients received the standard of care described in previous publications (23, 24). Briefly, patients were resuscitated according to the Galveston formula (5000 cc/m2 TBSA burned + 2000 cc/m2 TBSA lactated Ringer's solution given in increments) over the first 24 hours. Within 48 hours of admission, all patients underwent total burn wound excision and their wounds were covered with autograft. Any remaining open areas were covered with homograft. After the first operative procedure, patients were taken back to the operation theater when donor sites were healed. This procedure was repeated until all open wound areas were covered with autologous skin.
Nutritional intake is standardized for our hospital and was calculated as 1500 kcal/m2 body surface + 1500 kcal/m2 area burns previously described (25-27). The nutritional route of choice in our patient population was enteral nutrition via a naso-duodenal (Dobhoff) or nasogastric tube.
Patient demographics (age, date of burn and admission, sex, burn size, and depth of burn) and clinical data (concomitant injuries, inhalation injury, sepsis, morbidity, and mortality) were recorded throughout hospital course. Sepsis was defined as the combination of 1) a positive blood culture (≥105CFU) or pathologic identification of infectious agent from biopsy or post-mortem examination, and 2) at least three of the following: leucocytosis or leucopenia (>12,000 or <4,000/μl), hyperthermia or hypothermia (>38.5 or <36.5°C), tachycardia (>20% above normal value), refractory hypotension (systolic BP <20% below normal value), thrombocytopenia (platelets <50,000/mm3), hyperglycemia (serum glucose >240 mg/dl), and enteral feeding intolerance (residuals > 200 cc/hr or diarrhea >1 L/day) in accordance with the American Burn Association consensus definition on sepsis (28) MOF overall organ function (DENVER2) were assessed as previously published (29, 30).
Patient data was collected and recorded prospectively using the clinical information system Emtek by physicians, nurses, and supportive staff. Data was processed and analyzed with Microsoft Access® and Excel® (Microsoft Corporation Inc. Redmond, WA, USA).
Hormones, proteins, and cytokines
Blood and/or urine were collected from burn patients at admission and during the first 60 days post-burn for serum hormone, protein, cytokine, and urine hormone analysis. Blood was drawn in a serum-separator collection tube and centrifuged for 10 minutes at 1320 rpm; the serum was removed and stored at −70°C until assayed.
Serum hormones and acute phase proteins were determined using HPLC (31), nephelometry (BNII, Plasma Protein Analyzer Dade Behring, MD), and ELISA techniques as previously published (24). The Bio-Plex Human Cytokine 17-Plex panel was used with the Bio-Plex Suspension Array System (Bio-Rad, Hercules, CA) to profile expression of seventeen inflammatory cytokines and chemokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70,IL-13,IL-17, granulocyte colony stimulating factor, granulocytemacrophage colony stimulating factor, interferon-gamma (IFN-γ), monocyte chemo attractant protein-1, macrophage inflammatory protein-1beta, and TNF-α) as previously published by Finnerty et al. (5).
Ethics
The study was reviewed and approved by the Institutional Review Board of the University Texas Medical Branch, Galveston, Texas. Informed consent was obtained from each subject ≥18 years of age, parent or child's legal guardian. Assent was obtained from research subjects older than 7 years of age.
Data Analysis
For biochemical measurements, a Confidence Interval 2σ (CI= 0.9544997) was applied to improve reliability. Forward stepwise logistic regression, liner regression analysis, paired and unpaired Student's t-test, and Chi-square analysis tests were used where appropriate. For the cut-off analysis, Receiver Operating Characteristic (ROC) analysis was used. Statistical analysis was performed using Microsoft Excel® and Sigmastat® version 3.5 and Sigmaplot®, (Systat Software Inc., San Jose, CA, USA). Data are expressed as means±SD or SEM, where appropriate. Significance was accepted at p<0.05.
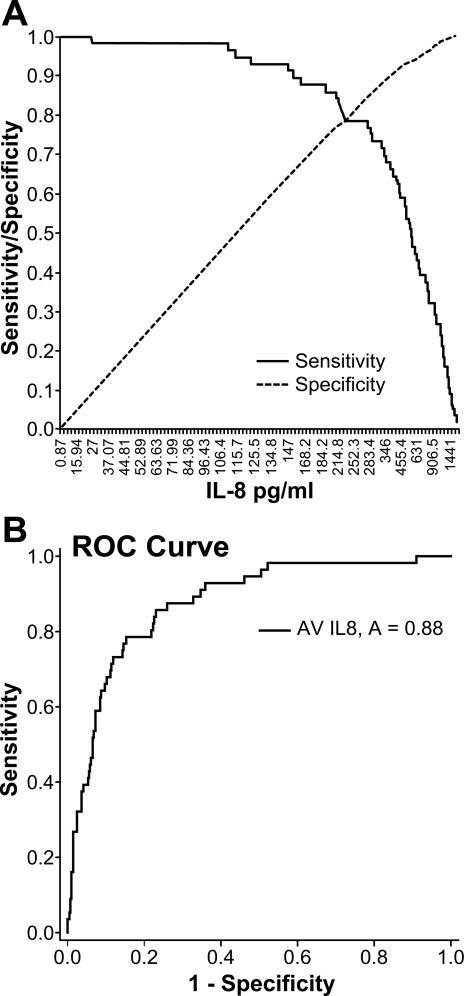
For determining the cut-off value, each patient's levels of IL-8 during the stay in the ICU were averaged. These values were processed with ROC analysis and a calculated cutoff value of 234 pg/ml of IL-8 was determined for highest sensitivity and specificity for survival. This value was then used to stratify the patients in the study. The calculated value is validated with an area under the curve (AUC) value of A=0.88 and considered having a good prognostic significance (Figure 1).
Figure 1. Cut-off determination using receiver operating characteristic (ROC) analysis.
Cut-off value at the intersection of sensitivity and specificity (A). Validated by ROC curve with A=0.88 (B).
RESULTS
Demographics and clinical outcome
Four-hundred sixty-eight pediatric burn patients were included in this study and were stratified according to the cut-off values described above in two groups (Table 1). The cohort with levels of IL-8 below 234 pg/ml IL-8 (low group: L) consisted of 335 patients. The second cohort of patients with IL-8 greater than 234 pg/ml consisted of 133 patients (high group: H). Both groups were similar for many parameters (gender, ethnic background, age, and burn mechanism). The H group had significantly higher incidence of inhalation injury and greater burn sizes (Table 1) (p<0.001). Patients (in the H group) with high IL-8 levels had a significantly longer length of stay, higher incidences of MOF, infections, sepsis, and mortality, and greater incidence of infections compared to patients in the L group (Table 2) (p<0.001).
Table 1.
Patients demographics.
| High IL-8 | Low IL-8 | P value | |
|---|---|---|---|
| n | 133 | 335 | |
| Gender | |||
| male | 84 | 243 | |
| female | 49 | 92 | |
| Ethnicity | |||
| African American | 6 | 15 | |
| Caucasian | 21 | 33 | |
| Hispanic | 105 | 283 | |
| Other | 1 | 4 | |
| Age at admit (years) | 8 ± 6 | 8 ± 5 | NS |
| Inhalation injury (%) | 73 (55) | 129 (39) | <0.01 |
| Type of burn | |||
| Flame n (%) | 104 (78) | 259 (77) | |
| Scald n (%) | 26 (20) | 51 (15) | |
| Other n (%) | 3 (2) | 25 (8) | |
| TBSA burn (%) | 67 ± 18 | 57± 15 | < 0.001 |
| TBSA second (%) | 20 ± 20 | 19 ± 19 | NS |
| TBSA third (%) | 55 ± 26 | 45 ± 22 | < 0.001 |
| Burn to admit (days) | 3 ± 3 | 4 ± 4 | <0.05 |
Table 2.
Patient outcome and critical events during stay in the ICU.
| High IL-8 | Low IL-8 | P value | |
|---|---|---|---|
| n | 133 | 335 | |
| Number of OR | 6 ± 4 | 4 ± 3 | < 0.001 |
| Time btw OR (days) | 5 ± 2 | 5 ± 3 | NS |
| LOS ICU (days) | 39 ± 32 | 32 ± 24 | <0.05 |
| LOS/TBSA | 0.6 ± 0.4 | 0.5 ± 0.3 | NS |
| died n (%) | 44 (33) | 12 (4) | <0.001 |
| Max DENVER2 | 5 ± 2 | 3 ± 1 | < 0.001 |
| MOF n (%) | 67 (50) | 49 (15) | < 0.001 |
| Sepsis n (%) | 41 (31) | 39 (12) | < 0.001 |
| Infections n | 3.3 ± 2.7 | 2.1 ± 2.3 | < 0.001 |
Patients with high IL-8 levels show a higher morbidity and mortality.
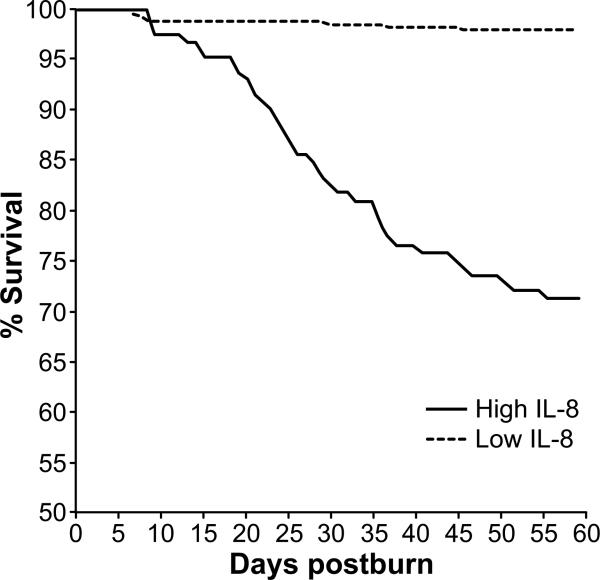
Mortality rates in patients with low IL-8 levels (L group) were significantly reduced when compared to the H group during the first 60 days after injury (Figure 2). The absolute mortality levels were lower in the L group as well (Table 2, p<0.001).
Figure 2. Kaplan Meier survival curve for the observed first 60 days after burn injury.
There is a significant higher mortality rate in the high IL-8 level group.
Predictive value of IL-8 levels
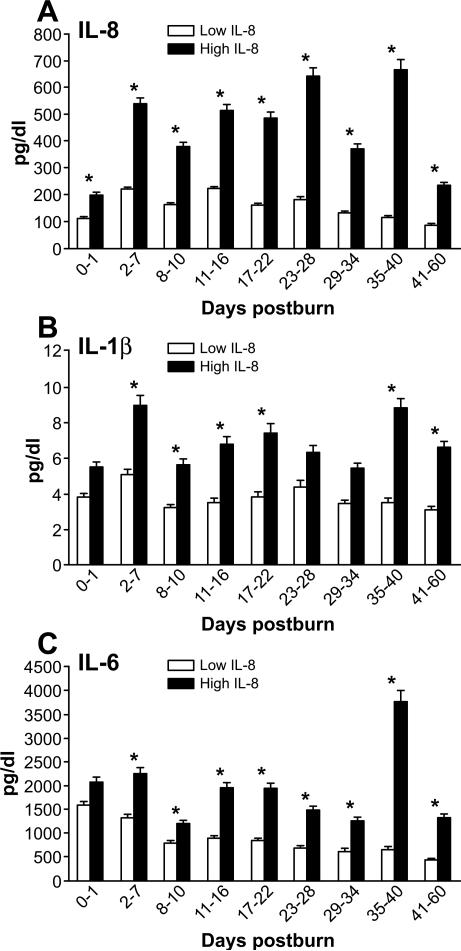
Validation of the patient stratification can be shown with measurements of IL-8 over time showing persistently elevated levels of IL-8 in the H group compared to the L group (Figure 3A, p<0.05).
Figure 3. Pro-inflammatory Cytokines and Chemokines.
IL-8 expression pattern shown over time (A). IL-1beta and IL-6 expression stratified based on IL-8 expression (B, C).
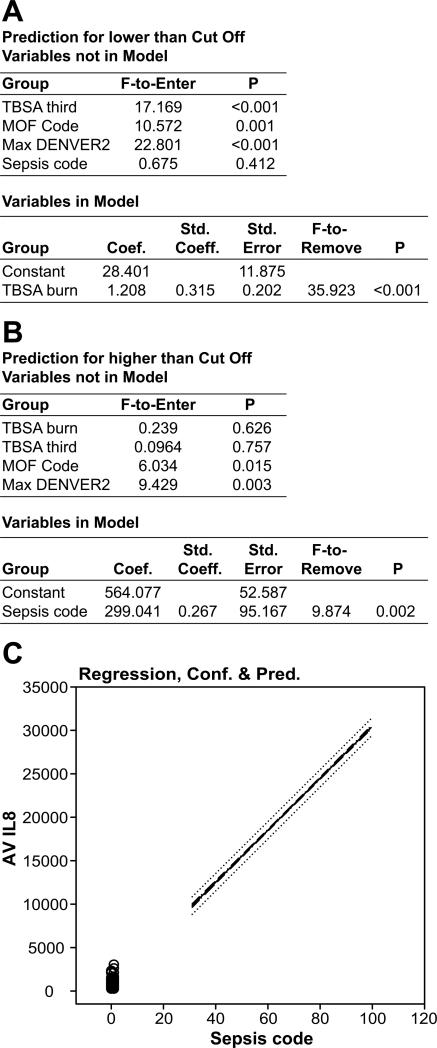
Analysis of the prediction value of IL-8 levels for the L group (Figure 4A) revealed that levels of this cytokine increase with burn size (p<0.001, highest F-to-Enter = 35.923). IL-8 is also specific (p<0.001) for prediction of MOF and the maximum DENVER2 score. There was no correlation with between the incidence of sepsis and IL-8 levels.
Figure 4. Forward stepwise logistic regression analysis model for the predictive value of IL-8.
In the low IL-8 group has only a significant relationship with burn size (A). In patients with higher levels than the determined cut-off IL-8 levels there is a significant correlation with the incidence of sepsis (B). IL-8: confidence, cutoff, and prediction of sepsis (C).
In the H group (Figure 4B) IL-8 levels correlated strongly with the incidence of sepsis (p=0.002; highest F-to-Enter = 9.874). IL-8 also correlated with the incidence of MOF and the maximum DENVER2 scores in the H group (p<0.005). Interestingly, burn size showed no correlation with IL-levels in the H-group. A nearly linear increase in the probability of sepsis with increasing IL-8 levels occurs, indicating that IL-8 levels are correlated with burn size, but to a greater extent with infection and sepsis, indicating that these patients are experiencing even more inflammation.
To identify possible confounders for the incidence of sepsis, we conducted a linear regression analysis for both groups. In patients below the cut off, a significant relationship (p<0.001) with third-degree burn size was found. In patients above the cut off, no relationship was found (Table 3). For all other variables such as age or time from burn to admit, no significant correlation was found in either group.
Table 3.
Linear regression analysis.
| Coefficient | Std. Error | t | P | VIF | |
|---|---|---|---|---|---|
| Linear Regression below cutoff for sepsis | |||||
| Constant | 0.0794 | 0.0729 | 1.089 | 0.277 | |
| AV IL8 | −0.000623 | 0.00038 | −1.641 | 0.102 | 1.094 |
| Age admit | 0.00171 | 0.00401 | 0.426 | 0.67 | 1.016 |
| Burn to Admit | −0.00593 | 0.00549 | −1.08 | 0.281 | 1.053 |
| TBSA third | 0.00412 | 0.000994 | 4.145 | <0.001 | 1.083 |
| Linear Regression above cutoff for sepsis | |||||
| Constant | 0.236 | 0.0951 | 2.486 | 0.014 | |
| AV IL8 | −0.0000126 | 0.0000624 | −0.202 | 0.84 | 1.003 |
| Age admit | −0.00728 | 0.00597 | −1.219 | 0.225 | 1.084 |
| Burn to Admit | −0.00956 | 0.0104 | −0.919 | 0.36 | 1.01 |
| TBSA third | 0.0003 | 0.0013 | 0.23 | 0.818 | 1.088 |
Inflammation is greater in patients with elevated IL-8
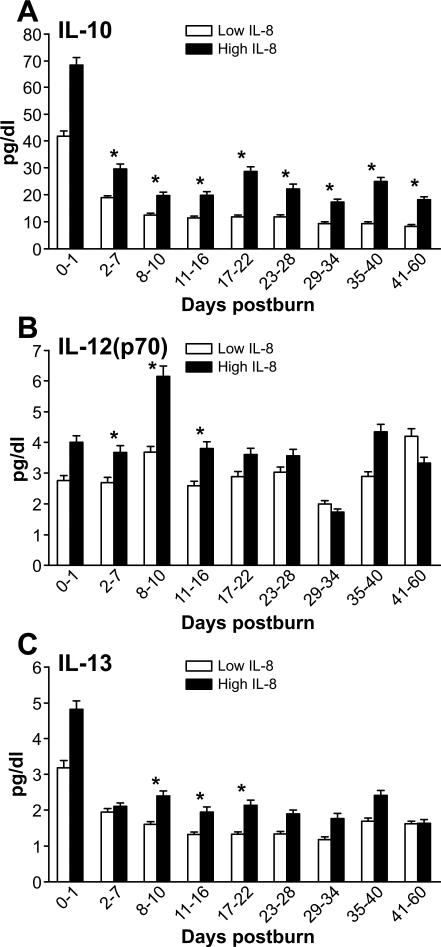
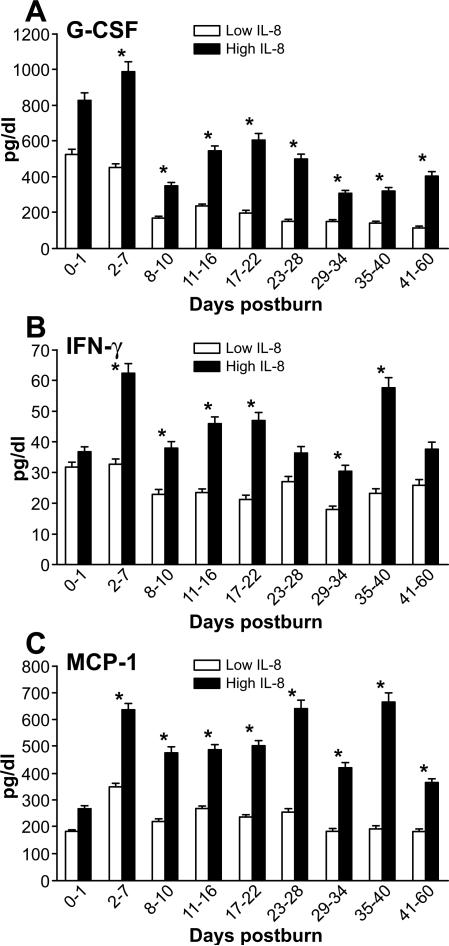
As previously reported, measured cytokine levels were elevated in both patient groups during the observed first sixty days post-burn. Significantly higher levels of IL-1β, IL-6, IL-10, IL12 (p70), and IL-13 can be found over time in the high IL-8 group compared to the low group, (p<0.05) (Figures 3, 5, 6A-B).
Figure 5. Profile of major regulatory cytokines.
Expression of IL-10, IL-12(p70), and IL-13 is associated with IL-8 expression (A, B, C).
Figure 6. Major regulatory mediators.
Expression of G-CSF, IFN-gamma, MCP-1 (markers for granulocyte activation (A), inflammation (B), and macrophage activation (C) is higher in the group with high IL-8 levels.
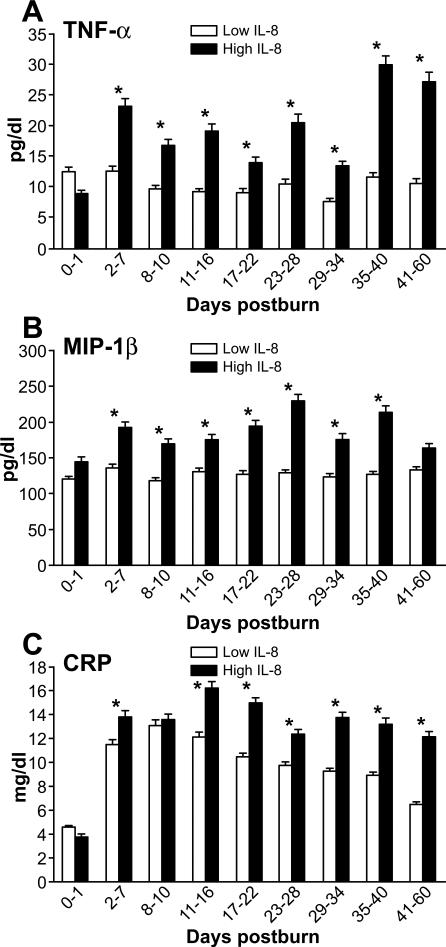
The established pro-inflammatory marker CRP (Figure 7C) showed tremendous increases after day 1 post-burn with prolonged elevation in the H group over the first 60 days compared to the L group (p<0.05).
Figure 7. Markers for Inflammation.
Regulatory mediator (TNF-alpha), chemokine (MIP-1beta) and the established marker for inflammation CRP (C) in the high IL-8 group (A, B, C).
Organ Function
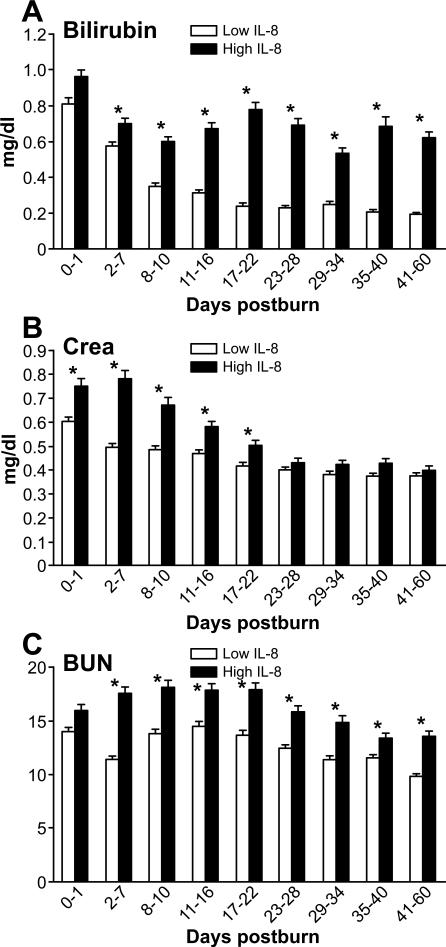
Liver dysfunction, represented by serum bilirubin, increased in both groups directly after burn injury with levels in the L group decreasing to normal after the first week, whereas the H group remained significantly elevated from day 2 until day 60 post-burn (p<0.05) (Figure 8A). Kidney dysfunction represented by creatinine (CRE) levels showed a significantly greater elevation in the high IL-8 group for the first 22 days (p<0.05). Measurements of blood urea nitrogen revealed a remarkable elevation in both groups with significantly increased levels in the high IL-8 group till day 60 (p<0.05) (Figure 8B, C).
Figure 8. Organ specific markers.
Elevation of organ specific markers for liver (A: bilirubin) and kidneys (B: Creatinine; C: BUN).
DISCUSSION
Sepsis and severe infections are major complications after severe burn injury that are associated with a high mortality (32). Therefore, it is important to detect infectious complications at an early stage in order to initiate appropriate treatments, decreasing morbidity and mortality. As the severely burned patient is in a hyper-inflammatory state due to physiological reactions to the trauma (33-35), established pro-inflammatory markers such as CRP or IL-6 may have limited utility as biomarkers for specific outcomes (36, 37). Currently, there are no established biomarkers that can detect or monitor infections or sepsis (9, 19).We therefore explored the possibility of the chemokine IL-8 as a predictor because IL-8 is known for being a major mediator of the inflammatory response. IL-8 plays a major role in the activation of neutrophils, general inflammatory processes, and tissue repair mechanisms such as angiogenesis and cell proliferation (16, 17, 20). Based on these findings, we suggest that IL-8 has an important role in two major processes that may determine survival after burn injury: tissue remodeling (15, 20) and inflammatory response (16, 21). We hypothesized that IL-8 serves as an indicator for the extent of burn due to tissue repair as well as the ability to quantify the inflammatory response induced by infection. We further hypothesized that following burn injury there is a standard inflammatory response which is exacerbated by the presence of infectious stimuli.
The first analysis in this study identified a cut-off concentration of 234 pg/ml IL-8 as the most specific and sensitive indicator for survival. Patients with high levels of IL-8 had a ten-fold higher in-hospital mortality rate compared to patients with lower IL-8 levels. We further identified inhalation injury, burn size, and the incidence of MOF, sepsis, and infections as factors that contributes significantly to these outcomes. We also found a relationship between the clinical trajectory stratified by IL-8 levels and the expression of other pro-inflammatory regulatory cytokines such as IFN-γ and TNF-α. Interestingly both, inflammatory and anti-inflammatory cytokines were elevated. This observation is in accordance with the results of other studies conducted in burn patients (5-7, 9). Markers of organ function showed that liver and kidney function were also significantly worse in patients with average values of IL-8 greater than 234 pg/ml. These findings indicate that IL-8 levels are influenced by the injury discriminating factors for survival post-burn which are: inhalation injury, burn size and infectious complications. Studies in burned patients have shown that the incidence of infectious complications increases the mortality rate by 40%.
To test the hypothesis that high levels of IL-8 are triggered by infectious stimuli, we conducted a stepwise regression analysis for the patient populations in each groups resulting from stratifying by the lower and higher cut-off values. We included the parameters TBSA burn size, MOF and sepsis in the statistical model. The findings for the low-level group correlate best with the TBSA burn size area and consecutively with the third-degree burn and the incidence of MOF. These results correlate with linear regression modeling considering possible confounding factors for sepsis. These findings are similar to other studies that revealed a correlation of IL-8 to endothelial cell survival, cell proliferation, and the expression of matrix metalloproteases (MMP) (38, 39). These are essential mechanisms for the cleavage and remodeling of tissue after burn injury. Stepwise logistic regression analysis of the high IL-8 group showed a significant correlation with the incidence of sepsis, significant greater maximum DENVER2 scores, and incidence of MOF. Burn size did not contribute to IL-8 levels in the high-level group. Logistic analysis shows a nearly linear correlation above the cut-off level of 234 pg/ml for IL-8 with the incidence of sepsis in the high IL-8 patient population.
Summarizing our results, we found that IL-8 is a sensitive and specific biomarker for burn size below a threshold of 234 pg/ml and that at higher levels, the degree of plasma IL-8 correlates strongly with the incidence of septic episodes. We conclude that IL-8 may serve as a useful biomarker to predict infections and septic events in burn victims.
ACKNOWLEDGMENTS
We thank all the individuals who participated in this clinical trial. We also would like to thank all the research staff, Maricela Pantoja for her technical assistance, and Eileen Figueroa and Steven Schuenke for their assistance in manuscript preparation.
Source of Funding: This work was supported by grants from Shriners Hospitals for Children: 71008, 84080, and 79135; National Institutes of Health: R01 GM56687, T32 GM008256, and P50 GM60338, and NIDRR H133A020102 (DNH), R01 GM087285-01) (MGJ), the Canadian Institutes of Health Research (123336) (MGJ), CFI Leader's Opportunity Fund (Project 25407) (MGJ), and Physicians’ Services Incorporated Foundation-Health Research Grant Program (MGJ).This study was conducted with the support of the Institute for Translational Sciences at the University of Texas Medical Branch, supported in part by a Clinical and Translational Science Award (UL1TR000071).
Footnotes
Conflicts of Interest
This trial was registered at www.clinicaltrials.gov as # NCT00675714.The authors have no conflicts of interest to declare.
REFERENCES
- 1.Herndon DN, Spies M. Modern burn care. Semin Pediatr Surg. 2001;10:28–31. doi: 10.1053/spsu.2001.19389. [DOI] [PubMed] [Google Scholar]
- 2.Jeschke MG, Norbury WB, Finnerty CC, Mlcak RP, Kulp GA, Branski LK, Gauglitz GG, Herndon B, Swick A, Herndon DN. Age differences in inflammatory and hypermetabolic postburn responses. Pediatrics. 2008;121:497–507. doi: 10.1542/peds.2007-1363. [DOI] [PubMed] [Google Scholar]
- 3.Pensler JM, Herndon DN, Ptak H, Bonds E, Rutan TC, Desai MH, Abston S. Fungal sepsis: an increasing problem in major thermal injuries. J Burn Care Rehabil. 1986;7:488–491. [PubMed] [Google Scholar]
- 4.Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19:403–434. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finnerty CC, Herndon DN, Przkora R, Pereira CT, Oliveira HM, Queiroz DM, Rocha AM, Jeschke MG. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26:13–19. doi: 10.1097/01.shk.0000223120.26394.7d. [DOI] [PubMed] [Google Scholar]
- 6.Finnerty CC, Jeschke MG, Herndon DN, Gamelli R, Gibran N, Klein M, Silver G, Arnoldo B, Remick D, Tompkins RG. Temporal cytokine profiles in severely burned patients: a comparison of adults and children. Mol Med. 2008;14:553–560. doi: 10.2119/2007-00132.Finnerty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia ZF, Coolbaugh MI, He F, Herndon DN, Papaconstantinou J. The effects of burn injury on the acute phase response. J Trauma. 1992;32:245–250. doi: 10.1097/00005373-199202000-00022. discussion 250-251. [DOI] [PubMed] [Google Scholar]
- 8.Klein MB, Silver G, Gamelli RL, Gibran NS, Herndon DN, Hunt JL, Tompkins RG. Inflammation and the host response to injury: an overview of the multicenter study of the genomic and proteomic response to burn injury. J Burn Care Res. 2006;27:448–451. doi: 10.1097/01.BCR.0000227477.33877.E6. [DOI] [PubMed] [Google Scholar]
- 9.Jeschke MG, Mlcak RP, Finnerty CC, Norbury WB, Gauglitz GG, Kulp GA, Herndon DN. Burn size determines the inflammatory and hypermetabolic response. Crit Care. 2007;11:R90. doi: 10.1186/cc6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gauglitz GG, Song J, Herndon DN, Finnerty CC, Boehning D, Barral JM, Jeschke MG. Characterization of the inflammatory response during acute and post-acute phases after severe burn. Shock. 2008;30:503–507. doi: 10.1097/SHK.0b013e31816e3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cairns BA, Barnes CM, Mlot S, Meyer AA, Maile R. Toll-like receptor 2 and 4 ligation results in complex altered cytokine profiles early and late after burn injury. J Trauma. 2008;64:1069–1077. doi: 10.1097/TA.0b013e318166b7d9. discussion 1077-1078. [DOI] [PubMed] [Google Scholar]
- 12.Finnerty CC, Herndon DN, Chinkes DL, Jeschke MG. Serum cytokine differences in severely burned children with and without sepsis. Shock. 2007;27:4–9. doi: 10.1097/01.shk.0000235138.20775.36. [DOI] [PubMed] [Google Scholar]
- 13.Finnerty CC, Jeschke MG, Qian WJ, Kaushal A, Xiao W, Liu T, Gritsenko MA, Moore RJ, Camp DG, 2nd, Moldawer LL, Elson C, Schoenfeld D, Gamelli R, Gibran N, Klein M, Arnoldo B, Remick D, Smith RD, Davis R, Tompkins RG, Herndon DN. Determination of burn patient outcome by large-scale quantitative discovery proteomics. Crit Care Med. 2013;41:1421–1434. doi: 10.1097/CCM.0b013e31827c072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gauglitz GG, Finnerty CC, Herndon DN, Mlcak RP, Jeschke MG. Are serum cytokines early predictors for the outcome of burn patients with inhalation injuries who do not survive? Crit Care. 2008;12:R81. doi: 10.1186/cc6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie TX, Xia Z, Zhang N, Gong W, Huang S. Constitutive NF-kappaB activity regulates the expression of VEGF and IL-8 and tumor angiogenesis of human glioblastoma. Oncol Rep. 2010;23:725–732. [PubMed] [Google Scholar]
- 16.Rajarathnam K, Sykes BD, Kay CM, Dewald B, Geiser T, Baggiolini M, Clark-Lewis I. Neutrophil activation by monomeric interleukin-8. Science. 1994;264:90–92. doi: 10.1126/science.8140420. [DOI] [PubMed] [Google Scholar]
- 17.Schinkel S, Schinkel C, Pollard V, Garofallo R, Heberle H, Reisner P, Papaconstantinou J, Herndon DN. Effects of endotoxin on serum chemokines in man. Eur J Med Res. 2005;10:76–80. [PubMed] [Google Scholar]
- 18.Cox RA, Burke AS, Traber DL, Herndon DN, Hawkins HK. Production of pro-inflammatory polypeptides by airway mucous glands and its potential significance. Pulm Pharmacol Ther. 2007;20:172–177. doi: 10.1016/j.pupt.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, Branski LK, Gauglitz GG, Mlcak RP, Herndon DN. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petzelbauer P, Watson CA, Pfau SE, Pober JS. IL-8 and angiogenesis: evidence that human endothelial cells lack receptors and do not respond to IL-8 in vitro. Cytokine. 1995;7:267–272. doi: 10.1006/cyto.1995.0031. [DOI] [PubMed] [Google Scholar]
- 21.Porreca E, Sergi R, Baccante G, Reale M, Orsini L, Febbo CD, Caselli G, Cuccurullo F, Bertini R. Peripheral blood mononuclear cell production of interleukin-8 and IL-8-dependent neutrophil function in hypercholesterolemic patients. Atherosclerosis. 1999;146:345–350. doi: 10.1016/s0021-9150(99)00160-4. [DOI] [PubMed] [Google Scholar]
- 22.Lowman HB, Fairbrother WJ, Slagle PH, Kabakoff R, Liu J, Shire S, Hebert CA. Monomeric variants of IL-8: effects of side chain substitutions and solution conditions upon dimer formation. Protein Sci. 1997;6:598–608. doi: 10.1002/pro.5560060309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finnerty CC, Ali A, McLean J, Benjamin N, Clayton RP, Andersen CR, Mlcak RP, Suman OE, Meyer W, Herndon DN. Impact of stress-induced diabetes on outcomes in severely burned children. J Am Coll Surg. 2014;218:783–795. doi: 10.1016/j.jamcollsurg.2014.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraft R, Herndon DN, Al-Mousawi AM, Williams FN, Finnerty CC, Jeschke MG. Burn size and survival probability in paediatric patients in modern burn care: a prospective observational cohort study. Lancet. 2012;379:1013–1021. doi: 10.1016/S0140-6736(11)61345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hart DW, Wolf SE, Chinkes DL, Gore DC, Mlcak RP, Beauford RB, Obeng MK, Lal S, Gold WF, Wolfe RR, Herndon DN. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232:455–465. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hart DW, Wolf SE, Mlcak R, Chinkes DL, Ramzy PI, Obeng MK, Ferrando AA, Wolfe RR, Herndon DN. Persistence of muscle catabolism after severe burn. Surgery. 2000;128:312–319. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 27.Mlcak RP, Jeschke MG, Barrow RE, Herndon DN. The influence of age and gender on resting energy expenditure in severely burned children. Ann Surg. 2006;244:121–130. doi: 10.1097/01.sla.0000217678.78472.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenhalgh DG, Saffle JR, Holmes JHt, Gamelli RL, Palmieri TL, Horton JW, Tompkins RG, Traber DL, Mozingo DW, Deitch EA, Goodwin CW, Herndon DN, Gallagher JJ, Sanford AP, Jeng JC, Ahrenholz DH, Neely AN, O'Mara MS, Wolf SE, Purdue GF, Garner WL, Yowler CJ, Latenser BA. American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res. 2007;28:776–790. doi: 10.1097/BCR.0b013e3181599bc9. [DOI] [PubMed] [Google Scholar]
- 29.Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992;101:1481–1483. doi: 10.1378/chest.101.6.1481. [DOI] [PubMed] [Google Scholar]
- 30.Kraft R, Herndon DN, Finnerty CC, Shahrokhi S, Jeschke MG. Occurrence of multiorgan dysfunction in pediatric burn patients: incidence and clinical outcome. Ann Surg. 2014;259:381–387. doi: 10.1097/SLA.0b013e31828c4d04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeschke MG, Finnerty CC, Suman OE, Kulp G, Mlcak RP, Herndon DN. The effect of oxandrolone on the endocrinologic, inflammatory, and hypermetabolic responses during the acute phase postburn. Ann Surg. 2007;246:351–360. doi: 10.1097/SLA.0b013e318146980e. discussion 360-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrow RE, Przkora R, Hawkins HK, Barrow LN, Jeschke MG, Herndon DN. Mortality related to gender, age, sepsis, and ethnicity in severely burned children. Shock. 2005;23:485–487. [PubMed] [Google Scholar]
- 33.Traber DL, Linares HA, Herndon DN, Prien T. The pathophysiology of inhalation injury--a review. Burns Incl Therm Inj. 1988;14:357–364. doi: 10.1016/0305-4179(88)90003-4. [DOI] [PubMed] [Google Scholar]
- 34.Barret JP, Herndon DN. Modulation of inflammatory and catabolic responses in severely burned children by early burn wound excision in the first 24 hours. Arch Surg. 2003;138:127–132. doi: 10.1001/archsurg.138.2.127. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi H, Tsuda Y, Takeuchi D, Kobayashi M, Herndon DN, Suzuki F. Influence of systemic inflammatory response syndrome on host resistance against bacterial infections. Crit Care Med. 2004;32:1879–1885. doi: 10.1097/01.ccm.0000139606.34631.61. [DOI] [PubMed] [Google Scholar]
- 36.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 37.Sikora JP, Chlebna-Sokol D, Andrzejewska E, Chrul S, Polakowska E, Wysocka A, Sikora A. Clinical evaluation of proinflammatory cytokine inhibitors (sTNFR I, sTNFR II, IL-1 ra), anti-inflammatory cytokines (IL-10, IL-13) and activation of neutrophils after burn-induced inflammation. Scand J Immunol. 2008;68:145–152. doi: 10.1111/j.1365-3083.2008.02126.x. [DOI] [PubMed] [Google Scholar]
- 38.Dasu MR, Spies M, Barrow RE, Herndon DN. Matrix metalloproteinases and their tissue inhibitors in severely burned children. Wound Repair Regen. 2003;11:177–180. doi: 10.1046/j.1524-475x.2003.11305.x. [DOI] [PubMed] [Google Scholar]
- 39.Dasu MR, Barrow RE, Spies M, Herndon DN. Matrix metalloproteinase expression in cytokine stimulated human dermal fibroblasts. Burns. 2003;29:527–531. doi: 10.1016/s0305-4179(03)00154-2. [DOI] [PubMed] [Google Scholar]