Abstract
Expansion of oil palm plantations across the humid tropics has precipitated massive loss of tropical forest habitats and their associated speciose biotas. Oil palm plantation monocultures have been identified as an emerging threat to Amazonian biodiversity, but there are no quantitative studies exploring the impact of these plantations on the biome’s biota. Understanding these impacts is extremely important given the rapid projected expansion of oil palm cultivation in the basin. Here we investigate the biodiversity value of oil palm plantations in comparison with other dominant regional land-uses in Eastern Amazonia. We carried out bird surveys in oil palm plantations of varying ages, primary and secondary forests, and cattle pastures. We found that oil palm plantations retained impoverished avian communities with a similar species composition to pastures and agrarian land-uses and did not offer habitat for most forest-associated species, including restricted range species and species of conservation concern. On the other hand, the forests that the oil palm companies are legally obliged to protect hosted a relatively species-rich community including several globally-threatened bird species. We consider oil palm to be no less detrimental to regional biodiversity than other agricultural land-uses and that political pressure exerted by large landowners to allow oil palm to count as a substitute for native forest vegetation in private landholdings with forest restoration deficits would have dire consequences for regional biodiversity.
Introduction
Globally the demand for food, animal feed, and fuel continues to increase at unprecedented rates, yet land available for agriculture is shrinking in many parts of the world [1], [2]. World food demand is forecast to more than double by 2050 [1], brought about both by a growing human population and even more rapid rises in meat consumption [3]. Together with the rapidly growing biofuel market [4]–[6], they represent one of the most pervasive threats to tropical biodiversity, driving conversion of natural ecosystems [1], [7], [8]. Oil palm (Elaeis spp.) plantations are now a dominant tropical land-use occupying over 16 million hectares [9]. It is estimated that 74% of global palm oil usage is for food products and 24% for industrial purposes, the latter predominantly for biodiesel [10]. Production is especially prevalent in Indo-Malaysia (80% of the global total), but plantation acreage is also increasing rapidly in the Afro- and Neotropics [11], [12]. This rapid expansion is likely to continue for decades given both high profitability and high demand. Proponents of palm oil emphasize that its main alternatives, including soy, sunflower, and canola oils, have production efficiencies just 10–20% as high as palm oil (on a per-hectare basis) and would therefore require much larger areas of cultivated land to have a similar benefit [13], moreover, the industry is also highly lucrative and could potentially create thousands of new jobs and raise regional standards of living.
Although widely flagged as a green fuel, from climate-change and biodiversity perspectives, such advantages are diminished should palm oil production contribute either directly or indirectly to deforestation [10], [11]. This has proven to be the case in many parts of the world, where expansion has come at the expense of both undisturbed and logged primary forest [8], [12], [14] despite the high biodiversity value associated with even degraded primary forests [15]. This loss is also partially due to plantation owners using timber revenues (from primary forests) to provide set-up costs for plantation establishment and maintenance [14]. Thus the question of how to make oil palm a more environmentally friendly crop becomes of critical conservation importance [16–18]. As mitigation measures, in situ practices to enhance local biodiversity; such as production of oil palm beneath shade trees, diverse agro-forestry on plantation boundaries, and maintenance of forest patches within plantations have been proposed [18–21]. Significant environmental progress has also been made under the auspices of the Roundtable on Sustainable Palm Oil (RSPO) certification program [22], a result of many of the largest palm oil producers desire to implement environmentally-friendly management.
The Brazilian government is planning a large increase in biofuel production over the next decade, driven by internal and external market demand (ethanol), as well as by government-enforced blending (biodiesel) [23–25]. Much of this expansion is forecast for the eastern Amazonian state of Pará where the palm oil acreage doubled between 2004 and 2010 [25]. Bastos et al. [26] suggested that up to 80% of the state would be suitable for oil palm cultivation, with degraded lands (such as abandoned cattle pasture, and mining areas) likely to host much of the growth in production. The Environmental Council of Pará State (COEMA) recently passed a resolution [27] that permits the designation of oil palm as a ‘low-impact’ land-use that may substitute native forest vegetation in the legally-mandated permanent protection areas (áreas de preservação permanente) ‘APPs’ and legal reserves (reservas legais) ‘RLs’ required of smallholder properties of less than 20 ha. With this precedent there is now a powerful rural lobby arguing for this dispensation to be available to all landowners, irrespective of property size [28], as a replacement for regenerating forest vegetation. Given that the forests of the Amazon basin, representing about 41% of the world's remaining tropical rainforest are already subject to deforestation rates fluctuating around half a million hectares per year [29], this expansion into Amazonia requires careful appraisal of the potential impacts of oil palm monocultures on the region’s rich biodiversity. Such impacts have been heavily studied in south-east Asia, where even wildlife-friendly management techniques have failed to conserve species of conservation concern e.g. [30], but there are no such quantitative studies from Amazonia. Decisions about how to balance land requirements for agriculture biofuels and biodiversity conservation will thus have profound effects on the conservation of Amazonian biodiversity, as well as economic development and poverty alleviation [31].
Here we evaluate the value of oil palm plantations for avian biodiversity in relation to other local land-uses (primary and secondary forests and cattle pastures) located in the 145,000 km2 Belém Area of Endemism (hereafter Belém AE), a region of eastern Amazonia delimited by the east bank of the Tocantins river and the western biogeographic limit of Amazonian terra firme forests in western Maranhão state [32]. Total forest loss in the Belém AE has reached at least 75% of the original extent and further extensive forest loss and concomitant global extinctions are forecast if effective forest conservation policies are not enforced [33], [34]. We investigate the avian biodiversity value of oil palm landscapes in terms of plantation age, tree species richness and distance to source habitats and compare these values with those of land-uses in the surrounding landscape matrix (pastoral systems, primary and secondary forests).
Materials and Methods
Study Region
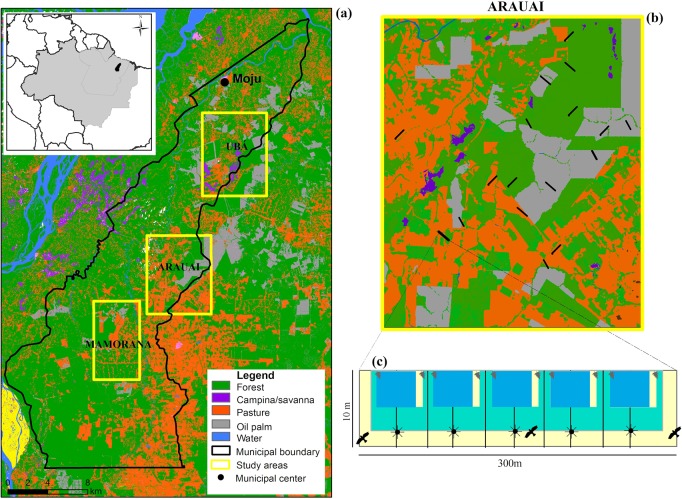
This study took place in the municipalities of Moju, Tailândia and Acará located circa 120 km south of Belém in Pará state. Mean annual temperature is 26.6 °C, mean annual precipitation is circa 2,500 mm [35] and local soils are highly weathered acid oxisols [36]. Forest canopy heights are typically in the range of 25–35 m and the understorey is dominated by plants from the families Lecythidaceae, Violaceae, Sapotaceae, Burseraceae, Moraceae and Leguminosae [37]. There are also small patches of natural open vegetation enclaves—campina formations on white sand soils which have a very distinct biodiversity [38]. By the year 2010, forest cover in Tailândia and Moju had been reduced by 45% to 4,989 km2 much of which is degraded primary or regenerating secondary forest (Fig 1 and [29]). These two municipalities form part of the oil palm ‘pole’ of Moju- Acará- Tailândia, where 124,700 ha of oil palm had been planted by 2009, predicted to increase to 370,500 ha by 2014 [39].
Fig 1. Map of the study region depicting (a) the three areas studied with the municipality of Moju and land-use types; the distribution (b) of transects across one study area (Arauai) and its land-uses; and c) transect design depicting vegetative sampling plots and sub-parcels and the position of point count stations.
Data collection
We selected three study regions occupied by different oil palm producers, as follows: Ubá (1,008 km2) in the north of the municipality of Moju including the source of the rio Ubá; Arauai (952 km2) located in the centre of the municipality including the source of the rio Arauai; and finally Mamorana (680 km2) located at the southern extreme of the municipality including the source of the rio Mamorana (for further details see [40]). Between 15–17 300 m transects were allocated to each region, distributed using a stratified-random sampling design (where a standard density of transects (1 per 400 ha) was distributed in proportion to the percentage cover of total forest and production areas) across each region to increase the likelihood that they would capture important internal heterogeneities in forest and/or production systems. To reduce the dependency between transects within each region, transects were separated by a minimum distance of 1.5 km. We also avoided placing transects within 200 m of any other land-use to control for potential edge effects.
In total we sampled 50 transects, 17 in primary forest, 4 in secondary forests—forests that developed after complete deforestation [41] of different ages (5, 7, 15 & 20 years old), 15 in variable-age oil palm plantations (1, 3, 4, 12, 14, 23, 24 & 25 years old),12 in cattle pasture and one in campina vegetation (although the latter was not used in later analyses except Fig. C in S1 File). Designation of secondary forest and plantation ages was done through visual inspection of a 20 year time-series of Landsat images for each transect, calibrated by interviews with local farmers. All primary forest sites had been subject to historic selective logging events but none have been burned in the last five years. All fieldwork took place on private lands and landowners in each region were visited prior to any fieldwork to introduce the project and secure permissions for surveys. Land-use classification was undertaken using ArcGis 9.3 and Envi 4.5 using both unsupervised (ISODATA) and supervised (MaxVer) classification protocols (ISODATA) [40]. The ISODATA (Interactive Self-organizing Data Analysis) method was used to determine land-use classes prior to fieldwork and employed minimum Euclidean distance measurements (in multidimensional space) to identify spectral clusters from the image and assign a class to each pixel. On returning from the field with our ground-truthed measurements we performed a supervised MaxVer (Maximum Likelihood Classification) analysis which assigns the probability of each pixel to pre-defined classes (based on our ground-truthing), assigning pixels to the class with the highest probability. To assess classification accuracy we used Cohen’s Kappa statistic [42], which provides an indication of the classification agreement between the classified and the ground-truthed maps that is not attributable to chance. The remote sensing analysis was performed using georeferenced data with Landsat TM-5 (Thematic Mapper) images with 30 m spatial resolution from the year 2010.
To sample the bird community we located three point count (PC) stations in each transect at 0, 150 and 300 m. A total of 288 PCs were conducted between the three regions. We (ACL & NGM) carried out two repetitions of three 15 minute, 75 m fixed radius PCs per transect, recording all species seen or heard. Repetitions ensured that temporal variation in avian vocal activity was minimized, and PCs were recorded using solid state recorders. The avifaunal surveys were complemented with sampling of woody plants and vegetative structure to see how habitat structural characteristics and botanic diversity might influence avian species richness. These surveys targeted trees and lianas and palms above 2 cm DBH (Diameter at Breast Height) and were conducted in 10 x 250 m plots, subdivided into 10 x (10 x 15 m) parcels following the protocols of Gardner et al. [43]. All individuals were identified to species or morphospecies by expert parabotanists (Nelson Rosa and Carlos Alberto Santos). Plant species which defied field identification were collected and deposited in the herbarium of the Museu Paraense Emílio Goeldi for later identification. To generate biomass estimates (as Mg.ha-1) we used allometric equations for all plant species with DBH ≥ 2 cm.
We used the equations of Chave et al. [44] for humid forest tree species:
where: AGB = above ground biomass for all species with DBH ≥ 2 cm; p = wood density (g/cm-3); and DBH = diameter at breast height (cm). We used a genera-specific equation for palms in the genus Cecropia, that of Nelson et al. [45]:
where DW = estimated biomass of Cercropia sp. trees. Finally to generate estimates of the biomass of oil palm Elaeis guineensis we used the age-specific formula of Corley & Tinker [46]:
where Bt = estimated biomass (kg) of Elaeis guineensis; r = radius (cm) of the palm’s trunk; Z = diameter of the palm base (a constant—0.777), ρ = height (m) of the trunk and ρ = trunk density (Kg/m3) which is calculated using the formula:
Where Id is the age of the oil palm in years.
Data analyses
We analysed the responses of total species richness as well as richness and turnover for the subset of ‘primary forest-associated birds’ (hereafter termed ‘forest birds’). These forest birds represent the core avifauna of undisturbed terra firme forests but not necessarily birds restricted to those habitats, as some core primary forest species also occur in human-modified forest and non-forest habitats (e.g. Blue-gray Tanager Tangara episcopus and Bananaquit Coereba flaveola). These categorizations were based on previously published classification of birds from the region e.g. [47] and [48]. Our taxonomy follows the checklist of Brazilian birds compiled by the Comitê Brasileiro de Registros Ornitológicos [49].
To compare sampling intensity and avian responses between different land-uses we used sample-based rarefaction curves, with 95% confidence intervals calculated using the ‘specaccum’ tool, included in the vegan package of the R software [50]. Comparisons between species richness in each land-use type were made using a one-way ANOVA test with intervals followed by a Tukey post-hoc test to check for significant pairwise differences. To explore univariate relationships between forest cover and forest bird species richness we performed linear regressions using percentage of total forest cover (primary and old (>15 years) secondary forests combined in a 1 km buffer), the percentage of primary forest cover in a 1 km buffer around the centroid of each transect (to standardise sampling unit size) and distance to the nearest primary forest as predictor variables for avian species richness.
To assess the relative importance of different environmental variables on bird species richness we first assessed for variable colinearity using Pearson correlation between the variables, edge distance, tree species richness, plants biomass and percentage of primary forest cover excluding variables with an unacceptably-high degree of colinearity (r ≥ 0.7). We then used generalized linear models (GLMs) with a Gaussian distribution and a log link function and model averaging using AICc weights [51] to generate subsets of top models and uncover the relative importance of different variables. All these analysis were done using R version 2.15.1 [50] with the ‘glmulti’ and ‘MuMin’ packages.
To assess the variation in species composition between land-use systems and different primary forest disturbance classes we produced non-metric multi-dimensional scaling ordinations (nMDS [52]) using the Sorensen similarity matrix for species presence-absence data. To assess the statistical significance of differences in assemblage composition between different land-use types and forest degradation classes we conducted a one-way PERMANOVA which uses pseudo-F values to compare among-group to within-group similarity and assesses significance by permutation. All multivariate assemblage analyses were carried out in Primer v.6 (PRIMER-E Ltd, Plymouth, UK, [53]). To facilitate comparison to a larger sample of different land-uses we compared our data from Moju-Tailândia-Acará with data from an extensive inventory of the neighbouring municipality of Paragominas [41]. This inventory used the same transect selection and avian sampling protocols and has the same source avifauna [54], [55] as the present study and was conducted by the same field ornithologists (ACL & NGM). In total we used avian data from 187 transects—97 in primary forest, 25 in secondary forest, 53 in cattle pasture and 12 from mechanised agriculture (soy bean fields in this case). We investigated relative contribution of individual species to the overall dissimilarity using the similarity percentages routine-SIMPER [50]. Owing to historic legacy effects of land purchasing (with smallholder properties typically closer to forest borders), oil palm plantations were on average situated farther from primary forest borders (mean = 972 m, SD = 267 m, range 545–1424 m) than cattle pastures (mean = 510 m, SD = 150 m, range 216–719 m). To control for this potential bias of leakage of forest species we compared oil palm forest bird richness with forest bird richness from cattle pastures (n = 23 mean = 1009 m, SD = 382 m, range 554–2069 m) in Paragominas situated over 550 m from the nearest primary forest border and assessed the significance of differences with a paired t-test using R.
Finally, we used a global phylogeny of birds [56] to visually compare the phylogenetic structure of the most speciose avian clades (families with >8 species) between the dominant regional land-uses (primary forest, pasture and oil palm). The resulting circular phylograms were visualized and edited using the FigTree v 1.4.1 software (http://tree.bio.ed.ac.uk/software/figtree/) to document non-random extinction processes.
Results
Regional land-use classification
Across the 2,588 km2 study region our remote-sensing analysis identified seven discrete land-use types: varyingly-degraded primary forest: 40.2% (1041 km2), secondary forest: 9.0% (234.9 km2), campina formations: 2.8% (71.8 km2), oil palm plantations: 8.3% (214.9 km2), cattle pasture: 39.2% (1016 km2), open water: 0.3% (9.0 km2) and cloud/shadow: 0.01% (0.15 km2). These results were associated with a Kappa coefficient of 0.85 (with a global accuracy of 89% of the pixels correctly classified). Following Monserud and Leemans [57] Kappa statistics values, from 0.7 to 0.85 represent very good agreement between images.
Species richness between land-use types
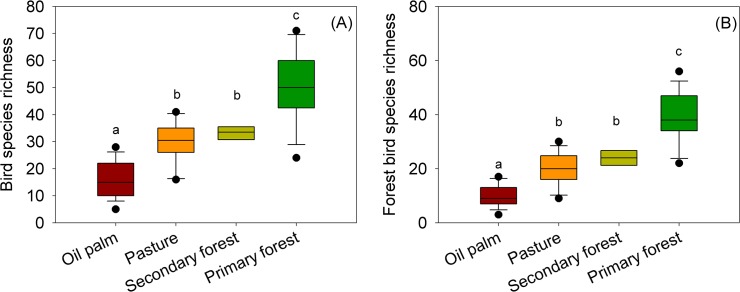
We recorded 3,090 detections of 249 bird species, of which 1982 were forest-associated species (for a full list see Table A in S1 File). The species accumulation curves indicated that surveys in most land-use types were near asymptotic (Fig A in S1 File). We recorded mean species richness per transect of 50.1 in primary forest (SD = 13.2, n = 16, total richness = 211, range = 24–69,), 33.25 in secondary forest (SD = 2.3, n = 4, total richness = 68, range = 30–36), 30.0 in cattle pasture (SD = 7.9, n = 12, total richness = 100, range = 17–41), and 16.3 in oil palm plantation (SD = 6.9, n = 15, total richness = 69, range = 6–28). These differences in species richness were significant between all land-use types considering the whole avifaunal community (Fig 2A, F = 32; df = 43; N = 4; P< 0.01) and for forest-associated birds (Fig 2B, F = 46; df = 43; N = 4; P< 0.01), with the exception of those between pasture and secondary forests for which the mean differences richness per transect was statistically non-significant (Fig 2). The difference in forest bird species richness was not significant between oil palm and cattle pastures (mean = 4.34, SD = 2.19, n = 23) situated over 500 m from the nearest primary forest in Paragominas (unpaired t-test, t = 1.7; df = 36; P = 0.09, Fig. C, in S1 File). We found that avian species was statistically different between old (>11 years, n = 6, mean species richness: 10.2) and young (<10 years, n = 8, mean species richness: 21.5) oil palm transects for both the entire avian community (F = 29.4; df = 13; P<0.01) and forest-associated birds only (F = 18.7; df = 13; P<0.01). Furthermore, avian communities in oil palm plantations of varying ages underwent avian community succession resulting in different community structure and richness between plantations of different ages (Fig C, in S1 File). Recently planted stands (≈ 1–2 years) had a very low vegetative biomass and were mostly occupied by birds typical of cattle pastures (or natural savannah enclaves—see [38]) such as Pale-breasted Spinetails Synallaxis albescens and Red-breasted Blackbirds Sturnella militaris. Plantations of ≈ 5 years had a more significant biomass comparable with young secondary forests, but had a similar community structure, albeit including some species more typical of edge/secondary forest such as Reddish Hermit Phaethornis ruber, Great Antshrike Taraba major and White-fringed Antwren Formicivora grisea.
Fig 2. Box plots comparing avian species richness between land-use types, using the entire avian assemblage (A) and just forest-associated birds (B).
Non-significant pairwise differences between land-use types are indicated by the presence of the same letter (according to a Tukey test 95%).
Environmental determinants of species richness
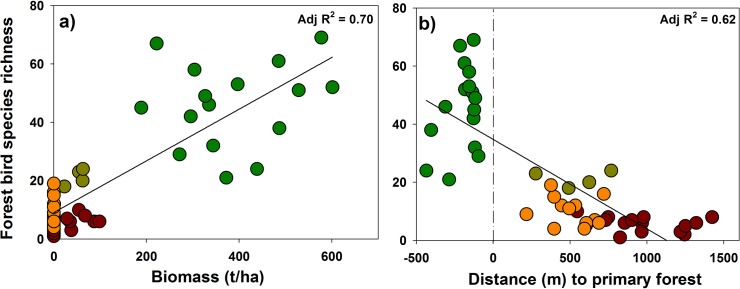
We found a significant positive and broadly linear relationship between the richness of all bird species and tree biomass (adj. R2 = 0.49, p < 0.001) which strengthened when only forest associated birds were included in the model (Fig 3, adj. R2 = 0.70, P < 0.001). We uncovered a significant negative relationship between distance to forest edge and species richness (adj. R2 = 0.56, P < 0.001) which was marginally more significant when only forest birds were retained (Fig 3, adj. R2 = 0.56, P < 0.001). The GLMs revealed that tree richness was the most important predictor variable influencing richness for the whole community (Fig 4 and Table B in S1 File) followed by distance to the forest border, forest cover and vegetative biomass for the whole community, whilst for forest associated birds the most important predictor variables were tree species richness, biomass, distance to the forest border and forest cover. The top model results showed that, considering the whole avian community, four models had low ΔAICc (<2), with the best model including distance to border and tree richness. Considering only forest-associated birds two models had low ΔAICc with the best retaining only tree species richness (Table B in S1 File).
Fig 3. Linear regressions between richness of forest bird species and a) tree biomass and b) distance to the nearest primary forest border.
Fig 4. Relative importance of environmental variables in explaining total avian species richness (A) and forest-associated bird richness (B).
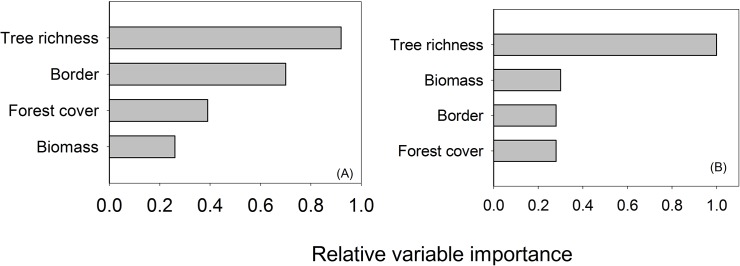
Signs indicate the direction of the effect of each variable. The explanatory variables include distance to the forest border (border), tree species richness, forest cover (% of primary forest cover) and biomass of trees (biomass).
Avian community structure across land-use types
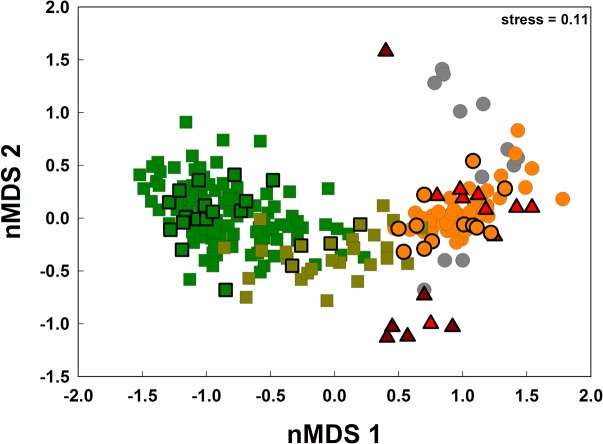
Avian species composition changed consistently and significantly along a gradient of human impacts between primary forests, secondary forests, cattle pastures and oil palm plantations Fig 5, Table C in S1 File, PERMANOVA, Pseudo-F = 8.1725, P < 0.001). All species assemblages were significantly different from each other (P < 0.001) with the exception of pastures and plantations for which P = 0.573. Community structure in primary forests, secondary forests and cattle pasture was broadly similar to that found in the neighbouring municipality of Paragominas (Fig 5) indicating low species turnover between the municipalities and emphasising the generalizability of the results across the Belém AE. Each land-use type played host to characteristic assemblages of species whose relative dominance contributed to community dissimilarity (Table 1), with communities in the oil palm plantations sharing more species with cattle pastures than they did with forest habitats. The phylograms (Fig 6) indicate that land-use mediated local extinctions were non-random, with greatest losses of species occurring from the diverse radiation of suboscine passerines (antbirds, tyrant flycatchers, ovenbirds and woodcreepers) for which few species persisted in cattle pastures or oil palm plantations. There was some turnover of species, with waterbirds such as crakes and rails and diverse granivorous oscine passerines typical of open areas colonising the pastures and oil palm plantations (Fig 6. Table A in S1 File).
Fig 5. NMDS plots of community structure of the avian community in Moju (polygons with heavy black borders) and Paragominas (narrow borders), primary forest transects are represented by dark green squares, secondary forests by light green squares, orange circles are cattle pastures, grey circles are mechanised agriculture and triangles are oil palm plantations (dark red = 15–20 years old, lighter red 0–6 years old).
Table 1. The top-ranked 15 bird species in each land-use type which contributed to the dissimilarity of species composition between oil palm plantations, cattle pastures, secondary forests and primary forests.
| Oil Palm | Pasture | ||||||
| Latin binomial | Av.Abund | Contrib% | Cumu.% | Latin binomial | Av.Abund | Contrib% | Cumu.% |
| Formicivora grisea 1 | 1 | 20.7 | 20.7 | Myiophobus fasciatus 2 | 0.92 | 7.54 | 7.54 |
| Tyrannus melancholicus | 0.8 | 11.89 | 32.59 | Tangara episcopus | 0.92 | 7.54 | 15.09 |
| Ramphocelus carbo 4 | 0.67 | 8.33 | 40.92 | Tyrannus melancholicus | 0.92 | 7.54 | 22.63 |
| Tangara palmarum | 0.53 | 5.48 | 46.41 | Troglodytes musculus 2 | 0.85 | 6.4 | 29.03 |
| Myiophobus fasciatus 2 | 0.6 | 5.19 | 51.6 | Elaenia flavogaster | 0.85 | 5.97 | 35 |
| Troglodytes musculus 2 | 0.53 | 4.76 | 56.36 | Phaeomyias murina | 0.85 | 5.97 | 40.97 |
| Pitangus sulphuratus | 0.47 | 3.79 | 60.15 | Ramphocelus carbo 4 | 0.85 | 5.41 | 46.38 |
| Turdus leucomelas | 0.4 | 3.48 | 63.62 | Synallaxis albescens 2 | 0.69 | 4.14 | 50.52 |
| Phaethornis ruber 3 | 0.4 | 3 | 66.63 | Tangara palmarum | 0.69 | 4.06 | 54.58 |
| Sturnella militaris 2 | 0.47 | 2.93 | 69.56 | Volatinia jacarina 2 | 0.69 | 3.93 | 58.51 |
| Volatinia jacarina 2 | 0.47 | 2.93 | 72.49 | Sturnella militaris 2 | 0.62 | 3.64 | 62.15 |
| Progne chalybea 3 | 0.4 | 2.68 | 75.17 | Formicivora grisea | 0.62 | 2.82 | 64.97 |
| Synallaxis albescens 2 | 0.4 | 2.11 | 77.28 | Progne chalybea 3 | 0.54 | 2.27 | 67.24 |
| Crotophaga ani | 0.4 | 2.02 | 79.3 | Tachyphonus rufus | 0.54 | 2.26 | 69.5 |
| Amazona amazonica | 0.33 | 1.78 | 81.09 | Myiarchus tyrannulus | 0.54 | 2.17 | 71.67 |
| Secondary Forest | Primary Forest | ||||||
| Latin binomial | Av.Abund | Contrib% | Cumu.% | Latin binomial | Av.Abund | Contrib% | Cumu.% |
| Coereba flaveola 2 | 1 | 6.11 | 6.11 | Cercomacra cinerascens | 1 | 4.54 | 4.54 |
| Formicivora grisea | 1 | 6.11 | 12.22 | Lophotriccus galeatus | 1 | 4.54 | 9.09 |
| Phaethornis ruber 3 | 1 | 6.11 | 18.34 | Phaethornis ruber 3 | 0.93 | 4.04 | 13.13 |
| Pheugopedius genibarbis | 1 | 6.11 | 24.45 | Myrmotherula axillaris 2 | 0.93 | 3.97 | 17.1 |
| Progne chalybea 2 | 1 | 6.11 | 30.56 | Pyriglena leuconota 2 | 0.93 | 3.94 | 21.03 |
| Pyriglena leuconota 2 | 1 | 6.11 | 36.67 | Glyphorynchus spirurus | 0.87 | 3.32 | 24.36 |
| Ramphocelus carbo 4 | 1 | 6.11 | 42.78 | Myiopagis gaimardii 2 | 0.8 | 2.97 | 27.33 |
| Thamnophilus amazonicus | 1 | 6.11 | 48.89 | Pheugopedius genibarbis | 0.8 | 2.78 | 30.11 |
| Amazona amazonica | 0.75 | 3.14 | 52.04 | Ramphocelus carbo 4 | 0.8 | 2.78 | 32.88 |
| Manacus manacus | 0.75 | 3.14 | 55.18 | Thamnomanes caesius | 0.8 | 2.62 | 35.5 |
| Patagioenas speciosa | 0.75 | 3.14 | 58.32 | Isleria hauxwelli | 0.73 | 2.25 | 37.75 |
| Saltator maximus | 0.75 | 3.14 | 61.46 | Coereba flaveola 2 | 0.73 | 2.22 | 39.97 |
| Lophotriccus galeatus | 0.75 | 3.08 | 64.53 | Zimmerius gracilipes | 0.73 | 2.15 | 42.12 |
| Myiopagis gaimardii 2 | 0.75 | 3.08 | 67.61 | Thamnophilus amazonicus | 0.67 | 2.11 | 44.23 |
| Myrmotherula axillaris 2 | 0.75 | 3.08 | 70.69 | Ramphastos vitellinus | 0.67 | 1.89 | 46.12 |
Numbered superscripts refer to the number of other habitats in which species were also top-ranked contributors to species similarity.
Fig 6. Circular phylograms illustrating avian community composition in a) primary forests b) cattle pasture and c) oil palm plantations, families with more than eight species are labelled.
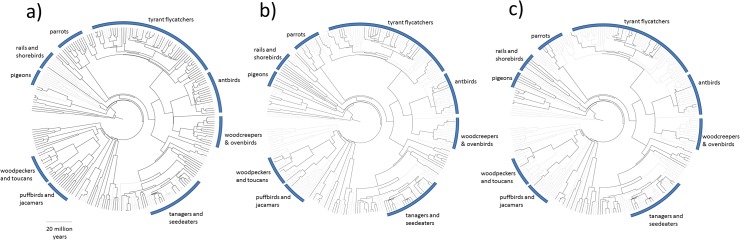
Bold lines indicate species presences in the given land-use type whereas pale lines denote species absences from that land-use type which were found in one or more other land-uses.
Occurrence of threatened and endemic species
Most of the bird species (96%) we detected are classified as Least Concern by BirdLife International [58] with the exception of nine species in the threat categories Endangered (EN), Vulnerable (VU) and Near Threatened (NT). These were as follows: White-crested Guan Penelope pileata (VU); Ruddy Pigeon Patagioenas subvinacea (VU); Golden Parakeet Guaruba guarouba (VU); White-bellied Parrot Pionites leucogaster (EN); Pearly Parakeet Pyrrhura lepida (VU); Vulturine Parrot Pyrilia vulturina (VU); (Eastern) Red-necked Aracari Pteroglossus bitorquatus (EN); Red-billed Toucan Ramphastos tucanus (VU); Channel-billed Toucan Ramphastos vitellinus (EN) (as Ariel Toucan Ramphastos ariel) and Long-tailed Woodcreeper Deconychura longicauda (NT). All these species were confined to primary forests; with the exception of a single Red-necked Aracari photographed in an arborescent pasture [59] and one record of Channel-billed Toucan from a secondary forest transect (Table A in S1 File). In addition we found a further four species which are narrowly endemic to the Belém AE (and a further four highly-differentiated endemic subspecies likely to be subject to future taxonomic upgrades to species status [60–62]) and six other species that are restricted to south-east Amazonia, east of the river Madeira and south of the river Amazon. We also recorded an undescribed species of Myiornis pygmy-tyrant from one primary forest transect, which is now known from several forest sites in north-east Brazil, see [38].
Discussion
We found that oil palm plantations in eastern Amazonia supported species-poor avian communities of comparable richness and composition to other non-forest land-uses such as cattle pasture. These communities in oil palm were principally composed of non-forest species of low conservation concern. This general conclusion is supported by results from similar studies of tropical oil palm plantations in formerly forested landscapes in Borneo [30], Peninsula Malaysia [63], Thailand [64], and Colombia [65]. Furthermore, we expect that the loss and turnover of the avian community in our landscape will be mirrored in diverse other taxonomic groups (e.g. mammals, reptiles, invertebrates). Responses of forest bird communities to oil palm plantations have been shown to be an excellent indicator of responses in other taxonomic groups in other countries [30], [66], [67] and forest birds have been shown to be excellent indicators of the responses of diverse taxonomic groups to other forms of land-use change in Amazonia [68]. As such, we consider that oil palm plantations cannot be considered to be ‘low impact’ land-uses and reinforces the current position of the COEMA [28] in not permitting oil palm to be used a substitute for native vegetation in APPs or RLs for large property owners.
Avian biodiversity in Amazonian oil palm plantations
We did not find any species of conservation concern within the plantations nor any species typically regarded as being indicators of terra firme forest habitats. Species occupying oil palm plantations were typically a subset of those that occupy other Amazonian non-forest land-uses (e.g. [55]). These communities are dominated by a small number of generalist species which disproportionately contribute to community dissimilarity from other land-uses. Likewise we did not find evidence for positive effects of proximity to large forest tracts for within-plantation biodiversity, although we avoided habitat edges by at least 200 m to control for inflation of species richness through spillover [68]. Although oil palm may be of low value for most forest birds, birds may be of value to oil palm producers, through insectivorous passerine top-down control of phytophagous insect herbivory and through predation on rodents by raptors [69]. We frequently encountered species like White-tailed Hawks Geranoaetus albicaudatus hunting in the plantations and local population densities of this and other raptors might be increased through provision of nest boxes and hunting perches.
Although we generally found avian communities in oil palm plantations to be typical of anthropogenic land-uses, a notable exception to this rule came from two transects which were planted on what was originally open habitat campina formations (rather than humid moist forest). These transects hosted some species regionally restricted to these non-forest enclaves e.g. Ocellated Crake Micropygias chomburgkii, Rusty-backed Antwren Formicivora rufa and Lesser Elaenia Elaenia chiriquensis, which we also encountered at several other non-degraded campina enclaves [38]. Owing to the poor sandy soil at these localities the palms were stunted and some of the original campina physiognomy had been retained between the rows of palms. The inclusion of these transects resulted in an inflation of the total richness in oil palm plantations and we note that campina formations are considered to be “Zonas Ambientalmente Sensíveis” (Environmentally sensitive areas), which may only be used with the adoption of technologies and with an intensity compatible with local environmental conditions [70].
Despite their higher basal area and presence of epiphytes we found older plantations (<15 years) to be even more depauperate than younger plantations, most of the species typical of pastures vanish, with a closed canopy leaving very few species typical of more arborescent (but still edge) habitats, such as Yellow-breasted Flycatcher Tolmomyias flaviventris and Gray-chested Greenlet Hylophilus semicinereus and abundant White-fringed Antwrens and Pale-breasted Thrushes Turdus leucomelas.
Avian biodiversity in the remaining forest matrix
Although we consider oil palm plantations to be very poor habitat for Amazonian forest bird species, we did however find significant avian biodiversity within the remaining skeletal forest matrix of the region. These included species of national and global conservation concern such as Golden Parakeet and Vulturine Parrot. Fieldwork by other ornithologists in the same fragments has even resulted in confirmation of breeding of top predators such as Harpy Eagle Harpia harpyja [71]. However, even the avifauna within these forest fragments represents a shifted avian biodiversity baseline as many forest-dependent species previously recorded by our surveys in high basal area transects in neighbouring Paragominas [53] such as Snethlage's Antpitta Hylopezus paraensis, Opal-crowned Manakin Lepidothix iris iris and Tawny-crowned Greenlet Hylophilus ochraceiceps rubrifrons were unrecorded in Moju-Tailândia both by our surveys and those of Silveira [72]. We infer their local extinction given their historical disappearance from degraded primary forests elsewhere in the region [73] and attribute this loss to habitat modification from selective logging and/or fire which afflicted these forest patches before they became established as legal forest reserves (RLs: Reservas Legais) by the oil palm growers. If connectivity to existing undisturbed forest nuclei can be restored, either by making sure that obligations to maintain APPs are fulfilled or setting aside conservation corridors, then it is possible that some of these disturbance-sensitive taxa may recolonize the forest fragments [74]. However, application of the new Brazilian Forest Code will result in the loss of ≈ 60% of forest vegetation from the APPs in the region [40].
We recorded very few game birds such as tinamous and cracids in the forest fragments and from this infer that these patches are subject to heavy hunting pressure. This assumption is backed up by regular encounters with human hunters and detection of hunter artefacts (trails, shot gun cartridges, elevated hunter ‘perches’). Large-bodied fauna is nominally protected in these areas by vehicle checkpoints and patrols operated by the oil palm companies and these initiatives by the companies are to be applauded and encouraged. However, although these measures may act as a significant deterrent for some hunters, policing such large areas is obviously extremely difficult. This effect of protection was most noticeable for Sporophila seedeaters which were recorded commonly within area managed by oil palm companies but very rarely outside due to widespread trapping of these species for the wild bird trade.
Implications of oil palm expansion for regional biodiversity
In comparison with an exhaustive survey of regional land-uses we do not consider oil palm to be of any greater value to birds than other land-uses in Amazonia such as cattle pasture, soybean and eucalyptus plantations (e.g. [55], [67], [75]). These conclusions are echoed throughout the world in studies of forest monocultures (e.g. [76], [77]). It is possible that oil palm may function as a more permeable matrix for some bird species than other non-forest land-uses such as soy bean plantations and this hypothesis merits testing with dispersal challenge experiments or radio-tagging experiments, although we note that recent research indicates that even secondary forest is an effective barrier to dispersal for many primary forest species [78].
Despite the poor habitat value for Amazonian biodiversity, we recognize that oil palm may be an important alternative for regional development given its positive role in the potential recovery of abandoned areas, income generation and in producing renewable energy [5], [6], [46]. However, sustainable production of palm oil must include solid promises that any expansion growth does not come at the expense of existing forest habitats through direct or indirect deforestation [79] in accord with Ecological-Economic Zoning initiatives [26], [70].There should be ample room for expansion on degraded pastures without putting pressure on existing forests, including secondary forests in early successional stages [80]. For example, data from the TerraClass [81] initiative reveals that there are 9.6 million hectares of abandoned pastures in the Legal Amazon of which 3.3 million are in the state of Pará. In contrast, there is only one strictly protected area in the Belém AE, the beleaguered Reserva Biológica do Gurupi, which protects just 1.4% of the land area in this biogeographic province [82]. As a consequence even small and medium-sized forest remnants in this region have high global conservation value.
Moreover, given the relatively strict Brazilian environmental regulations associated with the Forest Code there is potential to lever oil palm environmental standards globally through inter-continental competition for the ‘green’ oil palm market under the auspices of the RSPO. International pressure to comply with Brazil’s strict environmental minimal standards might benefit Amazonian biodiversity if oil palm companies prove to be better stewards of the remaining forests than other regional actors and participate in schemes to secure the long-term future of existing forests and improve landscape-level connectivity.
Supporting Information
Systematic list (following CBRO 2014) of bird species recorded in the land-uses: PF = primary forest, SF = secondary forest, CP = cattle pasture, OP = oil palm. The status column highlights both their global Red List status following Birdlife International (2014), where VU = Vulnerable and EN = Endangered and their endemicity (following HBW 2015), where END1 = full species endemic to the Belém AE (and adjacent forests of a similar physiognomy in north-east Brazil), END1* = subspecies endemic to the Belém AE and END2 = species endemic to south-east Amazonia, west of the river Madeira and south of the river Amazon. Table B. Top ranked model results from GLMs for the whole avian community and forest birds alone. The explanatory variables include distance to the forest border (Border), tree species richness (Tree richness), forest cover (% of primary forest cover) and biomass of trees (Biomass). For each model R2 is the proportion of variation explained, ΔAICc is the difference between AICc between this and the preceding model and weight is the Akaike weight for the given model. Table C. PERMANOVA Pseudo-F statistic values of the global test and P-value and t values of pair-wise comparison, P-values and mean similarity of bird community composition in different land-use types. Fig. A. Species rarefaction curves per point count considering the entire avian assemblage in land-uses (A) primary forest, (B) secondary forest, (C) cattle pasture & (D) oil palm. Fig. B. Relationship between distance to the nearest primary forest border and richness of forest bird species for nom primary forest transects around Moju (heavy dark border) and Paragominas (narrow dark border). Green circles denote secondary forest transects, orange circles = cattle pasture, grey circles = mechanised agriculture and red circles = oil palm. Oil palm transects have a comparable species richness to other non-forest land-uses. Fig. C. nMDS plot of community structure of the entire avian assemblage in Moju, primary forest transects are represented by dark green squares, secondary forests by light green squares, cattle pastures are yellow circles, the blue star is a natural campina formation, dark red triangles are older oil palm plantations (12–25 years), lighter red triangles are intermediate aged oil palm plantations (3–4 years) and orange triangles are recently-planted oil palm plantations (1–2 years). Polygon size is proportional to species richness.
(DOCX)
Acknowledgments
We are grateful to Nelson Rosa and Carlos Alberto Santos da Silva from the Museu Goeldi for plant identification. We thank the Oil palm producers Biopalma, Petrobras Biofuels (PBIO)-Project Belém and Agropalma (Agreement #01205000245/2013-26) for access to their lands and logistical support. Finally we thank one anonymous reviewer, David Edwards and Tor Haugaasen for comments and suggestions which improved the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
We are grateful to the Instituto Nacional de Ciência e Tecnologia, Biodiversidade e Uso da Terra na Amazônia (CNPq grant 574008/2008-0), for financial support; CNPq for the Science without Borders Program’s Fellowship to ACL and for a postdoctoral fellowship grant to NGM, as well as a CNPq for Research Productivity grant (CNPq 306368/2013-7) to ICGV. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Tilman D, Fargione J, Wolff B, Antonio CD, Dobson A, Howarth R, et al. Forecasting agriculturally driven global environmental change. Science. 2001;292: 281–284. [DOI] [PubMed] [Google Scholar]
- 2. Foley JA, Ramankutty N, Brauman KA, Cassidy ES, Gerber JS, Johnston M, et al. Solutions for a cultivated planet. Nature. 2011;478: 337–342. 10.1038/nature10452 [DOI] [PubMed] [Google Scholar]
- 3. Alexandratos N. World food and agriculture: Outlook for the medium and longer term. Proc. Natl. Acad. Sci. U S A. 1999;96: 5908–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koh LP. Potential habitat and biodiversity losses from intensified biodiesel feedstock production. Conserv Biol. 2007;21: 1373–1375. [DOI] [PubMed] [Google Scholar]
- 5. Koh LP, Ghazoul J. Biofuels, biodiversity, and people: understanding the conflicts and finding opportunities. Biol Conserv. 2008;141: 2450–2460. [Google Scholar]
- 6. Corley RHV. How much palm oil do we need? Environ Sci Policy. 2009;12: 134–139. [Google Scholar]
- 7. Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, et al. Biodiversity—Global biodiversity scenarios for the year 2100. Science. 2000;287: 1770–1774. [DOI] [PubMed] [Google Scholar]
- 8. Sodhi NS, Koh LP, Brook BW, Ng PKL. Southeast Asian biodiversity: an impending disaster. Trends Ecol Evol. 2004;19: 654–660. [DOI] [PubMed] [Google Scholar]
- 9.FAO. FAOSTAT Database Food and Agriculture Organization of the United Nations, Rome, Italy; 2013. Available: http://www.faostat.fao.org. Accessed 2015 Jan 16.
- 10. USDA. Indonesia: Rising Global Demand Fuels Palm Oil Expansion Commodity Inteligence Report. United States Department of Agriculture (USDA); 2010. Available: http://www.pecad.fas.usda.gov/highlights/2010/10/indonesia/. Accessed 2015 Jan 16. [Google Scholar]
- 11. Butler RA, Laurance WF. Is oil palm the next emerging threat to the Amazon? Trop Conserv Sci. 2009;2: 1–10. [Google Scholar]
- 12. Koh LP, Wilcove DS. Oil palm: disinformation enables deforestation. Trends Ecol Evol. 2009;24: 67–68. 10.1016/j.tree.2008.09.006 [DOI] [PubMed] [Google Scholar]
- 13.Basiron Y. Providing correct perspective of oil palm cultivation effects on land use. Presentation of Malaysian Palm Oil Council. ELTI Biofuels Conference 2009. Environmental Leadership and Training Initiative; 2009. Available: http://elti.fesprojects.net/2009AsiaBiofuels/sundram.pdf. Balboa, Panama.
- 14. Fitzherbert EB, Struebig M, Morel A, Danielsen F, Brühl CA, Donald PF, et al. How will oil palm expansion affect biodiversity? Trends Ecol Evol. 2008;23: 538–545. 10.1016/j.tree.2008.06.012 [DOI] [PubMed] [Google Scholar]
- 15. Edwards DP, Larsen TH, Docherty TD, Ansell FA, Hsu WW, Derhé MA, et al. Degraded lands worth protecting: the biological importance of Southeast Asia's repeatedly logged forests. Proc R Soc Lond [Biol]. 2011;278: 82–90. 10.1098/rspb.2010.1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gibbs HK, Johnston M, Foley J, Holloway T, Monfreda C, Ramankutty N, et al. Carbon-payback times for crop-based biofuel expansion in the tropics: the effects of changing yield and technology. Environ Res Lett. 2008;3: 034001. [Google Scholar]
- 17. Danielsen F, Beukema H, Burgess ND, Parish F, Brühl CA, Donald PF, et al. Biofuel plantations on forested lands: double jeopardy for biodiversity and climate. Conserv. Biol. 2009;23: 348–358. 10.1111/j.1523-1739.2008.01096.x [DOI] [PubMed] [Google Scholar]
- 18. Koh LP, Levang P, Ghazoul J. Designer landscapes for sustainable biofuels. Trends Ecol Evol. 2009;24: 431–438. 10.1016/j.tree.2009.03.012 [DOI] [PubMed] [Google Scholar]
- 19. Edwards DP, Fisher B, Wilcove DS. High Conservation Value or high confusion value? Sustainable agriculture and biodiversity conservation in the tropics. Conserv. Lett. 2012;5: 20–27. [Google Scholar]
- 20. Bhagwat SA, Willis KJ. Agroforestry as a solution to the oil-palm debate. Conserv. Biol. 2008;22: 1368–1370. 10.1111/j.1523-1739.2008.01026.x [DOI] [PubMed] [Google Scholar]
- 21. Koh LP. Can oil palm plantations be made more hospitable for forest butterflies and birds? J Appl Ecol. 2008;5: 1002–1009. [Google Scholar]
- 22.RSPO. Roundtable on sustainable palm oil (RSPO): principles and criteria for sustainable palm oil production. Including indicators and guidance; 2007. Available: http://www.rspo.org. Accessed 2013 Feb 12.
- 23.(EPE) Ministério de Minas e Energia and Empresa de Pesquisa Energética. Decadal plan for electrical energy expansion 2006–2015 [In Portuguese]; 2006. Available: http://portal2.tcu.gov.br/portal/pls/portal/docs/2062402.PDF. Rio de Janeiro, Brazil, EPE. Accessed 2013 Feb 12.
- 24. Pousa GP, Santos AL, Suarez PA. History and policy of biodiesel in Brazil. Energy Policy. 2007;35: 5393–5398. [Google Scholar]
- 25. Villela AA, Jaccoud D, Rosa LP, Freitas MV. Status and prospects of oil palm in the Brazilian Amazon. Biomass Bioenerg. 2014;67: 270–278. [Google Scholar]
- 26. Bastos TX, Muller AA, Pacheco NA, Sampaio SMN, Assad ED, Marques AFS. Zoneamento de riscos climáticos para a cultura do dendezeiro no estado do Pará. Rev Bras Agromet. 2001;9: 564–570. [Google Scholar]
- 27.SEMA. Define os critérios para Dispensa de Licenciamento Ambiental (DLA), de obra ou empreendimentos/atividades de baixo potencial poluidor/degradador e dá outras providencias. Secretaria de Estado de Meio Ambiente (SEMA); 2014. Available: http://www.sema.pa.gov.br/2013/03/12/10122/. Accessed 2014 Nov 17.
- 28. Lees AC, Vieira ICG. Oil-palm concerns in Brazilian Amazon. Nature: 2013;497: 188 10.1038/497188b [DOI] [PubMed] [Google Scholar]
- 29. INPE. Monitoramento da Floresta Amazônica Brasileira por Satélite. São José dos. Campos- SP: Projeto Prodes; 2014. Available: http://www.obt.inpe.br/prodes/index.html. Accessed 2014 Nov 12. [Google Scholar]
- 30. Edwards DP, Hodgson JA, Hamer KC, Mitchell SL, Ahmad AH, Cornell SJ, et al. Wildlife-friendly oil palm plantations fail to protect biodiversity effectively. Conserv Lett. 2010;3: 236–242. [Google Scholar]
- 31. Sachs JD, Baillie JEM, Sutherland WJ, Armsworth PR, Ash N, Beddington J, et al. Biodiversity conservation and the millennium development goals. Science. 2009;325: 1502–1503. 10.1126/science.1175035 [DOI] [PubMed] [Google Scholar]
- 32. Haffer J. Speciation in Amazonian forest birds. Science. 1969;165: 131–147. [DOI] [PubMed] [Google Scholar]
- 33. Soares-Filho BS, Nepstad DC, Curran LM, Cerqueira GC, Garcia RA, Ramos CA, et al. Modeling conservation in the Amazon basin. Nature. 2006;440: 520–523. [DOI] [PubMed] [Google Scholar]
- 34. Bird JP, Buchanan GM, Lees AC, Clay RP, Develey PF, Yépez I, et al. Integrating spatially explicit habitat projections into extinction risk assessments: a reassessment of Amazonian avifauna incorporating projected deforestation. Divers Distrib. 2012;18: 273–281. [Google Scholar]
- 35. Moraes BC, Costa JMN, Costa ACL, Costa MH. Variação espacial e temporal da precipitação no estado do Pará. Acta Amaz. 2005;35: 207–214. [Google Scholar]
- 36. Falesi ÍC, Santos WH, Vieira LS. Os Solos da Colônia Agrícola de Tomé-Açu (Boletim Técnico 44). Belém, Brazil: IPEAN; 1964. [Google Scholar]
- 37. Oliveira ECP, Lameira OA, Zoghbi MGB. Identificação da época de coleta do óleo-resina de copaíba (Copaifera spp.) no município de Moju, PA. Rev Bras Plantas Med. 2006;8: 14–23. [Google Scholar]
- 38. Lees AC, Moura NG, Almeida AS, Vieira ICG. Noteworthy ornithological records from the threatened campinas of the lower Tocantins, east Amazonian Brazil. Bull Br Orn Club. 2014;134: 247–258. [Google Scholar]
- 39. Droulers M, Venturieri A, Mourã M, Thalês M, Poccard R. Le palmier à huile: un avenir pour l'Amazonie? Confins. 2010; 10 10.4000/confins.6867 [DOI] [Google Scholar]
- 40. Almeida AS, Vieira ICG. Conflitos no uso da terra em Áreas de Preservação Permanente em um polo de produção de biodiesel no Estado do Pará. Rev Ambient Água. 2014;9: 476–487. [Google Scholar]
- 41. Putz FE, Redford KH. The importance of defining ‘forest’: tropical forest degradation, deforestation, long-term phase shifts, and further transitions. Biotropica. 2010;42: 10–20. [Google Scholar]
- 42. Cohen J. A coefficient of agreement for nominal scales. Edu Psychol Meas. 1960;20: 37–46. [Google Scholar]
- 43. Gardner TA, Ferreira J, Barlow J, Lees AC, Parry L, Vieira ICG, et al. A social and ecological assessment of tropical land uses at multiple scales: the Sustainable Amazon Network. Philos Trans R Soc Lond B Biol Sci. 2013;368: 1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chave J, Andalo C, Brown S, Cairns MA, Chambers JQ, Eamus D, et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia. 2005;45: 87–99. [DOI] [PubMed] [Google Scholar]
- 45. Nelson BW, Mesquita R, Pereira JLG, Souza SGA, Batista GT, Couto LB. Allometric regressions for improved estimate of secondary forest biomass in the central Amazon. Forest Ecol Manag. 1999;117: 149–167. [Google Scholar]
- 46. Corley RHV, Tinker PB. The Oil Palm. Oxford: Blackwell Science Ltd.; 2003. [Google Scholar]
- 47. Henriques LMP, Wunderle JM Jr, Willig MR. Birds of the Tapajós National Forest, Brazilian Amazon: a preliminary assessment. Ornitol Neotrop. 2003;14: 307–338. [Google Scholar]
- 48. Lees AC, Zimmer KJ, Marantz CM, Whittaker A, Davis BJW, Whitney BM. Alta Floresta revisited: an updated review of the avifauna of the most intensively surveyed site in south-central Amazonia. Bull Br Orn Club. 2013;133: 178–239. [Google Scholar]
- 49.CBRO—Comitê Brasileiro de Registros Ornitológicos. Listas das aves do Brasil, 11th Edition; 2014. Available: http://www.cbro.org.br. Accessed 2014 Nov 1.
- 50. R Development Core Team. R: a language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria, ISBN 3-900051-07-0; 2014. Available: http://www.R-project.org/. Accessed 2013 Jan 1. [Google Scholar]
- 51. Burnham KP, Anderson DR. Model selection and multimodel inference. a practical information-theoretic approach New York: Springer; 2003. [Google Scholar]
- 52. Clarke KR, Green RH. Statistical design and analysis for a biological effects study. Mar Ecol Prog Ser. 1988;46: 213–226. [Google Scholar]
- 53. Clarke KR, Gorley R. PRIMER Ver. 6. User Manual/Tutorial, Plymouth, UK: PRIMER-E; 2006. [Google Scholar]
- 54. Lees AC, Moura NG, Silva AS, Aleixo ALP, Barlow J, Berenguer E, et al. Paragominas: a quantitative baseline inventory of an eastern Amazonian avifauna. Rev Bras Orn. 2012;20: 93–118. [Google Scholar]
- 55. Moura NG, Lees AC, Andretti CB, Davis BJW, Solar RC, Aleixo A, et al. Avian biodiversity in multiple-use landscapes of the Brazilian Amazon. Biol Conserv. 2013;167: 339–348. [Google Scholar]
- 56. Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. The global diversity of birds in space and time. Nature. 2012;491: 444–448. 10.1038/nature11631 [DOI] [PubMed] [Google Scholar]
- 57. Monserud RA, Leemans R. Comparing global vegetation maps with the Kappa statistic. Ecol Model. 1992;62: 275–293. [Google Scholar]
- 58.Lees AC. WA1175355, Pteroglossus bitorquatus Vigors, 1826. Wiki Aves—A Enciclopédia das Aves do Brasil; 2013. Available: http://www.wikiaves.com/1175355. Accessed 2014 Nov 1.
- 59.BirdLife International. IUCN Red List of threatened birds; 2014. Available: http://www.birdlife.org/datazone/species Accessed 2014 Sep 1.
- 60. Zimmer KJ, Isler ML. Family Thamnophilidae (Typical antbirds) In: del Hoyo J, Elliott A, Christie DA, editors. Handbook of the birds of the World, Volume 8 Barcelona, Spain: Lynx Edicions; 2003. pp 448–592. [Google Scholar]
- 61. Remsen J. Family Furnariidae (Ovenbirds) In: Del Hoyo J, Elliott A, Christie DA, editors. Handbook of the birds of the World, Volume 8 Barcelona, Spain: Lynx Edicions; 2003. pp 162–357. [Google Scholar]
- 62. Snow DW (2004) Family Pipridae (manakins) In: del Hoyo J, Elliott A, Christie DA, editors. Handbook of the birds of the World, Volume 9 Barcelona, Spain: Lynx Edicions; 2004. pp 110–169. [Google Scholar]
- 63. Peh KSH, Sodhi NS, De Jong J, Sekercioglu CH, Yap CAM, Lim SLH. Conservation value of degraded habitats for forest birds in southern Peninsular Malaysia. Divers Distrib. 2006;12: 572–581. [Google Scholar]
- 64. Aratrakorn S, Thunhikorn S, Donald P. Changes in bird communities following conversion of lowland forest to oil palm and rubber plantations in southern Thailand. Bird Conserv Int. 2006;16: 71–82. [Google Scholar]
- 65.Gilroy JJ, Prescott GW, Cardenas JS, Castañeda PGDP, Sánchez A, Rojas‐Murcia LE, et al. Minimizing the biodiversity impact of Neotropical oil palm development. Glob Change Biol.; 2014. 10.1111/gcb.12696 [DOI] [PubMed] [Google Scholar]
- 66. Edwards DP, Magrach A, Woodcock P, Ji Y, Lim NTL, Edwards FA, et al. Selective-logging and oil palm: multitaxon impacts, biodiversity indicators, and trade-offs for conservation planning. Ecol. Appl. 2014;24: 2029–2049. [PubMed] [Google Scholar]
- 67. Barlow J, Gardner TA, Araujo IS, Ávila-Pires TC, Bonaldo AB, Costa JE, et al. Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc. Natl. Acad. Sci. U S A. 2007;104: 18555–18560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lucey JM, Tawatao N, Senior MJ, Chey VK, Benedick S, Hamer KC, et al. Tropical forest fragments contribute to species richness in adjacent oil palm plantations. Biol Conserv. 2014;169: 268–276. [Google Scholar]
- 69. Foster WA, Snaddon JL, Turner EC, Fayle TM, Cockeril TD. Establishing the evidence base for maintaining biodiversity and ecosystem function in the oil palm landscapes of South East Asia. Proc R Soc Lond [Biol]. 2011;366: 3277–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ferreira LV, Thales MC, Pereira JLG, Fernandes JAM, Furtado C, Chaves PP. Biodiversidade In: Monteiro MA, Menezes CRC, Galvão IMF, organisers. Zoneamento Ecológico-Econômico da Zona Leste e Calha Norte do Estado do Pará: Diagnóstico do Meio Físico-Biótico. Belém: Núcleo de Gerenciamento do Programa Pará Rural; 2010. pp 25–102. [Google Scholar]
- 71.Cerqueira PV. WA646981, Harpia harpyja (Linnaeus, 1758). Wiki Aves—A Enciclopédia das Aves do Brasil; 2012. Available: http://www.wikiaves.com/646981. Accessed 2014 Nov 3.
- 72.Silveira LF. Diversity of birds and monitoring of cynegetic species in the forest reserves of the Agropalma group, in Tailândia municipality, state of Pará. Technical Report to Agropalma, Pará; 2006. Available: http://www.ib.usp.br/~lfsilveira/agropalma2.pdf. Accessed 2014 Nov 10.
- 73. Moura NG, Lees AC, Aleixo A, Barlow J, Dantas SM, Ferreira J. et al. Two hundred years of local avian extinctions in Eastern Amazonia. Conserv. Biol. 2014;28: 1271–1281. 10.1111/cobi.12300 [DOI] [PubMed] [Google Scholar]
- 74. Lees AC, Peres CA. Conservation value of remnant riparian forest corridors of varying quality for Amazonian birds and mammals. Conserv Biol. 2008;22: 439–449. 10.1111/j.1523-1739.2007.00870.x [DOI] [PubMed] [Google Scholar]
- 75. Mahood SP, Lees AC, Peres CA. Amazonian countryside habitats provide limited avian conservation value. Biodivers Conserv. 2011;21: 385–405. [Google Scholar]
- 76. Brockerhoff EG, Jactel H, Parrotta JA, Quine CP, Sayer J. Plantation forests and biodiversity: oxymoron or opportunity? Biodivers Conserv. 2008;17: 925–951. [Google Scholar]
- 77. Gibson L, Lee TM, Koh LP, Brook BW, Gardner TA, Barlow J, et al. Primary forests are irreplaceable for sustaining tropical biodiversity. Nature. 2011;478: 378–381. 10.1038/nature10425 [DOI] [PubMed] [Google Scholar]
- 78. Powell LL, Stouffer PC, Johnson EI. Recovery of understory bird movement across the interface of primary and secondary Amazon rainforest. Auk 2013;130: 459–468. [Google Scholar]
- 79. Strassburg BB, Latawiec AE, Barioni LG, Nobre CA, da Silva VP, Valentim JF, et al. When enough should be enough: Improving the use of current agricultural lands could meet production demands and spare natural habitats in Brazil. Global Environ Chang. 2014;28: 84–97. [Google Scholar]
- 80. Vieira ICG, Gardner TA, Ferreira J, Lees AC, Barlow J. Challenges of governing second-growth forests: a case study from the Brazilian Amazonian state of Pará. Forests. 2014;5: 1737–1752. [Google Scholar]
- 81.PROJETO Terraclass. Mapeamento do uso e da cobertura da terra na Amazônia Legal brasileira; 2012. Available: http://www.inpe.br/cra/projetos_pesquisas/TerraClass_2012_26nov2014.pdf Accessed 2015 Jan 19.
- 82. Silva JMD, Rylands AB, Da Fonseca GAB. The fate of the Amazonian areas of endemism. Conserv Biol. 2005;19: 89–94. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Systematic list (following CBRO 2014) of bird species recorded in the land-uses: PF = primary forest, SF = secondary forest, CP = cattle pasture, OP = oil palm. The status column highlights both their global Red List status following Birdlife International (2014), where VU = Vulnerable and EN = Endangered and their endemicity (following HBW 2015), where END1 = full species endemic to the Belém AE (and adjacent forests of a similar physiognomy in north-east Brazil), END1* = subspecies endemic to the Belém AE and END2 = species endemic to south-east Amazonia, west of the river Madeira and south of the river Amazon. Table B. Top ranked model results from GLMs for the whole avian community and forest birds alone. The explanatory variables include distance to the forest border (Border), tree species richness (Tree richness), forest cover (% of primary forest cover) and biomass of trees (Biomass). For each model R2 is the proportion of variation explained, ΔAICc is the difference between AICc between this and the preceding model and weight is the Akaike weight for the given model. Table C. PERMANOVA Pseudo-F statistic values of the global test and P-value and t values of pair-wise comparison, P-values and mean similarity of bird community composition in different land-use types. Fig. A. Species rarefaction curves per point count considering the entire avian assemblage in land-uses (A) primary forest, (B) secondary forest, (C) cattle pasture & (D) oil palm. Fig. B. Relationship between distance to the nearest primary forest border and richness of forest bird species for nom primary forest transects around Moju (heavy dark border) and Paragominas (narrow dark border). Green circles denote secondary forest transects, orange circles = cattle pasture, grey circles = mechanised agriculture and red circles = oil palm. Oil palm transects have a comparable species richness to other non-forest land-uses. Fig. C. nMDS plot of community structure of the entire avian assemblage in Moju, primary forest transects are represented by dark green squares, secondary forests by light green squares, cattle pastures are yellow circles, the blue star is a natural campina formation, dark red triangles are older oil palm plantations (12–25 years), lighter red triangles are intermediate aged oil palm plantations (3–4 years) and orange triangles are recently-planted oil palm plantations (1–2 years). Polygon size is proportional to species richness.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.