Abstract
Gonadotropin-releasing hormone (GnRH) and activin regulate synthesis of FSH and ultimately fertility. Recent in vivo studies cast SMAD4 and FOXL2 as master transcriptional mediators of activin signaling that act together and independently of GnRH to regulate Fshb gene expression and female fertility. Ovarian hormones regulate GnRH and its receptor (GNRHR) through negative and positive feedback loops. In contrast, the role of ovarian hormones in regulating activin, activin receptors, and components of the activin signaling pathway, including SMAD4 and FOXL2, remains understudied. The widespread distribution of activin and many of its signaling intermediates complicates analysis of the effects of ovarian hormones on their synthesis in gonadotropes, one of five pituitary cell types. We circumvented this complication by using a transgenic model that allows isolation of polyribosomes selectively from gonadotropes of intact females and ovariectomized females treated with or without a GnRH antagonist. This paradigm allows assessment of ovarian hormonal feedback and distinguishes responses that are either independent or dependent on GnRH. Surprisingly, our results indicate that Foxl2 levels in gonadotropes decline significantly in the absence of ovarian input and independently of GnRH. Expression of the genes encoding other members of the activin signaling pathway are unaffected by loss of ovarian hormonal feedback, highlighting their selective effect on Foxl2. Expression of Gnrhr, a known target of FOXL2, also declines upon ovariectomy consistent with reduced expression of Foxl2 and loss of ovarian hormones. In contrast, Fshb mRNA increases dramatically post-ovariectomy due to increased compensatory input from GnRH. Together these data suggest that ovarian hormones regulate expression of Foxl2 thereby expanding the number of genes controlled by the hypothalamic-pituitary-gonadal axis that ultimately dictate reproductive fitness.
Introduction
Reproductive fitness requires tight regulation of the genes encoding LH and FSH. A complex network of signals emanate from the hypothalamic-pituitary-gonadal axis defining a characteristic temporal pattern of synthesis and secretion of LH and FSH that occurs over the course of the estrous cycle in rodents [1–3].
Peaks of LH and FSH that occur during late proestrus trigger ovulation [4]. This combined surge reflects primarily an increase in frequency and amplitude of pulses of GnRH from the hypothalamus [5–7]. Additional contributions from hypothalamic kisspeptin, pituitary adenylate cyclase-activating polypeptide, and pituitary bone morphogenetic proteins, as well as steroid feedback from the gonads, further define the frequency and amplitude of the preovulatory surge of LH and FSH [2, 8, 9].
A second surge of FSH occurs independently of LH during estrus. This secondary surge of FSH is less dependent on pulses of GnRH, which are slower and lower in amplitude during estrus, and instead reflects autocrine/paracrine increases in activin from pituitary gonadotropes and folliculostellate cells accompanied by reciprocal paracrine and endocrine decreases in follistatin from gonadotropes and folliculostellate cells, and inhibin from ovarian granulosa cells [2, 10–12]. The secondary surge of FSH signals the next round of follicular recruitment [10].
Growing evidence indicates that the activin signaling pathway may be the primary trigger that drives the secondary surge of FSH [2, 10–12]. As a member of the TGFβ superfamily, activin acts by binding to serine/threonine receptor kinases located on the plasma membrane of gonadotropes [3, 12, 13]. Activation of receptor kinases leads to phosphorylation of SMAD2/3, which releases from the receptor, associates with SMAD4, and translocates to the nucleus where the complex regulates transcription by binding to SMAD responsive elements [14]. While activin has been reported to regulate expression of both Lhb and Fshb [2], conditional, gonadotrope-specific knockout of SMAD4 led to a unique FSH deficient phenotype with normal levels of serum LH and Lhb mRNA [15]. This result is consistent with the notion that rising levels of activin during estrus may selectively regulate expression of Fshb that underlies the secondary surge of FSH.
The proximal promoter-regulatory region of the Fshb promoter contains conserved and species-specific regulatory elements that bind complexes of SMADs and the forkhead transcription factor FOXL2 [16–22]. These complexes form in response to activation of the activin signaling pathway and in vitro render the Fshb promoter responsive to the members of the TGFβ superfamily [2, 23–25]. Conditional, gonadotrope specific deletion of Foxl2 in mice impairs fertility in both male and female mice due to FSH deficiency caused by diminished transcription of Fshb mRNA [23]. The importance of interactions between SMAD4 and FOXL2 is further underscored upon conditional, gonadotrope specific deletion of both genes in mice. While males remain fertile, females are sterile due to complete absence of FSH and undetectable expression of Fshb mRNA [15].
Expression of Foxl2 is restricted to developing eyelids, ovarian granulosa cells, and pituitary thyrotropes and gonadotropes [26, 27]. Emerging evidence indicates that expression of Foxl2 may be controlled by sex steroids. For example, estrogen up regulates expression of Foxl2 in Southern catfish [28], rainbow trout [29], and the rare minnow, Gobiocyprus rarus [30]. In bovine endometrium, progesterone treatment decreases FOXL2 levels and its levels during the estrous cycle are inversely related to circulating progesterone levels [31]. Together, these studies suggest that the expression of Foxl2 in gonadotropes may be subject to regulation by the hypothalamic-pituitary-gonadal axis (HPG).
Examining gonadotrope gene expression in vivo is challenging given the comingled arrangement of at least five cell types in the pituitary. Gonadotropes make up 5–15% of the cells in the pituitary [32, 33]. Roughly 80% of gonadotropes express both LH and FSH and after castration the number of cells co-expressing both hormones increases to close to 100% [34]. Other specialized cell types include somatotropes, corticotropes, lactotropes, thyrotropes and folliculostellate cells. This cellular complexity complicates study of cell-restricted or ubiquitous transcripts that differentially regulate the glycoprotein hormone encoding genes of the gonadotrope. To circumvent this limitation, we established a new line of mice (Gonadotrope specific RiboTag or GRT) by crossing mice that express CRE recombinase specifically in gonadotropes (LHB-cre) [35] with RiboTag mice [36] thereby allowing gonadotrope specific transcripts to be isolated by immunoprecipitation.
Herein we report the use of GRT mice, in conjunction with an ovariectomy (OVX)/GnRH antagonist paradigm, to interrogate the impact of loss of ovarian function on a battery of genes that define the activin signaling pathway. Our in vivo data suggest that ovarian hormones regulate expression of gonadotrope Foxl2 thereby expanding the number of genes controlled by the HPG axis that ultimately dictate reproductive fitness.
Materials and Methods
Ethics Statement
All animal procedures were approved and carried out with strict accordance to guidelines from the Institutional Animal Care and Use Committee (IACUC, no. 4031) at Washington State University. Surgeries were performed under avertin anesthesia. Ketoprofen was used as the post-operative analgesic and all efforts were made to minimize animal suffering. Animals were euthanized using CO2.
Animals
RiboTag mice (Rpl22 HA/HA) mice were generously provided by Dr. Paul S. Amieux (University of Washington) and have been described previously [36]. RiboTag mice harbor a floxed allele of Rpl22 that encodes a component of the 60S ribosomal subunit. This allele was engineered to express RPL22 with a hemagglutinin (HA) tag upon CRE-mediated recombination. LhbCre (Tg(Lhb-cre)1Sac) mice were kindly provided by Dr. Sally Camper (University of Michigan) and have been previously described [35]. Tg(Lhb-cre)1Sac mice express Cre recombinase in gonadotropes due to Lhb promoter driven expression of Cre recombinase. This was confirmed by crossing Tg(Lhb-cre)1Sac mice with either a RosaGFP cre-reporter line or the Rosa26LacZ cre-reporter line. Both reporter genes were expressed primarily in gonadotropes and minimally in the testes of male mice along with a few cells in the cortex of the brain and the kidney [35]. This minimal expression in other tissues is nugatory due to the use of only the pituitary in the immunoprecipitations. Rpl22 HA/HA mice were mated with Tg(Lhb-cre)1Sac mice to produce GRT mice.
Genotyping was performed on tail sections that were digested overnight in 10mM NaOH and 0.2mM EDTA at 70°C then PCR was performed using this lysate and primers specific for either the tagged HA-RPL22 locus or LhbCre (primer sequences in [35, 36]). Offspring containing both the tagged RPL22HA allele as well as the Lhbcre transgene were used in the HA-RPL22 isolation experiments.
Ovariectomies were performed on 8 to 18 week old mice. Fifteen-21 age matched GRT females were split into sham + vehicle, OVX + vehicle, or OVX + antide groups. Starting on the day of surgeries, female mice were injected with the vehicle propylene glycol or 60 μg Antide in propylene glycol every other day for 10 days at which point pituitaries were harvested and pooled from 5 to 7 animals based on treatment group with each group containing the same number of pituitaries within a given experiment. Pooled samples were divided into aliquots for analysis of total versus immunopreciptated RNA by real time PCR. Each point on the graph represents a pooled sample from a single experiment.
Polyribosome immunoprecipitation
Immunoprecipitations were performed as described in Sanz et al. [36] with minor modifications. All steps were performed on ice. Briefly, magnetic Protein G beads (Dynabeads Protein G; Invitrogen) were conjugated to HA antibody (HA.11 Clone 16B12 monoclonal antibody; Covance) or Myc antibody (9E10; Santa Cruz) by rotation for one hour at room temperature. Beads were washed once in citrate buffer, twice in immunoprecipitation buffer, and then incubated with soluble pituitary extracts, as described below. Pituitaries were removed, pooled by treatment group (5–7 pituitaries per group), homogenized in ice-cold polysome buffer (400μL) [36], and centrifuged at 10,000g for 10 min. An aliquot of supernatant (input) was reserved with the remaining supernatant added to the HA-conjugated beads and incubated by rotation overnight at 4°C. The next day, beads were washed three times in high salt buffer for 5 min. The input fraction (pituitary) and the immunoprecipitated fraction (gonadotrope specific) was treated with 5U DNase (Fermentas) by incubation at 25°C for 15 min and then 37°C for 15 min followed by incubation on ice. Qiagen RLT buffer was then added to the DNase-treated samples and RNA isolated using the RNeasy MinElute Cleanup protocol (Qiagen). All samples were stored at -80°C. RNA was analyzed using either a NanoDrop Spectrophotometer (Thermo Scientific) or a Eukaryote Total RNA Pico Chip on the Agilent 2100 Bioanalyzer (Washington State University Laboratory for Bioanalysis and Biotechnology I).
cDNA synthesis and qPCR
cDNA was synthesized using qScript cDNA SuperMix (Quanta Biosciences) following the manufacturer’s protocol using the input fraction (total pituitary) or the immunoprecipitated fraction (gonadotrope) RNA. The synthesized cDNA was diluted 1:15, and 5 μL was used in each subsequent real time reaction using Fast SYBR Green Master Mix (Applied Biosystems) in a 20 μL final volume reaction on a 7500 Fast real time machine (Applied Biosystems). Cycling conditions were incubation at 95°C for 20 sec followed by 40 cycles of 95°C for 3 sec then 60°C for 30 sec followed by melt curve analysis. Primers were optimized for primer concentration, and primer efficiency was determined. Only primer sets with primer efficiencies between 95% and 105% were used. Primer sequences and concentrations are listed in Table 1. Rpl19 was used as the endogenous control and cDNA from LβT2 cells was used as the calibrator in the 2-ΔΔCt calculation [37]. qPCR for each primer set was performed in duplicate for each sample isolated and the ΔCt mean used in calculations. 2-ΔCt or 2-ΔΔCt values were graphed for experiments performed on different days with the median with interquartile range shown. Although infrequent, amplicon values with high Ct values that exceeded the detectable range as determined by standard curve analysis were excluded. Thus “n” values ranged from 3 to 5 for any given primer set.
Table 1. Transcripts, primer concentrations and primer sequences used in real time analysis.
| Transcript | Concentration | 5'→3' |
|---|---|---|
| Lhb | 300 nM | CACCTTCACCACCAGCATCT |
| GCACAGGAGGCAAAGCA | ||
| Gnrhr | 300 nM | TTGTCTTTGCAGGACCACAGTTAT |
| TGGGTCACACATTGCGAGAA | ||
| Cga | 150 nM | TGCTTCTCCAGGGCATATCC |
| CTTTGGAACCAGCATTGTCTTCT | ||
| Fshb | 300 nM | TGCCGTTTCTGCATAAGCAT |
| TCGTATACCAGCTCCTTGAAGGTA | ||
| Rpl19 | 150 nM | GGAAAAAGAAGGTCTGGTTGGA |
| TGATCTGCTGACGGGAGTTG | ||
| Gh | 150 nM | CCCAGGCTGCTTTCTGCTT |
| AGCAATTCCATGTCGGTTCTCT | ||
| Prl | 300 nM | CCCTGGCTACACCTGAAGACA |
| GACTGCACCAAACTGAGGATCA | ||
| Tshb | 300 nM | CACCATCTGTGCTGGGTATTGT |
| GTGCATATTTGGGAAGAAACAGTTT | ||
| Foxl2 | 150 nM | TCCGGCATCTACCAGTACATCA |
| TATTCTGCCAGCCCTTCTTGTT | ||
| Inhba | 150 nM | AGAAGGCAACCACACGACTTTT |
| CTTGCCAACAGAAATCCTCTCA | ||
| Inhbb | 300 nM | AGACATCGCATCCGCAAAC |
| GATGAAGAACTGTTGCCTGCAA | ||
| Inha | 300 nM | TGCACAGGACCTCTGAACCA |
| GGCACCTGTAGCTGGGAAAA | ||
| Smad2 | 300 nM | TTGTGCAGAGCCCCAACTG |
| CGGCTTCAAAACCCTGGTT | ||
| Smad3 | 300 nM | CTCTCCCCGAATCCGATGT |
| AGGCCGGCTCACAGTAGGT | ||
| Smad4 | 150 nM | AGACTACCCCAGGCAGAGCAT |
| GCTCGGTGAAGGTGAATCTCA | ||
| Smad7 | 300 nM | CCATCAAGGCTTTTGACTATGAGA |
| AAGCTGATCTGCACGGTGAA |
Statistical Analysis
Data from the separate experiments were combined and analyzed with the assumption that there was no significant interaction between the “experiment” and treatment group and no “effect of experiment.” While this approach introduces additional independent variables that can increase biological variability, it allows for a more conservative estimate of statistical significance.
Statistical analysis was performed using one-way parametric ANOVA followed by Tukey’s multiple comparison test (*<0.05; **<0.01; ***<0.001) with GraphPad software.
Results
Gonadotrope specific transcripts are enriched in polyribosomes isolated from GRT mice
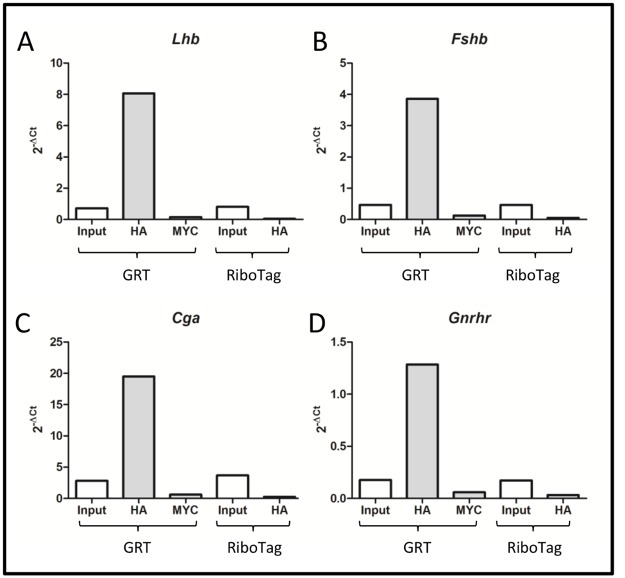
Gonadotrope specific RiboTag (GRT) mice express HA-tagged RPL22 protein exclusively in gonadotropes. To verify isolation of gonadotrope specific transcripts, pituitaries were harvested from GRT mice as well as RiboTag mice, which lack the Lhbcre transgene and serve as a control. Lysates were subject to immunoprecipitation with either HA or Myc antibody. Gonadotrope specific transcripts (Lhb, Fshb, Cga, and Gnrhr) were enriched (11-fold, 8-fold, 7-fold, and 7-fold, respectively) in HA pulldowns from GRT pituitary lysate (Fig 1). The enrichment is specific to the HA tag present on the RPL22 protein as neither immunoprecipitation with Myc antibody from pituitaries of GRT mice nor immunoprecipitation with HA antibody from control Ribotag mice showed enrichment over the input values (Fig 1).
Fig 1. Gonadotrope specific transcripts are enriched in polyribosomes isolated from GRT mice.
Pituitaries were isolated from five GRT or RiboTag female mice and pooled. Isolated RNA was subject to immunoprecipitation with either HA or Myc antibody. Real-time PCR was performed with primers specific to A.) Lhb; B.) Fshb; C.) Cga; and D.) Gnrhr. Rpl19 primers were used for normalization in the calculation of 2-ΔCt values.
GnRH responsive changes in gonadotrope specific mRNAs from HA-tagged polyribosomes mirror that observed in total pituitary RNA
Levels of Lhb, Fshb, and Cga mRNAs in murine pituitaries increase after castration [38] while Gnrhr mRNA levels decrease [39, 40]. Treatment with the GnRH antagonist Antide inhibits the post-castration rise of Lhb, Fshb, and Cga mRNA [41, 42], providing a measure of GnRH responsiveness. To determine whether the amount of polyribosomal associated transcripts follow these patterns, GRT mice were either sham operated or OVX followed by a 10-day treatment with either vehicle or Antide. Pituitaries were then harvested and transcripts isolated from both total pituitary RNA (input fraction) and HA-tagged polyribosomes (gonadotrope specific fraction).
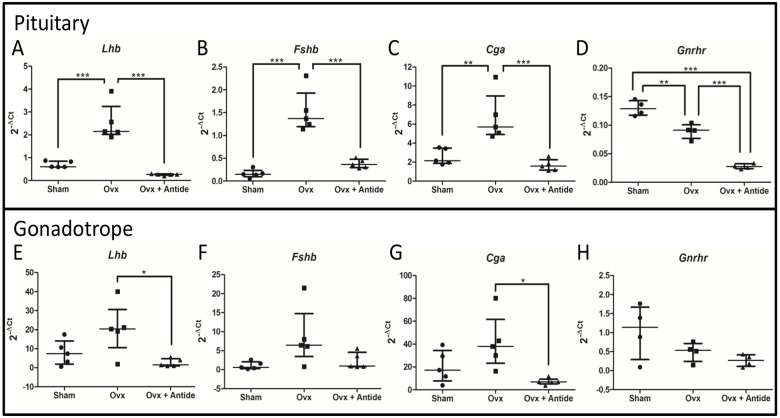
Changes in the levels of three gonadotrope-specific transcripts (Lhb, Fshb, and Cga) in total pituitary RNA mirrored changes of the three mRNAs in HA-tagged polyribosomal mRNA from gonadotropes (Fig 2; panels A-C compared with panels E-G). Gonadotrope levels showed more variance than total pituitary levels, possibly a result of differences in ribosome loading between samples or other technical differences associated with immunoprecipitation of small sample sizes. This variance prevented demonstration of statistical significance for some of the treatment groups. Nevertheless, the trends mirror those seen for total pituitary mRNA.
Fig 2. GnRH responsive transcripts increase after ovariectomy and decrease after Antide treatment.
Gonadotrope specific transcripts were isolated from female mice either sham operated or OVX and then treated with either vehicle or Antide. Real-time PCR 2-ΔCt values for five separate experiments (n = 5) were plotted showing the median with interquartile range of total pituitary levels of A.) Lhb; B.) Fshb; C.) Cga; and D.) Gnrhr and gonadotrope levels of E.) Lhb; F.) Fshb; G.) Cga; and H.) Gnrhr. Statistical differences were determined by a one-way ANOVA followed by Tukey’s multiple comparison test (*<0.05; **<0.01; ***<0.001). Each “n” represents 5–7 pooled pituitaries per treatment group.
As expected, mRNAs for Lhb, Fshb, and Cga increased after OVX, reflecting removal of steroid negative feedback that should be accompanied by a subsequent increase in GnRH secretion. Respective mRNA fold-changes (total versus polyribosomal) were 3.6- vs. 2.6-fold for Lhb, 9.4- vs. 8.0-fold for Fshb, and 2.6 vs. 2.0-fold for Cga. Similar mirrored changes in levels of Lhb, Fshb, Cga mRNAs from OVX + Antide treated mice were also observed when measured in either total RNA or in polyribosomes from gonadotropes. Indeed, Antide almost completely blocks the post-OVX rise in Lhb, Fshb, and Cga, which is expected if their increase is dependent primarily on GnRH.
In contrast to Lhb, Fshb, or Cga mRNA, Gnrhr mRNA exhibited a post-OVX decline that was similar when measured either in total pituitary RNA or in polyribosomes from gonadotropes (approximately 70% vs. 50%, respectively). Treatment of OVX animals with Antide led to an even further significant decrease in Gnrhr mRNA (30%) compared to OVX alone in samples of total pituitary RNA.
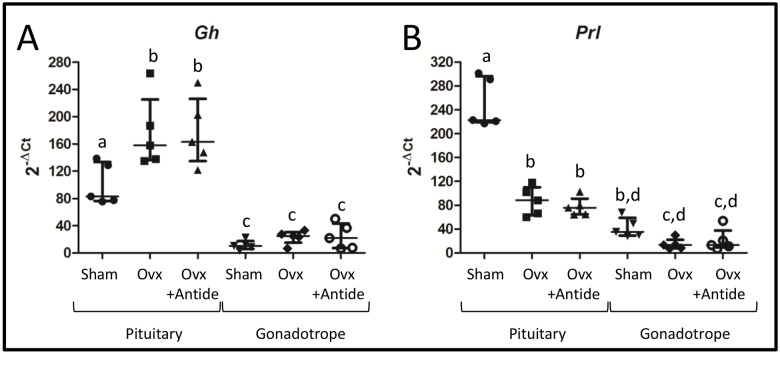
To further verify that HA-tagged polyribosomes are enriched with transcripts from gonadotropes, levels of the somatotrope specific Gh mRNA and the lactotrope specific Prl mRNA were also examined. Gh mRNA exhibits a post-OVX rise whereas Prl mRNA declines (Fig 3). This result is consistent with the known negative and positive effects of gonadal steroids on Gh and Prl mRNAs, respectively [43, 44]. Moreover, both of these non-gonadotrope transcripts were significantly depleted in HA-tagged polyribosomes when compared to their levels in total pituitary RNA (Fig 3). This result is expected if the polyribosomes are derived primarily from gonadotropes. Finally, levels of both mRNAs were refractory to treatment with Antide, which is expected since GnRH receptors are uniquely located in the pituitary on gonadotropes [45].
Fig 3. Gh and Prl mRNAs lack enrichment in HA-tagged polyribosomes.
Real-time PCR was performed on total RNA or HA-tagged polyribosomes with primers specific to A.) Gh and B.) Prl. Values were plotted with the median and interquartile range values indicated for both total pituitary and HA-tagged polyribosomes. Statistical differences (n = 5) were determined by a one-way ANOVA followed by a Tukey’s multiple comparison test. Groups identified with different letters indicate significantly different groups. Each “n” represents 5–7 pooled pituitaries per treatment group.
Activin and inhibin subunit transcript levels in gonadotropes are refractory to regulation by ovarian hormones or GnRH
Fshb expression is induced by activin [46]. Activin A and activin B are homodimers of the inhibin βA (Inhba) or inhibin βB (INHBB) subunits, respectively. Inhibin, an antagonist to activin signaling, is also present in two forms, inhibin A and inhibin B, heterodimers of a common α subunit (INHA) and either INHBA or INHBB subunits, respectively. These factors act as autocrine and paracrine signals in the pituitary [47–49]. Given that activin and inhibin are widely expressed in a variety of tissues including ovary, brain, placenta, kidney, adrenal, and bone marrow [47], GRT mice should be useful for measuring changes in their subunit mRNAs in the gonadotrope using the OVX/Antide paradigm as different responses to OVX have been reported [38, 40].
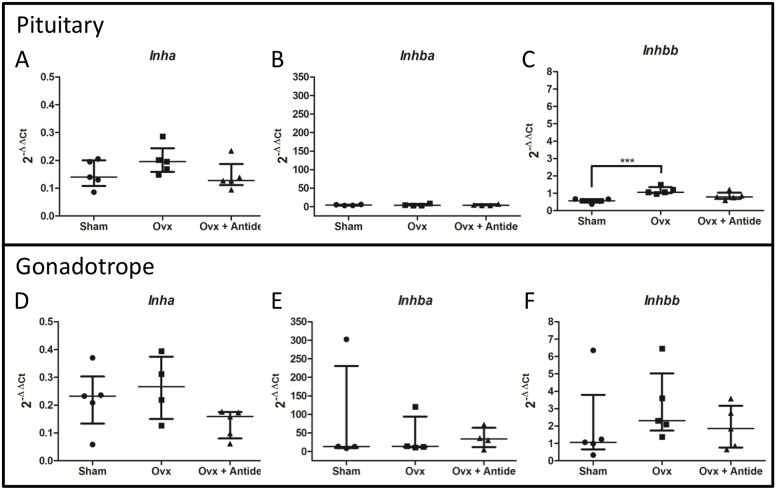
Results from the GRT mice indicate that OVX has a minimal impact on the levels of activin and inhibin subunit mRNAs in total pituitary extracts, with the exception of Inhbb mRNA increasing by two-fold after OVX (Fig 4A, 4B and 4C). Similarly in gonadotropes, levels of these subunit mRNAs appear to be independent of ovarian and GnRH input, although levels are more variable (Fig 4D, 4E and 4F). While comparison of the 2-ΔΔCt values suggests that Inhbb mRNA may be enriched by approximately two-fold in gonadotropes, analysis by the Wilcoxon-Mann-Whitney test indicates that the change is suggestive but inconclusive statistically (p = 0.095). Nevertheless, the putative enrichment of Inhbb mRNA is consistent with previous reports indicating that activin B is expressed at higher levels in gonadotropes compared to other cells of the pituitary [49].
Fig 4. Activin and inhibin subunit transcript levels are refractory to regulation by ovarian hormones or GnRH.
2-ΔΔCt values were calculated using total RNA from LβT2 cells as the calibrator. The median with the interquartile range is indicated for total pituitary levels of A.) Inha; B.) Inhba; and C.) Inhbb and gonadotrope levels of D.) Inha; E.) Inhba; and F.) Inhbb. Statistical differences (n = 5) were determined by a one-way ANOVA followed by Tukey’s multiple comparison test (***<0.001). Each “n” represents 5–7 pooled pituitaries per treatment group.
Together, our results suggest that even though activin and inhibin are important regulators of Fshb expression [2, 3, 12], their subunit mRNA levels in the gonadotrope appear to be independent of the ovary and refractory to the changes in GnRH that occur with the OVX/Antide paradigm.
Attempts were made to measure the level of follistatin, another target of Foxl2 and Smad3 [25] and itself a regulator of activin signaling, yet levels were too low in these samples to be readily detectable (data not shown) as also has been found by others [38].
Smad3 mRNA in gonadotropes may be ovarian dependent and GnRH independent
Activin signals through SMAD proteins and Smad3-deficient male mice have lower levels of Lhb and Fshb [50]. AP1 and SMAD proteins synergize to regulate Fshb [51], offering evidence of the interplay between GnRH and activin signaling in the control of Fshb expression. Like activin and inhibin, SMADs are widely expressed in cells and tissues, thus the GRT mice allow for assessment of effects of the ovarian hormones and GnRH on Smad mRNAs specifically in gonadotropes.
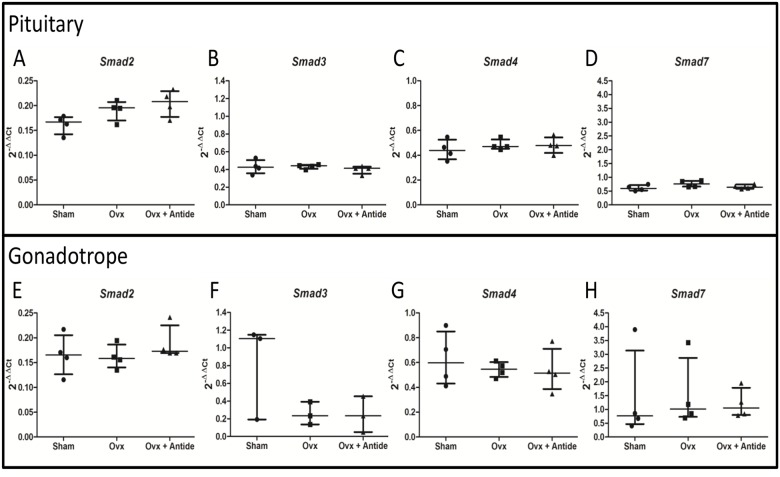
Removal of the ovary with or without Antide treatment has little impact on Smad3, Smad4, or Smad7 when sampled from total pituitary RNA (Fig 5B, 5C and 5D). Although, Smad2 mRNA levels trend higher after OVX with levels that remain unaffected after treatment with Antide (Fig 5A), the change lacks statistical significance. Changes in Smad mRNAs in gonadotropes also lacked statistical significance, although Smad3 mRNA may be dependent on the ovary and independent of GnRH given that one outlier prevented demonstration of statistical significance. The observation that Smad3 mRNA is enriched in the gonadotrope fraction when compared to total pituitary RNA (2-ΔΔCt values differ by a factor of two) further supports the notion that SMAD3 may have a greater role in gonadotropes than in other pituitary cell types.
Fig 5. Smad3 mRNA in gonadotropes may be ovarian dependent and GnRH independent.
2-ΔΔCt values were calculated using total RNA from LβT2 cells as the calibrator and these levels were plotted with the median indicated along with the interquartile range for pituitary A.) Smad2; B.) Smad3; C.) Smad4; and D.) Smad7 and the gonadotrope E.) Smad2; F.) Smad3; G.) Smad4; and H.) Smad7. Statistical differences were determined by a one-way ANOVA followed by Tukey’s multiple comparison test. Here n = 3 or 4, where “n” represents 5–7 pooled pituitaries per treatment group).
Foxl2 levels in gonadotropes are dependent on the ovary
Both gonadotropes and thyrotropes synthesize FOXL2 as evidenced by its immunohistochemical co-localization with both LHβ and TSHβ [25, 52]. Hence, the GRT mouse provides a useful tool for assessing the impact of ovarian hormones and GnRH on the presence of Foxl2 in polyribosomes from gonadotropes.
Foxl2 mRNA levels were enriched approximately five-fold in polyribosomal mRNA samples prepared from gonadotropes versus total pituitary RNA (Fig 6A and 6B). Lack of thyrotrope transcripts was confirmed by the absence of Tshb transcripts in the HA antibody pull-down (data not shown). Ovariectomy reduced Foxl2 mRNA ~42% in total RNA samples and ~76% in polyribosomal RNA from gonadotropes. This differential suggests that the decrease in Foxl2 mRNA occurs to a greater degree in gonadotropes than in thyrotropes. Antide had no impact on Foxl2 levels in either total pituitary RNA or total gonadotrope polyribosomal RNA, suggesting that ovarian hormones play an important role in maintenance of Foxl2 mRNA in gonadotropes. Interestingly, this decrease in Foxl2 levels after OVX appears to be specific to females as Foxl2 mRNA levels in intact males are not significantly different from levels in castrated males treated with vehicle or Antide (S1 Fig).
Fig 6. Foxl2 levels in gonadotropes are dependent on the ovary.
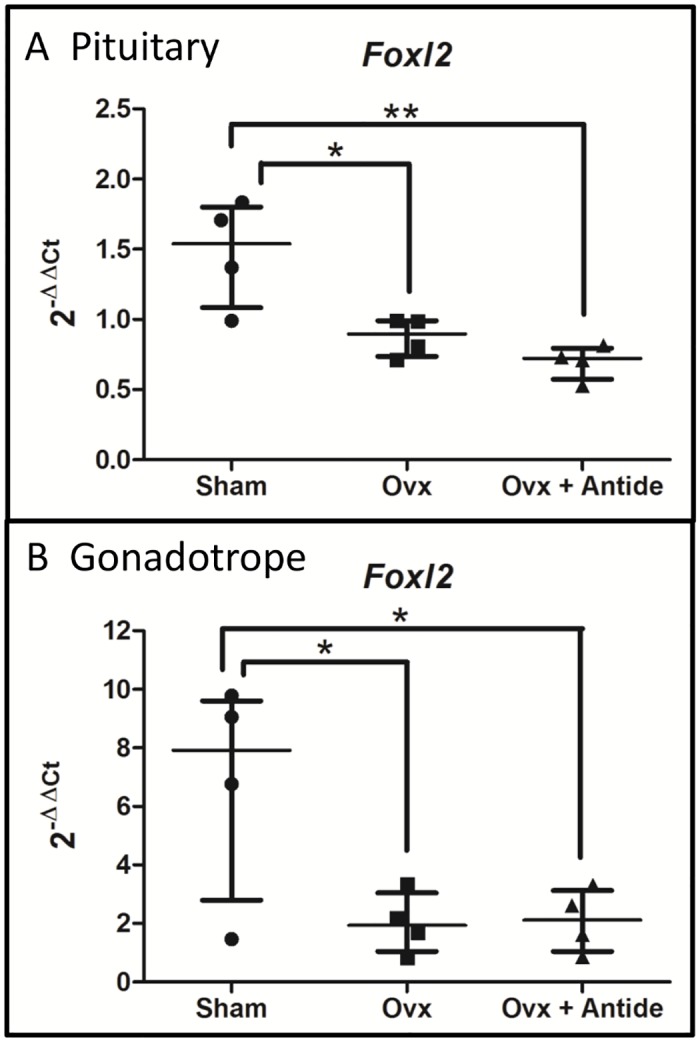
2-ΔΔCt values were calculated using total RNA from LβT2 cells as the calibrator. The median with interquartile range is shown for A.) total pituitary Foxl2 levels and B.) gonadotrope Foxl2 levels. Statistical differences were determined by a one-way ANOVA followed by Tukey’s multiple comparison test (*<0.05; **<0.01). N = 4, where each “n” represents 5–7 pooled pituitaries per treatment group.
Discussion
FOXL2, in partnership with SMAD4, has been characterized recently as an essential master regulator of fertility via its positive effects on Fshb gene expression in vivo [15]. Expression of Fshb in late embryonic gonadotropes also requires FOXL2 [22]. In vitro studies indicate that FOXL2 appears to act as a key transcriptional bridge that underlies the synergistic response of Fshb to GnRH, activin, glucocorticoids, and progestins [2, 22, 24, 53]. Together these studies offer compelling evidence supporting a central role for FOXL2 in regulating expression of Fshb in cycling females. Our current study suggests that levels of Foxl2 mRNA in gonadotropes are regulated by ovarian hormones, thereby placing this transcriptional factor under control of the HPG axis. In this regard, Foxl2 joins the immediate early genes (including Egr1, Jun, and Fos) as transcriptional targets of the HPG axis that play key roles in regulating gonadotrope gene expression.
Increased GnRH compensates for diminished expression of Foxl2 after ovariectomy
Expression of Fshb in gonadotropes increases by approximately eight-fold after OVX (Fig 2). Treatment with Antide almost completely blocks the post-OVX increase in Fshb mRNA. This result is consistent with the known increase in GnRH pulse frequency that occurs after OVX that is due to a loss of negative feedback from gonadal steroids [54, 55]. Although removal of the ovary also reduces serum inhibin, its loss appears to contribute only marginally to the increase in Fshb mRNA given the almost complete block of the post-OVX rise that occurs after Antide treatment.
Activin B has been linked with the rise in FSH secretion after OVX in the rat by use of passive immunoneutralization with monoclonal antibody generated against activin B [56, 57]. Nevertheless, our results suggest that activins produced in gonadotropes are unaffected by OVX as no significant changes occurred in the levels of Inha and Inhba mRNAs (Fig 4D and 4E). Even though OVX led to a modest increase in Inhbb mRNA, the robust reduction in Foxl2 mRNAs in gonadotropes makes it unlikely that changes in activin signaling account for the substantial increase in Fshb mRNA.
Although activin can act in an autocrine manner to induce Fshb in the pituitary [9, 57, 58], other tissue sources of activins and inhibin could also contribute to their serum levels. Yet, despite these possible sources of activins, the mRNA for the downstream signaling factor FOXL2 decreased after OVX and was refractory to changes in GnRH (Fig 6). In fact, Foxl2 expression decreased by almost 50% in total pituitary RNA and by an additional 50% in polyribosomal RNA from gonadotropes. Together, these changes suggest that activin signaling may be less important than GnRH in mediating the post-OVX rise in expression of Fshb. This notion is also consistent with previous findings indicating that the long-term post OVX rise in Fshb is GnRH dependent [59].
Maintenance of Foxl2 and Gnrhr expression requires ovarian hormones
While it is well known that loss of steroid negative feedback increases synthesis and secretion of GnRH [60], the impact of ovarian hormones on Foxl2 expression is less understood. In that regard, we were surprised that OVX led to substantial decreased expression of Foxl2 and that the decline occurred independently of GnRH (Fig 6). The dependence of Foxl2 expression on ovarian hormones suggests that their role may be to ensure that Foxl2 transcripts are sufficiently abundant during late proestrus to support the secondary surge of FSH secretion that occurs during estrus, a period when expression of Fshb is less dependent on GnRH and more dependent on activins.
To date, little is known about the regulation of Foxl2 transcription. The Foxl2 promoter is bidirectional [61] with the LIM homeodomain factors Lhx3 and Lhx4 [52], as well as Foxl2 itself, [62, 63] up-regulating its transcription. Nicotinamide, a SIRT1 inhibitor, has been shown to increase Foxl2 transcript and protein levels in granulosa cells [63]. In our OVX/Antide treatment paradigm, Foxl2 regulation appears to be primarily the result of ovarian factors as blocking GnRH activity with Antide treatment did not relieve the repression seen after OVX. It is also interesting that in males, orchiectomy did not change the level of Foxl2 mRNA (S1 Fig), further supporting the view that estrogens or progestins may be the ovarian hormones that maintain Foxl2 expression during the estrous cycle.
Expression of Gnrhr mRNA, when measured in total RNA, also declined after OVX and fell even further after treatment with Antide, suggesting that levels of the receptor mRNA are dependent on both the ovary and GnRH (Fig 2D). In contrast, when measured in HA-tagged polyribosomes, Gnrhr mRNA levels appeared completely dependent on the ovary and independent of GnRH. This outcome may reflect selective isolation of Gnrhr mRNA from a subpopulation of gonadotropes that only express Lhb mRNA since the LhbCre transgene would be silent in cells that only express Fshb. This possibility is consistent with previous reports indicating that GNRHR levels decrease by 50% after OVX [64] and that treatment of hpg mice with estradiol and exogenous GnRH can restore GNRHR to normal levels [65]. Additionally, treatment of OVX mice with GnRH antiserum led to even further decline in GNRHR [39].
FOXL2 also regulates expression of Gnrhr in αT3 cells, a gonadotrope-specific cell line [27]. Thus, the post-OVX decline in Gnrhr could be due to down regulation of Gnrhr mRNA caused by an increase in GnRH, or loss of a positive contribution from gonadal steroids that regulate FOXL2, or by a combination of changes in both. We favor the latter possibility as treatment of OVX animals with Antide led to an even further significant decrease in Gnrhr mRNA (30%) in samples of total pituitary RNA. This result suggests that levels of Gnrhr mRNA are dependent on gonadal steroids in intact animals and that elevated levels of GnRH that occur after OVX may provide partial compensation rather than down regulate the receptor encoding mRNA. Finally, treatment of OVX mice with estrogen and progesterone partially rescued GNRHR levels even in the presence of GnRH antiserum, a result which is consistent with our evidence that Gnrhr expression is dependent on ovarian steroids [39].
Regulated expression of Foxl2 during the rodent estrous cycle may function as a synergistic rheostat
Several regulatory elements within the proximal promoter of Fshb mediate responsiveness to GnRH and activin [2, 24]. These bind a plethora of transcriptional factors including glucocorticoid receptor, progesterone receptor, AP1, SMAD3, SMAD4, and FOXL2. All of these transcription factors are known to mediate the synergistic interactions between GnRH, activin, gonadal and adrenal steroids that ultimately define the transcriptional tone of Fshb [2, 15, 24].
At least two regulatory elements bind FOXL2 [2, 15, 19, 50]. These FOXL2 elements lie adjacent to or overlap with SMAD responsive elements [22]. Importantly FOXL2 and SMADS interact synergistically upon binding to their cognate response elements on the Fshb promoter [19]. Although GnRH signals primarily through an AP1 response element in the proximal Fshb promoter, recent in vitro studies indicate that cJUN interacts with FOXL2, with both proteins required for GnRH-responsiveness [22, 27]. Together these findings define a central role for FOXL2 as it serves as a bridge that joins both the GnRH and activin pathways that act positively to regulate transcription of Fshb over the estrous cycle.
Given that maximal expression of Foxl2 in gonadotropes requires ovarian hormones, we posit that the concentration of gonadotrope Foxl2 mRNA changes during the course of the rodent estrous cycle. While both estradiol and progesterone have been reported to regulate Foxl2 expression [28–31], we suspect that progesterone plays a predominate role in regulating Foxl2 expression in gonadotropes. Since the secondary surge of FSH is more dependent on activin than GnRH [2, 10–12], we predict that gonadotrope FOXL2 reaches maximal levels during late proestrus, a time when progesterone peaks and estradiol falls. If true, then the synergistic effect of FOXL2 and SMADs on expression of Fshb would be maximal at the time activin signaling becomes the primary driver of the secondary surge of FSH. As progesterone falls during estrus, FOXL2 levels may decline as well. One of the hallmarks of a synergistic interaction is that a small change in concentration of one of the interacting components can lead to a large biological effect. Thus, even modest changes in levels of Foxl2 expression and its cognate protein would be expected to have an impact on expression of Fshb given the known synergism between FOXL2 and members of the SMAD family of DNA-binding proteins [19]. Progesterone receptor also interacts synergistically with the GnRH and activin signaling pathways. Thus, changes in progesterone levels may act as a rheostat that provides a one-two punch that mediates fluctuations in expression of Foxl2 and activity of the progesterone receptor, both of which set the transcriptional tone of Fshb and ultimately fertility in females. Clearly future studies are needed to address the validity of this proposed model. Regardless of whether the model is correct, our collective data indicate that the HPG axis controls expression of Foxl2 in pituitary gonadotropes primarily through positive feedback from ovarian hormones.
Supporting Information
Male mice were either sham operated or orchiectomized and then treated with vehicle or Antide every other day for 10 days. Pituitaries were harvested and gonadotrope specific transcripts isolated. Real time PCR was performed with primers specific to Foxl2 and Rpl19, used for normalization. 2-ΔΔCt values were calculated using total RNA from LβT2 cells as the calibrator. The median with interquartile range is shown for A.) total pituitary Foxl2 levels and B.) gonadotrope Foxl2 levels. Statistical analysis was performed with a one-way ANOVA followed by Tukey’s multiple comparison test (*<0.05). N = 5, where each “n” represents 5–7 pooled pituitaries per treatment group)
(TIF)
Acknowledgments
The authors would like to thank Kelsey Brooks for performing the general animal husbandry and genotyping as well as the OVX procedures and Antide treatments. We would also like to thank the Laboratory for Bioanalysis and Biotechnology I at Washington State University for performing the bioanalyzer analysis and for use of the real time machines.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by a grant from the National Institutes of Health 5R01HD055776 awarded to JHN. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jorgensen JS, Quirk CC, Nilson JH. Multiple and overlapping combinatorial codes orchestrate hormonal responsiveness and dictate cell-specific expression of the genes encoding luteinizing hormone. Endocrine reviews. 2004;25(4):521–42. Epub 2004/08/06. 10.1210/er.2003-0029 . [DOI] [PubMed] [Google Scholar]
- 2. Thackray VG, Mellon PL, Coss D. Hormones in synergy: regulation of the pituitary gonadotropin genes. Molecular and cellular endocrinology. 2010;314(2):192–203. Epub 2009/09/15. 10.1016/j.mce.2009.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bilezikjian LM, Justice NJ, Blackler AN, Wiater E, Vale WW. Cell-type specific modulation of pituitary cells by activin, inhibin and follistatin. Molecular and cellular endocrinology. 2012;359(1–2):43–52. Epub 2012/02/15. 10.1016/j.mce.2012.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hunzicker-Dunn M, Mayo KE. Gonadotropin Signaling in the Ovary, an Update In: Neil JD, editor. The Physiology of Reproduction. 4 ed San Diego, CA: Elsevier; 2014. [Google Scholar]
- 5. Ferris HA, Shupnik MA. Mechanisms for pulsatile regulation of the gonadotropin subunit genes by GNRH1. Biology of reproduction. 2006;74(6):993–8. Epub 2006/02/17. 10.1095/biolreprod.105.049049 . [DOI] [PubMed] [Google Scholar]
- 6. Ciccone NA, Kaiser UB. The biology of gonadotroph regulation. Current opinion in endocrinology, diabetes, and obesity. 2009;16(4):321–7. Epub 2009/06/06. 10.1097/MED.0b013e32832d88fb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dalkin AC, Haisenleder DJ, Ortolano GA, Ellis TR, Marshall JC. The frequency of gonadotropin-releasing-hormone stimulation differentially regulates gonadotropin subunit messenger ribonucleic acid expression. Endocrinology. 1989;125(2):917–24. Epub 1989/08/01. 10.1210/endo-125-2-917 . [DOI] [PubMed] [Google Scholar]
- 8. Bliss SP, Navratil AM, Xie J, Roberson MS. GnRH signaling, the gonadotrope and endocrine control of fertility. Frontiers in neuroendocrinology. 2010;31(3):322–40. Epub 2010/05/11. 10.1016/j.yfrne.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Corrigan AZ, Bilezikjian LM, Carroll RS, Bald LN, Schmelzer CH, Fendly BM, et al. Evidence for an autocrine role of activin B within rat anterior pituitary cultures. Endocrinology. 1991;128(3):1682–4. Epub 1991/03/01. . [DOI] [PubMed] [Google Scholar]
- 10. Besecke LM, Guendner MJ, Sluss PA, Polak GG, Woodruff TK, Jameson JL, et al. Pituitary Follistatin Regulates Activin-Mediated Production of Follicle-Stimulating Hormone during the Rat Estrous Cycle. Endocrinology. 1997;138:2841–8. [DOI] [PubMed] [Google Scholar]
- 11. Haisenleder DJ, Ortolano GA, Jolly D, Dalkin AC, Landefeld TD, Vale WW, et al. Inhibin secretion during the rat estrous cycle: relationships to FSH secretion and FSH beta subunit mRNA concentrations. Life sciences. 1990;47(19):1769–73. Epub 1990/01/01. . [DOI] [PubMed] [Google Scholar]
- 12. Bilezikjian LM, Blount AL, Donaldson CJ, Vale WW. Pituitary actions of ligands of the TGF-beta family: activins and inhibins. Reproduction. 2006;132(2):207–15. Epub 2006/08/04. 10.1530/rep.1.01073 . [DOI] [PubMed] [Google Scholar]
- 13. Bernard DJ, Lee KB, Santos MM. Activin B can signal through both ALK4 and ALK7 in gonadotrope cells. Reproductive biology and endocrinology: RB&E. 2006;4:52 Epub 2006/10/17. 10.1186/1477-7827-4-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annual review of cell and developmental biology. 2005;21:659–93. 10.1146/annurev.cellbio.21.022404.142018 . [DOI] [PubMed] [Google Scholar]
- 15. Fortin J, Boehm U, Deng CX, Treier M, Bernard DJ. Follicle-stimulating hormone synthesis and fertility depend on SMAD4 and FOXL2. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2014;28(8):3396–410. 10.1096/fj.14-249532 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suszko MI, Balkin DM, Chen Y, Woodruff TK. Smad3 mediates activin-induced transcription of follicle-stimulating hormone beta-subunit gene. Molecular endocrinology (Baltimore, Md). 2005;19(7):1849–58. Epub 2005/03/12. 10.1210/me.2004-0475 . [DOI] [PubMed] [Google Scholar]
- 17. Lamba P, Fortin J, Tran S, Wang Y, Bernard DJ. A novel role for the forkhead transcription factor FOXL2 in activin A-regulated follicle-stimulating hormone beta subunit transcription. Molecular endocrinology (Baltimore, Md). 2009;23(7):1001–13. Epub 2009/03/28. 10.1210/me.2008-0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Corpuz PS, Lindaman LL, Mellon PL, Coss D. FoxL2 Is required for activin induction of the mouse and human follicle-stimulating hormone beta-subunit genes. Molecular endocrinology (Baltimore, Md). 2010;24(5):1037–51. Epub 2010/03/18. 10.1210/me.2009-0425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tran S, Lamba P, Wang Y, Bernard DJ. SMADs and FOXL2 synergistically regulate murine FSHbeta transcription via a conserved proximal promoter element. Molecular endocrinology (Baltimore, Md). 2011;25(7):1170–83. Epub 2011/05/31. 10.1210/me.2010-0480 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bernard DJ. Both SMAD2 and SMAD3 mediate activin-stimulated expression of the follicle-stimulating hormone beta subunit in mouse gonadotrope cells. Molecular endocrinology (Baltimore, Md). 2004;18(3):606–23. Epub 2004/01/01. 10.1210/me.2003-0264 . [DOI] [PubMed] [Google Scholar]
- 21. Lamba P, Santos MM, Philips DP, Bernard DJ. Acute regulation of murine follicle-stimulating hormone beta subunit transcription by activin A. Journal of molecular endocrinology. 2006;36(1):201–20. Epub 2006/02/08. 10.1677/jme.1.01961 . [DOI] [PubMed] [Google Scholar]
- 22. Roybal LL, Hambarchyan A, Meadows JD, Barakat NH, Pepa PA, Breen KM, et al. Roles of Binding Elements, FOXL2 Domains, and Interactions With cJUN and SMADs in Regulation of FSHbeta. Molecular endocrinology (Baltimore, Md). 2014;28(10):1640–55. Epub 2014/08/12. 10.1210/me.2014-1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tran S, Zhou X, Lafleur C, Calderon MJ, Ellsworth BS, Kimmins S, et al. Impaired fertility and FSH synthesis in gonadotrope-specific Foxl2 knockout mice. Molecular endocrinology (Baltimore, Md). 2013;27(3):407–21. 10.1210/me.2012-1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coss D, Mellon PL, Thackray VG. A FoxL in the Smad house: activin regulation of FSH. Trends in endocrinology and metabolism: TEM. 2010;21(9):562–8. Epub 2010/07/06. 10.1016/j.tem.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blount AL, Schmidt K, Justice NJ, Vale WW, Fischer WH, Bilezikjian LM. FoxL2 and Smad3 coordinately regulate follistatin gene transcription. The Journal of biological chemistry. 2009;284(12):7631–45. Epub 2008/12/25. 10.1074/jbc.M806676200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cocquet J, Pailhoux E, Jaubert F, Servel N, Xia X, Pannetier M, et al. Evolution and expression of FOXL2. Journal of medical genetics. 2002;39(12):916–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ellsworth BS, Burns AT, Escudero KW, Duval DL, Nelson SE, Clay CM. The gonadotropin releasing hormone (GnRH) receptor activating sequence (GRAS) is a composite regulatory element that interacts with multiple classes of transcription factors including Smads, AP-1 and a forkhead DNA binding protein. Molecular and cellular endocrinology. 2003;206(1–2):93–111. 10.1016/s0303-7207(03)00235-1 [DOI] [PubMed] [Google Scholar]
- 28. Liu Z, Wu F, Jiao B, Zhang X, Hu C, Huang B, et al. Molecular cloning of doublesex and mab-3-related transcription factor 1, forkhead transcription factor gene 2, and two types of cytochrome P450 aromatase in Southern catfish and their possible roles in sex differentiation. The Journal of endocrinology. 2007;194(1):223–41. Epub 2007/06/27. 10.1677/JOE-07-0135 . [DOI] [PubMed] [Google Scholar]
- 29. Baron D, Cocquet J, Xia X, Fellous M, Guiguen Y, Veitia RA. An evolutionary and functional analysis of FoxL2 in rainbow trout gonad differentiation. Journal of molecular endocrinology. 2004;33(3):705–15. Epub 2004/12/14. 10.1677/jme.1.01566 . [DOI] [PubMed] [Google Scholar]
- 30. Jiang W, Yang Y, Zhao D, Liu X, Duan J, Xie S, et al. Effects of sexual steroids on the expression of foxl2 in Gobiocypris rarus. Comparative biochemistry and physiology Part B, Biochemistry & molecular biology. 2011;160(4):187–93. Epub 2011/09/06. 10.1016/j.cbpb.2011.08.005 . [DOI] [PubMed] [Google Scholar]
- 31. Eozenou C, Vitorino Carvalho A, Forde N, Giraud-Delville C, Gall L, Lonergan P, et al. FOXL2 is regulated during the bovine estrous cycle and its expression in the endometrium is independent of conceptus-derived interferon tau. Biology of reproduction. 2012;87(2):32 10.1095/biolreprod.112.101584 . [DOI] [PubMed] [Google Scholar]
- 32. Le Tissier PR, Hodson DJ, Lafont C, Fontanaud P, Schaeffer M, Mollard P. Anterior pituitary cell networks. Frontiers in neuroendocrinology. 2012;33(3):252–66. Epub 2012/09/18. 10.1016/j.yfrne.2012.08.002 . [DOI] [PubMed] [Google Scholar]
- 33. Ibrahim SN, Moussa SM, Childs GV. Morphometric studies of rat anterior pituitary cells after gonadectomy: correlation of changes in gonadotropes with the serum levels of gonadotropins. Endocrinology. 1986;119(2):629–37. Epub 1986/08/01. . [DOI] [PubMed] [Google Scholar]
- 34. Childs GV, Lloyd JM, Unabia G, Gharib SD, Wierman ME, Chin WW. Detection of luteinizing hormone beta messenger ribonucleic acid (RNA) in individual gonadotropes after castration: use of a new in situ hybridization method with a photobiotinylated complementary RNA probe. Molecular endocrinology (Baltimore, Md). 1987;1(12):926–32. Epub 1987/12/01. 10.1210/mend-1-12-926 . [DOI] [PubMed] [Google Scholar]
- 35. Charles MA, Mortensen AH, Potok MA, Camper SA. Pitx2 deletion in pituitary gonadotropes is compatible with gonadal development, puberty, and fertility. Genesis. 2008;46(10):507–14. Epub 2008/09/20. 10.1002/dvg.20398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(33):13939–44. Epub 2009/08/12. 0907143106 [pii] 10.1073/pnas.0907143106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif). 2001;25(4):402–8. Epub 2002/02/16. 10.1006/meth.2001.1262 . [DOI] [PubMed] [Google Scholar]
- 38. Glidewell-Kenney C, Weiss J, Hurley LA, Levine JE, Jameson JL. Estrogen receptor alpha signaling pathways differentially regulate gonadotropin subunit gene expression and serum follicle-stimulating hormone in the female mouse. Endocrinology. 2008;149(8):4168–76. Epub 2008/05/10. 10.1210/en.2007-1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Naik SI, Young LS, Saade G, Kujore A, Charlton HM, Clayton RN. Role of GnRH in the regulation of pituitary GnRH receptors in female mice. Journal of reproduction and fertility. 1985;74(2):605–14. Epub 1985/07/01. . [DOI] [PubMed] [Google Scholar]
- 40. Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Molecular endocrinology (Baltimore, Md). 2003;17(6):1039–53. 10.1210/me.2002-0398 . [DOI] [PubMed] [Google Scholar]
- 41. Keri RA, Wolfe MW, Saunders TL, Anderson I, Kendall SK, Wagner T, et al. The proximal promoter of the bovine luteinizing hormone beta-subunit gene confers gonadotrope-specific expression and regulation by gonadotropin-releasing hormone, testosterone, and 17 beta-estradiol in transgenic mice. Molecular endocrinology (Baltimore, Md). 1994;8(12):1807–16. Epub 1994/12/01. . [DOI] [PubMed] [Google Scholar]
- 42. Fallest PC, Trader GL, Darrow JM, Shupnik MA. Regulation of rat luteinizing hormone beta gene expression in transgenic mice by steroids and a gonadotropin-releasing hormone antagonist. Biology of reproduction. 1995;53(1):103–9. Epub 1995/07/01. . [DOI] [PubMed] [Google Scholar]
- 43. Saade G, London DR, Lalloz MR, Clayton RN. Regulation of LH subunit and prolactin mRNA by gonadal hormones in mice. Journal of molecular endocrinology. 1989;2(3):213–24. Epub 1989/05/01. . [DOI] [PubMed] [Google Scholar]
- 44. Meinhardt UJ, Ho KK. Modulation of growth hormone action by sex steroids. Clinical endocrinology. 2006;65(4):413–22. 10.1111/j.1365-2265.2006.02676.x . [DOI] [PubMed] [Google Scholar]
- 45. Wen S, Schwarz JR, Niculescu D, Dinu C, Bauer CK, Hirdes W, et al. Functional characterization of genetically labeled gonadotropes. Endocrinology. 2008;149(6):2701–11. Epub 2008/03/08. 10.1210/en.2007-1502 . [DOI] [PubMed] [Google Scholar]
- 46. Gore AJ, Philips DP, Miller WL, Bernard DJ. Differential regulation of follicle stimulating hormone by activin A and TGFB1 in murine gonadotropes. Reproductive biology and endocrinology: RB&E. 2005;3:73 Epub 2005/12/31. 10.1186/1477-7827-3-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meunier H, Rivier C, Evans RM, Vale W. Gonadal and extragonadal expression of inhibin alpha, beta A, and beta B subunits in various tissues predicts diverse functions. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(1):247–51. Epub 1988/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bilezikjian LM, Corrigan AZ, Blount AL, Vale WW. Pituitary follistatin and inhibin subunit messenger ribonucleic acid levels are differentially regulated by local and hormonal factors. Endocrinology. 1996;137(10):4277–84. Epub 1996/10/01. 10.1210/endo.137.10.8828487 . [DOI] [PubMed] [Google Scholar]
- 49. Roberts V, Meunier H, Vaughan J, Rivier J, Rivier C, Vale W, et al. Production and regulation of inhibin subunits in pituitary gonadotropes. Endocrinology. 1989;124(1):552–4. Epub 1989/01/01. . [DOI] [PubMed] [Google Scholar]
- 50. Coss D, Thackray VG, Deng CX, Mellon PL. Activin regulates luteinizing hormone beta-subunit gene expression through Smad-binding and homeobox elements. Molecular endocrinology (Baltimore, Md). 2005;19(10):2610–23. Epub 2005/06/18. 10.1210/me.2005-0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang Y, Fortin J, Lamba P, Bonomi M, Persani L, Roberson MS, et al. Activator protein-1 and smad proteins synergistically regulate human follicle-stimulating hormone beta-promoter activity. Endocrinology. 2008;149(11):5577–91. Epub 2008/07/26. 10.1210/en.2008-0220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ellsworth BS, Egashira N, Haller JL, Butts DL, Cocquet J, Clay CM, et al. FOXL2 in the pituitary: molecular, genetic, and developmental analysis. Molecular endocrinology (Baltimore, Md). 2006;20(11):2796–805. Epub 2006/07/15. 10.1210/me.2005-0303 . [DOI] [PubMed] [Google Scholar]
- 53. Ghochani Y, Saini JK, Mellon PL, Thackray VG. FOXL2 is involved in the synergy between activin and progestins on the follicle-stimulating hormone beta-subunit promoter. Endocrinology. 2012;153(4):2023–33. 10.1210/en.2011-1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Elskus AA, Phelps AF, Schwartz NB. Acute sex differences in serum LH levels in gonadectomized rats: investigation of pituitary response to GnRH pulse frequency and prolactin secretion as etiological agents. Neuroendocrinology. 1995;61(3):301–9. . [DOI] [PubMed] [Google Scholar]
- 55. Luderer U, Schwartz NB. Sex differences in acute luteinizing hormone responses to gonadectomy remain after progesterone antagonist and dopamine agonist treatment. Biology of reproduction. 1991;45(6):918–26. . [DOI] [PubMed] [Google Scholar]
- 56. DePaolo LV. Hypersecretion of follicle-stimulating hormone (FSH) after ovariectomy of hypophysectomized, pituitary-grafted rats: implications for local regulatory control of FSH. Endocrinology. 1991;128(4):1731–40. Epub 1991/04/01. . [DOI] [PubMed] [Google Scholar]
- 57. DePaolo LV, Bald LN, Fendly BM. Passive immunoneutralization with a monoclonal antibody reveals a role for endogenous activin-B in mediating FSH hypersecretion during estrus and following ovariectomy of hypophysectomized, pituitary-grafted rats. Endocrinology. 1992;130(3):1741–3. Epub 1992/03/01. . [DOI] [PubMed] [Google Scholar]
- 58. Weiss J, Harris PE, Halvorson LM, Crowley WF Jr, Jameson JL. Dynamic regulation of follicle-stimulating hormone-beta messenger ribonucleic acid levels by activin and gonadotropin-releasing hormone in perifused rat pituitary cells. Endocrinology. 1992;131(3):1403–8. Epub 1992/09/11. . [DOI] [PubMed] [Google Scholar]
- 59. Burger LL, Dalkin AC, Aylor KW, Workman LJ, Haisenleder DJ, Marshall JC. Regulation of gonadotropin subunit transcription after ovariectomy in the rat: measurement of subunit primary transcripts reveals differential roles of GnRH and inhibin. Endocrinology. 2001;142(8):3435–42. [DOI] [PubMed] [Google Scholar]
- 60. Gharib SD, Wierman ME, Shupnik MA, Chin WW. Molecular biology of the pituitary gonadotropins. Endocrine reviews. 1990;11(1):177–99. Epub 1990/02/01. 10.1210/edrv-11-1-177 . [DOI] [PubMed] [Google Scholar]
- 61. Pannetier M, Renault L, Jolivet G, Cotinot C, Pailhoux E. Ovarian-specific expression of a new gene regulated by the goat PIS region and transcribed by a FOXL2 bidirectional promoter. Genomics. 2005;85(6):715–26. Epub 2005/05/12. 10.1016/j.ygeno.2005.02.011 . [DOI] [PubMed] [Google Scholar]
- 62. Moumne L, Dipietromaria A, Batista F, Kocer A, Fellous M, Pailhoux E, et al. Differential aggregation and functional impairment induced by polyalanine expansions in FOXL2, a transcription factor involved in cranio-facial and ovarian development. Human molecular genetics. 2008;17(7):1010–9. Epub 2007/12/26. 10.1093/hmg/ddm373 . [DOI] [PubMed] [Google Scholar]
- 63. Benayoun BA, Georges AB, L'Hote D, Andersson N, Dipietromaria A, Todeschini AL, et al. Transcription factor FOXL2 protects granulosa cells from stress and delays cell cycle: role of its regulation by the SIRT1 deacetylase. Human molecular genetics. 2011;20(9):1673–86. Epub 2011/02/04. 10.1093/hmg/ddr042 . [DOI] [PubMed] [Google Scholar]
- 64. Clayton RN, Detta A, Naik SI, Young LS, Charlton HM. Gonadotrophin releasing hormone receptor regulation in relationship to gonadotrophin secretion. Journal of steroid biochemistry. 1985;23(5B):691–702. Epub 1985/11/01. . [DOI] [PubMed] [Google Scholar]
- 65. Naik SI, Young LS, Charlton HM, Clayton RN. Evidence for a pituitary site of gonadal steroid stimulation of GnRH receptors in female mice. Journal of reproduction and fertility. 1985;74(2):615–24. Epub 1985/07/01. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Male mice were either sham operated or orchiectomized and then treated with vehicle or Antide every other day for 10 days. Pituitaries were harvested and gonadotrope specific transcripts isolated. Real time PCR was performed with primers specific to Foxl2 and Rpl19, used for normalization. 2-ΔΔCt values were calculated using total RNA from LβT2 cells as the calibrator. The median with interquartile range is shown for A.) total pituitary Foxl2 levels and B.) gonadotrope Foxl2 levels. Statistical analysis was performed with a one-way ANOVA followed by Tukey’s multiple comparison test (*<0.05). N = 5, where each “n” represents 5–7 pooled pituitaries per treatment group)
(TIF)
Data Availability Statement
All relevant data are within the paper.