Abstract
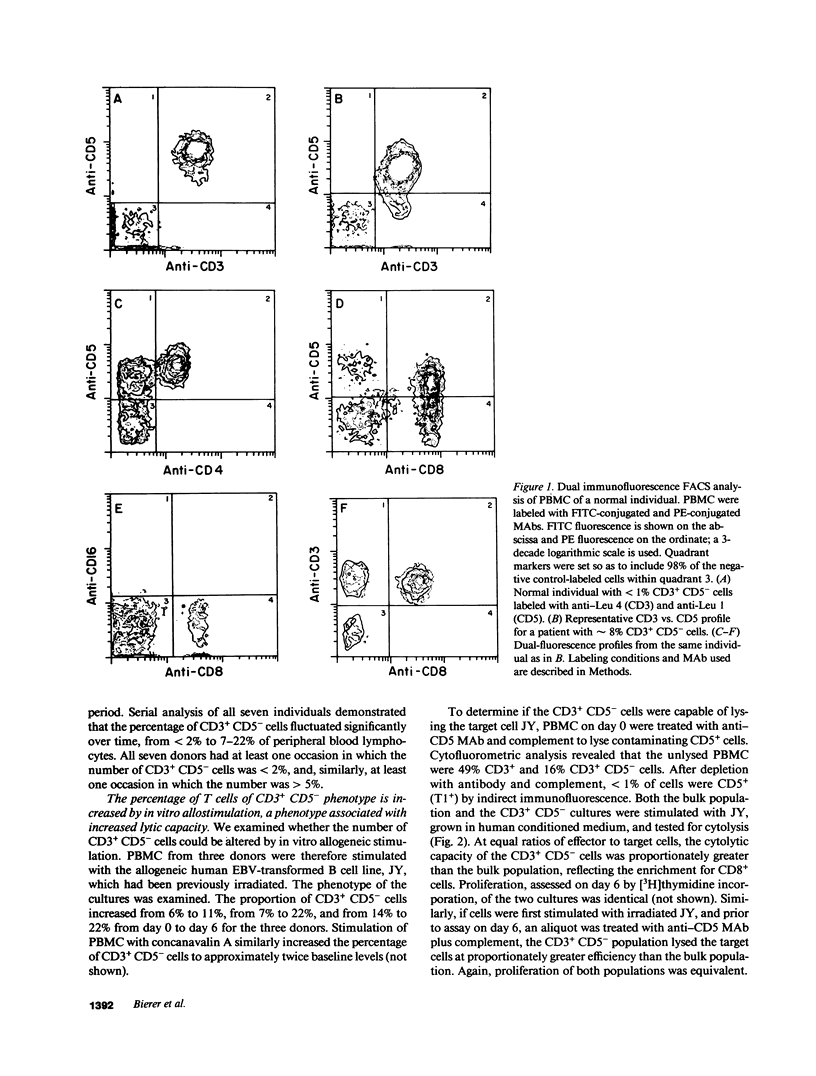
Although the CD5 (T1) antigen was initially described as a pan-T cell membrane glycoprotein, we report that 14 of 40 normal individuals were found to have 5% or greater of their blood mononuclear cells characterized as CD3 (T3)+ but CD5- by dual immunofluorescence flow cytometry. These cells expressed normal quantities of surface CD3 and CD2 but low levels of CD7, were CD8+ and CD4-, and CD16-. In order to determine whether cells of this phenotype were functional, six CD5- cytolytic T lymphocyte (CTL) clones isolated from normal individuals were studied. The CD5- CTL clones all demonstrated normal cytolytic activity against appropriate target cells. Monoclonal antibodies (MAbs) directed against CD3, CD8, CD2, and lymphocyte function-associated antigen 3, but not against CD5, inhibited cytolytic activity. Changes in intracellular calcium [( Ca2+]i) in response to anti-CD5 and anti-CD3 MAbs were measured. Stimulation by anti-CD5 MAb alone did not give rise to a change in [Ca2+]i. However, under conditions of limiting concentrations of anti-CD3 MAb, preincubation of normal CD5+, but not CD5-, clones with anti-CD5 MAb led to a dramatic enhancement in the ability of anti-CD3 MAb to elicit a rise in [Ca2+]i. We conclude that CD5- T lymphocytes represent a normal lymphoid phenotype. Although CD5 may be involved in T cell activation when present, these CD5- CTL clones appear to express normal cytolytic activity.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisenberg A. C., Wilkes B. M., Harris N. L., Ault K. A., Carey R. W. Chronic T-cell lymphocytosis with neutropenia: report of a case studied with monoclonal antibody. Blood. 1981 Oct;58(4):818–822. [PubMed] [Google Scholar]
- Ault K. A., Antin J. H., Ginsburg D., Orkin S. H., Rappeport J. M., Keohan M. L., Martin P., Smith B. R. Phenotype of recovering lymphoid cell populations after marrow transplantation. J Exp Med. 1985 Jun 1;161(6):1483–1502. doi: 10.1084/jem.161.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson R. J., Levitt L. J., Levy R., Miller R. A. Cellular immunoabsorption using monoclonal antibodies. Selective removal of T cells from peripheral blood and bone marrow. Transplantation. 1984 Aug;38(2):136–142. [PubMed] [Google Scholar]
- Boumsell L., Coppin H., Pham D., Raynal B., Lemerle J., Dausset J., Bernard A. An antigen shared by a human T cell subset and B cell chronic lymphocytic leukemic cells. Distribution on normal and malignant lymphoid cells. J Exp Med. 1980 Jul 1;152(1):229–234. doi: 10.1084/jem.152.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceuppens J. L., Baroja M. L. Monoclonal antibodies to the CD5 antigen can provide the necessary second signal for activation of isolated resting T cells by solid-phase-bound OKT3. J Immunol. 1986 Sep 15;137(6):1816–1821. [PubMed] [Google Scholar]
- Dillman R. O., Shawler D. L., Dillman J. B., Royston I. Therapy of chronic lymphocytic leukemia and cutaneous T-cell lymphoma with T101 monoclonal antibody. J Clin Oncol. 1984 Aug;2(8):881–891. doi: 10.1200/JCO.1984.2.8.881. [DOI] [PubMed] [Google Scholar]
- Engleman E. G., Warnke R., Fox R. I., Dilley J., Benike C. J., Levy R. Studies of a human T lymphocyte antigen recognized by a monoclonal antibody. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1791–1795. doi: 10.1073/pnas.78.3.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foon K. A., Schroff R. W., Bunn P. A., Mayer D., Abrams P. G., Fer M., Ochs J., Bottino G. C., Sherwin S. A., Carlo D. J. Effects of monoclonal antibody therapy in patients with chronic lymphocytic leukemia. Blood. 1984 Nov;64(5):1085–1093. [PubMed] [Google Scholar]
- Gorga J. C., Knudsen P. J., Foran J. A., Strominger J. L., Burakoff S. J. Immunochemically purified DR antigens in liposomes stimulate xenogeneic cytolytic T cells in secondary in vitro cultures. Cell Immunol. 1986 Nov;103(1):160–173. doi: 10.1016/0008-8749(86)90077-8. [DOI] [PubMed] [Google Scholar]
- Gorin N. C., Douay L., Laporte J. P., Lopez M., Zittoun R., Rio B., David R., Stachowiak J., Jansen J., Cazellas P. Autologous bone marrow transplantation with marrow decontaminated by immunotoxin T 101 in the treatment of leukemia and lymphoma: first clinical observations. Cancer Treat Rep. 1985 Sep;69(9):953–959. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hanjan S. N., Kearney J. F., Cooper M. D. A monoclonal antibody (MMA) that identifies a differentiation antigen on human myelomonocytic cells. Clin Immunol Immunopathol. 1982 May;23(2):172–188. doi: 10.1016/0090-1229(82)90106-4. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Hemler M. E., Mann D. L., Eisenbarth G. S., Shelhamer J., Mostowski H. S., Thomas C. A., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J Immunol. 1981 Apr;126(4):1409–1414. [PubMed] [Google Scholar]
- Hollander N., Pillemer E., Weissman I. L. Blocking effect of lyt-2 antibodies on T cell functions. J Exp Med. 1980 Sep 1;152(3):674–687. doi: 10.1084/jem.152.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander N., Pillemer E., Weissman I. L. Effects of Lyt antibodies on T-cell functions: augmentation by anti-Lyt-1 as opposed to inhibition by anti-Lyt-2. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1148–1151. doi: 10.1073/pnas.78.2.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Rabinovitch P. S., Martin P. J., Beatty P. G., Hansen J. A. Distinct patterns of transmembrane calcium flux and intracellular calcium mobilization after differentiation antigen cluster 2 (E rosette receptor) or 3 (T3) stimulation of human lymphocytes. J Clin Invest. 1986 Apr;77(4):1224–1232. doi: 10.1172/JCI112425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H., Rabinovitch P. S., Ledbetter J. A. CD5 antibodies increase intracellular ionized calcium concentration in T cells. J Immunol. 1987 May 1;138(9):2782–2792. [PubMed] [Google Scholar]
- Kernan N. A., Collins N. H., Juliano L., Cartagena T., Dupont B., O'Reilly R. J. Clonable T lymphocytes in T cell-depleted bone marrow transplants correlate with development of graft-v-host disease. Blood. 1986 Sep;68(3):770–773. [PubMed] [Google Scholar]
- Krensky A. M., Robbins E., Springer T. A., Burakoff S. J. LFA-1, LFA-2, and LFA-3 antigens are involved in CTL-target conjugation. J Immunol. 1984 May;132(5):2180–2182. [PubMed] [Google Scholar]
- Krensky A. M., Sanchez-Madrid F., Robbins E., Nagy J. A., Springer T. A., Burakoff S. J. The functional significance, distribution, and structure of LFA-1, LFA-2, and LFA-3: cell surface antigens associated with CTL-target interactions. J Immunol. 1983 Aug;131(2):611–616. [PubMed] [Google Scholar]
- Landegren U., Ramstedt U., Axberg I., Ullberg M., Jondal M., Wigzell H. Selective inhibition of human T cell cytotoxicity at levels of target recognition or initiation of lysis by monoclonal OKT3 and Leu-2a antibodies. J Exp Med. 1982 May 1;155(5):1579–1584. doi: 10.1084/jem.155.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., June C. H., Grosmaire L. S., Rabinovitch P. S. Crosslinking of surface antigens causes mobilization of intracellular ionized calcium in T lymphocytes. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1384–1388. doi: 10.1073/pnas.84.5.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., June C. H., Martin P. J., Spooner C. E., Hansen J. A., Meier K. E. Valency of CD3 binding and internalization of the CD3 cell-surface complex control T cell responses to second signals: distinction between effects on protein kinase C, cytoplasmic free calcium, and proliferation. J Immunol. 1986 Jun 1;136(11):3945–3952. [PubMed] [Google Scholar]
- Ledbetter J. A., Martin P. J., Spooner C. E., Wofsy D., Tsu T. T., Beatty P. G., Gladstone P. Antibodies to Tp67 and Tp44 augment and sustain proliferative responses of activated T cells. J Immunol. 1985 Oct;135(4):2331–2336. [PubMed] [Google Scholar]
- Ledbetter J. A., Rouse R. V., Micklem H. S., Herzenberg L. A. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J Exp Med. 1980 Aug 1;152(2):280–295. doi: 10.1084/jem.152.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. J., Hansen J. A., Buckner C. D., Sanders J. E., Deeg H. J., Stewart P., Appelbaum F. R., Clift R., Fefer A., Witherspoon R. P. Effects of in vitro depletion of T cells in HLA-identical allogeneic marrow grafts. Blood. 1985 Sep;66(3):664–672. [PubMed] [Google Scholar]
- Martin P. J., Hansen J. A., Nowinski R. C., Brown M. A. A new human T-cell differentiation antigen: unexpected expression on chronic lymphocytic leukemia cells. Immunogenetics. 1980;11(5):429–439. doi: 10.1007/BF01567812. [DOI] [PubMed] [Google Scholar]
- Mentzer S. J., Smith B. R., Barbosa J. A., Crimmins M. A., Herrmann S. H., Burakoff S. J. CTL adhesion and antigen recognition are discrete steps in the human CTL-target cell interaction. J Immunol. 1987 Mar 1;138(5):1325–1330. [PubMed] [Google Scholar]
- Parham P., Barnstable C. J., Bodmer W. F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol. 1979 Jul;123(1):342–349. [PubMed] [Google Scholar]
- Rabinovitch P. S., June C. H., Grossmann A., Ledbetter J. A. Heterogeneity among T cells in intracellular free calcium responses after mitogen stimulation with PHA or anti-CD3. Simultaneous use of indo-1 and immunofluorescence with flow cytometry. J Immunol. 1986 Aug 1;137(3):952–961. [PubMed] [Google Scholar]
- Racadot E., Hervé P., Beaujean F., Vernant J. P., Flesch M., Plouvier E., Andreu G., Rio B., Philippe N., Souillet G. Prevention of graft-versus-host disease in HLA-matched bone marrow transplantation for malignant diseases: a multicentric study of 62 patients using 3-pan-T monoclonal antibodies and rabbit complement. J Clin Oncol. 1987 Mar;5(3):426–435. doi: 10.1200/JCO.1987.5.3.426. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody with selective reactivity with functionally mature human thymocytes and all peripheral human T cells. J Immunol. 1979 Sep;123(3):1312–1317. [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Reynolds C. W., Foon K. A. T gamma-lymphoproliferative disease and related disorders in humans and experimental animals: a review of the clinical, cellular, and functional characteristics. Blood. 1984 Dec;64(6):1146–1158. [PubMed] [Google Scholar]
- Royston I., Majda J. A., Baird S. M., Meserve B. L., Griffiths J. C. Human T cell antigens defined by monoclonal antibodies: the 65,000-dalton antigen of T cells (T65) is also found on chronic lymphocytic leukemia cells bearing surface immunoglobulin. J Immunol. 1980 Aug;125(2):725–731. [PubMed] [Google Scholar]
- Rozans M. K., Smith B. R., Emerson S., Crimmins M., Laurent G., Reichert T., Burakoff S. J., Miller R. A. Functional assessment of T cell depletion from bone marrow prior to therapeutic transplantation using limiting dilution culture methods. Transplantation. 1986 Oct;42(4):380–387. doi: 10.1097/00007890-198610000-00010. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton T., Stevens T. L., Ledbetter J. A., Wofsy D. Anti-Ly-1 antibody induces interleukin 2 release from T cells. J Immunol. 1986 Mar 1;136(5):1734–1737. [PubMed] [Google Scholar]
- Stong R. C., Uckun F., Youle R. J., Kersey J. H., Vallera D. A. Use of multiple T cell-directed intact ricin immunotoxins for autologous bone marrow transplantation. Blood. 1985 Sep;66(3):627–635. [PubMed] [Google Scholar]
- Swain S. L. Significance of Lyt phenotypes: Lyt2 antibodies block activities of T cells that recognize class 1 major histocompatibility complex antigens regardless of their function. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7101–7105. doi: 10.1073/pnas.78.11.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas Y., Glickman E., DeMartino J., Wang J., Goldstein G., Chess L. Biologic functions of the OKT1 T cell surface antigen. I. The T1 molecule is involved in helper function. J Immunol. 1984 Aug;133(2):724–728. [PubMed] [Google Scholar]
- Tsoukas C. D., Carson D. A., Fong S., Vaughan J. H. Molecular interactions in human T cell-mediated cytotoxicity to EBV II. Monoclonal antibody OKT3 inhibits a post-killer-target recognition/adhesion step. J Immunol. 1982 Oct;129(4):1421–1425. [PubMed] [Google Scholar]
- Wang C. Y., Good R. A., Ammirati P., Dymbort G., Evans R. L. Identification of a p69,71 complex expressed on human T cells sharing determinants with B-type chronic lymphatic leukemic cells. J Exp Med. 1980 Jun 1;151(6):1539–1544. doi: 10.1084/jem.151.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Agthoven A., Terhorst C., Reinherz E., Schlossman S. Characterization of T cell surface glycoproteins T 1 and T 3 present on all human peripheral T lymphocytes and functionally mature thymocytes. Eur J Immunol. 1981 Jan;11(1):18–21. doi: 10.1002/eji.1830110105. [DOI] [PubMed] [Google Scholar]



