Recently Befroy and colleagues measured TCA cycle flux and anaplerosis in human liver by analyzing 13C enrichment in carbon 1 and carbon 5 of glutamate during [1-13C]acetate infusion1. The entry of [1-13C]acetyl-CoA into the cycle yields [5-13C]glutamate on each turn of the cycle but [1-13C]glutamate only on subsequent turns. Anaplerosis, for instance via pyruvate carboxylation, results in the influx of unlabeled oxaloacetate. The subsequent loss of 13C-oxaloacetate from the cycle to make phosphoenolpyruvate (PEP) decreases the enrichment in glutamate-C1 but not glutamate-C5. Hence, the steady-state ratio of [1-13C] to [5-13C]glutamate should reflect anaplerosis relative to TCA cycle activity (i.e. citrate synthase flux = 1). PEP formed from anaplerosis has at least two fates, either in gluconeogenesis (GNG) or to regenerate pyruvate via pyruvate kinase (PK). PK, and other pathways (e.g. malic enzyme), contribute to a futile pyruvate cycle whereby oxaloacetate → pyruvate → oxaloacetate. The relationship among these variables is simple: GNG = anaplerosis – PK flux. This pathway has been included in virtually all models of hepatic GNG2-9. In humans PK flux, relative to citrate synthase (CS), was 1.5 to 5.8 when determined using a [3-14C]lactate tracer with radioisotope detection,8 [3-13C]lactate tracer with mass isotope detection3 or [U-13C]propionate tracer with NMR detection6,9. In fact, pyruvate cycling accounted for the majority of anaplerosis in these human studies3,6,8,9, yet was excluded from anaplerosis by Befroy et al.1 This exclusion complicates the analysis considerably because pyruvate cycling in the presence of H13CO3-, as observed in the in vivo 13C NMR spectra1, regenerates [1-13C]glutamate. Hence, the steady-state ratio of [1-13C] to [5-13C]glutamate no longer reflects total anaplerosis. Given the implications, it is worth examining the effect of pyruvate cycling on 13C in glutamate C1 and C5 using the conditions reported by Befroy et al.
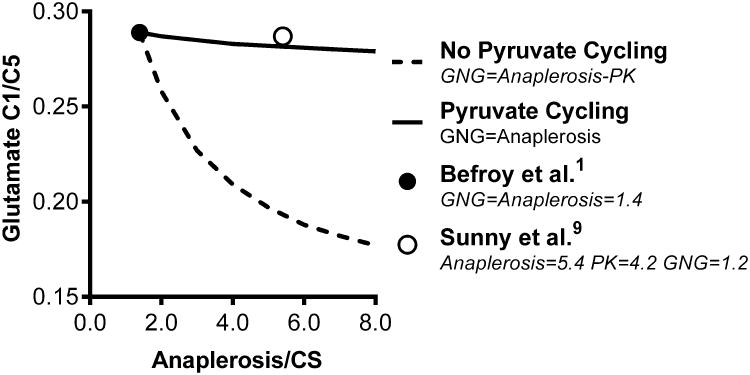
Carbon-13 labeling in glutamate is a complex function of pyruvate cycling, anaplerosis and enrichment in acetyl-CoA and bicarbonate 4,10. Consequently, simulations must be used to explore these interactions10. Using published relationships10 and the conditions reported by Befroy et al., glutamate 13C1/13C5 is sensitive to total anaplerosis if 13CO2 = 0 and pyruvate cycling = 0 but much less sensitive when 13CO2 = 6% 1 and pyruvate cycling is active2-9 (Figure 1). The glutamate 13C1/13C5 ratio reported by Befroy et al. is indeed consistent with substantial pyruvate cycling (Figure 1), as previously described in human liver3,6,8,9. However, the presence of pyruvate cycling also means that a 5-fold range in anaplerosis is essentially undetectable by the glutamate 13C1/13C5 ratio. Thus, this method cannot be used to support the conclusion by Befroy et al., that other methods have overestimated anaplerosis1, since the glutamate 13C1/13C5 ratio cannot detect total anaplerosis under these conditions (Figure 1).
Figure 1.
The effect of pyruvate cycling on the steady-state 13C1/13C5 of glutamate. Data was simulated using program tcaSIM (see source data). All simulations were conducted using an [1-13C]acetyl-CoA fractional enrichment of 0.22 and 13CO2 fractional enrichment of 0.06 as reported by Befroy et al.1 Anaplerosis relative to TCA cycle flux (CS) was varied from 1.4, as reported by Befroy et al.1, to 8. The 13C1/13C5 ratio decreases by 40% across this range under the assumption of no pyruvate cycling (e.g. PK) activity. The 13C1/13C5 ratio is unresponsive to anaplerosis when pyruvate cycling is concomitantly active because the resulting 13C pyruvate condenses with 13CO2 (i.e. PC) and ultimately re-generates [1-13C]glutamate. Most notably, the 13C1/13C5 method used by Befroy et al.1 cannot distinguish low anaplerosis from higher values9 criticized by Befroy et al.1
Futile cycles serve a role in the regulation of metabolism. Earlier studies, including those in humans, found that the futile cycle interconverting pyruvate and oxaloacetate is unquestionably active in liver regardless of whether lactate or propionate tracers are used2-9. Thus, it is best to assume that pyruvate cycling is always a feature of liver metabolism and to incorporate this possibility in data analysis.
Footnotes
Conflict of interests: The Authors declare no competing financial interests.
References
- 1.Befroy DE, et al. Nature medicine. 2014;20:98–102. doi: 10.1038/nm.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen SM. Biochemistry. 1987;26:573–580. doi: 10.1021/bi00376a032. [DOI] [PubMed] [Google Scholar]
- 3.Diraison F, Large V, Maugeais C, Krempf M, Beylot M. The American journal of physiology. 1999;277:E529–536. doi: 10.1152/ajpendo.1999.277.3.E529. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez CA, Des Rosiers C. The Journal of biological chemistry. 1995;270:10037–10042. doi: 10.1074/jbc.270.17.10037. [DOI] [PubMed] [Google Scholar]
- 5.Freidmann B, Goodman EH, Jr, Saunders HL, Kostos V, Weinhouse S. Archives of biochemistry and biophysics. 1971;143:566–578. doi: 10.1016/0003-9861(71)90241-4. [DOI] [PubMed] [Google Scholar]
- 6.Jones JG, Solomon MA, Cole SM, Sherry AD, Malloy CR. American journal of physiology Endocrinology and metabolism. 2001;281:E848–856. doi: 10.1152/ajpendo.2001.281.4.E848. [DOI] [PubMed] [Google Scholar]
- 7.Katz J, Wals P, Lee WN. The Journal of biological chemistry. 1993;268:25509–25521. [PubMed] [Google Scholar]
- 8.Magnusson I, et al. The Journal of biological chemistry. 1991;266:6975–6984. [PubMed] [Google Scholar]
- 9.Sunny NE, Parks EJ, Browning JD, Burgess SC. Cell metabolism. 2011;14:804–810. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeffrey FM, Storey CJ, Sherry AD, Malloy CR. The American journal of physiology. 1996;271:E788–799. doi: 10.1152/ajpendo.1996.271.4.E788. [DOI] [PubMed] [Google Scholar]