SUMMARY
Tia1/Pub1 is a stress granule component carrying a Q/N-rich prion domain. We provide direct evidence that Tia1 forms a prion in yeast. Moreover, Tia1/Pub1 acts co-operatively with release factor Sup35/eRF3 to establish a two-protein self-propagating state. This two-protein prion driven by the Q/N-rich prion domains of Sup35 and Tia1/Pub1 can be visualized as distinctive line structures along tubulin cytoskeleton. Furthermore, we find that tubulin-associated complex containing Pub1 and Sup35 oligomers normally exists in yeast, and its assembly depends on prion domains of Pub1 and Sup35. This Sup35/Pub1 complex, which also contains TUB1 mRNA and components of translation machinery, is important for the integrity of the tubulin cytoskeleton: PUB1 disruption and Sup35 depletion from the complex lead to cytoskeletal defects. We propose that the complex is implicated in protein synthesis at the site of microtubule assembly. Thus our study identifies the role for prion domains in the assembly of multi-protein complexes.
INTRODUCTION
Prions are self-propagating transmissible protein conformations. The original prion hypothesis proposed the existence of a proteinaceous infectious agent that caused lethal neurodegenerative diseases (Prusiner, 2013). Subsequent studies in our laboratory revealed that a neuronal form of the Aplysia CPEB protein, ApCPEB, exhibits prion-like properties when expressed in yeast. ApCPEB also oligomerizes in response to serotonin, a neurotransmitter released by learning in Aplysia, and this oligomerization is critical for the role of CPEB in regulating protein synthesis required for long-term synaptic plasticity (Si et al., 2003; Si et al., 2010). These findings support the idea that the aggregated state of prion-related proteins contributes to the functional state of the brain. Non-disease prions and amyloids for which the self-propagating state is functional and forms in response to cellular signaling have also been discovered in other organisms where they are involved in cell adhesion, heterokaryon incompatibility, melanosome biogenesis and antiviral immunity (Newby and Lindquist, 2013).
Prion concept was also extended to yeast Saccharomyces cerevisiae to explain the nature of [URE3] and [PSI+], two naturally occurring non-Mendelian factors transmitted to mitotic or meiotic progeny with cytoplasm (Wickner et al., 2013). Studies of [PSI+] and [URE3] revealed considerable similarity between genetically unrelated prion proteins. In addition to a conserved functional domain responsible for the protein’s cellular function, they encompass a highly divergent prion domain that is necessary and sufficient for the establishment and maintenance of the prion state. The switch to a prion conformation starts with the formation of small seeds where protein molecules undergo initial conversion into highly structured β-rich SDS-resistant amyloid aggregates. Subsequent propagation involves aggregate growth by the addition of monomers, and breaking of aggregates to create more seeds. Amino acid composition of prion domains facilitates prionization: low complexity and paucity of structure-promoting charged residues keeps them unstructured in the non-prion state, and extreme richness in polar uncharged glutamine and asparagine (Q/N) residues promotes aggregation. Cellular chaperone machinery aids in aggregate fragmentation (Liebman and Chernoff, 2012).
Understanding the common features of prion-forming proteins has allowed to identify several new prions (Wickner et al., 2013) and many more prion candidates (Michelitsch and Weissman, 2000; Derkatch et al., 2001; Alberti et al., 2009). However, it remains unclear why so many proteins maintain prion domains and ability to attain prion conformations. Whereas a gain of function is associated with formation of CPEB prion in brain, most yeast prion phenotypes reflect partial functional inactivation of prion-forming proteins due to aggregation. This has fueled a long-term debate on whether most prions are diseases, egoistic elements or functional components of the cell (True and Lindquist, 2000; Halfmann et al., 2012; Kelly et al., 2012; Newby and Lindquist, 2013; Wickner et al., 2013).
We here elucidate the functional role of prion domains. Based on studies of Tia1/Pub1 and Sup35 proteins we propose that heterologous prion domains interact to create multi-protein self-propagating functional structures that can modulate the location or activity of the prion-forming proteins.
Tia1 and its yeast homolog Pub1 are RNA-binding proteins and components of stress granules implicated in mRNA turnover and regulation of translation (Anderson and Kedersha, 2008; Decker and Parker, 2012). Both proteins carry Q/N-rich domains at their C-termini and were identified as candidate prions based on properties of these domains (Kedersha et al., 1999; Gilks et al., 2004; Alberti et al., 2009). Also, suggesting another functional prion in the brain, Tia1 is critical for normal synaptic plasticity (J.R. and E.R.K, unpublished data).
Sup35 is an eRF3-type translation termination factor and a prion-forming protein for [PSI+] (Inge-Vechtomov et al., 2003; Liebman and Chernoff 2012). The C-terminal functional domain of Sup35, Sup35C, is essential and sufficient for translation termination, and the Q/N-rich prion domain, Sup35NM, is sufficient for the formation of [PSI+] (ibid.).
We chose Sup35 and Tia1/Pub1 to explore functional prion-prion interactions. Indeed, (i) through its prion domain, Sup35 is involved in prion-prion interactions leading to the de novo formation and elimination of [PSI+] and other prions (Derkatch and Liebman, 2007); (ii) Sup35 interactome is enriched with RNA-binding proteins involved in mRNA processing (Inge-Vechtomov et al., 2003; www.yeastgenome.org); (iii) formation of a non-heritable Pub1 amyloid is promoted by [PSI+] (Urakov et al., 2010).
We present evidence that two proteins, Tia1/Pub1 and Sup35/Gspt2, form three distinct homo- and hetero-protein self-propagating conformations. We also isolate a microtubule-associated complex that is assembled through prion domains of Pub1 and Sup35, contains prion-like SDS-resistant oligomers of Sup35, includes components of translational machinery and TUB1 mRNA, and is implicated in maintaining the integrity of the tubulin cytoskeleton.
RESULTS
Tia1 forms prion-like cytoplasmic aggregates in yeast and amyloid fibers in vitro
According to earlier studies, prion domain of Tia1 forms protease-resistant aggregates in cultured mammalian cells, but there is no evidence for prion-like aggregation of full-length Tia1 (Kedersha et al., 1999; Gilks et al., 2004). Thus we first asked if full-length Tia1 attains a self-propagating aggregated state in yeast.
Expression of Tia1-Yfp from a copper-inducible construct induces multiple punctate fluorescent cytoplasmic foci (Fig. 1A). Similar to the de novo appearance of the [PSI+] prion induced by overexpression of Sup35 (Zhou et al., 2001), initial formation of Tia1-Yfp foci is promoted by entrance into late-log / early stationary phase. When Tia1-Yfp expression is started in a freshly diluted culture, intense fluorescence indicates Tia1-Yfp accumulation in most cells by early-log (Fig. S1A), while the proportion of foci-containing cells remains low in early- and midlog, sharply increases in late-log, and reaches 90-95% when cultures enter stationary phase (Fig. 1B). Furthermore, in cultures maintained in early- to mid-log by regular dilutions into fresh medium, the proportion of cells with foci is significantly lower than in continuously growing cultures (Fig. 1C). However, foci are not strictly phase-specific: once formed, they do not disappear during subsequent dilutions (data not shown).
Figure 1. Tia1 forms self-perpetuating SDS-resistant cytoplasmic aggregates in yeast.
(A) Tia1-Yfp forms punctate fluorescent foci. Transformants of [pin−][psi−] 74-D694 carrying pCUPTIA1-YFP (or pCUP-YFP control) were pre-cultured in SD-Ura for 2 days, then diluted to OD600 0.1 into SD-Ura+50 μM CuSO4. Images were taken after 40 hrs of Tia1-Yfp expression.
(B) Formation of Tia1-Yfp foci increases upon entry into late-log and stationary phase. Cultures were grown as in (A).
(C) Preventing the entry into late-log delays Tia1 aggregate formation. Cultures started as in (A) were either grown continuously in SD-Ura+50 μM CuSO4 for 34 hrs, or diluted to OD600 0.1 into SD-Ura+50 μM CuSO4 every 10-13 hrs.
(D) Prion-like nature of Tia1 aggregates. Cells pre-cultured in SD-Ura for 2 days were diluted to OD600 0.1 into either SD-Ura+50 μM CuSO4 (INI) or SD-Ura (NNI). After 2 days all cultures were diluted into SD-Ura and grown for 2 days with regular dilutions into fresh SD-Ura to maintain logarithmic growth; then - diluted into SD-Ura+50 μM CuSO4 where Tia1-Yfp foci were scored at different time points (10 and 20 hr data are shown). See Fig. S2A for Tia1 expression.
(E) Formation of Tia1-Yfp foci is Hsp104-dependent. Growth and imaging (at 50 hrs) were as in (A) but in the isogenic hsp104-Δ strain.
(F) Tia1 forms SDS-resistant aggregates, and pre-induced Tia1 seeds facilitate aggregate formation. INI and NNI cultures were grown as in (D). Proteins were extracted 12 hrs after the start of the final Tia1-Yfp induction. Extracts were incubated in 2% SDS at room temperature (RT) or at 100°C (Boil) and separated by SDS-AGE. Tia1-Yfp was detected with anti-Gfp.
(G) Transmission electron micrographs of negatively stained Tia1 fibers (see Supplemental Experimental Procedures).
(H) Tia1 aggregates co-localize with Dcp2. Growth and imaging (at 30 hrs) were as in (A), but in the isogenic strain where DCP2-YFP replaces the DCP2 ORF, and with the pCUP-TIA1-mCherry plasmid. Arrow and arrowhead point, respectively, at a Dcp2 focus lacking Tia1, and a Tia1 focus lacking Dcp2.
Graphs show averages and standard deviations (AVE +/− SD) based on 5 cultures and 500 cells for each culture for each time point.
See also Fig. S1.
To test if structures visualized as fluorescent foci could self-perpetuate in a prion-like manner, Tia1-Yfp foci were induced in the medium with 50 μM CuSO4; then cultures were diluted into the medium not containing excess CuSO4, and then, after ~20 generations, diluted back into the Tia1-Yfp inducing medium (Fig. 1D). We hypothesized that in the no excess copper medium low-level Tia1-Yfp expression would not induce new Tia1 foci, but would allow for maintaining the existing self-propagating structures. Indeed, earlier appearance and much higher proportion of foci-containing cells was observed in these Induction-No induction-Induction (INI) cultures compared to the control where yeast were passaged in the no excess copper medium prior to the final induction (No induction-No induction-Induction, NNI; Figs. 1D, S1B, S1C). However, even in the INI cultures, foci-containing cells accumulated gradually during the final Tia1-Yfp induction (compare 10 and 20 hrs in Fig. 1D). Thus, while Tia1-Yfp forms self-propagating structures and seeds are retained for many generations, the seeds are not equivalent to visible foci.
Self-perpetuating nature of Tia1 foci is further supported by their dependence on Hsp104, a chaperone essential for the propagation of yeast prions with Q/N-rich prion domains. Tia1 aggregation was essentially abolished in the hsp104-Δ strain (Figs. 1E, S1D).
Another characteristic trait of prions is SDS-resistant oligomers that are hypothesized to be equivalent to prion seeds (Kryndushkin et al., 2003). SDS-resistant oligomers were observed for both Tia1-Yfp used in previous experiments (Fig. 1F) and untagged full-length Tia1, proving that Tia1 aggregation is not due to the Yfp tag (Fig. S1E). Strikingly, presence of the oligomers coincides with the establishment of a self-propagating state. During initial Tia1-Yfp induction, oligomers are not detected until mid- or late-log. But they are seen much earlier in the INI experiment when Tia1-Yfp is re-induced in the seed-containing culture (Fig. 1F; note that cultures in Fig. 1F are in early-log).
In vivo evidence that Tia1 forms amyloid-like structures with an extensive network of intermolecular hydrogen bonds is consistent with the formation of amyloid fibers by purified Tia1 in vitro (Fig. 1G).
As seen from Figure 1H, most Tia1-Yfp fluorescent foci co-localize with Dcp2, a marker for P-bodies and a majority of stress granules, an RNP complex where Tia1 functions (Buchan et al., 2008). However, occasional independent Tia1 and Dcp2 foci are also observed, sometimes in the same cell, and Dcp2 granules are still detected in hsp104-Δ cells where Tia1 foci are absent (Figs. 1H, S1F). Pub1, a yeast homolog of Tia1, is also detected in Tia1-Yfp foci (Fig. S1G).
A new type of self-propagating aggregates forms upon co-expression of Tia1 and Sup35NM
We next asked if Tia1 engages in interaction with Sup35 and its prion form, [PSI+].
In positive prion-prion interactions, pre-existing prions facilitate the de novo appearance of other, heterologous prions (Derkatch and Liebman, 2007). No such interactions were found for Tia1. Tia1 foci formed efficiently in [psi−][pin−] cells (see Fig. 1 where all experiments were performed in [psi−][pin−] strains), and neither the time, nor the rate of foci appearance was increased by the presence of [PSI+] or [PIN+] prions (data not shown). And in the reverse experiment, we could not induce the de novo appearance of [PSI+] in [pin−] cells expressing Tia1 (Fig. 2A; note: [PSI+] usually forms only in the presence of [PIN+], but many Q/N-rich prions and prion-like proteins can substitute for [PIN+]; Derkatch et al., 1997, 2001).
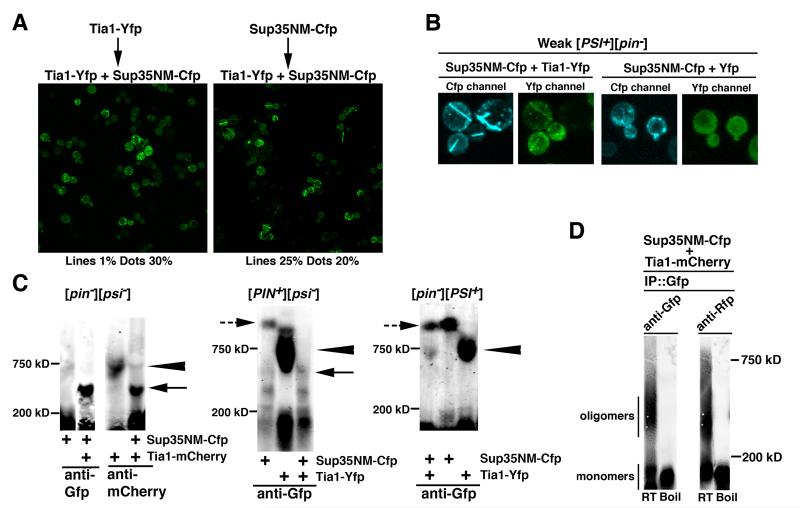
Figure 2. Self-propagating line aggregates form upon co-expression of Tia1 and Sup35NM.
(A) Tia1 aggregates do not seed [PSI+] formation in [pin−] and inhibit [PSI+] induction in [PIN+]. Transformants of [pin−][psi−] and [PIN+][psi−] 74-D694 with plasmids encoding the indicated proteins were grown on solid SD-Ura,Leu+20 μM CuSO4 for ~15 generations and then replica plated to SD-Ade where growth is indicative of the de novo appearance of [PSI+]. Images show growth on SD-Ade after 14 days at 22°C.
(B) Line aggregates in cells co-expressing Tia1 and Sup35NM. Left: co-localization of Tia1-Yfp and Sup35NM-Cfp lines. Right: line aggregates in mother-daughter pairs.
(C) Formation of line aggregates is increased upon entry into late-log and stationary phase.
(D) Prion-like heritability of Tia1-Yfp / Sup35NM-Cfp line aggregates.
(E) Formation of Tia1-Yfp / Sup35NM-Cfp lines depends on Hsp104.
(F) Line formation upon co-expression of full-length Sup35-Cfp and Tia1-Yfp.
In (B, C, D, E and F) experiments were preformed, respectively, as in Figs. 1A, 1B, 1D, 1E and 1A except that cells were co-transformed with pCUP-TIA1-YFP and pCUP-SUP35NM-CFP (B - E), or with pCUP-TIA1-YFP and pCUP-SUP35-CFP (F). Graphs show AVE +/− SD based on 3 patches (A), or 4-5 cultures and ~1000 cells counted for each time point (C-E).
See also Fig. S2. Specifically, see Figs. S2A, S2B for protein levels and growth curves.
In negative prion-prion interactions, prions interfere with each other’s de novo formation or destabilize each other (Derkatch and Liebman, 2007). Indeed, Tia1 inhibited the de novo formation of [PSI+] in a [PIN+][psi−] strain (Fig. 2A). However, no destabilization of pre-existing [PSI+]s was detected (Fig. S2C).
To test if the inhibition of [PSI+] formation by Tia1 was due to an interaction of the Tia1 and Sup35 proteins, we monitored localization of Tia1-Yfp and the Cfp-tagged prion domain of Sup35, Sup35NM-Cfp, co-expressed from the CUP-driven constructs. Two distinct types of fluorescent structures were observed in [psi−][pin−] cells: (i) dot-shaped foci that contained only Tia1-Yfp and were indistinguishable from the foci forming when Tia1-Yfp is expressed alone, and (ii) straight lines that crossed the entire cell and frequently penetrated into daughter cells (Fig. 2B). The line structures incorporated both Tia1-Yfp and Sup35NM-Cfp, but while most of Tia1-Yfp was in the lines, most of Sup35NM-Cfp remained in the cytoplasm. The number of cells with dots and lines was approximately equal, and most cells and cell clusters contained only one type of aggregates. Consistent with the lack of [PSI+] induction in [psi−][pin−] cultures co-expressing Tia1-Yfp and Sup35NM-Cfp (Fig. 2A), straight and rigid line structures had no resemblance to ring-and curve-shaped newly forming [PSI+] aggregates (Zhou et al., 2001).
Similar to Tia1-Yfp foci, formation of Tia1-Yfp/Sup35NM-Cfp lines is facilitated by entrance into late-log (Figs. 2C, S2D), with no strict phase-specificity in subsequent dilutions. Furthermore, according to the INI experiment (see description in Figs. 1D, S1B), propensity to form lines is heritable (Fig. 2D), and gradual appearance of lines during the final induction of Tia1-Yfp/Sup35NM-Cfp co-expression (Fig. 2D) indicates that the self-propagating seeds maintained during the “no induction” stage are distinct from visible line aggregates. Finally, formation of Tia1-Yfp/Sup35NM-Cfp lines is Hsp104-dependent (Figs. 2E, S2E).
Underscoring physiological relevance of the interaction between Tia1 and Sup35NM, full-length Sup35 protein that can function in translation termination also forms lines when co-expressed with Tia1-Yfp (Fig. 2F). Also, the observed aggregation is not due to fluorescent tags: lines form when Tia1-Yfp is co-expressed with untagged Sup35, and Sup35NM-Cfp co-aggregates with Tia1 regardless of what fluorophore is attached to it, YFP or mCherry (Figs. S2F, S2G).
Thus, together Tia1 and Sup35 form a prion-like structure (lines) that is different from self-propagating structures attained by Tia1 alone (dot foci) or Sup35 alone ([PSI+]).
Competitive relationship between Tia1, Sup35 and Tia1/Sup35 self-propagating structures
To further explore the relationship between Tia1, Sup35 and Tia1/Sup35 self-propagating structures, we transformed yeast with plasmids where Tia1-Yfp and Sup35NM-Cfp are controlled by different promoters, CUP or GAL, and expressed first only one of the proteins and then both of them together. Expressing Tia1-Yfp prior to the Tia1-Yfp/Sup35NM-Cfp co-expression strongly inhibited line formation, while the percentage of cells with dot foci was slightly increased (Fig. 3A left). Thus, (i) self-propagating seeds that form Tia1 dots do not promote line formation, i.e. the seeds for Tia1 and Tia1/Sup35 aggregates are different; (ii) there is no evidence for the conversion of dots into lines; (iii) inhibition of line formation is likely due to sequestration of freshly synthesized Tia1 into the already formed Tia1 dots. In a reverse experiment, when promoters were swapped, pre-expression of Sup35NM-Cfp shifted the balance toward line aggregates (Fig. 3A right). But, expectedly, the change was not as dramatic as the inhibition of line formation above: in [psi−][pin−] cells used for this experiment, Sup35NM alone cannot form a self-propagating structure that would later sequester most of newly synthesized Sup35NM.
Figure 3. Tia1, Tia1 / Sup35 and Sup35 self-propagating structures have distinct seeds and SDS-resistant oligomers.
(A) Tia1 dots compete with Tia1 / Sup35 lines. Transformants of [pin−][psi−] 74-D694 with either pCUP-TIA1-YFP and pGAL-SUP35NM-CFP (left) or pGAL-TIA1-YFP and pCUP-SUP35NM-CFP (right) were pre-cultured in SD-Ura,Leu for 2 days and diluted to OD600 0.1 into SD-Ura,Leu+50 μM CuSO4, to induce the CUP-driven constructs. After 20 hrs cells were washed and diluted to OD600 0.5 into SGalSR-Ura,Leu+50 μM CuSO4, to induce both CUP- and GAL-driven constructs. In this medium cells with dot and line aggregates were counted after 10 and 30 hrs (30 hr data are shown; 5 cultures were analyzed for each plasmid combination).
(B) Atypical Tia1 / Sup35NM lines form in [PSI+] cells. A weak [PSI+][pin−] strain co-expressing Sup35NM-Cfp and Tia1-Yfp (or Yfp control) was grown as in Fig. 1A. Images were taken after 20 hrs of induction. Similar data for strong [PSI+] not shown.
(C) Distinct SDS-resistant oligomers for Tia1 / Sup35 lines, Tia1 dots and [PSI+]. Proteins were extracted from the indicated strains 36 hrs after the induction of protein expression and separated by SDS-AGE. “+” indicates expressed proteins. Antibodies used for detection are listed under the panels. Arrow, arrowhead and dashed arrow show, respectively, oligomers corresponding to Tia1 / Sup35 lines, Tia1 dots and [PSI+]. Left panel: all lanes are from the same gel, control lanes are not shown. Middle and right panels are from the same gel; see Fig. S3B.
(D) Co-immunoprecipitation (co-IP) of SDS-resistant aggregates of Tia1-mCherry and Sup35NMCfp in line-containing cells. Experiment was performed as in (C, left panel) except that IP with anti-Gfp preceded SDS-AGE analysis. RT and Boil – see Fig. 1F legend.
See also Fig. S3.
Formation of Tia1/Sup35 self-propagating structures also interferes with the induction of the de novo appearance of the Sup35-based [PSI+] prion in [psi−][PIN+] cells (Fig. 2A). Accordingly, in microscopy, the co-expressing cells accumulated Tia1 dots and Tia1/Sup35 lines, whereas Sup35NM rings characteristic of the de novo formation of [PSI+] were rare (data not shown).
Finally, in [PSI+] cells, the Sup35-based prion interfered with the formation of typical Tia1-Yfp/Sup35NM-Cfp lines. While short lines appeared in ~70% co-expressing cells in early-log, frequently along the dot-shaped [PSI+] aggregates, most of these lines remained short, not as straight, and containing much more Sup35NM-Cfp than Tia1-Yfp, compared to lines in [psi−] cells (Fig. 3B).
The three self-propagating structures formed by Tia1 or Sup35 alone, and jointly by Tia1 and Sup35 are characterized by distinct SDS-resistant oligomers
To test if Tia1/Sup35 lines indeed manifest a distinct self-propagating structure, we analyzed SDS-resistant oligomers formed by Tia1, Tia1/Sup35 and Sup35.
The left panel of Figure 3C shows data for [pin−][psi−] cells either co-expressing Tia1-mCherry and Sup35NM-Cfp (Tia1 dots and Tia1/Sup35NM lines are present), or expressing each protein alone (Tia1 forms dots, and Sup35NM does not form aggregates). For Tia1-mCherry, two types of oligomers are detected in the co-expressing cultures. Abundant smaller-size oligomers are specific for these cultures. Less abundant larger oligomers are also present, and abundant, when Tia1-mCherry is expressed alone. The only type of oligomers detected for Sup35NM-Cfp in co-expressing cultures overlaps in size with the smaller species of Tia1 oligomers, and, as expected, no SDS-resistant oligomers are formed by Sup35NM-Cfp expressed alone. When SDS-AGE analysis of co-expressing cultures was preceded by immunoprecipitation (IP) with the Sup35NM-Cfp-specific antibody, both Sup35NM-Cfp and the small subtype of Tia1-mCherry oligomers were detected in the IPs (Figs. 3D, S3C, S3D). These data indicate that lines are formed by Tia1/Sup35NM co-aggregates, in which Tia1-containing SDS-resistant oligomers are distinct from oligomers formed by Tia1 alone.
According to analysis of [PIN+][psi−] and [pin−][PSI+] cultures, Tia1/Sup35NM oligomers are also distinct from oligomers formed by Sup35 alone (Figs. 3C center and right; S3B). In [PIN+][psi-], Sup35NM-Cfp expressed alone forms large oligomers, similar in size to [PSI+]. In co-expression cultures these large oligomers are hardly detectable (consistent with reduced [PSI+] formation in Fig. 2A), and the prevailing species is similar in size to Tia1/Sup35 oligomers in [pin−][psi−]. In [pin-][PSI+] cultures, Tia1-Yfp and Sup35NM-Cfp co-expression leads to the accumulation of Tia1-size oligomers, but smaller Tia1/Sup35-size oligomers are not detected (consistent with the lack of typical lines in Fig. 3B).
Interaction between Sup35 and Tia1 is specific and evolutionarily conserved
We next tested if Pub1, the yeast Tia1 homolog, forms prion-like aggregates alone and jointly with Sup35. Indeed, Pub1-Yfp expressed from a CUP-driven construct coalesced into small fluorescent foci, and co-expression of Pub1-Yfp and Sup35NM-Cfp also promoted line formation (Figs. 4A, S4A). SDS-resistant Pub1-Yfp oligomers were detected in cultures expressing Pub1 either alone or together with Sup35NM. In the co-expressing cultures, Pub1 and Sup35NM oligomers were of the same size. Oligomers formed by Pub1 alone were smaller (Figs. 4B, S4B, S4C). Overall, Pub1 aggregation properties are very similar to those described above for Tia1, except that Pub1 lines and dots are thinner / smaller and not as bright because a higher proportion of Pub1 remains in the cytoplasm. Possibly, being an endogenous protein, Pub1 has more interacting partners that prevent its sequestration into the aggregates.
Figure 4. Evolutionary conservation and specificity of Tia1 / Sup35 interaction.
(A) Pub1-Yfp forms dot aggregates when overexpressed alone, and dot and line aggregates when co-expressed with Sup35NM.
(B) Different species of SDS-resistant Pub1 oligomers in cells expressing Pub1 alone or co-expressing Pub1 and Sup35NM. SDS-AGE was performed as in Fig. 3C. Arrowhead and arrow indicate, respectively Pub1 and Pub1 / Sup35 oligomers. RT and Boil – see Fig. 1F legend.
(C) Co-expression of Tia1 and Gspt2 leads to the formation of straight (left) and imperfect (middle) lines, and of clumpy dot-shaped structures (right).
(D) Substituting the prion domain of Ure2 for Sup35N blocks interaction of Sup35 and Tia1 that led to line formation.
For (A) – (D): cultures of [psi−][pin−] 74-D694 transformants expressing the indicated proteins were grown as in Figs. 1A and 2B. Protein extractions and imaging were done in late-log.
See also Fig. S4.
We then asked if the ability to interact with Tia1 is retained by mouse Sup35 homologs, Gspt1 and Gspt2. Gspt1, apparently a major translation release factor, is expressed in most tissues in a proliferation-dependent manner. Gspt2 is brain-specific and does not respond to growth stimulation (Hoshino et al., 1998). Paradoxically, only Gspt2 can restore the viability of sup35-Δ yeast (Le Goff et al., 2002). Strikingly, we also detected frequently co-localizing aggregates only for the Tia1 and Gspt2 co-expression pair. Lines formed by Gspt2 and Tia1 were either straight, like those formed by Sup35/Tia1, or wiggly, sometimes with several lines originating from the same point. While Gspt2 was evenly distributed along these lines, Tia1 distribution resembled beads on the string (Figs. 4C, S4D). Tia1 and Gspt2 also formed clumpy dots that frequently co-localized (Figs. 4C, S4D). Co-expression of Tia1 and Gspt1 induced only dot-like foci, which rarely co-localized (data not shown).
Specificity of Tia1/Sup35 interaction was probed in experiments where proteins with other aggregation-prone Q/N-rich domains were substituted for either Tia1 or Sup35NM. Swapping Sup35N for aa 1–65 of Ure2, a prion-forming domain for the [URE3] prion, or for the first exon of huntingtin with polyQ stretches of different length, blocked line formation, while Tia1 dots accumulated as in cultures expressing Tia1 alone (Figs. 4D, S4E). Reciprocally, there were no lines in cells co-expressing Sup35NM with Rnq1, Ure2, Swi1, Cyc8, Cpeb3 or several candidate prions identified in Derkatch et al. (2001): we either detected no Sup35 aggregates, or only rings and dots indicative of the de novo formation of [PSI+] (data not shown).
Importance of prion domains of Tia1 and Sup35, and specifically of the Sup35M KE-rich stretches, for the formation of line aggregates
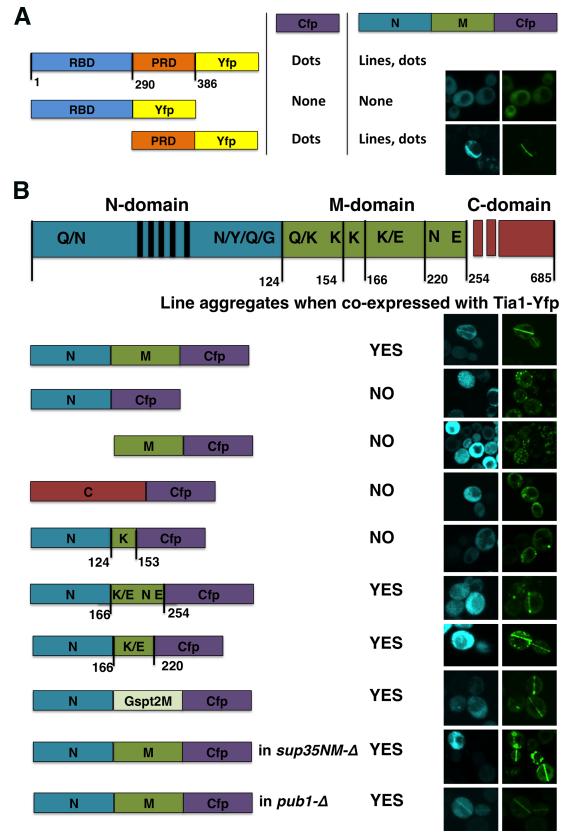
Consistent with most known Q/N-rich prion-forming proteins being modular, with Q/N-rich prion domains necessary and sufficient for the establishment and maintenance of the prion state, the Q/N-rich C-terminal domain of Tia1 (PRD) is sufficient for the formation of both Tia1 dots and Tia1/Sup35 lines (Fig. 5A). The Tia1 RNA-binding domain (RBD) alone forms neither punctate dots nor lines (ibid.).
Figure 5. Importance of prion domains of Tia1 and Sup35 for line formation.
(A) The Q/N-rich domain of Tia1 is required and sufficient for the formation of Tia dots and Tia1 / Sup35 lines, Numbers on the scheme indicate the limits of Tia1 RBD (aa 1-289) and PRD (aa 290-386). Listed are aggregate types observed in the Yfp (Tia1) channel. See (B) for the Sup35-NM-Cfp / Tia1-Yfp control.
(B) Sup35N and aa 166-220 of Sup35M are implicated in the formation of lines with Tia1. On the scheme N, M and C indicate Sup35N (aa 1-123), Sup35M (aa 124-253) and Sup35C (aa 254-685). For regions within Sup35N and Sup35M – see text and Table S1. Vertical lines within Sup35N indicate oligopeptide repeats.
Experiments were performed in [pin−][psi−] 74-D694, except for two last lines in (B) where isogenic sup35-ΔNM and pub1-Δ mutants were used. Cultures expressing the indicated constructs (A), or the indicated Sup35 constructs and Tia1-Yfp (B) were grown as in Figs. 1A and 2B. Images were taken in late-log.
Prion domain of Sup35, Sup35NM, is traditionally divided into two parts (Table S1). The N-terminal Q/N-rich Sup35N is necessary and sufficient for the formation and maintenance of [PSI+]. The heavily charged middle region, Sup35M, can improve both stability of [PSI+] and faithful transmission of weak and strong [PSI+] variants, so it is viewed as an auxiliary part of the prion domain (Liebman and Chernoff, 2012). Analysis of Tia1 co-expression with Sup35N and Sup35M revealed that neither part of the Sup35 prion domain could alone promote the formation of line aggregates with Tia1, indicating that this requires both Sup35N and Sup35M (Fig. 5B).
Substituting other Q/N-rich aggregation-prone sequences for Sup35N disrupted line formation with Tia1 (Figs. 4D, S4E). This further indicates that line formation involves one or several determinants of prionogenicity within Sup35N, e.g. a Q/N stretch at the beginning of Sup35N, a series of Q/N/Y/G-rich oligopeptide repeats, or a moderately N/Y/Q/G-rich sequence at the end of Sup35N (Table S1).
To elucidate the role of Sup35M in line formation, we designed a series of deletion constructs based on compositionally different regions of Sup35M (Table S1): (i) aa 124-166, directly interacts with the Hsp104 chaperone, positively charged, includes a Q/K-rich stretch (aa 130-135) and a K-rich sequence (aa 139-164); (ii) aa 167–222, includes several mixed K/E-rich stretches; (iii) aa 223-253, negatively charged, incudes an N-rich sequence (aa 226-231) and an E/D-rich stretch (aa 241-253). We also included a construct terminating at aa 153, as it has been extensively studied before (Table S1). Analysis of co-expression of these deletion constructs with Tia1 indicates that line formation depends upon the K/E-rich region between aa 167-220 (Fig. 5B and not shown). Importance of K/E–rich stretches within this region was confirmed by the finding that deleting the mixed K/E sequences in the aa 167-220 region completely abolishes line formation (Fig S5A). Noteworthy, lines formed by Sup35 fragments lacking the beginning of the M-domain were not as straight, the presence of Sup35 in these lines was barely detectable, and Tia1 distribution had a tight bead-on-a-string pattern. This may be because other parts of Sup35M are also engaged in line formation, or because internal deletions distort conformations of nearby regions.
Of the two mammalian homologs of Sup35, Gspt1 is unable to form lines with Tia1, and Gspt2 retains the line-forming ability (Fig. 4C), but neither has a region similar to Sup35N: their N-termini are not Q/N-rich and have no oligopeptide repeats. Yet, both Gspt1 and Gspt2 carry regions that are rich in charged amino acids, similar to Sup35M. Furthermore, while the M-domain of Gspt1 is mostly E-rich, Gspt2M includes an E/K-rich region with mixed E/K stretches, very much like the aa 167-220 part of Sup35M (Table S1). Concomitantly, Sup35N-Gspt2M-Cfp, where Gspt2M was substituted for Sup35M, retains the line-forming ability (Fig. 5B).
In all previously described experiments, Sup35 or its fragments were co-expressed with Tia1 in a strain that also expressed the endogenous Sup35 protein. To confirm that Sup35NM and its deletion variants can alone form self-propagating line aggregates with Tia1, we repeated the experiments in the sup35-ΔNM strain where the Sup35NM-encoding part of the endogenous SUP35 ORF is deleted, and only Sup35C, essential for viability, is produced. Indeed, the lack of full-length Sup35 did not interfere with line formation (Figs. 5B, S5B). Analogously, Tia1 and Sup35NM could form lines when co-expressed in the pub1-Δ strain (Fig. 5B).
Self-propagating aggregates of Sup35 and Tia1/Pub1 are associated with tubulin cytoskeleton
Straight and rigid appearance of Tia1/Pub1 and Sup35 lines, their orientation toward bud necks in mother-daughter pairs (Fig. 2B), and towards bud scars in non-budding cells (Fig. 6A) strikingly resemble interpolar microtubules that form in yeast during mitosis (Kilmartin and Adams, 1984). Thus, we hypothesized that self-propagating structures formed by Tia1 and Sup35 are associated with the tubulin cytoskeleton, with lines forming along microtubular structures.
Figure 6. Sup35 and Tia1/Pub1 interact with Tub1 and depend on microtubules for line formation.
(A) Tia1 / Sup35 lines are directed towards bud scars. Bud scars were stained with calcoflour A and observed with DAPI filter set.
(B) Line formation depends upon the integrity of tubulin cytoskeleton: benomyl inhibits line formation. Benomyl (20 μg/ml final concentration) was added when cultures were diluted to OD600 0.1 and expression of Tia1-Yfp and Sup35NM-Cfp was induced. Line formation was monitored for 2 days; images at 36 hrs are shown. Inset shows a cell with an imperfect line.
(C) Co-IP of Sup35NM and Tia1 with anti-Tub1 antibody. Top: Tub1 in protein extracts used for IP. Bottom: Sup35NM-Cfp (arrow) and Tia1-mCherry (arrowhead) in IPs.
(D) Endogenous Sup35 and chromosomally-encoded Pub1-Gfp expressed at physiological levels interact with α-tubulin.
(E) Microtubule-associated Sup35 is in an oligomeric state. SDS-AGE was preceded by an IP with anti-Tub1. RT and Boil – see Fig. 1F legend.
(F) Sup35 prion domain and, specifically, Sup35N is sufficient for the association of Sup35 with Tub1. Arrows, dashed arrows and black arrowhead point at, respectively, Sup35NM-Cfp, Sup35N-Cfp and Sup35M-Cfp. Grey arrowhead points at the expected position of Sup35M-Cfp in the IP. In the graph amounts of the proteins are normalized to the amounts of Tub1 pulled down by anti-Tub1; AVE +/− SD are based on 3 experiments.
(G) Prion domain of Sup35 is essential for its association with Tub1. Cultures of [pin−] [psi−] 74-D694 or of the isogenic sup35-Δ strain carrying either pSUP35-SUP35 or pSUP35-SUP35ΔN were grown in YPD to late-log. Arrows and black arrowhead indicate the full length Sup35 and Sup35-ΔN, respectively. Grey arrowhead points at the expected position of Sup35-ΔN in the IP. In (A) – (C) and (F): cultures of [pin−][psi−] 74-D694 (A –C) and sup35-ΔNM (F) transformants expressing the indicated proteins were grown as in Figs. 1A and 2B till late log.
In (D) and (E): cultures of [pin−][psi−] 74-D694 (D left, E) or BY4147 where PUB1-GFP substitutes the PUB1 ORF under the control of the endogenous PUB1 promoter (D right) were grown in YPD till late-log. C indicates “empty bead” controls.
See also Fig. S6. For “empty bead controls” see Figs. S6E, S6G, S6C, and Fig. S3C and its legend.
Indicative of the importance of the integrity of the tubulin cytoskeleton for the formation of Tia1/Sup35 lines, the percentage of line-containing cells was sharply reduced in the presence of a microtubule-disrupting drug benomyl (at least 10-fold at any time point), and the rare lines that still formed appeared abnormal (Figs. 6B, S6A). Contrariwise, formation of Tia1 dot aggregates was not strongly affected. Also, the integrity of actin cytoskeleton is not critical for the Tia1/Sup35 line formation: Latrunculin A, an actin-depolymerizing drug, did not inhibit their appearance (Fig. S6B). This underscores the independence of Tia1/Sup35 co-aggregates from [PSI+] (Fig. 3C), as [PSI+] interacts with actin cytoskeleton (see Fig. S6B legend).
To test if Tia1 and Sup35NM directly interact with the microtubule cytoskeleton, we asked if these proteins co-IP with α-tubulin, Tub1. Indeed, in cultures co-expressing Tia1 and Sup35NM, both proteins were pulled down by the anti-Tub1 antibody (Figs. 6C, S6C). When Tia1 or Sup35NM were expressed alone, they also co-IPed with anti-Tub1, and their amounts in the pull-gowns were not reduced compared to co-expression cultures (ibid.). Thus, Tia1 and Sup35NM interact with cytoskeleton even when visible line structures are absent. However, this result does not indicate that Tia1 and Sup35 bind to Tub1 independently: in this experiment interaction of Sup35NM and Tia1 with Tub1 could be mediated by endogenously encoded Sup35 and Pub1.
Sup35 and Pub1 bind to α-tubulin when expressed from chromosomal ORFs at physiological level
In the previous section interaction between tubulin and the prion domain of Sup35 was established when it was expressed as a protein fragment, Sup35NM-Cfp, and at ~ 5-fold level compared to the endogenous Sup35. Here we confirm that endogenous Sup35 IPs with α-tubulin in a strain not expressing any genetically engineered constructs (Fig. 6D left). Most experiments in subsequent sections are performed in such native conditions; and we further refer to this strain wild as type 74-D694. Analogously, by substituting PUB1-GFP for the PUB1 ORF in its original location, we confirm that Pub1-Gfp expressed at a physiological level is still a component of the tubulin-associated complex (Fig. 6D right). Of note, neither Sup35 nor Pub1 co-IP with Act1, a major component of the actin cytoskeleton (Fig. S6D).
Analysis of wild type cell extracts on sucrose gradients is consistent with Sup35/Pub1 participating in a microtubule-associated complex: while some Sup35 stays on the top of the gradient with soluble proteins, the rest of it is distributed between the fractions that contain most of the α-tubulin (Fig. S6F).
The native tubulin-associated complex contains Sup35 in a prion-like conformation
Previous SDS-AGE analyses of the 74-D694 strain derivatives lacking the Sup35-based prion [PSI+] detected only monomeric Sup35 (Bagriantsev and Liebman, 2004), and we confirmed this for total cell extracts of [pin−][psi−] 74-D694 (data not shown). However, SDS-AGE analysis of the anti-Tub1 IPs of these extracts revealed that essentially all Sup35 in the pull-downs was in temperature-liable SDS-resistant oligomers (Fig. 6E). Thus, an SDS-resistant aggregated conformation of Sup35 is present specifically in the microtubule-associated complex, while the reminder of cellular Sup35 remains in a soluble state.
Formation of the tubulin-associated complex is mediated by prion domains of Sup35 and Pub1, and binding of Sup35 depends on Pub1
To probe the importance of the Sup35 prion domain in the formation of the tubulin-associated complex, we tested if Sup35NM co-IPs with α-tubulin in the sup35-ΔNM strain where only the Sup35C-encding part of the SUP35 ORF is retained. Sup35C does not interact with Sup35NM (Paushkin et al., 1996), so Sup35NM would not be recruited into the tubulin-associated complex if the complex formed exclusively through Sup35C. As anti-Tub1 pulls down Sup35NM even from sup35-ΔNM extracts (Fig. 6F), the Sup35NM domain is engaged in complex formation. This is in agreement with the formation of Sup35NM/Tia1 lines in the sup35-ΔNM strain (Figs. 5B, S5B), but while both Sup35N and Sup35M are essential for the formation of line structures, Sup35N alone can join the tubulin-associated complex (Fig. 6F).
To test if Sup35 lacking Sup35N can join the complex, anti-Tub1 co-IP was performed in the sup35-Δ strain where viability was maintained by centromeric plasmids carrying either full-length SUP35 or sup35-ΔN controlled by the original SUP35 promoter. Only full-length Sup35 and not Sup35-ΔN was detected in co-IPs (Fig. 6G).
Thus, Sup35 prion domain is essential and sufficient for the association with microtubule cytoskeleton. As the Pub1/Sup35-including Tub1-associated complex is present in the cells under normal growth conditions, our data suggest a function for the Sup35 prion domain, which is distinct from the formation of the Sup35-based [PSI+] prion: to mediate the interaction of eRF3 with the cytoskeleton.
Importance of the Pub1 prion domain for the association with α-tubulin was analyzed in the pub1-Δ strain expressing full-length Pub1 or its fragments. In addition to the Pub1(1-245) fragment completely lacking the prion domain and carrying only the RBD, we also tested Pub1(1-419) that retains most of the prion domain except the Q-rich sequence at the extreme C-terminus. Different from Sup35, removal of the Pub1 prion domain did not abolish its association with tubulin but only slightly reduced it (Fig. 7A), suggesting that Pub1 may be also recruited to the complex through RBD.
Figure 7. Composition of the Tub1 / Pub1 / Sup35 complex and phenotypes of pub1-Δ and sup35 mutants suggest that the complex is involved in the α-tubulin synthesis.
(A) Formation of the Tia1 / Sup35 tubulin-associated complex is modulated by the Pub1 prion domain. Transformants of [pin−][psi−] 74-D694 pub1-Δ with plasmids expressing CUP-driven PUB1 or PUB1 fragments tagged with YFP were grown as in Fig. 1A till late-log. Numbers indicate Pub1 aa encoded by the construct. Arrows point at Pub1-Yfp and its fragments, arrowhead – at Sup35, dashed arrow – at Tub1. See Fig. S7A for the analysis of Sup35 binding to Tub1 in isogenic wild type and pub1-Δ strains not carrying overexpression constructs.
(B) Initiation factor eIF4E is precipitated by anti-Tub1, and amounts of eIF4E in the IPs are reduced in pub1-Δ and sup35-ΔNM strains.
(C) Ribosomal protein Rpl28 is pulled down by anti-Tub1. IN, IP and C indicate, respectively, cell lysate input, immunoprecipitate and “empty bead” control.
(D) Mature 26S ribosomal RNA but not rRNA precursor are pulled down by anti-Tub1. Shown are RT PCR data for the 125 bp fragment within the D1 region of the 26S rRNA (26S), and the 841 bp rRNA fragment of a precursor starting in the internal transcribed spacer ITS1 and ending in D1 (ITS). Controls: -RT - no reverse transcriptase with 26S primers; C - “empty bead”.
(E) Microtubule-associated Sup35 / Pub1 complex is specifically enriched with theTUB1 mRNA, and its recruitment is mediated by Pub1. Left: RT-PCR from anti-Tub1 IP; two sets of RT-PCR primers were used for TUB1 and SUP35; all panels are from the same gel. Right: RT-PCR of TUB1 from the indicated strains from total RNA (top left), or from anti-Tub1 IP (top right), and quantification of this experiment (bottom). -RT control is with TUB1 primers.
(F) Disruption of the PUB1 gene increases sensitivity to benomyl.
(G) Deletion of the SUP35 region encoding the Sup35NM prion domain increases sensitivity to benomyl.
In (F) and (G): Shown are 10-fold serial dilutions of the indicated strains on YPD medium and on YPD+30 μg/ml benomyl. Images were taken after 2 days of growth at 30°C.
(H) Depletion of Sup35 protein from Tub1-associated complex in BenS sup35 and sup45 mutants. Mutation positions are: E266Stop in Sup45 for 45n, R372K in Sup35 for 35m and Q70Stop in Sup35 for 35n.
(I) Abnormal growth of a BenS 35m sup35 mutant (R372K).
In (B) – (H): cultures of [pin−][psi−] 74-D694 or the indicated mutants were grown in YPD till late log.
AVE +/− SD in bar graphs are based on 3-5 experiments. In (A), (B) and (H), amounts of proteins are normalized to the amounts of Tub1 pulled down by anti-Tub1. In (E), amount of TUB1 mRNA in IPs is normalized to TUB1 mRNA in total mRNA.
See also Fig. S7.
Do Sup35 and Pub 1 depend on each other for the association with α-tubulin? Amounts of Sup35 co-IPed with Tub1 dropped dramatically in the pub1-Δ strain (Figs. 7A, S7A). Re-introduction of full-length PUB1 or PUB1(1-419) restored Sup35 levels in the pull-downs (Fig. 7A), but no such restoration was observed for PUB1(1-245) (Fig. 7A). Consistent with evolutionary conservation of Tia1/Pub1 interaction with Sup35, introduction of TIA1 also restored the association of Sup35 with Tub1 in the pub1-Δ strain (Fig S7B). Together with other data this suggests that Sup35 is recruited into the complex through the interaction between Q/N-rich sequences in Sup35 and Pub1.
The Tub1/Pub1/Sup35 complex contains components of translational machinery and TUB1 mRNA, and their recruitment depends on Pub1 and Sup35 prion domain
To elucidate the physiological role of the microtubule-associated complex incorporating Sup35 and Pub1, we first asked if Sup35 could recruit other translational machinery to the complex. In the anti-Tub1 co-IPs we detected eIF4E, an initiation factor (Figs. 7B, S7C), Pab1, a poly-A binding protein mediating interactions between the 5′ cap and 3′ mRNA tail (Fig. S7D), Rpl28, a large ribosome subunit protein (Fig. 7C), and the large ribosome subunit 26S rRNA (Figs. 7D, S7E). This is consistent with the association of both ribosomal subunits with α-tubulin. Furthermore, binding of the translational machinery with tubulin was reduced in the pub1-Δ and sup35-ΔNM strains (Fig. 7B and not shown) indicating that ribosomes are a part of the Tub1/Pub1/Sup35 complex, and that Pub1 and Sup35 prion domains participate in recruitment of ribosomal components.
Analysis of the mRNA content of the complex revealed the presence of the TUB1 mRNA (Figs. 7E left, S7F). As with the components of translational machinery, smaller amounts of the TUB1 mRNA were co-IPed with Tub1 in the pub1-Δ strain compared to wild type 74-D694, even though levels of total TUB1 mRNA were not affected by the disruption (Fig 7E right). Several other highly abundant mRNAs, e.g. for histones H4 (HHF2) and H2A (HTA1), and components of translational machinery Sup35, Sup45, Rpl28 and eEF-1A (TEF1) were absent from the complex (Figs. 7E left, S7G and not shown).
We also ruled out that components of the complex were held together by RNA molecules, akin to some ribonucleoprotein complexes. Indeed, in cell lysates treated with RNAse A, Sup35, Pub1 and eIF4E were still associated with tubulin (Fig. S7H). Only Rpl28 was completely removed, which is consistent with Rpl28 joining as part of a ribosomal subunit held together by ribosomal RNA (Fig. S7H).
Genetic evidence for a function of the microtubule-associated complex in maintaining the integrity of the microtubule cytoskeleton
Genetic approaches were used to obtain an independent evidence for the biological importance of the complex identified by biochemical methods. If the complex maintains the integrity of tubulin cytoskeleton, then disruption of the complex should result in phenotypes observed in microtubule deficient mutants. Indeed, compared to wild type 74-D694, both pub1-Δ and sup35-ΔNM strains have increased sensitivity to benomyl, BenS (Figs. 7F, 7G). Also, some mutations in Sup35 and in Sup45, another key translation termination factor component have been previously shown to be BenS (Tikhomirova and Inge-Vechtomov, 1996), and Sup35 depletion was linked to phenotypes that could reflect microtubular defects (Valouev et al., 2002).
To test if BenS sup35 and sup45 mutants are deficient in the ability to join the Tub1-associated complex, we isolated spontaneous sup35 and sup45 mutants, and selected those most sensitive to benomyl. Approximately half of these BenS mutants formed pseudohyphal cell chains, a defect in cell separation indicative of a microtubular deficiency. Strikingly, in these pseudohyphal mutants, Sup35 was essentially completely depleted from the Tub1-associated complex. Furthermore, while the amount of Sup35 was reduced in cellular lysates in most sup35 mutants, at least in some of them depletion of Sup35 from the Tub1-associated complex was much more profound than a drop in the level of cellular Sup35 (Figs. 7H, 7I and not shown). Thus microtubular defects of sup35 and sup45 mutants likely result from a specific deficiency in the microtubule-associated complex rather than from a general defect in translation termination.
DISCUSSION
Possible role of Sup35/Pub1 complex in maintaining the integrity of tubulin cytoskeleton
We considered two possibilities for why Sup35/Pub1 complex is associated with tubulin cytoskeleton: (i) cytoskeleton is used as a scaffold or a transport highway for the complex; (ii) the complex is recruited to microtubules because it is implicated in cytoskeletal biogenesis or function. While the first possibility cannot be excluded, earlier evidence and our results support the second. Mutations or depletion of Sup35 lead to phenotypes indicative of microtubule defects, such as benomyl sensitivity, and abnormal spindle assembly, chromosome segregation, karyogamy and mitosis (Tikhomirova and Inge-Vechtomov, 1996; Basu et al., 1998; Borchsenius et al., 2000; Valouev et al., 2002; True and Lindquist, 2002). Furthermore, we find that pub1-Δ and sup35-ΔNM mutants are BenS, and sensitivity of these and other sup35 mutants to benomyl is linked to Sup35 depletion from the complex.
The specific role of the Sup35 and Pub1 in maintaining the integrity of tubulin cytoskeleton could be through gene sharing, when proteins perform functions distinct from their main activities in the cell, e.g. Sup35 acts not as a translation factor. Alternatively, Sup35 and Pub1 are recruited to this specific location in connection with their regular cellular function, protein synthesis. Our data are more consistent with the second possibility. (i) The complex contains components of translation machinery indicative of the presence of ribosomes - eIF4E, Pab1, Rpl28, 26S rRNA. (ii) Phenotypes characteristic of microtubular defects have also been linked to at least one more component of translational machinery, Sup45 (Borchsenius et al., 2000; Borchsenius et al., 2005), and we find that Sup35 is depleted from tubulin-associated complex in a BenS sup45 mutant.
What could be the translation-related function of the complex? Pub1 is a component of stress granules that regulate mRNA processing, from storage to degradation, to delivery to translation initiation sites (Decker and Parker, 2012). So Pub1/Sup35 complex can be associated with storage of translational components (and Sup35 is a stored component), or with active protein synthesis (and Pub1 delivers mRNA and initiation complexes to the translation site). Two findings favor active site-specific protein synthesis. (i) The composition of the complex is not that of a typical stress granule, which usually consist of mRNA, initiation factors and small ribosomal subunits (Decker and Parker, 2012); may occasionally incorporarte eRF1 and eRF3 (Dori and Choder, 2007); but never include large ribosomal subunit components, such as Rpl28 and 26S rRNA. (ii) The complex is specifically enriched with TUB1 mRNA and devoid of mRNAs for other major housekeeping proteins and translation factors. Thus we hypothesize that we identified the complex engaged in the tubulin synthesis localized to the site of microtubular assembly.
Our experiments with mouse homologs of Pub1 and Sup35 connote that propensity to interact and form tubulin-associated aggregates is evolutionarily conserved for the Pub1/Tia1 and Sup35/Gspt2 protein pair. For Pub1/Tia1 the interaction depends on the Q/N-rich prion domain conserved from yeast to higher eukaryotes. In Sup35, two parts of the Sup35NM prion domain are involved. One, the Q/N-rich Sup35N, is not conserved in higher eukaryotes. But the other, a highly charged Sup35M, is present right upstream of the termination domain in all species except the most primitive metazoans (Inagaki and Doolittle, 2000). Within Sup35M the K/E sequences containing both positively and negatively charged residues are implicated in the formation of tubulin-associated Sup35/Tia1 lines. Strikingly, these K/E patches are present in Gsp2, the one of two mouse homologs of Sup35 that retains the line-forming ablity. We hypothesize that K/E-rich stretches modulate Sup35/Gspt2 interaction with microtubules. Indeed, similar K/E intermixing has been found in two microtubule-associated proteins, MAP2β and Kar3 (Tikhomirova and Inge-Vechtomov, 1996).
It is also likely that interaction between Tia1 and Gspt2 plays a specific role in the nervous system underscoring the role of functional prions in the brain. Indeed, Gspt2 is expressed predominantly in neurons (Hoshino et al., 1998), and for Tia1, a ubiquitously expressed protein, studies in our lab indicate a specific neuronal function in synaptic plasticity in relationship to stress (J.R. and E.R.K., unpublished data).
A conceptual framework for functional prions
We show that the same protein can participate in different self-perpetuating structures, and that an RNP complex assembled through prion domains of two prion-forming proteins is implicated in maintaining the integrity of microtubule cytoskeleton. These two findings bring the concept of functional prions to a new level and provide experimental foundation for the idea of dynamic prion-based complexes. Indeed, engagement of an RNA-binding protein into multiple mono- and hetero-protein prion-like aggregates offers a simple solution to the dynamic transfer of mRNAs between a network of RNA-processing complexes, rapid switching between RNA storage and degradation, and between inhibition and activation of protein synthesis.
Analysis of the Sup35/Pub1 microtubule-associated complex supports the idea that prion domain-based self-assembly is important in the formation of RNP complexes and that this self-assembly involves cooperative action of different prion-forming proteins. Indeed, recruitment of Sup35 depends on Sup35 and Pub1 prion domains, and Sup35 prion domain facilitates the joining of translational machinery. Also, Sup35 associated with the complex is in the SDS-resistant oligomeric state, indicative of a role of prion-like mechanism in complex formation.
Capability to take on prion-like conformations is common among proteins involved in the biogenesis, turnover and cellular distribution of mRNAs. Known prions and prion candidates are over-represented among RNA-binding proteins and components of RNP complexes regulating transcription and nuclear and cytoplasmic maturation, transport and turnover of mRNA (Michelitsch and Weissman, 2000; Kato et al., 2012; Wickner et al., 2013). Furthermore, for several Q/N-rich proteins prion domains are required for their localization into RNP complexes (Gilks et al., 2004; Decker et al., 2007; Reijns et al., 2008; Kato et al., 2012).
Discovery that the same protein may join different self-propagating complexes hints at how the network of such RNP complexes can form. Analysis of visible aggregates and SDS-resistant oligomers indicates that Tia1/Pub1 and Sup35 are involved in the formation of at least three self-propagating structures. One, induced by co-expression of Pub1/Tia1 with Sup35/Gspt2, is a co-aggregate of the two proteins visualized as lines formed along microtubules. We link it to the Pub1/Sup35 microtubule-associated complex. The other, manifested by dots induced by expressing Tia1 alone, co-localizes with Dcp2, consistent with Tia1 forming a mono-protein prion, or a hetero-protein prion with a yet unidentified partner in Dcp2-containing granules. The only other prion known for Sup35 is [PSI+], a Sup35 homopolymer for which no association with RNP complexes has been reported, and which affects the cell through partial inactivation of translation termination due to Sup35 aggregation (Newby and Lindquist, 2013). However, it is feasible that Sup35 forms another hetero-protein prion in an RNP complex: Sup35 interacts with several RNA-binding proteins found in P-bodies and stress granules (www.yeastgenome.org), and eRF3 was detected in a subset of mammalian P-bodies (Dori and Choder, 2007).
While our study provides the first example for a two-protein Q/N-rich prion, this is likely to be a widespread phenomenon. Prion-prion interactions involving cross-seeding and occasional formation of heterologous prion aggregates have been reported for a number of prions and amyloids (Derkatch and Liebman, 2007), and recent studies find that many low complexity sequences, including Q/N-rich domains, heteropolymerize in biogels in vitro (Kato et al., 2012). Also, one yeast prion, [GAR+], is a complex of at least two proteins, Pma1 and Std1, but as neither of the proteins encompasses a Q/N-rich prion domain, it is unclear whether this phenomenon involves Q/N heteropolymerization (Brown and Lindquist, 2009).
Identification of both a functional and a loss of function prion for the Sup35 protein raises the question of whether these two types of prions have different features. In many aspects Tia1/Sup35 and [PSI+] are similar and resemble other Q/N-rich prions: induction of the prion state by transient overexpression of prion-forming proteins, formation of SDS-resistant oligomers, and even facilitation of prion formation by the entrance into late-log (Zhou et al., 2001; Wickner et al., 2013). One notable difference is that [PSI+] aggregates remain visible when Sup35-Gfp is expressed at a physiological level, or can be seen immediately after the start of its high-level expression (Patino et al., 1996; Satpute-Krishnan and Serio, 2005). For Tia1 and Tia1/Sup35 the aggregation-prone state is propagated through smaller seeds distinct from visible aggregates. In our opinion, such small seeds are expected for functional prions engaged in the assembly of large complexes: low-level oligomerization is less disruptive to the interaction with other cellular components and allows for more dynamic rearrangement. Noteworthy, maintenance by small seeds was previously reported for a prion formed in yeast by the Het-s protein that forms a gain of function [Het-s] prion in P. anserina (Mathur et al., 2010).
EXPERIMENTAL PROCEDURES
Strains and plasmids
Unless otherwise stated strains are isogenic derivatives of 74-D694: a ade1-14(UGA) his3-Δ200 ura3-52 leu2-3, 112 trp1-289(UAG), and [pin−][psi−] was used unless the presence of [PIN+] or [PSI+] is indicated.
Unless specifically mentioned, constructs are CEN and pRS400-based, and ORFs are controlled by the CUP promoter. Plasmids with TIA1, PUB1 and their fragments are URA3-marked. Plasmids with SUP35, its fragments and GSPTs are LEU2 marked. Sources for PCR-amplifications: TIA1 – the C57BL6/J mouse brain; PUB1 - the 74-D694 strain; mouse GSPT1 and GSPT2 – respectively, BC031640.1 and BC117825.1 clones purchased from Open Biosystems.
Yeast cultivation
Growth of transformants with copper-inducible constructs is described in Fig. 1A legend. Otherwise yeast were grown in YPD. See Figure Legends for details of specific experiments.
Methods
See Supplemental Experimental Procedures for a description of genetic and biochemical methods and the list of antibodies.
Microscopy and imaging
Cells were observed using a confocal laser scanning microscope FV1000 (Olympus) with a 60x / 1.42 NA oil immersion objective. Images were analyzed with FV10-ASW software. TEM was performed at the NYU Langone Medical Imaging Core Facility. Odyssey (LI-COR Inc) system was used for imaging and analysis of gels and Western blots.
Data analysis
Sample sizes, number of repeats and normalizations used for calculating AVE +/− SD are stated in Figure Legends.
Supplementary Material
Highlights.
Tia1/Pub1 and Sup35 form mono-protein prions as well as a distinct two-protein prion
We discover a tubulin-bound complex including Pub1, Sup35 and translational machinery
The complex depends on Sup35 and Pub1 prion domains and includes oligomerized Sup35
The complex is enriched in Tub1 mRNA and preserves microtubule cytoskeleton integrity
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grant 7 R01 GM070934-06 (I.L.D.), Howard Hughes Medical Institute (E.R.K.) and Kavli Institute for Brain Science (E.R.K). We thank Catherine Potenski and Sergei Zadorski for plasmids and strains, Kevin Karl and Joe Stephan for technical help, and Joe Stephan, Stefan Kassabov and Luana Fioriti for helpful discussions.
Footnotes
SUPPLEMENTAL INFORMATION Supplemental Information includes Supplemental Experimental Procedures, 7 figures and 1 table.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alberti S, Halfmann R, King O, Kapila A, Lindquist S. A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell. 2009;137:146–158. doi: 10.1016/j.cell.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P, Kedersha N. Stress granules: the Tao of RNA triage. Trends Biochem Sci. 2008;33:141–150. doi: 10.1016/j.tibs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Bagriantsev S, Liebman SW. Specificity of prion assembly in vivo. [PSI+] and [PIN+] form separate structures in yeast. J Biol Chem. 2004;279:51042–51048. doi: 10.1074/jbc.M410611200. [DOI] [PubMed] [Google Scholar]
- Basu J, Williams BC, Li Z, Williams EV, Goldberg ML. Depletion of a Drosophila homolog of yeast Sup35p disrupts spindle assembly, chromosome segregation, and cytokinesis during male meiosis. Cell Motil Cytoskeleton. 1998;39:286–302. doi: 10.1002/(SICI)1097-0169(1998)39:4<286::AID-CM4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Borchsenius AS, Repnevskaia MV, Kurischko C, Inge-Vechtomov SG. Association between defects of karyogamy and translation termination in yeast Saccharomyces cerevisiae. Genetika. 2005;41:178–186. [PubMed] [Google Scholar]
- Borchsenius AS, Tchourikova AA, Inge-Vechtomov SG. Recessive mutations in SUP35 and SUP45 genes coding for translation release factors affect chromosome stability in Saccharomyces cerevisiae. Curr Genet. 2000;37:285–291. doi: 10.1007/s002940050529. [DOI] [PubMed] [Google Scholar]
- Brown JC, Lindquist S. A heritable switch in carbon source utilization driven by an unusual yeast prion. Genes Dev. 2009;23:2320–2332. doi: 10.1101/gad.1839109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan JR, Muhlrad D, Parker R. P-bodies promote stress-granule assembly in Saccharomyces cerevisiae. J Cell Biol. 2008;183:441–455. doi: 10.1083/jcb.200807043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker CJ, Teixeira D, Parker R. Edc3p and a glutamine/asparagine-rich domain of Lsm4p function in processing body assembly in Saccharomyces cerevisiae. J Cell Biol. 2007;179:437–449. doi: 10.1083/jcb.200704147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker CJ, Parker R. P-bodies and stress granules: possible roles in the control of translation and mRNA degradation. Cold Spring Harb Perspect Biol. 2012;4:a012286. doi: 10.1101/cshperspect.a012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkatch IL, Bradley ME, Hong JY, Liebman SW. Prions affect the appearance of other prions: the story of [PIN+] Cell. 2001;106:171–182. doi: 10.1016/s0092-8674(01)00427-5. [DOI] [PubMed] [Google Scholar]
- Derkatch IL, Bradley ME, Zhou P, Chernoff YO, Liebman SW. Genetic and environmental factors affecting the de novo appearance of the [PSI+] prion in Saccharomyces cerevisiae. Genetics. 1997;147:507–519. doi: 10.1093/genetics/147.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkatch IL, Liebman SW. Prion-prion interactions. Prion. 2007;1:161–169. doi: 10.4161/pri.1.3.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dori D, Choder M. Conceptual modeling in systems biology fosters empirical findings: the mRNA lifecycle. PLoS One. 2007;2:e872. doi: 10.1371/journal.pone.0000872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, Anderson P. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell. 2004;15:5383–5398. doi: 10.1091/mbc.E04-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann R, Jarosz DF, Jones SK, Chang A, Lancaster AK, Lindquist S. Prions are a common mechanism for phenotypic inheritance in wild yeasts. Nature. 2012;482:363–368. doi: 10.1038/nature10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino S, Imai M, Mizutani M, Kikuchi Y, Hanaoka F, Ui M, Katada T. Molecular cloning of a novel member of the eukaryotic polypeptide chain-releasing factors (eRF). Its identification as eRF3 interacting with eRF1. J Biol Chem. 1998;273:22254–22259. doi: 10.1074/jbc.273.35.22254. [DOI] [PubMed] [Google Scholar]
- Inagaki Y, Doolittle WF. Evolution of the eukaryotic translation termination system: origins of release factors. Mol Biol Evol. 2000;17:882–889. doi: 10.1093/oxfordjournals.molbev.a026368. [DOI] [PubMed] [Google Scholar]
- Inge-Vechtomov S, Zhouravleva G, Philippe M. Eukaryotic release factors (eRFs) history. Biol Cell. 2003;95:195–209. doi: 10.1016/s0248-4900(03)00035-2. [DOI] [PubMed] [Google Scholar]
- Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, Mirzaei H, Goldsmith EJ, Longgood J, Pei J, et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell. 2012;149:753–767. doi: 10.1016/j.cell.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha NL, Gupta M, Li W, Miller I, Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol. 1999;147:1431–1442. doi: 10.1083/jcb.147.7.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AC, Shewmaker FP, Kryndushkin D, Wickner RB. Sex, prions, and plasmids in yeast. Proc Natl Acad Sci U S A. 2012;109:E2683–2690. doi: 10.1073/pnas.1213449109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin JV, Adams AE. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J Cell Biol. 1984;98:922–933. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryndushkin DS, Alexandrov IM, Ter-Avanesyan MD, Kushnirov VV. Yeast [PSI+] prion aggregates are formed by small Sup35 polymers fragmented by Hsp104. J Biol Chem. 2003;278:49636–49643. doi: 10.1074/jbc.M307996200. [DOI] [PubMed] [Google Scholar]
- Le Goff C, Zemlyanko O, Moskalenko S, Berkova N, Inge-Vechtomov S, Philippe M, Zhouravleva G. Mouse GSPT2, but not GSPT1, can substitute for yeast eRF3 in vivo. Genes Cells. 2002;7:1043–1057. doi: 10.1046/j.1365-2443.2002.00585.x. [DOI] [PubMed] [Google Scholar]
- Liebman SW, Chernoff YO. Prions in yeast. Genetics. 2012;191:1041–1072. doi: 10.1534/genetics.111.137760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur V, Taneja V, Sun Y, Liebman SW. Analyzing the birth and propagation of two distinct prions, [PSI+] and [Het-s](y), in yeast. Mol Biol Cell. 2010;21:1449–1461. doi: 10.1091/mbc.E09-11-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelitsch MD, Weissman JS. A census of glutamine/asparagine-rich regions: implications for their conserved function and the prediction of novel prions. Proc Natl Acad Sci U S A. 2000;97:11910–11915. doi: 10.1073/pnas.97.22.11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby GA, Lindquist S. Blessings in disguise: biological benefits of prion-like mechanisms. Trends Cell Biol. 2013;23:251–259. doi: 10.1016/j.tcb.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Patino MM, Liu JJ, Glover JR, Lindquist S. Support for the prion hypothesis for inheritance of a phenotypic trait in yeast. Science. 1996;273:622–626. doi: 10.1126/science.273.5275.622. [DOI] [PubMed] [Google Scholar]
- Paushkin SV, Kushnirov VV, Smirnov VN, Ter-Avanesyan MD. Propagation of the yeast prion-like [PSI+] determinant is mediated by oligomerization of the SUP35-encoded polypeptide chain release factor. EMBO J. 1996;15:3127–3134. [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB. Biology and genetics of prions causing neurodegeneration. Annu Rev Genet. 2013;47:601–623. doi: 10.1146/annurev-genet-110711-155524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijns MA, Alexander RD, Spiller MP, Beggs JD. A role for Q/N-rich aggregation-prone regions in P-body localization. J Cell Sci. 2008;121:2463–2472. doi: 10.1242/jcs.024976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpute-Krishnan P, Serio TR. Prion protein remodelling confers an immediate phenotypic switch. Nature. 2005;437:262–265. doi: 10.1038/nature03981. [DOI] [PubMed] [Google Scholar]
- Si K, Choi YB, White-Grindley E, Majumdar A, Kandel ER. Aplysia CPEB can form prion-like multimers in sensory neurons that contribute to long-term facilitation. Cell. 2010;140:421–435. doi: 10.1016/j.cell.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Si K, Lindquist S, Kandel ER. A neuronal isoform of the aplysia CPEB has prion-like properties. Cell. 2003b;115:879–891. doi: 10.1016/s0092-8674(03)01020-1. [DOI] [PubMed] [Google Scholar]
- Tikhomirova VL, Inge-Vechtomov SG. Sensitivity of sup35 and sup45 suppressor mutants in Saccharomyces cerevisiae to the anti-microtubule drug benomyl. Curr Genet. 1996;30:44–49. doi: 10.1007/s002940050098. [DOI] [PubMed] [Google Scholar]
- True HL, Lindquist SL. A yeast prion provides a mechanism for genetic variation and phenotypic diversity. Nature. 2000;407:477–483. doi: 10.1038/35035005. [DOI] [PubMed] [Google Scholar]
- Urakov VN, Vishnevskaya AB, Alexandrov IM, Kushnirov VV, Smirnov VN, Ter-Avanesyan MD. Interdependence of amyloid formation in yeast: implications for polyglutamine disorders and biological functions. Prion. 2010;4:45–52. doi: 10.4161/pri.4.1.11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valouev IA, Kushnirov VV, Ter-Avanesyan MD. Yeast polypeptide chain release factors eRF1 and eRF3 are involved in cytoskeleton organization and cell cycle regulation. Cell Motil Cytoskeleton. 2002;52:161–173. doi: 10.1002/cm.10040. [DOI] [PubMed] [Google Scholar]
- Wickner RB, Edskes HK, Bateman DA, Kelly AC, Gorkovskiy A, Dayani Y, Zhou A. Amyloids and yeast prion biology. Biochemistry. 2013;52:1514–1527. doi: 10.1021/bi301686a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Derkatch IL, Liebman SW. The relationship between visible intracellular aggregates that appear after overexpression of Sup35 and the yeast prion-like elements [PSI+] and [PIN+] Mol Microbiol. 2001;39:37–46. doi: 10.1046/j.1365-2958.2001.02224.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.