Abstract
Substantial increases in grain yield of cereal crops are required to feed a growing human population. Here we show that a natural variant of SEMIDWARF AND HIGH-TILLERING (SDT) increases harvest index and grain productivity in rice. Gain-of-function sdt mutation has a shortened polyadenylation tail on the OsmiR156h microRNA precursor, which cause the up-regulation of OsmiR156h. The plants carrying the semidominant sdt allele exhibit reduced plant height, enhanced lodging resistance, increased tiller numbers per plant, and resulting in an increased grain yield. We also show that combining the sdt allele with the OsSPL14WFP allele can be effective in simultaneously improving tillering capacity and panicle branching, thereby leading to higher harvest index and grain yield. Most importantly, pyramiding of the sdt allele and the green revolution gene sd1 enhances grain yield by about 20% in hybrid rice breeding. Our results suggest that the manipulation of the polyadenylation status of OsmiR156 represents a novel strategy for improving the yield potential of rice over what is currently achievable.
Introduction
In the cereals, grain productivity is currently heavily dependent on the application of nitrogenous fertilizer. However, over-fertilization with nitrogen causes lodging (stem collapse prior to harvest) with a consequent loss of yield. A rice semi-dwarfing gene, sd1, known as the "Green Revolution” gene [1,2], has been extensively used in rice breeding programs over the past 50 years [3]. The continuing growth of the world's population and the limited arable land resources require that grain yield capacity of rice will have to be raised yet further [4–6]. However, grain yield loss due to lodging remains a problem in many high-yielding varieties carrying the Green Revolution sd1 gene [7], and the predominant use of this gene by famers and breeders is due to the lack of other useful semi-dwarfing genes, leading to narrowing down the genetic base of modern cultivars [8, 9].
It has been shown that miR156 involves in many plant growth and developmental processes [10]. The SQUAMOSA-PROMOTER BINDING PROTEIN-LIKE (SPL) transcription factor is the direct targets of miR156 in various plant species [10,11], which regulates flowering time, plastochron length, trichome patterning, tiller number and panicle branching [12–16]. In particular, the natural variants of the OsSPL14 gene (e.g. IPA and FWP) play the important role in the regulation of plant architecture and panicle branching in rice [14,15], where OsSPL16 has been reported to be involved in the control of grain size, shape and quality in rice [17]. There is a well-established negative correlation between tillering capacity and panicle branching, meaning that achieving a simultaneous genetic gain in both tiller numbers per plant and grain numbers per panicle represents a major challenge for rice breeders. Here, we show that we identified a natural variant of SEMIDWARF AND HIGH-TILLERING (SDT), which reduces plant height, increases tiller numbers per plant and enhances grain yield in rice. The further positional cloning and genetic complementation analysis demonstrate that the sdt allele is involved an alternative polyadenylation of the OsmiR156h microRNA precursor, and the up-regulation of OsmiR156h promotes tillering capacity and enhances grain yield.
Results
A natural allelic variant of sdt influences plant height and tillering
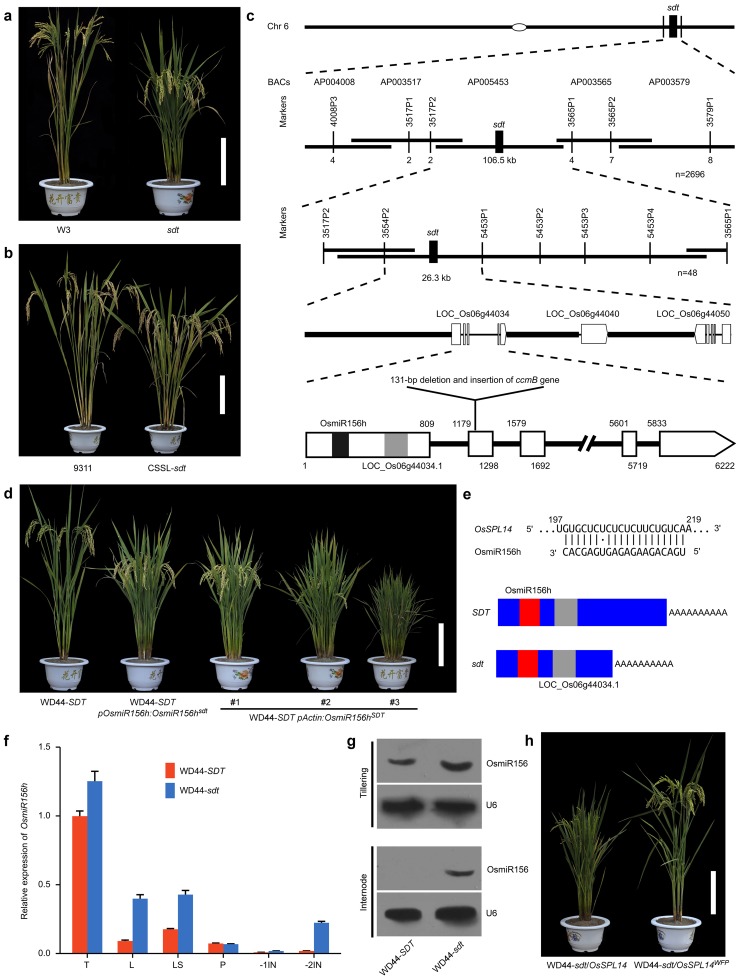
To investigate novel approaches for improving lodging resistance to support heavy panicles under the high nitrogen conditions, a spontaneous rice semidwarf and high-tillering (sdt) mutant was selected based on different responses to nitrogen fertilization. The sdt mutant plants displayed the reduced plant height and an increased number of tiller per plant when compared with a typical indica variety Wan3 (W3) (Fig 1a). Among a set of chromosome segment substitution lines (CSSLs) derived from the cross between the sdt mutant (the donor parent) and the indica variety 9311 (the recurrent parent), one CSSL line exhibited the similar semidwarf and high-tillering phenotypes as the sdt mutant, which we named as CSSL-sdt (Fig 1b). A subsequent genetic analysis of the selfed progeny of the backcross (CSSL-sdt ×9311)×9311 identified a major quantitative trait locus qsdt responsible for semidwarfism and high tillering that was mapped to the long arm of chromosome 6 (Fig 1c). The phenotype of BC2F2 segregants heterozygous at this locus was intermediate between that of the two alternative homozygotes (S1 Fig), which indicated that the sdt allele is semidominant.
Fig 1. Positional cloning of sdt.
(a) The phenotype of parental plants. Scale bar: 20 cm. (b) Mature plant appearance of CSSLs. Scale bar: 20 cm. (c) In the fine-scale map, the QTL falls in the candidate region between markers 3517P2 and 3565P2 using 2,696 BC1F2 segregants, and the progeny test of homozygous recombinant individuals further narrowed to a ~26.3 kb segment flanked by markers 3545P2 and 5453P1. The numbers below the line indicate the number of recombinants between sdt and the marker shown. Open bars represent the exons of the predicted genes, and filled bars represent putative transcripts for, respectively, LOC_Os06g44034.1 and OsmiR156h precursor. (d) Mature plant phenotype of the transgenic plants expressing OsmiR156h. Scale bar: 20 mm. (e) Allelic variation of 3’-UTR in the SDT locus. Red and gray rectangles represent the transcripts for, respectively, OsmiR156h stem-loop sequence and LOC_Os06g44034.1. (f) Expression of OsmiR156h in WD44-SDT and NIL-sdt. Expression levels of OsmiR156h were analyzed by stem-loop real-time RT-PCR. T: developing tiller buds; L: flag leaf; LS: leaf sheath; P: young panicle. -1IN: the first internode (the uppermost internode); -2IN: the second internode. Expression levels are expressed as the relative copies per 1000 copies of rice actin3. Data given as mean ± SE (n = 3). (g) Northern blot analysis of OsmiR156 levels in the NILs plants. Blots of total RNA extracted from young tillers of 55-day-old plants and the second internodes of 80-day-old plants, respectively. U6 was used as a loading control for OsmiR156. (h) The phenotypes of the WD44-sdt/OsSPL14 WFP and WD44-sdt/OsSPL14 plants. Scale bar: 20 mm.
Positional cloning of sdt
A high resolution genetic map based on 2,696 BC1F2 individuals bred from the cross CSSL-sdt × W3 narrowed the genomic location of qsdt to a ~106.5-kb region defined by molecular markers 3517P2 and 3565P2. The progeny test of homozygous recombinant individuals further narrowed to a ~26.3-kb segment flanked by molecular markers 3545P2 and 5453P1, a segment which contains three predicted genes (Fig 1c). A sequence comparison of parental copies of the three putative genes showed that there wasn’t any single nucleotide polymorphism (SNP) with respect to either LOC_Os06g44040 or LOC_Os06g44050, but that a 131-bp segment present in the second exon of LOC_Os06g44034 of the W3 allele had been replaced in CSSL-sdt by an inverted fragment of the mitochondrial gene ccmB (cytochrome c biogenesis B) [18] (Fig 1c and S2 Fig). LOC_Os06g44034 contains five exons and four introns, the first exon encodes an OsmiR156h microRNA precursor and a protein-coding transcript (LOC_Os06g44034.1) (Fig 1c), while the other exons generate a long 3'-UTR. Quantitative RT-PCR analysis showed that the increased transcription level of LOC_Os06g44034 was dependent on the presence of the insertion polymorphism (S3 Fig).
OsmiR156h is associated with semidwarf and high-tillering phenotype
To perform genetic complementation analysis, we developed a near-isogenic line, WD44-sdt, which is homozygous for the sdt mutant allele in the high-yielding japonica variety Wandao44 background, whereas WD44-SDT is homozygous for the Wandao44 SDT allele. The transgenic WD44-SDT plants, in which the LOC_Os06g44034.1 cDNA from WD44-sdt was constitutively overexpressed, had no visible alteration in either plant height or the number of tillers per plant (S4 Fig). However, the transgenic WD44-SDT plants expressing the WD44-sdt OsmiR156h sequence under the control of its native promoter exhibited reduced plant height and increased tiller numbers when compared with non-transgenic WD44-SDT plants (Fig 1d). The transgenic WD44-SDT plants carrying the transgene construct harbored the WD44-SDT OsmiR156h also displayed increased tiller numbers and dwarfed phenotypes, with transcript abundance of OsmiR156h being positively correlated with the extent of phenotypic change [10] (Fig 1d and S5 Fig). In addition, we found that down-regulation of OsSPL14 (SQUAMOSA PROMOTER BINDING PROTEIN- LIKE14), which is a direct target of OsmiR156h [14,15,19] (Fig 1e), led to increases in tiller numbers per plant and reduction of plant stature (S6 Fig). These results suggested that OsmiR156h-OsSPL14 regulatory module plays an important role in the regulation of tillering capacity. The miR156-SPL module has been suggested to be conserved in land plant evolution [15,20–22]. We also found that the transgenic wheat plants constitutively expressing OsmiR156h exhibited increased tiller numbers and the dwarf stature (S7 Fig). Thus, OsmiR156h is the sequence responsible for the sdt QTL.
Alternative polyadenylation of OsmiR156h contributes to the sdt phenotype
A 3’-RACE analysis targeting the OsmiR156h precursor was performed to investigate whether OsmiR156h expression was affected by alternative 3’-UTR polyadenylation of OsmiR156h precursor transcript [23,24]. The predicted 1,552-bp fragment was amplifiable from WD44-SDT, which has the same exon borders as the annotated exons. However, a transcript shortened at the 3'-UTR was present in WD44-sdt (Fig 1e and S8 Fig). The shortening of 3’-UTR was due to the loss of a 643-nt segment which corresponded to the sequences of exons 3 through 5, together with an additional 76-nt upstream of the poly(A) tail. The shortened 3'-UTR included a poly(A) signal sequence (AATAAA) located 54-nt upstream of the poly(A) itself (S9 Fig). OsmiR156h precursor transcripts were detectable in various organs in rice. A higher abundance was noted in developing tiller buds, while a much lower abundance was detected in the leaf, leaf sheath, culm and young panicle (Fig 1f). Nevertheless, the abundance of both the OsmiR156h precursor and the mature transcripts were higher in WD44-sdt than in WD44-SDT (Fig 1f and 1g).
The OsSPL genes are targeted by OsmiR156 [14,15,17], the transcriptional levels of a number of such genes were compared between WD44-sdt and WD44-SDT. We found that OsmiR156h-targeted OsSPL genes proved to be down-regulated in various parts of WD44-sdt plants, such as OsSPL14 [14,15] and OsSPL16 [17] (S10 Fig). It is known that the OsSLP14 WFP allele produces a higher abundance of OsSPL14 transcript [15]. When the OsSLP14 WFP allele was introduced into WD44-sdt, the forming phenotype was similar to that of WD44-SDT (Fig 1h). Taken together, these genetic results indicated that the up-regulation of OsmiR156h through the alternative 3’-UTR polyadenylation produces the sdt phenotype.
Introduction of the sdt allele into the elite rice variety improves grain yield
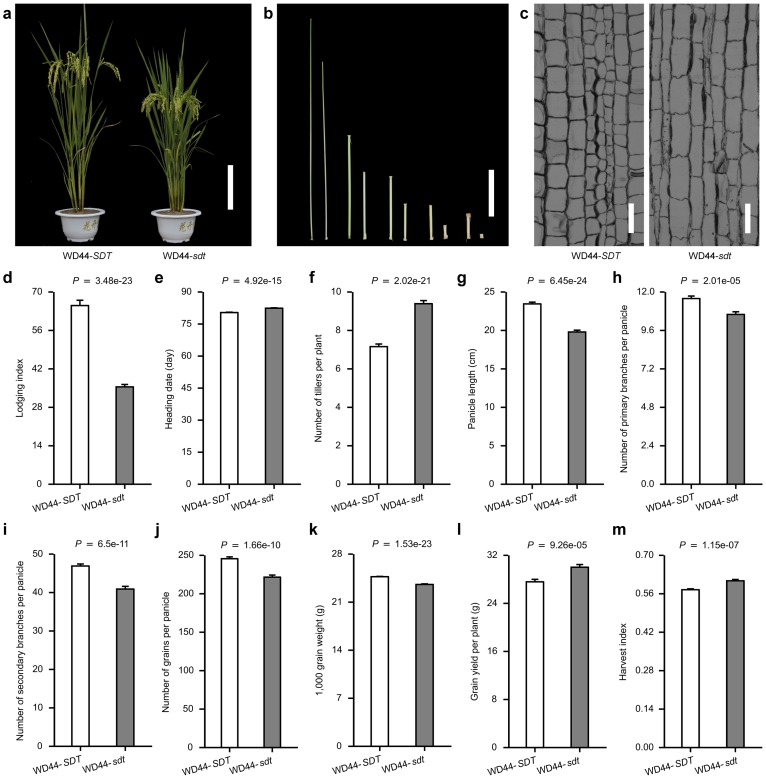
WD44-sdt plants were semidwarf in stature (Fig 2a and 2b), but lacked some of the negative pleiotropic effects when compared to those transgenic plants associated with the constitutive expression of OsmiR156, such as the strongly reduced panicle size and delayed heading date (Fig 1d). To investigate effect of the sdt allele on grain yield, the field performance of WD44-SDT and WD44-sdt was compared under normal cultivation conditions [25]. The two lines did not differ from one another with respect to their heading date (Fig 2e), whereas the number of tillers per plant of WD44-sdt was about 30% greater than that of WD44-SDT (Fig 2f). WD44-sdt plants produced shorter panicles and a lower number of both primary and secondary branches, which together resulted in the reduction in the number of grains formed per panicle as well as the production of smaller grains (Fig 2g–2k). However, the overall grain yield per plant of WD44-sdt was about 9% above that of WD44-SDT (Fig 2l), and its harvest index (ratio of grain weight to whole plant above ground biomass) was about 5% higher (Fig 2m). Thus, the sdt allele has the potential to both improve harvest index and the overall grain yield in rice. We also found that the longitudinal sections of the uppermost internode showed WD44-sdt internode cells were longer than those in WD44-SDT, but the length of each internode was less in WD-sdt than in WD44-SDT (Fig 2c). This outcome suggested that sdt functions as a negative regulator of cell proliferation in the stem, but it enhanced lodging resistance [26] (Fig 2d).
Fig 2. The contrasting phenotype of WD44-SDT and WD44-sdt.
(a) Mature plant appearance. Scale bar: 20 cm. (b) Culm internode length. Scale bar: 20 cm. (c) The effect of the sdt allele on cell proliferation: longitudinal sections of the uppermost internode. Scale bar: 0.1 mm. (d) Lodging index [30] (e) Heading date. (f) Tiller numbers per plant. (g) Panicle length. (h) Number of primary branches per panicle. (i) Number of secondary branches per panicle. (j) Number of grains per panicle. (k) 1,000 grain weight. (l) Grain yield per plant. (m) Harvest index. All phenotypic data were measured from the plants in randomized complete block design with three replications, which were grown with a distance of 20 × 20 cm in paddies under normal cultivation conditions. Data represented mean ± SE (n = 120). A Student’s t-test was used to generate the P values.
Combining sdt with OsSPL14 WFP can be effective in simultaneously improving tillering capacity and panicle branching
There is a well-established negative correlation between tillering capacity and panicle branching, meaning that achieving a simultaneous genetic gain in both tiller numbers per plant and grain numbers per panicle represents a major challenge for rice breeders [27,28]. Both the OsSLP14 WFP and OsSPL14 ipa alleles have been shown to promote panicle branching [14,15], but the up-regulation of OsSPL14 substantially reduces the number of tiller per plant [29] (S11 Fig). The ability of the sdt allele to compensate for this negative effect was tested by breeding the near-isogenic line NIL-OsSLP14 WFP /sdt in a background of the indica variety NP174. Field-grown NIL-OsSLP14 WFP/sdt plants were shorter, and produced about 55% more tillering than that formed by NIL-OsSLP14 WFP/SDT plants (Table 1 and S12 Fig). Although NIL-OsSLP14 WFP/sdt plants set fewer grains per panicle than that formed by NIL-OsSLP14 WFP/SDT plants, the combination of sdt with OsSLP14 WFP increased lodging resistance and overall grain yield by about 20%, and its harvest index was about 25% higher than that of plants carrying a single mutated allele (Table 1).
Table 1. Comparison of grain yield performance between NIL-OsSPL14 WFP /SDT and NIL-OsSPL14 WFP /SDT plants.
| Traits | NIL-OsSPL14 WFP/SDT | NIL-OsSPL14 WFP/sdt |
|---|---|---|
| Number of tillers per plant | 7.83 ± 0.09 | 12.24 ± 0.14 |
| Number of primary rachis branches per panicle | 16.40 ± 0.14 | 19.40 ± 0.32 |
| Number of secondary rachis branches per panicle | 83.60 ± 1.07 | 60.25 ± 0.86 |
| Number of grains per panicle | 274.69 ± 3.55 | 194.04 ± 1.45 |
| 1,000 grain weight (g) | 23.74 ± 0.24 | 26.56 ± 0.47 |
| Lodging index | 149.63 ± 3.65 | 63.62 ± 1.86 |
| Grain yield per plant (g) | 34.95 ± 0.59 | 42.08 ± 0.34 |
| Harvest index | 0.48 ± 0.01 | 0.60 ± 0.01 |
All phenotypic data were measured from the NILs plants in randomized complete block design with three replications, which were grown with a distance of 20 × 20 cm in paddies under normal cultivation conditions. Data given as mean ± SE (n = 120).
Pyramiding of the sdt and sd1 alleles improves grain yield in hybrid rice breeding
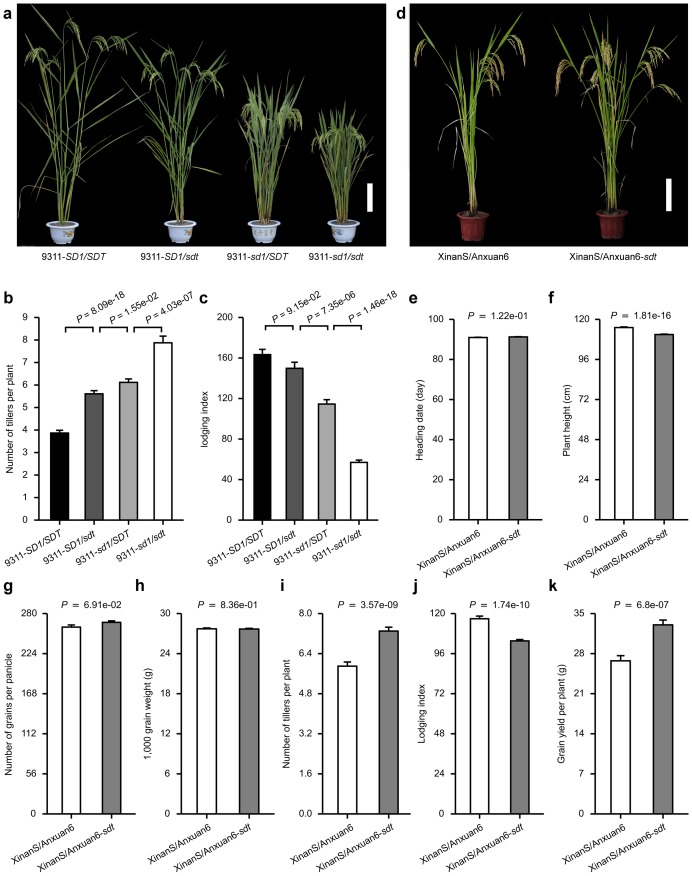
The Green Revolution rice semi-dwarfing gene (sd1) has been the backbone of the major increase in grain yield achieved over the past 50 years. The interaction between sd1 and sdt was explored by comparing a set of four near-isogenic lines in a background of the high-yielding indica variety 9311 (Fig 3a). Short stature and high tillering were conferred by the presence of either sd1 or sdt, but plants carrying both sd1 and sdt were shorter, tillered more profusely and were less susceptible to lodging than those plants carrying only single semi-dwarfing gene (Fig 3a–3c). We next evaluated whether pyramiding of sdt and sd1 alleles could be used to improve grain yield and lodging resistance, particularly in super hybrid rice breeding [30]. A two-line hybrid rice combination XinanS/Anxuan6 (Xinliangyou 6) developed from the cross between XinanS (the photo-thermo sensitive genic male sterile line) and Anxuan6 (the restorer line carrying the sd1 allele) has been predominately cultivated in Yangtze River area in China, the utility of a NIL line Anxuan6-sdt carrying both sd1 and sdt was investigated (Fig 3d). No perceptible effect on heading date, plant height, and the number of grains per panicle or grain weight were evident between the hybrid combination XinanS/Anxuan6 and XinanS/Anxuan6-sdt (Fig 3e–3g). However, the presence of the sdt allele increased tiller numbers per plant and lodging resistance (Fig 3h and 3i), and improved gain yield by 20% (Fig 3j). Therefore, the manipulation of OsmiR156 expression through alternative polyadenylation represents a useful strategy for breaking the unfavorable correlation between tillering capacity and panicle branching, which in turn offers a route to higher grain yield over that of existing high-yielding inbred and hybrid rice varieties.
Fig 3. Pyramiding of sdt and sd1 alleles improves lodging resistance and grain yield.
(a) Mature plant phenotype of four near-isogenic lines in a background of the indica variety 9311. Scale bar: 20 cm. (b) The allelic combination of sdt and sd1 increases tiller numbers per plant. Data represented mean ± SE (n = 60). (c) Combining sdt with sd1 enhances lodging resistance [30]. Data represented mean ± SE (n = 60). (d-j) The contrasting phenotype and grain yield of F1 hybrid plants. (d) Mature plant appearance. Scale bar, 20 cm; (e) Plant height; (f) Number of grains per panicle; (g) 1,000 grain weight; (h) Number of tillers per plant; (i) Lodging index; (j) Grain yield per plant. Data were measured from the plants in randomized complete block design with three replications, which were grown with a distance of 20 × 20 cm in paddies under normal cultivation conditions. Data represented mean ± SE (n = 120). A Student’s t-test was used to generate the P values.
Discussion
The gain-of-function rice sdt mutant has increased tiller number, which is the key factor accounting for the improvement of grain yield per plant, while the number of grain number per panicle and 1,000-grain weight are all reduced. Although the WD44-sdt internode cells were longer than those in WD44-SDT, the length of each internode was less in WD44-sdt than that in WD44-SDT, suggesting that sdt functions as a negative regulator of cell proliferation in the stem, and consequently resulting in enhanced lodging resistance. The previous studies have shown that mutated IPA1 (Ideal Plant Architecture 1) allele of OsSPL14 can promote panicle branching and reduce the formation of tillering, which defines the "ideal plant architecture" phenotype and potentially improves grain yield [14,15]. Although more panicle branches have better potential to increase the yield, plants with that trait tend to have low seed setting percentage since nutrition is limited. In fact, the total panicle number of rice population is the guarantee of high yield in rice production, those varieties with more tillers can realize the stable grain yield easily under the ambient environmental conditions.
The over-expression of the miR156-insensitive ipa1 allele of the OsSPL14 gene led to an ideal plant architecture in rice by reducing the number of tillers per plant and increasing the number of grain per panicle [14,15]. On contrast, Xie at al. showed that over-expression of OsmiR156 exhibited higher branches, but it caused the reduction of grain numbers [10]. We also found that over-expression of OsmiR156h under the rice Actin promoter does not lead to favorable phenotypes because the transgenic plants produced too many tillering. We also found that higher expression of OsSPL14 also resulted in the decreases in panicle branching (S11 Fig), suggesting that the up-regulation of miR156 could lead to different rice plant phenotypes: promote or repress panicle branching. These results indicates that the moderate transcriptional level or the expression patterns of OsmiR156h is critical for modulating the balance between panicle branching and tillering capacity and improving grain yield in rice.
The sdt allele is involved in shortening the polyadenylation tail of OsmiR156h microRNA precursor. As described above, a segment located at the second exon of LOC_Os06g44034 was replaced by an inverted fragment of ccmB in the sdt mutant, and this insertion mutation causes shortened 3'UTR of the OsmiR156h precursor transcript, which in turn increases the abundance of miR156h. Most importantly, pyramiding of sdt and OsSPL14 WFP elite alleles overcame the reduction in tiller numbers associated with overexpression of OsSPL14, and increased the overall grain yield by about 20%. This result indicated that combining sdt with OsSPL14 WFP can be effective in simultaneously improving tillering capacity and panicle branching, leading to higher overall grain yield. Taken together, the manipulation of the polyadenylation status of OsmiR156 represents a novel strategy to coordinately regulate the balance between panicle branching and tiller numbers in rice. The miR156-SPL regulatory module will be useful for farmers and breeders to improve grain yield potential of rice over what is currently achievable.
Materials and Methods
Plant materials
The CSSL population was generated by single seed descent from the cross sdt mutant × 9311, and the NILs by backcrossing CSSL-sdt with either WD44, NP174, 9311 or Anxuan6. Paddy-grown rice plants were raised during the standard rice growing season at an experimental station in Institute of Technical Biology and Agriculture Engineering, Hefei Institutes of Physical Science, Chinese Academy of Sciences (Anhui Province).
Mapping of sdt
Fine mapping was based on a set of 2696 BC1F2 progeny bred from the backcross (CSSL-sdt × W3) × W3. The sequences of the three predicted genes in the candidate region were compared among the mapping parents. Markers used for genotyping are listed in S1 Table.
Transgene constructs
The full-length LOC_Os06g44034.1 cDNA was amplified from leaf tissues of WD44-sdt plants, and then cloned into pActin::ocs vector. Constructs driving the constitutive expression of the putative OsmiR156h precursor were generated by introducing the genomic sequence containing the OsmiR156h precursor into pCAMBIA2300 vector [17]. Transgenic rice and wheat plants were produced by Agrobacterium-mediated transformation [17,31]. Relevant primer sequences and vectors are showed in S2 Table and S13 Fig.
qRT-PCR analysis
Total RNA was extracted from various parts of the rice plant using the TRIzol reagent (Invitrogen), converted to cDNA and used as a template for real-time PCR as described elsewhere [32]. Each experiment was repeated at least three times, with the rice actin3 gene used as a reference sequence. Relevant PCR primer sequences are given in S3 Table.
3’-RACE
3’-RACE PCR was carried out using the 3’-RACE Kit (Takara D314) following the manufacturer's instructions. The PCR relied on the nested adaptor primer and specific primers for the first exon of LOC_Os06g44034. Relevant PCR primer sequences are given in S4 Table.
RNA blots
Total RNA was separated by electrophoresis through a 19% denaturing polyacrylamide gels, and transferred to a Hybond N+ nylon membrane (Amersham Bioscience, GE Healthcare) as described previously [33]. After hybridization at 40°C in ULTRAhyb-oligo hybridization buffer (Ambion, Austin, TX) with a biotin-labeled (Invitrogen) mixed probe (OsmiR156 and U6), the membranes were washed twice at 40°C in 2×SSC and 0.5% SDS for 30 minutes before scanning.
Supporting Information
Segregation of the BC2F2 population derived from the backcross between the selected BC1F2 progeny carrying the sdt allele and 9311 plant. Comparisons of plant height among homozygotes of the sdt allele, heterozygotes of the sdt and the SDT allele, and homozygotes of the SDT allele. Data represented as mean ± SE (n = 30).
(TIF)
Allelic variation at the sdt locus, including a one-nucleotide substitution (g.1205C>G), a 131-bp deletion (g.1280_1401del) and an insertion of DNA fragment of the mitochondrial gene ccmB. The numbers indicate the position of the genomic sequence counted from the transcription start site of LOC_Os06g44034, and the lines above DNA sequences represent the location of the exons.
(TIF)
The transcriptional levels were determined by qRT-PCR using young leaf tissues. Expression levels are expressed as the relative copies per 1000 copies of rice actin3. Data given as mean ± SE (n = 3).
(TIF)
Mature plant appearance of the transgenic WD44-SDT plants overexpressing LOC_Os06g44034.1 under the control of rice Actin promoter. Scale bar: 20 cm.
(TIF)
(a) The transcriptional levels of OsmiR156h were determined by qRT-PCR. Expression levels are expressed as the relative copies per 1000 copies of rice actin3. Data given as mean ± SE (n = 3). (b) Plant height. (c) Tiller numbers per plant. The transgenic plants were shown in Fig 1d.
(TIF)
(a) Mature plant appearance. Scale bar: 20 cm. (b) Plant height. (c) Tiller numbers per plant. Data given as mean ± SE (n = 10). A Student’s t-test was used to generate the P values.
(TIF)
A winter wheat variety KN199 was used to generate the transgenic plants carrying the p35S::OsmiR156h sdt construct. Scale bar: 20 cm.
(TIF)
The fragment of OsmiR156h precursor was amplified using the nested adaptor primer and specific primers for the first exon of LOC_Os06g44034.
(TIF)
Comparative DNA sequence of 3’-UTR was analyzed using 3’-RACE. The identical nucleotide sequences were showed by dark boxes, variant nucleotide sequence were shown by light boxes, and the polyA signal (“AATAAA”) was indicated by red boxes.
(TIF)
(a) Young tillers of 55-day-old plants. (b) Second topmost internodes of 80-day-old plants. (c) Flag leaf tissues of 80-day-old plants. The transcriptional levels of OsSPLs were determined by qRT-PCR. Transcript abundance relative to the level of the WD44-SDT plants set to be one. Data shown as mean ± SE (n = 3).
(TIF)
Mature plant appearance of the transgenic WD44-SDT plants carrying the pActin::OsSPL14 construct. Scale bar: 20 cm.
(TIF)
Mature plant appearance of field-grown two NILs plants. Scale bar: 20 cm.
(TIF)
All vectors have pCAMBIA2300 backbone.
(TIF)
(DOCX)
(DOC)
(DOC)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from National Natural Science Foundation of China (31130070, 91335207 and 31301297), the Ministry of Science and Technology of China (2012AA10A301) and Chinese Academy of Sciences (KSCX2-EW-N-01, KJCX2-EW-N05 and KJCX2-YW-N34).
References
- 1. Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, et al. Green revolution: a mutant gibberellin-synthesis gene in rice. Nat. 2002; 416:701–702. [DOI] [PubMed] [Google Scholar]
- 2. Spielmeyer W, Ellis MH, Chandler PM. Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proc Natl Acad Sci USA. 2002; 99: 9043–9048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khush GS. Green revolution: preparing for the 21st century. Genome. 1999; 42: 646–655. [PubMed] [Google Scholar]
- 4. Squicciarini MP, Guariso A, Swinnen J. Global hunger: Food crisis spurs aid for poverty. Nat. 2013; 501:492. [DOI] [PubMed] [Google Scholar]
- 5. Porkka M, Kummu M, Siebert S, Varis O. From food insufficiency towards trade dependency: a historical analysis of global food availability. PLoS One. 2013; 8 (12):e82714 10.1371/journal.pone.0082714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCouch S, Baute GJ, Bradeen J, Bramel P, Bretting PK, Buckler E, et al. Agriculture: Feeding the future. Nat. 2013; 499:23–24. 10.1038/499023a [DOI] [PubMed] [Google Scholar]
- 7. Ookawa T, Hobo T, Yano M, Murata K, Ando T, Miura H, et al. New approach for rice improvement using a pleiotropic QTL gene for lodging resistance and yield. Nat Commun. 2010; 1:132 10.1038/ncomms1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heal GM, Walker B, Levin S, Arrow K, Dasgupta P, Daily G, et al. Genetic diversity and interdependent crop choices in agriculture. Resour Ebergy Econ. 2004; 26:175–184. [Google Scholar]
- 9. Nagano H, Onishi K, Ogasawara M, Horiuchi Y, Sano Y. Genealogy of the "Green Revolution" gene in rice. Genes Genet Syst. 2005; 80:351–356. [DOI] [PubMed] [Google Scholar]
- 10. Xie K, Wu C, Xiong L. Genomic organization, differential expression, and interaction of squamosa promoter-binding-like transcription factors and microRNA156 in rice. Plant Physiol. 2006; 142:280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang JW, Park MY, Wang LJ, Koo Y, Chen XY, Weigel D, et al. MiRNA control of vegetative phase change in trees. PLoS Genet. 2011; 7:e1002012 10.1371/journal.pgen.1002012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu G, Park MY, Conway SR, Wang JW, Weigel D, Poethig RS. The sequential action of MIR156 and MIR172 regulates developmental timing in Arabidopsis . Cell. 2009; 138:750–759. 10.1016/j.cell.2009.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang JW, Schwab R, Czech B, Mica E, Weigel D. Dual effects of miR156-targeted SPL genes and cyp78a5/kluh on plastochron length and organ size in Arabidopsis thaliana . Plant Cell. 2008; 20:1231–1243. 10.1105/tpc.108.058180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiao YQ, Wang YH, Xue DW, Wang J, Yan MX, Liu GF, et al. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat Genet. 2010; 42: 541–544. 10.1038/ng.591 [DOI] [PubMed] [Google Scholar]
- 15. Miura K, Ikeda M, Matsubara A, Song XJ, Ito M, Asano K, et al. OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat Genet. 2010; 42: 545–549. 10.1038/ng.592 [DOI] [PubMed] [Google Scholar]
- 16. Yu N, Cai WJ, Wang S, Shan CM, Wang LJ, Chen XY. Temporal control of trichome distribution by microrna156-targeted spl genes in Arabidopsis Thaliana . Plant Cell. 2010; 22:2322–2335. 10.1105/tpc.109.072579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang S, Wu K, Yuan Q, Liu X, Liu Z, Lin X, et al. Control of grain size, shape and quality by OsSPL16 in rice. Nat Genet. 2010; 44: 950–954. [DOI] [PubMed] [Google Scholar]
- 18. Tian X, Zheng J, Hu S, Yu J. The rice mitochondrial genomes and their variations. Plant Physiol. 2005; 140: 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reinhart BJ, Weinstein EG, Rhoades MW, Bartel B, Bartel DP. MicroRNAs in plants. Gen Dev. 2002; 16:1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jones-Rhoades MW, Bartel DP, Bartel B. MicroRNAs and their regulatory roles in plants. Annu Rev Plant Biol. 2006; 57:19–53. [DOI] [PubMed] [Google Scholar]
- 21. Arazi T, Talmor-Neiman M, Stav R, Riese M, Huijser P, Baulcombe DC. Cloning and characterization of micro-RNAs from moss. Plant J. 2005; 43:837–848. [DOI] [PubMed] [Google Scholar]
- 22. Kantar M, Lucas SJ, Budak H. MiRNA expression patterns of Triticum dicoccoides in response to shock drought stress. Planta. 2011; 233: 471–484. 10.1007/s00425-010-1309-4 [DOI] [PubMed] [Google Scholar]
- 23. Hornyik C, Terzi LC, Simpson GG. The spen family protein FPA controls alternative cleavage and polyadenylation of RNA. Dev Cell. 2010; 18:203–213. 10.1016/j.devcel.2009.12.009 [DOI] [PubMed] [Google Scholar]
- 24. Liu F, Marquardt S, Lister C, Swiezewski S, Dean C. Targeted 3' processing of antisense transcripts triggers Arabidopsis FLC chromatin silencing. Science. 2010; 327: 94–97. 10.1126/science.1180278 [DOI] [PubMed] [Google Scholar]
- 25. Huang X, Qian Q, Liu Z, Sun H, He S, Luo D, et al. Natural variation in the DEP1 locus enhances grain yield in rice. Nat Genet. 2009; 41: 494–497. 10.1038/ng.352 [DOI] [PubMed] [Google Scholar]
- 26. Islam MS, Peng S, Visperas RM, Ereful N, Bhuiya MSU, Julfiquar AW. Lodging-related morphological traits of hybrid rice in a tropical irrigated ecosystem. Field Crops Res. 2007; 101:240–248. [Google Scholar]
- 27. Sakamoto T, Matsuoka M. Identifying and exploiting grain yield genes in rice. Curr Opin Plant Bio. 2008; l11: 209–214. 10.1016/j.pbi.2008.01.009 [DOI] [PubMed] [Google Scholar]
- 28. Ikeda-Kawakatsu K, Yasuno N, Oikawa T, Iida S, Nagato Y, Maekawa M, et al. Expression level of ABERRANT PANICLE ORGANIZATION1 determines rice inflorescence form through control of cell proliferation in the meristem. Plant Physiol. 2009; 150:736–747. 10.1104/pp.109.136739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luo L, Li W, Miura K, Ashikari M, Kyozuka J. Control of tiller growth of rice by OsSPL14 and Strigolactones, which work in two independent pathways. Plant Cell Physiol. 2012; 53: 1793–1801. 10.1093/pcp/pcs122 [DOI] [PubMed] [Google Scholar]
- 30. Yuan L. Hybrid rice breeding for super high yield. Hybrid Rice. 1997; 12:1–6. [Google Scholar]
- 31. Hiei Y, Ohta S, Komari T, Kumashiro T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994; 6: 271–282. [DOI] [PubMed] [Google Scholar]
- 32. Jiang C, Gao X, Liao L, Harberd NP, Fu X. Phosphate-starvation root architecture and anthocyanin accumulation of phosphate starvation responses are modulated by the GA-DELLA signaling pathway in Arabidopsis . Plant Physiol. 2007; 145:1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu G, Poethig RS. Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3 . Development. 2006; 133: 3539–3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Segregation of the BC2F2 population derived from the backcross between the selected BC1F2 progeny carrying the sdt allele and 9311 plant. Comparisons of plant height among homozygotes of the sdt allele, heterozygotes of the sdt and the SDT allele, and homozygotes of the SDT allele. Data represented as mean ± SE (n = 30).
(TIF)
Allelic variation at the sdt locus, including a one-nucleotide substitution (g.1205C>G), a 131-bp deletion (g.1280_1401del) and an insertion of DNA fragment of the mitochondrial gene ccmB. The numbers indicate the position of the genomic sequence counted from the transcription start site of LOC_Os06g44034, and the lines above DNA sequences represent the location of the exons.
(TIF)
The transcriptional levels were determined by qRT-PCR using young leaf tissues. Expression levels are expressed as the relative copies per 1000 copies of rice actin3. Data given as mean ± SE (n = 3).
(TIF)
Mature plant appearance of the transgenic WD44-SDT plants overexpressing LOC_Os06g44034.1 under the control of rice Actin promoter. Scale bar: 20 cm.
(TIF)
(a) The transcriptional levels of OsmiR156h were determined by qRT-PCR. Expression levels are expressed as the relative copies per 1000 copies of rice actin3. Data given as mean ± SE (n = 3). (b) Plant height. (c) Tiller numbers per plant. The transgenic plants were shown in Fig 1d.
(TIF)
(a) Mature plant appearance. Scale bar: 20 cm. (b) Plant height. (c) Tiller numbers per plant. Data given as mean ± SE (n = 10). A Student’s t-test was used to generate the P values.
(TIF)
A winter wheat variety KN199 was used to generate the transgenic plants carrying the p35S::OsmiR156h sdt construct. Scale bar: 20 cm.
(TIF)
The fragment of OsmiR156h precursor was amplified using the nested adaptor primer and specific primers for the first exon of LOC_Os06g44034.
(TIF)
Comparative DNA sequence of 3’-UTR was analyzed using 3’-RACE. The identical nucleotide sequences were showed by dark boxes, variant nucleotide sequence were shown by light boxes, and the polyA signal (“AATAAA”) was indicated by red boxes.
(TIF)
(a) Young tillers of 55-day-old plants. (b) Second topmost internodes of 80-day-old plants. (c) Flag leaf tissues of 80-day-old plants. The transcriptional levels of OsSPLs were determined by qRT-PCR. Transcript abundance relative to the level of the WD44-SDT plants set to be one. Data shown as mean ± SE (n = 3).
(TIF)
Mature plant appearance of the transgenic WD44-SDT plants carrying the pActin::OsSPL14 construct. Scale bar: 20 cm.
(TIF)
Mature plant appearance of field-grown two NILs plants. Scale bar: 20 cm.
(TIF)
All vectors have pCAMBIA2300 backbone.
(TIF)
(DOCX)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.