Abstract
When retinal cell cultures were mechanically scratched, cell growth over the empty area was observed. Only dividing and migrating, 2 M6-positive glial cells were detected. Incubation of cultures with apyrase (APY), suramin, or Reactive Blue 2 (RB-2), but not MRS 2179, significantly attenuated the growth of glial cells, suggesting that nucleotide receptors other than P2Y1 are involved in the growth of glial cells. UTPγS but not ADPβS antagonized apyrase-induced growth inhibition in scratched cultures, suggesting the participation of UTP-sensitive receptors. No decrease in proliferating cell nuclear antigen (PCNA+) cells was observed at the border of the scratch in apyrase-treated cultures, suggesting that glial proliferation was not affected. In apyrase-treated cultures, glial cytoplasm protrusions were smaller and unstable. Actin filaments were less organized and alfa-tubulin-labeled microtubules were mainly parallel to scratch. In contrast to control cultures, very few vinculin-labeled adhesion sites could be noticed in these cultures. Increased Akt and ERK phosphorylation was observed in UTP-treated cultures, effect that was inhibited by SRC inhibitor 1 and PI3K blocker LY294002. These inhibitors and the FAK inhibitor PF573228 also decreased glial growth over the scratch, suggesting participation of SRC, PI3K, and FAK in UTP-induced growth of glial cells in scratched cultures. RB-2 decreased dissociated glial cell attachment to fibronectin-coated dishes and migration through transwell membranes, suggesting that nucleotides regulated adhesion and migration of glial cells. In conclusion, mechanical scratch of retinal cell cultures induces growth of glial cells over the empty area through a mechanism that is dependent on activation of UTP-sensitive receptors, SRC, PI3K, and FAK.
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-015-9444-9) contains supplementary material, which is available to authorized users.
Keywords: Müller glia, Nucleotides, P2Y receptors, Adhesion, Migration, Cytoskeleton
Introduction
The vertebrate retina is widely used for approaching the development of the CNS, and genesis of all cell types during its development is well characterized [1]. In this tissue, Müller cells are the principal glia that interacts with the majority of neurons [2]. They have supportive function for retinal neurons, responding to and releasing a variety of molecules during development and in the adult tissue [3]. These cells regulate extracellular levels of K+, H+, and neurotransmitters, release vasoactive agents and D-serine, and inhibit cell swelling under hypotonic conditions, among other functions [4, 5].
Müller cells are generated from late developing progenitors within the retina. These progenitors leave cell cycle after most ganglion, amacrine, and horizontal cells, as well as photoreceptors, have already ceased mitosis and are differentiating [6]. Although, during normal development of the retina, Müller cells stop dividing, they are capable of re-entering the cell cycle during “reactive gliosis” in response to retinal damage or disease [4, 7, 8].
In the retina, ATP can be released through a calcium-dependent mechanism by application of several depolarizing stimuli such as light, KCl, and glutamate agonists [9–12]. ATP can also be released from pigmented epithelium (PE) by the opening of connexin 43 hemmichanels [13] or NMDA receptor stimulation [14]. Recently, the release of ATP in the retina or in cultures of retinal cells was observed in pathological conditions such as high glucose [15] or elevated intraocular pressure [16].
Both P1 and P2 purinergic receptors are expressed in retinal Müller cells. Although with variability between species, Müller cells express A1, A2A, and A2B adenosine receptors, as well as some P2Y and P2X nucleotide receptor subtypes [17]. Evidence for the presence of P2Y1, P2Y2, P2Y4, and P2Y6 in Müller cells from salamander, human, guinea pig, or rodent retina was obtained by elicitation of calcium responses with agonists, immunocytochemistry against receptor protein, or hybridization with cDNA probes for gene transcripts [18, 19].
During development, evidence for the presence of P2Y1 receptors in glial progenitors was obtained in chick embryo retinal cultures and early postnatal rodent retina [20–23]. Activation of these receptors by ATP or ADP induces the proliferation of glial progenitors by a mechanism involving PKC, MAPK, and PI3K/AKT pathways [23–26]. As opposed to early developing neuronal progenitors that are sensitive to UTP, no proliferation of late developing glial progenitors is observed by activation of UTP-sensitive nucleotide receptors in monolayer cultures or cultured explants of chick embryo retinas [20, 24]. The proliferative response of glial cells in culture to ATP decreases, as the cultured cells differentiate, and no effect of this nucleotide is observed 4 days after the onset of cultures obtained from retinas of 7-day-old chick embryos or 1-day-old mice [21, 23].
Besides proliferation, nucleotides also regulate migration and/or chemotaxis of a broad range of cell types, including immune cells, cardiac fibroblasts, epithelial, endothelial, or tumor cells, keratinocytes, and microglia [27]. During CNS development, nucleotides induce the migration of astrocyte progenitors [28] and neural stem cells [29]. When these cells are stimulated with ATP, UTP, or EGF, an increase in cell spreading and formation of stress fibers is observed [30].
In the present work, we investigated if nucleotides regulate the growth of Müller cells in scratched chick embryo retinal monolayer cultures. Our results show that activation of UTP—but not ADP-sensitive nucleotide receptors—induces glial cell growth in the scratched area by a mechanism that also involves PI3K, SRC, and FAK signaling pathways. Glia adhesion and migration, but not proliferation, are modulated by nucleotides.
Materials and methods
All procedures were performed according to the Guidelines for the Care and Use of Laboratory Animals, as described in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and by the National Institute of Health, and approved by the commission of animal care from Fluminense Federal University (CEPA/PROPPi-00132/09).
Materials
Fertilized White Leghorn chicken eggs were obtained from a local hatchery and incubated at 38 °C in a humidified atmosphere up to the appropriate stage. ADPβS, ADP, suramin, Reactive Blue 2 (RB-2), MRS 2179, anti-beta-tubulin III (TUJ1), anti-α-tubulin, pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid (PPADS), and UTP were from Sigma-Aldrich (St. Louis, MO, USA); [3H]-thymidine (5 Ci/mmol) was from PerkinElmer (São Paulo, SP, Brazil). Antibodies against proliferating cell nuclear antigen (PCNA, catalog # 2586), phospho-AKT (SER 473, catalog # 4060), and phospho-ERK 1/2 were from Cell Signaling Technology (MA, USA). Anti-transitin (catalog # EAP3) and anti-vinculin (catalog # VN 3-24) were from DSHB (IA, USA). Anti-P2Y2 (catalog # APR 010) and anti-P2Y4 (catalog # APR-006) were from Alomone Labs (Jerusalem, Israel). Monoclonal anti-2M6 was kindly provided by Dr. B. Schlosshauer (Max-Planck-Institute, Tübingen, Germany). Minimum Essential Medium (MEM) and fetal calf serum were from Life Technologies (São Paulo, SP, Brazil). Fluo-3 AM and phalloidin-Alexa 568 were from Molecular Probes (São Paulo, SP, Brazil). All other reagents were of analytical grade.
Retinal cell monolayer cultures
Chick embryos at embryonic day 8 (E8) were killed instantaneously by decapitation, and the eyes were removed and immediately transferred to Ca2+- and Mg2+-free balanced salt solution (CMF) where the retinas were dissected from other structures. Trypsin, at a final concentration of 0.1 %, was then added to the tissues and the suspension incubated at 37 °C for 20–25 min. Trypsin solution was removed and the retinas suspended in MEM containing 5 % fetal calf serum, 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Tissues were mechanically dissociated by successive aspirations of the medium and cells counted in a Neubauer Chamber. For scratch injury and [3H]-thymidine incorporation experiments, cells were seeded on culture dishes (internal diameter = 34 mm) at a density of 107 cells/dish (1.04 × 104 cells/mm2) and 5 × 106 cells/dish (5 × 103 cells/mm2), respectively. Cells were then incubated at 37 °C for the indicated periods of time, in humidified atmosphere of 95 % air/5 % CO2. The culture medium was exchanged every other day.
Enriched glia cultures
Purified cultures of glia were obtained as described by Loiola and Ventura [12] and maintained for about 3 weeks. The initial density of cells was 5.0 × 106 cells/dish and medium was changed regularly two times a week. Cultures were used after 20–22 days, when almost all neurons died and the cultures contained only glial cells.
Scratch injury
Cultures of retinal cells obtained from 8-day-old embryos and maintained for 7 days (E8C7) were injured one time with a 10-μL pipette tip (Axygen) by scratching out the monolayer and generating an area devoid of cells. Culture medium was changed, and cultures were immediately treated with apyrase or nucleotide agonists or antagonists for 3 days. Medium was changed every other day and drugs added again to the cultures. The area of scratch in the cultures was photographed under phase contrast illumination on a Nikon Eclipse microscope (×10) and the area devoid of cells determined by the Image J software previously calibrated to 1.06 pixel/μm. Ten fields of 0.325 mm2 per culture were photographed each day, and the growth of cells estimated as the decrease in the area devoid of cells. In some experiments, the scratch border area covered by glial cells between neurons and the center of the scratch was also estimated using the same procedure.
Immunofluorescence
Retinal cultures from 8-day-old embryos maintained for 10 days (E8C10) containing 107 cells/dish and scratched on day 7 were washed with phosphate-buffered saline (PBS) and fixed for 15 min in 0.16 M phosphate buffer, pH 7.6 with 4 % paraformaldehyde. After three washes of 5 min with PBS, pH 7.6, cells were permeabilized with 0.25 % triton X-100 for 30 min. Nonspecific sites were blocked by incubating cells for 60 min in PBS/Triton X-100 containing 0.1 % NGS and 5 % bovine serum albumin (BSA). Cells were incubated overnight at 4 °C with anti-2M6 (1:200), anti-beta-tubulin III (1:100), anti-alfa-tubulin (1:2000), or anti-vinculin (1:50) primary antibodies. Cultures were washed and incubated with Alexa secondary antibody (1:200) for 2 h at room temperature. Nuclei were counterstained with DAPI and cells examined and photographed with Leica SP5 confocal microscope. For PCNA labeling, scratched cultures were fixed, washed with PBS, and incubated with 10 mM citrate buffer (pH 6.0) for 10 min at 100 °C before the blockade of nonspecific sites. For visualization of actin filaments, scratched cultures on glass bottom dishes (FluoroDish, World Precision Instruments, Inc.) were incubated with phalloidin-Alexa 568 for 30 min at 4 °C and visualized on a Leica SP5 confocal microscope.
Live imaging
Retinal cells were seeded on glass bottom dishes (FluoroDish, WPI Inc.) and cultured for 7 days, when the cultures were scratched. After 2 days, culture medium was changed to MEM buffered with 25 mM HEPES (pH 7.4) plus serum and antibiotics and cultures mounted on a Leica SP5 confocal microscope equipped with a culture chamber. Cultures were maintained at 37 °C and photographed every 5 min under differential interference contrast illumination using a ×20 objective during 6 to 24 h.
Cell adhesion assay
Glia purified cultures were incubated with dissociation reagent (Tryple TM select, Life Tech. Inc.) for 3 min, at 37 °C. Dissociation medium was carefully removed and the cells dissociated in MEM with 5 % serum. Cells, at a density of 75,000 cells/well were seeded on 12-well dishes previously coated with 10 μg/mL of fibronectin and incubated for 2 h in the presence or absence of 2.5 U/mL of apyrase or 50–100 μM Reactive Blue-2 (RB-2). Cultures were washed four times with PBS to remove unattached cells and fixed with methanol. Ten fields along the diameter of each culture were photographed on a Nikon Eclipse microscope under phase contrast illumination using a ×10 objective. Cells were counted with the aid of the ImageJ software.
Chemotaxis assay
Glial cells obtained as described above were re-suspended in MEM with 5 % serum at 3 × 105 cells/mL. Corning transwell chambers (Sigma-Aldrich Inc.) with 8-μm pore polyester membrane inserts were coated with 10 μg/mL of fibronectin and placed into well of 24-well culture plates. The lower transwell chamber was filled with 600 μL of MEM-5 % serum containing 20 μM UTPγS or 100 μM RB-2. An aliquot of 100 μL of cell suspension was added to the upper chamber, and cells were further incubated at 37 °C. After 16–18 h in a 5 % CO2/95 % air atmosphere, the remaining cells in the upper chamber were removed by scraping the membrane with a cotton swab and cells attached to the bottom surface fixed with ice-cold methanol for 15 min. After two washes with PBS, cells were stained with hematoxylin for 30 min, washed, and counted in photographs of 10 fields obtained by sliding a ×20 objective of a Nikon inverted microscope along the diameter of the membrane insert.
[3H]-thymidine incorporation
Treated cultures were incubated with [3H]-thymidine (0.5 μCi) for 60 min, at 37 °C. Cultures were then washed four times with 2 mL BME buffered with 25 mM HEPES, pH 7.4, and the cells dissolved with 0.2 mL of 0.4 N NaOH. After dilution of the samples with 3 mL H2O, 0.6 mL of 50 % trichloroacetic acid (TCA) was added and the mixtures incubated, at 4 °C, for at least 30 min. The samples were filtered through Whatmann GF/B glass fiber filters and washed three times with 5 % TCA. Filters were dried and the radioactivity determined by scintillation spectroscopy.
Calcium imaging
The Fluo-3 AM indicator was used, following previous published protocols [31, 32]. In brief, cultures were incubated for 1 h in complete MEM medium containing 5 μM Fluo-3 AM, 0.2 % (v/v) Pluronic F-127, 0.5 % (v/v) DMSO, and 2.5 mM probenecid. Cells were washed with HBSS with probenecid and incubated for an additional 15 min to allow complete de-esterification of the AM ester. Live calcium imaging was carried out on a Leica TCS SP5 II confocal microscope, using a 488 laser line for excitation, images sizes of 256 × 256 pixels, and acquisition rates of 1 frame/s. Emitted fluorescence was recorded at wavelengths between 530 and 565 nm. Data were expressed as the ratio F/Fo with F and Fo representing the maximal Fluo-3 AM fluorescence emission during stimulation and the mean fluorescence emission before stimulation of the cells, respectively. Mean basal, non-stimulated level of Fluo-3 AM fluorescence was 6.8 ± 0.2 arbitrary units (N = 68 cells).
Western blotting
Retinal cells cultured as monolayers for 8 days (E8C8) were washed with MEM-HEPES, pH 7.4, pre-incubated for 30 min with 1 μM Src Inhibitor or 25 μM LY 294002, and then incubated with 100 μM UTP for 5 min at 37 °C. After stimulation, sample buffer without bromophenol blue (62.5 mM Tris-HCl, pH 6.8, containing 10 % glycerol, 2 % sodium dodecyl sulfate (SDS), and 5 % 2-mercaptoethanol) was added and cell extracts boiled for 10 min. Protein content in 2-μL samples of extracts was estimated by the Bradford protein assay, using BSA solution plus 2 μL sample buffer as standard. After addition of bromophenol blue (0.02 %), protein extracts (50 mg/lane) were size-fractionated on 9 % SDS polyacrylamide gel, transferred to PVDF membranes (GE Healthcare), stained with Ponceau red, and blocked in Tris-buffered saline (pH 7.6) with 0.1 % Tween-20 and 5 % non-fat milk. Membranes were incubated with anti-phospho-Akt or anti-phospho-ERK overnight, at 4 °C. Blots were developed using a secondary antiserum conjugated to horseradish peroxidase (Bio-Rad Labs Inc.) and enhanced chemiluminescence, according to the manufacturer’s protocol (ECL prime, GE Healthcare). In selected experiments, membranes were stripped and re-probed with anti-Akt or anti-ERK. Membranes containing proteins from extracts of purified glial cultures were incubated with anti-P2Y2 and anti-P2Y4 overnight, and blots were developed in the same way. The intensities of labeled bands in Western blot experiments were quantified by using TotalLab TL120 1D.
TUNEL assays
Retinal cultures treated or not with 2.5 U/mL of apyrase were fixed with 4 % paraformaldehyde for 1 h and washed three times with PBS. Apoptotic cells were labeled with the APO-BrdU ™ TUNEL Assay Kit (Molecular Probes) according to the provided protocol. Labeled cultures were photographed on a Leica SP5 confocal microscope. Experiments were replicated three times with similar results.
Statistical analysis
The statistical analysis was performed by ANOVA and the Bonferroni’s multiple comparison test.
Results
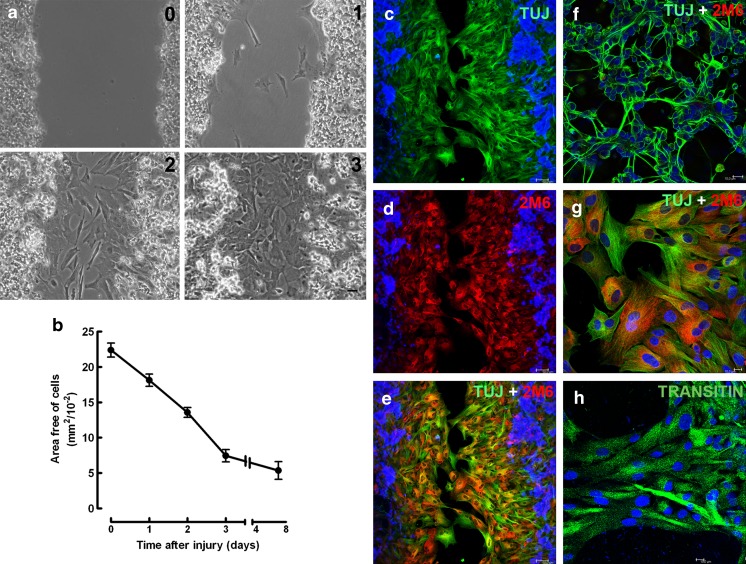
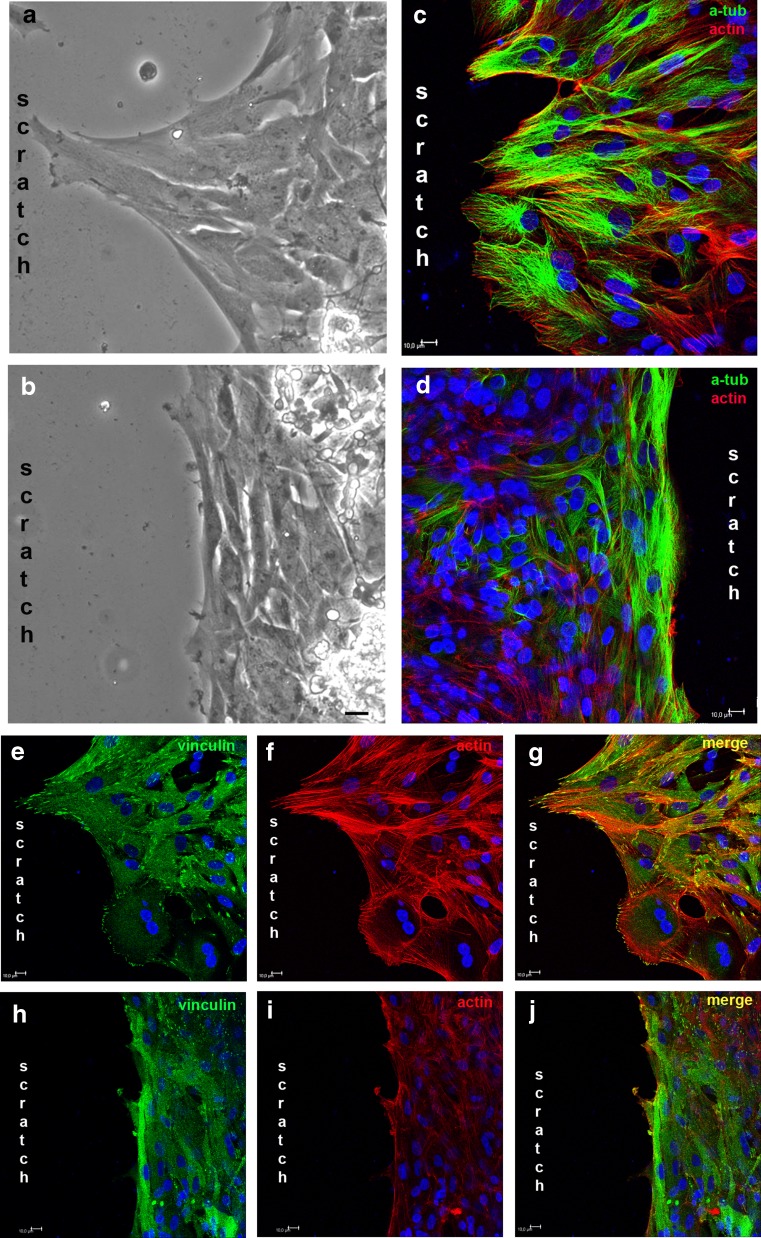
When retinal cultures obtained from 8-day-old embryos and cultivated for 7 days (E8C7) were scratched with a pipette tip, an area devoid of cells of 22.3 ± 1.0 mm2/10−2 could be determined in micrographs of 0.325-mm2 fields of the cultures (Fig. 1a, b). The area free of cells decreased progressively on the subsequent days, and 3 days after the scratch, the injured portion of the culture was populated with cells, with the empty area representing only 34.5 % of the original area (7.7 ± 0.9 mm2/10−2). After 7 days, the area devoid of cells decreased slightly further to 5.3 ± 1.2 mm2/10−2. Only glial cells labeled with anti-2M6 antiserum could be noticed in the scratched area. Although anti-β-tubulin III (TUJ) labeling was also observed over glial cells located in the scratched area (Fig. 1g), most of the β-tubulin III positive, 2M6 negative neurons were observed only at the margins of the scratched area or at regions away from the scratch (Fig. 1f). Moreover, very few neuronal processes were observed crossing or growing toward the scratched area, and glial cells located in this area were also positive for the progenitor marker transitin, the avian homolog of nestin (Fig. 1h).
Fig. 1.
Time course of glial growth over the scratched area in chick embryo retinal monolayer cultures. Retinal cell cultures from 8-day-old chick embryos maintained for 7 days (E8C7) were scratched, the medium changed, and cultures cultivated for several days. a Phase contrast micrographs showing the scratched area 0, 1, 2, or 3 days after the scratch of the cultures. b Quantification of the area free of cells as a function of time after the scratch. On the day indicated, 3 to 10 fields of 0.325 mm2 per culture were photographed under phase contrast illumination each day and the growth of cells estimated as the decrease in the area devoid of cells. c–e Three days after the scratch of the monolayer, immunocytochemistry against the neuronal marker beta-tubulin III (green) and the glial antigen 2 M6 (red) was performed. Cell nuclei were stained with DAPI (blue). f Photomicrograph of the cultures showing that neurons are beta-tubulin III positive, but 2M6 negative. g Confocal micrograph of the scratched area showing that 2 M6-positive glial cells (red) are also labeled with TUJ1 antiserum (green). h Photomicrograph of glial cells of the scratched area labeled for transitin (green). Quantitative data represent the mean ± SEM in mm2/10−2 of three to five separate experiments. Bar = 50 μm (a, c, d, e) and 10 μm (f, g, h)
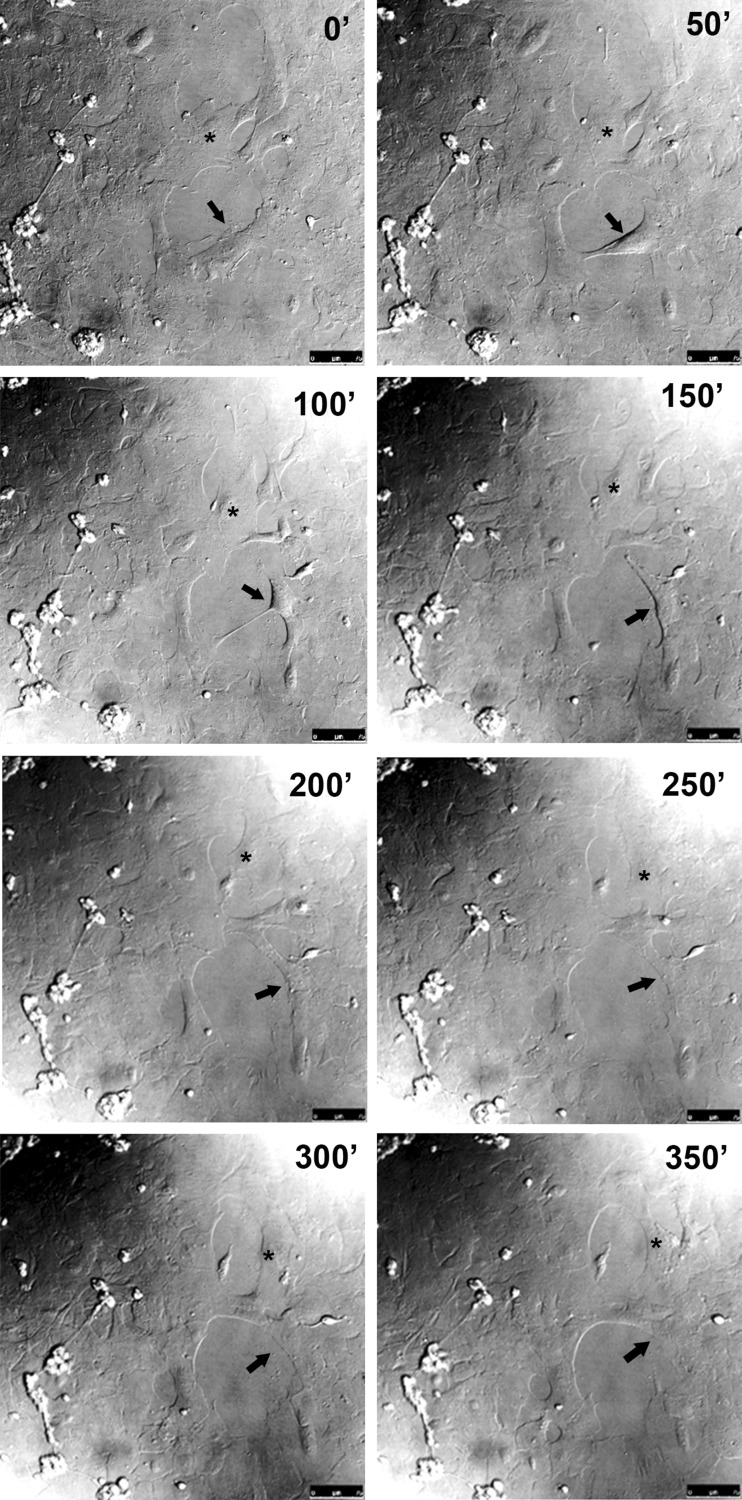
In order to verify if the filling of the scratched area involved glial cell proliferation and/or migration, the growth of cells over scratched areas of the cultures were recorded by time-lapse live imaging on the third day after scratching the cultures. Micrographs were taken every 5 min under differential interference contrast illumination. Both division (arrow) and movement of the glial cells (asterisk) were observed in the scratched areas as illustrated by the sequential micrographs in Fig. 2 and by the Online Resource 1.
Fig. 2.
Glial cell migration and division in the scratched area of retinal monolayer cultures. Retinal cell cultures at E8C7 were scratched, and after 3 days, culture medium was changed to MEM buffered with 25 mM HEPES (pH 7.4) plus serum and antibiotics and cultures mounted on a confocal microscope with a culture chamber. Cultures were photographed at the indicated time periods under differential interference contrast illumination using ×20 objective. A migrating (asterisk) and a dividing (arrow) glial cell are shown. Bar = 75 μm
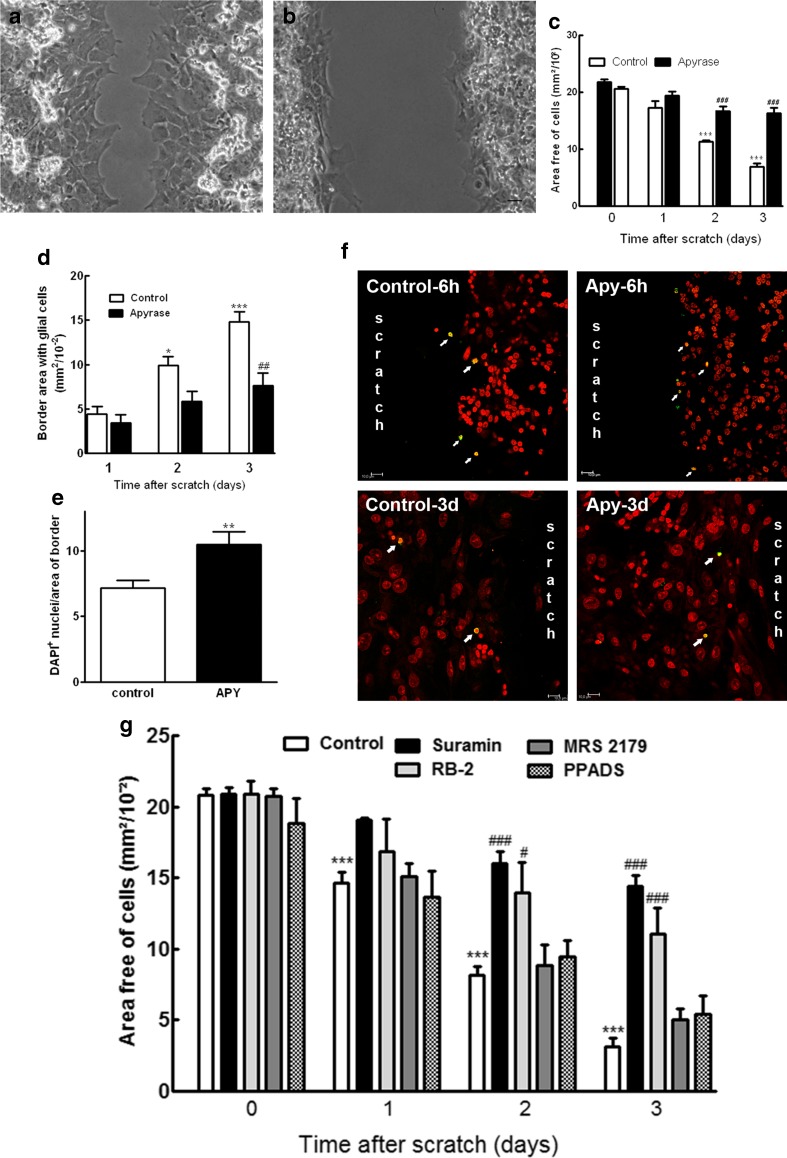
Previously, Sanches et al. [20] demonstrated that ATP through the activation of P2Y1 receptors induces the proliferation of retinal progenitors in culture, an effect that could be observed until the fourth day after the onset of retinal cell cultures [21]. However, besides their effect on the proliferation of glial progenitors, several experimental data suggest that nucleotides are involved in the growth of glial cells in the reactive gliosis that occurs when adult nervous tissue is injured [33, 34]. In order to investigate if nucleotides were involved in the growth of glial cells over the scratched area of the monolayer retinal cultures, scratched cultures at E8C7 were incubated with 2.5 U/mL of apyrase, an enzyme that hydrolyses nucleoside triphosphates and diphosphates (Fig. 3). While in control cultures a significant growth of glial cells was observed and the area devoid of cells decreased from 21.8 ± 0.4 mm2/10−2 at the time cultures were scratched to 6.8 ± 0.6 mm2/10−2 after 3 days (31 % of the original area), in apyrase-treated cultures, the area free of cells decreased only slightly, from 20.6 ± 0.4 mm2/10−2 to 16.3 ± 0.9 mm2/10−2 (79.1 % of the original area), suggesting that the addition of this enzyme significantly attenuated the growth of glial cells over the area of scratch (Fig. 3a–c). The effect of apyrase on the growth of glial cells after the scratch was also noticed when the area of the growing border was determined (Fig. 3d). While in control cultures the border area increased to 14.8 ± 1.1 mm2/10−2 3 days after the scratch, in apyrase-treated cultures, the area of the border was only 7.6 ± 1.4 mm2/10−2. Moreover, the inhibition of glial growth induced by apyrase was accompanied by an increase of ~46 % in the density of glia nuclei at the border of the scratch (Fig. 3e), but not by an increase in cell death. No increase in the number of TUNEL-positive cells was noticed in apyrase-treated cultures 6 h after the scratch or 3 days later (Fig. 3f)
Fig. 3.
Effect of apyrase and nucleotide P2 receptor antagonists on the growth and survival of glial cells in the scratched area of retinal monolayer cultures. Retinal cultures at E8C7 were scratched and treated with 2.5 U/mL apyrase. a, b Representative micrographs of scratched cultures cultivated in the absence or presence of apyrase for 3 days. c Effect of apyrase on the growth of glial cells over the scratched area as determined by quantifying the progressive decrease in the area free of cells in the scratched cultures. d Inhibition of the growth of glial cells induced by apyrase as determined by measuring the progressive increase of the border area with glial cells in scratched cultures. e Density of glia nuclei at the growing border of the cultures 3 days after the scratch. Density of nuclei was determined by the number of DAPI+ nuclei divided by the area of the border. f Representative micrographs showing apoptotic cells (arrows) in scratched cultures that were treated with 2.5 U/mL of apyrase for 6 h or 3 days, fixed, and processed by TUNEL assays as described in the “Materials and methods” section. Anti-BrdU (green) and propidium iodide (red) were used to label cells. g Effect of P2 receptor antagonists on the growth of glial cells over the scratched area. Retinal cultures at E8C7 were scratched and treated with 100 μM suramin or PPADS, 40 μM Reactive Blue 2 (RB-2), or 30 μM MRS 2179 for the next 3 days. The area free of cells was determined in 10 fields per culture as described in the “Materials and methods” section. Data represent the mean ± SEM of six separate experiments performed in duplicate (c) or three to eight separate experiments performed in duplicate (d, e, g). ***p < 0.001 compared to control cultures just after the scratch at time 0 (c, g) or control cultures 1 day after the scratch (d). # p < 0.5, ## p < 0.01, and ### p < 0.001 compared to control cultures at the corresponding time after the scratch of the cultures. Scale bar = 30 μm in a and b and 10 μm in f
The effect of P2 receptor antagonists on the growth of glial cells over the scratched area in injured cultures is shown in Fig. 3g. Both suramin and RB-2, two general P2 antagonists, significantly attenuated the decrease in the area free of cells observed in control cultures. The attenuation induced by these compounds was progressive, and 3 days after the scratch of the cultures, the area free of cells decreased to 14.8 % of the original area in the control cultures but represented 69.3 and 53.1 % of the original area in suramin- and RB-2-treated cultures, respectively. In contrast to suramin and RB-2, no significant effect of the specific P2Y1 receptor antagonist MRS 2179 (30 μM) or 100 μM PPADS was detected. Three days after the scratch, the area free of cells decreased to 24.0 and 25.9 % of the original area free of cells, respectively, values similar to the area observed in the control cultures. Although MRS 2179 did not affect the growth of glial cells over the scratched area in cultures at the stages between E8C7 and E8C10, this compound blocked, in a dose-dependent manner, the increase in the incorporation of [3H]-thymidine induced by 500 μM ADP in retinal cultures at E7C2 (Online Resource 2). Moreover, no effect of 50 μM ADPβS on the incorporation of [3H]-thymidine in retinal cultures at E8C8 was observed, corroborating previous evidences showing that activation of P2Y1 receptors by ATP induces the proliferation of glial progenitors in early developing, but not in more differentiated retinal cultures [20, 21].
The presence of P2Y receptors sensitive to ADP or UTP in glial cells growing over the scratched area was investigated by calcium imaging assays (Fig. 4). Retinal cultures at E8C7 were scratched and cultivated for 2 days. At E8C9, cultures were loaded with the calcium indicator Fluo-3 AM as described in the “Materials and methods” section and stimulated with 100 μM final concentration of ADP (Fig. 4, panels A, C, E, and G) or UTP (Fig. 4, panels B, D, F, and H). Both nucleotides evoked transient increases in [Ca2+]i in glial cells growing at the border of the scratched area. Peak values of the transient increases in [Ca2+]i represented 200 % (n = 15 cells) and 220 % (n = 16 cells) of the resting levels of [Ca2+]I for ADP and UTP, respectively.
Fig. 4.
Effect of ADP and UTP on the intracellular calcium content of glial cells growing in the scratched area of the cultures. Retinal cultures at E8C7 were scratched, cultivated for 2 days, and loaded with the calcium indicator Fluo3-AM as described in the “Materials and methods” section. a, b Confocal micrographs of glial cells at the border of the scratched area before stimulation with nucleotides. c, d Confocal micrographs of the same glial cells at the border of the scratched areas shown in a and b after stimulation with 100 μM ADP (c) or UTP (d). e, f Representative traces of [Ca2+]I transients, measured as peak heights, after ADP and UTP stimulation (arrows). g, h Intensity of fluorescence before (control) and peak intensity of fluorescence after ADP and UTP stimulation of the cultures. Data in g and h represent the mean ± SEM of fluorescence intensity of 15 and 16 glial cells recorded in three separate experiments. Bar = 30 μm
Suramin and RB-2 are antagonists that block several P2 receptor subtypes. In order to investigate further the nature of the P2 receptors involved in the glial cell growth over the scratched area, retinal cultures were injured at E8C7 and incubated in the following days with 2.5 U/mL of apyrase in the presence of 10 μM UTPγS (Fig. 5a) or 50 μM ADPβS (Fig. 5b), two hydrolysis-resistant analogs of UTP and ADP, respectively. While in control cultures the area free of cells decreased from 21.1 ± 0.6 mm2/10−2 to 5.1 ± 1.2 mm2/10−2, the corresponding area in apyrase-treated cultures decreased from 20.6 ± 0.6 mm2/10−2 to 15.1 ± 1.0 mm2/10−2 after 3 days of treatment. The addition of UTPγS to apyrase-treated cultures induced a significant decrease in the area free of cells after 3 days of incubation (from 20.6 ± 0.6 mm2/10−2 to 6.5 ± 1.9 mm2/10−2), an area very similar to the one observed in control cultures. In contrast, no decrease in the apyrase blockade of glial growth over the scratched area was observed with ADPβS. The area free of cells decreased from 21.9 ± 1.9 mm2/10−2 to 13.2 ± 0.6 mm2/10−2 in the cultures treated with apyrase plus ADPβS, a value similar to the decrease observed in cultures incubated only with apyrase (from 22.2 ± 1.4 mm2/10−2 to 13.9 ± 1.4 mm2/10−2).
Fig. 5.
Effect of UTPγS and ADPβS on the apyrase-mediated inhibition of glial growth in the scratched area of the cultures. Retinal cell cultures at E8C7 were scratched and incubated with 2.5 U/mL of apyrase in the absence or presence of 10 μM UTPγS (a) or 50 μM ADPβS (b) for the next 3 days. Data represent the mean ± SEM in mm2/10−2 of four or five separate experiments performed in duplicate. ***p < 0.001, compared to control cultures just after the scratch (time 0). # p < 0.05, ## p < 0.01, and ### p < 0.001, compared to control cultures at the corresponding time after scratch. §§§ p < 0.001, compared to apyrase-treated cultures
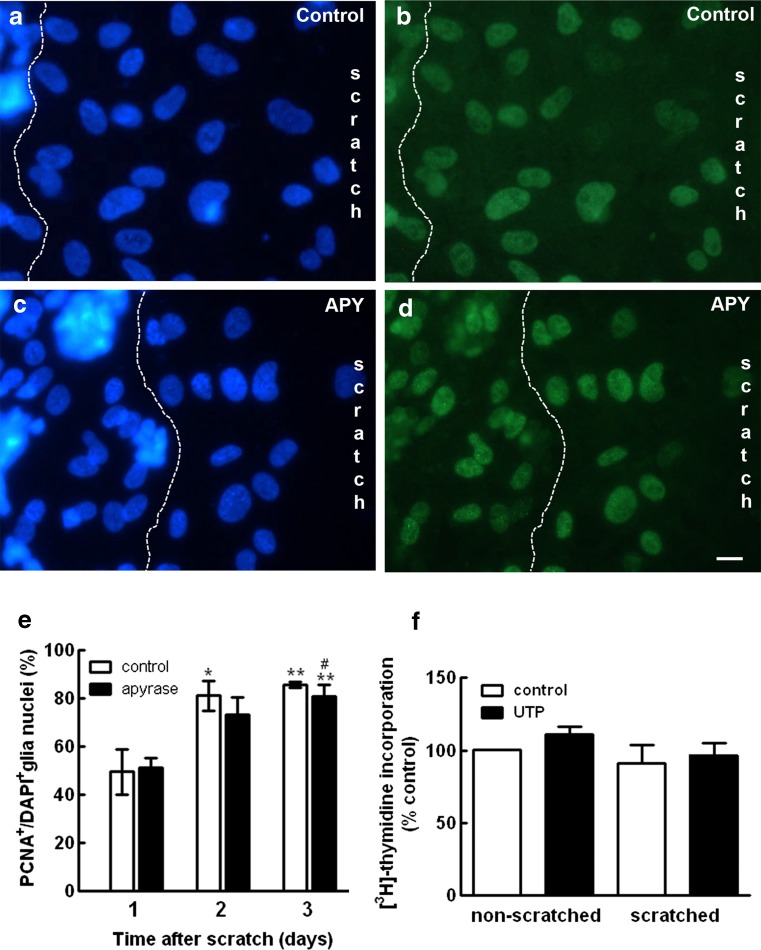
Although affecting different cell populations, both ADP and UTP induce cell proliferation in the early developing retina [13, 20, 21, 23, 24]. UTP also stimulates the proliferation of astrocytes from the cerebral cortex [35]. In order to verify if nucleotides were stimulating the proliferation of glial cells in the scratched area of the retinal cultures, the effect of apyrase on the number of proliferating PCNA-positive glial cells at the edge of the scratched area was evaluated (Fig. 6). Retinal cultures at E8C7 were scratched and incubated in the following days with or without 2.5 U/mL of apyrase. Cultures were fixed and labeled with anti-PCNA as described in the “Materials and methods” section. Neuronal and glial nuclei were labeled with DAPI and the edge of the scratched area photographed. The number of glial nuclei labeled for PCNA and/or DAPI was determined at the area between neurons and the center of the scratch in 10 micrographs of each culture as exemplified in Fig. 6a–d. While in control cultures the proportion of PCNA+/DAPI+ nuclei at the edge of the scratched area increased to 85.5 ± 1.2 % 3 days after the scratch (Fig. 6e), no significant decrease in the proportion of PCNA+/ DAPI+ nuclei was observed in the apyrase-treated cultures. Three days after the scratch, this ratio decreased only slightly to 80.8 ± 4.6 % in apyrase-treated cultures.
Fig. 6.
Effect of apyrase on glial cell proliferation at the edge of the scratched area of the cultures. Retinal cultures were scratched and incubated in the absence (a, b) or presence (c, d) of 2.5 U/mL of apyrase for 3 days. Cell nuclei were stained with DAPI (a, c) or with antiserum against PCNA (b, d). e Ratio of PCNA+/DAPI+ glial nuclei determined in micrographs of the edge of the scratch. The area of neurons was excluded with a dashed line as shown in a–d, and glia nuclei were counted at the area between neurons and the center of the scratch. PCNA+/DAPI+ ratios were determined 1, 2, or 3 days after scratching the cultures. f Cultures at E8C8 that were scratched or not at E8C7 were incubated with 100 μM UTP for 24 h and the incorporation of [3H]-thymidine determined as described in the “Materials and methods” section. Data were expressed as % control of non-scratched cultures and represent the mean ± SEM of three to six separate experiments performed in duplicate. Bar = 10 μm
Since UTPγS antagonized the inhibition of the glial growth induced by apyrase in the scratched area of the retinal cultures (Fig. 5a), the effect of UTP on the incorporation of [3H]-thymidine in the cultures was also evaluated (Fig. 6f). Cultures at E8C8 that were scratched or not at E8C7 were incubated with 100 μM UTP for 24 h and the incorporation of [3H]-thymidine determined as described in the “Materials and methods” section. Similar levels of [3H]-thymidine incorporation were observed in cultures that were scratched or not at E8C7. Moreover, no significant increase in the incorporation of [3H]-thymidine was observed by treating both types of cultures with UTP.
In cultured murine neural stem cells, nucleotide- and EGF-dependent cell migration and actin cytoskeletal rearrangements were associated with the phosphorylation of FAK and Akt [30]. Moreover, activation of the UTP-sensitive nucleotide P2Y2 receptor, a protein that contains two Src-homology-3 (SH3) binding domains that interact with Src [36], induces the migration of rat astrocytes in culture [37]. In order to identify potential intracellular signaling pathways involved in nucleotide-induced growth of glial cells in the scratched area, the effect of UTP on the phosphorylation of Akt and ERKs was investigated. Retinal cultures at E8C8 were incubated for 5 min with 100 μM UTP, in the absence or presence of 1 μM Src inhibitor-1 or 25 μM LY294002. As revealed by the Western blotting experiments shown in Fig. 7, UTP induced a significant increase in the phosphorylation of Akt and ERK in the cultures, effects that were significantly decreased by the Src and PI3K inhibitors.
Fig. 7.
UTP-induced phosphorylation of Akt and ERK in retinal cultures: inhibition by Src and PI3K inhibitors. Retinal cultures at E8C8 were incubated with 100 μM UTP for 5 min in the absence or presence of 1 μM Src inhibitor-1 or 25 μM LY294002. a Representative blots of three independent experiments performed in duplicate. b, c Blots were quantified by densitometry and expressed as mean ± SEM (arbitrary units). ***p < 0.001, compared to control cultures. ## p < 0.01 and ### p < 0.001, compared to UTP-treated cultures
The effect of inhibitors of intracellular signaling pathways on the growth of glial cells over the scratched area was also evaluated. Retinal cultures at E8C7 were scratched and incubated in the following days with the Src inhibitor-1 (0.2 μM), the PI3K inhibitor LY294002 (10 μM), the FAK inhibitor PF 573228 (10 μM), or with the MEK inhibitors PD98059 (10 μM) and U0126 (10 μM) (Fig. 8). Attenuations of glial growth by Src inhibitor-1, PF 573228, and LY294002 were statistically significant and around 70, 52, and 41 %, respectively. Although both MEK inhibitors showed some attenuation of glial growth in the scratched area (25.1 and 34.2 % for PD98059 and U0126, respectively), their effects were not statistically significant.
Fig. 8.
Effect of intracellular signaling inhibitors on the growth of glial cells over the scratched area of retinal monolayer cultures. Retinal cultures at E8C7 were scratched and incubated with 0.2 μM Src inhibitor-1, 10 μM PF 573228, 10 μM LY294002, 10 μM PD 98059, or 10 μM U0126 for the next 3 days. Data are expressed as mean ± SEM in mm2/10−2 of three to five separate experiments performed in duplicate. ***p < 0.001 or **p < 0.01, compared to control cultures just after the scratch (time 0). # p < 0.05, ## p < 0.01, and ### p < 0.001, compared to control cultures at the corresponding time after scratch
The effect of apyrase on the morphology and cytoskeleton of glial cells at the border of the scratched area is shown in Fig. 9. While scratched control cultures showed glial cells with cytoplasm protrusions toward the empty area, glial cells from scratched apyrase-treated cultures were smaller, many of them with the cytoplasm parallel to the border of the scratch and without protrusions oriented to the empty area (Fig. 9a, b). Moreover, while in control cultures many glial cells showed lamellipodia-like protrusions with phalloidin-labeled stress fibers and alfa-tubulin network spreading toward the scratched area (Fig. 9c), in apyrase-treated cultures, a lower phalloidin labeling in glial cells could be noticed and alfa-tubulin formed bundles parallel to the border of the scratched area (Fig. 9d). The same pattern of disorganized phalloidin-labeled actin filaments was observed when cultures were treated with 0.2 μM Src inhibitor-1 (data not shown).
Fig. 9.
Effect of apyrase on the morphology and cytoskeletal arrangement of glial cells at the edge of the scratched area. Cultures at E8C7 were scratched and incubated with 2.5 U/mL of apyrase for 3 days. Then, cultures were fixed and incubated with anti-α-tubulin or anti-vinculin. Phalloidin-Alexa 568 was used to label actin filaments. a, b Micrographs photographed under phase contrast illumination of control and apyrase-treated cultures showing the morphology of glial cells at the border of the scratched area. c, d Alfa-tubulin and actin labeling in control (c) and apyrase-treated (d) cultures. e, f Labeling against vinculin (green) and actin (red) in control cultures. h, i Labeling against vinculin (green) and actin (red) in apyrase-treated cultures. Merged figures are shown in (g) and (h). Bar = 20 μm (a, b) and 10 μm (c–j)
The parallel arrangement of α-tubulin and absence of cytoplasmic protrusions in glial cells at the border of the scratch in the apyrase-treated cultures could be due to a lower adhesion of these cells to the substrate. In order to investigate this possibility, labeling of vinculin, a protein associated with adhesion sites in migrating cells, was performed in scratched cultures that were cultivated in the presence or absence of 2.5 U/mL of apyrase for 3 days (Fig. 9e–j). Phalloidin was used to label actin fibers. Several vinculin-expressing puncta located at the end of stress fibers in cytoplasmic protrusions of glial cells growing toward the scratch could be noticed in control cultures (Fig. 9e–g). In contrast, vinculin-labeled puncta were rarely distinguished in glial cells in apyrase-treated cultures (Fig. 9h–j).
In order to characterize further the effect of apyrase on the growth of glial cells in the scratched area, the border of the scratch in apyrase-treated cultures were recorded by time-lapse live imaging between the second and the third days after scratching the cultures. Micrographs were taken every 5 min under differential interference contrast illumination. As illustrated by the sequential micrographs in Fig. 10 and by the Online Resource 3, glial cell protrusions toward the empty area were smaller and unstable, soon retracting toward the cell soma at the border of the scratch. As opposed to control cultures, no glial cell moving away from other glial cells at the border of the scratch was noticed in apyrase-treated cultures. However, as in control, glial cell division was frequently noticed in these cultures. Similar observations were obtained in RB-2-treated cultures (data not shown).
Fig. 10.
Absence of glial cell migration at the scratched area of retinal cultures treated with apyrase. Retinal cell cultures at E8C7 were scratched and treated with 2.5 U/mL of apyrase for 2 days. Culture medium was changed to MEM buffered with 25 mM HEPES (pH 7.4) plus apyrase, serum, and antibiotics and cultures mounted on a confocal microscope with a culture chamber. Cultures were photographed at the indicated time periods under differential interference contrast illumination using ×20 objective. Note the small and unstable glia protrusion (white arrow). Bar = 30 μm
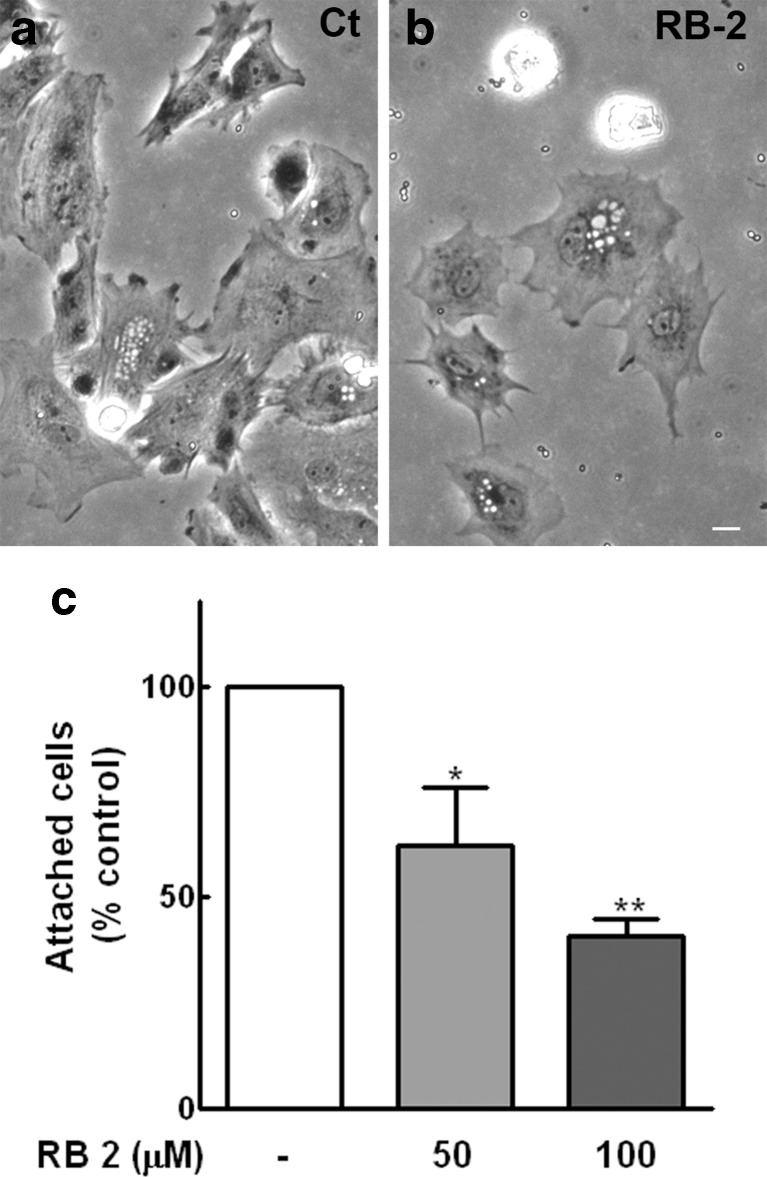
Cell migration is a complex, multistep process that encompasses cell polarization, protrusion, adhesion, and forward cell displacement [38]. Since vinculin-labeled puncta over glia were decreased and the displacement of these cells in apyrase-treated cultures seemed not to be complete, the effect of RB-2 on the attachment of glial cells to fibronectin-coated dishes was performed. Dissociated glial cells at a density of 75.000/well were added to 12-well culture dishes previously coated with 10 μg/mL of fibronectin in the presence or absence of RB-2 at 50 or 100 μM concentrations. After 2 h, cultures were extensively washed, fixed, and photographed, and the number of attached cells was estimated. While only a small and not significant decrease of 13.5 % in the amount of attached cells was observed in apyrase-treated cultures, treatment of glial cells with 50 or 100 μM RB-2 decreased significantly the number of attached cells by 37.5 and 58.9 %, respectively (Fig. 11c). The morphology of the attached glial cells in the cultures is shown in Fig. 11a, b.
Fig. 11.
Effect of RB-2 on glial cell adhesion to fibronectin-coated culture dishes. Glial cells from purified cultures with 20–22 days were dissociated and seeded at a density of 75,000 cells/well on 12-well culture dishes previously coated with 10 μg/mL of fibronectin, in the presence or absence of RB-2 at 50 or 100 μM concentrations. After 2 h, cultures were extensively washed, fixed, and photographed, and the number of attached cells counted. Representative micrographs of control (a) and 100 μM RB-2-treated cultures (b). c Quantification of cell attachment. Cells in 10 fields along the diameter of each dish were counted. Data represent the mean ± SEM (% of control) of three experiments performed in duplicate. *p < 0.05 and **p < 0.01 compared to control. Bar = 20 μm
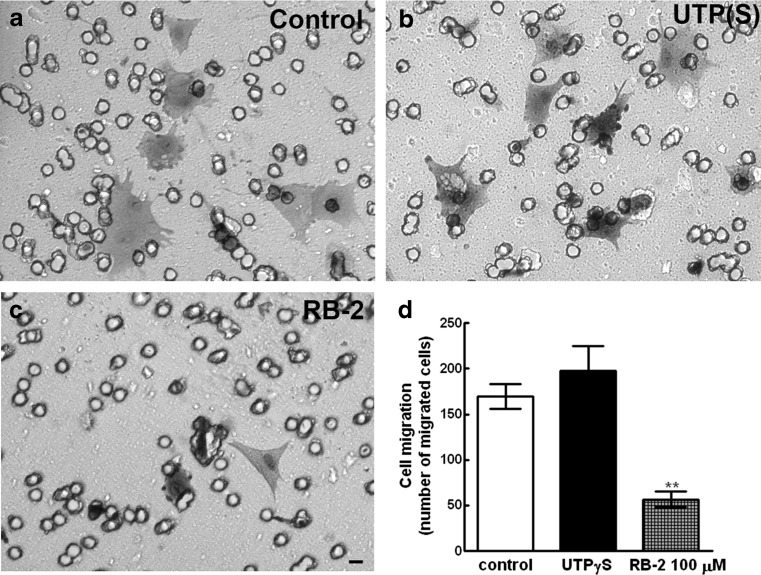
Retinal glial cell migration was also evaluated using transwell inserts in chemotaxis assays (Fig. 12). Addition of 100 μM RB-2 to the lower chamber of cell culture dishes containing inserts with 8-μm polyester membranes caused a significant decrease of approximately 66.7 % in the number of cells at the lower side of the membrane (Fig. 12d). In contrast, addition of 20 μM UTPγS increased the number of cells at the lower side of the insert by ~17 %. However, this increase was not statistically significant.
Fig. 12.
Effect of UTPγS and RB-2 on glial cell migration. Transwell inserts with 10 μg/mL fibronectin-coated polyester membranes were used to separate two chambers in 24-well dishes. Glial cells from purified cultures with 20–22 days were dissociated and seeded in serum-containing medium (3 × 104 cells/chamber) onto membrane in the upper chamber, whereas the lower chamber contained 20 μM UTPγS or 100 μM RB-2. After 4.5 h at 37 °C, cells at the lower side of the membranes were fixed, stained, and counted in 10 fields along the diameter of the membrane as described in the “Materials and methods” section. a–c Representative micrographs of migrated cells in control, UTPγS-, and RB-2-treated cultures. d Quantification of migrated cells. Data represent the mean ± SEM of four experiments performed in duplicate. Bar = 20 μm
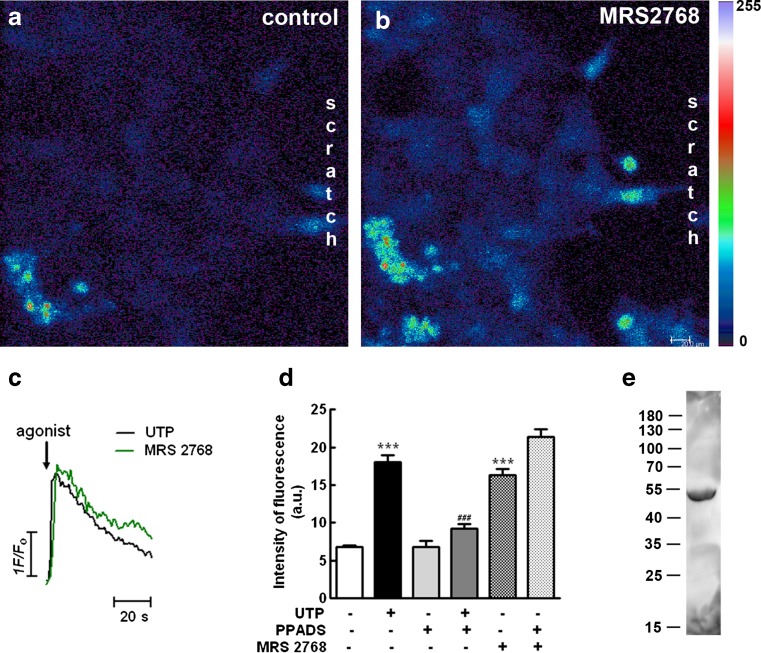
The UTP-sensitive P2Y2 receptor was implicated in migration of cultured astrocytes [37]. In order to determine the presence of this receptor subtype in glial cells growing toward the scratched area, retinal cultures at E8C7 were scratched and cultivated for 3 days. At E8C10, cultures were loaded with the calcium indicator Fluo-3 AM as described in the “Materials and methods” section and stimulated with 20-μM final concentration of the P2Y2-specific agonist MRS2768 or 100 μM UTP, in the absence or presence of 100 μM PPADS, a relative weak antagonist for the P2Y4 receptor (Fig. 13a–d). Both agonists evoked transient increases in [Ca2+]i in glial cells growing at the border of the scratched area. Peak values of the transient increases in [Ca2+]i represented 240 % (n = 20 cells) and 265 % (n = 36 cells) of the resting levels of [Ca2+]i for MRS 2768 and UTP, respectively. Moreover, while the antagonist PPADS decreased the calcium response induced by UTP to 135 % of the resting levels of [Ca2+]i, no significant effect of this antagonist was observed on the calcium response induced by MRS 2768, and levels of [Ca2+]i represented 315 % of the resting levels.
Fig. 13.
Effect of the P2Y2 receptor agonist MRS 2768 on the intracellular calcium content of glial cells growing in the scratched area of the cultures and expression of P2Y2 receptor protein in purified glial cultures. Retinal cultures at E8C7 were scratched, cultivated for 3 days, and loaded with the calcium indicator Fluo3-AM as described in the “Materials and methods” section. a Confocal micrograph of glial cells at the border of the scratched area before stimulation with agonist. b Confocal micrograph of the same glial cells at the border of the scratched areas shown in a after stimulation with 20 μM MRS 2768. c Representative traces of [Ca2+]I transients, measured as peak heights, after 100 μM UTP or 20 MRS 2768 stimulation (arrow). d Intensity of fluorescence before (control) and peak intensity of fluorescence after stimulation of the cultures with 100 μM UTP or 20 μM MRS 2768 in the presence or not of 100 μM PPADS. e Detection of P2Y2 receptor subtype in extracts from purified glial cultures. The numbers on the left represent molecular weights in kilodalton. Data in d represent the mean ± SEM of fluorescence intensity of 15 to 68 glial cells recorded in two to six separate experiments. Bar = 20 μm
Immunoblotting experiments using an antiserum against the rat P2Y2 receptor revealed the presence of this receptor protein in extracts from purified retinal glial cultures (Fig. 13e). The estimated molecular weight for the chicken receptor protein was ~50 kDa, a value very similar to the value described for the rat P2Y2 receptor by the manufacturer. In these preparations, no labeled band was detected using the antiserum #APR-006 from Alomone against the rat P2Y4 receptor (data not shown).
Discussion
In the present work, we show that only retinal glial cells grow progressively toward the area devoid of cells in retinal cultures that were mechanically scratched. This response was blocked by apyrase, suggesting that nucleotides participate in the growth of glial cells. This idea is reinforced by the observation that suramin or Reactive Blue 2, two general P2 nucleotide receptor antagonists, blocked the growth of glial cells.
Live imaging experiments revealed that glial cells proliferate, expand cytoplasmic protrusions, and migrate intensively at the scratched area. Since proliferation of retinal progenitors in the chick developing retina is stimulated by nucleotides like ADP and UTP [13, 20, 21, 24], the growth inhibitory effect of apyrase and P2 receptor antagonists could be due to inhibition of glia proliferation in the scratched area. However, no effect of apyrase on the number of PCNA+ cells was observed at the border of the scratch, suggesting that nucleotides did not affect the proliferation of these cells. In good agreement with this hypothesis is our observation that neither UTP nor ADP stimulated the incorporation of [3H]-thymidine in the cultures, no matter if they were scratched or not. Moreover, previous data showed that both UTP- and ADP-mediated increase in retinal cell proliferation occurs only in early developing tissues, during a stage where progenitors are still proliferating [13, 20, 21]. In retinal tissues from embryos older than 9-day-old or in retinal cultures from 7-day-old animals cultivated for 4 or more days, no effect of ATP on cell proliferation was observed in previous studies [20, 21], and the proliferative activity of glial cells at the border of the scratch in our cultures most likely is regulated by trophic factors other than nucleotides. An interesting possibility that deserves to be investigated further is whether growth factors such as EGF, IGF-1, or FGF can modulate the proliferation of glial cells in scratched retinal cultures. It was established that these factors induce the proliferation of Müller glial cells in adult chick retinas, submitted or not to chemical injury [39, 40].
Inhibition of the growth of glial cells in scratched cultures by apyrase was antagonized by the UTP hydrolysis-resistant analog UTPγS, but not by ADPβS, suggesting that nucleotide-dependent growth of glial cells is related to UTP—but not to ADP-sensitive P2Y receptor subtypes. ADP-sensitive P2Y1 receptors were previously described in Müller glial cells [22], a finding that is corroborated by our present observation that ADP induces intracellular calcium increases in Müller cells growing at the edge of the scratch in the cultures. Moreover, although MRS2179, a specific antagonist of P2Y1 receptors, significantly decreased ADP-induced proliferation of glial progenitors in early developing retinal cultures, this compound did not affect the growth of glia in the scratched cultures, reinforcing our hypothesis that UTP preferring P2Y2/P2Y4 receptors are the sites involved in the growth of glia to the scratched area. However, we cannot exclude completely the involvement of other P2 receptors in glial cell growth, since suramin and RB-2 are not selective antagonists for P2Y2/P2Y4 receptors.
While several reports have shown that nucleotides can induce the migration of different cell types in culture [27], cell migration is a complex coordinated process of cell polarization, protrusion of the cytoplasm, adhesion, and forward displacement of the cell [38]. In the present study, live imaging experiments revealed that in cultures that were treated with apyrase, glial cells at the border of the scratch formed only small cytoplasmic protrusions that soon retracted toward the cell soma. Moreover, as opposed to control cultures, detachment of glial cell bodies away from the rest of glial cells of the border of the scratch was never observed in these apyrase-treated cultures, suggesting that adhesion and/or migration of glial cells required nucleotide receptor activation to occur properly. In good agreement with this possibility is our observation that addition of the P2 receptor antagonist RB-2 attenuated significantly dissociated glial cell adhesion to fibronectin-coated culture dishes, as well as glia migration through fibronectin-coated membranes of trans-well inserts. The observations that, in apyrase-treated cultures, phalloidin-labeled actin cytoskeleton was less organized, α-tubulin labeled microtubules were arranged in parallel orientation relative to the scratched area, and vinculin-labeled adhesion sites were hardly found, also support this hypothesis. However, since the effect of RB-2 on cell adhesion was characterized using dissociated glial cells, we cannot exclude the possibility that apyrase and antagonists affected additional mechanisms other than glial cell attachment to the substrate and migration in the scratched cultures. In this latter preparation, glial cells are attached to each other at the border of the scratch. It was demonstrated previously that cultured migrating astrocytes establish lateral junctions containing N-cadherin that regulate cell orientation in the direction of the free cell edge [41]. Since nucleotides induce the expression of N-cadherin in cultured astrocytes [42] as well as in transformed cells [43], one interesting point that deserves to be further investigated is whether nucleotides alter the expression of cell adhesion molecules in glial cells growing in scratched retinal cultures.
Cell migration entails activation of a variety of molecular and cellular events that converge in morphological changes and cell displacement. Different intracellular signaling pathways activated by membrane receptors were implicated in these events, including nucleotide receptors [27]. For example, phosphorylation of Akt and FAK induced by nucleotides and EGF were associated with migration of neural stem cells [30]. PI3K/Akt or MEK/ERK pathways were found to mediate UTP-induced migration of astrocytes [37] or smooth muscle cells [44]. In the present study, we found that, in contrast to the MEK inhibitors U0126 and PD98059 that showed small effects, inhibitors of PI3K and SRC as well as the FAK inhibitor PF573228 attenuated the decrease in the area free of cells in scratched cultures, suggesting that growth of glia toward the scratch occurs through a mechanism involving the activation of PI3K, SRC, and FAK and possibly the ERK pathway. Noteworthy, adhesion and migration of dissociated glial cells were blocked by the antagonist RB-2, and glial growth in scratched cultures was attenuated by apyrase and nucleotide antagonists. Therefore, one interesting possibility would be that nucleotide-dependent adhesion and/or migration of glial cells was mediated by PI3K, SRC, and FAK activated by nucleotide receptors. Accordingly, UTP significantly stimulated the phosphorylation of Akt in the retinal cultures, an effect that was also blocked by the PI3K and SRC inhibitors. However, activation of these intracellular pathways could be related to other receptors or additional mechanisms also required for the growth of glial cells in the scratched cultures. These possibilities deserve to be further explored.
Finally, although Muller cells from adult human and rat retinas express transcripts for both P2Y2 and P2Y4 receptors [17], only immunoreactivity for the P2Y4 receptor protein was detected in Muller cells from the rodent retina [22], suggesting that these cells express only the P2Y4 UTP-sensitive receptor subtype. In the present work, we detected the P2Y2 receptor protein with an antiserum against the rat P2Y2 receptor in extracts from purified chick retinal glial cultures. Moreover, besides UTP, the P2Y2 receptor specific agonist MRS 2768 induced intracellular calcium increases in Muller cells at the border of the scratch, suggesting the presence of functional P2Y2 receptors that could mediate UTP-induced growth of glial cells in scratched cultures. In good agreement with this possibility are previous evidences implicating this receptor in the migration of cultured astrocytes [37]. However, due to the poor specificity of the commercially available antagonists for P2Y2 and P2Y4 receptors, our data cannot exclude the involvement of the P2Y4 receptor in the growth of glial cells in scratched cultures. While both suramin and RB-2 blocked the growth of glial cells toward the scratched area, PPADS, a compound that, like RB-2, is considered as a weak antagonist for P2Y4 receptor, had no effect. Since PPADS blocked the increase in intracellular calcium induced by UTP but not by the P2Y2 receptor agonist MRS 2768 in glial cells growing at the border of the scratch, our data, collectively, support the idea that both P2Y2 and P2Y4 receptors are expressed in these cells. Additional experiments with more specific agonists and antagonists will help to define which UTP-preferring receptor is involved in adhesion and migration of glia cells in scratched retinal cultures.
Electronic supplementary material
Glial cell division and migration in control scratched cultures. Cultures at E8C7 were scratched and after 3 days, medium was changed to MEM buffered with 25 mM HEPES (pH 7.4) plus serum and antibiotics and cultures mounted on a Leica SP5 confocal microscope. Time-lapse live imaging were performed by photographing cultures every 5 min during 6 h under differential interference contrast illumination using a 20x objective plus 1.5 x zoom. Bar = 75 μm. (MPG 2150 kb)
Effect of P2Y1 receptor antagonist and ADPβS on the incorporation of [ 3 H]-thymidine in retinal cell cultures. Retinal cultures were treated for 24 h with MRS2179 or ADPβS and the incorporation of [3H]-thymidine determined as described in the section of methods. (A) Retinal cultures at E7C1 were incubated with 500 μM ADP in the absence or presence of 5, 25 or 50 μM MRS 2179. (B) Retinal cultures at E8C8 that were scratched or not (control) were incubated with 50 μM ADPβS. Data represent the mean ± SEM of 3 to 5 independent experiments performed in duplicate. *** p < 0.001, compared to control cultures. ## p < 0.01 and ### p < 0.001, compared to ADP-treated cultures. (PPTX 67 kb)
Glial cell activity at the edge of the scratch in apyrase-treated cultures. Cultures at E8C7 were scratched and treated with 2.5 U/mL apyrase. After 3 days, medium was changed to MEM buffered with 25 mM HEPES (pH 7.4) plus apyrase, serum and antibiotics and cultures mounted on a Leica SP5 confocal microscope. Time-lapse live imaging were performed by photographing cultures every 5 min during ~8 h under differential interference contrast illumination using a 20x objective plus 1.5 x zoom. (MPG 3014 kb)
Acknowledgments
We would like to thank Maria Leite Eduardo and Sarah A. Rodrigues for technical assistance. This work was supported by grants from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Coordenação de Aperfeiçoamento de Pessoal do Ensino Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Pró-reitoria de Pesquisa, Pós-graduação e Inovação (Proppi-UFF). I.M.O is the recipient of post-doctoral fellowship from PNPD-CAPES. Transitin (EAP3) and vinculin (VN 3-24) monoclonal antibodies developed respectively by G.J. Cole and Shinsuke Saga were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242. H.U. acknowledges grant support from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and CNPq, Brazil.
Conflict of interest
I declare that all authors have no conflict of interest.
References
- 1.Adler R. A model of retinal cell differentiation in the chick embryo. Prog Retin Eye Res. 2000;19(5):529–557. doi: 10.1016/S1350-9462(00)00008-2. [DOI] [PubMed] [Google Scholar]
- 2.Sarthy V, Ripps H. The retinal Müller cell - structure and function. New York: Kluwer Academic / Plenum Publishers; 2001. [Google Scholar]
- 3.Reis RAM, Ventura ALM, Schitine CS, de Mello MC, de Mello FG. Müller glia as an active compartment modulating nervous activity in the vertebrate retina: neurotransmitters and trophic factors. Neurochem Res. 2008;33:1466–1474. doi: 10.1007/s11064-008-9604-1. [DOI] [PubMed] [Google Scholar]
- 4.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25(4):397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Reichenbach A, Bringmann A. New functions of Müller cells. Glia. 2013;61(5):651–678. doi: 10.1002/glia.22477. [DOI] [PubMed] [Google Scholar]
- 6.Martins RA, Pearson RA. Control of cell proliferation by neurotransmitters in the developing vertebrate retina. Brain Res. 2008;1192:37–60. doi: 10.1016/j.brainres.2007.04.076. [DOI] [PubMed] [Google Scholar]
- 7.Bringmann A, Wiederman P. Müller glia cells in retinal disease. Ophthalmologica. 2011;227(1):1–19. doi: 10.1159/000328979. [DOI] [PubMed] [Google Scholar]
- 8.Dyer MA, Cepko CL. Regulating proliferation during retinal development. Nat Rev Neurosci. 2001;2:333–342. doi: 10.1038/35072555. [DOI] [PubMed] [Google Scholar]
- 9.Perez MT, Ehinger BE, Lindström K, Fredholm BB. Release of endogenous and radioactive purines from the rabbit retina. Brain Res. 1986;398(1):106–112. doi: 10.1016/0006-8993(86)91255-2. [DOI] [PubMed] [Google Scholar]
- 10.Santos PF, Caramelo OL, Carvalho AP, Duarte CB. Characterization of ATP release from cultures enriched in cholinergic amacrine-like neurons. J Neurobiol. 1999;41(3):340–348. doi: 10.1002/(SICI)1097-4695(19991115)41:3<340::AID-NEU3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 11.Newman EA. Calcium increases in retinal glial cells evoked by light-induced neuronal activity. J Neurosci. 2005;25(23):5502–5510. doi: 10.1523/JNEUROSCI.1354-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loiola EC, Ventura ALM. Release of ATP from avian Müller glia cell in culture. Neurochem Int. 2011;58:414–422. doi: 10.1016/j.neuint.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Pearson RA, Dale N, Llaudet E, Mobbs P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005;46(5):731–744. doi: 10.1016/j.neuron.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 14.Reigada D, Lu W, Mitchell CH. Glutamate acts at NMDA receptors on fresh bovine and on cultured human retinal pigment epithelial cells to trigger release of ATP. J Physiol. 2006;575:707–720. doi: 10.1113/jphysiol.2006.114439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa G, Pereira T, Neto AM, Cristóvão AJ, Ambrósio AF, Santos PF. High glucose changes extracellular adenosine triphosphate levels in rat retinal cultures. J Neurosci Res. 2009;87:1375–1380. doi: 10.1002/jnr.21956. [DOI] [PubMed] [Google Scholar]
- 16.Resta V, Novelli E, Vozzi G, Scarpa C, Caleo M, Ahluwalia A, Solini A, Santini E, Parisi V, Di Virgilio F, Galli-Resta L. Acute retinal ganglion cell injury caused by intraocular pressure spikes is mediated by endogenous extracellular ATP. Eur J Neurosci. 2007;25:2741–2754. doi: 10.1111/j.1460-9568.2007.05528.x. [DOI] [PubMed] [Google Scholar]
- 17.Wurm A, Pannicke T, Iandiev I, Francke M, Hollborn M, Wiedemann P, Reichenbach A, Osborne NN, Bringmann A. Purinergic signaling involved in Müller cell function in the mammalian retina. Prog Retin Eye Res. 2011;30:324–342. doi: 10.1016/j.preteyeres.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Guzman-Aranguez A, Santano C, Martin-Gil A, Fonseca B, Pintor J. Nucleotides in the eye: focus on functional aspects and therapeutic perspectives. J Pharmacol Exp Ther. 2013;345:331–341. doi: 10.1124/jpet.112.202473. [DOI] [PubMed] [Google Scholar]
- 19.Housley GD, Bringmann A, Reichenbach A. Purinergic signaling in special senses. Trends Neurosci. 2009;32(3):128–141. doi: 10.1016/j.tins.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Sanches G, Alencar LS, Ventura ALM. ATP induces proliferation of retinal cells in culture via activation of PKC and extracellular signal-regulated kinase cascade. Int J Dev Neurosci. 2002;20(1):21–27. doi: 10.1016/S0736-5748(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 21.França GR, Freitas RCC, Ventura ALM. ATP-induced proliferation of developing retinal cells: regulation by factors released from postmitotic cells in culture. Int J Dev Neurosci. 2007;25(5):283–291. doi: 10.1016/j.ijdevneu.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Wurm A, Erdmann I, Bringmann A, Reichenbach A, Pannicke T. Expression and function of P2Y receptors on Müller cells of the postnatal rat retina. Glia. 2009;57:1680–1690. doi: 10.1002/glia.20883. [DOI] [PubMed] [Google Scholar]
- 23.Sholl-Franco A, Fragel-Madeira L, Macama AC, Linden R, Ventura ALM. ATP controls cell cycle and induces proliferation in the mouse developing retina. Inl J Dev Neurosci. 2010;28(1):63–73. doi: 10.1016/j.ijdevneu.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Nunes PHC, Calaza KC, Albuquerque LM, Fragel-Madeira L, Sholl-Franco A, Ventura ALM. Signal transduction pathways associated with ATP-induced proliferation of cell progenitors in the intact embryonic retina. Int J Dev Neurosci. 2007;25:499–508. doi: 10.1016/j.ijdevneu.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Ornelas IM, Ventura ALM. Involvement of the PI3K/Akt pathway in ATP-induced proliferation of developing retinal cells in culture. Int J Dev Neurosci. 2010;28(6):503–511. doi: 10.1016/j.ijdevneu.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Ornelas IM, Martins Silva T, Ventura ALM. PI3K/Akt pathway regulates mitotic progression of progenitor cells in the avian embryonic retina. PLoS ONE. 2013;8(1):e53517. doi: 10.1371/journal.pone.0053517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corriden R, Insel PA. New insights regarding the regulation of chemotaxis by nucleotides, adenosine, and their receptors. Purinergic Signal. 2012;8:587–598. doi: 10.1007/s11302-012-9311-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Striedinger K, Meda P, Scemes E. Exocytosis of ATP from astrocyte progenitors modulates spontaneous Ca2+ oscillations and cell migration. Glia. 2007;55(6):652–662. doi: 10.1002/glia.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scemes E, Duval N, Meda P. Reduced expression of P2Y1 receptors in Connexin43–null mice alters calcium signaling and migration of neural progenitor cells. J Neurosci. 2003;23(36):11444–11452. doi: 10.1523/JNEUROSCI.23-36-11444.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grimm I, Ullsperger SN, Zimmermann H. Nucleotides and epidermal growth factor induce parallel cytoskeletal rearrangements and migration in cultured adult murine neural stem cells. Acta Physiol. 2010;199:181–189. doi: 10.1111/j.1748-1716.2010.02092.x. [DOI] [PubMed] [Google Scholar]
- 31.Gonçalves JC, Silveira AL, de Souza HD, Nery AA, Prado VF, Prado MA, Ulrich H, Araújo DA. The monoterpene (-)-carvone: a novel agonist of TRPV1 channels. Cytometry A. 2013;83:212–219. doi: 10.1002/cyto.a.22236. [DOI] [PubMed] [Google Scholar]
- 32.Glaser T, de Oliveira SL, Cheffer A, Beco R, Martins P, Fornazari M, Lameu C, Junior HM, Coutinho-Silva R, Ulrich H. Modulation of mouse embryonic stem cell proliferation and neural differentiation by the P2X7 receptor. PLoS One. 2014;9(5):e96281. doi: 10.1371/journal.pone.0096281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neary JT, Zhu Q, Kang Y, Dash PK. Extracellular ATP induces formation of AP-1 complexes in astrocytes via P2 purinoceptors. Neuroreport. 1996;7:2893–2896. doi: 10.1097/00001756-199611250-00017. [DOI] [PubMed] [Google Scholar]
- 34.Rathbone MP, Middlemiss PJ, Gysbers JW, Andrew C, Herman MA, Reed JK, Ciccarelli R, Di Iorio P, Caciagli F. Trophic effects of purines in neurons and glial cells. Prog Neurobiol. 1999;59:663–690. doi: 10.1016/S0301-0082(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 35.Neary JT, Kang Y, Shi YF. Cell cycle regulation of astrocytes by extracellular nucleotides and fibroblast growth factor-2. Purinergic Signal. 2005;1(4):329–336. doi: 10.1007/s11302-005-8075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Liao Z, Camden J, Griffin KD, Garrad RC, Santiago-Pérez LI, González FA, Seye CI, Weisman GA, Erb L. Src homology 3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors. J Biol Chem. 2004;279(9):8212–8218. doi: 10.1074/jbc.M312230200. [DOI] [PubMed] [Google Scholar]
- 37.Wang M, Kong Q, Gonzalez FA, Sun G, Erb L, Seye C, Weisman GA. P2Y nucleotide receptor interaction with alpha integrin mediates astrocyte migration. J Neurochem. 2005;95(3):630–640. doi: 10.1111/j.1471-4159.2005.03408.x. [DOI] [PubMed] [Google Scholar]
- 38.Cárdenas A, Kong M, Alvarez A, Maldonado H, Leyton L. Signaling pathways in neuron-astrocyte adhesion and migration. Curr Mol Med. 2014;14:275–290. doi: 10.2174/1566524014666140128113311. [DOI] [PubMed] [Google Scholar]
- 39.Fischer AJ, Mcguire CR, Dierks BD, Reh TA. Insulin and fibroblast growth factor 2 activate a neurogenic program in Müller glia of the chicken retina. J Neurosci. 2002;22:9387–9398. doi: 10.1523/JNEUROSCI.22-21-09387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Close JL, Liu J, Gumuscu B, Reh TA. Epidermal growth factor receptor expression regulates proliferation in the postnatal rat retina. GLIA. 2006;54:94–104. doi: 10.1002/glia.20361. [DOI] [PubMed] [Google Scholar]
- 41.Dupin I, Camand E, Etienne-Manneville S. Classical cadherins control nucleus and centrosome position and cell polarity. J Cell Biol. 2009;185(5):779–786. doi: 10.1083/jcb.200812034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tran MD, Wanner IB, Neary JT. Purinergic receptor signaling regulates N-cadherin expression in primary astrocyte cultures. J Neurochem. 2008;105:272–286. doi: 10.1111/j.1471-4159.2008.05214.x. [DOI] [PubMed] [Google Scholar]
- 43.Martiáñez T, Lamarca A, Casals N, Gella A. N-cadherin expression is regulated by UTP in schwannoma cells. Purinergic Signal. 2013;9(2):259–270. doi: 10.1007/s11302-012-9348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaulet H, Desgranges C, Renault MA, Dupuch F, Ezan G, Peiretti F, Loirand G, Pacaud P, Gadeau AP. Extracellular nucleotides induce arterial smooth muscle cell migration via osteopontin. Circ Res. 2001;89:772–778. doi: 10.1161/hh2101.098617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Glial cell division and migration in control scratched cultures. Cultures at E8C7 were scratched and after 3 days, medium was changed to MEM buffered with 25 mM HEPES (pH 7.4) plus serum and antibiotics and cultures mounted on a Leica SP5 confocal microscope. Time-lapse live imaging were performed by photographing cultures every 5 min during 6 h under differential interference contrast illumination using a 20x objective plus 1.5 x zoom. Bar = 75 μm. (MPG 2150 kb)
Effect of P2Y1 receptor antagonist and ADPβS on the incorporation of [ 3 H]-thymidine in retinal cell cultures. Retinal cultures were treated for 24 h with MRS2179 or ADPβS and the incorporation of [3H]-thymidine determined as described in the section of methods. (A) Retinal cultures at E7C1 were incubated with 500 μM ADP in the absence or presence of 5, 25 or 50 μM MRS 2179. (B) Retinal cultures at E8C8 that were scratched or not (control) were incubated with 50 μM ADPβS. Data represent the mean ± SEM of 3 to 5 independent experiments performed in duplicate. *** p < 0.001, compared to control cultures. ## p < 0.01 and ### p < 0.001, compared to ADP-treated cultures. (PPTX 67 kb)
Glial cell activity at the edge of the scratch in apyrase-treated cultures. Cultures at E8C7 were scratched and treated with 2.5 U/mL apyrase. After 3 days, medium was changed to MEM buffered with 25 mM HEPES (pH 7.4) plus apyrase, serum and antibiotics and cultures mounted on a Leica SP5 confocal microscope. Time-lapse live imaging were performed by photographing cultures every 5 min during ~8 h under differential interference contrast illumination using a 20x objective plus 1.5 x zoom. (MPG 3014 kb)