Abstract
Here, we describe a molecular switch associated with opioid receptors-linked signalling cascades that provides a dual opioid control over P2X3 purinoceptor in sensory neurones. Leu-enkephalin inhibited P2X3-mediated currents with IC50 ~10 nM in ~25 % of small nociceptive rat dorsal root ganglion (DRG) neurones. In contrast, in neurones pretreated with pertussis toxin leu-enkephalin produced stable and significant increase of P2X3 currents. All effects of opioid were abolished by selective μ-opioid receptor antagonist D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP), nonselective inhibitor naloxone, and by PLC inhibitor U73122. Thus, we discovered a dual link between purinoceptors and μ-opioid receptors: the latter exert both inhibitory (pertussis toxin-sensitive) and stimulatory (pertussis toxin-insensitive) actions on P2X3 receptors through phospholipase C (PLC)-dependent pathways. This dual opioid control of P2X3 receptors may provide a molecular explanation for dichotomy of opioid therapy. Pharmacological control of this newly identified facilitation/inhibition switch may open new perspectives for the adequate medical use of opioids, the most powerful pain-killing agents known today.
Keywords: Pain, Sensory neurones, Nociceptive neurones, P2X3 receptors, Opioid receptors, Leu-enkephalin
Introduction
Neurones located in the dorsal root ganglia (DRG) are principal cells involved in transmitting information of various somatosensory modalities. Small- and medium-sized neurones localized in the DRGs initiate the sensation of pain and hence are classified as nociceptive neurones [1]. Numerous studies have indicated that homomeric P2X3 ATP receptors expressed in the membrane of sensory neurones contribute to various types of pain behaviour, as well as pathological forms of nociception represented by hyperalgesia and allodynia [2–4].
Opioids are commonly employed for pharmacological control of pain [5], although their therapeutic usage is limited by multiple adverse effects and societal issues. It is generally acknowledged that opioids induce analgesia through their action on the central nervous system [6]. This perception, however, has been challenged with accumulation of evidence indicating that a considerable part of opioid analgesia is mediated by peripheral μ-, κ- and δ-opioid receptors [7–9]. Numerous clinical studies demonstrated that conventional and peripherally restricted opioid receptor agonists exhibit similar analgesic potency [10, 8]. The μ-opioid receptors (MORs) that belong to 7-transmembrane domain G protein-coupled receptors (GPCRs) coupled with pertussis toxin (PTX)-sensitive Gi/Go proteins are predominantly expressed in small (nociceptive) DRG neurones [1]. Activation of MORs inhibits adenylate cyclase (AC) by the Gα subunit, whereas Gβγ subunits activate phospholipase C (PLC) with consequent production of second messengers diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (InsP3) [11, 12]. Opioid-induced inhibition of AC- and cyclic adenosine monophosphate (cAMP)-dependent protein kinase A (PKA) activity in sensory neurones reduces currents mediated by transient receptor potential vanilloid 1 (TRPV1) channels [13, 14]. Furthermore, opioids acting through MORs suppress voltage-gated Ca2+ channels and stimulate G protein-mediated gating of inward-rectifier K+ channels [15–18]. Inhibition of MORs by naloxone was also reported to remove the antinociceptive action of P2X3 receptors antagonist A-317491 in rat models of chemogenic and inflammatory pain [19]. All these pathways represent peripheral sites of opioid analgesia, which curtails nociceptive signalling [20, 21].
In the course of chronic treatment, analgesic effects of opioids are reduced (opioid tolerance) or even reversed resulting in hyperalgesia [22–24]. At the cellular level, opioid-induced hyperalgesia has been considered as a transition from classical PTX-sensitive inhibition of AC to PTX-insensitive stimulation of AC with consequent increase in the intracellular level of cAMP [25, 26].
In the present study, we show that homomeric P2X3 receptors are regulated by opioid signalling in a subpopulation of sensory neurones. Furthermore, we demonstrate a novel molecular mechanism for dual action of opioids on purinoceptors by presenting the data that stimulation of MORs exerts PTX-sensitive inhibitory and PTX-insensitive stimulatory effects on P2X3 receptors.
Materials and methods
Cell culture
Lumbar dorsal root ganglia (DRG; L1 to L5) were removed following decapitation of 8-day-old Wistar rats. After isolation, ganglia were treated with trypsine (1/250-2 mg/ml) and collagenase (type IV, 1 mg/ml) at 32 °C for 35–40 min. Throughout the entire procedure, the medium (MEM) was continuously saturated with 95%O2 + 5 % CO2 gas mixture (pH 7.4). Neurones dissociated by trituration were plated on glass coverslips and incubated in MEM medium supplemented with 10 % foetal bovine serum at 37 °C. The cells were used for electrophysiological experiments after 2–3 days in vitro.
Electrophysiology
Whole-cell patch-clamp recordings were performed at room temperature (22 ± 1 °C) using a patch-clamp amplifier (Axopatch 200B; Axon Instruments, Union City, CA, USA). Signals were filtered at 2 kHz with the in-built filter, digitized at 5 kHz and stored in a laboratory computer using Digidata 1200B interface and pCLAMP 8.0 software (Molecular Devices, California, USA). All recordings were made at a holding potential of −100 mV. The extracellular solution contained (in mM) 130 NaCl, 2 CaCl2, 5 KCl, 10 glucose and 10 HEPES (pH was adjusted to 7.4). The patch pipette solution (artificial intracellular medium) contained (in mM) 130 KCl, 10 HEPES, 10 EGTA, 0.5 GTP and 5 Mg-ATP (pH was adjusted to 7.3). The resistance of the pipettes was 5–7 MOhm.
Agonists of P2X3 receptors were applied for 500 ms by a rapid U-tube system [27] positioned at a distance between 100 and 150 μm from the cell. The time of solution exchange was approximately 20–30 ms as confirmed by liquid junction potential measurements. The time needed for subsequent washout of applied saline was 100–150 ms (a more distantly located second U-tube was used for this purpose). The P2X-receptor agonist α,β–methylene-ATP (meATP), lithium salt was dissolved in external solution immediately before the experiments. The endogenous opioid peptide neurotransmitter leu-enkephalin (Enk) and the selective μ-opioid receptor agonist endomorphin-1 were used as opioid receptor agonists. All chemicals were obtained from Sigma (Grand Island, USA).
[Ca2+]i recordings
Changes in intracellular concentration of ionized calcium ([Ca2+]i) in isolated DRG neurones were imaged using the high-affinity fluorescent Ca2+ indicator fluo-4, as previously described [28]. Briefly, the dye was loaded by 1-h incubation of the DRG neurones with 3 μM fluo-4 acetoxymethyl ester (fluo-4 AM; diluted from a stock containing 2 mM fluo-3 AM and 0.025 % (w/v) pluronic F-127 in dimethyl sulphoxide) followed by a 1-h wash in Ringer’s solution to allow time for de-esterification. The cells were imaged using a LSM 5 PASCAL laser scanning confocal microscope (Carl Zeiss, Jena, Germany). The x–y confocal images were acquired at 0.5–2 Hz using a Zeiss Plan-Apochromat 40× 1.3 NA oil-immersion objective. The excitation beam was produced by the 488-nm line of a 200-mW argon ion laser, and illumination intensity was attenuated to 0.5–0.8 %. Fluo-4 fluorescence was captured at wavelengths above 505 nm. To optimize signal quality, the pinhole was set to provide a confocal optical section below 2 μm (measured with 0.2-μm fluorescent beads). The focus was adjusted to acquire the images from the middle of the cell depth. In the figures, the intensity of fluo-4 fluorescence was normalized to the average fluorescence intensity in the images captured before agonist application (F/F0). The temporal profiles of the agonist-induced [Ca2+]i transients are illustrated by the plots showing the normalized fluo-4 fluorescence intensity (F/F0) averaged within an entire confocal optical slice of the cell.
Data analysis
Data are presented as mean ± S.D. (where n is a number of tested opioid sensitive cells). The IC50 is the concentration of a Enk that inhibited P2X3 currents by 50 %. Commercially available software Origin 8.1 (OriginLab Corporation, USA) was used for data analysis. Statistical significance was evaluated using Student’s t test. A probability value of P < 0.05 was considered statistically significant (when necessary, marked by asterisks on the graphs).
Results
Leu-enkephalin inhibits P2X3 receptors
Small (15–25 μm in diameter) DRG neurones were selected at the start of each experiment according to their responses to meATP [29]. Only neurones with fast and completely desensitizing (within 300–400 ms) ion currents characteristic for P2X3 receptor were selected for subsequent study.
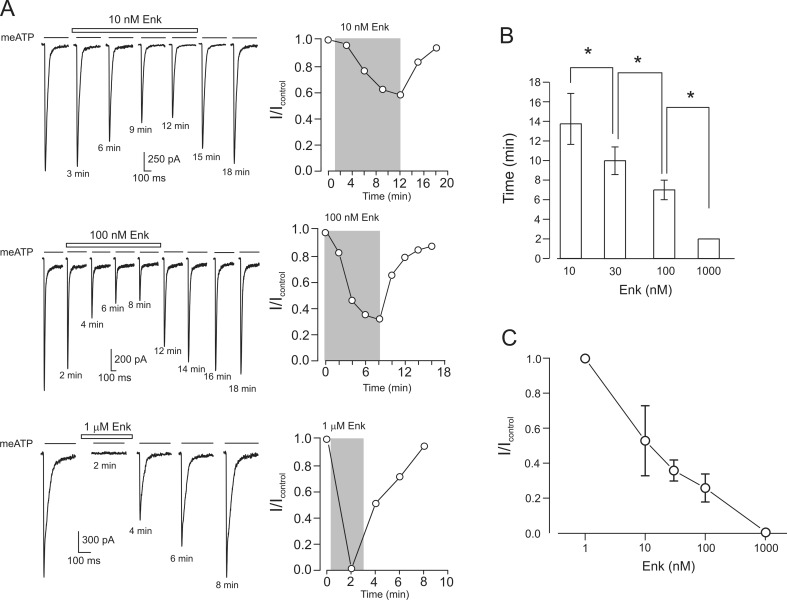
P2X3-mediated currents were evoked by 0.5 s applications of 30 μM meATP, which were delivered every 2–3 min. This concentration of meATP is close to saturating for the activation of P2X3 receptors, whereas 2- to 3-min intervals between agonist applications ensure >90 % recovery of P2X3 receptors from desensitization [30]. In all experiments, application of Enk was preceded by 2–3 applications of meATP performed to obtain a stable control current. Application of Enk resulted in two distinct outcomes: either it produced no effect (127 cells out of 171 individual neurones) or it inhibited P2X3-mediated currents (Fig. 1; 44 neurones or 25.8 % of the selected cells). The inhibition was accelerated when the concentration of Enk was increased (Fig. 1b), which indicates that kinetics of Enk binding to MORs rather than the rate of signal transduction is a limiting step for the inhibition of P2X3 receptors. The inhibitory effect of Enk was dose-dependent and reversible (Fig. 1a, c). Enk in the concentration of 1 nM did not produce any change in the P2X3 current, but subsequent application of 1 μM Enk to the same cell resulted in complete current inhibition. After washout of Enk, the amplitude of P2X3-mediated current fully recovered within 6–8 min (Fig. 1). Of note, application of 1 μM Enk completely (by 0.99 ± 0.01, n = 4) inhibited P2X3 receptor-mediated currents in all opioid-sensitive neurones. Similarly, selective μ-opioid receptor agonist endomorphin-1 at 100 nM almost completely inhibited P2X3 currents (by 0.97 ± 0.02, n = 3, data not shown).
Fig. 1.
Leu-enkephalin (Enk) reversibly inhibits P2X3-mediated currents in a time- and concentration-dependent manner. a Current traces recorded from different neurones (left) and time course of the changes in the amplitude of the peak current (right) in control and in the presence of different concentrations of Enk. Here and below, drug application protocol is indicated on the graph, and the time under each current trace indicates the time from the start of the experiment. In graphs depicting the time course of peak current changes, the Enk application is indicated by the grey area. b Time to reach steady-state level of P2X3 current inhibition depends on the opioid concentration (indicated below bars); *P < 0.05, statistically significant difference (n = 4–9). c Dose–response relationship for the inhibition of P2X3 currents by Enk. The data are presented as the ratio I/I contr where I contr is the amplitude of P2X3 current before the drug application, and I is the current at the steady-state of inhibitory action. Each data point was obtained by averaging data from 4–6 cells
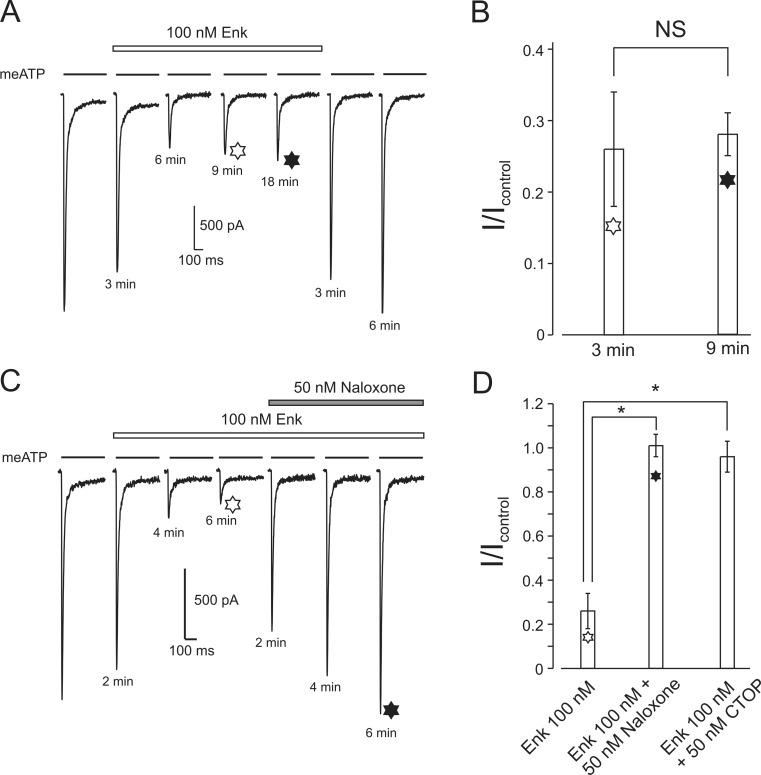
Slow recovery from desensitization is a unique property of P2X3 receptors. Its rate depends on the agonist (with recovery being the slowest when ATP is used for activation [30]) and the temperature [31]; the recovery from desensitization is inhibited by purotoxin-1 from spider venom [32]. To reveal possible effects of Enk on P2X3 receptors recovery from desensitization, the meATP application protocol was modified. Initially, the cells were stimulated every 3 min by meATP before and during application of Enk (100 nM). When Enk reached its steady-state inhibition (about 65 %), the interval between meATP applications was increased to 9 min. The amplitude of the Enk-inhibited current remained unchanged (Fig. 2a, b). Therefore, we concluded that Enk-mediated inhibition of P2X3 receptors is not associated with modulation of the recovery from desensitization.
Fig. 2.
Inhibitory effect of Enk is not related to P2X3 receptor desensitization and is reversed by μ-opioid receptor antagonists. a, b Increasing an interval between agonist applications from 3 min (white six-point star) to 9 min (black six-point star) does not affect the amplitude of current partially inhibited by Enk. Mean values for four experiments are presented in b. The time after every solution change is indicated under the current traces. NS indicates no significant difference (n = 4). c, d Naloxone reverses the inhibitory action of Enk on P2X3-mediated currents; μ-opioid specific antagonist CTOP produced the same effect. Mean values for naloxone (n = 4) and CTOP (n = 3) are presented in d; *P < 0.05, statistically significant difference
Prolonged application of Enk (18 min as shown on Fig. 2a and up to 20 min in other experiments) did not desensitize/internalize opioid receptors: the amplitude of inhibited P2X3 current did not recover in the presence of Enk.
Inhibition of P2X3 receptors is mediated by μ-opioid receptors
Naloxone is a competitive opioid antagonist, which prevents opioid–receptor interaction thus blocking opioid signalling. Naloxone alone (50 nM to 1 μM) did not produce any changes in P2X3 receptor-mediated currents (in cells incubated with 1 μM naloxone for 8 min, the average amplitude of P2X3 current was 0.99 ± 0.03, n = 3 of the control value). Exposure to naloxone, however, removed Enk-dependent inhibition almost completely within 6–8 min (I/Icontr = 0.95 ± 0.05, n = 3; Fig. 2c, d). The D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP), the most selective antagonist of MOR [33] applied at 50 nM completely abolished inhibitory effect of 100 nM Enk (I/Icontr = 0.97 ± 0.07, n = 3, Fig. 2d). Similarly to naloxone, exposure of DRG neurones to stop alone did not change the amplitude of P2X3 currents (the average amplitude of P2X3 current was 0.97 ± 0.02, n = 3 of the control value in cells exposed to 1 μM CTOP for 8 min).
High doses of opioid antagonists transiently augment P2X3 receptor currents
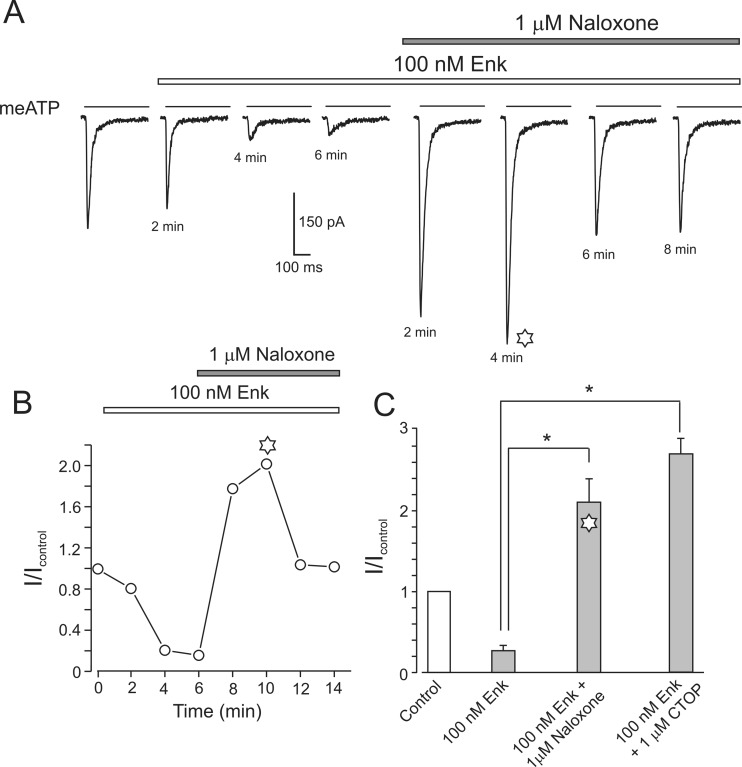
Exposure to 1 μM of naloxone not only removed MOR-mediated inhibition of P2X3 current but also the amplitude of the P2X3 current (in the presence of 100 nM Enk) exhibited an almost twofold and transient increase (I/Icontr = 2.1 ± 0.3, n = 4). A similar transient increase was obtained following application of a high dose (1 μM) of СTOP: the I/Icontr was 2.7 ± 0.2 (n = 4) (Fig. 3).
Fig. 3.
Application of high concentrations of naloxone or CTOP removes Enk inhibition and results in transient overstimulation of the P2X3 currents. a When applied in the presence of 100 nM Enk, a high dose of naloxone (1 μM) causes a transient increase in P2X3 receptor current which subsequently returns to a control level. b Time course of the current changes. c Bar graph representing average data for similar experiments with naloxone (1 μM, n = 4) and CTOP (1 μM, n = 3); *P < 0.05, statistically significant difference
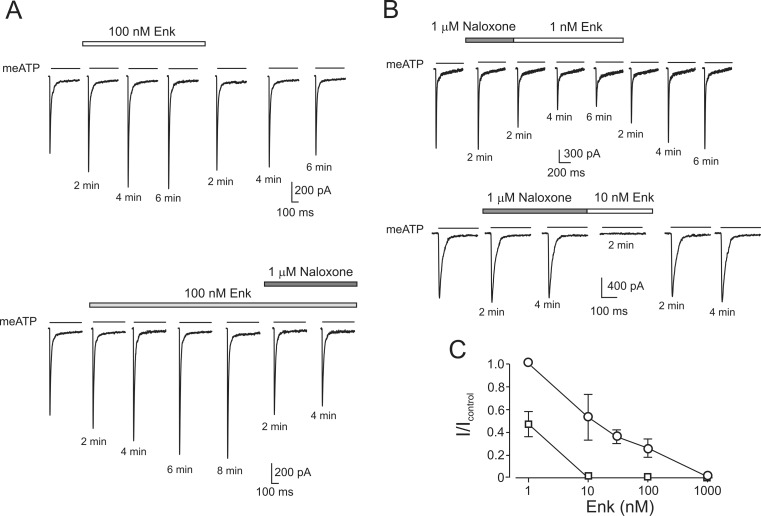
This transient increase in the amplitude of P2X3 current hinted for possible stimulatory effect of opioids (by analogy with the data on nodose neurones [34]). In DRG neurones, pre-incubated with PTX (300 ng/ml) for 17–24 h application of Enk (10 nM–1 μM) resulted exclusively in facilitating effect on the P2X3 current amplitude in all opioid-sensitive cells tested (n = 11; Fig. 4a). This facilitatory effect was completely removed by an application of naloxone (n = 3, Fig. 4a, lower panel).
Fig. 4.
Inhibitory action of opioid on P2X3 receptors is reversed after PTX treatment and is dramatically enhanced by pretreatment with naloxone. a Application of Enk and naloxone to PTX-treated neurone. (Upper panel) Effect of Enk on P2X3-mediated currents. (Lower panel) Effect of subsequent treatment with Enk and naloxone on P2X3 currents. b Current traces were recorded in the presence of naloxone and after subsequent application of Enk (1 nM, upper panel and 10 nM, lower panel; in each panel, recordings were made from different cells). c Dose–response relationships in normal conditions (circles, the same plot as in Fig. 1) and after pretreatment of DRG neurone with naloxone (squares). Pairwise comparison of data points (1 and 10 nM of Enk) are significantly (P < 0.05) different
When DRG neurones were exposed to naloxone (1 μM) for 2–4 min and subsequently naloxone solution was substituted by Enk, a substantial increase in the efficacy of the opioid inhibition of P2X3 receptors was observed. In control settings, 1 nM Enk had no effect, whereas after pre-incubation with naloxone, the same concentration of Enk produced ~50 % inhibition of P2X3 currents within 10–12 min (Fig. 4b, upper panel). Similarly, while 10 nM Enk inhibited P2X3 currents by ~50 % in control conditions (cf. Fig. 1) after pre-incubation with naloxone, the same concentration of Enk led to a complete block of the P2X3 currents within 2 min (Fig. 4b, lower panel). In summary, pre-incubation with naloxone decreases IC50 for the inhibitory action of Enk from 10 to 1 nM (Fig. 4c).
Role of PLC and PKC in opioid-mediated regulation of P2X3 receptors
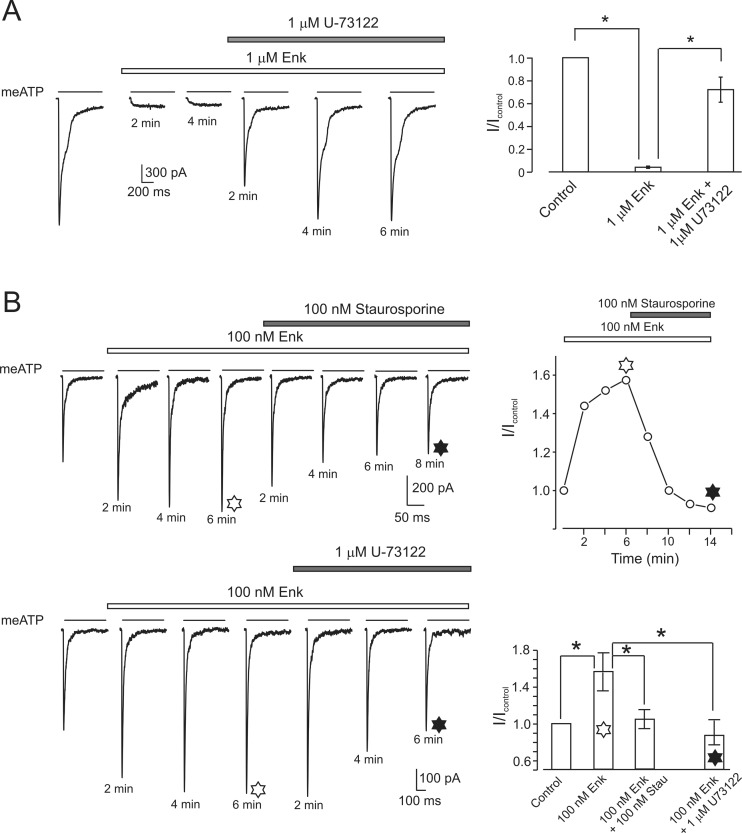
Exposure of DRG neurones to PLC inhibitor U73122 at 1 μM concentration for 6–8 min did not affect P2X3 currents (n = 4; in the presence of the drug, the average amplitude of P2X3 current was 0.98 ± 0.04 of the control value). However, when U73122 was applied to the cells in which P2X3 currents were completely blocked by a high dose of Enk, the current was restored to 0.87 ± 0.11 (n = 4) of the control (Fig. 5a).
Fig. 5.
PLC and PKC are involved in stimulatory and inhibitory actions of opioid on P2X3 receptors. a Left panel, P2X3-mediated current is inhibited by Enk and restored by subsequent application of PLC blocker U73122. Average data from five cells are shown in the right. *P < 0.05, statistically significant difference. b Upper panel, representative current traces recorded after PTX treatment in the presence of opioid alone and after addition of staurosporine. The time course of the changes in the peak amplitude of P2X3-mediated current is shown on the right. Lower left panel shows P2X3-mediated currents recorded from PTX-treated neurones in control, in the presence of Enk and after addition of PLC inhibitor U73122. Right panel represents a summary of the experiments, showing average values of P2X3 current amplitudes measured in control and in the presence of opioids and different inhibitors. Exposure of PTX-treated cells to Enk increased the peak amplitude of P2X3 currents, which action was antagonized by staurosporine and U73122. *P < 0.05, statistically significant difference (n = 5)
In DRG neurones pretreated with PTX for 17–24 h, both U73122 and PKC inhibitor staurosporine suppressed Enk-induced potentiation of P2X3-mediated currents (Fig. 5b). Exposure of PTX-treated cells to Enk increased the peak amplitude of P2X3 currents (I/Icontr = 1.56 ± 0.2, n = 7,* P < 0.05); this stimulation was reduced to a control level by staurosporine (I/Icontr = 1.04 ± 0.1, n = 3, P < 0.05) and by U73122 (0.84 ± 0.15, n = 4). At the same time (and similarly to PTX-untreated cells), application of U73122 and staurosporine alone for 6–8 min did not affect P2X3 currents (n = 5).
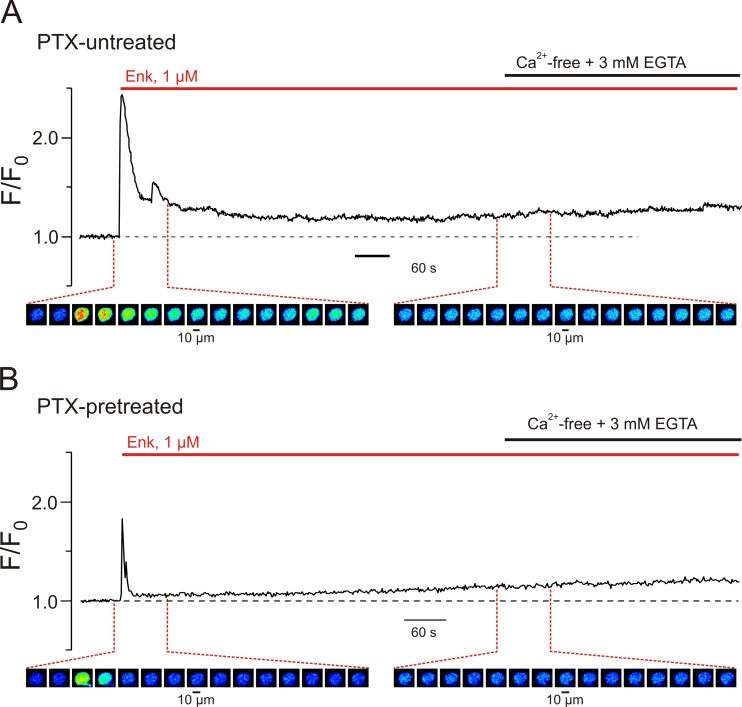
Recordings of [Ca2+]i dynamics performed on these nociceptive neurones have shown that Enk-induced Ca2+ signals are associated with intracellular Ca2+ release from the ER stores: the Enk-induced [Ca2+]i transients did not require extracellular Ca2+ (Fig. 6a). Treatment of these neurones with PTX for 17–24 h prior to the experiment substantially reduced but did not abolish Ca2+ responses stimulated by Enk (Fig. 6b), indicating that a subpopulation of PTX-insensitive G proteins link MORs to PLC; the Ca2+ responses were observed in all six neurones studied.
Fig. 6.
Leu-enkephalin-induced Ca2+ release from intracellular stores in DRG neurones is activated via Gi/o-independent signalling pathway. Traces of self-normalized fluo-4 fluorescence (F/F0) reflect [Ca2+]i changes induced by 1 μM Enk in DRG neurones a under control conditions and b following 24-h pretreatment with pertussis toxin (PTX). F0 is F averaged before Enk application. Colour-coded images below the plots were captured during the periods depicted in the plots (every 5th image is shown). The initial rapid phase of Enk-induced [Ca2+]i transient was substantially reduced following PTX pretreatment, whereas the sustained component of the response (that was not affected by the removal of extracellular Ca2+ indicating intracellular release) was attenuated to a much lesser degree
Discussion
Dual effect of opioids on P2X3 receptors
In this study, we demonstrate that activation of MORs exerts a dual (inhibitory and stimulatory) effect on P2X3 currents in DRG neurones. In naïve cells, the application of opioid significantly inhibits P2X3 currents (Fig. 1). In contrast, when opioids were presented to the cells pretreated with PTX, P2X3 current amplitude was markedly increased (Figs. 4 and 5b). We can therefore suggest that MORs signalling directed at P2X3 receptors is mediated by both PTX-sensitive and PTX-insensitive G proteins that mediate opposite effects. In normal conditions, the inhibitory effect prevails, while stimulatory is either hidden or absent. The functional expression of PTX-insensitive metabotropic pathway was also confirmed by [Ca2+]i recordings: a portion of ER-originating Ca2+ release in response to Enk was preserved in PTX-treated neurones (Fig. 6).
Biochemical studies indicate that MORs, like many other GPCRs, are capable of adopting several distinct active conformations coupled to different G proteins [35]. This feature of MORs-mediated signalling underlies for example dual influences on AC [36]. We may therefore assume that the dual action of MORs on P2X3 receptors is similarly mediated by different MOR conformation states distinctly coupled to PTX-sensitive/insensitive G proteins. Thus, opioid-induced stimulation/inhibition of P2X3 receptors is associated with distinct intracellular pathways linked to different G proteins.
Both inhibitory and stimulatory effects of MORs on P2X3 receptors are mediated by the PLC
Membrane proteins and ion channels (including P2X3 receptor) are regulated by a minor lipid constituent of plasma membrane, the phosphatidylinositol 4,5-bisphosphate or PIP2. Depletion of the membrane pool of PIP2 leads to the inhibition of the P2X3 current, which can be restored by addition of PIP2 into the cytosol [37]; activation of MOR leads to PLC-mediated hydrolyses of PIP2 thus depleting the membrane pool of the latter. Therefore, opioid-induced PIP2 hydrolysis is likely to be responsible for the inhibition of P2X3 currents. The role for PLC is further corroborated by the observation that the specific blocker, U73122, completely abolished opioid-induced inhibition of P2X3 receptors (Fig. 5a). Suppression of inhibitory component of dual opioid action on P2X3 receptors could unmask normally hidden stimulatory component. However, in all the cells tested (n = 4), U73122 restored the current inhibited by opioid back to the control level. This observation prompted a possibility that both opioid-induced stimulation and inhibition of P2X3 receptors are mediated by PLC-dependent pathway. Experiments presented on Fig. 5b corroborate this conclusion: stimulatory action of opioid on P2X3 receptors in PTX-treated cells is fully inhibited by U73122.
This, seemingly paradoxical, conclusion is in agreement with data on the regulation of P2X3 receptors by other metabotropic pathways. For example, activation of P2Y2 receptors inhibits P2X3 via PTX-sensitive Gi/Go linked to PLC, whereas bradykinin and substance P receptors coupled to PTX-insensitive Gq proteins stimulate P2X3 currents through PLC/PIP2/DAG/PKC cascade [38–40]. Assuming that similar pathways are used by MORs, one could expect that PKC is involved in the stimulatory action of MORs on P3X3 receptors as well.
PKC is involved in the stimulatory action of MORs on P2X3 receptors
Activation of PLC/PIP2 pathway results in the generation of two second messengers, InsP3 and DAG, with the phosphokinase C (PKC) being the ubiquitous target for the latter. Regulation of P2X3 receptors by PKC is well documented [41]. We found that inhibition of PKC by staurosporine completely abolished opioid-induced stimulation of P2X3 currents in DRG neurones treated with PTX (Fig. 5b). The PKC can be activated either by DAG or by an increase in cytoplasmic Ca2+. Since concentration of the latter was clamped in our experimental conditions, it is likely that PLC/PIP2/DAG/PKC pathway is involved in the stimulation of P2X3 receptors by MORs. That is, opioid-induced activation of PLC mediates not only inhibition but also stimulation of P2X3 receptors.
The paradox of divergent PIP2 signalling in the same membrane areas has been discussed in the past. It was proposed that molecular components of G protein/PLC signalling pathways and their final targets can be segregated between spatially separated microdomains [42], while distinct pools of PIP2 in plasma membrane have been identified by electron microscopy [43]. Depletion of the PIP2 pool associated with P2X3 receptors leads to the inhibition of P2X3 current [37]. We suggest that hydrolysis of another PIP2 pool separated from P2X3 receptors induces their stimulation via the DAG/PKC pathway (Fig. 7). Involvement of PKC in the stimulatory effect of MORs on nociceptors (putative hyperalgesia) found in our in vitro study is in conceptual agreement with in vivo findings according to which opioid-induced PLC/PKC signalling contributes to hyperalgesic effects of opioids. For example, supraspinal analgesic effects of morphine are enhanced in PKC-null mice [44]. In another study, morphine applied in extremely low dose produced hyperalgesia in the hot plate test, which effect involved PLC/PKC signalling pathway [45].
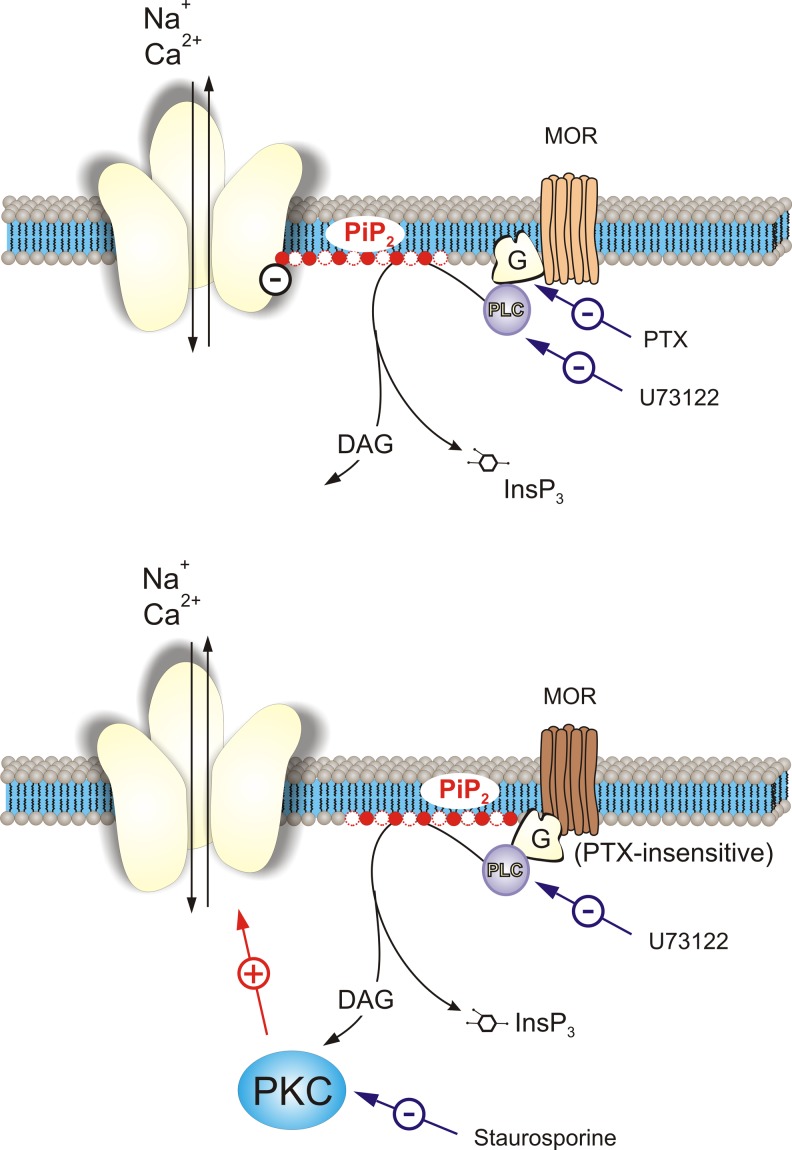
Fig. 7.
Proposed bimodal signalling pathways activated by opioid and leading to inhibition and enhancement of P2X3 receptor-mediated current in DRG neurones. Activated MORs may acquire two distinct states associated either with G proteins. Two distinct PIP2 pools linked with P2X3 receptor (upper panel) or separated from it (lower panel), are both hydrolyzed by PLC (that can be inhibited by U73122), which is activated either by Gi/o (sensitive to PTX) or by (yet unidentified) PTX-insensitive G proteins. Hydrolysis of the PIP2 pool associated with P2X3 receptor results in the inhibition of the latter. Hydrolysis of PIP2 pool separated from P2X3 receptor results in the generation of second messenger DAG, which activates PKC (that can be inhibited by staurosporine); activated PKC in turn may stimulate P2X3 receptors. Thus, P2X3 receptor is subjected to the two opposite regulatory influences: inhibition via Gi/o/PLC/PIP2 pathway and stimulation through Gq/PLC/PIP2/DAG/PKC pathway
Conformation-dependent interaction of MORs with antagonists separates intracellular pathways regulating P2X3 receptors
We propose that in the subpopulation of DRG neurones, P2X3 receptors are subjected to inhibitory and excitatory regulation mediated by diverse MOR conformation states coupled to different G proteins. In physiological conditions, an inhibition of P2X3 receptors prevails and hence masks the effect of stimulatory arm. However, when MOR inhibitors (naloxone or CTOP) are applied at a high dose, the stimulation of P2X3 receptors becomes transiently visible (as shown on Fig. 3). This may reflect the higher rate of interaction of antagonists with “inhibitory” MOR conformation. This phenomenon is in qualitative agreement with strong increase in efficiency of opioid inhibition after naloxone pretreatment (Fig. 4b): MOR receptors tend to bind the antagonist when being in the “inhibitory” conformation. The latter dominates when the antagonist is replaced by the agonist. Incidentally, after naloxone is washed out, MORs maintain a persistent (exceeding several minutes) “memory” for the increased opioid efficiency (Fig. 4b). A similar long-lasting MOR ‘memory’ has been described recently in other types of experiments: it was found that the affinity of MORs to an agonist is increased dramatically by prior agonist exposure [46]. These findings and results of our experiments indicate that interactions with agonists and antagonists can produce long-term changes in the conformation of MORs.
To summarize possible MOR/P2X3 interactions (Fig. 7), we propose that MORs can acquire distinct conformational states differentially coupled to PTX-sensitive (Gi/o) and PTX-insensitive (most probably Gq) G proteins. The conformational state coupled to Gi/o proteins activates PLC, which in turn mediates hydrolysis of the PIP2 pool associated with P2X3 receptors. Depletion of this PIP2 pool results in the inhibition of P2X3 currents. In contrast, the alternative (possibly Gq-mediated) pathway hydrolyses a separate pool of PIP2, which is not associated with P2X3 receptors. We suggest that DAG which is synthesized during this hydrolysis may activate PKC that stimulates P2X3 receptors. Indeed, inhibition of PKC with staurosporine eliminates stimulation of P2X3 receptors. Naturally, we have to further suggest that DAG appears in both microdomains; however, inhibitory action of PIP2 depletion prevails and thus overpowers the PKC-dependent stimulation. As a result, several modes of P2X3 receptors coexist in opioids-sensitive sensory neurones: some receptors appear in the inhibited state, some in stimulated and some can be simultaneously inhibited and stimulated. Balancing of the inhibitory and stimulatory arms defines the receptor availability and nociceptive phenotype of the neurone; when under chronic opioids treatment or in response to opioid receptor antagonist therapy, the loss of this balance may contribute to pathological nociception.
In conclusion, activation of MORs normally inhibits P2X3 responses in DRG neurones; when treated, however, with high doses of MOR antagonists, the stimulation of P2X3 receptors can transiently occur. Although the functional significance of the stimulatory action of opioids needs further investigation, it is possible that corresponding G protein machinery may contribute to the pro-nociceptive effects of opioids occurring in pathological conditions such as opioid-induced hyperalgesia. Another adverse effect of opioids, namely antagonist-induced opioid hypersensitivity is similarly in concordance with the phenomena observed at a single cell level. Search for pharmacological control of the mechanisms operating inhibition/excitation switch may be important for the future development of antinociceptive therapies.
Acknowledgments
This work was supported by Ukrainian Fund for Fundamental Studies (grant DFFD F46.2/001) and by NIH (grant NIH 1R03TW008228-01A1).
Conflict of interest
The authors declare no conflict of interest.
Abbreviations
- AC
adenylate cyclase
- [Ca2+]i
free cytosolic Ca2+ concentration
- cAMP
cyclic adenosine monophosphate
- DRG
dorsal root ganglion
- CTOP
D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2
- DAG
diacylglycerol
- Enk
leu-enkephalin
- GPCRs
G protein-coupled receptors
- InsP3
inositol 1,4,5-trisphosphate
- meATP
α,β–methylene-ATP
- MORs
μ-opioid receptors
- PKA
protein kinase A
- PKC
protein kinase C
- PLC
phospholipase C
- PTX
pertussis toxin
- TRPV1
transient receptor potential vanilloid 1
Contributor Information
Alexei Verkhratsky, Phone: 44 (0)161 275 5414, Email: Alexej.Verkhratsky@manchester.ac.uk.
Oleg Krishtal, Phone: + 380 44 253 24 66, Email: krishtal@biph.kiev.ua.
References
- 1.Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, Lu YJ, Zhang ZN, He SQ, Zheng HC, Wu SX, Hokfelt TG, Bao L, Zhang X. Coexpression of δ- and μ-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci U S A. 2010;107:13117–13122. doi: 10.1073/pnas.1008382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnstock G. A unifying purinergic hypothesis for the initiation of pain. Lancet. 1996;347:1604–1605. doi: 10.1016/S0140-6736(96)91082-X. [DOI] [PubMed] [Google Scholar]
- 3.Burnstock G. Purinergic mechanisms and pain—an update. Eur J Pharmacol. 2013;716:24–40. doi: 10.1016/j.ejphar.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 4.Prado FC, Araldi D, Vieira AS, Oliveira-Fusaro MC, Tambeli CH, Parada CA. Neuronal P2X3 receptor activation is essential to the hyperalgesia induced by prostaglandins and sympathomimetic amines released during inflammation. Neuropharmacology. 2013;67:252–258. doi: 10.1016/j.neuropharm.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson TJ, Schatman ME, Fudin J. The damage done by the war on opioids: the pendulum has swung too far. J Pain Res. 2014;7:265–268. doi: 10.2147/JPR.S65581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sehgal N, Smith HS, Manchikanti L. Peripherally acting opioids and clinical implications for pain control. Pain Physician. 2011;14:249–258. [PubMed] [Google Scholar]
- 7.Reichert JA, Daughters RS, Rivard R, Simone DA. Peripheral and preemptive opioid antinociception in a mouse visceral pain model. Pain. 2001;89:221–227. doi: 10.1016/S0304-3959(00)00365-1. [DOI] [PubMed] [Google Scholar]
- 8.Shannon HE, Lutz EA. Comparison of the peripheral and central effects of the opioid agonists loperamide and morphine in the formalin test in rats. Neuropharmacology. 2002;42:253–261. doi: 10.1016/S0028-3908(01)00173-3. [DOI] [PubMed] [Google Scholar]
- 9.Stein C, Schafer M, Machelska H. Attacking pain at its source: new perspectives on opioids. Nat Med. 2003;9:1003–1008. doi: 10.1038/nm908. [DOI] [PubMed] [Google Scholar]
- 10.Hanna MH, Elliott KM, Fung M. Randomized, double-blind study of the analgesic efficacy of morphine-6-glucuronide versus morphine sulfate for postoperative pain in major surgery. Anesthesiology. 2005;102:815–821. doi: 10.1097/00000542-200504000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Dhawan BN, Cesselin F, Raghubir R, Reisine T, Bradley PB, Portoghese PS, Hamon M. International Union of Pharmacology. XII. Classification of opioid receptors. Pharmacol Rev. 1996;48:567–592. [PubMed] [Google Scholar]
- 12.Murthy KS, Makhlouf GM. Opioid mu, delta, and kappa receptor-induced activation of phospholipase C-β 3 and inhibition of adenylyl cyclase is mediated by Gi2 and Go in smooth muscle. Mol Pharmacol. 1996;50:870–877. [PubMed] [Google Scholar]
- 13.Endres-Becker J, Heppenstall PA, Mousa SA, Labuz D, Oksche A, Schafer M, Stein C, Zollner C. μ-opioid receptor activation modulates transient receptor potential vanilloid 1 (TRPV1) currents in sensory neurons in a model of inflammatory pain. Mol Pharmacol. 2007;71:12–18. doi: 10.1124/mol.106.026740. [DOI] [PubMed] [Google Scholar]
- 14.Vetter I, Wyse BD, Monteith GR, Roberts-Thomson SJ, Cabot PJ. The μ opioid agonist morphine modulates potentiation of capsaicin-evoked TRPV1 responses through a cyclic AMP-dependent protein kinase A pathway. Mol Pain. 2006;2:22. doi: 10.1186/1744-8069-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marker CL, Lujan R, Loh HH, Wickman K. Spinal G-protein-gated potassium channels contribute in a dose-dependent manner to the analgesic effect of μ- and δ- but not κ-opioids. J Neurosci. 2005;25:3551–3559. doi: 10.1523/JNEUROSCI.4899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ocana M, Cendan CM, Cobos EJ, Entrena JM, Baeyens JM. Potassium channels and pain: present realities and future opportunities. Eur J Pharmacol. 2004;500:203–219. doi: 10.1016/j.ejphar.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 17.Schroeder JE, McCleskey EW. Inhibition of Ca2+ currents by a μ-opioid in a defined subset of rat sensory neurons. J Neurosci. 1993;13:867–873. doi: 10.1523/JNEUROSCI.13-02-00867.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu ZZ, Chen SR, Pan HL. Differential sensitivity of N- and P/Q-type Ca2+ channel currents to a mu opioid in isolectin B4-positive and -negative dorsal root ganglion neurons. J Pharmacol Exp Ther. 2004;311:939–947. doi: 10.1124/jpet.104.073429. [DOI] [PubMed] [Google Scholar]
- 19.McGaraughty S, Honore P, Wismer CT, Mikusa J, Zhu CZ, McDonald HA, Bianchi B, Faltynek CR, Jarvis MF. Endogenous opioid mechanisms partially mediate P2X3/P2X2/3-related antinociception in rat models of inflammatory and chemogenic pain but not neuropathic pain. Br J Pharmacol. 2005;146:180–188. doi: 10.1038/sj.bjp.0706346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stein C. Opioids, sensory systems and chronic pain. Eur J Pharmacol. 2013;716(1-3):179–187. doi: 10.1016/j.ejphar.2013.01.076. [DOI] [PubMed] [Google Scholar]
- 21.Stein C, Lang LJ. Peripheral mechanisms of opioid analgesia. Curr Opin Pharmacol. 2009;9:3–8. doi: 10.1016/j.coph.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 23.DuPen A, Shen D, Ersek M. Mechanisms of opioid-induced tolerance and hyperalgesia. Pain Manag Nurs. 2007;8:113–121. doi: 10.1016/j.pmn.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14:145–161. [PubMed] [Google Scholar]
- 25.Chakrabarti S, Gintzler AR. Phosphorylation of Galphas influences its association with the micro-opioid receptor and is modulated by long-term morphine exposure. Mol Pharmacol. 2007;72:753–760. doi: 10.1124/mol.107.036145. [DOI] [PubMed] [Google Scholar]
- 26.Chakrabarti S, Regec A, Gintzler AR. Biochemical demonstration of mu-opioid receptor association with Gsalpha: enhancement following morphine exposure. Brain Res Mol Brain Res. 2005;135:217–224. doi: 10.1016/j.molbrainres.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 27.Pankratov Y, Lalo UV, Dashkin AN, Krishtal A. Heterogeneity of the functional expression of P2X3 and P2X2/3 receptors in the primary nociceptive neurons of rat. Neurochem Res. 2001;26:993–1000. doi: 10.1023/A:1012344803672. [DOI] [PubMed] [Google Scholar]
- 28.Shapovalov G, Gkika D, Devilliers M, Kondratskyi A, Gordienko D, Busserolles J, Bokhobza A, Eschalier A, Skryma R, Prevarskaya N. Opiates modulate thermosensation by internalizing cold receptor TRPM8. Cell Rep. 2013;4:504–515. doi: 10.1016/j.celrep.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 29.North RA. P2X3 receptors and peripheral pain mechanisms. J Physiol Lond. 2004;554:301–308. doi: 10.1113/jphysiol.2003.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pratt EB, Brink TS, Bergson P, Voigt MM, Cook SP. Use-dependent inhibition of P2X3 receptors by nanomolar agonist. J Neurosci. 2005;25:7359–7365. doi: 10.1523/JNEUROSCI.5189-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khmyz V, Maximyuk O, Teslenko V, Verkhratsky A, Krishtal O. P2X3 receptor gating near normal body temperature. Pflugers Arch. 2008;456:339–347. doi: 10.1007/s00424-007-0376-2. [DOI] [PubMed] [Google Scholar]
- 32.Grishin EV, Savchenko GA, Vassilevski AA, Korolkova YV, Boychuk YA, Viatchenko-Karpinski VY, Nadezhdin KD, Arseniev AS, Pluzhnikov KA, Kulyk VB, Voitenko NV, Krishtal OO. Novel peptide from spider venom inhibits P2X3 receptors and inflammatory pain. Ann Neurol. 2010;67:680–683. doi: 10.1002/ana.21949. [DOI] [PubMed] [Google Scholar]
- 33.Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T. Pharmacological characterization of the cloned κ-, δ-, and μ-opioid receptors. Mol Pharmacol. 1994;45:330–334. [PubMed] [Google Scholar]
- 34.Chizhmakov I, Yudin Y, Mamenko N, Prudnikov I, Tamarova Z, Krishtal O. Opioids inhibit purinergic nociceptors in the sensory neurons and fibres of rat via a G protein-dependent mechanism. Neuropharmacology. 2005;48:639–647. doi: 10.1016/j.neuropharm.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Blazquez P, Gomez-Serranillos P, Garzon J. Agonists determine the pattern of G-protein activation in μ-opioid receptor-mediated supraspinal analgesia. Brain Res Bull. 2001;54:229–235. doi: 10.1016/S0361-9230(00)00448-2. [DOI] [PubMed] [Google Scholar]
- 36.Szucs M, Boda K, Gintzler AR. Dual effects of DAMGO [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin and CTAP (D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2) on adenylyl cyclase activity: implications for μ-opioid receptor Gs coupling. J Pharmacol Exp Ther. 2004;310:256–262. doi: 10.1124/jpet.104.066837. [DOI] [PubMed] [Google Scholar]
- 37.Mo G, Bernier LP, Zhao Q, Chabot-Dore AJ, Ase AR, Logothetis D, Cao CQ, Seguela P. Subtype-specific regulation of P2X3 and P2X2/3 receptors by phosphoinositides in peripheral nociceptors. Mol Pain. 2009;5:47. doi: 10.1186/1744-8069-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mo G, Peleshok JC, Cao CQ, Ribeiro-da-Silva A, Seguela P. Control of P2X3 channel function by metabotropic P2Y2 utp receptors in primary sensory neurons. Mol Pharmacol. 2013;83:640–647. doi: 10.1124/mol.112.082099. [DOI] [PubMed] [Google Scholar]
- 39.Park CK, Bae JH, Kim HY, Jo HJ, Kim YH, Jung SJ, Kim JS, Oh SB. Substance P sensitizes P2X3 in nociceptive trigeminal neurons. J Dent Res. 2010;89:1154–1159. doi: 10.1177/0022034510377094. [DOI] [PubMed] [Google Scholar]
- 40.Paukert M, Osteroth R, Geisler HS, Brandle U, Glowatzki E, Ruppersberg JP, Grunder S. Inflammatory mediators potentiate ATP-gated channels through the P2X3 subunit. J Biol Chem. 2001;276:21077–21082. doi: 10.1074/jbc.M101465200. [DOI] [PubMed] [Google Scholar]
- 41.Brown DA, Yule DI. Protein kinase C regulation of P2X3 receptors is unlikely to involve direct receptor phosphorylation. Biochim Biophys Acta. 2007;1773:166–175. doi: 10.1016/j.bbamcr.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delmas P, Crest M, Brown DA. Functional organization of PLC signaling microdomains in neurons. Trends Neurosci. 2004;27:41–47. doi: 10.1016/j.tins.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Fujita A, Cheng J, Tauchi-Sato K, Takenawa T, Fujimoto T. A distinct pool of phosphatidylinositol 4,5-bisphosphate in caveolae revealed by a nanoscale labeling technique. Proc Natl Acad Sci U S A. 2009;106:9256–9261. doi: 10.1073/pnas.0900216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newton PM, Kim JA, McGeehan AJ, Paredes JP, Chu K, Wallace MJ, Roberts AJ, Hodge CW, Messing RO. Increased response to morphine in mice lacking protein kinase C epsilon. Genes Brain Behav. 2007;6:329–338. doi: 10.1111/j.1601-183X.2006.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galeotti N, Stefano GB, Guarna M, Bianchi E, Ghelardini C. Signaling pathway of morphine induced acute thermal hyperalgesia in mice. Pain. 2006;123:294–305. doi: 10.1016/j.pain.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Birdsong WT, Arttamangkul S, Clark MJ, Cheng K, Rice KC, Traynor JR, Williams JT. Increased agonist affinity at the μ-opioid receptor induced by prolonged agonist exposure. J Neurosci. 2013;33:4118–4127. doi: 10.1523/JNEUROSCI.4187-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]