Abstract
PURPOSE
The optimal method of reconstruction following mastectomy for breast cancer patients receiving radiation (RT) is controversial. This study evaluated patient satisfaction and complication rates among patients who received implant-based breast reconstruction.
METHODS AND MATERIALS
The specific treatment algorithm analyzed included patients receiving mastectomy and immediate temporary tissue expander (TE), followed by placement of a permanent breast implant (PI). If indicated, radiation therapy (RT) was delivered to the fully expanded TE. Records of 218 consecutive patients with 222 invasive (85%) or in-situ (15%) breast lesions from the Salt Lake City region treated between 1998 and 2009 were retrospectively reviewed, 28% of whom received RT. Median RT dose was 50.4 Gy, and 41% received a scar boost at a median dose of 10 Gy. Kaplan-Meier analyses were performed to evaluate the cumulative incidence of surgical complications, including permanent PI removal. Risk factors associated with surgical events were analyzed. To evaluate cosmetic results and patient satisfaction, an anonymous survey was administered.
RESULTS
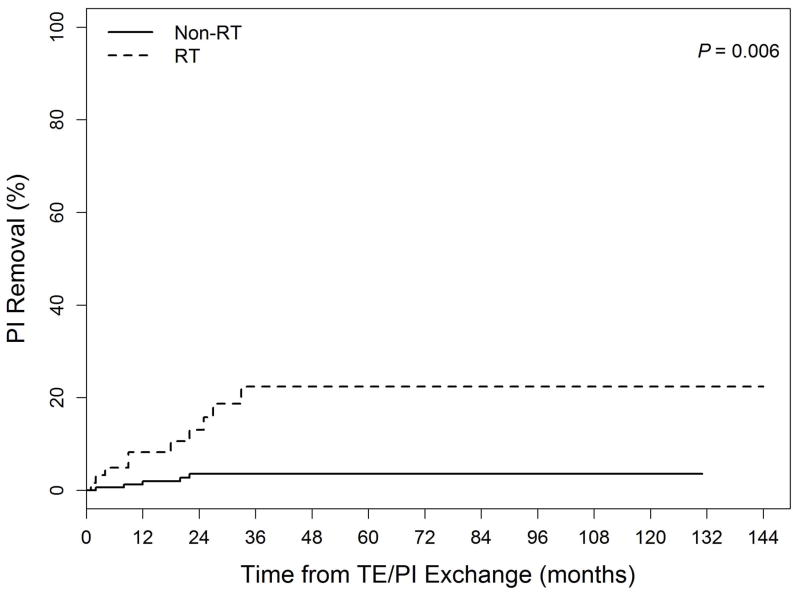
Mean follow-up was 44 months (range, 6 – 144). Actuarial five-year PI removal rates for Non-RT and RT patients were 4% and 22%, respectively. On multivariate analysis (MVA), the only factor associated with PI removal was RT (p=0.009). Surveys were returned describing the outcomes of 149 breasts. For the Non-RT and RT groups, those who rated their breast appearance as good or better were 63% vs. 62%, respectively. Under 1/3 of each group was dissatisfied with their reconstruction.
CONCLUSIONS
RT did not significantly affect patient satisfaction scores, but on MVA RT was the only factor associated with increased PI removal. This reconstruction technique may be considered an acceptable option even if RT is needed, but the increased complication risk with RT must be recognized.
Keywords: radiation, breast reconstruction, patient satisfaction, complications
INTRODUCTION
Delayed autologous tissue reconstruction may have the potential for superior aesthetic results compared to implant based reconstruction. This appears especially true in patients requiring radiation therapy (RT)and therefore autologous reconstruction is often recommended when RT is planned. However, the potential for less morbidity and shorter operative and recovery time make implants an appealing choice to many patients. Although there has been concern in the past for suboptimal dosimetry when radiating breast implants,(1) subsequent dosimetric evaluations(2, 3) and clinical outcome studies support the safety of such practices.(4, 5) Since implant based reconstruction accounts for the majority of reconstructions performed post-mastectomy(6), an increase in the sparse data available regarding expected aesthetic and clinical outcomes after RT is essential.
The timing of RT with implant based reconstructions is controversial, and most available data describes RT delivered to the permanent implant (PI).(5, 7–10) Outcomes for patients treated this way have resulted in generally good outcomes; however, data are from a very small number of institutions thereby potentially limiting their applicability. We evaluated the most common PI-based treatment sequence used at multiple Salt Lake City based institutions serving patients from across the Intermountain West. It involves: (1) mastectomy with immediate subpectoral tissue expander (TE) placement, (2) completion of tissue expansion, (3) radiation if indicated, and (4) exchange of the TE for a PI. To date there are only data describing crude rates of complications using this technique.(10–12) This underestimates the true risks since problems may occur many years following PI placement. The current analyses were undertaken to define actuarial rates of surgical complications for this technique, and to add to the currently sparse data regarding patient satisfaction and complication risk factors.
METHODS
Patient Population
A retrospective review was performed to identify patients from the Salt Lake City region treated between 1998 and 2009 who underwent mastectomy (M) and immediate subpectoral tissue expander (TE) placement followed by subsequent subpectoral permanent implant (PI) placement. Patients were required to have at least 6 months of follow up after PI placement for inclusion in this study. It is our practice to offer immediate TE placement to most patients undergoing mastectomy, even those requiring post mastectomy RT. In some patients, the need for post mastectomy RT was determined only upon final pathology from their mastectomy/node procedure. We do not make intraoperative decisions to convert to autologous flap reconstruction. If patients require RT after TE placement, they go on to complete RT with the TE in place and fully expanded. At the completion of RT, patients may then go on to PI only reconstruction, autologous tissue only reconstruction, or a combination of PI and latissimus flap. This is a decision made by both the surgeon and the patient. Patient preference, quality of tissue after RT and availability of alternative tissue sources are all considered when making this decision.
Definitions of complications
PI Replacement was defined as PI explantation and replacement with another PI during the same procedure. PI Removal was explantation of the PI without replacement. PIRR (PI removal or replacement) was any PI explantation with or without PI replacement. Non-PIRR events were any surgical interventions that did not result in PI removal or replacement. CRSI (complication requiring surgical intervention) was any unplanned complication that led to surgical intervention. Reasons for Non-PIRR and CRSI complications included asymmetry/poor cosmesis/contracture, pain from contracture, necrosis/dehiscence/extrusion, infection, seroma/hematoma, poor nipple cosmesis other than planned reconstruction, and recurrent disease.
Patient Satisfaction
Patients were mailed an anonymous survey involving a 5-point Likert scale. If a response was not received, the survey was resent a maximum of three times. When grading reconstructed breast appearance, possible answers included “Excellent,” “Very Good,” “Good,” “Fair,” and “Poor.” Patients answering “Good” or better were scored as having positive views of their appearance. Regarding satisfaction level questions, potential answers included “Strongly Agree,” “Agree,” “Neutral,” “Disagree,” and “Strongly Disagree.” To maximize survey response rate, the survey was limited to four key questions. Complication-free intervals (i.e. PI Replacement, PI Failure, PIRR, Non-PIRR, and CRSI) were assessed until either an event occurred or a survey was returned so that survey and surgical event follow-up was concordant.
Follow-up and statistical analysis
RT and Non-RT cohorts were compared using Fisher’s exact test for categorical variables and t-test for continuous variables, with p<0.05 considered significant. Times to operative events were calculated from initial PI placement date to the relevant surgical intervention date. Kaplan-Meier methods were used to estimate cumulative actuarial incidence rates of complications. Univariate analyses (UVA) were used to examine the following: age (as a continuous variable and for ≤45 vs >45 years), body mass index (BMI) (as a continuous variable and for ≤30 vs. >30), hypertension, diabetes, smoking history, overall stage, whether a mastectomy involved skin and/or nipple sparing, whether SLN biopsy and/or axillary dissection were done, whether a dermal substitute was used, whether a flap was performed, treating institution, chemotherapy and/or hormone therapy use, implant type (saline vs. silicone) and whether nipple reconstruction was performed. For the RT subset, additional variables assessed included time from RT to TE/PI exchange (as a continuous variable and for >6 or ≤6 months), use of a posterior axillary boost (PAB), scar boost, or bolus, and whether the IMC (internal mammary chain), supraclavicular (SCV), or SCV plus axillary apex regions were radiated. The Cox proportional hazards model was used for time to event outcomes. Fisher’s exact test was used for categorical outcomes. Factors significant on UVA (p<0.05) were also evaluated in multivariate analyses (MVA).
Regarding survey data, patient and treatment characteristics were assessed for potential correlations with survey answers. Categorical predictors were analyzed using Fisher’s exact test, and continuous predictors were analyzed using a likelihood ratio test of trend. “R” statistical computing software, version 2.15.0, was used for all statistical analyses.
RESULTS
Two hundred eighteen consecutive patients with 222 invasive (85%) or in-situ (15%) breast lesions were identified who underwent ≥6 months of follow-up after PI placement. The median age for all patients was 50 years (range, 25 – 83) (Table 1). Most patients (87%) received surgical evaluation of the axilla in the form of SLN biopsy and/or axillary dissection. Approximately one third of the patients (37%) received an acellular dermal matrix sling at the time of TE placement. Capsulotomy was performed at the time of PI placement, and a latissimus dorsi flap was used in 4 patients at the time of PI placement. The majority of patients received chemotherapy (51%) and/or hormone therapy (70%).
Table 1.
Patient & Surgical Characteristics
| Non-RT† | RT | Total | P | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Total number of patients | 157 | 61 | 218 | ||||
| Total number of reconstructed breasts | 161 | 61 | 222 | ||||
| Age | <0.0001 | ||||||
| Median | 51.0 | 46.5 | 50.0 | ||||
| Range | 25.0 – 79.9 | 26.3 – 82.9 | 25.0 – 82.9 | ||||
| BMI†† | 0.99 | ||||||
| Median | 23.8 | 24.2 | 24.0 | ||||
| Range | 15.6 – 43.7 | 17.7 – 40.9 | 15.6 – 43.7 | ||||
| Hypertension | 50 | 32% | 8 | 13% | 58 | 27% | 0.006 |
| Diabetes | 10 | 6% | 1 | 2% | 11 | 5% | 0.30 |
| Smoker | 25 | 16% | 2 | 3% | 27 | 12% | 0.01 |
| Bilateral Cancer | 4 | 3% | 0 | 0% | 4 | 2% | 0.59 |
| Stage* | <0.0001 | ||||||
| 0 | 32 | 20% | 1 | 2% | 33 | 15% | |
| I | 74 | 46% | 3 | 5% | 77 | 35% | |
| II | 52 | 32% | 40 | 66% | 92 | 41% | |
| III | 3 | 2% | 17 | 28% | 20 | 9% | |
| Chemotherapy | <0.0001 | ||||||
| Yes | 55 | 35% | 56 | 92% | 111 | 51% | |
| No | 96 | 61% | 5 | 8% | 101 | 46% | |
| Unknown | 6 | 4% | 0 | 0% | 6 | 3% | |
| Hormone Therapy | 0.13 | ||||||
| Yes | 104 | 66% | 48 | 79% | 152 | 70% | |
| No | 50 | 32% | 13 | 21% | 63 | 29% | |
| Unknown | 3 | 2% | 0 | 0% | 3 | 1% | |
| Surgical Details* | |||||||
| Skin Sparing Mastectomy | 34 | 21% | 22 | 35% | 56 | 25% | 0.04 |
| Nipple Sparing Mastectomy | 15 | 9% | 10 | 16% | 25 | 11% | 0.16 |
| SLN§ Biopsy and/or Axillary Dissection | 133 | 83% | 60 | 98% | 193 | 87% | 0.004 |
| SLN Biopsy Only | 67 | 42% | 15 | 25% | 82 | 37% | |
| Axillary Dissection Only | 55 | 34% | 17 | 28% | 72 | 32% | |
| Alloderm/DermaMatrix Usage | 56 | 35% | 26 | 42% | 82 | 37% | 0.35 |
| Permanent Implant Procedure* | 0.002 | ||||||
| Saline | 87 | 54% | 24 | 39% | 111 | 50% | |
| Silicone | 74 | 46% | 37 | 61% | 111 | 50% | |
| Flap Performed | 0 | 0% | 4 | 6% | 4 | 2% | |
| Elective Surgeries | |||||||
| Contralateral Prophylactic Mastectomy | 40 | 25% | 16 | 26% | 56 | 26% | 1.00 |
| Any Contralateral Procedure* | 113 | 70% | 41 | 67% | 154 | 69% | 0.51 |
| Ipsilateral Nipple Reconstruction* | 76 | 47% | 23 | 37% | 99 | 45% | 0.17 |
KEY:
= Radiation Therapy,
= Body Mass Index,
= Sentinel Lymph Node,
indicates categories where denominator is total number of breasts with disease reconstructed with a tissue expander and permanent implant.
Radiotherapy
Sixty-one patients (28%) received RT, all of whom had unilateral disease. Median RT dose and fractionation to the TE was 50.4 Gy in 28 fractions, and 41% of radiated patients received a scar boost at a median dose of 10 Gy. Only 1 patient received hypofractionated RT to 40.05 Gy in 15 fractions, which was followed by a boost of 10 Gy in 5 fractions. The majority of patients received RT to the SCV region (n = 48), most of whom also had the axillary apex included (n = 37), and chest wall bolus (0.5 to 1 cm every other day) was frequently used during RT (n = 36). Use of a PAB (n = 9) and RT to the IMC nodes (n = 3) were infrequent.
Complications
Median and mean follow-up of patients with an intact PI were 38 and 44 months, respectively (range, 6 – 144). Median time from mastectomy to TE/PI exchange was 6.0 months (range, 2 – 20). Median time from RT completion to TE/PI exchange was 3.5 months (range, 1–11). Twenty-four complications occurred in 20 patients (9%) after mastectomy/TE placement but before TE/PI exchange. For the Non-RT group, 12 complications requiring surgery occurred in 9 patients before placement of a PI. Reasons for surgery included necrosis/dehiscence [5], TE exposure in 2 patients with 1 requiring TE removal and replacement, hematoma/seroma drainage [2], asymmetry in 2 patients with one having exchange for a larger TE, and infection [1]. Only one of these patients eventually had removal of their PI. For the RT group, 11 patients had 12 complications requiring surgery, of which 10 occurred before their RT (necrosis/dehiscence [5], seroma/hematoma drainage [3], TE deflation [1], and asymmetry [1]). The 2 post RT complications were due to deflation and infection. Of these patients, 3 went on to have PI Removal, only 1 of which had the complication following RT.
Eighty-four complications in 68 patients occurred following PI/TE exchange (Table 2). Most complications were related to asymmetry/cosmesis/contracture. There were 3 patients with PI Removal who subsequently had autologous reconstructions (1 TRAM and 2 DIEP free-flap reconstructions), all of whom received RT. No complications occurred following these autologous procedures. The 1 patient who received a hypofractionated RT course did not have any surgical complications after 25 months of follow-up.
Table 2.
Crude Complication Rates
| PI† Removal | PI Replacement | PIRR†† | Non-PIRR§ | CRSI⌷ | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-RT¶ | RT | Non-RT | RT | Non-RT | RT | Non-RT | RT | Non-RT | RT | |||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Any Complication* | 5 | 3% | 10 | 16% | 23 | 14% | 10 | 16% | 28 | 17% | 19 | 33% | 14 | 9% | 11 | 18% | 36 | 24% | 27 | 44% |
| Asymmetry/Cosmesis Contracture | 0 | 0% | 1 | 2% | 23 | 14% | 8 | 13% | 23 | 14% | 9 | 15% | 9 | 6% | 6 | 10% | 31 | 19% | 16 | 26% |
| Pain/ Contracture | 2 | 1% | 3 | 5% | 0 | 0% | 1 | 2% | 2 | 1% | 4 | 7% | 2 | 1% | 1 | 2% | 4 | 2% | 5 | 8% |
| Necrosis/Dehiscence/ Extrusion | 0 | 0% | 3 | 5% | 0 | 0% | 2 | 3% | 0 | 0% | 5 | 8% | 0 | 0% | 1 | 2% | 0 | 0% | 6 | 10% |
| Infection | 1 | 1% | 3 | 5% | 0 | 0% | 0 | 0% | 1 | 1% | 3 | 5% | 0 | 0% | 1 | 2% | 1 | 1% | 4 | 7% |
| Seroma/Hematoma | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 1% | 0 | 0% | 1 | 1% | 0 | 0% |
| Nipple Cosmesis | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 1% | 1 | 2% | 1 | 1% | 1 | 2% |
| Recurrence | 2 | 1% | 0 | 0% | 0 | 0% | 0 | 0% | 2 | 1% | 0 | 0% | 1 | 1% | 0 | 0% | 3 | 2% | 0 | 0% |
KEY:
= Permanent Implant,
= Permanent Implant Removal or Replacement,
= Complication Requiring Surgery other than Permanent Implant Removal or Replacement,
= Complication Requiring Surgical Intervention,
= Radiation Therapy,
= Overall crude complication rates per column may be less than the sum of the subcategories, as patients may have had >1 complication
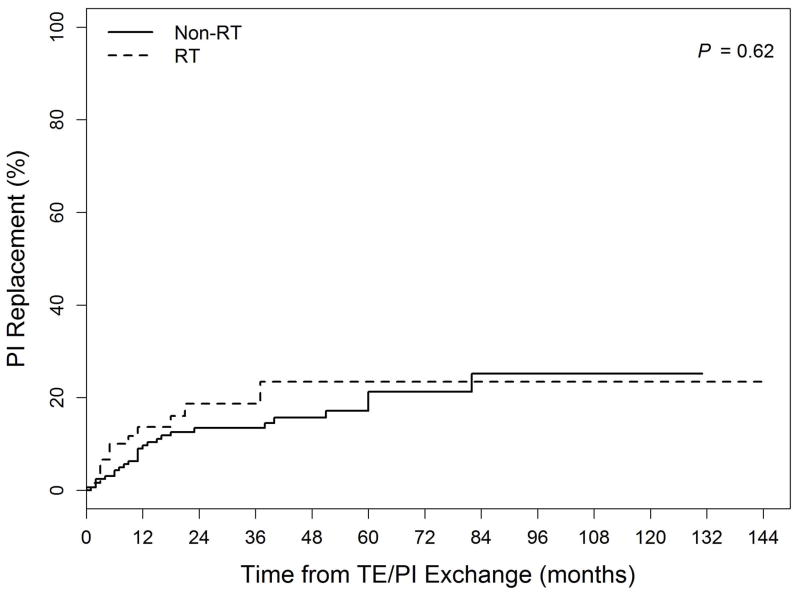
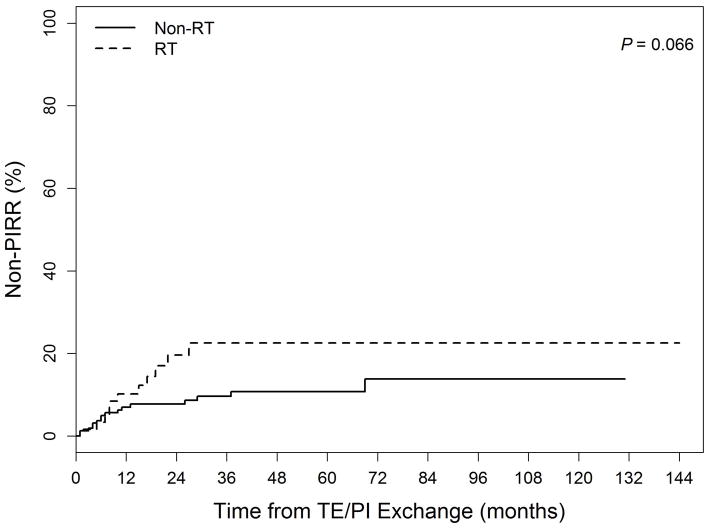
Actuarial cumulative incidence curves for PI Removal, PI Replacement, and Non-PIRR events are shown in the Figure. The 5-year PI Removal rates were 3.6% for the Non-RT group and 22.4% for the RT group (p=0.006). Five-year PI Replacement rates did not differ between the groups (Non-RT: 21.3% vs. RT: 23.5%, p=0.62). Non-PIRR events were numerically but not statistically significantly higher in RT vs. Non-RT patients at 5 years (22.5 vs. 10.8%, p=0.066). Two and 5-year PIRR rates for group Non-RT were 17% and 24%, as compared to 31% and 42% for group RT(UVA p=0.009, MVA p=0.42). For CRSI, 2 and 5-year rates were 23% and 33% for Non-RT and 41% and 50% for RT patients (UVA p=0.007, MVA p=0.23).
Figure.
Cumulative incidence of complications related to: (1a) PI Removal (Permanent implant explantation without replacement) (2b) PI Replacement (Permanent implant removal and immediate replacement with another implant) (3c) Non-PIRR (Surgical complications not including permanent implant removal or replacement) Legend: TE = tissue expander
Table 3 lists factors significant for predicting complications on UVA and MVA. For PI Removal, RT use (p = 0.006) and surgical complications before TE/PI exchange (p = 0.013) were correlated with increased complications on UVA, but only RT remained significant on MVA (p = 0.003). For PI Replacement, younger age (p = 0.021) predicted worse outcomes on UVA only, while silicone implants (p = 0.012) and nipple reconstruction (p = 0.027) were also significant on MVA. For PIRR, chemotherapy (p = 0.018) and stage (p = 0.033) were significant but only on UVA. The only factor correlated with Non-PIRR was nipple reconstruction (p = 0.0016). For CRSI, factors that remained significant on MVA were silicone implants (p = 0.003) and nipple reconstruction (p = 0.001).
Table 3.
Entire patient cohort risk factors for PI Removal, PI Replacement, PIRR, Non-PIRR and CRSI
| PI† Removal | PI Replacement | PIRR †† | Non-PIRR§ | CRSI⌷ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UVA** | MVA††† | UVA | MVA | UVA | MVA | UVA | MVA | UVA | MVA | |
| HR§§ (p) | HR (p) | HR (p) | HR (p) | HR (p) | HR (p) | HR (p) | HR (p) | HR (p) | HR (p) | |
| RT¶ Used | 5.52 (0.006) | 5.42 (0.003) | 1.20 (0.62) | n/a | 2.12 (0.009) | 1.35 (0.42) | 2.02 (0.066) | n/a | 1.96 (0.007) | 1.48 (0.23) |
| Surgical Complication before TE# /PI Exchange | 3.89 (0.013) | 2.28 (0.17) | 0.95 (0.93) | n/a | 1.61 (0.24) | n/a | 0.36 (0.29) | n/a | 1.26 (0.53) | n/a |
| Silicone vs. Saline Implant | 1.21 (0.71) | n/a | 2.25 (0.012) | 2.80 (0.006) | 1.93 (0.025) | 1.65 (0.10) | 2.07 (0.057) | n/a | 2.08 (0.004) | 2.23 (0.003) |
| Nipple Reconstruction Performed | 0.48 (0.18) | n/a | 2.1 (0.027) | 2.61 (0.006) | 1.49 (0.16) | n/a | 3.64 (0.0016) | n/a | 1.84 (0.012) | 2.31 (0.001) |
| Age ≤45 Years vs. >45 Years | 1.01 (0.61) | n/a | 1.96 (0.021) | 1.69 (0.11) | 1.03 (0.015) | 1.02 (0.16) | 1.15 (0.74) | n/a | 1.63 (0.041) | 1.02 (0.18) |
| Chemotherapy Used | 2.20 (0.22) | n/a | 1.79 (0.13) | n/a | 2.02 (0.018) | 1.45 (0.29) | 1.29 (0.74) | n/a | 1.68 (0.037) | 1.27 (0.42) |
| Stage III vs. 0/I/II | 4.10 (0.25) | n/a | 1.04 (0.95) | n/a | 3.43 (0.033) | 1.27 (0.59) | 0.88 (0.86) | n/a | 4.33 (0.032) | 1.23 (0.61) |
KEY:
= Permanent Implant,
= Permanent Implant Replacement or Removal,
= Complication Requiring Surgery other than Implant Replacement or Removal,
= Complication Requiring Surgical Intervention,
= Radiation Therapy,
= Tissue Expander,
= Univariate Analysis,
= Multivariate Analysis,
= Hazard Ratio.
Significant findings are bolded and italicized.
For the RT subgroup, hypertension was significant only on UVA for CRSI. However, hypertension remained significant on MVA for PI Replacement (p=0.036), and it was the only factor that predicted for Non-PIRR (p = 0.024). Hormonal treatment was associated with lower rates of PI Replacement (p = 0.041) and CRSI on UVA only (HR 0.39, p = 0.035), and it correlated for decreased PIRR on both UVA (HR 0.33, p = 0.026) and MVA (HR 0.35, p = 0.038). Use of a PAB correlated with increased chance of PI Removal on UVA (HR 4.49, p <0.05) but not on MVA (HR 4.43, p = 0.054). Other RT technique variables did not correlate with complication rates.
Satisfaction
One hundred forty-five survey responses were received, with a total of 149 of 222 breasts scored for a 67% response rate (Table 4). Most patients were satisfied in both groups, and RT did not statistically correlate with dissatisfaction for any question. A positive breast appearance score depended on BMI (p = 0.028) and treating institution (p = 0.04). Overall satisfaction was only dependent on lower BMI (p = 0.03). Patients more likely to answer favorably to the question “Knowing what I know today, I would definitely choose to have breast reconstruction” had a lower BMI (p = 0.009), had no axillary dissection (p = 0.0002), and received a dermal substitute (p = 0.023). A lower BMI (p = 0.006) and skin sparing mastectomy (p = 0.023) were correlated with favorable answers to “Knowing what I know today, I would definitely choose to have the same type of breast reconstruction.”. Having a PI Removal, PIRR, or CRSI event correlated with significantly decreased satisfaction in each survey question, but PI Replacement and Non-PIRR events were not significantly related to satisfaction.
Table 4.
Survey Results
| Non-RT† | RT | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q††1: Positive Q2-4: Agree |
Q1: Good Q2-4: Neutral |
Q1: Negative Q2-4: Disagree |
Q1: Positive Q2-4: Agree |
Q1:Good Q2-4: Neutral |
Q1: Negative Q2-4: Disagree |
|||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Q1. Please rate your reconstructed breast’s appearance. | 55 | 50% | 14 | 13% | 41 | 37% | 14 | 36% | 10 | 26% | 15 | 38% |
| Q2. Overall, I am satisfied with my breast reconstruction. | 60 | 55% | 15 | 14% | 35 | 32% | 20 | 51% | 7 | 18% | 12 | 31% |
| Q3. Knowing what I know today, I would definitely choose to have breast reconstruction. | 85 | 77% | 14 | 13% | 11 | 10% | 28 | 72% | 3 | 8% | 8 | 21% |
| Q4. Knowing what I know today, I would definitely choose to have the same type of breast reconstruction. | 60 | 55% | 23 | 21% | 26 | 24% | 22 | 56% | 5 | 13% | 12 | 31% |
Key:
= Radiation Therapy,
= Question
DISCUSSION
Most patients can have good outcomes with TE/PI based reconstructions, despite the higher PI Removal rate we found associated with RT (5-year: 22% vs. 4%). Other reports involving RT delivered to the TE have also described poorer outcomes compared to unirradiated patients,(10,13) and our crude PI Removal rate of 16% in RT patients compares favorably to rates of 16–25% documented elsewhere.(10, 12, 13) In a series of 141 patients receiving RT to a fully expanded TE with a median follow-up of 37 months, Cowen et al. noted a lower but comparable crude PIRR rate (denoted in their manuscript as implant failure) of 23% versus our 31%.(11) Our RT CRSI crude rate of 44% was similar to the 45% rate noted by Brooks et al. following their mean follow-up of 41 months (denoted in their manuscript as severe complications).(13) Of note is that 27 of the 97 RT patients in the Brooks et al. series received RT prior to mastectomy, and expansion post-RT occurred in an unspecified number of patients, both of which could have increased complications.
Regarding the issue of whether RT should be delivered to a fully expanded TE or to the PI, at a median follow-up of 50 months Nava et al. noted a significantly lower crude PI removal rate when RT was delivered to the PI (7 of 109 patients) rather than the TE (10 of 40 patients).(10) In a series of 151 patients who received RT to the PI following TE/PI exchange, Ho et al. reported a 7-year PI Removal rate of just 13.3% after a median follow-up of 86 months.(5) Other outcomes included 7-year PI Replacement and PIRR rates of 17.1% and 29%, respectively. Although these data regarding RT to PIs appears superior to our outcomes, direct comparisons using retrospective analyses are difficult, and differences may be due to other patient and treatment characteristics, including reluctance to reoperate on a previously radiated PI.
We found no correlation between BMI and complications, whereas for Brooks et al BMI was significant even on MVA for CRSI. (13)We found younger age (both as a categorical and continuous variable) associated with significantly higher PI Replacement and CRSI but only on UVA. Brooks et al. also found age associated with CRSI only on UVA, however the increased risk was instead for older patients. Our finding in younger patients may be due to a willingness of younger patients to have additional procedures to improve their aesthetic outcome, as the majority of the additional surgeries were performed for cosmetic reasons..
Although hypertension has been linked to increased TE/PI based complications,(9) no correlation has yet been found for patients receiving RT to the TE.(13) We found hypertension significantly associated with PI Replacement and Non-PIRR complications only in the RT subgroup. Cowen et al. found PIRR related to stage as well as smoking status on MVA.(11) For our study, on MVA stage did not remain correlated with PIRR, and we did not find any correlation between smoking and complications although this may be due to the low number of smokers. In a series of 884 patients (of which 84 received RT, all to the PI), McCarthy et al. found age ≥65, hypertension, smoking, and BMI >30 all correlated with PI removal on UVA; however, no MVA was performed.(9) In contrast, another series of 1639 patients treated at the same institution (MSKCC), all of whom received RT to the PI, revealed no association between patient characteristics and PIRR.(5) Although we did not find any correlation between complications and the timing of TE/PI exchange following RT, at a mean follow-up of 31 months Peled et al. noted a crude PI removal rate of 22.4% versus 7.7% for those patients that had PI exchange earlier than versus after 6 months following RT, respectively.(12) We found the use of silicone implants significantly associated with PI replacement on MVA. This correlation has not been previously found, but the effects of silicone vs. saline implants have rarely been evaluated.(5) This finding may in part be explained by the increased deformability of silicone implants compared to fully filled or overfilled saline implants in the setting of contracture. We also noted that nipple reconstruction was associated with both increased PI Replacement and CRSI on MVA. This may be because the additional surgical procedure required to create the nipple led to further surgical complications that would not have occurred without this elective procedure. While adjuvant hormone therapy has been associated with increased capsular contracture,(11) neither hormone therapy nor chemotherapy have been associated with increased PIRR events in patients receiving RT to either the TE(11) or the PI.(5) In our series, chemotherapy did not reach significance on MVA as a predictor for PIRR complications. Hormonal treatment was actually associated with decreased complications on MVA, but only for RT patients. Considering all available data, systemic treatment does not appear to increase complication risks. Also, similar to Ho et al., we did not find any strong correlation between RT technique and complications.(5) It is unsurprising that PAB was not associated with complications on MVA, as the PI is located outside the PAB field.
Based on anonymous surveys returned by over two thirds of patients, RT did not decrease patient satisfaction. These results are contrary to prior findings involving both RT to the TE(10) or PI.(7, 8) In our series, approximately two thirds of both the Non-RT and RT groups were pleased with their reconstructed breast appearance, and just over one half of each group was satisfied overall with their reconstruction. The lack of a significant influence of RT on satisfaction may be due to adequate management of patient expectations in the setting of RT. Nava found more people satisfied if RT was delivered to the PI (52.2%) instead of the TE (46.2%) (p = 0.04), whereas 68.1% of unirradiated patients were satisfied.(10) At 33 months mean follow-up, Cordeiro et al. found that unirradiated patients were significantly more satisfied than those who received RT to their PI (88% vs. 67%, p = 0.004).(8) However, RT was not a significant predictor of cosmetic outcome. The lower cosmetic ratings in our study are likely in part because they are based on patient surveys, not surgeon assessment as was done by Cordeiro et al.
As expected, PI Removal correlated with worse patient scores for all evaluated questions, as did PIRR and CRSI. Cowen et al. did not find PIRR associated with patient satisfaction.(11) However, they did find satisfaction depended on surgeon (p < 0.001), similar to our finding of satisfaction associated with treating institution. We found BMI highly correlated with patient satisfaction, contrary to Cordeiro et al. who did not find BMI predictive of aesthetic outcome.(7) However, physicians scored appearance in their study, and 12 of 16 patients who expressed dissatisfaction were scored by their surgeons as having a good to excellent breast appearance. This discrepancy underscores the importance of having both patient and physician reported outcomes.
This study is limited by its retrospective design. This prohibited us from reliably assessing physician-reported metrics such as aesthetic appearance and contracture, factors evaluated in retrospective analyses of prospectively gathered data in other series.(7, 8, 10–12) We instead relied on anonymous patient surveys. Our follow-up duration is comparable to other published series involving RT delivered to TEs; however, complication rates and satisfaction continue to change over time, and updated results with longer follow-up are warranted. Although the heterogeneity of our multiple physicians’ surgical and RT techniques could be considered a limitation, we feel this instead strengthens our results as it makes them more broadly applicable.
CONCLUSIONS
We have demonstrated that acceptable clinical outcomes are possible with the algorithm of TE placement at the time of mastectomy, full tissue expansion, RT if indicated, and then exchange of the TE for a PI. Although RT was significantly associated with increased PI Removal, our PI Removal rate following RT to the TE is comparable to crude rates published elsewhere. To our knowledge we are the first to report actuarial rates of surgical complications following RT to TEs. We expanded on the categories of complications first defined by Ho et al. to include surgical complications not involving PI Removal or Replacement (Non-PIRR), and added a category including all surgical complications (CRSI). We encourage the use of the categories defined in this series to facilitate future comparisons between publications.
The majority of patients were satisfied with their results, but there is a need for improvement in complication rates in the setting of RT. Compared with prior retrospective analyses involving RT to the PI following TE/PI exchange, our outcomes with RT delivered to the TE followed by TE/PI exchange appear slightly worse.(5, 7, 8, 10) However, the question of optimal RT timing remains unanswered, and it can only be determined with a prospective randomized study comparing the two methods.
Acknowledgments
Jayant Agarwal receives grant funding from Lifecell Inc, Synthes Inc, and Mentor Inc.
The project described used the “Study Design and Biostatistics” shared resource at Huntsman Cancer Institute, which is supported by Award Number P30CA042014 from the National Cancer Institute.
Footnotes
Presented at the American Radium Society, West Palm Beach, Florida, May 2011
Conflicts of interest / Disclosures:
No additional disclosures or conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. All authors except Vilija Avizonis (not affiliated with HCI) disclose this grant.
References
- 1.Motwani SB, Strom EA, Schechter NR, et al. The impact of immediate breast reconstruction on the technical delivery of postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:76–82. doi: 10.1016/j.ijrobp.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 2.Ohri N, Cordeiro PG, Keam J, et al. Quantifying the impact of immediate reconstruction in postmastectomy radiation: a large, dose-volume histogram-based analysis. Int J Radiat Oncol Biol Phys. 2012;84:e153–159. doi: 10.1016/j.ijrobp.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 3.Chung E, Marsh RB, Griffith KA, et al. Quantifying dose to the reconstructed breast: can we adequately treat? Med Dosim. 2013;38:55–59. doi: 10.1016/j.meddos.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Hazard L, Miercort C, Gaffney D, et al. Local-regional radiation therapy after breast reconstruction: what is the appropriate target volume? A case-control study of patients treated with electron arc radiotherapy and review of the literature. Am J Clin Oncol. 2004;27:555–564. doi: 10.1097/01.coc.0000135923.57073.7a. [DOI] [PubMed] [Google Scholar]
- 5.Ho A, Cordeiro P, Disa J, et al. Long-term outcomes in breast cancer patients undergoing immediate 2-stage expander/implant reconstruction and postmastectomy radiation. Cancer. 2012;118:2552–2559. doi: 10.1002/cncr.26521. [DOI] [PubMed] [Google Scholar]
- 6.American Socieity of Plastic Surgeons Webpage. Available at http://www.plasticsurgery.org/news-and-resources/statistics.html.
- 7.Cordeiro PG, McCarthy CM. A single surgeon's 12-year experience with tissue expander/implant breast reconstruction: part II. An analysis of long-term complications, aesthetic outcomes, and patient satisfaction. Plast Reconstr Surg. 2006;118:832–839. doi: 10.1097/01.prs.0000232397.14818.0e. [DOI] [PubMed] [Google Scholar]
- 8.Cordeiro PG, Pusic AL, Disa JJ, et al. Irradiation after immediate tissue expander/implant breast reconstruction: outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plast Reconstr Surg. 2004;113:877–881. doi: 10.1097/01.prs.0000105689.84930.e5. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy CM, Pusic AL, Disa JJ, et al. Unilateral postoperative chest wall radiotherapy in bilateral tissue expander/implant reconstruction patients: a prospective outcomes analysis. Plast Reconstr Surg. 2005;116:1642–1647. doi: 10.1097/01.prs.0000187794.79464.23. [DOI] [PubMed] [Google Scholar]
- 10.Nava MB, Pennati AE, Lozza L, et al. Outcome of different timings of radiotherapy in implant-based breast reconstructions. Plast Reconstr Surg. 2011;128:353–359. doi: 10.1097/PRS.0b013e31821e6c10. [DOI] [PubMed] [Google Scholar]
- 11.Cowen D, Gross E, Rouannet P, et al. Immediate post-mastectomy breast reconstruction followed by radiotherapy: risk factors for complications. Breast Cancer Res Treat. 2010;121:627–634. doi: 10.1007/s10549-010-0791-5. [DOI] [PubMed] [Google Scholar]
- 12.Peled AW, Foster RD, Esserman LJ, et al. Increasing the time to expander-implant exchange after postmastectomy radiation therapy reduces expander-implant failure. Plast Reconstr Surg. 2012;130:503–509. doi: 10.1097/PRS.0b013e31825dbf15. [DOI] [PubMed] [Google Scholar]
- 13.Brooks S, Djohan R, Tendulkar R, et al. Risk factors for complications of radiation therapy on tissue expander breast reconstructions. Breast J. 2011;18:28–34. doi: 10.1111/j.1524-4741.2011.01182.x. [DOI] [PubMed] [Google Scholar]