Abstract
Gastric diverticula are rare and usually asymptomatic. They are most frequently located on the posterior wall of the stomach. Many of them were reported as adrenal masses. Here, we present a 48-year-old male with a gastric fundus diverticulum that was misdiagnosed as a left adrenal mass on magnetic resonance imaging (MRI). Laparoscopic resection of the diverticulum was successfully performed, and histopathological examination revealed a true gastric diverticulum with moderate chronic gastritis. Although most cases of gastric diverticula are asymptomatic, to the best of our knowledge, this is the first reported case of chronic gastritis that developed in a gastric diverticulum.
Keywords: Gastric diverticulum, Chronic gastritis, Adrenal mass, MRI
Introduction
Gastric diverticula are the least common type of diverticular disease of the gastrointestinal tract. Most of the time they are asymptomatic [1]. Occasionally, they may present with upper abdominal discomfort, dyspepsia, or a feeling of fullness after meals. Rarely, their presentation might be the result of their complications [2]. Many cases of gastric diverticula were misdiagnosed as a left adrenal tumor during upper abdominal imaging, and exploratory laparotomy was performed for some of them [1].
Case Report
A 48-year-old male patient was referred to our hospital for evaluation of a left adrenal mass diagnosed by magnetic resonance imaging (MRI). The patient had a long history of recurrent abdominal pain, vomiting, and abdominal distension for the last few years. Physical examination was negative and the past medical and family histories were unremarkable. Abdominal ultrasound showed a heterogeneous hypoechoic mass lesion at the anatomical site of the left adrenal gland. Contrast enhanced abdominal MRI was requested which was interpreted as either an infected or a degenerated adrenal mass.
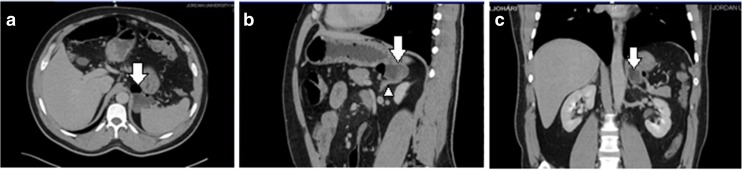
At our hospital, abdominal computerized tomography (CT) with intravenous (IV) contrast media and negative oral contrast media (water) showed an approximately 4 × 4.5 cm non-enhancing thin-walled cystic lesion with an air-fluid level in close contact to the gastric fundus posteriorly, but direct communication with the gastrointestinal tract was not distinctly exhibited; the lesion also exerted a mass effect on the left adrenal gland but seemed to be separated from it. The CT appearances were suggestive of a gastric diverticulum versus an enteric duplicating cyst (Fig. 1), and a complementary barium meal showed a wide-neck smooth outpouching from the gastric fundal wall posteriorly. The laboratory investigations were normal. After discussing the case with the surgery team, laparoscopic resection of the gastric diverticulum was done.
Fig. 1.
Axial (a), coronal (b), and sagittal MPR (c) showed a small heterogeneous mass lesion with an air-fluid level, seen in close contact to the gastric fundus posteriorly (white arrows); the left adrenal gland (white arrow head)
The final histological diagnosis was true gastric diverticulum with chronic inflammatory changes (Fig. 2). After the surgery, the patient was doing well and was discharged home. The patient was followed up and remained symptom free 3 months after surgery.
Fig. 2.
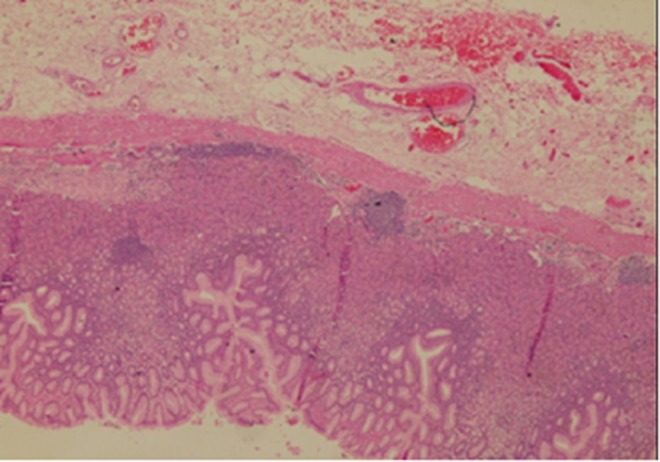
Gastric mucosa showed moderate chronic gastritis with attenuated muscularis propria and lymphoid aggregate (H&E stain, ×200)
Discussion
Gastric diverticula are usually solitary, with most of them measuring 1–6 cm in diameter, and can be multiple [3]. They are classified into congenital (true diverticula), involving all layers of the gastric wall, and acquired (false diverticula) which lack the muscularis or serosal layer [4].
Congenital diverticula constitute about 75 % of gastric diverticula. Typically, they are located in the posterior wall of the fundus of the stomach, approximately 2 cm below the gastroesophageal junction and 3 cm from the lesser curve [5]. Acquired gastric diverticula are far less common and are typically located in the antrum and prepyloric region. They usually exist in association with other gastrointestinal pathologies, such as peptic ulcer disease, malignancy, and gastric outlet obstruction, and following surgical procedures on the stomach including Roux-en-Y gastric bypass surgery [5]. Lately, gastric diverticula have also been reported in patients with Caroli’s disease [6].
Estimates of the prevalence of gastric diverticula were varied depending on the method used to observe them. This ranged from 0.04 % in contrast study radiographs to 0.01–0.11 % by endoscopic examination and 0.1–2.6 % of autopsies [5]. They usually present in the fifth and sixth decades of life, with no sex predominance. However, about 4 % of gastric diverticula occur in patients younger than 20 years and have been described in newborns where they are usually associated with pyloric obstruction [5]. Clinically, most patients with gastric diverticula are asymptomatic, but in the rare symptomatic cases, they may present with a long history of vague upper abdominal discomfort and fullness especially after meals, nausea, vomiting, or dyspeptic symptoms [2]. Occasionally, the presentation of gastric diverticula can be dramatically related to their complications like intra-peritoneal or intra-luminal hemorrhage, perforation, and torsion requiring immediate surgical intervention [7]. Other rare complications including malignant transformation within the diverticulum, gastrohepatopleural fistula, and linitis plastica due to gastric diverticulosis have also been reported [3, 4, 8].
Diverticula larger than 4 cm are more likely to be symptomatic, more prone to produce complications, and tend to respond less to medical treatment [4]. The differential diagnosis will include left adrenal mass, pancreatic and renal cysts as well as duplication cyst. When gas is existing in the diverticulum, it should be differentiated from an abscess or a necrotic tumor [9].
The literature contains many cases of gastric diverticula that were seriously misdiagnosed as adrenal masses, with exploratory laparotomy being performed for some of them [10]. Therefore, an accurate preoperative diagnosis of gastric diverticulum is important, so that an unnecessary exploratory laparotomy can be avoided. Moreover, although most gastric diverticula are asymptomatic and need no treatment, an accurate diagnosis is essential given the risk of their rare but severe complications.
An MDCT scanner with the capability for very thin (submillimetric) slice thickness and multiplanner reformation images using intravenous and oral contrast media proved to be very a useful test in the identification of gastric diverticula and in differentiating them from other lesions particularly adrenal tumors and pseudotumors.
The appropriate management of gastric diverticula depends on the presentation and complications of the diverticula. No specific treatment is usually needed for symptomatic gastric diverticula. Surgical resection is recommended when the diverticulum is complicated by bleeding, perforation, or malignancy and in symptomatic gastric diverticula that presented again with refractory symptoms of dyspepsia and abdominal pain that did not respond appropriately to medical treatment. Both open and laparoscopic resection yield good results [5].
Acknowledgments
Conflict of Interest
None to declare
References
- 1.Araki A, Shinohara M, Yamakawa J, et al. Gastric diverticulum preoperatively diagnosed as one of two left adrenal adenomas. Int J Urol. 2006;13(1):64–6. doi: 10.1111/j.1442-2042.2006.01236.x. [DOI] [PubMed] [Google Scholar]
- 2.Maduforo, N.C.N.a.C.O Gastric diverticulum in a Nigerian: a case report. J Med Med Sci. 2011;2(7):946–948. [Google Scholar]
- 3.Patel SD, Semeraro D, Hall RI. Linitis plastica due to gastric diverticulosis. J R Soc Med. 2005;98(9):416–7. doi: 10.1258/jrsm.98.9.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donkervoort SC, Baak LC, Blaauwgeers JL, et al. Laparoscopic resection of a symptomatic gastric diverticulum: a minimally invasive solution. JSLS. 2006;10(4):525–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Rashid F, Aber A, Iftikhar SY. A review on gastric diverticulum. World J Emerg Surg. 2012;7(1):1. doi: 10.1186/1749-7922-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naraynsingh V, Maharaj D, Busby GO, et al. Caroli’s disease associated with a gastric diverticulum. West Indian Med J. 2000;49(2):175–6. [PubMed] [Google Scholar]
- 7.Mnif L, Amouri A, Masmoudi MA, et al. Large gastric diverticulum. Tunis Med. 2010;88(10):765–6. [PubMed] [Google Scholar]
- 8.Fabio RPO, Lirussi F. Gastrohepatopleural fistula complicating a gastric diverticulum: a rare event and a difficult diagnosis. Internet J Gastroenterol. 2008;7(2):9. [Google Scholar]
- 9.Schwartz AN, Goiney RC, Graney DO. Gastric diverticulum simulating an adrenal mass: CT appearance and embryogenesis. AJR Am J Roentgenol. 1986;146(3):553–4. doi: 10.2214/ajr.146.3.553. [DOI] [PubMed] [Google Scholar]
- 10.Nakajo Masatoyo, TS., Umanodan Tomokazu, Nakajo Masataka (2002) A case of bilateral adrenal pseudotumors caused by hepatic cavernous hemangioma and gastric divericulum. Japanese Journal of Clinical Radiology: p. 573–577