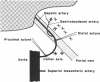
Abstract
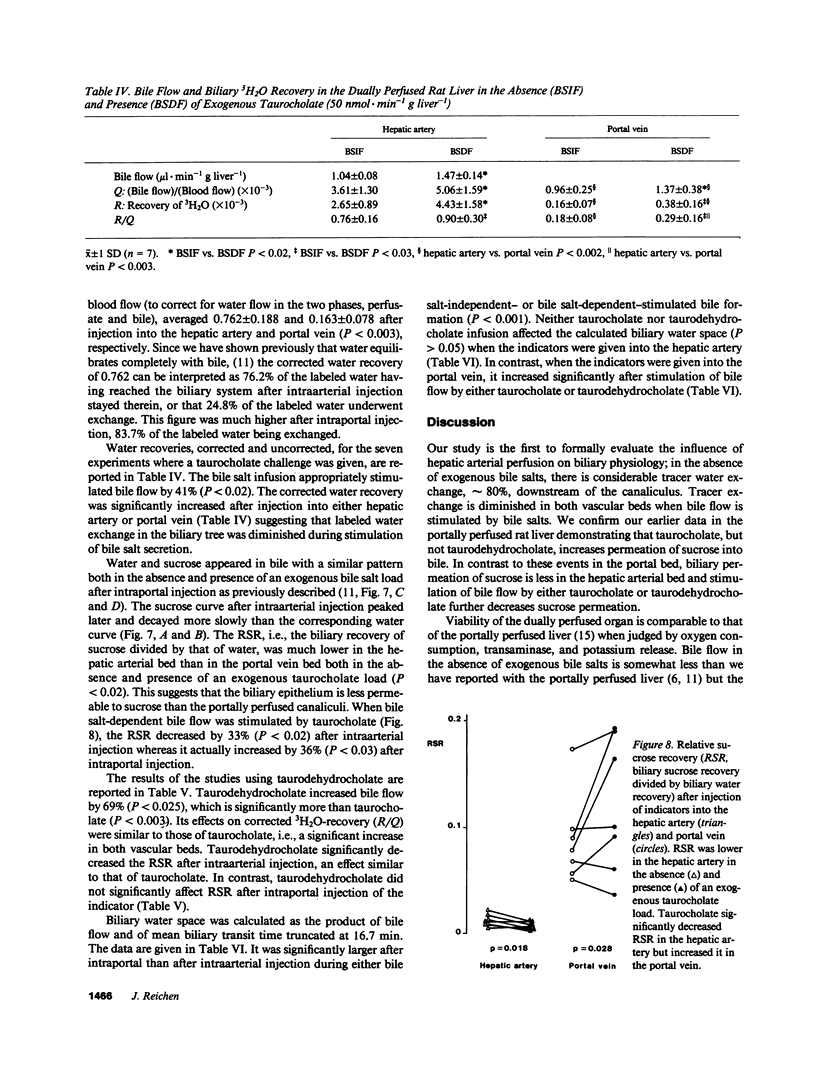
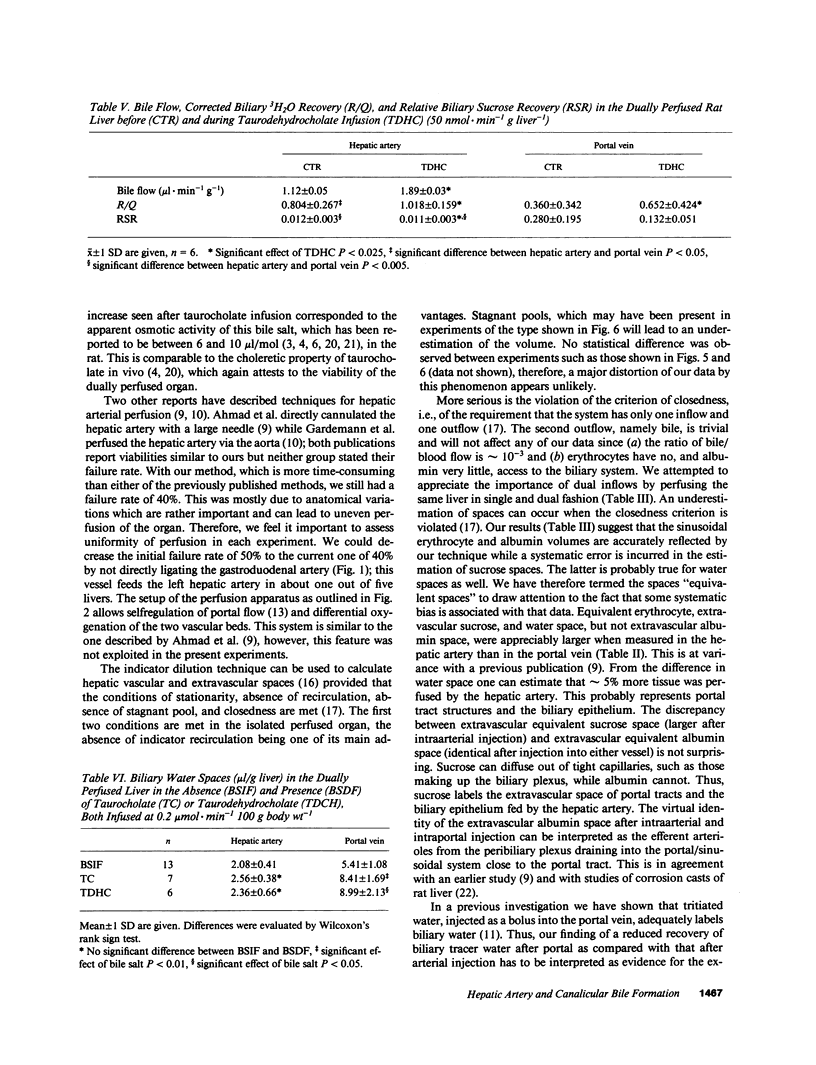
The role of the hepatic artery in tracer water exchange and regulation of permeation of small solutes during canalicular bile formation was studied in the rat using a system that permitted perfusion of both hepatic artery and portal vein. Hepatic vein and biliary multiple indicator dilution curves were obtained after injection of indicators into either vessel. The main difference in hepatic venous dilution curves was a 3.1-fold longer t0 (time spent in nonexchanging vessels) and a 5% larger equivalent water space after injection into the hepatic artery. Biliary tracer recovery of water was markedly higher after arterial injection than after portal vein injection. Both taurocholate and taurodehydrocholate stimulated bile flow and increased biliary tracer recovery after injection into either vessel. The biliary recovery of sucrose relative to that of water, which is a measure of biliary sucrose permeation, was much lower when given into the hepatic artery than when given into the portal vein. During taurocholate infusion, it decreased by 33% in the hepatic artery but increased 36% in the portal vein. Taurodehydrocholate, by contrast, did not affect permeation of sucrose given into the portal vein. Our studies demonstrate marked exchange of tracer water in the biliary epithelium. Taurocholate, but not taurodehydrocholate, increases permeation of sucrose into bile in the portal vein bed while both bile salts decrease it in the arterial bed.
Full text
PDF
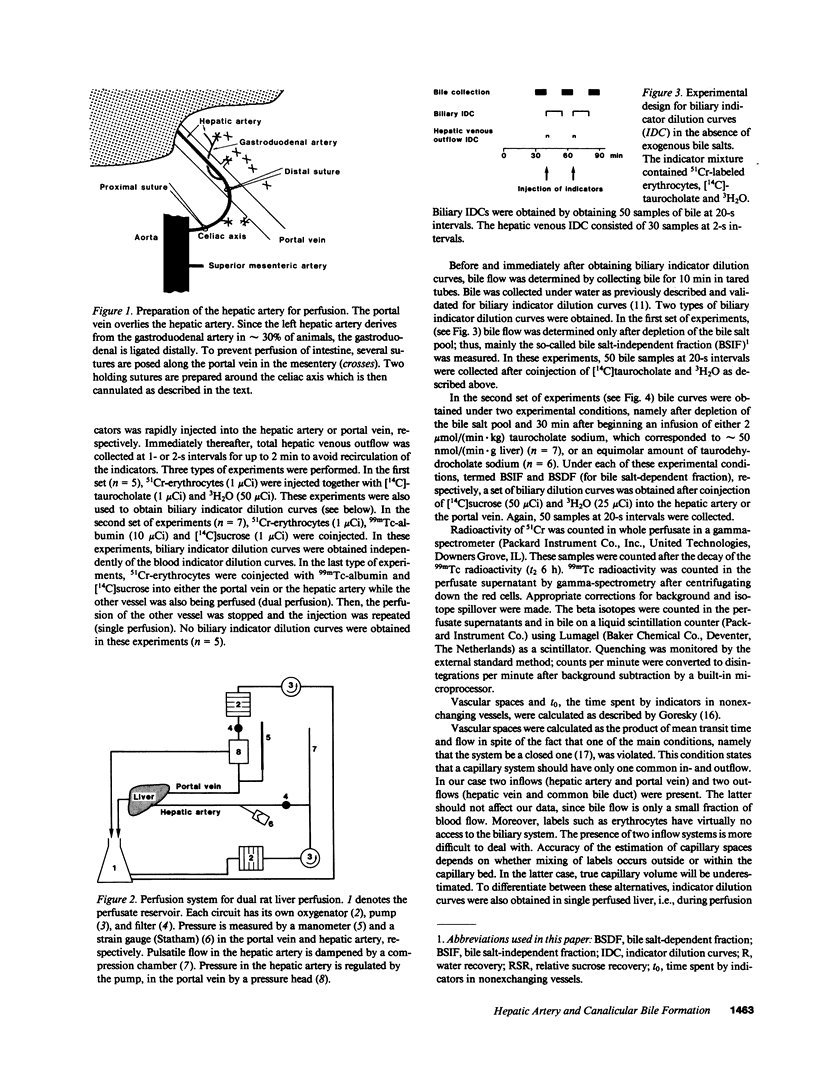
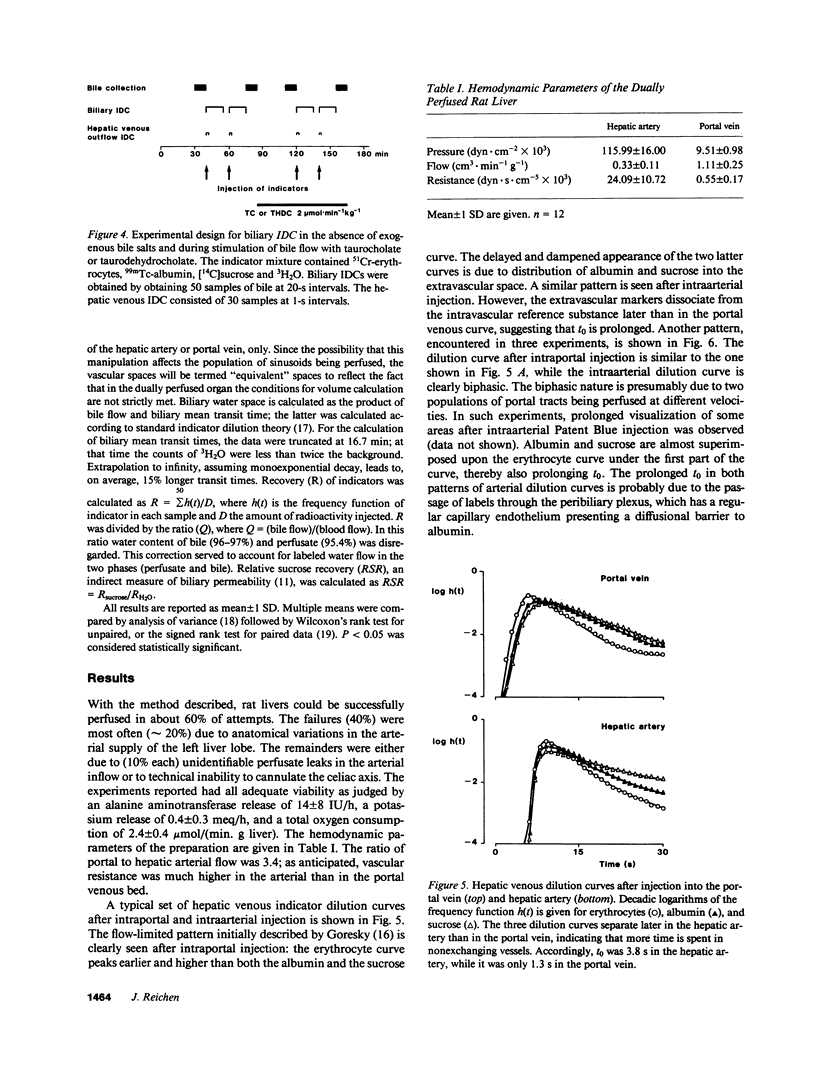
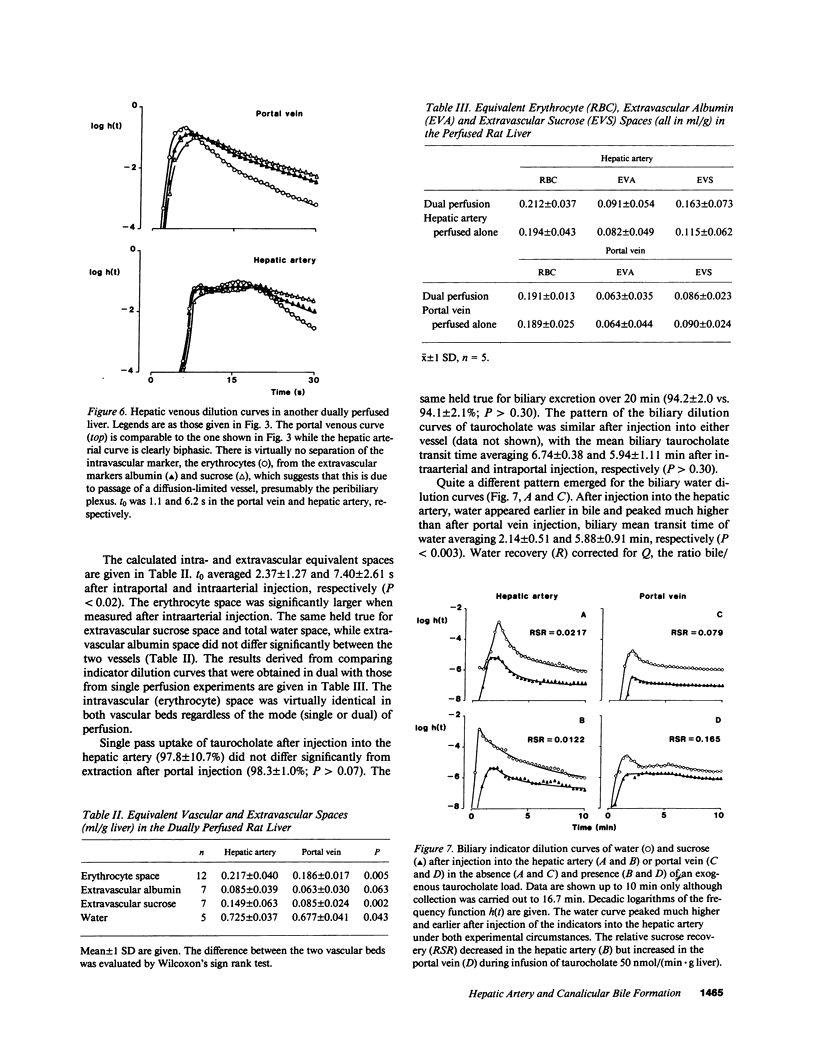


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad A. B., Bennett P. N., Rowland M. Influence of route of hepatic administration on drug availability. J Pharmacol Exp Ther. 1984 Sep;230(3):718–725. [PubMed] [Google Scholar]
- Anwer M. S., Hegner D. Importance of solvent drag and diffusion in bile acid-dependent bile formation: ion substitution studies in isolated perfused rat liver. Hepatology. 1982 Sep-Oct;2(5):580–586. doi: 10.1002/hep.1840020511. [DOI] [PubMed] [Google Scholar]
- Balabaud C., Kron K. A., Gumucio J. J. The assessment of the bile salt-nondependent fraction of canalicular bile water in the rat. J Lab Clin Med. 1977 Feb;89(2):393–399. [PubMed] [Google Scholar]
- Boyer J. L. Canalicular bile formation in the isolated perfused rat liver. Am J Physiol. 1971 Oct;221(4):1156–1163. doi: 10.1152/ajplegacy.1971.221.4.1156. [DOI] [PubMed] [Google Scholar]
- ENEROTH P. THIN-LAYER CHROMATOGRAPHY OF BILE ACIDS. J Lipid Res. 1963 Jan;4:11–16. [PubMed] [Google Scholar]
- Finck M. H., Reichen J., Vierling J. M., Kloppel T. M., Brown W. R. Hepatic uptake and disposition of human polymeric IgA1 in perfused rat liver: evidence for incomplete biliary excretion and intrahepatic degradation. Am J Physiol. 1985 Apr;248(4 Pt 1):G450–G455. doi: 10.1152/ajpgi.1985.248.4.G450. [DOI] [PubMed] [Google Scholar]
- Forker E. L. Bile formation in guinea pigs: analysis with inert solutes of graded molecular radius. Am J Physiol. 1968 Jul;215(1):56–62. doi: 10.1152/ajplegacy.1968.215.1.56. [DOI] [PubMed] [Google Scholar]
- GORESKY C. A. A linear method for determining liver sinusoidal and extravascular volumes. Am J Physiol. 1963 Apr;204:626–640. doi: 10.1152/ajplegacy.1963.204.4.626. [DOI] [PubMed] [Google Scholar]
- Gardemann A., Strulik H., Jungermann K. A portal-arterial glucose concentration gradient as a signal for an insulin-dependent net glucose uptake in perfused rat liver. FEBS Lett. 1986 Jul 7;202(2):255–259. doi: 10.1016/0014-5793(86)80697-4. [DOI] [PubMed] [Google Scholar]
- Hamilton R. L., Berry M. N., Williams M. C., Severinghaus E. M. A simple and inexpensive membrane "lung" for small organ perfusion. J Lipid Res. 1974 Mar;15(2):182–186. [PubMed] [Google Scholar]
- Häcki W., Paumgartner G. Determination of the biliary dead space using 14C-taurocholate as a marker. Experientia. 1973 Sep 15;29(9):1091–1093. doi: 10.1007/BF01946737. [DOI] [PubMed] [Google Scholar]
- Jones A. L., Schmucker D. L., Mooney J. S., Ockner R. K., Adler R. D. Alterations in hepatic pericanalicular cytoplasm during enhanced bile secretory activity. Lab Invest. 1979 Apr;40(4):512–517. [PubMed] [Google Scholar]
- Lake J. R., Licko V., Van Dyke R. W., Scharschmidt B. F. Biliary secretion of fluid-phase markers by the isolated perfused rat liver. Role of transcellular vesicular transport. J Clin Invest. 1985 Aug;76(2):676–684. doi: 10.1172/JCI112021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layden T. J., Boyer J. L. Influence of bile acids on bile canalicular membrane morphology and the lobular gradient in canalicular size. Lab Invest. 1978 Aug;39(2):110–119. [PubMed] [Google Scholar]
- Layden T. J., Elias E., Boyer J. L. Bile formation in the rat: the role of the paracellular shunt pathway. J Clin Invest. 1978 Dec;62(6):1375–1385. doi: 10.1172/JCI109258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzini I., Sakisaka S., Meier P. J., Boyer J. L. Demonstration of a transcellular vesicle pathway for biliary excretion of inulin in rat liver. Gastroenterology. 1986 Nov;91(5):1278–1288. doi: 10.1016/s0016-5085(86)80028-2. [DOI] [PubMed] [Google Scholar]
- MEIER P., ZIERLER K. L. On the theory of the indicator-dilution method for measurement of blood flow and volume. J Appl Physiol. 1954 Jun;6(12):731–744. doi: 10.1152/jappl.1954.6.12.731. [DOI] [PubMed] [Google Scholar]
- Paumgartner G., Herz R., Sauter K., Schwarz H. P. Taurocholate excertion and bile formation in the isolated perfused rat liver. An in vitro-in vivo comparison. Naunyn Schmiedebergs Arch Pharmacol. 1974;285(2):165–174. doi: 10.1007/BF00501151. [DOI] [PubMed] [Google Scholar]
- Reichen J., Le M. Taurocholate, but not taurodehydrocholate, increases biliary permeability to sucrose. Am J Physiol. 1983 Nov;245(5 Pt 1):G651–G655. doi: 10.1152/ajpgi.1983.245.5.G651. [DOI] [PubMed] [Google Scholar]
- Reichen J., Le M. Verapamil favorably influences hepatic microvascular exchange and function in rats with cirrhosis of the liver. J Clin Invest. 1986 Aug;78(2):448–455. doi: 10.1172/JCI112596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichen J., Paumgartner G. Uptake of bile acids by perfused rat liver. Am J Physiol. 1976 Sep;231(3):734–742. doi: 10.1152/ajplegacy.1976.231.3.734. [DOI] [PubMed] [Google Scholar]
- Reichen J., Simon F. R. Mechanisms of cholestasis. Int Rev Exp Pathol. 1984;26:231–274. [PubMed] [Google Scholar]
- Schmucker D. L., Gilbert R., Jones A. L., Hradek G. T., Bazin H. Effect of aging on the hepatobiliary transport of dimeric immunoglobulin A in the male Fischer rat. Gastroenterology. 1985 Feb;88(2):436–443. doi: 10.1016/0016-5085(85)90504-9. [DOI] [PubMed] [Google Scholar]
- Smith N. D., Boyer J. L. Permeability characteristics of bile duct in the rat. Am J Physiol. 1982 Jan;242(1):G52–G57. doi: 10.1152/ajpgi.1982.242.1.G52. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Stäubli W., Gnägi H. R., Hess F. A. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969 Jul;42(1):68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Sherman I., Phillips M. J., Fisher M. M. Three-dimensional observations of the hepatic arterial terminations in rat, hamster and human liver by scanning electron microscopy of microvascular casts. Hepatology. 1985 May-Jun;5(3):452–456. doi: 10.1002/hep.1840050318. [DOI] [PubMed] [Google Scholar]