Abstract
Autism spectrum disorder (ASD) affects a significant number of individuals worldwide with the prevalence continuing to grow. It is becoming clear that a large subgroup of individuals with ASD demonstrate abnormalities in mitochondrial function as well as gastrointestinal (GI) symptoms. Interestingly, GI disturbances are common in individuals with mitochondrial disorders and have been reported to be highly prevalent in individuals with co-occurring ASD and mitochondrial disease. The majority of individuals with ASD and mitochondrial disorders do not manifest a primary genetic mutation, raising the possibility that their mitochondrial disorder is acquired or, at least, results from a combination of genetic susceptibility interacting with a wide range of environmental triggers. Mitochondria are very sensitive to both endogenous and exogenous environmental stressors such as toxicants, iatrogenic medications, immune activation, and metabolic disturbances. Many of these same environmental stressors have been associated with ASD, suggesting that the mitochondria could be the biological link between environmental stressors and neurometabolic abnormalities associated with ASD. This paper reviews the possible links between GI abnormalities, mitochondria, and ASD. First, we review the link between GI symptoms and abnormalities in mitochondrial function. Second, we review the evidence supporting the notion that environmental stressors linked to ASD can also adversely affect both mitochondria and GI function. Third, we review the evidence that enteric bacteria that are overrepresented in children with ASD, particularly Clostridia spp., produce short-chain fatty acid metabolites that are potentially toxic to the mitochondria. We provide an example of this gut–brain connection by highlighting the propionic acid rodent model of ASD and the clinical evidence that supports this animal model. Lastly, we discuss the potential therapeutic approaches that could be helpful for GI symptoms in ASD and mitochondrial disorders. To this end, this review aims to help better understand the underlying pathophysiology associated with ASD that may be related to concurrent mitochondrial and GI dysfunction.
Keywords: autism spectrum disorders, Clostridia spp., electron transport chain, enteric bacteria, fatty acid metabolism, gastrointestinal, mitochondrial dysfunction, oxidative stress, propionic acid, short-chain fatty acids
The autism spectrum disorders (ASDs) are neurodevelopmental disorders characterized by impairments in communication and social interactions along with restrictive and repetitive behaviors (1). In the United States, ASD is now estimated to affect 1 in 68 individuals (2). Even though ASD is formally defined by a specific set of behaviors, the disorder has a wide heterogeneous spectrum of behavioral manifestations. Despite the behavioral definition, many children with ASD have co-morbid medical conditions including gastrointestinal (GI) symptoms (3), seizures and epilepsy (4), attention deficits (5), anxiety (6), allergies (7) and mitochondrial disease (8, 9).
The etiology of ASD is not known at this time. Even though only a minority of individuals with ASD has inherited single gene or chromosomal defects, a great deal of ASD research has traditionally concentrated on genetic causes of ASD (10). Recently, other areas of research have led to the increasing recognition that several physiological abnormalities are related to ASD. For example, an increasing number of research studies support evidence that metabolic disturbances, immune dysregulation, oxidative stress, and toxicant exposures could be linked to ASD (11, 12). Identification of these abnormalities is important as they may lead to screening, treatment and possibly even prevention strategies. For example, understanding which toxicant exposures could be linked to ASD could help identify important genetic vulnerabilities and could possibly lead to the development of prevention strategies (13). Furthermore, understanding metabolic disturbances could lead to identification of biomarkers and targeted treatments for specific metabolic abnormalities, which is important since treatments for metabolic disturbances have been shown to foster improvements in ASD symptoms (14–17).
Abnormalities in mitochondrial function are one of the most prevalent metabolic disturbances associated with ASD. A meta-analysis estimated that a significant subset of children with ASD manifest biomarkers of mitochondrial dysfunction (8) with some studies estimating that as many as 50%+ of children with ASD may manifest biomarkers of mitochondrial dysfunction when unique biomarkers, such as specific patterns of acyl-carnitine abnormalities, are included (18, 19). Other studies that carefully examined electron transport chain (ETC) function in immune cells derived from children with ASD suggest that up to 80% of children with ASD exhibit some degree of abnormal ETC function (20, 21).
Mitochondrial dysfunction appears pervasive in ASD pathophysiology. Studies have demonstrated physiologic and genetic markers of mitochondrial dysfunction in the postmortem ASD brain (22–27). Mitochondrial dysfunction may be associated with several genetic syndromes that are highly associated with ASD, including Rett syndrome (28–30), Phelan–McDermid syndrome (31), 15q11-q13 duplication syndrome (32, 33), Septo-optic dysplasia (34) Down's syndrome (35, 36), and organic acidemias (37, 38). Many animal models of ASD also demonstrate the pervasive nature of mitochondrial dysfunction and its putative role in the pathophysiology of the disorder. Interestingly, mitochondrial dysfunction has been demonstrated in animal models induced by exogenous toxicants such as the propionic acid adult rodent model (39, 40) and the prenatal valproic acid exposure rodent model (41), as well as in genetic animal models of ASD, including the Rett syndrome (28), phosphatase and tensin homolog gene haploinsufficiency (42), and Angelman syndrome (43) rodent models of ASD.
The literature contains several reports of novel mitochondrial disorders in individuals with ASD. Several reports have documented that ETC activity is markedly greater than normal for complex I in muscle (44) and complex IV in muscle (45, 46), skin (19), and brain (47). Related to this, studies from our laboratory have demonstrated that a subset of approximately one-third of the lymphoblastoid cell lines derived from ASD patients has increased mitochondrial respiratory activity (48, 49) as compared to control and other ASD lymphoblastoid cell lines. This subset of ASD cell lines exhibit increased vulnerability to oxidative challenges as compared to other ASD cell lines and controls, suggesting that these cell lines may be more vulnerable to endogenous and exogenous stressors that increase reactive species. Another novel mitochondrial disorder associated with ASD that is possibly linked to the enteric microbiome (19) will be described in this review.
Mitochondria are best known for their role in producing cellular energy. Thus, in individuals with disorders of mitochondrial function, their most affected body organs and systems are those that have the highest energy demand, including the central and peripheral nervous system, GI tract, muscles, and immune system. Interestingly, these are some of the same organs and systems commonly affected in children with ASD (9). Individuals with ASD who also have mitochondrial dysfunction are reported to have more severe behavioral and cognitive disabilities and are prone to neurodevelopmental regression compared to those with ASD without mitochondrial dysfunction (8, 50–52). Mitochondrial dysfunction may also explain the wide variety of medical abnormalities associated with ASD (9). In a recent meta-analysis, a review of all published cases of individuals with ASD and mitochondrial disease demonstrated that certain particular medical abnormalities are seen with a strikingly high prevalence in children with ASD and mitochondrial disease (8). In fact, while the prevalence of GI disorders in the general ASD populations was found to be 20%, the prevalence of GI disorders in individuals reported to have ASD with mitochondrial disease was 74%. This was also higher than children with mitochondrial disease without ASD, of which 39% were reported to have GI disorders. Thus, there may be a unique link between mitochondrial disease, GI disorders, and ASD.
This review will highlight the connections between GI disorders and mitochondrial abnormalities with reference to ASD. There appears to be at least three possible connections between the GI tract and mitochondrial abnormalities specific to ASD, which need not be mutually exclusive. First, mitochondrial dysfunction itself could result in GI dysfunction. Second, there are common exposures to environmental stressors that are associated with ASD that can affect both the mitochondria and the GI tract. Third, cell wall agents (i.e. lipopolysaccharide) (53) or metabolites from enteric bacteria (19, 40) could disrupt mitochondrial function. Each of these connections will be discussed in detail. We will also discuss the potential treatments that may be effective for improving GI symptoms, given the mitochondrial pathophysiology we have highlighted.
Mitochondrial disease is associated with GI disorders
There is a strong association between GI symptoms and mitochondrial disease. In this section, we will review the evidence for the association between mitochondrial disease and GI disorders without specific reference to ASD. This will provide a framework to further discuss the link between GI symptomatology and mitochondrial dysfunction in ASD discussed later in this article. Several well-defined mitochondrial diseases with genetic underpinnings are strongly associated with GI abnormalities. We review the link between specific mitochondrial diseases and GI disorders, the importance of mitochondrial function for enterocyte function, and the evidence for GI abnormalities in mitochondrial disease in general.
Mitochondrial neurogastrointestinal encephalopathy syndrome is characterized by progressive GI dysmotility leading to pseudo-obstruction and severe GI symptomatology. This disorder is caused by a mutation in the TYMP gene, a gene for thymidine phosphorylase, which results in nucleotide pool imbalances and mitochondrial deoxyribonucleic acid (mtDNA) depletion. Interestingly, pseudo-obstruction is also caused by mutations in the nuclear POLG and TMEM70 genes. The POLG gene codes for the mtDNA polymerase gamma that is responsible for replication of human mtDNA, and mutations in POLG also cause mtDNA depletion. The TMEM70 gene codes for ETC complex V; complex V is responsible for making the energy carrier of the cell known as adenosine triphosphate (ATP). Researchers examining the prevalence of mitochondrial dysfunction in adults with chronic intestinal pseudo-obstruction have found that 15 of the 80 adult patients studied (19%) demonstrated biochemical and/or histological findings consistent with a mitochondrial disease (54). Five demonstrated TYMP mutations, five demonstrated POLG mutations, and two demonstrated mtDNA mutations consistent with mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes syndrome (MELAS). Genetic defects were not found in the remaining three patients with biochemical and/or histological evidence of mitochondrial disease. Almost all of the patients identified with a mitochondrial disorder also demonstrated neurological abnormalities. Another study examining eight children with chronic intestinal pseudo-obstruction could not find a genetic mutation that could account for a mitochondrial disease (55) so it was presumed unlikely in this cohort. However, the small sample size was a significant limitation to this latter study. In addition, the absence of a genetic mutation does not rule out mitochondrial disease. Indeed, functional testing of mitochondrial enzymes, which was not performed, would be needed to evaluate the true function of the mitochondria.
Another GI disorder that has been linked to mitochondrial dysfunction is cyclic vomiting syndrome. Cyclic vomiting syndrome has been associated with MELAS (56, 57), Kearns–Sayre syndrome (58), large mtDNA deletions (59), 3010A and 16519T mtDNA polymorphisms (60, 61), the A3243G mtDNA mutation (62), and greater homoplasmic sequence variants in the mtDNA termination associated sequence (63). It also has a pattern of maternal inheritance consistent with mitochondrial disease (64). Several studies have suggested that the genetic basis of this disorder is different in children as compared to adults (64, 65). One study that looked at the prevalence of mitochondrial disorders in patients with cyclic vomiting syndrome found that 38% of patients had abnormalities in blood and/or urine suggesting evidence of mitochondrial dysfunction (66). Finally, effective treatments for cyclic vomiting syndrome overlap treatments used for mitochondrial disease including co-enzyme Q10, riboflavin, niacin, l-carnitine, and lipoic acid (67–69). Thus, cyclic vomiting syndrome appears to be strongly linked to mitochondrial dysfunction in a significant number of cases.
Several mitochondrial diseases are associated with progressive liver dysfunction (70). In fact, about 10–20% of childhood mitochondrial diseases involve liver dysfunction (71, 72). Perhaps, the most well known is Alpers–Huttenlocher syndrome, a syndrome caused by POLG mutations that presents with refractory seizures, developmental regression, and liver dysfunction (73). This disorder is well known because valproic acid can trigger acute liver failure leading to death (74). Like POLG mutations, other genetic abnormalities that impair mtDNA replication, including C10orf2, DGUOK, MPV17, PEO1, and SUCLG1, are part of the hepatocerebral mitochondrial DNA depletion syndromes, which are syndromes that include prominent liver failure (70, 75, 76). Genetic defects resulting in defects of mitochondrial protein synthesis also cause liver failure (70, 77). One of these mitochondrial protein synthesis abnormalities caused by TRMU gene mutations is unique in that the infantile-onset acute liver failure can spontaneously remit in many cases (78).
Pancreatic dysfunction is also a significant feature of some mitochondrial disorders (70). The best known of these is, perhaps, Pearson syndrome, which is a mitochondrial disorder caused by large-scale mtDNA rearrangements, which are characterized by exocrine pancreatic insufficiency, macrocytic anemia, and lactic acidosis (79). However, other mitochondrial disorders caused by mtDNA mutations have also been reported to involve pancreatic dysfunction (80). Endocrine pancreatic dysfunction in the form of diabetes mellitus has also been linked to several mitochondrial diseases (81, 82) and research has suggested that insulin resistance, the basic abnormality in type 2 diabetes, involves mitochondrial dysfunction (83).
Interestingly, the organic acidurias, which are disorders involving metabolic enzymatic defects resulting in accumulation of organic acids, appear to directly or indirectly involve mitochondrial dysfunction (38) and commonly involve GI symptoms. For example, propionic acidemia and other branched-chain organic acidurias, such as methylmalonic aciduria, isovaleric aciduria, and maple syrup urine disease, commonly manifest vomiting during acute crises and can manifest pancreatitis in severe cases (84–86), whereas ethylmalonic encephalopathy is characterized by chronic diarrhea (87). Interestingly, propionic acid, which is significantly elevated in propionic acidemia, can have direct effects on GI physiology. In animal models, propionic acid reduces gastric motility (88), directly stimulates longitudinal colonic smooth muscle contractions (89), induces rapid large amplitude phasic contractions followed by tonic contractions in the distal colon via serotonin and prostaglandin release (90), and dilates colonic arteries resulting in a trophic effect on intestinal mucosa (91). This suggests that non-specific GI symptoms associated with propionic acidemia such as feeding refusal, vomiting, weight loss, and abdominal distension could be a direct effect of propionic acid on GI motility through a disruption in mitochondrial function.
Mitochondrial function may also be important at the level of the enterocytes. Perturbed enterocyte mitochondrial function is believed to initiate inflammation and relapses in inflammatory bowel disease, potentially through disrupting the balance between the enterocyte and the endogenous enteric microbiome (92) or by disrupting the active transport of luminal substrates or ion flux across the cell membrane. Disruption of mitochondrial function may be responsible for a wide range of disorders involved in enterocyte dysfunction, including (a) enterocyte dysfunction following traumatic brain injury (93, 94); (b) small bowel injury related to non-steroidal anti-inflammatory drug exposure (95–97), surgical manipulation (98, 99), intestinal allergic reaction (100), and carnitine deficiency (101); (c) cyclooxygenase inhibitor-induced enteropathy (102); (d) small bowel dysfunction in chronic alcoholics with liver disease (103), and rodent models of cirrhosis (104) and obstructive jaundice (105); (e) Clostridium perfringens enterotoxin-mediated enterocyte cell death (106, 107); (f) Clostridium difficile toxin A (108); (g) methotrexate-induced enteritis (109); and finally, (h) cases of villous atrophy (110, 111).
Several studies have taken a broader look at GI function in mitochondrial disorders in children. A case series described six children who initially presented with GI dysmotility within 2 weeks of life who experienced later onset of neurological symptoms. All were found to have significant ETC abnormalities without identifiable mtDNA mutations or neuropathic GI abnormalities (112). In another case series of 36 children with diagnosed mitochondrial disorders, 20 (56%) were found to have GI abnormalities (113). Lastly, a rather elegant study found that, of 26 children with mitochondrial disorders, gastric emptying was delayed in 69% and intestinal transit time was prolonged in 46% (114). Thus, even in general mitochondrial disorders, GI symptoms seem to be common.
Interestingly, variations in mitochondrial function that do not cause frank disease may also be related to GI abnormalities. For example, one study found that specific mtDNA polymorphisms were associated with variations in satiation, rate of gastric emptying, GI pain symptoms, and specific types of irritable bowel syndrome (115). Thus, the connection between mitochondrial dysfunction and GI abnormalities is rather strong. Given that it is likely that a large proportion of children with ASD have abnormal mitochondrial function, it is possible that many of the comorbid GI abnormalities associated with ASD could be related, at least in part, to mitochondrial dysfunction.
Environmental and iatrogenic exposures can disrupt both GI and mitochondrial function
Both the mitochondria and GI tract are sensitive to environmental and iatrogenic exposures, and some of the exposures that can affect both the mitochondria and GI tract have been linked to ASD. In this section, we will review the known iatrogenic and environmental exposures that are associated with ASD and affect both GI and mitochondrial function.
There are many examples of iatrogenic exposures that have been linked to GI abnormalities, mitochondrial dysfunction, and ASD. Valproic acid is also known to cause hepatotoxicity (116) and pancreatitis (117) as well as being linked to mitochondrial disease and dysfunction (118), and exposure in utero increases the risk of developing ASD (119). In fact, the prenatal valproic acid exposure rodent model of ASD has been shown to manifest mitochondrial dysfunction (41). However, valproic acid is one of the few medications with good evidence for treating seizures and behavioral symptoms in children with ASD with a good safety profile (4), so its mechanism and effect on the mitochondria are likely to be complex and dependent on age or stage of development. Both prenatal and perinatal acetaminophen exposure (120) as well as acetaminophen exposure during childhood (121) have been associated with the development of ASD. Acetaminophen has also been associated with GI problems, including acute upper GI emergencies (122) and liver toxicity (123), and is believed to cause hepatotoxicity through its effect on the mitochondria (124). Exposures to antibiotics, either early in life (125) or during pregnancy (126), have been linked to the later developmental of ASD, and some have suggested that early antibiotic exposure could cause ASD (127, 128). Many believe that mitochondria are evolutionarily derived from bacteria, leading to the potential for some antibacterial agents such as antibiotics to negatively influence host mitochondria (129–131). Indeed, antibiotics, particularly quinolones, aminoglycosides, and beta-lactams, some of which are not uncommonly used to treat childhood and maternal infections, can cause mitochondrial dysfunction (132) and are well known to cause adverse GI effects as well as altering the developing infant gut microbiome (132–134). Lastly, proton-pump inhibitors, which are used to treat gastroesophageal reflex, a disorder not uncommonly associated with ASD, impair the transportation of carnitine, potentially interrupting carnitine metabolism (135). In addition, proton-pump inhibitors can alter the pH of the GI tract. Since various bacteria grow optimally at a narrow pH range, proton-pump inhibitors can alter the enteric microbiome.
Several environmental exposures that have been linked to ASD can cause mitochondrial dysfunction and GI abnormalities. Pesticides and heavy metals that have been linked to ASD (13) also cause mitochondrial dysfunction and GI abnormalities. For example, in rodents, chlorpyrifos induces mitochondrial ultrastructure changes (136) and reduces mitochondrial enzyme function (137, 138), while chronic chlorpyrifos exposure increases gut permeability and bacterial translocation in rodents (139). Mercury induces mitochondrial-dependent apoptosis in human (140) and murine (141) cells, induces mitochondrial dysfunction in rodent liver and brain tissue (142), impairs the carnitine transporter (143), and reduces mitochondrial enzyme function in cells from murine (141, 144, 145) and zebrafish (146). Mercury causes dysfunction of human intestinal epithelium cells (147) and has been linked to chronic atrophic gastritis (148) while it also appears to significantly change the bacterial community structures in the gut microbiome in animal models (149).
Thus, from this brief review of some environmental exposures that have been linked to ASD, it is clear that certain such exposures have also been associated with both mitochondrial dysfunction and GI abnormalities. Although most studies have not looked at a causative link between GI abnormalities and mitochondrial function, given the fact that mitochondrial dysfunction can cause GI abnormalities, including gastric dysmotility, hepatic and pancreatic dysfunction, and abnormalities in enterocyte function, it is possible that mitochondrial dysfunction is the primary abnormality leading to the GI dysfunction associated with these exposures.
Alterations in the enteric microbiome may induce mitochondrial dysfunction
The human digestive tract is host to a complex array of intestinal bacterial florae that outnumber host cells by a factor of at least 10 to 1, and over 100 to 1 regarding the amount of genetic material. This ecosystem, termed the enteric microbiome, behaves as a functional organ and produces a diverse array of bioactive metabolic products capable of entering the systemic circulation. It is important to note that the enteric microbiome and its metabolic products are not static and can be altered throughout the life cycle of the individual, with the first 18 months of life being an especially important time for the development of a stable enteric microbial ecosystem (150). The metabolic products from the enteric microbiome can have profound and dynamic effects on host metabolism, immune function, and gene expression in many organ systems, including the gut and brain (151–153).
Many authors have proposed a connection between the gut microbiome and ASD (39, 133, 134, 151, 154–156). There are several studies that point to overrepresentation of enteric bacteria, particularly Clostridia spp., in children with ASD (157–161), especially those with a regressive ASD phenotype (162, 163) and/or those children who present with GI symptoms at or before the onset of ASD symptoms (164). In addition, a small clinical trial demonstrated that treatment with vancomycin, an antibiotic aimed at decreasing Clostridia spp., is transiently effective in treating ASD symptoms (165).
Clostridia spp. are producers of the short-chain fatty-acid propionic acid (39, 40) following the fermentation of dietary carbohydrates and some proteins. Propionic acid, as well as other short-chain fatty-acid bacterial fermentation products (e.g. butyric and acetic acid), are compounds that are increasingly recognized as being important in the maintenance of health and have been implicated as possible contributing factors for certain disease processes (40, 166, 167). In particular, propionic acid can modulate cell signaling (e.g. specific free fatty acid G-protein-coupled receptors) (168, 169), cell–cell interactions (e.g. gap junctions) (170), gene expression (e.g. histone deacetylase inhibition) (171, 172), immune function (173), and neurotransmitter synthesis and release (174) as well as influence mitochondrial (175) and lipid (176, 177) metabolism. Interestingly, neurodevelopmental abnormalities that include ASD features are seen in individuals with impaired propionic acid metabolism (175, 178, 179). Furthermore, elevated propionic acid levels are present in the stool from individuals with ASD (180).
Interestingly, propionic acid is also endogenously present or added as a food preservative to a wide variety of foods including refined wheat and dairy products (181–187). Of note, propionic acid and its chemical derivatives have increasing use in agriculture and the food industry (166) and occur naturally in many foods (e.g. Swiss cheese). It is a major animal silage and food preservative in wheat and dairy products, either as a sodium or calcium salt (188, 189). Propionic acid is also produced by adding high fructose corn syrup substrate to propionibacteria cultures, which are then inoculated into foods. Inulin propionate has recently been suggested as a weight loss agent (190, 191), and aspartame is known to increase propionic acid levels in rodent gut flora. Nitropropionic acid, a derivative of propionic acid produced by many plants and fungi, is a potential contaminant of processed rice and also produced in ruminant gut. It is a potent mitochondrial toxin, capable of causing neurotoxicity and administration in rodents is an acceptable model for Huntington's chorea (192). In addition, propionate is an important naturally occurring intermediate of odd-chain length fatty-acid oxidation.
Recently, we have developed an animal model of ASD (39, 40). In the initial model, brief intracerebroventricular pulsed infusions of propionic acid into adult animals produced reversible (~30 min) bouts of ASD-type behaviors including altered social interactions (193), stereotyped behavior (194), tics (194), and hyperactivity (194, 195) as well as other cognitive and sensorimotor deficits (196) [see Fig. 1 of (40) for behavioral videos of propionic acid rodent model of ASD, link: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3747729/figure/F0001/]. The propionic acid rodent model of ASD also demonstrates biological abnormalities associated with ASD such as reactive astrocytosis and activated microglia (193, 194, 196) as well as abnormalities in redox, lipid, phosphatidylethanolamine, mitochondrial, acyl-carnitine, and carnitine metabolism (177, 194, 195, 197). Electrographic abnormalities are also seen, specifically epileptiform-like spikes in the hippocampus, neocortex, and basal ganglia, with discharges in the basal ganglia associated with measurable extrapyramidal behavioral abnormalities (194).
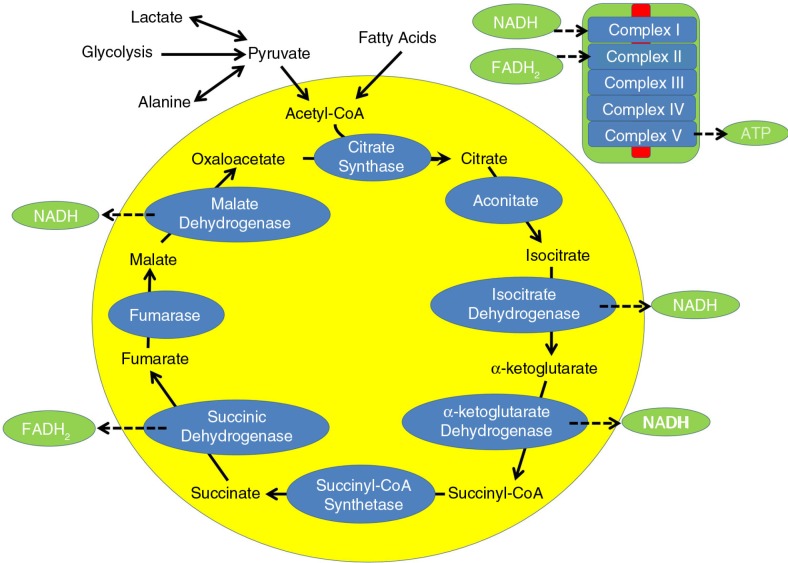
Fig. 1.
The tricarboxylic acid cycle during typical metabolism. Carbohydrates and fatty acids enter the cycle as acetyl-CoA and through a series of enzymatic steps produce energy utilizing two electron carriers, nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2). NADH and FADH2 are metabolized by complex I and complex II, respectively, of the electron transport chain (ETC). Complex V of the ETC produces adenosine triphosphate (ATP), the energy carrier of the cell.
Further development of this model shows that brief intracerebroventricular infusions of propionic acid into adolescent rats results in similar abnormalities in behavior, including abnormal restricted and repetitive behavior, altered social behavior, object vs. social preference, and cognitive abnormalities as well as ASD-like immunohistochemical evidence of innate neuroinflammation (198). To further validate this developmental model of ASD, animals were briefly exposed systemically to propionic acid prenatally and postnatally. Such exposure was found to alter conditioned taste and place avoidance (199), acoustic startle response and pre-pulse inhibition (200) and induced an increase in anxiety, repetitive, and impaired social behavior (201, 202), in rats as adolescents in a sexually dimorphic manner. In addition, propionic acid, and to a lesser extent butyric acid, modulates the expression of genes associated with ASD, including cell adhesion molecules (neurexin 1 and neuroligin), neurotransmitter systems, mitochondrial, redox, immune, and FMR1 genes in PC12 cells (172).
One of the unique biochemical markers of this rodent model was also independently reported in children with ASD (18), suggesting that this model has predictive validity. A pattern of abnormalities in fatty-acid metabolism characterized by elevations in short-chain and long-chain, but not medium-chain, acyl-carnitines were found in brain tissue of adult rats intracerebroventricularly infused with propionic acid (177). A similar pattern of elevation in acyl-carnitines was reported in children with ASD in a study that reviewed a wide range of metabolic markers from 133 consecutive patients evaluated in a medically based autism clinic (18). A standardized metabolic screening algorithm was used (203, 204) and abnormalities were verified with repeat testing (203). The workup included screening for fatty-acid oxidation defects by measuring a fasting acyl-carnitine panel. Consistent acyl-carnitine abnormalities were present in 24% of the patient sample. When the individuals with acyl-carnitine abnormalities were pooled, a specific pattern of abnormalities in acyl-carnitines was found that paralleled the acyl-carnitine elevation in the adult rodent propionic acid model of ASD: short-chain and long-chain acyl-carnitines but not medium-chain acyl-carnitines were elevated. Interestingly, 67% of this subset of children with ASD demonstrated neurodevelopmental regression, which is a particularly high rate compared to the general ASD population [about 25% (8)].
To further verify this pattern of abnormalities, we reviewed 213 patients with ASD seen in a medically based clinic who underwent a metabolic evaluation similar to the one noted above (19). Of the 213 patients screened, 17% were found to have consistent elevations in acyl-carnitines. When the data were combined across patients, C4OH, C14, and C16:1 were found to be significantly elevated at 186%, 226%, and 131% of the upper limit of normal. This subset of ASD patients with consistent elevations in short and long acyl-carnitines (CESLAC) was evaluated further.
Further testing of individuals with ASD and CESLAC ruled-out secondary causes of fatty-acid oxidation deficiencies including multiple carboxylase deficiencies, zinc deficiency, elevated copper, generalized hyperlipidemia or hypercholesterolemia, and hypoglycemia. Other biochemical markers for mitochondrial disorders were found in some CESLAC patients. Citrate and/or isocitrate was elevated in most CESLAC patients, and lactate was elevated in about half of the CESLAC patients. Genetic testing demonstrated a 22q13.1-q13.33 duplication in one (31) and suspicious novel maternally inherited homoplasmic variants of unknown significance in the cytochrome b gene in two (205). Thus, overall, no clear genetic factors seem to be consistent within these CESLAC patients that would explain their pattern of metabolic abnormalities.
Five CESLAC patients who underwent muscle biopsy had abnormal muscle histology, and electron microscopy of the muscle demonstrated abnormal mitochondria. ETC studies conducted on the muscle (206) demonstrated a partial defect in ETC complex I and complex I + III. The reason for the pattern of ETC abnormalities, particularly a decrease in ETC complex I function, is not exactly clear, but one of the hypothesized mechanisms in the context of elevated propionic acid has been outlined in our recent publication (19). The significant inhibition of ETC complex I may be closely related to a decrease in the production of the reduced form of nicotinamide adenine dinucleotide (NADH) since NADH is the driving force for ETC complex I (Fig. 1). We believe that this is due to decreased activity of two enzymes in the citric acid cycle that produce NADH, specifically, isocitrate dehydrogenase and α-ketoglutarate dehydrogenase (Fig. 1). The reason for this is outlined below.
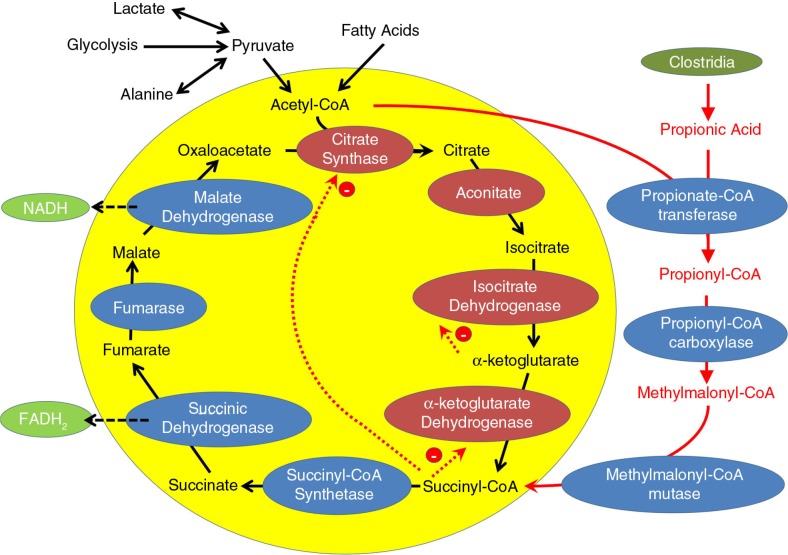
Using acetyl-CoA, propionic acid can produce propionyl-CoA, a compound that can be further metabolized to methylmalonyl-CoA (Fig. 2). Methylmalonyl-CoA is important because it can enter the citric acid cycle after being metabolized into succinyl-CoA (Fig. 2). Thus, high levels of propionic acid can be used to bypass the first four enzymes of the citric acid cycle, thereby functionally ‘short circuiting’ the citric acid cycle (Fig. 2). This results in a bypass of important steps of the citric acid cycle (including two enzymes that make NADH) and elevates succinyl-CoA concentrations that can further negatively modulate these bypassed enzymes through several mechanisms. First, high levels of succinyl-CoA will result in high levels of citric acid cycle intermediates that precede it in the cycle, such as α-ketoglutarate, isocitrate, and citrate (the latter two were found to be elevated in this ASD patient cohort) because their breakdown is stoichiometrically inhibited by high levels of their breakdown products. Second, succinyl-CoA directly inhibits citrate synthase, the first step in the citric acid cycle (Fig. 2) as well as α-ketoglutarate dehydrogenase, the citric acid cycle enzyme that produces it and an enzyme that produces NADH (Fig. 2). In addition, elevated levels of α-ketoglutarate will inhibit isocitrate dehydrogenase, the citric acid cycle enzyme that produces it and another enzyme that produces NADH (Fig. 2).
Fig. 2.
The tricarboxylic acid cycle during high levels of propionic acid. Propionic acid, presumably derived from Clostridia spp., is metabolized to propionyl-CoA using acetyl-CoA. Propionyl-CoA is further metabolized into methylmalonyl-CoA, which enters the tricarboxylic acid cycle as succinyl-CoA. Succinyl-CoA inhibits the first and fourth enzyme in the tricarboxylic acid cycle. In this manner, propionic acid may ‘short circuit’ the tricarboxylic acid cycle, thereby reducing the production of nicotinamide adenine dinucleotide (NADH). This decrease in NADH is hypothesized to cause the decrease in complex I activity measured in the patients with consistent elevations in short and long acyl-carnitines (CESLAC).
Thus, an increased succinyl-CoA concentration as a result of high levels of propionic acid can shut down the first half of the citric acid cycle and prevent the production of two of the three NADH molecules typically produced by the citric acid cycle. As ETC complex I transfers electrons from NADH, whereas ETC complex II transfers electrons from flavin adenine dinucleotide in the hydroquinone form (FADH2), it is not surprising that ETC complex I is found to be underactive (especially relative to ETC complex II) in the muscle of CESLAC patients.
The elevations in acyl-carnitines in CESLAC patients suggest that at least some specific parts of fatty-acid oxidation are inhibited. This is important as each turn of β-oxidation (the key reaction of fatty acid oxidation) produces three NADH for each two FADH2, so inhibition of β-oxidation will further decrease NADH relative to FADH2. The products of fatty-acid oxidation and how they enter the citric acid cycle are dependent on the length of the fatty acid, specifically whether the fatty acid is odd or even in length. Each turn of β-oxidation shortens the fatty acid by a length of two carbons and produces one acetyl-CoA. This is the same for both odd and even length fatty acids until the last step when the fatty acid is reduced to either a two- or three-carbon fatty acid. If the fatty acid is even in length, then the last step in the breakdown of the fatty acid produces an acetyl-CoA, while the last step in the breakdown of an odd length fatty acid results in propionyl-CoA. The significant elevations in the acyl-carnitines in this cohort were composed of even length fatty acids, suggesting that the breakdown of acetyl-CoA is inhibited relative to propionyl-CoA. This is consistent with the notion that the alternative pathway that ‘short circuits’ the citric acid cycle is upregulated. The fact that acetyl-CoA breakdown may be dependent on the availability of propionic acid when this alternative pathway is upregulated means that β-oxidation may be stoichiometrically inhibited if propionic acid is not available. If the source of propionic acid is the metabolic fermentation products of certain enteric bacteria, then the level of propionic acid will depend on enteric factors that may vary from day-to-day and hour-to-hour, such as diet, transcolonal uptake, or GI transport time. The reason why medium-chain fatty acids are not particularly elevated in this cohort is not clear at this time. Examination of the β-oxidation pathway in fibroblasts did not demonstrate any consistent abnormalities across patients examined (207), again pointing to an acquired functional inhibition of the fatty-acid oxidation pathway rather than a fixed deficit in this pathway. Overall, the elevation in acyl-carnitines in CESLAC patients appear to not be due to enzymatic defects but rather due to a functional abnormality related to altered metabolism.
CESLAC patients also demonstrated significant changes in glutathione metabolism suggesting increased oxidative stress and increased cellular vulnerability to reactive oxygen and nitrogen species. This finding could be related to the findings of mitochondrial dysfunction. First, aconitase, the second enzyme in the citric acid cycle, is redox sensitive and its activity has been shown to correlate with the glutathione redox ratio in the brain of individuals with ASD (22). Second, ETC complexes I and III are inhibited by reactive oxygen species. Thus, the abnormal redox state of the cell could also be inhibiting the specific mitochondrial enzymes that appear to be dysfunctional in CESLAC patients and in a similar manner could affect the mitochondria in the brain tissue in the propionic acid animal model of ASD (19, 40, 195).
Interestingly, ETC function in fibroblasts from the CESLAC patients was almost opposite from the results from the muscle, with complex I activity being elevated rather than depressed. However, fibroblasts are grown in culture for approximately 6 weeks, thereby representing mitochondrial function without the influences of any systemic metabolites or modulators, whereas muscle ETC function is derived from freshly frozen muscle that essentially reflects the influences of the body in situ. Thus, the fibroblast results suggest that the enzymes themselves are not dysfunctional, but rather that they are being actively inhibited by the in situ environment of the human body.
Thus, the detailed examination of CESLAC patients reveals unique changes in ETC and citric acid cycle function consistent with the excess metabolic flux of propionic acid (19). Theoretically, propionic acid can be overproduced by the overrepresented species of Clostridia found in the GI tract of children with ASD (39, 157, 180) and could result in mitochondrial dysfunction. Systematic effects of propionic acidemia, an organic acidemia in which propionic acid builds up excessively, are being recognized to be due, in part, to mitochondrial dysfunction (38).
Several lines of evidence link propionic acid with ASD. The propionic acid rodent model of ASD demonstrates ASD-like behavioral abnormalities as well as other biochemical and immune abnormalities associated with ASD (40). Our recent study demonstrated that short-chain fatty acids, such as propionic acid, can modulate the expression of many ASD implicated genes, including those involved in mitochondrial function (172). Propionic acid can have direct effects on GI motility (88–90) and perfusion (91) reminiscent of GI abnormalities associated with ASD (40, 194). Interestingly, behavioral and GI symptoms in ASD have been reported to improve when simple carbohydrates, which can be fermented by enteric bacteria to produce propionic acid, are eliminated from the diet (40, 208) or when propionic acid producing bacteria are transiently eradicated by antibiotics (165). This is clearly a novel area of ASD research that requires further study in patients with ASD and with additional animal studies.
Leading toward potential treatment
Given these insights into the relationship between mitochondrial dysfunction and GI abnormalities in ASD, there may be therapeutic avenues that can be explored for at least some children with ASD and these underlying conditions.
Given that propionic acid is a short-chain fatty acid that can complex with l-carnitine, increased propionic acid production could also potentially explain the common relative carnitine deficiency documented in children with ASD (19, 39, 40). Interestingly, clinical trials, including two double-blind placebo-controlled trials, have demonstrated that ASD symptoms can be improved with l-carnitine treatment (33, 209–215), with one trial demonstrating that the improvement in symptoms was related to the improvement in the l-carnitine levels in the blood. However, l-carnitine also increases the transport of fatty acids across the enterocytes into the body and has been suggested to be potentially detrimental if unhealthy fatty acids are ingested (216). Thus, it is important to consider that supplementation with l-carnitine could increase the flux of propionic acid from the gut into the body in the presence of bacteria that produce this metabolite. This could theoretically explain some of the adverse effects seen with l-carnitine clinical trials in some children with ASD. Acetyl l-carnitine, which transports the ‘beneficial’ acetate moiety for driving TCA function, may theoretically be superior, but this remains to be explored. Still, it is possible that propionic acid could potentially be used as a fuel for the mitochondria, if mitochondrial metabolism was able to adapt accordingly, and this might be dependent on the form in which it is delivered. For example, propionyl-l-carnitine, but not l-carnitine or propionate, is therapeutic to intestinal pathology in ulcerative colitis (217). Lastly, the GI pathology in a rodent model of colitis induced by early exposure to propionic acid or trinitrobenzene sulfonic acid was reduced by pre-administration of l-carnitine (218, 219).
Interestingly, the ketogenic diet has been shown to improve ASD-like symptoms in mouse models of ASD, including the BTBR (220), EL (221), and prenatal valproic acid exposure (41) mouse models. The ketogenic diet has been rated as very effective for controlling seizures and improving behavior and cognition in children with ASD (222–224) and has been recommended for children with ASD, especially those with epilepsy, in several systematic and expert literature reviews (4, 225). The ketogenic diet has been found to regulate key signaling pathways that both enhance mitochondrial function and promote neuroprotection (226) and is recommended for a wide variety of mitochondrial disorders (227, 228). With special reference to the novel mitochondrial disorder we have described in the CESLAC patients, several lines of research suggest that the ketogenic diet may be particularly useful for complex I defects (229, 230). Interestingly, the finding that the main high affinity blood–brain barrier transporter of propionic acid can be competitively inhibited by hydroxybutyrate (231) gives a potential mechanism where ketogenic diets may work through competitive inhibition of propionic acid transport-mediated entry into the brain.
Effective treatments for cyclic vomiting syndrome, a disorder that most likely has heavy mitochondrial underpinnings, especially in children, overlap with treatments used for mitochondrial disease including co-enzyme Q10, riboflavin, niacin, l-carnitine, and lipoic acid (67–69). Some of these same treatments have reportedly been found to improve ASD symptoms, including l-carnitine (33, 209–215), a multivitamin containing riboflavin, niacin and coenzyme Q10 (232, 233), and ubiquinol (234).
Several heavy metals have been associated with ASD (13). There is growing interest in using typical enteric microbiome flora such as Lactobacillus, which have a natural ability to bind to toxic heavy metals as a means for protecting against exposures (235). In an open-label trial, Lactobacillus rhamnosus GR was protective against increasing levels of blood mercury and arsenic in pregnant women, but not children (236). Given the wide use of probiotics in children with ASD and the growing excitement as a result of recent animal models of ASD (237), further research in this area might be promising.
Melatonin is widely used for assisting with sleep initiation in children with ASD and has been shown to be effective for improving sleep in several controlled clinical trials (238, 239). Besides the hormonal effects of melatonin, it also appears to have antioxidant and anti-inflammatory properties as well as modulating mitochondrial function (240). Interestingly, melatonin is produced in and has receptors throughout the GI tract (240) and has been shown to modulate serotonin-induced contractions and gastric glandular mucosal blood flow (241). Melatonin is being investigated for its role in protection and healing of the GI tract (242). Thus, melatonin could be especially helpful for children with ASD who have comorbid GI and mitochondrial abnormalities.
Small bowel injury related to surgical manipulation appears to involve mitochondrial dysfunction within enterocytes (98, 99). Several animal studies have suggested that supplementation with metabolic intermediates can protect the bowel from injury in animal models through a mitochondrial mechanism. Supplementation with α-ketoglutarate improves intestinal morphology, antioxidant capacity, and parameters of mitochondrial energy production in the lipopolysaccharide-induced intestinal damage animal model (243). Interestingly, α-ketoglutarate can be converted into glutamate, another metabolic intermediate and a neurotransmitter, by mitochondrial glutamate dehydrogenase in the GI tract. Glutamine is an important nutrient and fuel for the GI tract during illness, stress, and injury and can be produced by glutamate and ammonia. Pretreatment with glutamine prior to surgical gut manipulation in rodents prevents gut and lung injury due to oxidative and inflammatory processes and protects enterocyte mitochondria (244). Interestingly, the protective effect of enterocyte mitochondria during surgical stress may also be related to nitric oxide production as pretreatment with l-arginine protects enterocyte mitochondria from the effects of intestinal manipulation, but not when nitric oxide synthase is inhibited (245).
Conclusion
This manuscript outlines several of the connections between mitochondrial dysfunction, enteric microbiome abnormalities, and GI abnormalities in relation to ASD. There are many potential interactions between basic biological mechanisms and disease etiologies, which can result in symptoms of ASD and GI tract abnormalities as well as mitochondrial dysfunction. Considering the effect of the microbiome and its metabolic products adds another layer of complexity onto an already complex story. Understanding the interaction between ASD and GI symptoms in the context of mitochondrial dysfunction can provide a greater understanding of the underlying pathophysiology and how biological abnormalities are related to the complex behavioral manifestations characteristic of ASD. Considering the potential etiologies that can cause mitochondrial dysfunction, ASD and/or GI tract dysfunction can provide insight into the possible etiological factors contributing to ASD. Lastly, considering treatments that can address these underlying abnormalities may lead to the development of new treatments that reduce symptoms and improve cellular metabolism.
Acknowledgments
The authors express their heartfelt thanks to countless parents and caregivers of persons with autism and to David Patchell-Evans, for his tireless devotion to persons with autism, and his daughter, Kilee Patchell-Evans.
Conflict of interest and funding
The authors have no conflicts of interest or financial disclosures to declare. This research was supported by the Arkansas Biomedical Institute (REF), Autism Canada (DFM), Autism Research Institute (DFM, REF), and GoodLife Children's Charities (DFM).
References
- 1.APA. 4th ed. Washington, DC: American Psychiatric Association; 1994. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 2.Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators, Centers for Disease Control and Prevention. Prevalence of autism spectrum disorder among children aged 8 years – autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63:1–21. [PubMed] [Google Scholar]
- 3.Chaidez V, Hansen RL, Hertz-Picciotto I. Gastrointestinal problems in children with autism, developmental delays or typical development. J Autism Dev Disord. 2013;44:1117–27. doi: 10.1007/s10803-013-1973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frye RE, Rossignol D, Casanova MF, Brown GL, Martin V, Edelson S, et al. A review of traditional and novel treatments for seizures in autism spectrum disorder: findings from a systematic review and expert panel. Front Public Health. 2013;1:31. doi: 10.3389/fpubh.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taurines R, Schwenck C, Westerwald E, Sachse M, Siniatchkin M, Freitag C. ADHD and autism: differential diagnosis or overlapping traits? A selective review. Atten Defic Hyperact Disord. 2012;4:115–39. doi: 10.1007/s12402-012-0086-2. [DOI] [PubMed] [Google Scholar]
- 6.Sukhodolsky DG, Bloch MH, Panza KE, Reichow B. Cognitive-behavioral therapy for anxiety in children with high-functioning autism: a meta-analysis. Pediatrics. 2013;132:e1341–50. doi: 10.1542/peds.2013-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angelidou A, Alysandratos KD, Asadi S, Zhang B, Francis K, Vasiadi M, et al. Brief report: “allergic symptoms” in children with autism spectrum disorders. More than meets the eye? J Autism Dev Disord. 2011;41:1579–85. doi: 10.1007/s10803-010-1171-z. [DOI] [PubMed] [Google Scholar]
- 8.Rossignol DA, Frye RE. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol Psychiatry. 2012;17:290–314. doi: 10.1038/mp.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frye RE, Rossignol DA. Mitochondrial dysfunction can connect the diverse medical symptoms associated with autism spectrum disorders. Pediatr Res. 2011;69:41R–7R. doi: 10.1203/PDR.0b013e318212f16b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaefer GB, Mendelsohn NJ, Professional P, Guidelines C. Clinical genetics evaluation in identifying the etiology of autism spectrum disorders: 2013 guideline revisions. Genet Med. 2013;15:399–407. doi: 10.1038/gim.2013.32. [DOI] [PubMed] [Google Scholar]
- 11.Rossignol DA, Frye RE. A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol Psychiatry. 2012;17:389–401. doi: 10.1038/mp.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frye RE, James SJ. Metabolic pathology of autism in relation to redox metabolism. Biomark Med. 2014;8:321–30. doi: 10.2217/bmm.13.158. [DOI] [PubMed] [Google Scholar]
- 13.Rossignol DA, Genuis SJ, Frye RE. Environmental toxicants and autism spectrum disorders: a systematic review. Transl Psychiatry. 2014;4:e360. doi: 10.1038/tp.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frye RE, Rossignol DA. Treatments for biomedical abnormalities associated with autism spectrum disorder. Front Pediatr. 2014;2:66. doi: 10.3389/fped.2014.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frye RE, Sequeira JM, Quadros EV, James SJ, Rossignol DA. Cerebral folate receptor autoantibodies in autism spectrum disorder. Mol Psychiatry. 2013;18:369–81. doi: 10.1038/mp.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frye RE, Melnyk S, Fuchs G, Reid T, Jernigan S, Pavliv O, et al. Effectiveness of methylcobalamin and folinic acid treatment on adaptive behavior in children with autistic disorder is related to glutathione redox status. Autism Res Treat. 2013;2013:609705. doi: 10.1155/2013/609705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frye RE, DeLatorre R, Taylor HB, Slattery J, Melnyk S, Chowdhury N, et al. Metabolic effects of sapropterin treatment in autism spectrum disorder: a preliminary study. Transl Psychiatry. 2013;3:e237. doi: 10.1038/tp.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frye RE. Biomarker of abnormal energy metabolism in children with autism spectrum disorder. N Am J Med Sci. 2012;5:141–7. [Google Scholar]
- 19.Frye RE, Melnyk S, Macfabe DF. Unique acyl-carnitine profiles are potential biomarkers for acquired mitochondrial disease in autism spectrum disorder. Transl Psychiatry. 2013;3:e220. doi: 10.1038/tp.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giulivi C, Zhang YF, Omanska-Klusek A, Ross-Inta C, Wong S, Hertz-Picciotto I, et al. Mitochondrial dysfunction in autism. JAMA. 2010;304:2389–96. doi: 10.1001/jama.2010.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Napoli E, Wong S, Hertz-Picciotto I, Giulivi C. Deficits in bioenergetics and impaired immune response in granulocytes from children with autism. Pediatrics. 2014;133:e1405–10. doi: 10.1542/peds.2013-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose S, Melnyk S, Pavliv O, Bai S, Nick TG, Frye RE, et al. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl Psychiatry. 2012;2:e134. doi: 10.1038/tp.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chauhan A, Gu F, Essa MM, Wegiel J, Kaur K, Brown WT, et al. Brain region-specific deficit in mitochondrial electron transport chain complexes in children with autism. J Neurochem. 2011;117:209–20. doi: 10.1111/j.1471-4159.2011.07189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang G, Gutierrez Rios P, Kuo SH, Akman HO, Rosoklija G, Tanji K, et al. Mitochondrial abnormalities in temporal lobe of autistic brain. Neurobiol Dis. 2013;54:349–61. doi: 10.1016/j.nbd.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anitha A, Nakamura K, Thanseem I, Yamada K, Iwayama Y, Toyota T, et al. Brain region-specific altered expression and association of mitochondria-related genes in autism. Mol Autism. 2012;3:12. doi: 10.1186/2040-2392-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anitha A, Nakamura K, Thanseem I, Matsuzaki H, Miyachi T, Tsujii M, et al. Downregulation of the expression of mitochondrial electron transport complex genes in autism brains. Brain Pathol. 2013;23:294–302. doi: 10.1111/bpa.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossignol DA, Frye RE. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front Physiol. 2014;5:150. doi: 10.3389/fphys.2014.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grosser E, Hirt U, Janc OA, Menzfeld C, Fischer M, Kempkes B, et al. Oxidative burden and mitochondrial dysfunction in a mouse model of Rett syndrome. Neurobiol Dis. 2012;48:102–14. doi: 10.1016/j.nbd.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Gibson JH, Slobedman B, Harikrishnan KN, Williamson SL, Minchenko D, El-Osta A, et al. Downstream targets of methyl CpG binding protein 2 and their abnormal expression in the frontal cortex of the human Rett syndrome brain. BMC Neurosci. 2010;11:53. doi: 10.1186/1471-2202-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Condie J, Goldstein J, Wainwright MS. Acquired microcephaly, regression of milestones, mitochondrial dysfunction, and episodic rigidity in a 46,XY male with a de novo MECP2 gene mutation. J Child Neurol. 2010;25:633–6. doi: 10.1177/0883073809342004. [DOI] [PubMed] [Google Scholar]
- 31.Frye RE. Mitochondrial disease in 22q13 duplication syndrome. J Child Neurol. 2012;27:942–9. doi: 10.1177/0883073811429858. [DOI] [PubMed] [Google Scholar]
- 32.Frye RE. 15q11.2-13 duplication, mitochondrial dysfunction, and developmental disorders. J Child Neurol. 2009;24:1316–20. doi: 10.1177/0883073809333531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filipek PA, Juranek J, Smith M, Mays LZ, Ramos ER, Bocian M, et al. Mitochondrial dysfunction in autistic patients with 15q inverted duplication. Ann Neurol. 2003;53:801–4. doi: 10.1002/ana.10596. [DOI] [PubMed] [Google Scholar]
- 34.Schuelke M, Krude H, Finckh B, Mayatepek E, Janssen A, Schmelz M, et al. Septo-optic dysplasia associated with a new mitochondrial cytochrome b mutation. Ann Neurol. 2002;51:388–92. doi: 10.1002/ana.10151. [DOI] [PubMed] [Google Scholar]
- 35.Pagano G, Castello G. Oxidative stress and mitochondrial dysfunction in Down syndrome. Adv Exp Med Biol. 2012;724:291–9. doi: 10.1007/978-1-4614-0653-2_22. [DOI] [PubMed] [Google Scholar]
- 36.Pallardo FV, Lloret A, Lebel M, d'Ischia M, Cogger VC, Le Couteur DG, et al. Mitochondrial dysfunction in some oxidative stress-related genetic diseases: ataxia-telangiectasia, Down syndrome, Fanconi anaemia and Werner syndrome. Biogerontology. 2010;11:401–19. doi: 10.1007/s10522-010-9269-4. [DOI] [PubMed] [Google Scholar]
- 37.Al-Owain M, Kaya N, Al-Shamrani H, Al-Bakheet A, Qari A, Al-Muaigl S, et al. Autism spectrum disorder in a child with propionic acidemia. JIMD Rep. 2013;7:63–6. doi: 10.1007/8904_2012_143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wajner M, Goodman SI. Disruption of mitochondrial homeostasis in organic acidurias: insights from human and animal studies. J Bioenerg Biomembr. 2011;43:31–8. doi: 10.1007/s10863-011-9324-0. [DOI] [PubMed] [Google Scholar]
- 39.Macfabe D. Autism: metabolism, mitochondria, and the microbiome. Glob Adv Health Med. 2013;2:52–66. doi: 10.7453/gahmj.2013.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacFabe DF. Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb Ecol Health Dis. 2012;23 doi: 10.3402/mehd.v23i0.19260. 19260, doi: http://dx.doi.org/10.3402/mehd.v23i0.19260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahn Y, Narous M, Tobias R, Rho JM, Mychasiuk R. The ketogenic diet modifies social and metabolic alterations identified in the prenatal valproic acid model of autism spectrum disorder. Dev Neurosci. 2014;36:371–80. doi: 10.1159/000362645. [DOI] [PubMed] [Google Scholar]
- 42.Napoli E, Ross-Inta C, Wong S, Hung C, Fujisawa Y, Sakaguchi D, et al. Mitochondrial dysfunction in Pten haplo-insufficient mice with social deficits and repetitive behavior: interplay between Pten and p53. PLoS One. 2012;7:e42504. doi: 10.1371/journal.pone.0042504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su H, Fan W, Coskun PE, Vesa J, Gold JA, Jiang YH, et al. Mitochondrial dysfunction in CA1 hippocampal neurons of the UBE3A deficient mouse model for Angelman syndrome. Neurosci Lett. 2011;487:129–33. doi: 10.1016/j.neulet.2009.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graf WD, Marin-Garcia J, Gao HG, Pizzo S, Naviaux RK, Markusic D, et al. Autism associated with the mitochondrial DNA G8363A transfer RNALys mutation. J Child Neurol. 2000;15:357–61. doi: 10.1177/088307380001500601. [DOI] [PubMed] [Google Scholar]
- 45.Frye RE, Naviaux RK. Autistic disorder with complex IV overactivity: a new mitochondrial syndrome. J Pediatr Neurol. 2011;9:427–34. [Google Scholar]
- 46.Frye RE. Novel cytochrome B gene mutations causing mitochondrial disease in autism. J Pediatr Neurol. 2012;10:35–40. [Google Scholar]
- 47.Palmieri L, Papaleo V, Porcelli V, Scarcia P, Gaita L, Sacco R, et al. Altered calcium homeostasis in autism-spectrum disorders: evidence from biochemical and genetic studies of the mitochondrial aspartate/glutamate carrier AGC1. Mol Psychiatry. 2010;15:38–52. doi: 10.1038/mp.2008.63. [DOI] [PubMed] [Google Scholar]
- 48.Rose S, Frye RE, Slattery J, Wynne R, Tippett M, Pavliv O, et al. Oxidative stress induces mitochondrial dysfunction in a subset of autism lymphoblastoid cell lines in a well-matched case control cohort. PLoS One. 2014;9:e85436. doi: 10.1371/journal.pone.0085436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rose S, Frye RE, Slattery J, Wynne R, Tippett M, Melnyk S, et al. Oxidative stress induces mitochondrial dysfunction in a subset of autistic lymphoblastoid cell lines. Transl Psychiatry. 2014;4:e377. doi: 10.1038/tp.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minshew NJ, Goldstein G, Dombrowski SM, Panchalingam K, Pettegrew JW. A preliminary 31P MRS study of autism: evidence for undersynthesis and increased degradation of brain membranes. Biol Psychiatry. 1993;33:762–73. doi: 10.1016/0006-3223(93)90017-8. [DOI] [PubMed] [Google Scholar]
- 51.Mostafa GA, El-Gamal HA, El-Wakkad ASE, El-Shorbagy OE, Hamza MM. Polyunsaturated fatty acids, carnitine and lactate as biological markers of brain energy in autistic children. Int J Child Neuropsychiatry. 2005;2:179–88. [Google Scholar]
- 52.Frye RE, Delatorre R, Taylor H, Slattery J, Melnyk S, Chowdhury N, et al. Redox metabolism abnormalities in autistic children associated with mitochondrial disease. Transl Psychiatry. 2013;3:e273. doi: 10.1038/tp.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bullon P, Roman-Malo L, Marin-Aguilar F, Alvarez-Suarez JM, Giampieri F, Battino M, et al. Lipophilic antioxidants prevent lipopolysaccharide-induced mitochondrial dysfunction through mitochondrial biogenesis improvement. Pharmacol Res. 2015;91:1–8. doi: 10.1016/j.phrs.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 54.Amiot A, Tchikviladze M, Joly F, Slama A, Hatem DC, Jardel C, et al. Frequency of mitochondrial defects in patients with chronic intestinal pseudo-obstruction. Gastroenterology. 2009;137:101–9. doi: 10.1053/j.gastro.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 55.Galmiche L, Jaubert F, Sauvat F, Sarnacki S, Goulet O, Assouline Z, et al. Normal oxidative phosphorylation in intestinal smooth muscle of childhood chronic intestinal pseudo-obstruction. Neurogastroenterol Motil. 2011;23:24–9, e1. doi: 10.1111/j.1365-2982.2010.01595.x. [DOI] [PubMed] [Google Scholar]
- 56.Nishizawa M, Tanaka K, Shinozawa K, Kuwabara T, Atsumi T, Miyatake T, et al. A mitochondrial encephalomyopathy with cardiomyopathy. A case revealing a defect of complex I in the respiratory chain. J Neurol Sci. 1987;78:189–201. doi: 10.1016/0022-510x(87)90060-8. [DOI] [PubMed] [Google Scholar]
- 57.Koga Y, Akita Y, Nishioka J, Yatsuga S, Povalko N, Tanabe Y, et al. L-arginine improves the symptoms of strokelike episodes in MELAS. Neurology. 2005;64:710–2. doi: 10.1212/01.WNL.0000151976.60624.01. [DOI] [PubMed] [Google Scholar]
- 58.Boles RG, Baldwin EE, Prezant TR. Combined cyclic vomiting and Kearns–Sayre syndromes. Pediatr Neurol. 2007;36:135–6. doi: 10.1016/j.pediatrneurol.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 59.Boles RG, Chun N, Senadheera D, Wong LJ. Cyclic vomiting syndrome and mitochondrial DNA mutations. Lancet. 1997;350:1299–300. doi: 10.1016/S0140-6736(05)62477-4. [DOI] [PubMed] [Google Scholar]
- 60.Boles RG, Adams K, Li BU. Maternal inheritance in cyclic vomiting syndrome. Am J Med Genet A. 2005;133a:71–7. doi: 10.1002/ajmg.a.30524. [DOI] [PubMed] [Google Scholar]
- 61.Zaki EA, Freilinger T, Klopstock T, Baldwin EE, Heisner KR, Adams K, et al. Two common mitochondrial DNA polymorphisms are highly associated with migraine headache and cyclic vomiting syndrome. Cephalalgia. 2009;29:719–28. doi: 10.1111/j.1468-2982.2008.01793.x. [DOI] [PubMed] [Google Scholar]
- 62.Salpietro CD, Briuglia S, Merlino MV, Di Bella C, Rigoli L. A mitochondrial DNA mutation (A3243G mtDNA) in a family with cyclic vomiting. Eur J Pediatr. 2003;162:727–8. doi: 10.1007/s00431-003-1280-1. [DOI] [PubMed] [Google Scholar]
- 63.Wang Q, Ito M, Adams K, Li BU, Klopstock T, Maslim A, et al. Mitochondrial DNA control region sequence variation in migraine headache and cyclic vomiting syndrome. Am J Med Genet A. 2004;131:50–8. doi: 10.1002/ajmg.a.30323. [DOI] [PubMed] [Google Scholar]
- 64.Venkatesan T, Zaki EA, Kumar N, Sengupta J, Ali M, Malik B, et al. Quantitative pedigree analysis and mitochondrial DNA sequence variants in adults with cyclic vomiting syndrome. BMC Gastroenterol. 2014;14:181. doi: 10.1186/1471-230X-14-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boles RG, Zaki EA, Lavenbarg T, Hejazi R, Foran P, Freeborn J, et al. Are pediatric and adult-onset cyclic vomiting syndrome (CVS) biologically different conditions? Relationship of adult-onset CVS with the migraine and pediatric CVS-associated common mtDNA polymorphisms 16519T and 3010A. Neurogastroenterol Motil. 2009;21:936–e72. doi: 10.1111/j.1365-2982.2009.01305.x. [DOI] [PubMed] [Google Scholar]
- 66.Moses J, Keilman A, Worley S, Radhakrishnan K, Rothner AD, Parikh S. Approach to the diagnosis and treatment of cyclic vomiting syndrome: a large single-center experience with 106 patients. Pediatr Neurol. 2014;50:569–73. doi: 10.1016/j.pediatrneurol.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 67.Yorns WR, Jr., Hardison HH. Mitochondrial dysfunction in migraine. Semin Pediatr Neurol. 2013;20:188–93. doi: 10.1016/j.spen.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 68.Boles RG. High degree of efficacy in the treatment of cyclic vomiting syndrome with combined co-enzyme Q10, l-carnitine and amitriptyline, a case series. BMC Neurol. 2011;11:102. doi: 10.1186/1471-2377-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boles RG, Lovett-Barr MR, Preston A, Li BU, Adams K. Treatment of cyclic vomiting syndrome with co-enzyme Q10 and amitriptyline, a retrospective study. BMC Neurol. 2010;10:10. doi: 10.1186/1471-2377-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rahman S. Gastrointestinal and hepatic manifestations of mitochondrial disorders. J Inherit Metab Dis. 2013;36:659–73. doi: 10.1007/s10545-013-9614-2. [DOI] [PubMed] [Google Scholar]
- 71.Cormier-Daire V, Chretien D, Rustin P, Rotig A, Dubuisson C, Jacquemin E, et al. Neonatal and delayed-onset liver involvement in disorders of oxidative phosphorylation. J Pediatr. 1997;130:817–22. doi: 10.1016/s0022-3476(97)80027-3. [DOI] [PubMed] [Google Scholar]
- 72.Darin N, Oldfors A, Moslemi AR, Holme E, Tulinius M. The incidence of mitochondrial encephalomyopathies in childhood: clinical features and morphological, biochemical, and DNA anbormalities. Ann Neurol. 2001;49:377–83. [PubMed] [Google Scholar]
- 73.Saneto RP, Cohen BH, Copeland WC, Naviaux RK. Alpers-Huttenlocher syndrome. Pediatr Neurol. 2013;48:167–78. doi: 10.1016/j.pediatrneurol.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nanau RM, Neuman MG. Adverse drug reactions induced by valproic acid. Clin Biochem. 2013;46:1323–38. doi: 10.1016/j.clinbiochem.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 75.El-Hattab AW, Scaglia F. Mitochondrial DNA depletion syndromes: review and updates of genetic basis, manifestations, and therapeutic options. Neurotherapeutics. 2013;10:186–98. doi: 10.1007/s13311-013-0177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Copeland WC. Defects in mitochondrial DNA replication and human disease. Crit Rev Biochem Mol Biol. 2012;47:64–74. doi: 10.3109/10409238.2011.632763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kemp JP, Smith PM, Pyle A, Neeve VC, Tuppen HA, Schara U, et al. Nuclear factors involved in mitochondrial translation cause a subgroup of combined respiratory chain deficiency. Brain. 2011;134:183–95. doi: 10.1093/brain/awq320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zeharia A, Shaag A, Pappo O, Mager-Heckel AM, Saada A, Beinat M, et al. Acute infantile liver failure due to mutations in the TRMU gene. Am J Hum Genet. 2009;85:401–7. doi: 10.1016/j.ajhg.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Broomfield A, Sweeney MG, Woodward CE, Fratter C, Morris AM, Leonard JV, et al. Paediatric single mitochondrial DNA deletion disorders: an overlapping spectrum of disease. J Inherit Metab Dis. 2014 doi: 10.1007/s10545-014-9778-4. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ishiyama A, Komaki H, Saito T, Saito Y, Nakagawa E, Sugai K, et al. Unusual exocrine complication of pancreatitis in mitochondrial disease. Brain Dev. 2013;35:654–9. doi: 10.1016/j.braindev.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 81.Karaa A, Goldstein A. The spectrum of clinical presentation, diagnosis, and management of mitochondrial forms of diabetes. Pediatr Diabetes. 2015;16:1–9. doi: 10.1111/pedi.12223. [DOI] [PubMed] [Google Scholar]
- 82.Schaefer AM, Walker M, Turnbull DM, Taylor RW. Endocrine disorders in mitochondrial disease. Mol Cell Endocrinol. 2013;379:2–11. doi: 10.1016/j.mce.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Montgomery MK, Turner N. Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect. 2015;4:R1–15. doi: 10.1530/EC-14-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chapman KA, Gropman A, MacLeod E, Stagni K, Summar ML, Ueda K, et al. Acute management of propionic acidemia. Mol Genet Metab. 2012;105:16–25. doi: 10.1016/j.ymgme.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marquard J, El Scheich T, Klee D, Schmitt M, Meissner T, Mayatepek E, et al. Chronic pancreatitis in branched-chain organic acidurias – a case of methylmalonic aciduria and an overview of the literature. Eur J Pediatr. 2011;170:241–5. doi: 10.1007/s00431-010-1313-5. [DOI] [PubMed] [Google Scholar]
- 86.Deodato F, Boenzi S, Santorelli FM, Dionisi-Vici C. Methylmalonic and propionic aciduria. Am J Med Genet C Semin Med Genet. 2006;142c:104–12. doi: 10.1002/ajmg.c.30090. [DOI] [PubMed] [Google Scholar]
- 87.Tiranti V, D'Adamo P, Briem E, Ferrari G, Mineri R, Lamantea E, et al. Ethylmalonic encephalopathy is caused by mutations in ETHE1, a gene encoding a mitochondrial matrix protein. Am J Hum Genet. 2004;74:239–52. doi: 10.1086/381653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cuche G, Malbert CH. Short-chain fatty acids present in the ileum inhibit fasting gastrointestinal motility in conscious pigs. Neurogastroenterol Motil. 1999;11:219–25. doi: 10.1046/j.1365-2982.1999.00149.x. [DOI] [PubMed] [Google Scholar]
- 89.McManus CM, Michel KE, Simon DM, Washabau RJ. Effect of short-chain fatty acids on contraction of smooth muscle in the canine colon. Am J Vet Res. 2002;63:295–300. doi: 10.2460/ajvr.2002.63.295. [DOI] [PubMed] [Google Scholar]
- 90.Mitsui R, Ono S, Karaki S, Kuwahara A. Neural and non-neural mediation of propionate-induced contractile responses in the rat distal colon. Neurogastroenterol Motil. 2005;17:585–94. doi: 10.1111/j.1365-2982.2005.00669.x. [DOI] [PubMed] [Google Scholar]
- 91.Mortensen FV, Nielsen H, Mulvany MJ, Hessov I. Short chain fatty acids dilate isolated human colonic resistance arteries. Gut. 1990;31:1391–4. doi: 10.1136/gut.31.12.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schoultz I, Soderholm JD, McKay DM. Is metabolic stress a common denominator in inflammatory bowel disease? Inflamm Bowel Dis. 2011;17:2008–18. doi: 10.1002/ibd.21556. [DOI] [PubMed] [Google Scholar]
- 93.Zhu KJ, Huang H, Chu H, Yu H, Zhang SM. Alterations in enterocyte mitochondrial respiratory function and enzyme activities in gastrointestinal dysfunction following brain injury. World J Gastroenterol. 2014;20:9585–91. doi: 10.3748/wjg.v20.i28.9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hang CH, Shi JX, Li JS, Wu W, Yin HX. Alterations of intestinal mucosa structure and barrier function following traumatic brain injury in rats. World J Gastroenterol. 2003;9:2776–81. doi: 10.3748/wjg.v9.i12.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boelsterli UA, Redinbo MR, Saitta KS. Multiple NSAID-induced hits injure the small intestine: underlying mechanisms and novel strategies. Toxicol Sci. 2013;131:654–67. doi: 10.1093/toxsci/kfs310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Basivireddy J, Vasudevan A, Jacob M, Balasubramanian KA. Indomethacin-induced mitochondrial dysfunction and oxidative stress in villus enterocytes. Biochem Pharmacol. 2002;64:339–49. doi: 10.1016/s0006-2952(02)01067-5. [DOI] [PubMed] [Google Scholar]
- 97.Somasundaram S, Sigthorsson G, Simpson RJ, Watts J, Jacob M, Tavares IA, et al. Uncoupling of intestinal mitochondrial oxidative phosphorylation and inhibition of cyclooxygenase are required for the development of NSAID-enteropathy in the rat. Aliment Pharmacol Ther. 2000;14:639–50. doi: 10.1046/j.1365-2036.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- 98.Simmy T, Anup R, Prabhu R, Balasubramanian KA. Effect of surgical manipulation of the rat intestine on enterocyte populations. Surgery. 2001;130:479–88. doi: 10.1067/msy.2001.115832. [DOI] [PubMed] [Google Scholar]
- 99.Ramachandran A, Patra S, Balasubramanian KA. Intestinal mitochondrial dysfunction in surgical stress. J Surg Res. 2001;99:120–8. doi: 10.1006/jsre.2001.6104. [DOI] [PubMed] [Google Scholar]
- 100.Yang PC, Berin MC, Yu L, Perdue MH. Mucosal pathophysiology and inflammatory changes in the late phase of the intestinal allergic reaction in the rat. Am J Pathol. 2001;158:681–90. doi: 10.1016/S0002-9440(10)64010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sonne S, Shekhawat PS, Matern D, Ganapathy V, Ignatowicz L. Carnitine deficiency in OCTN2-/- newborn mice leads to a severe gut and immune phenotype with widespread atrophy, apoptosis and a pro-inflammatory response. PLoS One. 2012;7:e47729. doi: 10.1371/journal.pone.0047729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Montrose DC, Kadaveru K, Ilsley JN, Root SH, Rajan TV, Ramesh M, et al. cPLA2 is protective against COX inhibitor-induced intestinal damage. Toxicol Sci. 2010;117:122–32. doi: 10.1093/toxsci/kfq184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bhonchal S, Nain CK, Prasad KK, Nada R, Sharma AK, Sinha SK, et al. Functional and morphological alterations in small intestine mucosa of chronic alcoholics. J Gastroenterol Hepatol. 2008;23:e43–8. doi: 10.1111/j.1440-1746.2007.05080.x. [DOI] [PubMed] [Google Scholar]
- 104.Ramachandran A, Prabhu R, Thomas S, Reddy JB, Pulimood A, Balasubramanian KA. Intestinal mucosal alterations in experimental cirrhosis in the rat: role of oxygen free radicals. Hepatology. 2002;35:622–9. doi: 10.1053/jhep.2002.31656. [DOI] [PubMed] [Google Scholar]
- 105.Parks RW, Stuart Cameron CH, Gannon CD, Pope C, Diamond T, Rowlands BJ. Changes in gastrointestinal morphology associated with obstructive jaundice. J Pathol. 2000;192:526–32. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH787>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 106.Chakrabarti G, McClane BA. The importance of calcium influx, calpain and calmodulin for the activation of CaCo-2 cell death pathways by Clostridium perfringens enterotoxin. Cell Microbiol. 2005;7:129–46. doi: 10.1111/j.1462-5822.2004.00442.x. [DOI] [PubMed] [Google Scholar]
- 107.Chakrabarti G, Zhou X, McClane BA. Death pathways activated in CaCo-2 cells by Clostridium perfringens enterotoxin. Infect Immun. 2003;71:4260–70. doi: 10.1128/IAI.71.8.4260-4270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zeiser J, Gerhard R, Just I, Pich A. Substrate specificity of clostridial glucosylating toxins and their function on colonocytes analyzed by proteomics techniques. J Proteome Res. 2013;12:1604–18. doi: 10.1021/pr300973q. [DOI] [PubMed] [Google Scholar]
- 109.Kolli VK, Natarajan K, Isaac B, Selvakumar D, Abraham P. Mitochondrial dysfunction and respiratory chain defects in a rodent model of methotrexate-induced enteritis. Hum Exp Toxicol. 2014;33:1051–65. doi: 10.1177/0960327113515503. [DOI] [PubMed] [Google Scholar]
- 110.Bonnemains C, Berthelot J, Mousson de Camaret B, Chomienne F, Duveau E, Ginies JL. Mitochondrial cytopathy: an unusual infantile cause of total villous atrophy. Arch Pediatr. 2004;11:118–21. doi: 10.1016/j.arcped.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 111.Cormier-Daire V, Bonnefont JP, Rustin P, Maurage C, Ogler H, Schmitz J, et al. Mitochondrial DNA rearrangements with onset as chronic diarrhea with villous atrophy. J Pediatr. 1994;124:63–70. doi: 10.1016/s0022-3476(94)70255-1. [DOI] [PubMed] [Google Scholar]
- 112.Chitkara DK, Nurko S, Shoffner JM, Buie T, Flores A. Abnormalities in gastrointestinal motility are associated with diseases of oxidative phosphorylation in children. Am J Gastroenterol. 2003;98:871–7. doi: 10.1111/j.1572-0241.2003.07385.x. [DOI] [PubMed] [Google Scholar]
- 113.Nissenkorn A, Zeharia A, Lev D, Fatal-Valevski A, Barash V, Gutman A, et al. Multiple presentation of mitochondrial disorders. Arch Dis Child. 1999;81:209–14. doi: 10.1136/adc.81.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bhardwaj J, Wan DQ, Koenig MK, Liu Y, Hashmi SS, Rhoads JM. Impaired gastric emptying and small bowel transit in children with mitochondrial disorders. J Pediatr Gastroenterol Nutr. 2012;55:194–9. doi: 10.1097/MPG.0b013e3182514805. [DOI] [PubMed] [Google Scholar]
- 115.Camilleri M, Carlson P, Zinsmeister AR, McKinzie S, Busciglio I, Burton D, et al. Mitochondrial DNA and gastrointestinal motor and sensory functions in health and functional gastrointestinal disorders. Am J Physiol Gastrointest Liver Physiol. 2009;296:G510–6. doi: 10.1152/ajpgi.90650.2008. [DOI] [PubMed] [Google Scholar]
- 116.Star K, Edwards IR, Choonara I. Valproic acid and fatalities in children: a review of individual case safety reports in VigiBase. PLoS One. 2014;9:e108970. doi: 10.1371/journal.pone.0108970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gerstner T, Busing D, Bell N, Longin E, Kasper JM, Klostermann W, et al. Valproic acid-induced pancreatitis: 16 new cases and a review of the literature. J Gastroenterol. 2007;42:39–48. doi: 10.1007/s00535-006-1961-4. [DOI] [PubMed] [Google Scholar]
- 118.Finsterer J, Segall L. Drugs interfering with mitochondrial disorders. Drug Chem Toxicol. 2010;33:138–51. doi: 10.3109/01480540903207076. [DOI] [PubMed] [Google Scholar]
- 119.Roullet FI, Lai JK, Foster JA. In utero exposure to valproic acid and autism – a current review of clinical and animal studies. Neurotoxicol Teratol. 2013;36:47–56. doi: 10.1016/j.ntt.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 120.Bauer AZ, Kriebel D. Prenatal and perinatal analgesic exposure and autism: an ecological link. Environ Health. 2013;12:41. doi: 10.1186/1476-069X-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schultz ST, Klonoff-Cohen HS, Wingard DL, Akshoomoff NA, Macera CA, Ji M. Acetaminophen (paracetamol) use, measles-mumps-rubella vaccination, and autistic disorder: the results of a parent survey. Autism. 2008;12:293–307. doi: 10.1177/1362361307089518. [DOI] [PubMed] [Google Scholar]
- 122.Bianciotto M, Chiappini E, Raffaldi I, Gabiano C, Tovo PA, Sollai S, et al. Drug use and upper gastrointestinal complications in children: a case–control study. Arch Dis Child. 2013;98:218–21. doi: 10.1136/archdischild-2012-302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jozwiak-Bebenista M, Nowak JZ. Paracetamol: mechanism of action, applications and safety concern. Acta Pol Pharm. 2014;71:11–23. [PubMed] [Google Scholar]
- 124.Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Niehus R, Lord C. Early medical history of children with autism spectrum disorders. J Dev Behav Pediatr. 2006;27:S120–7. doi: 10.1097/00004703-200604002-00010. [DOI] [PubMed] [Google Scholar]
- 126.Atladottir HO, Henriksen TB, Schendel DE, Parner ET. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics. 2012;130:e1447–54. doi: 10.1542/peds.2012-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fallon J. Could one of the most widely prescribed antibiotics amoxicillin/clavulanate “augmentin” be a risk factor for autism? Med Hypotheses. 2005;64:312–5. doi: 10.1016/j.mehy.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 128.Mezzelani A, Landini M, Facchiano F, Raggi ME, Villa L, Molteni M, et al. Environment, dysbiosis, immunity and sex-specific susceptibility: a translational hypothesis for regressive autism pathogenesis. Nutr Neurosci. 2015;18:145–61. doi: 10.1179/1476830513Y.0000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zorov DB, Plotnikov EY, Silachev DN, Zorova LD, Pevzner IB, Zorov SD, et al. Microbiota and mitobiota. Putting an equal sign between mitochondria and bacteria. Biochemistry (Mosc) 2014;79:1017–31. doi: 10.1134/S0006297914100046. [DOI] [PubMed] [Google Scholar]
- 130.Naviaux RK. Oxidative shielding or oxidative stress? J Pharmacol Exp Ther. 2012;342:608–18. doi: 10.1124/jpet.112.192120. [DOI] [PubMed] [Google Scholar]
- 131.Naviaux RK. Metabolic features of the cell danger response. Mitochondrion. 2014;16:7–17. doi: 10.1016/j.mito.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 132.Kalghatgi S, Spina CS, Costello JC, Liesa M, Morones-Ramirez JR, Slomovic S, et al. Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in Mammalian cells. Sci Transl Med. 2013;5:192ra85. doi: 10.1126/scitranslmed.3006055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Borre YE, O'Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. 2014;20:509–18. doi: 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 134.Wang S, Hibberd ML, Pettersson S, Lee YK. Enterococcus faecalis from healthy infants modulates inflammation through MAPK signaling pathways. PLoS One. 2014;9:E97523. doi: 10.1371/journal.pone.0097523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pochini L, Scalise M, Indiveri C. Inactivation by omeprazole of the carnitine transporter (OCTN2) reconstituted in liposomes. Chem Biol Interact. 2009;179:394–401. doi: 10.1016/j.cbi.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 136.Middlemore-Risher ML, Adam BL, Lambert NA, Terry AV., Jr. Effects of chlorpyrifos and chlorpyrifos-oxon on the dynamics and movement of mitochondria in rat cortical neurons. J Pharmacol Exp Ther. 2011;339:341–9. doi: 10.1124/jpet.111.184762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Basha PM, Poojary A. Mitochondrial dysfunction in aging rat brain regions upon chlorpyrifos toxicity and cold stress: an interactive study. Cell Mol Neurobiol. 2014;34:737–56. doi: 10.1007/s10571-014-0056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee JE, Park JH, Shin IC, Koh HC. Reactive oxygen species regulated mitochondria-mediated apoptosis in PC12 cells exposed to chlorpyrifos. Toxicol Appl Pharmacol. 2012;263:148–62. doi: 10.1016/j.taap.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 139.Joly Condette C, Khorsi-Cauet H, Morliere P, Zabijak L, Reygner J, Bach V, et al. Increased gut permeability and bacterial translocation after chronic chlorpyrifos exposure in rats. PLoS One. 2014;9:e102217. doi: 10.1371/journal.pone.0102217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hornos Carneiro MF, Morais C, Small DM, Vesey DA, Barbosa F, Jr., Gobe GC. Thimerosal induces apoptotic and fibrotic changes to kidney epithelial cells in vitro. Environ Toxicol. 2014 doi: 10.1002/tox.22012. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 141.Huang CF, Liu SH, Lin-Shiau SY. Pyrrolidine dithiocarbamate augments Hg(2 + )-mediated induction of macrophage cell death via oxidative stress-induced apoptosis and necrosis signaling pathways. Toxicol Lett. 2012;214:33–45. doi: 10.1016/j.toxlet.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 142.Dalla Corte CL, Wagner C, Sudati JH, Comparsi B, Leite GO, Busanello A, et al. Effects of diphenyl diselenide on methylmercury toxicity in rats. Biomed Res Int. 2013;2013:983821. doi: 10.1155/2013/983821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pochini L, Scalise M, Galluccio M, Indiveri C. OCTN cation transporters in health and disease: role as drug targets and assay development. J Biomol Screen. 2013;18:851–67. doi: 10.1177/1087057113493006. [DOI] [PubMed] [Google Scholar]
- 144.Meinerz DF, de Paula MT, Comparsi B, Silva MU, Schmitz AE, Braga HC, et al. Protective effects of organoselenium compounds against methylmercury-induced oxidative stress in mouse brain mitochondrial-enriched fractions. Braz J Med Biol Res. 2011;44:1156–63. doi: 10.1590/s0100-879x2011007500136. [DOI] [PubMed] [Google Scholar]
- 145.Franco JL, Posser T, Missau F, Pizzolatti MG, Dos Santos AR, Souza DO, et al. Structure-activity relationship of flavonoids derived from medicinal plants in preventing methylmercury-induced mitochondrial dysfunction. Environ Toxicol Pharmacol. 2010;30:272–8. doi: 10.1016/j.etap.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bourdineaud JP, Rossignol R, Brethes D. Zebrafish: a model animal for analyzing the impact of environmental pollutants on muscle and brain mitochondrial bioenergetics. Int J Biochem Cell Biol. 2013;45:16–22. doi: 10.1016/j.biocel.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 147.Vazquez M, Velez D, Devesa V. In vitro evaluation of inorganic mercury and methylmercury effects on the intestinal epithelium permeability. Food Chem Toxicol. 2014;74c:349–59. doi: 10.1016/j.fct.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 148.Xue Z, Xue H, Jiang J, Lin B, Zeng S, Huang X, et al. Chronic atrophic gastritis in association with hair mercury level. Tumour Biol. 2014;35:11391–8. doi: 10.1007/s13277-014-2475-y. [DOI] [PubMed] [Google Scholar]
- 149.Lapanje A, Rupnik M, Drobne D. Gut bacterial community structure (Porcellio scaber, Isopoda, Crustacea) as a measure of community level response to long-term and short-term metal pollution. Environ Toxicol Chem. 2007;26:755–63. doi: 10.1897/06-099r.1. [DOI] [PubMed] [Google Scholar]
- 150.White RA, Bjornholt JV, Baird DD, Midtvedt T, Harris JR, Pagano M, et al. Novel developmental analyses identify longitudinal patterns of early gut microbiota that affect infant growth. PLoS Comput Biol. 2013;9:e1003042. doi: 10.1371/journal.pcbi.1003042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Midtvedt T. The gut: a triggering place for autism – possibilities and challenges. Microb Ecol Health Dis. 2012;23 doi: 10.3402/mehd.v23i0.18982. 18984, doi: http://dx.doi.org/10.3402/mehd.v23i0.18984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–7. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 153.Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108:3047–52. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Clayton TA. Metabolic differences underlying two distinct rat urinary phenotypes, a suggested role for gut microbial metabolism of phenylalanine and a possible connection to autism. FEBS Lett. 2012;586:956–61. doi: 10.1016/j.febslet.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 155.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–63. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sharon G, Garg N, Debelius J, Knight R, Dorrestein PC, Mazmanian SK. Specialized metabolites from the microbiome in health and disease. Cell Metab. 2014;20:719–30. doi: 10.1016/j.cmet.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, et al. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One. 2013;8:e76993. doi: 10.1371/journal.pone.0076993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kesli R, Gokcen C, Bulug U, Terzi Y. Investigation of the relation between anaerobic bacteria genus clostridium and late-onset autism etiology in children. J Immunoassay Immunochem. 2014;35:101–9. doi: 10.1080/15321819.2013.792834. [DOI] [PubMed] [Google Scholar]
- 159.Finegold SM. Therapy and epidemiology of autism – clostridial spores as key elements. Med Hypotheses. 2008;70:508–11. doi: 10.1016/j.mehy.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 160.Parracho HM, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol. 2005;54:987–91. doi: 10.1099/jmm.0.46101-0. [DOI] [PubMed] [Google Scholar]
- 161.Song Y, Liu C, Finegold SM. Real-time PCR quantitation of clostridia in feces of autistic children. Appl Environ Microbiol. 2004;70:6459–65. doi: 10.1128/AEM.70.11.6459-6465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Finegold SM, Downes J, Summanen PH. Microbiology of regressive autism. Anaerobe. 2012;18:260–2. doi: 10.1016/j.anaerobe.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 163.Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002;35:S6–16. doi: 10.1086/341914. [DOI] [PubMed] [Google Scholar]
- 164.Williams BL, Hornig M, Buie T, Bauman ML, Cho Paik M, Wick I, et al. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One. 2011;6:e24585. doi: 10.1371/journal.pone.0024585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Vaisanen ML, et al. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol. 2000;15:429–35. doi: 10.1177/088307380001500701. [DOI] [PubMed] [Google Scholar]
- 166.Al-Lahham SH, Peppelenbosch MP, Roelofsen H, Vonk RJ, Venema K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim Biophys Acta. 2010;1801:1175–83. doi: 10.1016/j.bbalip.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 167.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–40. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nakao S, Moriya Y, Furuyama S, Niederman R, Sugiya H. Propionic acid stimulates superoxide generation in human neutrophils. Cell Biol Int. 1998;22:331–7. doi: 10.1006/cbir.1998.0263. [DOI] [PubMed] [Google Scholar]
- 169.Han JH, Kim IS, Jung SH, Lee SG, Son HY, Myung CS. The effects of propionate and valerate on insulin responsiveness for glucose uptake in 3T3-L1 adipocytes and C2C12 myotubes via G protein-coupled receptor 41. PLoS One. 2014;9:e95268. doi: 10.1371/journal.pone.0095268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rorig B, Klausa G, Sutor B. Intracellular acidification reduced gap junction coupling between immature rat neocortical pyramidal neurones. J Physiol. 1996;490(Pt 1):31–49. doi: 10.1113/jphysiol.1996.sp021125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nguyen NH, Morland C, Gonzalez SV, Rise F, Storm-Mathisen J, Gundersen V, et al. Propionate increases neuronal histone acetylation, but is metabolized oxidatively by glia. Relevance for propionic acidemia. J Neurochem. 2007;101:806–14. doi: 10.1111/j.1471-4159.2006.04397.x. [DOI] [PubMed] [Google Scholar]
- 172.Nankova BB, Agarwal R, MacFabe DF, La Gamma EF. Enteric bacterial metabolites propionic and butyric acid modulate gene expression, including CREB-dependent catecholaminergic neurotransmission, in PC12 cells – possible relevance to autism spectrum disorders. PLoS One. 2014;9:e103740. doi: 10.1371/journal.pone.0103740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–9. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 174.DeCastro M, Nankova BB, Shah P, Patel P, Mally PV, Mishra R, et al. Short chain fatty acids regulate tyrosine hydroxylase gene expression through a cAMP-dependent signaling pathway. Brain Res Mol Brain Res. 2005;142:28–38. doi: 10.1016/j.molbrainres.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 175.Wajner M, Latini A, Wyse AT, Dutra-Filho CS. The role of oxidative damage in the neuropathology of organic acidurias: insights from animal studies. J Inherit Metab Dis. 2004;27:427–48. doi: 10.1023/B:BOLI.0000037353.13085.e2. [DOI] [PubMed] [Google Scholar]
- 176.Hara H, Haga S, Aoyama Y, Kiriyama S. Short-chain fatty acids suppress cholesterol synthesis in rat liver and intestine. J Nutr. 1999;129:942–8. doi: 10.1093/jn/129.5.942. [DOI] [PubMed] [Google Scholar]
- 177.Thomas RH, Foley KA, Mepham JR, Tichenoff LJ, Possmayer F, MacFabe DF. Altered brain phospholipid and acylcarnitine profiles in propionic acid infused rodents: further development of a potential model of autism spectrum disorders. J Neurochem. 2010;113:515–29. doi: 10.1111/j.1471-4159.2010.06614.x. [DOI] [PubMed] [Google Scholar]
- 178.Coulter DL. Carnitine, valproate, and toxicity. J Child Neurol. 1991;6:7–14. doi: 10.1177/088307389100600102. [DOI] [PubMed] [Google Scholar]
- 179.Calabrese V, Rizza V. Formation of propionate after short-term ethanol treatment and its interaction with the carnitine pool in rat. Alcohol. 1999;19:169–76. doi: 10.1016/s0741-8329(99)00036-1. [DOI] [PubMed] [Google Scholar]
- 180.Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig Dis Sci. 2012;57:2096–102. doi: 10.1007/s10620-012-2167-7. [DOI] [PubMed] [Google Scholar]
- 181.Brock M, Buckel W. On the mechanism of action of the antifungal agent propionate. Eur J Biochem. 2004;271:3227–41. doi: 10.1111/j.1432-1033.2004.04255.x. [DOI] [PubMed] [Google Scholar]
- 182.Coblentz WK, Bertram MG. Effects of a propionic acid-based preservative on storage characteristics, nutritive value, and energy content for alfalfa hays packaged in large round bales. J Dairy Sci. 2012;95:340–52. doi: 10.3168/jds.2011-4496. [DOI] [PubMed] [Google Scholar]
- 183.Coblentz WK, Coffey KP, Young AN, Bertram MG. Storage characteristics, nutritive value, energy content, and in vivo digestibility of moist, large rectangular bales of alfalfa-orchardgrass hay treated with a propionic acid-based preservative. J Dairy Sci. 2013;96:2521–35. doi: 10.3168/jds.2012-6145. [DOI] [PubMed] [Google Scholar]
- 184.Couallier EM, Payot T, Bertin AP, Lameloise ML. Recycling of distillery effluents in alcoholic fermentation: role in inhibition of 10 organic molecules. Appl Biochem Biotechnol. 2006;133:217–38. doi: 10.1385/abab:133:3:217. [DOI] [PubMed] [Google Scholar]
- 185.Darzi J, Frost GS, Robertson MD. Effects of a novel propionate-rich sourdough bread on appetite and food intake. Eur J Clin Nutr. 2012;66:789–94. doi: 10.1038/ejcn.2012.1. [DOI] [PubMed] [Google Scholar]
- 186.Fernandez U, Vodovotz Y, Courtney P, Pascall MA. Extended shelf life of soy bread using modified atmosphere packaging. J Food Prot. 2006;69:693–8. doi: 10.4315/0362-028x-69.3.693. [DOI] [PubMed] [Google Scholar]
- 187.Quitmann H, Fan R, Czermak P. Acidic organic compounds in beverage, food, and feed production. Adv Biochem Eng Biotechnol. 2014;143:91–141. doi: 10.1007/10_2013_262. [DOI] [PubMed] [Google Scholar]
- 188.Scotter MJ, Thorpe SA, Reynolds SL, Wilson LA, Strutt PR. Survey of baked goods for propionic acid and propionates. Food Addit Contam. 1996;13:133–9. doi: 10.1080/02652039609374391. [DOI] [PubMed] [Google Scholar]
- 189.Lind H, Jonsson H, Schnurer J. Antifungal effect of dairy propionibacteria – contribution of organic acids. Int J Food Microbiol. 2005;98:157–65. doi: 10.1016/j.ijfoodmicro.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 190.Palmnas MS, Cowan TE, Bomhof MR, Su J, Reimer RA, Vogel HJ, et al. Low-dose aspartame consumption differentially affects gut microbiota–host metabolic interactions in the diet-induced obese rat. PLoS One. 2014;9:e109841. doi: 10.1371/journal.pone.0109841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SE, et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2014 doi: 10.1136/gutjnl-2014-307913. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Francis K, Smitherman C, Nishino SF, Spain JC, Gadda G. The biochemistry of the metabolic poison propionate 3-nitronate and its conjugate acid, 3-nitropropionate. IUBMB Life. 2013;65:759–68. doi: 10.1002/iub.1195. [DOI] [PubMed] [Google Scholar]
- 193.Shultz SR, MacFabe DF, Ossenkopp KP, Scratch S, Whelan J, Taylor R, et al. Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: implications for an animal model of autism. Neuropharmacology. 2008;54:901–11. doi: 10.1016/j.neuropharm.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 194.MacFabe DF, Cain DP, Rodriguez-Capote K, Franklin AE, Hoffman JE, Boon F, et al. Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav Brain Res. 2007;176:149–69. doi: 10.1016/j.bbr.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 195.Thomas RH, Meeking MM, Mepham JR, Tichenoff L, Possmayer F, Liu S, et al. The enteric bacterial metabolite propionic acid alters brain and plasma phospholipid molecular species: further development of a rodent model of autism spectrum disorders. J Neuroinflammation. 2012;9:153. doi: 10.1186/1742-2094-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shultz SR, Macfabe DF, Martin S, Jackson J, Taylor R, Boon F, et al. Intracerebroventricular injections of the enteric bacterial metabolic product propionic acid impair cognition and sensorimotor ability in the Long-Evans rat: further development of a rodent model of autism. Behav Brain Res. 2009;200:33–41. doi: 10.1016/j.bbr.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 197.MacFabe DF, Rodríguez-Capote K, Hoffman JE, Franklin AE, Mohammad-Asef Y, Taylor AR, et al. A novel rodent model of autism: intraventricular infusions of propionic acid increase locomotor activity and induce neuroinflammation and oxidative stress in discrete regions of adult rat brain. Am J Biochem Biotechnol. 2008;4:146–66. [Google Scholar]
- 198.MacFabe DF, Cain NE, Boon F, Ossenkopp KP, Cain DP. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: relevance to autism spectrum disorder. Behav Brain Res. 2011;217:47–54. doi: 10.1016/j.bbr.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 199.Ossenkopp KP, Foley KA, Gibson J, Fudge MA, Kavaliers M, Cain DP, et al. Systemic treatment with the enteric bacterial fermentation product, propionic acid, produces both conditioned taste avoidance and conditioned place avoidance in rats. Behav Brain Res. 2012;227:134–41. doi: 10.1016/j.bbr.2011.10.045. [DOI] [PubMed] [Google Scholar]
- 200.Foley KA, MacFabe DF, Kavaliers M, Ossenkopp KP. Sexually dimorphic effects of prenatal exposure to lipopolysaccharide, and prenatal and postnatal exposure to propionic acid, on acoustic startle response and prepulse inhibition in adolescent rats: relevance to autism spectrum disorders. Behav Brain Res. 2015;278:244–56. doi: 10.1016/j.bbr.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 201.Foley KA, Ossenkopp KP, Kavaliers M, Macfabe DF. Pre- and neonatal exposure to lipopolysaccharide or the enteric metabolite, propionic acid, alters development and behavior in adolescent rats in a sexually dimorphic manner. PLoS One. 2014;9:e87072. doi: 10.1371/journal.pone.0087072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Foley KA, MacFabe DF, Vaz A, Ossenkopp KP, Kavaliers M. Sexually dimorphic effects of prenatal exposure to propionic acid and lipopolysaccharide on social behavior in neonatal, adolescent, and adult rats: implications for autism spectrum disorders. Int J Dev Neurosci. 2014;39:68–78. doi: 10.1016/j.ijdevneu.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 203.Munnich A, Rustin P. Clinical spectrum and diagnosis of mitochondrial disorders. Am J Med Genet. 2001;106:4–17. doi: 10.1002/ajmg.1391. [DOI] [PubMed] [Google Scholar]
- 204.Mitochondrial Medicine Society's Committee on Diagnosis. Haas RH, Parikh S, Falk MJ, Saneto RP, Wolf NI, et al. The in-depth evaluation of suspected mitochondrial disease. Mol Genet Metab. 2008;94:16–37. doi: 10.1016/j.ymgme.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Frye RE. Novel mitochondrial cytochrome b gene polymorphisms associated with autism. J Pediatr Neurol. 2012;10:35–40. [Google Scholar]
- 206.Kirby DM, Thorburn DR, Turnbull DM, Taylor RW. Biochemical assays of respiratory chain complex activity. Methods Cell Biol. 2007;80:93–119. doi: 10.1016/S0091-679X(06)80004-X. [DOI] [PubMed] [Google Scholar]
- 207.Roe CR, Roe DS. Recent developments in the investigation of inherited metabolic disorders using cultured human cells. Mol Genet Metab. 1999;68:243–57. doi: 10.1006/mgme.1999.2911. [DOI] [PubMed] [Google Scholar]
- 208.Horvath K, Papadimitriou JC, Rabsztyn A, Drachenberg C, Tildon JT. Gastrointestinal abnormalities in children with autistic disorder. J Pediatr. 1999;135:559–63. doi: 10.1016/s0022-3476(99)70052-1. [DOI] [PubMed] [Google Scholar]
- 209.Geier DA, Kern JK, Davis G, King PG, Adams JB, Young JL, et al. A prospective double-blind, randomized clinical trial of levocarnitine to treat autism spectrum disorders. Med Sci Monit. 2011;17:PI15–23. doi: 10.12659/MSM.881792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fahmy SF, El-hamamsy MH, Zaki OK, Badary OA. l-Carnitine supplementation improves the behavioral symptoms in autistic children. Res Autism Spectr Disord. 2013;7:159–66. [Google Scholar]
- 211.Pastural E, Ritchie S, Lu Y, Jin W, Kavianpour A, Khine Su-Myat K, et al. Novel plasma phospholipid biomarkers of autism: mitochondrial dysfunction as a putative causative mechanism. Prostaglandins Leukot Essent Fatty Acids. 2009;81:253–64. doi: 10.1016/j.plefa.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 212.Gargus JJ, Lerner MA. Familial autism with primary carnitine deficiency, sudden death, hypotonia and hypochromic anemia. Am J Human Gen. 1997;61:A98. [Google Scholar]
- 213.Gargus JJ, Imtiaz F. Mitochondrial energy-deficient endophenotype in autism. Am J Biochem Biotech. 2008;4:198–207. [Google Scholar]
- 214.Poling JS, Frye RE, Shoffner J, Zimmerman AW. Developmental regression and mitochondrial dysfunction in a child with autism. J Child Neurol. 2006;21:170–2. doi: 10.2310/7010.2006.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ezugha H, Goldenthal M, Valencia I, Anderson CE, Legido A, Marks H. 5q14.3 deletion manifesting as mitochondrial disease and autism: case report. J Child Neurol. 2010;25:1232–5. doi: 10.1177/0883073809361165. [DOI] [PubMed] [Google Scholar]
- 216.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Scioli MG, Stasi MA, Passeri D, Doldo E, Costanza G, Camerini R, et al. Propionyl-L-carnitine is efficacious in ulcerative colitis through its action on the immune function and microvasculature. Clin Transl Gastroenterol. 2014;5:e55. doi: 10.1038/ctg.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nafday SM, Chen W, Peng L, Babyatsky MW, Holzman IR, Lin J. Short-chain fatty acids induce colonic mucosal injury in rats with various postnatal ages. Pediatr Res. 2005;57:201–4. doi: 10.1203/01.PDR.0000150721.83224.89. [DOI] [PubMed] [Google Scholar]
- 219.Fortin G, Yurchenko K, Collette C, Rubio M, Villani AC, Bitton A, et al. L-carnitine, a diet component and organic cation transporter OCTN ligand, displays immunosuppressive properties and abrogates intestinal inflammation. Clin Exp Immunol. 2009;156:161–71. doi: 10.1111/j.1365-2249.2009.03879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ruskin DN, Svedova J, Cote JL, Sandau U, Rho JM, Kawamura M, Jr., et al. Ketogenic diet improves core symptoms of autism in BTBR mice. PLoS One. 2013;8:e65021. doi: 10.1371/journal.pone.0065021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mantis JG, Meidenbauer JJ, Zimick NC, Centeno NA, Seyfried TN. Glucose reduces the anticonvulsant effects of the ketogenic diet in EL mice. Epilepsy Res. 2014;108:1137–44. doi: 10.1016/j.eplepsyres.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 222.Frye RE, Sreenivasula S, Adams JB. Traditional and non-traditional treatments for autism spectrum disorder with seizures: an on-line survey. BMC Pediatr. 2011;11:37. doi: 10.1186/1471-2431-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Spilioti M, Evangeliou AE, Tramma D, Theodoridou Z, Metaxas S, Michailidi E, et al. Evidence for treatable inborn errors of metabolism in a cohort of 187 Greek patients with autism spectrum disorder (ASD) Front Hum Neurosci. 2013;7:858. doi: 10.3389/fnhum.2013.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Herbert MR, Buckley JA. Autism and dietary therapy: case report and review of the literature. J Child Neurol. 2013;28:975–82. doi: 10.1177/0883073813488668. [DOI] [PubMed] [Google Scholar]
- 225.Napoli E, Duenas N, Giulivi C. Potential therapeutic use of the ketogenic diet in autism spectrum disorders. Front Pediatr. 2014;2:69. doi: 10.3389/fped.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gano LB, Patel M, Rho JM. Ketogenic diets, mitochondria, and neurological diseases. J Lipid Res. 2014;55:2211–28. doi: 10.1194/jlr.R048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Schiff M, Benit P, Coulibaly A, Loublier S, El-Khoury R, Rustin P. Mitochondrial response to controlled nutrition in health and disease. Nutr Rev. 2011;69:65–75. doi: 10.1111/j.1753-4887.2010.00363.x. [DOI] [PubMed] [Google Scholar]
- 228.Milder J, Patel M. Modulation of oxidative stress and mitochondrial function by the ketogenic diet. Epilepsy Res. 2012;100:295–303. doi: 10.1016/j.eplepsyres.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hughes SD, Kanabus M, Anderson G, Hargreaves IP, Rutherford T, O'Donnell M, et al. The ketogenic diet component decanoic acid increases mitochondrial citrate synthase and complex I activity in neuronal cells. J Neurochem. 2014;129:426–33. doi: 10.1111/jnc.12646. [DOI] [PubMed] [Google Scholar]
- 230.Kang HC, Lee YM, Kim HD, Lee JS, Slama A. Safe and effective use of the ketogenic diet in children with epilepsy and mitochondrial respiratory chain complex defects. Epilepsia. 2007;48:82–8. doi: 10.1111/j.1528-1167.2006.00906.x. [DOI] [PubMed] [Google Scholar]
- 231.Conn AR, Fell DI, Steele RD. Characterization of alpha-keto acid transport across blood-brain barrier in rats. Am J Physiol. 1983;245:E253–60. doi: 10.1152/ajpendo.1983.245.3.E253. [DOI] [PubMed] [Google Scholar]
- 232.Adams JB, Holloway C. Pilot study of a moderate dose multivitamin/mineral supplement for children with autistic spectrum disorder. J Altern Complement Med. 2004;10:1033–9. doi: 10.1089/acm.2004.10.1033. [DOI] [PubMed] [Google Scholar]
- 233.Adams JB, Audhya T, McDonough-Means S, Rubin RA, Quig D, Geis E, et al. Effect of a vitamin/mineral supplement on children and adults with autism. BMC Pediatr. 2011;11:111. doi: 10.1186/1471-2431-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Gvozdjakova A, Kucharska J, Ostatnikova D, Babinska K, Nakladal D, Crane FL. Ubiquinol improves symptoms in children with autism. Oxid Med Cell Longev. 2014;2014:798957. doi: 10.1155/2014/798957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Monachese M, Burton JP, Reid G. Bioremediation and tolerance of humans to heavy metals through microbial processes: a potential role for probiotics? Appl Environ Microbiol. 2012;78:6397–404. doi: 10.1128/AEM.01665-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bisanz JE, Enos MK, Mwanga JR, Changalucha J, Burton JP, Gloor GB, et al. Randomized open-label pilot study of the influence of probiotics and the gut microbiome on toxic metal levels in Tanzanian pregnant women and school children. mBio. 2014;5:e01580–14. doi: 10.1128/mBio.01580-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Gilbert JA, Krajmalnik-Brown R, Porazinska DL, Weiss SJ, Knight R. Toward effective probiotics for autism and other neurodevelopmental disorders. Cell. 2013;155:1446–8. doi: 10.1016/j.cell.2013.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rossignol DA, Frye RE. Melatonin in autism spectrum disorders: a systematic review and meta-analysis. Dev Med Child Neurol. 2011;53:783–92. doi: 10.1111/j.1469-8749.2011.03980.x. [DOI] [PubMed] [Google Scholar]
- 239.Rossignol DA, Frye RE. Melatonin in autism spectrum disorders. Curr Clin Pharmacol. 2014;9:326–34. doi: 10.2174/15748847113086660072. [DOI] [PubMed] [Google Scholar]
- 240.Acuna-Castroviejo D, Escames G, Venegas C, Diaz-Casado ME, Lima-Cabello E, Lopez LC, et al. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014;71:2997–3025. doi: 10.1007/s00018-014-1579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lee PP, Pang SF. Melatonin and its receptors in the gastrointestinal tract. Biol Signals. 1993;2:181–93. doi: 10.1159/000109491. [DOI] [PubMed] [Google Scholar]
- 242.Brzozowska I, Strzalka M, Drozdowicz D, Konturek SJ, Brzozowski T. Mechanisms of esophageal protection, gastroprotection and ulcer healing by melatonin. Implications for the therapeutic use of melatonin in gastroesophageal reflux disease (GERD) and peptic ulcer disease. Curr Pharm Des. 2014;20:4807–15. doi: 10.2174/1381612819666131119110258. [DOI] [PubMed] [Google Scholar]
- 243.Hou Y, Wang L, Ding B, Liu Y, Zhu H, Liu J, et al. Alpha-Ketoglutarate and intestinal function. Front Biosci (Landmark Ed) 2011;16:1186–96. doi: 10.2741/3783. [DOI] [PubMed] [Google Scholar]
- 244.Thomas S, Prabhu R, Balasubramanian KA. Surgical manipulation of the intestine and distant organ damage-protection by oral glutamine supplementation. Surgery. 2005;137:48–55. doi: 10.1016/j.surg.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 245.Thomas S, Anup R, Susama P, Balasubramanian KA. Nitric oxide prevents intestinal mitochondrial dysfunction induced by surgical stress. Br J Surg. 2001;88:393–9. doi: 10.1046/j.1365-2168.2001.01683.x. [DOI] [PubMed] [Google Scholar]