Abstract
Neuromyelitis optica (NMO) is an inflammatory autoimmune disease of the central nervous system that preferentially targets the optic nerves and spinal cord. The clinical presentation may suggest multiple sclerosis (MS), but a highly specific serum autoantibody against the astrocytic water channel aquaporin-4 present in up to 80% of NMO patients enables distinction from MS. Optic neuritis may occur in either condition resulting in neuro-anatomical retinal changes. Optical coherence tomography (OCT) has become a useful tool for analyzing retinal damage both in MS and NMO. Numerous studies showed that optic neuritis in NMO typically results in more severe retinal nerve fiber layer (RNFL) and ganglion cell layer thinning and more frequent development of microcystic macular edema than in MS. Furthermore, while patients’ RNFL thinning also occurs in the absence of optic neuritis in MS, subclinical damage seems to be rare in NMO. Thus, OCT might be useful in differentiating NMO from MS and serve as an outcome parameter in clinical studies.
Keywords: Neuromyelitis optica, optical coherence tomography, multiple sclerosis, optic neuritis, retinal nerve fiber layer, ganglion cell layer
Introduction
Neuromyelitis optica (NMO) is an immune-mediated disorder of the central nervous system (CNS) in which the optic nerves and the spinal cord are preferentially involved.1 The disease-specific serum immunoglobulin (Ig)G targeting the astrocyte water channel aquaporin-4 (AQP4)2,3 has facilitated differentiation of NMO from multiple sclerosis (MS) and recognition of a broad phenotypic spectrum referred to as neuromyelitis optica spectrum disorders (NMOSD).4 The use of the AQP4-IgG autoantibody in various in vivo and ex vivo models has led to an initial understanding of the pathogenic mechanisms that contribute to optic nerve injury.5 While additional autoantibodies against myelin oligodendrocyte glycoprotein (anti-MOG) and aquaporin-1 have been reported in a small number of NMO patients, the specificity of these autoantibodies to NMO and their relationship to disease pathogenesis remain unclear.6–8
Techniques that provide information on the optic nerve structure and function are likely to prove useful to clinicians dealing with CNS diseases. Whereas a number of techniques evaluate visual function such as visual acuity, contrast sensitivity, color vision, visual fields, evoked potentials and pattern electroretinogram, others such as magnetic resonance imaging (MRI), optic coherence tomography (OCT), confocal scanning laser ophthalmoscopy (CSLO), and scanning laser polarimetry with variable corneal compensation (GDx-VCC), assess the anatomical integrity of the optic nerve and retina (Figure 1). OCT may have the advantage of requiring shorter acquisition times than CSLO,9,10 and is more sensitive than GDx-VCC11 in detecting retinal nerve fiber layer (RNFL) thinning in nasal and temporal sectors. It has the additional capability of measuring retinal segmental thickness.12,13
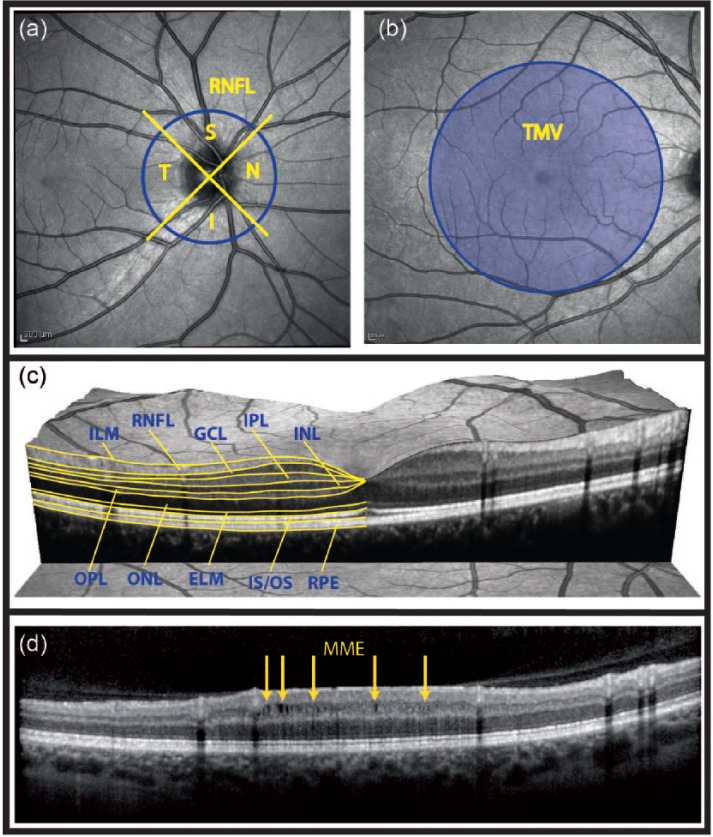
Figure 1.
Retinal parameters acquired by OCT.
(a) Fundus image showing the acquisition of the peripapillary RNFL thickness. OCT records a ring scan of 3.4 mm diameter around the optic nerve head, which is divided into quadrants. (b) The total macular volume is derived from a volume scan and contains all retinal layers in a 6 mm diameter cylinder around the fovea centralis. (c) Intra-retinal layer segmentation in a spectral domain OCT image. (d) MME in a patient with optic neuritis. MME locations are marked by yellow arrows.
OCT: optical coherence tomography; RNFL: retinal nerve fiber layer; S: superior; N: nasal; I: inferior; T: temporal; TMV: total macular volume; GCL; ganglion cell layer; IPL: inner plexiform layer; INL: inner nuclear layer; OPL: outer plexiform layer; ONL: outer nuclear layer; ELM: external limiting membrane; IS/OS: inner segments/outer segments of the photoreceptor layer; RPE: retinal pigment epithelium; MME: microcystic macular edema.
OCT is an analog of B-mode ultrasonography but uses infrared light instead of ultrasound to produce images based on the differential optical reflectivity. It can provide data on peripapillary and macular RNFL thicknesses and generate macular maps with segmental thicknesses and volumes (Figure 1). The recent development of spectral domain OCT (SD-OCT) allows enhanced resolution (2 µm), shorter acquisition times, three-dimensional scans, and video imaging.14,15 Also, eye tracking systems permit almost perfect repositioning in longitudinal studies, allowing investigators to capture subtle changes on the order of a few micrometers. Investigations in MS have demonstrated that OCT is easy to perform, reproducible, and provides downstream measures of secondary neurodegeneration (RNFL and macular thinning).12,16–19
Optic neuritis (ON) is a common condition involving primary inflammation, demyelination, and axonal injury in the optic nerve.20 This process may lead to retinal ganglion cell (RGC) death, decreased macular volume, and to visual dysfunction or permanent visual loss. ON usually presents as an acute episode of unilateral or less frequently bilateral optic nerve inflammation, accompanied by ocular pain and decreased vision. ON is immune mediated21 and, in NMOSD, is associated with detectable AQP4-IgG.22 The pathological features of NMOSD include deposition of IgG and activated complement, loss of AQP4 expression, astrocytopathy, neutrophil accumulation and demyelination with axon loss.23,24 The frequent involvement of the optic nerve in MS and NMO may be caused by a more reduced blood-brain barrier (BBB) function.25,26 Furthermore, the optic nerve expresses high levels of supramolecular aggregates of AQP4.27 The combination of enhanced AQP4 supramolecular aggregation and heightened BBB permeability may contribute to the specific pattern of tissue damage in NMOSD. Objective measures of the severity and etiology of optic nerve injury is important for the diagnosis, management, and treatment of ON. NMOSD-ON differs clinically from MS-ON; bilateral involvement is more common, and recurrent ON and severe residual visual dysfunction more likely.7,28–32 Common MRI imaging features include lesions extending over one-half the length of the optic nerve, posterior nerve involvement, and chiasmal inflammation.33,34 Whereas many characteristics of OCT in MS have already been established and are consistent with its pathophysiology,35–40 OCT features in NMOSD are currently ill-defined and the relationship of these abnormalities with disease pathophysiology remains unclear.41–51
This review analyzes the published data on OCT in NMOSD so that its ability to quantify optic nerve damage, facilitate diagnosis, monitor disease progression, evaluate therapeutic efficacy, and detect novel pathology might be assessed.
Methods
A literature search of Ovid MEDLINE (1946–2014) was conducted using the search terms “neuromyelitis optica” combined with “optical coherence tomography.” Given that OCT is a newer technology, all articles were current (2008–present). Review articles and case reports were excluded; however, the references contained in such articles were reviewed for completeness. PubMed was also searched using similar methodology. Only studies available in English were included. Authors were not contacted for unpublished data. A comprehensive overview of the individual studies is given in the supplementary table.
OCT findings in NMOSD
RNFL and macula
OCT has been applied to NMOSD cohorts, initially in cross-sectional studies46–48 and, more recently, in prospective longitudinal investigations.52 Cross-sectional studies have consistently shown that the RNFL is significantly altered in NMOSD patients with ON compared to healthy controls and that RNFL thinning may be an early and frequent phenomenon. NMOSD-ON affects the entire peripapillary RNFL with particular involvement of the superior and inferior quadrants 43,45 (Table 1 and Figure 2). This may reflect a lower preference for small-diameter axons, which are more abundant in the temporal quadrant and are preferentially affected in MS-ON.36,53 Macular thinning is more severe in NMOSD with ON than in MS-ON, in line with poorer visual recovery observed following ON in NMOSD. NMOSD patients with a history of ON tend to have significantly lower RNFL thicknesses than patients with MS-ON42–45 (Table 1 and Figure 2). A meta-analysis showed that ON significantly affects RNFL integrity and, on average, leads to a loss of approximately 20 μm in the affected eye in relapsing–remitting MS (RRMS) compared to healthy controls.36 A recent study by Green and Cree showed an average RNFL loss in MS-ON of 17.6 μm compared to an average 31.1 μm reduction in NMOSD-ON.44 Several studies showed that RNFL thickness in NMOSD patients after ON is reduced to 55–83 µm, compared to 93–108 µm in the respective control groups.42,43,45–48,50,54–57
Table 1.
Summary of neuro-ophthalmological parameters in neuromyelitis optica compared to multiple sclerosis.
| NMO-ON | MS-ON | HCs | Comments | |
|---|---|---|---|---|
| Visual impairment | Severe | Moderate | – | Visual acuity and contrast sensitivity recovery after ON attacks in NMO is lower than in MS, and blindness is not uncommon in NMO; altitudinal loss may be eventually seen in NMO, but not in MS |
| Funduscopy | Disc atrophy and vascular changes with ‘frosting’ | Segmental disc atrophy without venous sheathing | – | Vascular changes seen in eyes with ON in NMO: attenuation of arterioles in the peripapillary retina, often with accompanying venous changes |
| Optic nerve OCTa | ||||
| Average RNFL thickness | 55–83 µm | 74–95 µm | 93–108 µm | Reduction of peripapillary RNFL thickness in NMO is basically attack-related; MS patients may have RNFL reduction in non-ON eyes. Superior and inferior RNFL predominantly affected in NMO compared to temporal RNFL in MS. |
| Superior | 66–100 µm | 90–117 µm | 121–136 µm | |
| Inferior | 64–99 µm | 92–117 µm | 127–138 µm | |
| Temporal | 39–63 µm | 50–67 µm | 67–79 µm | |
| Nasal | 29–75 µm | 42–88 µm | 74–97 µm | |
| Maculab | Retinal thickness, total macular volume, and GCL/GC+IPL thickness are usually lower in affected eyes from NMO than MS, while INL/INL+OPL is often thicker in NMO. | |||
| Microcystic macular edema (MME) | 20–26% | 5–6% | 0% | MME eyes have lower pRNFL thickness, and VA than non-MME eyes |
RNFL segmentation from different optical coherence tomography (OCT) devices is slightly different, but comparable. bMacula thickness, total macular volume and intra-retinal layer segmentation varies in different machines; thus, results cannot be compared. NMO: neuromyelitis optica; MS: multiple sclerosis; HCs: healthy controls; ON: optic neuritis: RNFL: retinal nerve fiber layer; GCL: ganglion cell layer; IPL: inner plexiform layer; INL: inner nuclear layer; OPL: outer nuclear layer.
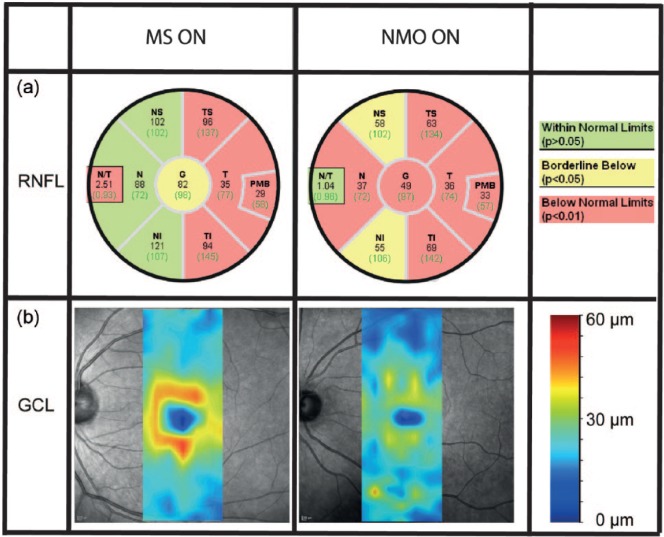
Figure 2.
Typical differences in retinal damage between NMO-ON and MS-ON.
(a) RNFL thickness values for different locations of the peripapillary ring scans including comparison to a healthy reference group. (b) Thickness map of the retinal GCL, derived with help of a semiautomatic segmentation software. The NMO-ON patient shows more severe thinning both in the RNFL and GCL.
MS: multiple sclerosis; ON: optic neuritis; NMO: neuromyelitis optica; RNFL: retinal nerve fiber layer; GCL: ganglion cell layer.
OCT measures and visual function
As in patients with MS, OCT in NMOSD correlated well with results from visual acuity testing.42,45 Using 1.25% low-contrast visual acuity charts in monocular testing, the average number of correct letters read by NMOSD patients with ON was four; MS-ON patients and healthy controls averaged 6.5 and 16, respectively.42 The same study indicated that high-contrast acuity became very poor in eyes of NMOSD patients with ON when the average RNFL thickness fell below 60 µm.42 More recent studies applying macular multilayer segmentation analyses have provided evidence that not only peripapillary RNFL but also neuronal layers like the ganglion cell layer (GCL) or the combined ganglion cell/inner plexiform layer (GCIP) are significantly thinned in patients with MS-ON and NMOSD-ON; the NMOSD patients showed greater thinning57,58 (Figure 2).
OCT measures in NMOSD eyes without ON
While in eyes of MS patients without a history of ON RNFL thickness is on average reduced by 7 µm,36 several studies showed that NMOSD patients without a history of ON had normal RNFLs.42,46,48,50 This suggests that subclinical ON in NMOSD is uncommon and contrasts with both the subclinical visual-evoked potential (VEP) abnormalities and OCT abnormalities recognized in clinically isolated syndrome (CIS) patients with or without clinical ON.19 Interestingly, another study reported that NMOSD eyes without a history of ON had prolonged P100 latencies on VEP compared to normal controls; however, the absolute latencies were still within the normal range.59 Other groups have reported thinning of the combined GCIP in NMOSD eyes without a history of ON.55,56 This discrepancy may result from difficulties in accurately ascertaining clinical ON events in a retrospective approach, differences in OCT devices, segmentation techniques, and the smaller variance of the GCIP metric. Some differences in segmental retinal thinning has been observed in NMOSD-ON and MS-ON eyes, suggesting that SD-OCT may be useful in distinguishing NMO from MS following ON.51 Additional investigations are needed, however, to confirm these preliminary observations.
Macular changes including microcystic macular edema (MME)
Some but not all OCT studies have also highlighted macular changes following MS-ON and RNFL changes following ON in NMOSD. These differences could reflect different pathogenic processes.54 MME has recently been described in patients with MS and other optic neuropathies (Figure 1(d)). In MS patients, MME seems to be associated with higher levels of clinical disability60 and disease activity as measured by the frequency of clinical relapses and MRI activity (T2- and gadolinium-enhancing T1 lesions).61 Although the underlying mechanisms of MME are still a matter of debate,62 clinical reports have shown that MME is not MS-ON specific but can also be detected in NMOSD-ON eyes and other optic neuropathies (chronic relapsing inflammatory optic neuropathy, ischemic, Leber disease, etc.). Microcystic inner nuclear layer abnormalities can be detected in 20%–26% of NMOSD patients,49,56,58 and in up to 40% of ON-affected eyes in AQP4-IgG-positive patients but not in unaffected eyes.63 This is much higher than the 5% previously reported in MS patients.60,49
OCT measures and clinical disability
Similar to studies of MS-ON, visual function correlates well with RNFL thickness36,46–48 in NMOSD patients. However, in contrast to some studies of MS-ON,13,64,65 RNFL thickness in NMOSD has only sporadically correlated with the overall Expanded Disability Status Scale (EDSS).46 Although this may not be surprising in light of the limited amount of visual function captured in the EDSS, the weaker correlation between RNFL thickness and EDSS in NMOSD as compared to MS may be the product of both distinct pathophysiology and involvement of a more limited spectrum of the CNS neuroaxis. The development of a validated disability scale specific to NMOSD will be important in determining whether OCT parameters may extend beyond the visual system to prove a clinical biomarker of disease activity.
ON and OCT in pediatric NMO
While most clinical characteristics of pediatric NMO are similar to adult-onset disease, a comprehensive clinical evaluation of NMOSD-ON in children has not been conducted. Approximately 50% of pediatric NMO cases have severe residual visual impairment in one or both eyes.66 However, the vast majority of cases involve recurrent episodes of ON. Since MRI examinations and VEPs can be challenging in children because of a lack of patient cooperation, OCT may be a promising tool to differentiate pediatric NMO from other causes of ON. A longitudinal analysis of NMOSD-ON in the pediatric population with accompanying OCT analysis will be critical for understanding whether visual outcomes and corresponding neuro-anatomic measures of injury vary in the pediatric age group and powering clinical trials for acute and prophylactic therapies.
Association of OCT measures with brain volume
Animal models have demonstrated that RNFL thinning as measured by OCT reflects retinal axonal loss.67–69 Recent studies on OCT/MRI correlations in MS have shown good correlation between RNFL measures and both white and gray matter atrophy17,70 and brain parenchymal fraction.71 Another recent study in a cohort of CIS and early RRMS patients with short disease duration (3.2 years) and mild levels of disability (median EDSS 1.5) indicated that in early stages of relapsing forms of the disease, OCT-derived retinal measures reflect white matter damage, with variability in gray matter being an age-related effect.72 von Glehn et al. demonstrated cortical thinning in NMO patients (1.55 mm) compared to healthy controls (1.62 mm, p = 0.027) and a positive correlation between RNFL and cortical thickness.73 When stratified by disease duration, RNFL and cortical thinning both progressed with time. Additional MRI analyses demonstrated global white and gray matter volume loss.
Effect of relapse treatment on OCT measures and visual function
Recent therapeutic studies found that relapse activity in NMO patients responded better to immunosuppressive drugs than to immunomodulators.74–77 Immunosuppressive drugs are known to have anti-inflammatory effects, but to date there are no data regarding their effect on neuronal and axonal loss. One study compared intravenous methylprednisolone (IVMP, 2 g per day for three to five days) to IVMP in conjunction with plasma exchange (PE, five consecutive exchanges) in individuals with a first attack of ON due to NMO or NMOSD.78 In the PE group, 75% recovered to 20/40 or better. While the Snellen equivalent did not improve in the IVMP-only group (20/400 at initial and end of study exams), the visual acuity in the PE group improved from 20/400 at baseline to 20/50 at the final visit. While high-contrast visual acuity scores were better in the PE group, there were no statistical differences in RNFL thickness (mean thickness 63 µm in the IVMP group vs. 70 µm in the IVMP plus PE group, p = 0.16). A second study performed in patients who had failed to improve with high-dose corticosteroids also examined the use of PE.79 In this small study, RNFL thickness was preserved at one year, with one patient followed longitudinally and demonstrating stable RNFL thickness over four years. Another small retrospective study from Japan suggested that early IVMP treatment after an acute ON event may help preserve RNFL thickness in NMO.45 Eyes with RNFL thicknesses exceeding 71.41 µm had a significantly earlier treatment with IVMP than those eyes below this cutoff. Average RNFL thickness after an ON attack was inversely correlated with the period from onset of clinical symptoms to IVMP therapy. Thus, because of its sensitivity, OCT is a noninvasive tool that is ideally suited to provide information on potential neuroprotection in ON clinical trials.45,80
Value of OCT for differential diagnosis
In light of different treatment strategies for NMOSD and MS, an early and accurate diagnosis is key for optimal patient management but may remain challenging in cases of seronegative NMO. In this regard, OCT may be of potential value to help the clinician discriminate between MS-ON and NMOSD-ON, particularly when ON is the initial clinical presentation. Some studies have analyzed the ability of OCT measures to distinguish between MS and NMOSD. Naismith et al. reported that the odds of falling into the NMOSD group increased by 8% for every 1 µm decrease in RNFL thickness.43 In a multilayer segmentation study, Park et al. found that ONL thickness greater than 83 µm at the inner temporal location (from the foveal center) and GCIP thickness of less than 62 µm at the outer superior location (from the foveal center) were suggestive of NMO.57 Schneider et al. reported that both peripapillary RNFL thickness and the ratio of nasal to temporal peripapillary RNFL thickness may be helpful in distinguishing NMOSD-ON and MS-ON.50 However, each of these findings has to be interpreted with caution given the low sample sizes and the exploratory nature of the analyses. Confirmatory studies using larger patient populations are needed before they can be used to guide clinical decision making.
Future prospects
Major advancements in the management of brain disease are likely to depend on the identification of imaging or molecular biomarkers. Such biomarkers are needed for an improved understanding of the pathogenesis, the stratification of patients based on the prognosis or response to therapy, and as surrogate endpoints in clinical trials. As such, one of the main initiatives from the National Institutes of Health (NIH) in Alzheimer’s disease is a multicenter study for validating biomarkers (MRI, positron-emission tomography (PET), beta-amyloid or Tau in cerebrospinal fluid (CSF)). For this reason, OCT for quantifying and monitoring axon damage of the optic nerve and the related retrograde degeneration of the GCL is likely to improve assessment of optic nerve tissue damage in NMO-ON. Although OCT will not capture non-optic nerve damage, its exquisite ability to quantify changes in the retina after optic nerve damage may allow prediction of visual recovery, general assessment of therapeutic efficacy as a surrogate of disability after inflammatory attacks or as a measure of neuroprotection or regeneration, and stratification of patients based on different patterns of damage.
Multimodal and comprehensive evaluation of the anterior visual pathway will provide even better understanding of NMOSD patients with ON. It may deliver clinically useful instruments for patient analysis. The good agreement between morphology captured by OCT, functional assessments such as multifocal visual-evoked potentials, and clinical outcomes such as visual fields, visual acuity and visual quality of life, may enable a comprehensive assessment of ON in patients with NMOSD. Moreover, multimodal assessments of patients provide the opportunity to integrate all factors participating in the disease, from molecular and cellular processes to visual system performance. New laser technologies are being developed that will allow molecular analysis of the retina changes, single-cell visualization of the RNFL and RGC, assessment of neural activity by imaging (which can be integrated with electroretinography), changes in blood flow and other retina fluids. NMO, an AQP4-astrocytopathy that commonly affects the optic nerves, is likely to benefit from these developments. For this reason, NMO can effectively indicate the potential of this new technology in assessing CNS damage and of neuroprotective or regenerative treatments.
Supplementary Material
Acknowledgments
The authors thank The Guthy-Jackson Charitable Foundation for its support in organizing the NMO International Clinical Consortium & Biorepository.
Footnotes
Conflict of interest: None declared.
Members of The Guthy-Jackson Charitable Foundation (GCJF) NMO International Clinical Consortium and Biorepository (ICC&BR) and the GJCF NMO Biorepository Oversight Committee (BOC) are recognized as affiliated authors of this study and listed below in alphabetic order by institution:
Catholic University, Rome, Italy: Raffaele Iorio
Charité University Berlin, Germany: Friedemann Paul, Jens Wuerfel
CHU de Fort de France, Martinique: Philippe Cabre
CHU, Lyon, France: Romain Marignier
CHU Strasbourg, France: Jérôme de Seze
Dr Juan P. Garrahan National Pediatric Hospital, Argentina: Silvia Tenembaum
IDIBAPS Barcelona, Spain: Albert Saiz, Pablo Villoslada
Johns Hopkins University, Baltimore, MD, USA: Michael Levy
Massachusetts General Hospital, Boston, MA, USA: Tanuja Chitnis, Eric C. Klawiter
Mayo Clinic, Scottsdale, AZ, USA: Dean Wingerchuk; Brian Weinshenker
Research Institute and Hospital of National Cancer Center, Goyang, Korea: Ho Jin Kim
Nitte University Mangalore, India: Lekha Pandit
Oxford University Hospitals National Health Service Trust UK: Maria Isabel Leite, Jacqueline Palace
Portland VA Medical Center, VA Medical Center and Oregon Health Science Center, OR, USA: Jack Simon
Prasat Neurological Institute Bangkok, Thailand: Metha Apiwattanakul
Ruhr University, Bochum, Germany: Ingo Kleiter
School of Medicine, Griffith University, Australia: Simon Broadley
Siriraj Hospital, Mahidol University, Bangkok, Thailand: Naraporn Prayoonwiwat
Stanford University School of Medicine, Palo Alto, CA, USA: May Han
St. Josef Hospital Bochum, Germany: Kerstin Hellwig
The Children’s Hospital of Philadelphia, PA, USA: Brenda Banwell
The Guthy Jackson Charitable Foundation, San Diego, CA, USA: Katja van Herle
The Mount Sinai Hospital, New York, NY, USA: Gareth John
Thomas Jefferson University, Philadelphia, PA, USA: D. Craig Hooper
Tohoku University Sendai, Japan: Kazuo Fujihara, Ichiro Nakashima, Douglas Sato
Universidade Federal de Sao Paulo, Sao Paulo, Brazil: Denis Bichuetti
University of California, Los Angeles: Michael R. Yeaman
University of California, San Francisco, CA, USA: Emmanuelle Waubant, Scott Zamvil
University of Colorado, Denver, CO, USA: Jeffrey Bennett
University of Michigan Medical School, Ann Arbor, MI, USA: Terry Smith
University of Minas Gerais, Belo Horizonte, Brazil: Marco Lana-Peixoto
University of Texas, Southwestern, Dallas, TX, USA: Olaf Stuve; Benjamin Greenberg
University of Düsseldorf, Germany: Orhan Aktas
University of Goettingen, Germany: Jens Wuerfel
University of Southern Denmark, Denmark: Nasrin Asgari
Walton Center, Liverpool, UK: Anu Jacob
Yale University School of Medicine, Department of Neurology, New Haven, CT, USA: Kevin O’Connor
Funding: This work was supported by the German Ministry for Education and Research (Competence Network Multiple Sclerosis) (to FP).
Contributor Information
JL Bennett, Departments of Neurology and Ophthalmology, University of Colorado, Denver, Colorado, USA.
J de Seze, Neurology Service, University Hospital of Strasbourg, France.
M Lana-Peixoto, CIEM MS Research Center, University of Minas Gerais Medical School, Belo Horizonte Brazil.
J Palace, Department of Neurology, Oxford University Hospitals National Health Service Trust, Oxford, UK.
A Waldman, Division of Neurology, Department of Pediatrics, The Children’s Hospital of Philadelphia, University of Pennsylvania, USA.
S Schippling, Neuroimmunology and Multiple Sclerosis Research Section, Department of Neurology, University Hospital Zürich, Switzerland.
S Tenembaum, Department of Neurology, National Pediatric Hospital Dr Juan P. Garrahan, Buenos Aires, Argentina.
B Banwell, Division of Neurology, Department of Pediatrics, The Children’s Hospital of Philadelphia, University of Pennsylvania, USA.
B Greenberg, Departments of Neurology & Neurotherapeutics, University of Texas Southwestern Medical Center at Dallas, Texas, USA.
M Levy, Department of Neurology, Johns Hopkins University, Baltimore, Maryland USA.
K Fujihara, Department of Multiple Sclerosis Therapeutics, Tohoku University Graduate School of Medicine, Sendai, Japan.
KH Chan, University Department of Medicine, LKS Faculty of Medicine, The University of Hong Kong, Hong Kong.
HJ Kim, Research Institute and Hospital of National Cancer Center Goyang Republic of Korea.
N Asgari, Institute of Molecular Medicine, University of Southern Denmark, and Department of Neurology, Vejle Hospital, Odense, Denmark.
DK Sato, Department of Neurology, Tohoku University School of Medicine, Sendai, Japan.
A Saiz, Center of Neuroimmunology, Service of Neurology, Hospital Clinic and Institute of Biomedical Research August Pi Sunyer, Barcelona, Spain.
J Wuerfel, NeuroCure Clinical Research Center, Charité – Universitätsmedizin Berlin, and Institute of Interventional and Diagnostic Neuroradiology, University Medicine Göttingen, Germany.
H Zimmermann, NeuroCure Clinical Research Center, Charité – Universitätsmedizin Berlin, Germany.
A Green, Multiple Sclerosis Center, UCSF Department of Neurology and Neuro-ophthalmology Service, UCSF Department of Ophthalmology, San Francisco, USA.
P Villoslada, Center of Neuroimmunology, Service of Neurology, Hospital Clinic and Institute of Biomedical Research August Pi Sunyer, Barcelona, Spain.
F Paul, NeuroCure Clinical Research and Department of Neurology, Charité – Universitätsmedizin Berlin and Experimental and Clinical Research Center, Charité – Universitätsmedizin Berlin and Max-Delbrück-Center for Molecular Medicine, Berlin, Germany.
References
- 1. Wingerchuk DM, Hogancamp WF, O’Brien PC, et al. The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology 1999; 53: 1107–1114. [DOI] [PubMed] [Google Scholar]
- 2. Lennon VA, Kryzer TJ, Pittock SJ, et al. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 2005; 202: 473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: Distinction from multiple sclerosis. Lancet 2004; 364: 2106–2112. [DOI] [PubMed] [Google Scholar]
- 4. Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol 2007; 6: 805–815. [DOI] [PubMed] [Google Scholar]
- 5. Papadopoulos MC, Verkman A. Aquaporin 4 and neuromyelitis optica. Lancet Neurol 2012; 11: 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tzartos JS, Stergiou C, Kilidireas K, et al. Anti-aquaporin-1 autoantibodies in patients with neuromyelitis optica spectrum disorders. PLoS One 2013; 8: e74773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kitley J, Leite MI, Nakashima I, et al. Prognostic factors and disease course in aquaporin-4 antibody-positive patients with neuromyelitis optica spectrum disorder from the United Kingdom and Japan. Brain 2012; 135: 1834–1849. [DOI] [PubMed] [Google Scholar]
- 8. Sato DK, Callegaro D, Lana-Peixoto MA, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology 2014; 82: 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frohman EM, Fujimoto JG, Frohman TC, et al. Optical coherence tomography: A window into the mechanisms of multiple sclerosis. Nat Clin Pract Neurol 2008; 4: 664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bertuzzi F, Suzani M, Tagliabue E, et al. Diagnostic validity of optic disc and retinal nerve fiber layer evaluations in detecting structural changes after optic neuritis. Ophthalmology 2010; 117: 1256–1264.e1. [DOI] [PubMed] [Google Scholar]
- 11. Zaveri MS, Conger A, Salter A, et al. Retinal imaging by laser polarimetry and optical coherence tomography evidence of axonal degeneration in multiple sclerosis. Arch Neurol 2008; 65: 924–928. [DOI] [PubMed] [Google Scholar]
- 12. Frohman E, Costello F, Zivadinov R, et al. Optical coherence tomography in multiple sclerosis. Lancet Neurol 2006; 5: 853–863. [DOI] [PubMed] [Google Scholar]
- 13. Saidha S, Syc SB, Durbin MK, et al. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult Scler 2011; 17: 1449–1463. [DOI] [PubMed] [Google Scholar]
- 14. Chen TC, Zeng A, Sun W, et al. Spectral domain optical coherence tomography and glaucoma. Int Ophthalmol Clin 2008; 48: 29–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bock M, Brandt AU, Dörr J, et al. Time domain and spectral domain optical coherence tomography in multiple sclerosis: A comparative cross-sectional study. Mult Scler 2010; 16: 893–896. [DOI] [PubMed] [Google Scholar]
- 16. Kallenbach K, Frederiksen J. Optical coherence tomography in optic neuritis and multiple sclerosis: A review. Eur J Neurol 2007; 14: 841–849. [DOI] [PubMed] [Google Scholar]
- 17. Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, et al. Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology 2007; 68: 1488–1494. [DOI] [PubMed] [Google Scholar]
- 18. Oberwahrenbrock T, Schippling S, Ringelstein M, et al. Retinal damage in multiple sclerosis disease subtypes measured by high-resolution optical coherence tomography. Mult Scler Int 2012; 2012: 530305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oberwahrenbrock T, Ringelstein M, Jentschke S, et al. Retinal ganglion cell and inner plexiform layer thinning in clinically isolated syndrome. Mult Scler 2013; 19: 1887–1895. [DOI] [PubMed] [Google Scholar]
- 20. Frohman EM, Frohman TC, Zee DS, et al. The neuro-ophthalmology of multiple sclerosis. Lancet Neurol 2005; 4: 111–121. [DOI] [PubMed] [Google Scholar]
- 21. Tsoi VL, Hill KE, Carlson NG, et al. Immunohistochemical evidence of inducible nitric oxide synthase and nitrotyrosine in a case of clinically isolated optic neuritis. J Neuroophthalmol 2006; 26: 87–94. [DOI] [PubMed] [Google Scholar]
- 22. Matiello M, Lennon VA, Jacob A, et al. NMO-IgG predicts the outcome of recurrent optic neuritis. Neurology 2008; 70: 2197–2200. [DOI] [PubMed] [Google Scholar]
- 23. Roemer SF, Parisi JE, Lennon VA, et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain 2007; 130: 1194–1205. [DOI] [PubMed] [Google Scholar]
- 24. Asavapanumas N, Ratelade J, Papadopoulos MC, et al. Experimental mouse model of optic neuritis with inflammatory demyelination produced by passive transfer of neuromyelitis optica-immunoglobulin G. J Neuroinflammation 2014; 11: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hofman P, Hoyng P, vanderWerf F, et al. Lack of blood-brain barrier properties in microvessels of the prelaminar optic nerve head. Invest Ophthalmol Vis Sci 2001; 42: 895–901. [PubMed] [Google Scholar]
- 26. Liu LY, Zheng H, Xiao HL, et al. Comparison of blood-nerve barrier disruption and matrix metalloprotease-9 expression in injured central and peripheral nerves in mice. Neurosci Lett 2008; 434: 155–159. [DOI] [PubMed] [Google Scholar]
- 27. Matiello M, Schaefer-Klein J, Sun D, et al. Aquaporin 4 expression and tissue susceptibility to neuromyelitis optica. JAMA Neurol 2013; 70: 1118–1125. [DOI] [PubMed] [Google Scholar]
- 28. Merle H, Olindo S, Bonnan M, et al. Natural history of the visual impairment of relapsing neuromyelitis optica. Ophthalmology 2007; 114: 810–815.e2. [DOI] [PubMed] [Google Scholar]
- 29. Kitley JL, Leite M, Matthews LE, et al. Use of mitoxantrone in neuromyelitis optica. Arch Neurol 2011; 68: 1086–1087. [DOI] [PubMed] [Google Scholar]
- 30. Fernandes DB, Ramos R de IP, Falcochio C, et al. Comparison of visual acuity and automated perimetry findings in patients with neuromyelitis optica or multiple sclerosis after single or multiple attacks of optic neuritis. J Neuroophthalmol 2012; 32: 102–106. [DOI] [PubMed] [Google Scholar]
- 31. Merle H, Olindo S, Jeannin S, et al. Visual field characteristics in neuromyelitis optica in absence of and after one episode of optic neuritis. Clin Ophthalmol 2013; 7: 1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pfueller CF, Paul F. Imaging the visual pathway in neuromyelitis optica. Mult Scler Int 2011; 2011: 869814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khanna S, Sharma A, Huecker J, et al. Magnetic resonance imaging of optic neuritis in patients with neuromyelitis optica versus multiple sclerosis. J Neuroophthalmol 2012; 32: 216–220. [DOI] [PubMed] [Google Scholar]
- 34. Storoni M, Davagnanam I, Radon M, et al. Distinguishing optic neuritis in neuromyelitis optica spectrum disease from multiple sclerosis: A novel magnetic resonance imaging scoring system. J Neuroophthalmol 2013; 33: 123–127. [DOI] [PubMed] [Google Scholar]
- 35. Parisi V, Manni G, Spadaro M, et al. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci 1999; 40: 2520–2527. [PubMed] [Google Scholar]
- 36. Petzold A, de Boer JF, Schippling S, et al. Optical coherence tomography in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol 2010; 9: 921–932. [DOI] [PubMed] [Google Scholar]
- 37. Fisher JB, Jacobs DA, Markowitz CE, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology 2006; 113: 324–332. [DOI] [PubMed] [Google Scholar]
- 38. Henderson AP, Trip SA, Schlottmann PG, et al. A preliminary longitudinal study of the retinal nerve fiber layer in progressive multiple sclerosis. J Neurol 2010; 257: 1083–1091. [DOI] [PubMed] [Google Scholar]
- 39. Talman LS, Bisker ER, Sackel DJ, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol 2010; 67: 749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saidha S, Syc SB, Ibrahim MA, et al. Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain 2011; 134 (Pt 2): 518–533. [DOI] [PubMed] [Google Scholar]
- 41. Levin MH, Bennett JL, Verkman AS. Optic neuritis in neuromyelitis optica. Prog Retin Eye Res 2013; 36: 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ratchford JN, Quigg ME, Conger A, et al. Optical coherence tomography helps differentiate neuromyelitis optica and MS optic neuropathies. Neurology 2009; 73: 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Naismith RT, Tutlam NT, Xu J, et al. Optical coherence tomography differs in neuromyelitis optica compared with multiple sclerosis. Neurology 2009; 72: 1077–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Green AJ, Cree BA. Distinctive retinal nerve fibre layer and vascular changes in neuromyelitis optica following optic neuritis. J Neurol Neurosurg Psychiatry 2009; 80: 1002–1005. [DOI] [PubMed] [Google Scholar]
- 45. Nakamura M, Nakazawa T, Doi H, et al. Early high-dose intravenous methylprednisolone is effective in preserving retinal nerve fiber layer thickness in patients with neuromyelitis optica. Graefes Arch Clin Exp Ophthalmol 2010; 248: 1777–1785. [DOI] [PubMed] [Google Scholar]
- 46. de Seze J, Blanc F, Jeanjean L, et al. Optical coherence tomography in neuromyelitis optica. Arch Neurol 2008; 65: 920–923. [DOI] [PubMed] [Google Scholar]
- 47. Merle H, Olindo S, Donnio A, et al. Retinal peripapillary nerve fiber layer thickness in neuromyelitis optica. Invest Ophthalmol Vis Sci 2008; 49: 4412–4417. [DOI] [PubMed] [Google Scholar]
- 48. Lange AP, Sadjadi R, Zhu F, et al. Spectral-domain optical coherence tomography of retinal nerve fiber layer thickness in NMO patients. J Neuroophthalmol 2013; 33: 213–219. [DOI] [PubMed] [Google Scholar]
- 49. Gelfand JM, Cree BA, Nolan R, et al. Microcystic inner nuclear layer abnormalities and neuromyelitis optica. JAMA Neurol 2013; 70: 629–633. [DOI] [PubMed] [Google Scholar]
- 50. Schneider E, Zimmermann H, Oberwahrenbrock T, et al. Optical coherence tomography reveals distinct patterns of retinal damage in neuromyelitis optica and multiple sclerosis. PloS One 2013; 8: e66151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fernandes DB, Raza AS, Nogueira RG, et al. Evaluation of inner retinal layers in patients with multiple sclerosis or neuromyelitis optica using optical coherence tomography. Ophthalmology 2013; 120: 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bouyon M, Collongues N, Zéphir H, et al. Longitudinal follow-up of vision in a neuromyelitis optica cohort. Mult Scler 2013; 19: 1320–1322. [DOI] [PubMed] [Google Scholar]
- 53. Bock M, Brandt AU, Dörr J, et al. Patterns of retinal nerve fiber layer loss in multiple sclerosis patients with or without optic neuritis and glaucoma patients. Clin Neurol Neurosurg 2010; 112: 647–652. [DOI] [PubMed] [Google Scholar]
- 54. Monteiro MLR, Fernandes DB, Apóstolos-Pereira SL, et al. Quantification of retinal neural loss in patients with neuromyelitis optica and multiple sclerosis with or without optic neuritis using Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci 2012; 53: 3959–3966. [DOI] [PubMed] [Google Scholar]
- 55. Syc SB, Saidha S, Newsome SD, et al. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain 2012; 135: 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sotirchos ES, Saidha S, Byraiah G, et al. In vivo identification of morphologic retinal abnormalities in neuromyelitis optica. Neurology 2013; 80: 1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Park KA, Kim J, Oh SY. Analysis of spectral domain optical coherence tomography measurements in optic neuritis: Differences in neuromyelitis optica, multiple sclerosis, isolated optic neuritis and normal healthy controls. Acta Ophthalmol 2014; 92: e57–e65. [DOI] [PubMed] [Google Scholar]
- 58. Kaufhold F, Zimmermann H, Schneider E, et al. Optic neuritis is associated with inner nuclear layer thickening and microcystic macular edema independently of multiple sclerosis. PLoS One 2013; 8: e71145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ringelstein M, Kleiter I, Ayzenberg I, et al. Visual evoked potentials in neuromyelitis optica and its spectrum disorders. Mult Scler 2014; 20: 617–620. [DOI] [PubMed] [Google Scholar]
- 60. Gelfand JM, Nolan R, Schwartz DM, et al. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain J Neurol 2012; 135 (Pt 6): 1786–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Saidha S, Sotirchos ES, Ibrahim MA, et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: A retrospective study. Lancet Neurol 2012; 11: 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brandt AU, Oberwahrenbrock T, Kadas EM, et al. Dynamic formation of macular microcysts independent of vitreous traction changes. Neurology 2014; 83: 73–77. [DOI] [PubMed] [Google Scholar]
- 63. George J, Kitley J, Leite M, et al. Microcystic inner nuclear layer pathology in aquaporin-4 antibody-positive patients. Poster In: 29th Congress of the European Committee for Research and Treatment in Multiple Sclerosis (ECTRIMS) Copenhagen, Denmark, 2–5 October 2013. [Google Scholar]
- 64. Albrecht P, Fröhlich R, Hartung HP, et al. Optical coherence tomography measures axonal loss in multiple sclerosis independently of optic neuritis. J Neurol 2007; 254: 1595–1596. [DOI] [PubMed] [Google Scholar]
- 65. Toledo J, Sepulcre J, Salinas-Alaman A, et al. Retinal nerve fiber layer atrophy is associated with physical and cognitive disability in multiple sclerosis. Mult Scler 2008; 14: 906–912. [DOI] [PubMed] [Google Scholar]
- 66. McKeon A, Lennon VA, Lotze T, et al. CNS aquaporin-4 autoimmunity in children. Neurology 2008; 71: 93–100. [DOI] [PubMed] [Google Scholar]
- 67. Li Q, Timmers AM, Hunter K, et al. Noninvasive imaging by optical coherence tomography to monitor retinal degeneration in the mouse. Invest Ophthalmol Vis Sci 2001; 42: 2981–2989. [PubMed] [Google Scholar]
- 68. Horio N, Kachi S, Hori K, et al. Progressive change of optical coherence tomography scans in retinal degeneration slow mice. Arch Ophthalmol 2001; 119: 1329–1332. [DOI] [PubMed] [Google Scholar]
- 69. Grieve K, Paques M, Dubois A, et al. Ocular tissue imaging using ultrahigh-resolution, full-field optical coherence tomography. Invest Ophthalmol Vis Sci 2004; 45: 4126–4131. [DOI] [PubMed] [Google Scholar]
- 70. Zimmermann H, Freing A, Kaufhold F, et al. Optic neuritis interferes with optical coherence tomography and magnetic resonance imaging correlations. Mult Scler 2013; 19: 443–450. [DOI] [PubMed] [Google Scholar]
- 71. Dörr J, Wernecke KD, Bock M, et al. Association of retinal and macular damage with brain atrophy in multiple sclerosis. PloS One 2011; 6: e18132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Young KL, Brandt AU, Petzold A, et al. Loss of retinal nerve fibre layer axons indicates white but not grey matter damage in early multiple sclerosis. Eur J Neurol 2013; 20: 803–811. [DOI] [PubMed] [Google Scholar]
- 73. von Glehn F, Jarius S, Cavalcanti Lira RP, et al. Structural brain abnormalities are related to retinal nerve fiber layer thinning and disease duration in neuromyelitis optica spectrum disorders. Mult Scler 2014; 20: 1189–1197. [DOI] [PubMed] [Google Scholar]
- 74. Kim SH, Kim W, Li XF, et al. Does interferon beta treatment exacerbate neuromyelitis optica spectrum disorder? Mult Scler 2012; 18: 1480–1483. [DOI] [PubMed] [Google Scholar]
- 75. Palace J, Leite M, Nairne A, et al. Interferon beta treatment in neuromyelitis optica: Increase in relapses and aquaporin 4 antibody titers. Arch Neurol 2010; 67: 1016–1017. [DOI] [PubMed] [Google Scholar]
- 76. Mealy MA, Wingerchuk DM, Palace J, et al. Comparison of relapse and treatment failure rates among patients with neuromyelitis optica: Multicenter study of treatment efficacy. JAMA Neurol 2014; 71: 324–330. [DOI] [PubMed] [Google Scholar]
- 77. Kleiter I, Hellwig K, Berthele A, et al. Failure of natalizumab to prevent relapses in neuromyelitis optica. Arch Neurol 2012; 69: 239–245. [DOI] [PubMed] [Google Scholar]
- 78. Merle H, Olindo S, Jeannin S, et al. Treatment of optic neuritis by plasma exchange (add-on) in neuromyelitis optica. Arch Ophthalmol 2012; 130: 858–862. [DOI] [PubMed] [Google Scholar]
- 79. Khatri BO, Kramer J, Dukic M, et al. Maintenance plasma exchange therapy for steroid-refractory neuromyelitis optica. J Clin Apheresis 2012; 27: 183–192. [DOI] [PubMed] [Google Scholar]
- 80. Sühs KW, Hein K, Sättler MB, et al. A randomized, double-blind, phase 2 study of erythropoietin in optic neuritis. Ann Neurol 2012; 72: 199–210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.