Abstract
Patients suffering from aortic arch aneurysms continue to encounter few treatment options. Because of co-morbidities, most are deemed to not be open surgical candidates. The two cases presented here demonstrate a novel endovascular approach in the care of an arch aneurysm complicated by dissection. Even though final graft configurations differed slightly between the two cases, all three great vessels were successfully de-branched through the combination of standard endovascular aneurysm repair techniques and modifications to off-the-shelf devices. Aortic flow was compartmentalized in the ascending aorta at or near the level of the sinotubular junction. This was done with a physician-assembled endografts. One of these lumens was dedicated to the descending aorta, while the other was further divided into three channels used to stent the great vessels. Completion angiography demonstrated patency in the arch, great vessels, and descending aorta. No endoleaks have been reported. Although data is limited, this approach appears promising.
Keywords: Aortic arch aneurysm, endovascular de-branch, thoracic manifold, endovascular aneurysm repair, great vessels, off-the-shelf devices
Introduction
Open repair of aortic arch aneurysms requires minimizing blood loss, cerebral perfusion or deep circulatory arrest, and relatively low-risk patients. An alternative endovascular approach for repairing the diseased aortic arch with a triple side-branch device was pioneered by Dr Inohoue.1 His innovative device was implanted through a trans-femoral approach. Unfortunately, one of the two patients treated with this device experienced significant cerebrovascular accident. More recently, Dr Abraham also used a trans-femoral delivered multi-branched stent graft to treat six patients. Again, he reported two incidences of stroke and cerebrovascular accident.2
Soon after Dr Inohoue’s innovative device was reported, Dr Chuter began working on a device that could be implanted through the innominate artery. After several iterations of improving his prototype, he arrived at his optimal solution. After a carotid–carotid and carotid–subclavian bypass, the device would be delivered from the innominate artery and landed in the ascending arch. One branch would stent the innominate while the other would be extended to the descending aorta. The device was implanted in one patient with good results.3
Here, we would like to propose a modular stent graft system that can be delivered from the subclavian or axillary artery and that can stent all three great vessels and the descending aorta. The device can theoretically be used to treat virtually any aneurysmal or dissected anatomy while stenting all three great vessels and maintaining continuous perfusion to the brain as well as the descending aorta.
Case report
We present two patients who were denied open repair as an option due to comorbidities and extensive dissection. The dissection in these two patients extended from the ascending arch to the iliac arteries. The dissection also extended up into all three great vessels. This degree of dissection may complicate the process of creating open bypasses, because it can be challenging to determine true from false lumen when creating the anastomoses. An endovascular approach allows for continuous perfusion and the use of angiography and ultrasound to ensure access in true lumen of the great vessels prior to stenting. In both of the cases, an endovascular technique using modified off-the-shelf devices was used after appropriate patient consent for physician-assembled off-label device use. Retrospective chart review was performed to collect case details with IRB approval.
Patient 1
A 47-year-old female with a history of systolic heart failure, hypertension, and multiple sclerosis was seen by our services following the denial for repair of a thoracoabdominal aortic aneurysm (TAAA) secondary to aortic dissection at several other medical centers. Further imaging of the chest demonstrated aneurysmal disease throughout the arch and into the three great vessels. The dissection actually extended from the ascending aorta through the arch down into the left iliac. All visceral vessels and iliacs were fed by true lumen. A max diameter of 58 mm was noted just distal to the left subclavian artery with progressive back pain.
To repair this lesion, two PAEGs were created, a double barrel (Figure 1(a)) and a manifold (Figure 1(b)). The thoracic double barrel was created by shortening two 24-mm Valiants to 50 mm in length and sewing them together on the proximal end with a common seam. Then they were sewn in an end-to-end anastomosis to a 46-mm Valiant, which was also shortened to 50 mm. The manifold was created by first sewing two 10-mm diameter and 30-mm long Viabahns together with a common seam on the proximal end. Then a 25 × 16 × 124 mm3 Endurant had its ipsilateral limb shortened to be 10 mm shorter than the contralateral limb. The double 10-mm Viabahns were sewed to the ipsilateral limb in an end-to-end anastomosis. The two PAEGs were then re-sheathed for deployment.
Figure 1.
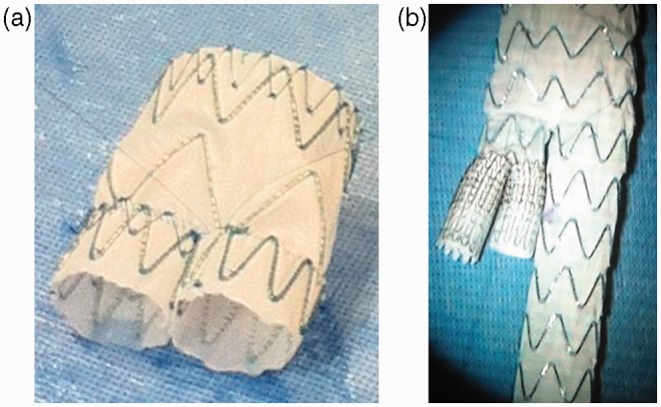
Physician-assembled endografts used to treat patient 1: (a) thoracic double barrel and (b) thoracic manifold.
In both of the above constructs, the grafts were fully constrained by wrapping with 20 gage surgical wire. To resheath the constructs, the wire was unwound as the outer sheath was re-advanced over the newly constructed grafts (Captivia Delivery System, Endurant Delivery System, Medtronic, Minneapolis, MN).
Right subclavian, left carotid, left brachial, and bilateral femoral accesses were performed. Ten millimeter conduits were attached to the right subclavian and one femoral artery. A Premium 6500 unipolar temporary myocardial pacing lead (Medtronic Inc., Minneapolis, MN) for overdrive pacing was placed in the right ventricle from a right femoral venous access. After access and angiograms were performed, the thoracic double barrel stent graft was positioned and deployed at the level of the sinotubular junction from the right subclavian conduit (Figure 2(a)). During placement, respirations were held and overdrive pacing was used. The thoracic manifold was then positioned and deployed in one of the two 24-mm limbs of the thoracic double barrel via the subclavian conduit (Figure 2(b)). Once again overdrive pacing was used during deployment.
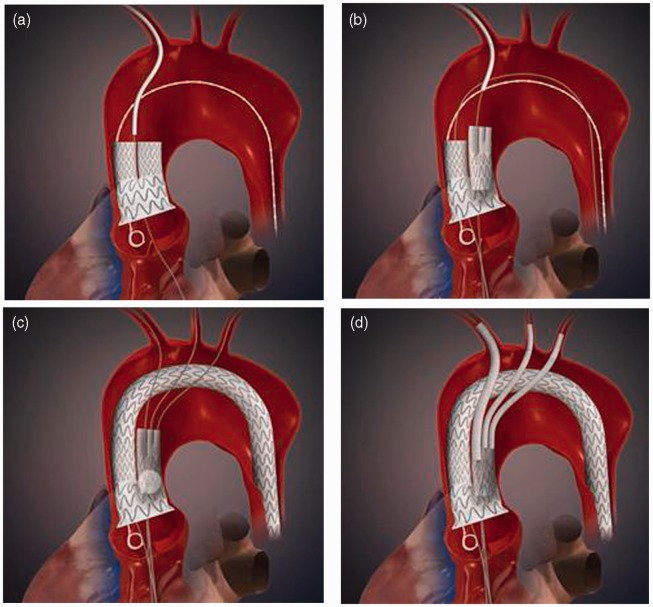
Figure 2.
Animation describing the sequential steps of implanting the graft system in patient 1: (a) the double barrel is placed through the right subclavian access; (b) the manifold is placed in the ipsilateral limb of the double barrel through the right subclavian access; (c) the transverse limb is brought up and over the arch and placed in the contralateral limb of the double barrel after wire access is secured in the left subclavian and left common carotid; and (d) the three great vessels are stented.
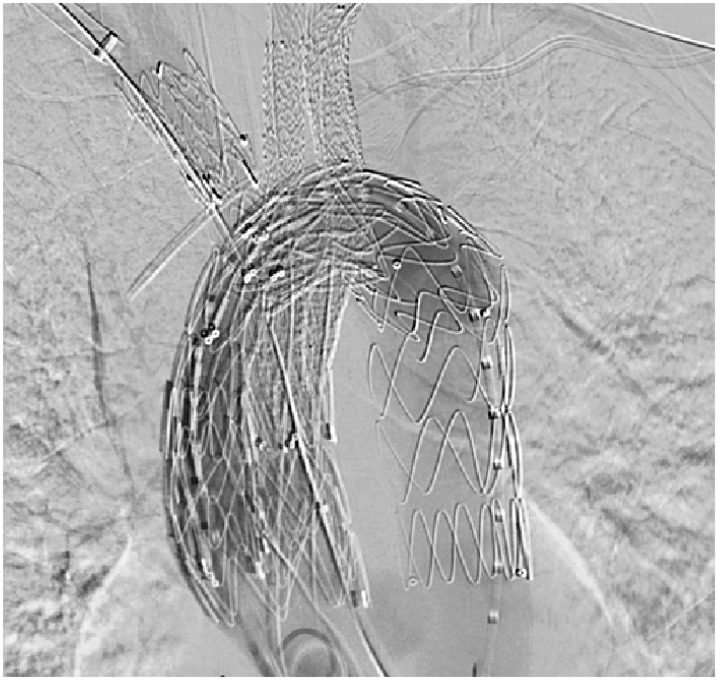
Figure 3.
Completion angiogram showing stents into each of the three great vessels.
Before we brought up the arch and descending thoracic graft, we established retrograde wire access in the other two limbs of the thoracic manifold. It is to be noted that access to the innominate was via our delivery wire from the right subclavian artery. The arch and descending thoracic grafts were brought up-and-over the arch and into the open 24-mm limb of the thoracic double barrel from a femoral conduit. The graft was positioned and deployed, effectively resulting in exclusion of the aneurysmal sac from half of the aortic blood flow (Figure 2(c)).
Next, we de-branched the left common carotid artery and left subclavian artery with iCast balloon-expandable covered stents (Figure 2(d)). These were both lined with Protégé self-expanding stents (eV3; Plymouth, MN). Lastly, the innominate was de-branched. Using a 16 × 20 × 82 mm3 Endurant limb which was shortened to 55 mm, the innominate was stented. A 28-mm Gore cuff was then used to extend seal into the innominate.
Completion angiography demonstrated a patent aortic arch and patent great vessels. Flow was retained in the innominate, right- and left-common carotids, and bilateral subclavian arteries (Figure 3). All contact points were balloon angioplastied. The procedure required 147 cc of contrast and 105 min of fluoroscopy. For an unknown reason, the patient experienced significant pulmonary issues and required two additional days on the ventilator. She was discharged to home on post-op day 13, and has been followed at 1 month, 6 months, and 12 months. Post-operative imaging showed no endoleaks (Figure 4).
Figure 4.
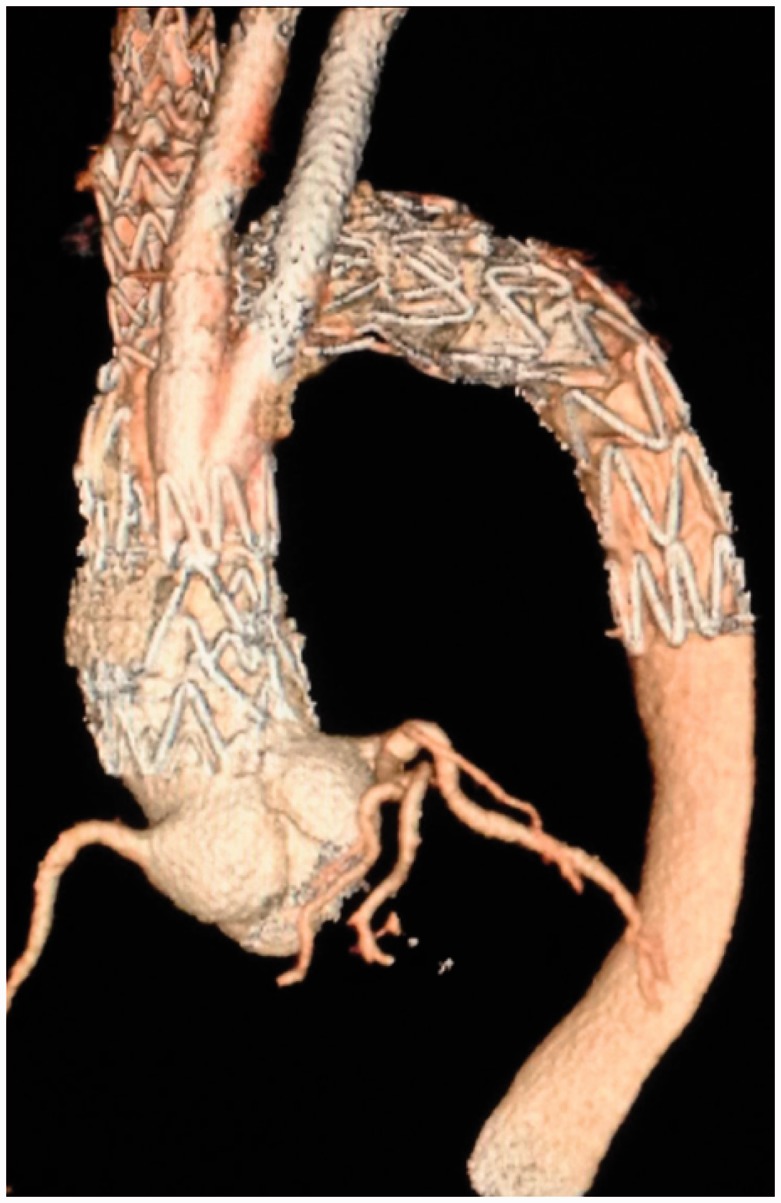
3D reconstruction of contrasted CT scans of Patient 1 from the 1 month follow-up.
Figure 5.
Physician-assembled endografts used to treat patient 2.
Patient 2
A 70-year-old male was referred to our service to evaluate a large transverse and descending aneurysm. This individual had previously undergone open surgical repair of the ascending aorta secondary to a Type A dissection four years prior. Imaging revealed aneurysmal disease throughout the arch with dissection extending into the great vessels and descending aorta to the iliacs. Patient 2’s left renal artery came off false lumen. When we did split perfusion angiography, we found that the left kidney was atrophic and non-functional.
He was denied open repair, and again because of the extent of his dissection in the great vessels, we avoided hybrid repair.
The patient underwent a similar procedure as described for Patient 1. The main body graft, as used on Patient 1, was determined to be too long for Patient 2 due to his shorter ascending arch. It was decided that joining the two grafts into a unitary configuration would be a better fit (Figure 5). The unitary manifold was created by first shortening the ipsilateral limb of a standard 28 × 16 × 124 mm3 Endurant main body stent graft. This limb was shortened to the crotch of the Endurant main body. Attached to the shortened ipsilateral limb were two 10-mm Viabahn covered stent grafts which were shortened to 30 mm. The contralateral limb was trimmed to 35 mm. A 24 × 24 × 82 mm3 Endurant iliac limb extension was then sutured to the modified Endurant main body forming a common seam; this limb was also trimmed to 35 mm. A 46 × 46 × 100 mm3 Valiant stent graft was deployed and trimmed to 30 mm. A running lock-stitch suture was used to connect the Valiant and modified Endurant thereby forming the unitary manifold (Figure 5). The entire length of the manifold graft was 60 mm. The thoracic manifold was then re-constrained into its original sheath.
Figure 6.
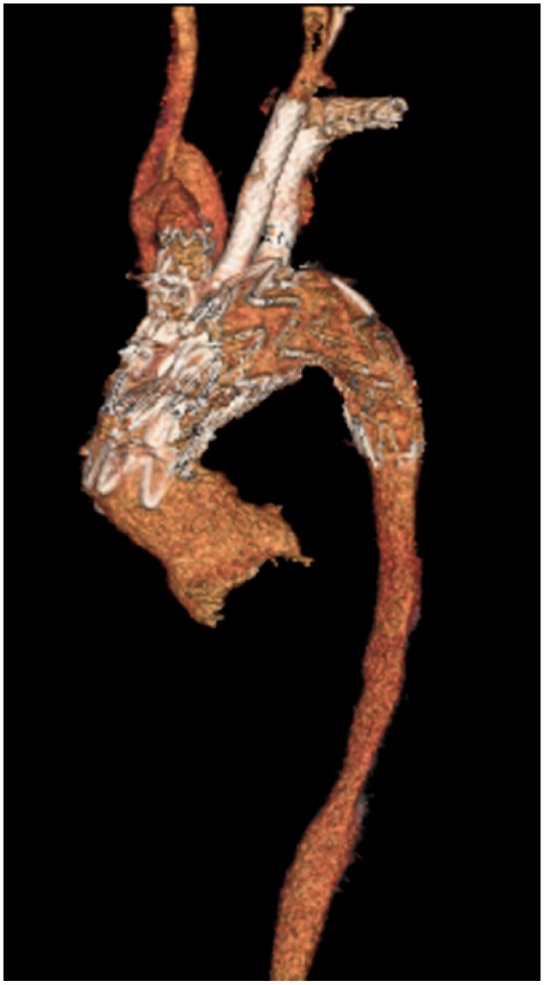
3D reconstructions of contrasted CT scan of Patient 2 from the 1 month follow-up.
The procedure used to treat Patient 2 was similar to the procedure used to treat Patient 1, with the main exception being the main body graft configuration. The procedure for Patient 2 required 61 cc of contrast and 83.1 min of fluoroscopy. Patient 2 had an uneventful postoperative period and was discharged to home on postoperative day 4. There are no noted endoleaks seen on postoperative imaging (Figure 6). The patient has been seen for his 1-month and 6-month follow-up since surgery.
Discussion
The benefit of snorkels and chimneys is that they have parallel take offs and gently sweeping centerlines in their bridging stents. We feel this lends itself to smooth laminar blood flow and long-term patency. Unfortunately, snorkels and chimneys do not provide for circumferential seal or fixation. Hence they may be prone to endoleaks. In the aortic arch, circulatory motion combined with high flow rates may increase the risk of endoleaks. We believe our proposed system lends itself well to long-term bridging stent patency while also providing for circumferential seal and fixation. What’s more is that the flow of chimney stents are often taken from the outer edge of aortic flow, which is the slowest flow of the aorta which has a parabolic velocity profile. Our bridging stents obtain more of their flow from central portions of the aorta, which provides for higher flow rates and greater perfusion of the great vessels.
As mentioned above, aortic arch main body grafts that are delivered from a trans-femoral location may carry an increased risk of peri-operative stroke. Dr Abraham elaborates more on the possible reason for the increased risk in his manuscript. As the relatively stiff delivery catheter and branched graft construct pass through significant aortic thrombus, they can mobilize emboli. As these stiff devices are driven up-and-over the arch, these mobilized emboli can shower the great vessels leading to stroke. Because of this risk, we decided to deliver our main body grafts through the subclavian artery. We feel this approach pioneered by Dr Chuter is preferable. The straight shot into the ascending arch from the subclavian and innominate also makes alignment of the main body graft in zone 0 more controllable.
By compartmentalizing flow at the level of the sinotubular junction, we were able to endovascularly de-branch all three great vessels of the arch while maintaining flow to the descending aorta throughout the procedure. Again the main body grafts are delivered through the right subclavian access, which allows for us to secure wire access in the innominate artery from the beginning. This is important, because it allows for surgical options in the event that we cannot secure wire access from the left common carotid or left subclavian artery. Once the innominate is stented, we always have the option to perform open debranch of the left common carotid and left subclavian arteries as needed. We are also very careful to establish and maintain wire access in all three great vessels before we deploy the arch graft which provides flow to the descending aorta. We feel this is important so that the arch graft does not jail the great vessels. Once the arch graft is positioned and deployed, we now make the critical connections between the arch manifold and the native great vessels. In case where there is significant thrombus formation in the arch, stenting the great vessels prior to delivery of the arch and descending graft remains an option. This acts to protect the great vessels from embolic debris. We feel the ability to stent all three great vessels is an important next step in the evolution of endovascular arch repair. If only one of the great vessels is repaired, such as the innominate, and accompanied by concomitant bypasses, then the entire cerebrovascular system becomes vulnerable. If the health of that single vessel becomes compromised, the whole cerebrovascular system will be compromised. Stenting all three great vessels maintains the natural redundancy of the three great vessels.
In both of these cases the goal was to cover the entry tear. The descending thoracic aorta was covered down to the height of the mid-thoracic aorta, and the great vessels were excluded from the dissected arch aneurysm. Beings dissections tend to be a life-long issues, these patients will be monitored carefully for the rest of their lives. Thus far, neither patient has required significant reintervention. In the event it is determined they require reintervention, it will be important to take into account the multiple fenestrations in the membrane separating the true and false lumen as well as any lumbars feeding the false lumen.
Conclusion
The proposed procedure appears to be a suitable alternative to open and hybrid repair. It is less invasive and has the potential for a significant reduction in hospital stay. The final stent configuration and procedural design can accommodate many aneurysmal and patient anatomies while maintaining flow to the brain and periphery throughout the procedure. Furthermore, this technique offers the surgeon great flexibility to stage the intervention to best fit the patient’s tolerance. Even though this technique has only been performed on two patients, the results show promise. A larger sample size with prospective examination of patient outcomes and graft durability is a logical next step. The author is currently working toward obtaining a Sponsor-Investigator IDE.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Habara T, Ishii H, Fukuda K. Anesthetic management of total aortic arch reconstruction by transluminally placed Inoue endovascular branched stent graft. Masui 2006; 55(3): 353–357. [PubMed] [Google Scholar]
- 2.Neequaye S, Abraham CZ. Total endograft replacement of aortic arch. Ann Cardiothorac Surg 2013; 2(3): 362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chuter TA, Schneider DB, Reilly LM, et al. Modular branched stent graft for endovascular repair of aortic arch aneurysm and dissection. J Vasc Surg 2003; 38(4): 859–863. [DOI] [PubMed] [Google Scholar]